1. Introduction
Light olefins (including ethylene, propylene, butylene, and butadiene) are extensively used across a variety of industries. The global demand for light olefins has been rising steadily, driven by the rapid growth of polymer markets, packaging materials, and downstream chemical industries, making olefins among the most sought-after commodities worldwide [
1]. The volatility of crude oil markets, combined with mounting pressure to reduce carbon emissions, has created a strong motivation to seek alternative olefin production technologies [
2,
3].
Conventional production of light olefins relies on two primary routes: steam cracking and fluid catalytic cracking (FCC). Steam cracking involves the thermal decomposition of feedstocks like ethane in the presence of steam at elevated temperatures, while FCC uses a hot circulating catalyst to convert heavier hydrocarbon fractions into lighter products such as olefins. However, these conventional methods are highly energy-intensive and heavily reliant on fossil-derived feedstocks, exposing the olefin market to price volatility and environmental concerns associated with greenhouse gas emissions [
4,
5].
The catalytic dehydration of bioethanol to ethylene is an emerging alternative that has reached a moderate Technology Readiness Level (TRL 6–7), as demonstrated by industrial projects like Braskem’s bio-based ethylene facility. Although it offers potential for renewable feedstocks, the scalability of MTO for large-scale olefin production is limited by biomass availability and competition with fuel markets, restricting its applicability across various carbon sources.
The methanol-to-olefins (MTO) process has emerged as a promising alternative for producing ethylene and propylene. It operates at lower temperatures with higher selectivity toward light olefins and offers greater flexibility in adjusting the ethylene-to-propylene output ratio [
6]. MTO technology converts methanol derived from natural gas, coal, or biomass into these olefins [
7,
8]. Because methanol can be synthesized from such diverse carbon sources, MTO reduces the petrochemical industry’s reliance on oil-based feedstocks [
9,
10]. In comparison to the output of a traditional thermal cracking process, the hydrocarbon fractions released by an MTO reactor tend to be lighter. Furthermore, the abundance and low cost of coal and natural gas as methanol feedstocks enhance the economic appeal of MTO.
Catalyst selection is critical in the MTO process. SAPO-34, a silicoaluminophosphate molecular sieve with small pores, has demonstrated superior performance as an MTO catalyst, achieving high methanol conversion and strong selectivity for light olefins [
6,
11]. Early studies demonstrated that zeolites like ZSM-5 can catalyze methanol conversion to hydrocarbons [
12], but the discovery of SAPO-34 was a major breakthrough due to its superior selectivity for light olefins [
13,
14]. Metal modifications of SAPO-34 (e.g., Mn-SAPO-34) have shown improved long-term stability over the unmodified catalyst [
15]. However, SAPO-34 (like other zeolite catalysts) suffers from gradual deactivation due to coke deposition, as carbonaceous by-products accumulate in its micropores and hinder catalytic activity and selectivity, ultimately limiting reactor lifetimes and increasing operational costs [
16,
17]. To mitigate coking, industrial MTO processes often use circulating fluidized-bed reactors, which provide excellent heat transfer and allow continuous catalyst regeneration [
18,
19].
Developing an accurate kinetic model for the MTO reaction is challenging, as the underlying mechanism is still debated [
20]. Park and Forment [
21] developed a kinetic model for MTO over HZSM-5. Sedighi et al. [
11] proposed a kinetic scheme for SAPO-34 and found that higher catalyst contact time and temperature both increased light olefin yields. Zhuang et al. [
22] employed computational fluid dynamics to analyze a fixed-bed MTO reactor and found that coke deposition correlates with declining methanol conversion. In addition, advanced optimization techniques have been used to refine MTO kinetics; for example, Taheri Najafabadi et al. [
6] applied a genetic algorithm to tune kinetic parameters, achieving good agreement between experimental and predicted results.
Optimizing the energy-intensive cryogenic distillation sequence and integrating it with heat recovery networks (e.g., via Pinch analysis) is critical for improving the MTO process’s energy efficiency and environmental performance [
23]. Despite extensive literature on individual aspects of MTO, few studies provide a comprehensive, plant-wide design framework that combines reactor modeling, separation, and heat integration into a single, optimized process. To address this gap, a systematic process selection analysis was conducted, and its findings were used to guide the integrated MTO plant design presented in this study.
This work presents the detailed design, modeling, and optimization of an MTO process, including material and energy balances, equipment design, and sizing. The study integrates rigorous thermodynamic analysis using the Ideal Gas, Virial, and Peng-Robinson equations of state with process simulation and optimization techniques. A fast fluidized bed reactor configuration was adopted to sustain catalyst activity and minimize deactivation, while systematic design methods were applied to the separation and purification systems. Comprehensive heat integration (via Pinch analysis) and recycling strategies were implemented to enhance energy recovery, reduce utility consumption, and improve process sustainability. Finally, an economic evaluation covering equipment, raw material, and utility costs was performed to assess the plant’s technical and financial feasibility, establishing the proposed MTO configuration as both efficient and economically viable.
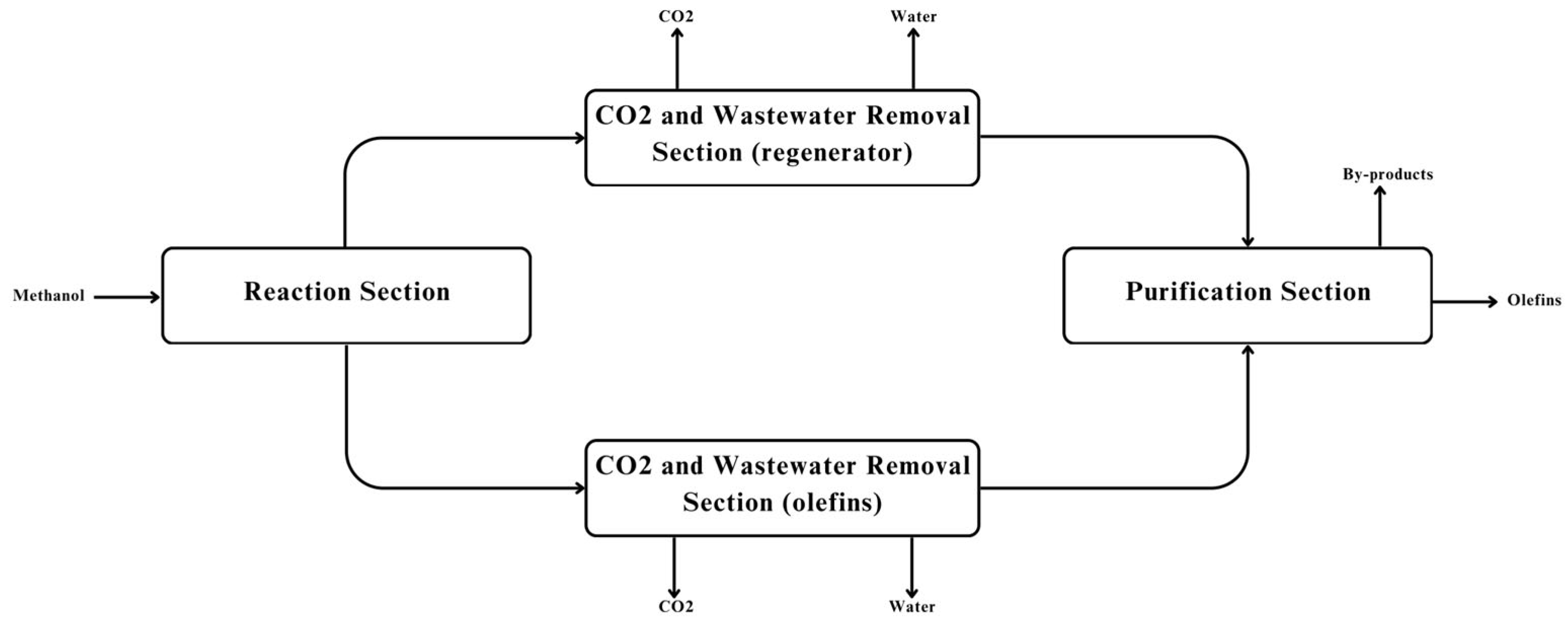
4. Process Integration
Sustainability in process industries entails meeting present needs without limiting future generations, with sustainable manufacturing serving as a key implementation by integrating economic efficiency, environmental responsibility, energy optimization, and operational safety. This approach minimizes environmental burdens while maintaining competitiveness through effective by-product management, efficient heating and cooling, and reduced hazardous waste. However, the MTO process is constrained by high utility demands from extensive external heating and cooling. To address this, pinch analysis was employed to optimize energy integration by identifying process streams, constructing temperature interval diagrams, and applying the cascade method to determine minimum utility requirements, culminating in a heat exchanger network that significantly reduces energy consumption and improves process efficiency.
4.1. Pinch Analysis and Heat Exchanger Network
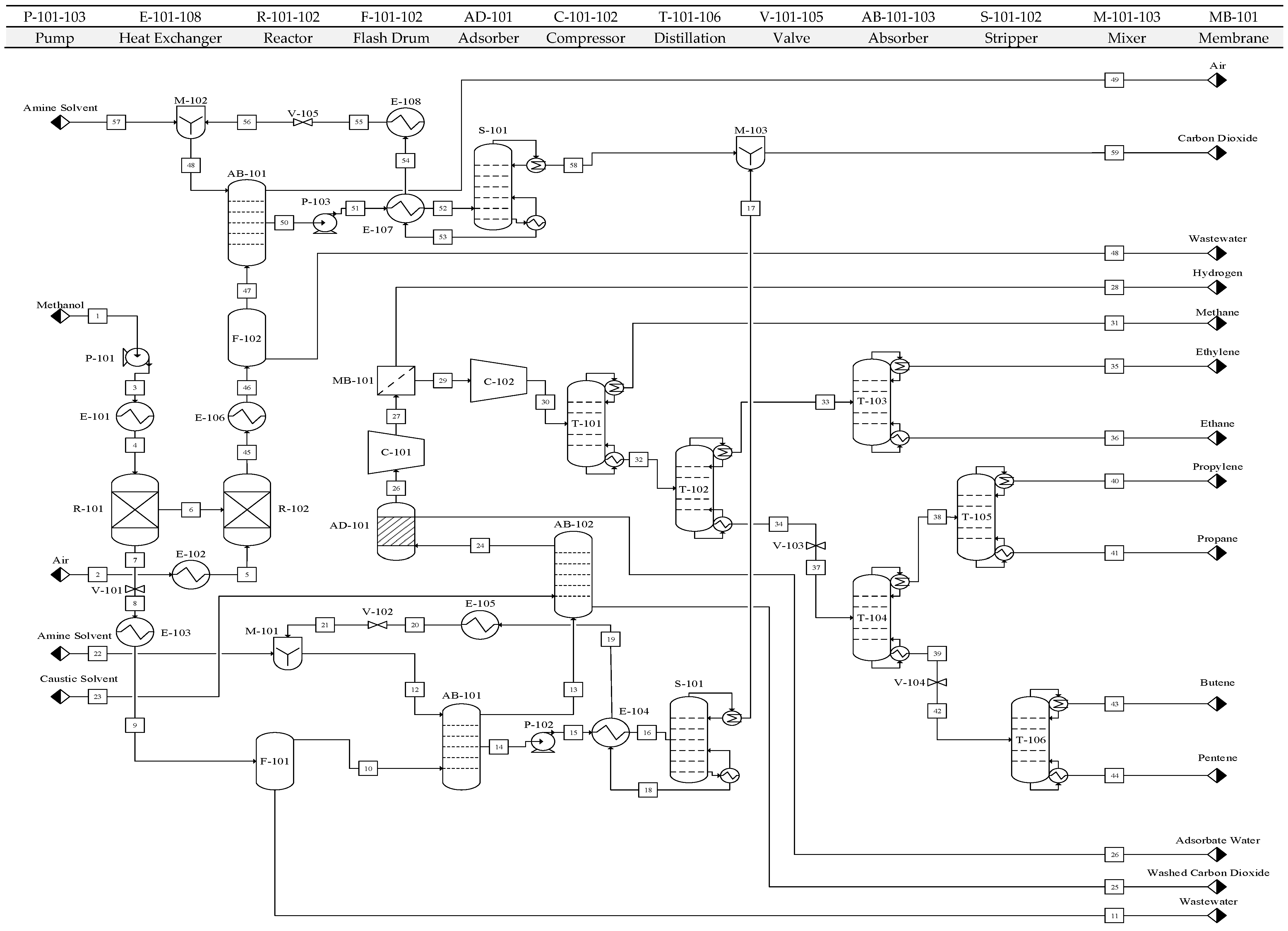
Hot and cold streams were identified, and their respective heat loads and average molar heat capacities were determined. Total heat duties, heat capacity flow rates, and supply and target temperatures for each stream are summarized in
Tables S3 and S4. The methanol feed (Stream 3) entering heat exchanger E-101 underwent into three heating stages—sensible, latent, and sensible (final)—and was divided into three segments to accurately represent its thermal behavior.
Hot and cold composite curves were superimposed to identify the pinch point, where the curves become tangent, representing the thermodynamic limit of heat recovery. At 107.12 °C, heat load reduction was insufficient because water boiled below methanol due to pressure reduction at valve V-101. To address this, the valve placement was modified, and Stream 7 was divided into two sub-segments with a valve inserted to prevent pentene liquefaction. This adjustment eliminated additional latent heat loads, requiring revision of the hot stream specifications, summarized in
Table S5.
The composite curves were generated by combining hot and cold stream cumulative heating loads over each temperature interval.
Figure S6 illustrates the revised composite curve after the modification. Pinch point at 501.85 °C.
The minimum temperature difference (ΔT
min) between hot and cold streams represents a trade-off between heat exchanger capital cost and utility operating cost, with optimal values in petrochemical processes typically ranging from 10–20 °C. Using the Aspen Energy Analyzer, 20 scenarios were evaluated to identify the optimum ΔT corresponding to the minimum annualized cost.
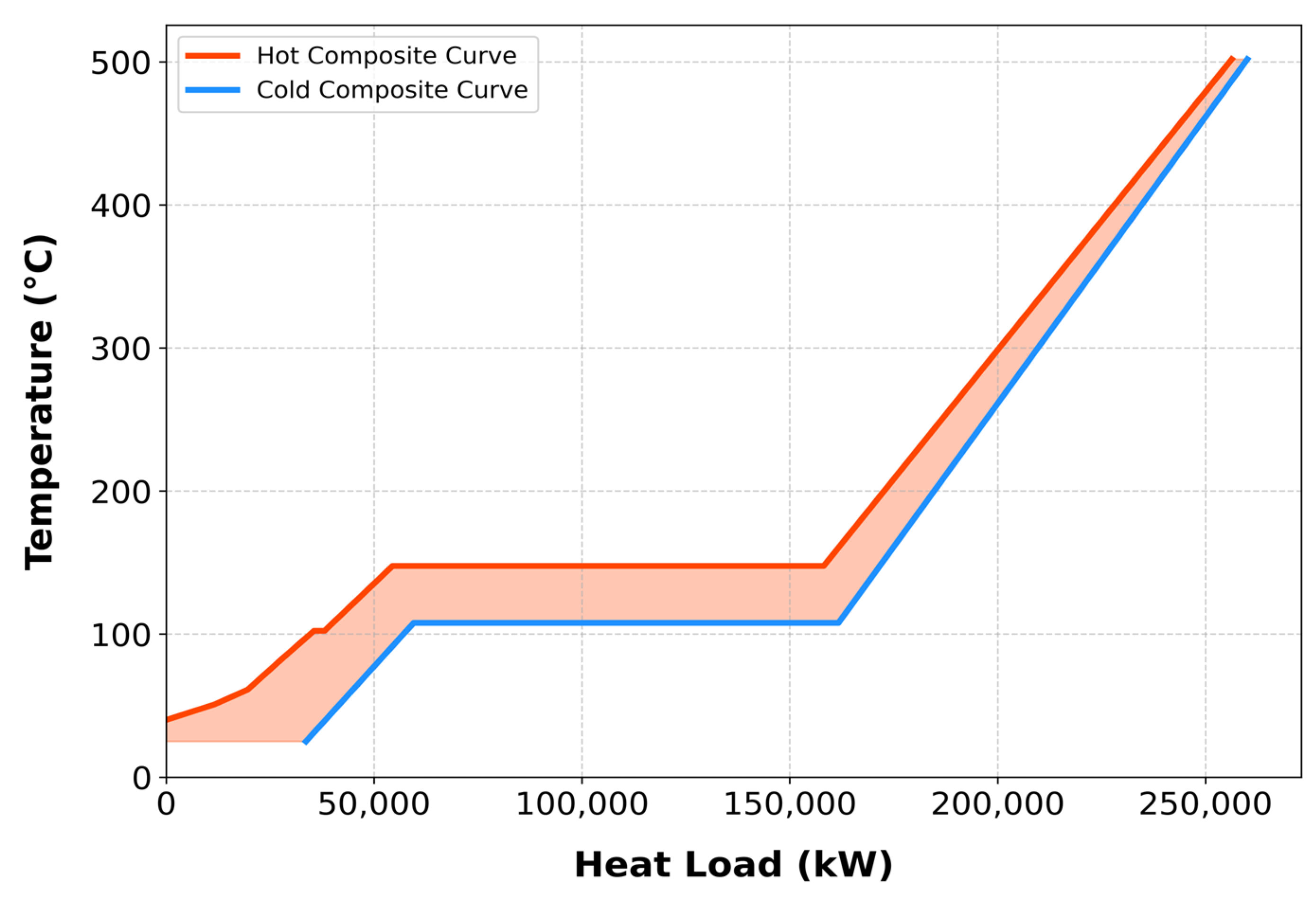
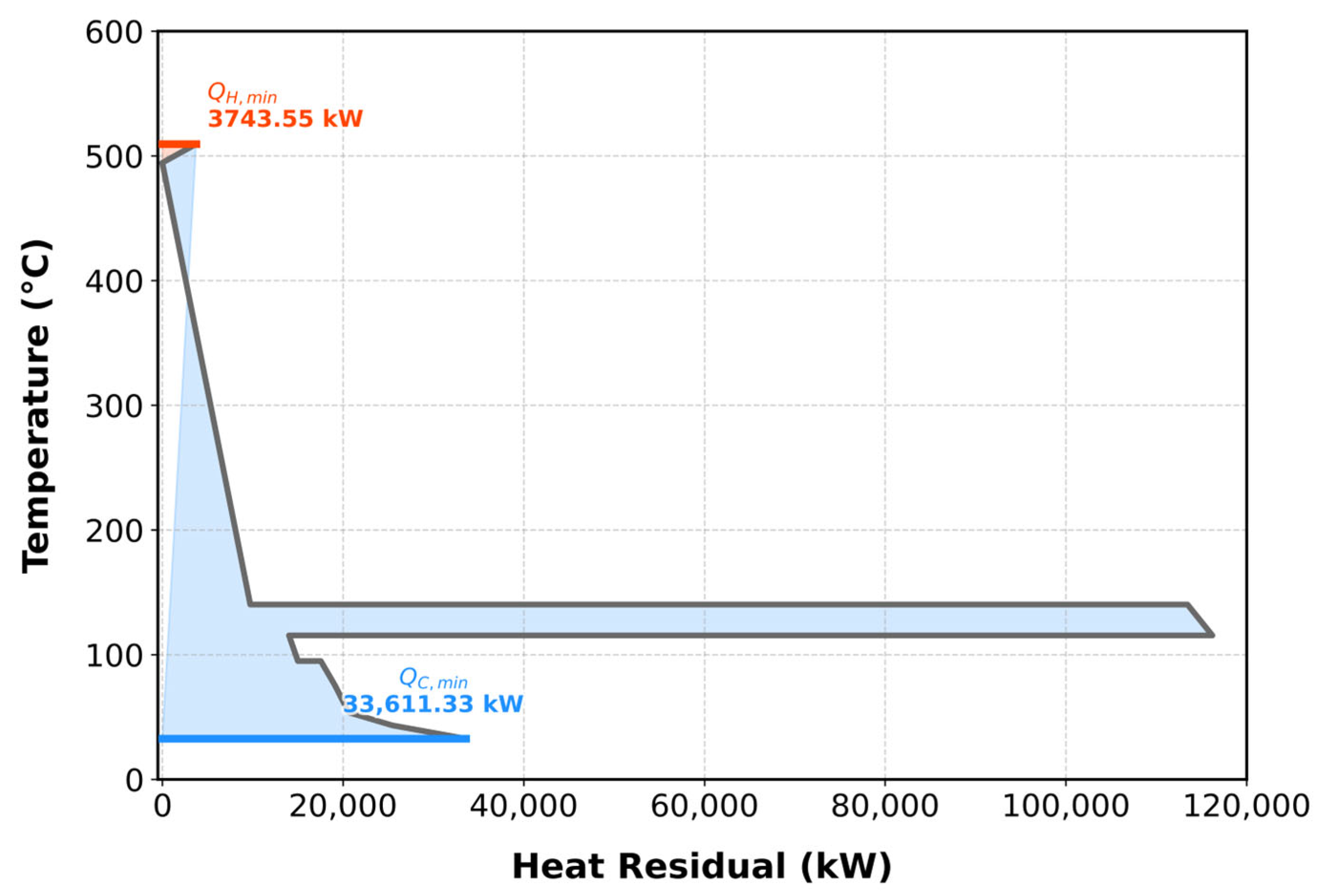
Figure S7 summarizes the cost index evaluations, while the T–H diagram (
Figure 3) shows that the initial overlap of composite curves indicates the minimum cooling utility, and their final separation corresponds to the minimum heating utility. At an optimal ΔT
min of 15 °C, the minimum heating and cooling utilities were determined as
_heating = 3743.55 kW and
_cooling = 33,611.33 kW, respectively.
The algebraic cascade diagram was developed using a temperature interval diagram to ensure thermodynamic feasibility. Heat loads for each interval were calculated by adjusting temperatures and accounting for residual heat from the preceding interval, resulting in 11 temperature intervals, as shown in
Figure S8. Multiple utilities were screened using the grand composite curve, constructed by plotting shifted temperatures representing the average of hot and cold stream temperatures against the revised residual heat loads. The highest negative residual value defines the minimum external heating requirement.
Table S6 summarizes the shifted temperatures and corresponding residual loads, while
Figure 4 illustrates the grand composite curve identifying the need for fired heaters and cooling water as external utilities.
The HEN was developed by matching hot and cold streams to meet minimum heating and cooling utility targets while minimizing the number of exchangers. The required network configuration was determined, ensuring compliance with both energy integration and utility requirements. The analysis concludes that two fired heaters and a total of 13 heat exchangers, including those associated with utilities, are required.
Figure S9 depicts the heat exchanger network configuration that fulfills all energy targets following the stream-matching process. Theoretical reductions in utility heat load consumption were compared with the design and sizing of all heat transfer equipment using actual heat load values, demonstrating reductions of 98.35% in heating demand and 86.89% in cooling demand, respectively.
4.2. By Product Utilization Through Direct Recycling
Mass integration, commonly known as direct recycling, involves reintroducing unreacted feed or by-products into the process to reduce waste and improve overall productivity. This strategy enhances material utilization, decreases fresh feed consumption, and supports sustainable process operation by minimizing environmental impact. Three actions are proposed here: (i) direct recycling of methane, (ii) carbon dioxide and water removal section revision, and (iii) direct recycling of wastewater.
(i) In fact, the process employs two fired heaters that utilize methane as fuel, sourced from column T-101. The methane stream produced in the process, containing 99 mol% methane with minor ethylene impurities, was utilized directly as fuel for the fired heaters. The presence of ethylene enhances the mixture’s heating value, improving fuel efficiency and reducing the required fuel flow rate. Before entering heat exchangers H-101 and H-102, the methane stream is conditioned to 1.1 bar and 298.15 K. An energy balance on the adiabatic throttling valve V-201 reduces the pressure from 30 bar to 1.1 bar, resulting in an outlet temperature of 133.02 K, followed by reheating to 298.15 K.
Table S7 summarizes the corresponding calculations.
(ii) Integrating the existing carbon capture unit on the regenerator side is more practical, as the fired heater effluent comprising air, moisture, and carbon dioxide has a lower flow rate than the R-102 outlet. Mixing both streams before AB-103 enables capture, requiring only a 4.28% increase in solvent molar flow. Establishing a new capture section would be economically unfeasible due to higher fixed costs. Therefore, redirecting the fired heater flue gases, which have a composition like the regenerator outlet, into the existing unit is optimal. Mixer M-201 was added to combine the streams before E-202, ensuring inlet conditions of 313.15 K and 1.1 bar consistent with the original capture unit.
(iii) Finally, the carbon capture section design required water makeup to maintain stable operation, presenting an opportunity to utilize process-generated wastewater to partially meet this demand. Material balance calculations for flashes F-101, F-102, and F-201 confirmed adequate water purity for reuse. Although F-101 may contain trace methanol before recycling Stream 11, these traces are expected to exit through condenser S-101 due to methanol’s higher volatility, preventing accumulation in the system.
Table S8 summarizes the amount of recycled wastewater allocated to each carbon capture section.
4.3. By Product Utilization Through Indirect Recycling
Indirect recycling utilizes by-products as process utilities to reduce energy consumption and utility costs. It contributes to economic optimization by recovering useful energy or material from secondary streams.
4.3.1. Indirect Recycling of Wastewater
The wastewater stream generated within the process was determined, from material and energy balance calculations, to consist of almost pure water. Although indirect recycling is not feasible due to its operating conditions, converting this stream into steam provides a practical and flexible alternative that enhances opportunities for energy integration. In standard utility systems, high-pressure steam is produced through sequential preheating and furnace heating to meet process heating requirements; the same concept can be applied by utilizing heat release from highly exothermic reactions via reactor cooling jackets [
41].
Table 6 summarizes the associated heat duties and operating temperatures for the relevant reactors.
The wastewater stream consists primarily of reaction co-product water, making up over 99.5 wt%, with trace impurities including residual methanol at less than 0.3 wt%, dissolved light hydrocarbons at under 0.1 wt%, and MEA at below 0.1 wt%. These impurities have minimal impact on the thermophysical properties and heat transfer calculations, with deviations from pure water properties remaining under 2% at these concentrations. The heat exchangers are built with standard fouling factors (0.0002 m2·K/W) to accommodate potential organic deposits from thermal decomposition. A continuous purge stream of 1–2% of the flow is recommended to prevent long-term accumulation of non-volatile species.
The use of wastewater in high-pressure (HP) steam generation is not yet common in all olefins plants; however, it is consistent with established steam-cracking practices. In these practices, process condensates such as quench water, dilution steam generator return, and decoking effluent are routinely recycled as boiler feedwater. Industry examples include the ExxonMobil Singapore Olefins Plant and several modern ethylene crackers that employ condensate recovery and boiler feed integration strategies [
42]. These established precedents confirm the technical viability of our proposed HP steam generation using treated process water.
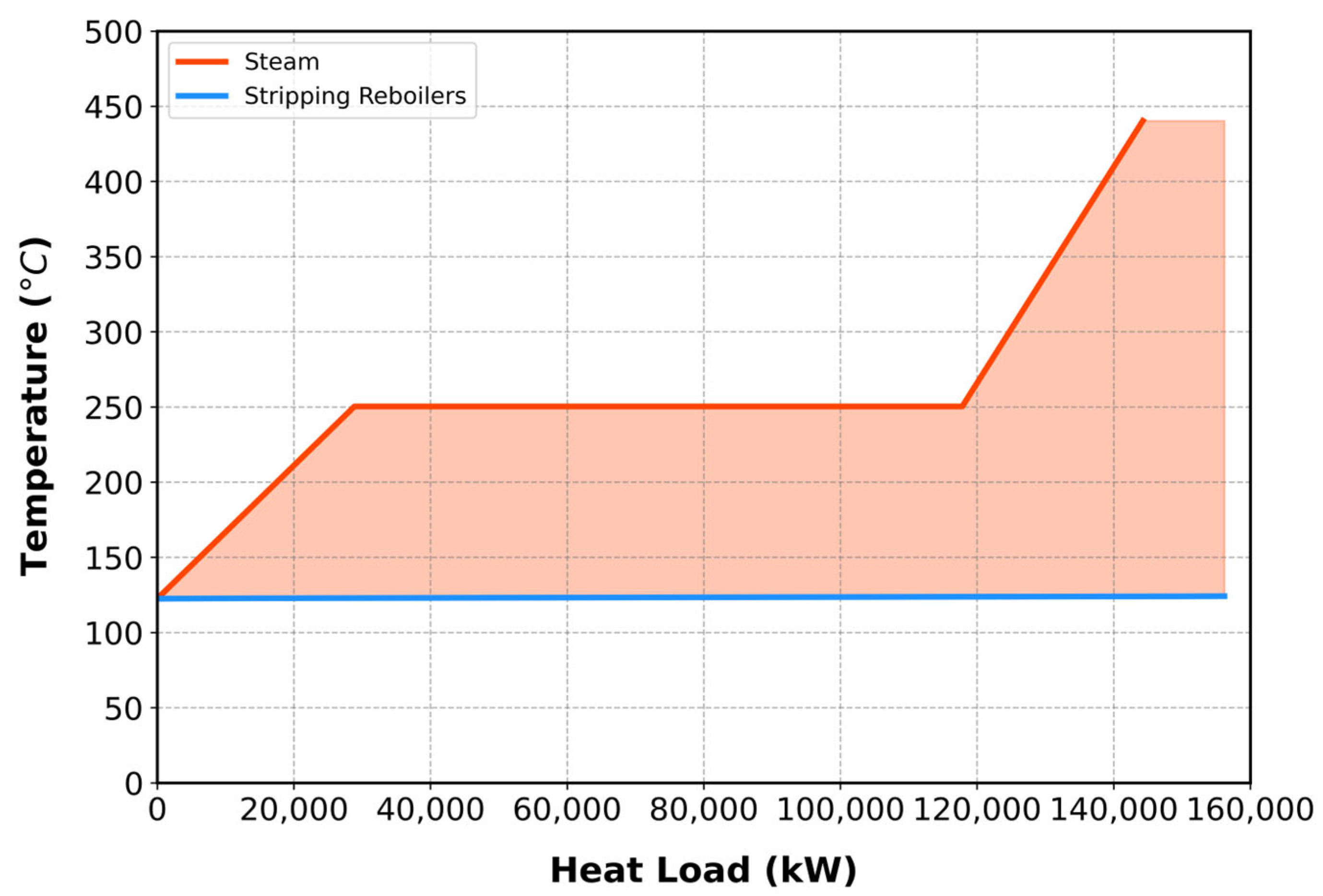
Steam generation occurs in two stages: liquid wastewater is first blended in mixer M-204 and pressurized from 1.1 to 40 bar by pump P-201, then heated via reactor cooling jackets to produce superheated steam for process heating. Molar flow rates are converted to mass flow rates to enable steam table calculations under isobaric conditions. At 40 bar, the steam temperature reaches 440.3 °C, indicating efficient heating below the reactor’s operational limit. To avoid freezing at elevated pressures, the outlet water temperature is limited to 2 °C. The total heat duty comprises sensible heating, latent vaporization, and sensible cooling, each evaluated separately. Using steam tables, the heat capacity and heat load were extracted for pinch analysis, treating water as a hot stream.
Table S9 presents the steam stream data used for heat integration to minimize energy consumption and the number of exchangers in distillation and stripping reboilers, while
Table S10 summarizes the cold streams.
The superheated steam generated from the process was integrated with boilers S-101 and S-102. Thermal pinch analysis, shown in
Figure 5, identified a pinch point at 122.45 °C. Using the Aspen Energy Analyzer, 20 scenarios were assessed to determine the optimal condensed water outlet temperature of 126.45 °C, corresponding to the minimum annualized cost.
Figure S10 presents the cost index evaluation results.
The integration of the water utility stream with reboilers S-101 and S-102 was successfully achieved, reducing dependence on external steam sources.
Figure S11 presents the heat exchanger network illustrating the stream-to-boiler integration, where the heat is reduced by 91.78%.
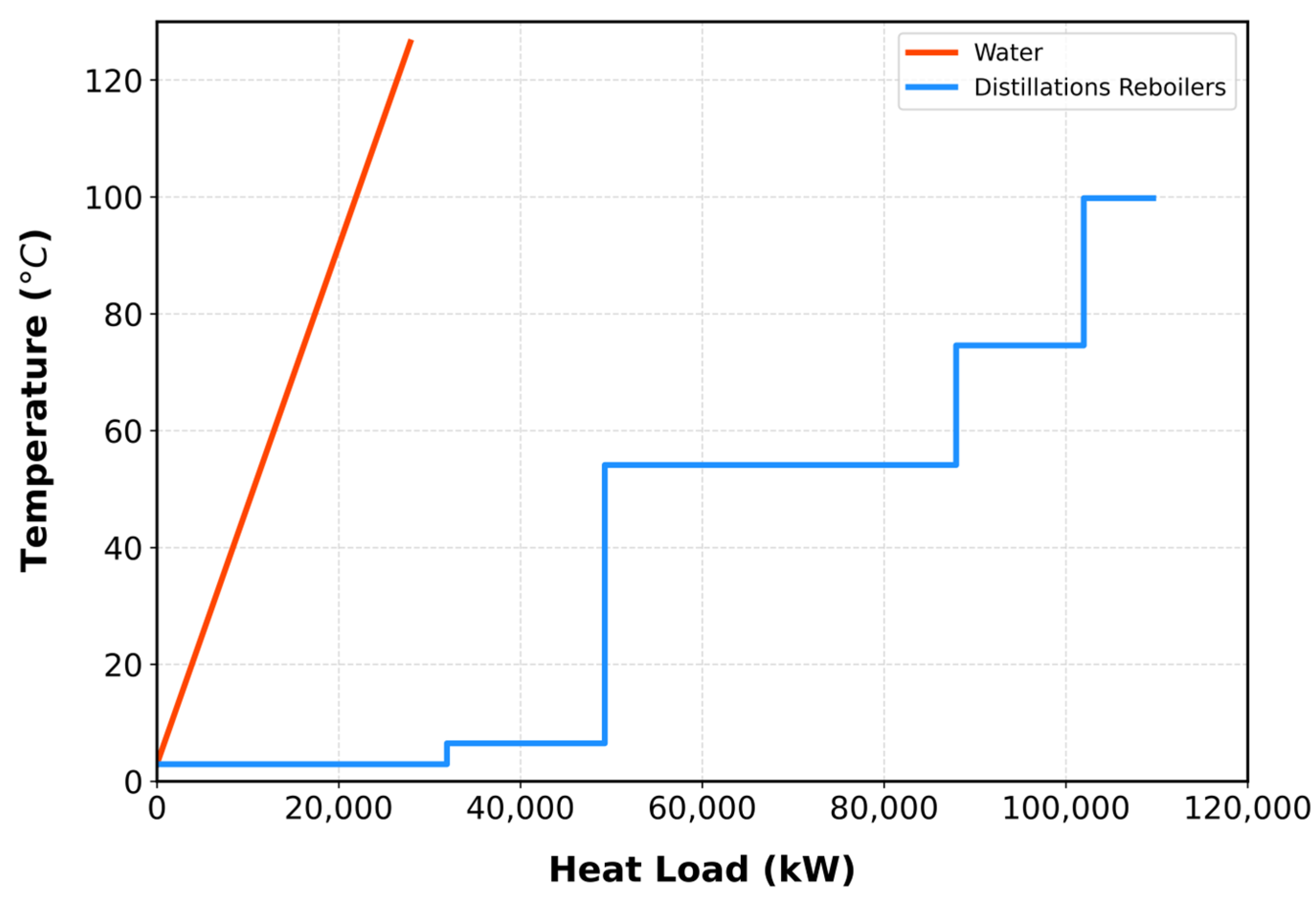
Further heat integration was achieved by utilizing the remaining portion of the water utility stream. As shown in
Figure 6, the utility stream acted as a hot stream, while the distillation reboilers served as cold streams. Pinch point analysis identified a thermal pinch with a temperature difference of 2.91 °C. Using the Aspen Energy Analyzer, the optimal outlet temperature was determined to be 7.91 °C, as summarized in
Figure S12.
Figure S13 illustrates the heat exchanger network integrating the utility stream with the T-101 reboiler, achieving a 24.11% energy reduction for T-101 and an overall 63.68% reduction in energy consumption across all columns.
4.3.2. Indirect Recycling of Hydrogen-Captured Carbon Dioxide
Hydrogen is indirectly recycled to provide the heat necessary for elevating methane to the fired heaters’ operating conditions. High-temperature hydrogen from unit MB-101 is utilized to preheat the methane stream, enhancing overall energy efficiency without affecting the streams’ physical or chemical characteristics. To satisfy a thermal duty of 36.181 kW, the hydrogen outlet temperature was maintained at 622.14 K.
Similarly, the captured CO
2 stream exiting condensers S-101 and S-102 at high flow rate and low temperature serves as a low-grade heat source, effectively used to cool flue gases prior to their entry into AB-103. Using the same methodology applied to the hydrogen stream, the CO
2 outlet temperature was determined. As the flue gas undergoes partial condensation, pinch analysis was conducted to detect possible thermal constraints. The analysis revealed no pinch points, confirming the elimination of additional cooling utilities.
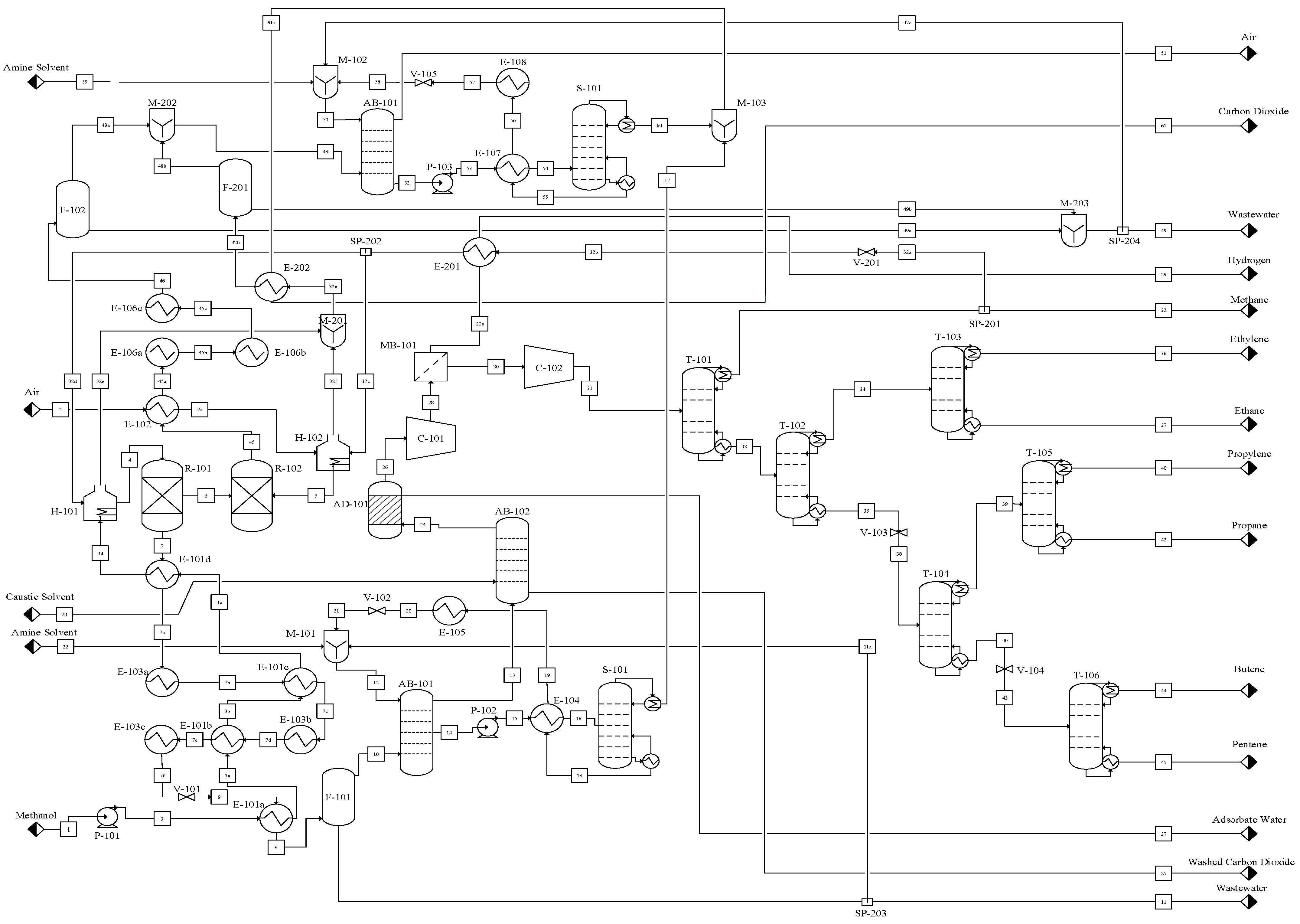
Figure S14 presents the composite curves for the flue gas and cooling streams, illustrating heat integration potential, while
Figure 7 shows the revised MTO process flowsheet reflecting the process integration, and
Figure S15 reflects the continued utility process flow diagram of indirect recycling of wastewater.
5. Economic Analysis
With the design phase completed, covering material and energy requirements, equipment design, and compliance with local and international safety and environmental standards. The project now proceeds to one of its later stages: the economic analysis of the proposed plant.
5.1. Equipment, Raw Materials, and Utilities Costs
Estimating equipment cost is the initial step in determining the plant’s capital cost. Various methods are available, differing in accuracy. The most reliable thing is obtaining vendor quotations. Alternatively, past purchase orders can be used with appropriate cost index adjustments. Published data and software tools offer less accurate but acceptable estimates when other sources are unavailable. In this work, equipment costs were estimated using correlations from Analysis, Synthesis, and Design of Chemical Processes by Turton.
Table S11 represents the summary of the purchased equipment, showing a total CAPEX of USD 550.30 million.
It is important to note that the costs associated with CO2 emissions, such as carbon taxes or sequestration fees, were excluded from this analysis. This exclusion is justified because a comprehensive CO2 capture infrastructure is required primarily for internal process needs—specifically, to prevent freezing in downstream cryogenic separation units. Additionally, this infrastructure functions as an internal cooling utility for economic optimization, rather than being used solely for environmental compliance.
Raw material cost represents a major portion of total manufacturing expenses and is calculated annually to reflect steady-state operation. Methanol serves as the main feedstock with approximately 2,953,500 tonnes/year (8950 tonnes/day), producing around 585,800 tonnes/year (1775 tonnes/day) of ethylene and 414,800 tonnes/year (1257 tonnes/day) of propylene under steady-state operation (based on 330 operating days per year). In comparison, sodium hydroxide (NaOH) is required for the caustic wash with 119,080 tonnes/year (360 tonnes/day). For this preliminary analysis, price estimates were obtained from Chemanalyst and ICIS. A 30% tooling discount is applied to methanol [
43], reflecting local incentives to promote downstream olefins production, resulting in a total OPEX of USD 719.04 million.
In addition, utility costs constitute a significant portion of operating expenses, primarily covering the consumption of cooling water, steam, electricity, and refrigerants.
Table 7 presents the utility costs applied in this analysis [
29], while
Table S12 details the utility cost distribution per equipment.
5.2. Profitability Analysis
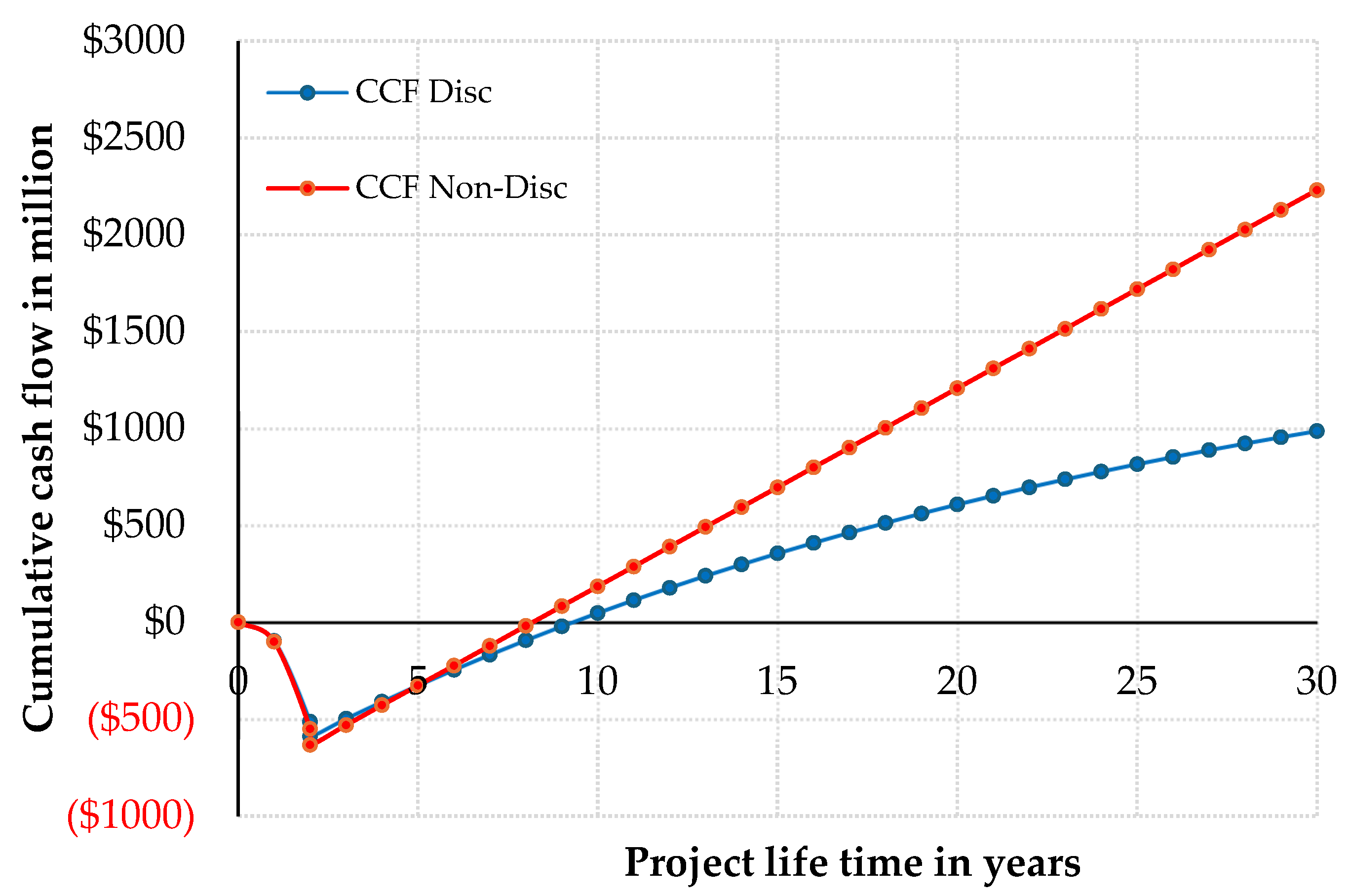
A cash flow diagram is used to represent the movement of money across the project life cycle, as shown in
Figure 8.
The economic evaluation yielded a payback period of 5.38 years, calculated as the ratio of total capital investment to annual profit. The discounted payback period, obtained by interpolating the cumulative discounted cash flow between Years 9 and 10, was 6.82 years. The rate of return on investment reached 15.75%, while the discounted cash-flow rate of return determined using the zero-net present value condition was 15.85%. The project also achieved a net present value of 1069.538 million USD and a cumulative cash position of 2517.757 million USD at Year 32, demonstrating strong profitability and resilience over the project lifetime.
5.3. Comparison with Conventional Technologies
The CAPEX for the grassroots plant is estimated at USD 550.30 million, and OPEX is estimated at USD 719.04 million. The analysis of economic and performance indicators shows that the integrated MTO process is highly competitive compared to conventional olefin production methods. The design presented here demonstrates the ability to produce light olefins at a cost competitive with that of a conventional naphtha steam cracker of equivalent capacity. The analysis indicates that the production cost of ethylene and propylene in the MTO plant, under current assumptions for methanol and product prices, is estimated between USD 800 and USD 1000 per tonne of olefin [
44]. This cost falls within the typical range for olefins produced from a naphtha cracker at moderate crude oil prices and may be lower when methanol is available at a reduced cost. The capital investment required for the MTO plant is significantly lower than that for a conventional world-scale naphtha cracker. The financial requirement for the MTO facility is estimated at several hundred million USD, due to the use of less complex, smaller-scale equipment and the absence of high-severity furnaces. In contrast, investment for a steam cracker with similar olefin output typically reaches multi-billion USD levels. The analysis shows that operating costs are favorable for the MTO route when low-cost methanol is available, as assumed in this study. Methanol can be sourced economically from natural gas or coal, unlike naphtha, which is subject to price fluctuations tied to crude oil markets.
The integrated MTO process developed in this study demonstrates production efficiency and economic viability, positioning it as a strong competitor to traditional olefin technologies. The combination of high conversion rates and selectivity, energy integration that achieves more than a 50% reduction in external energy consumption, and efficient stream recycling results in a process capable of producing polymer-grade ethylene and propylene while maintaining a favorable profit margin. The calculated payback period of approximately 5.4 years and an internal rate of return between 15% and 16% match or exceed the performance metrics commonly observed in conventional petrochemical projects. This highlights the potential of MTO as a viable alternative source for olefins. The findings of this study support the industrial viability and competitiveness of the integrated MTO process for producing sustainable olefins, especially considering the flexibility in feedstock sourcing and the increasing need to reduce carbon emissions in the petrochemical industry.
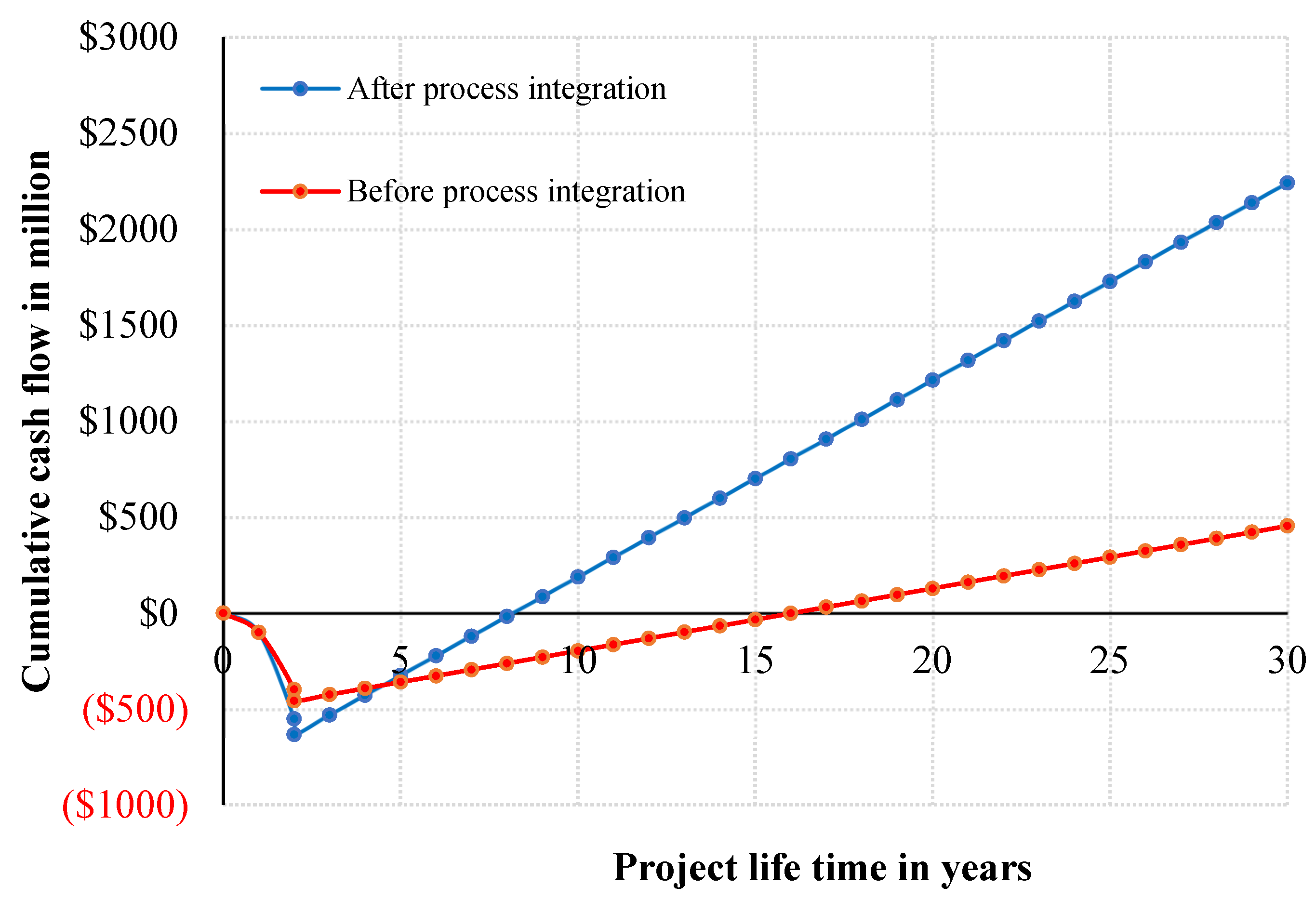
5.4. Process Integration Effect on Profitability
Process integration plays a critical role in enhancing the economic performance of the MTO plant. Without integration, the utility cost was approximately $147 million per year, whereas after heat and mass integration, this figure was reduced substantially to $66.4 million per year, achieving an annual saving of nearly $80 million.
Figure 9 compares the non-discounted cumulative cash flow at base case conditions for both scenarios. The integrated process not only reaches the breakeven point faster but also continues to generate substantially higher returns over the project’s lifetime.