Effect of Dairy Powders and Sorbitol-Based Encapsulation Systems on Functional, Thermal, and Microstructural Quality of Probiotic Ice Cream
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Method
2.2.1. Production of Lyophilized L. acidophilus Cultures
Preparation of Microorganism Inoculum
Preparation of Protective Media
Lyophilization Process
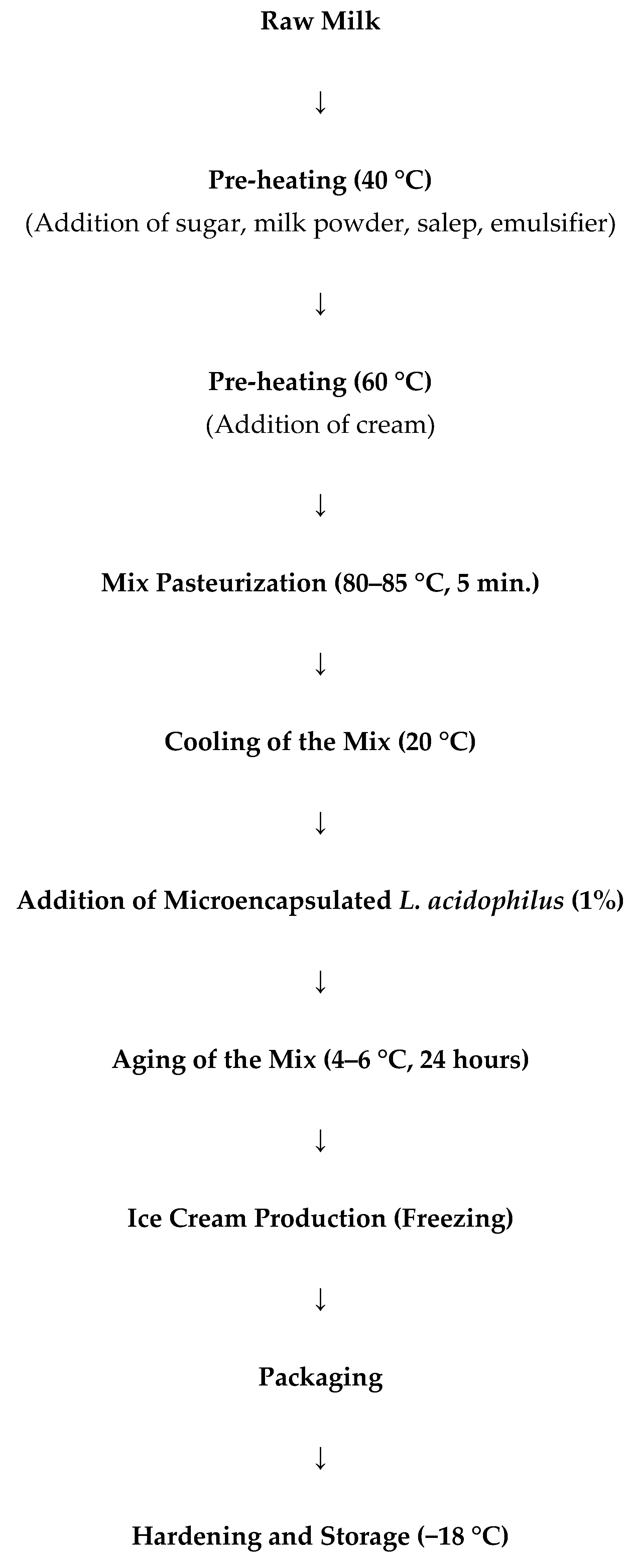
2.2.2. Ice Cream Production
2.2.3. Enumeration of Microencapsulated L. acidophilus
2.2.4. Texture Analysis of the Ice Cream Mix
2.2.5. Analyses Performed on Ice Cream
Physicochemical Analyses
Dry Matter
Protein
pH
Titratable Acidity
First Drip Time
Complete Melting Time
Overrun
Color Analysis
Hardness Measurement
Rheological Properties
Differential Scanning Calorimetry (DSC)
Cryo-SEM Analysis
Statistical Analysis
3. Results and Discussion
3.1. Textural Properties of the Ice Cream Mix
3.2. Physicochemical Properties of the Ice Creams
3.2.1. Dry Matter
3.2.2. Protein
3.2.3. TA and pH
3.2.4. Color Stability (L, a, b*)**
3.2.5. Melting Behavior
3.2.6. Overrun
3.2.7. Hardness
3.2.8. Differential Scanning Calorimetry (DSC)
3.2.9. Rheological Properties
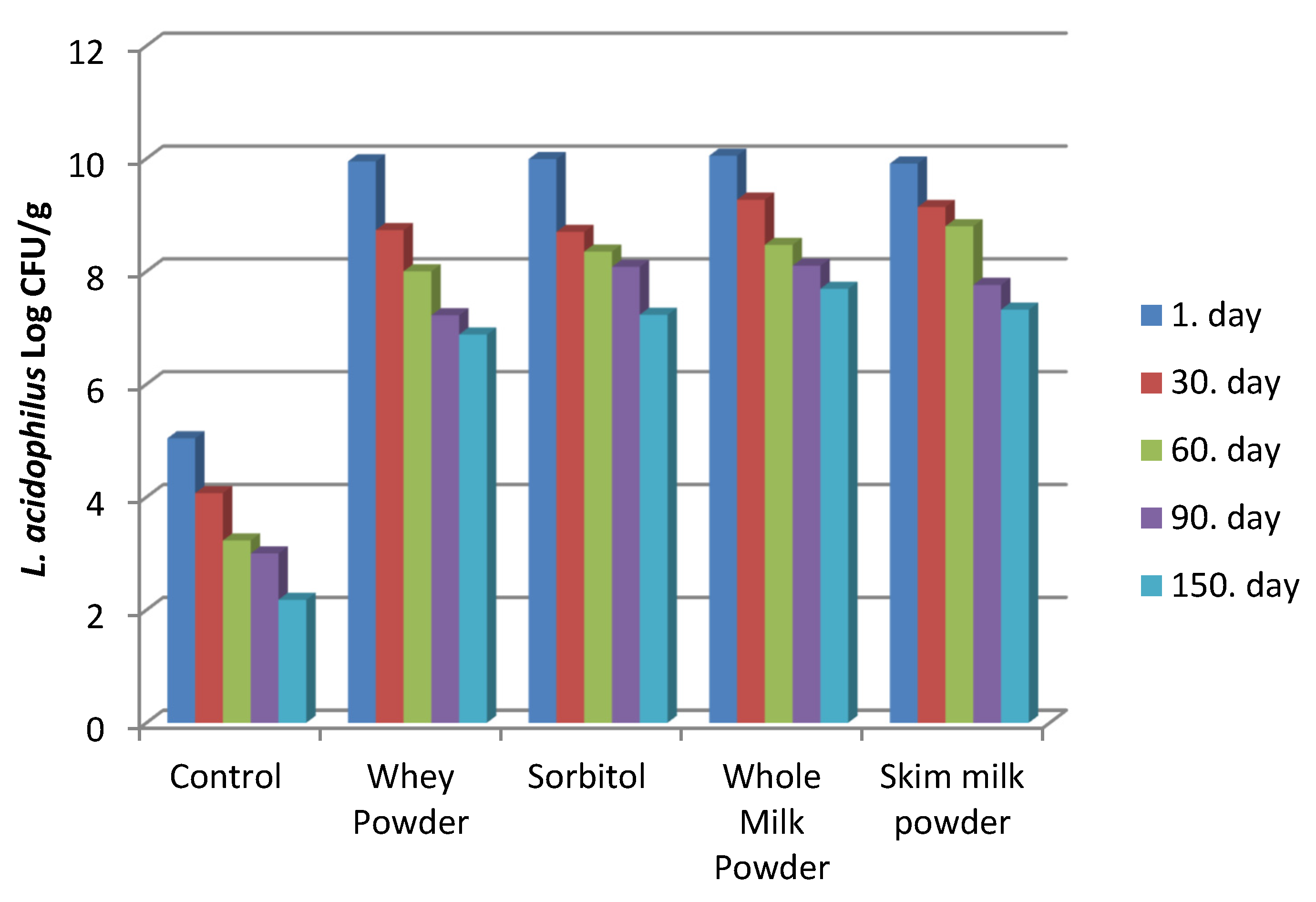
3.2.10. L. acidophilus Count and Viability Rates
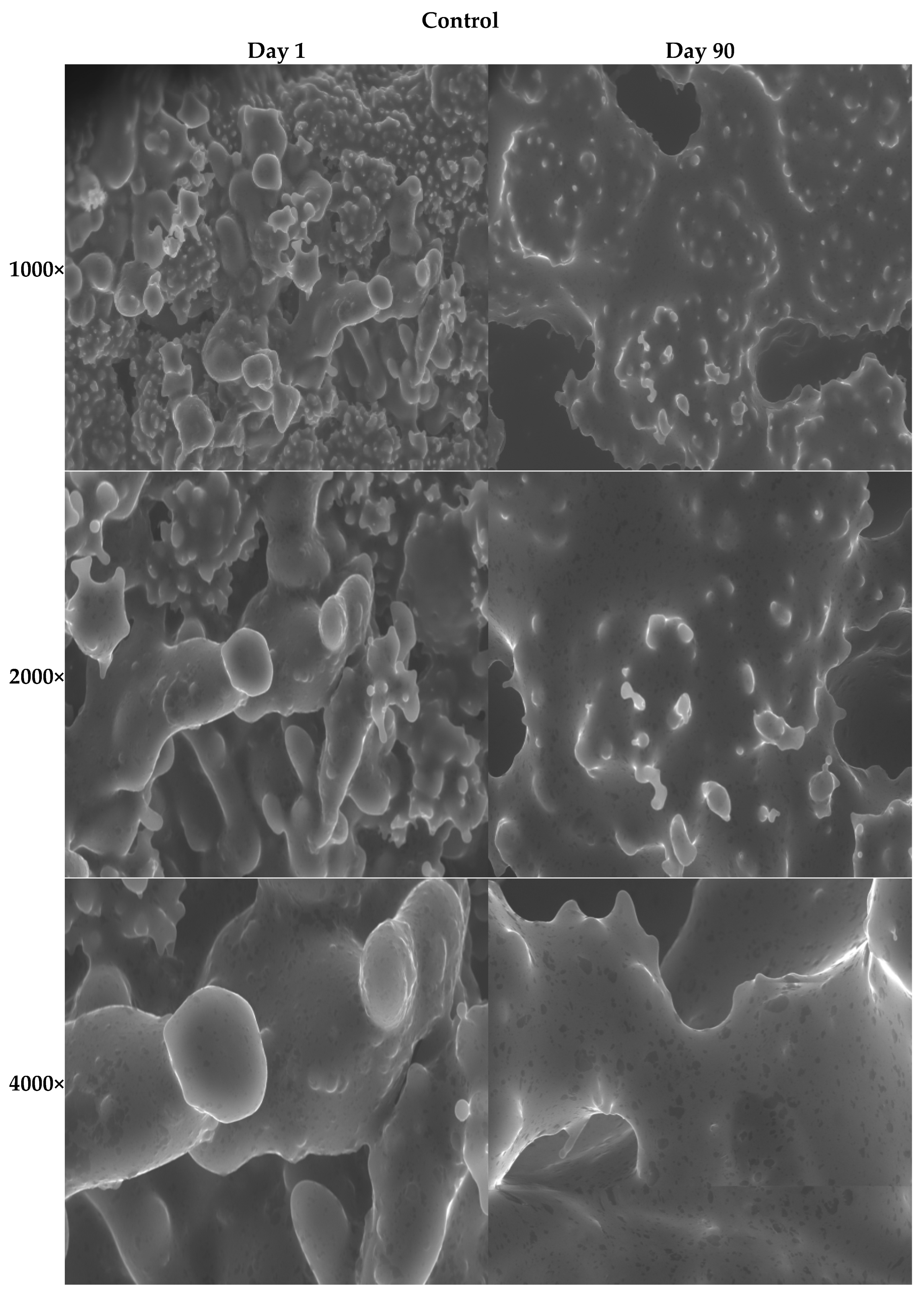
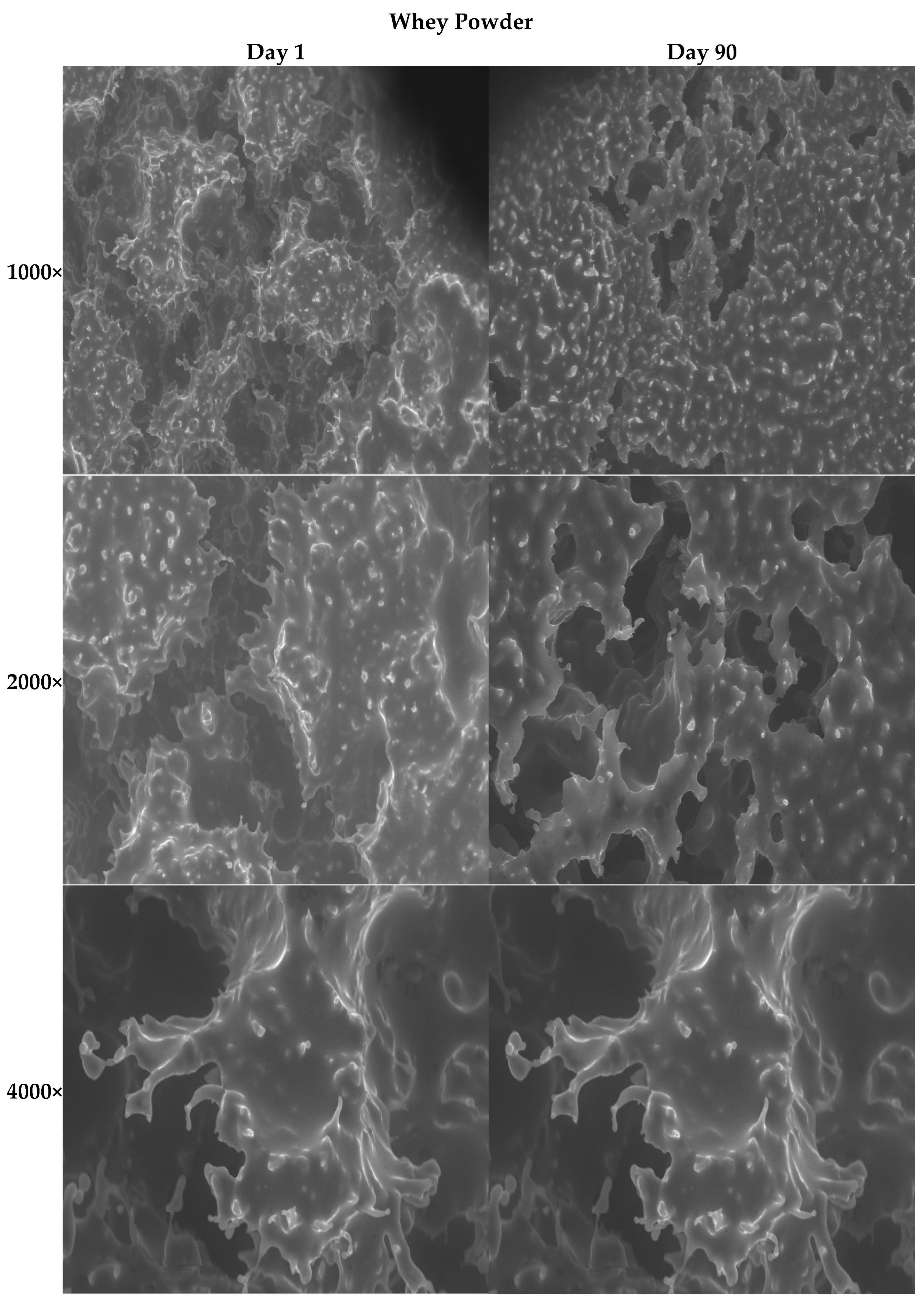
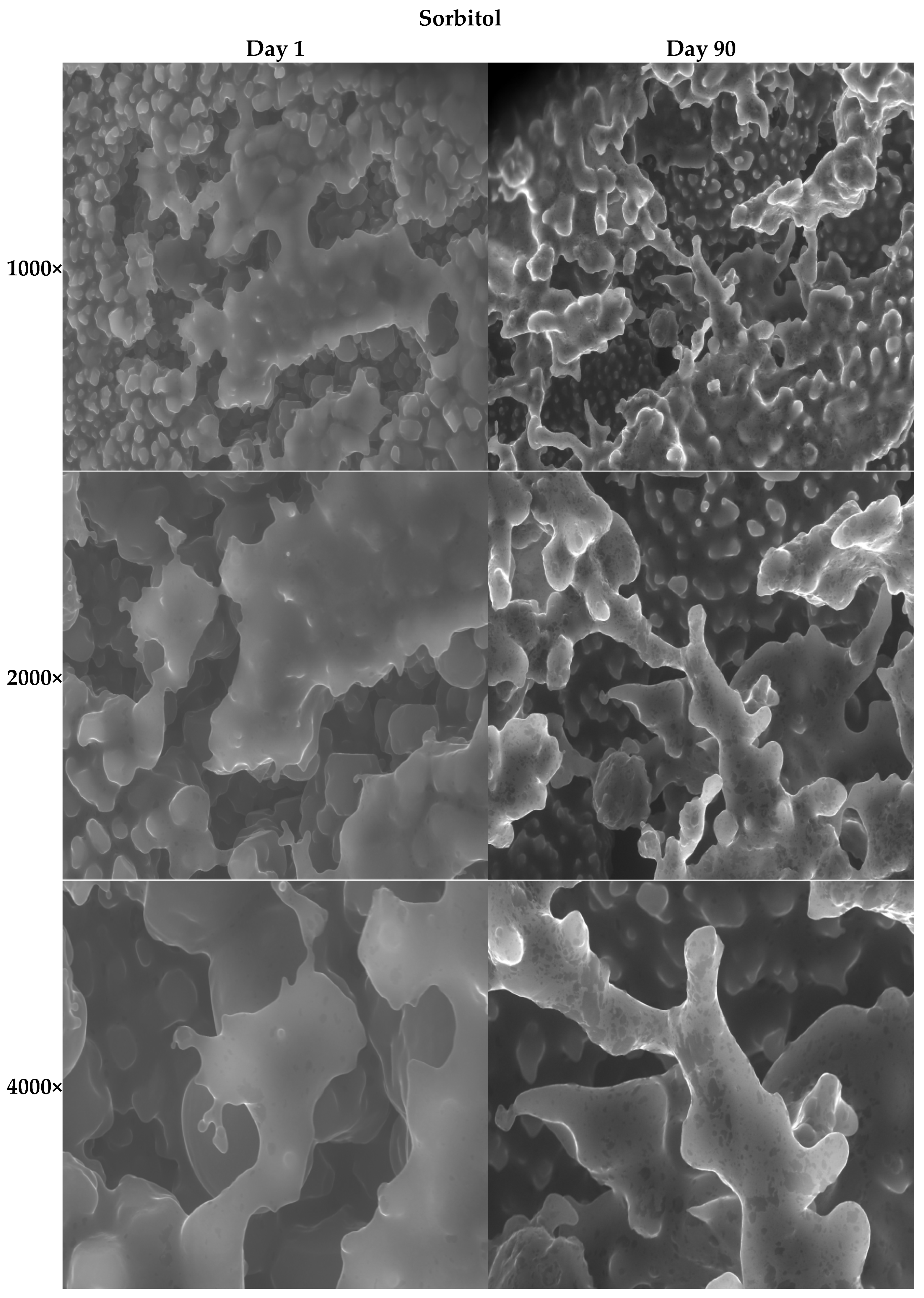
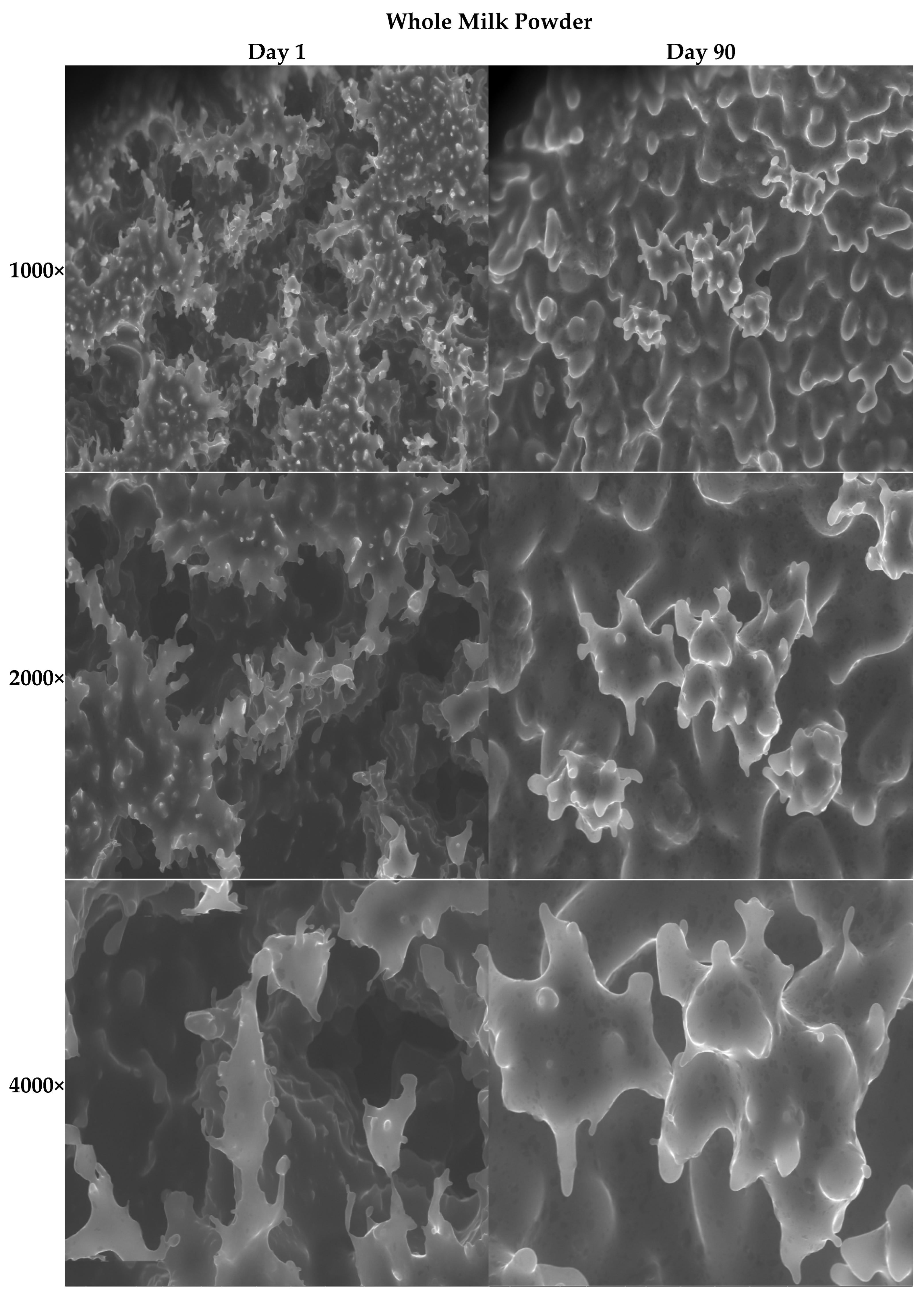
3.2.11. SEM Images of the Ice Creams
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Adhikari, K.; Mustapha, A.; Grun, I.U.; Fernando, L.N. Viability of microencapsulated bifidobacteria in set yogurt during refrigerated storage. J. Dairy Sci. 2000, 83, 1946–1951. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, X.; Li, X.; Zhang, L.; Wang, Y.; Chen, J.; Zhou, H. Health-Promoting Effects of Lactobacillus acidophilus and Its Applications in Food and Beverage. Fermentation 2024, 10, 380. [Google Scholar] [CrossRef]
- Lisboa, H.M.; Sarinho, V.; Barros, M.; Pascoal, A.; Dias, C. Probiotic Ice Cream: Formulation Innovations, Stability Challenges, and Future Perspectives. Food Humanit. 2025, 5, 100780. [Google Scholar] [CrossRef]
- Zeashan, M.; Afzaal, M.; Saeed, F.; Ahmed, A.; Tufail, T.; Ahmed, A.; Anjum, F.M. Survival and Behavior of Free and Encapsulated Probiotic Bacteria under Simulated Human Gastrointestinal and Technological Conditions. Food Sci. Nutr. 2020, 8, 2419–2426. [Google Scholar] [CrossRef] [PubMed]
- Akalın, A.S.; Fenderya, S.; Akbulut, N. Viability and activity of bifidobacteria in yogurt containing fructo-oligosaccharide during refrigerated storage. Int. J. Food Sci. Technol. 2004, 39, 613–621. [Google Scholar] [CrossRef]
- Kareem, R.A.; Razavi, S.H.; Mousavi, Z. Survival of Free and Encapsulated Probiotics in Healthy Gummy Candy as a Carrier for Probiotic Capsules. Arch. Food Nutr. Sci. 2024, 8, 29–37. [Google Scholar] [CrossRef]
- Akalın, A.S.; Erisir, D. Effects of Inulin and Oligofructose on the Rheological Characteristics and Probiotic Culture Survival in Low-Fat Probiotic Ice Cream. J. Food Sci. 2008, 73, 184–188. [Google Scholar] [CrossRef]
- Akalın, A.S.; Karagözlü, C.; Ender, G.; Ünal, G. Effects of Aging Time and Storage Temperature on the Rheological and Sensory Characteristics of Whole Ice Cream. Milchwiss. —Milk Sci. Int. 2008, 63, 293–295. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/20083201016 (accessed on 27 October 2025).
- AOAC. Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1990; ISBN 0-935584-42-0. Available online: https://www.aoac.org/official-methods-of-analysis/ (accessed on 27 October 2025).
- Yu, S.; Wang, Z.; Li, Q.; Zhao, W. The First Di-D-Fructofuranose 1,2′:2,3′-Dianhydride Hydrolase (DFA-IIIase) from the Genus of Non-Arthrobacter (Pathogen Salmonella enterica subsp. enterica Serovar Mbandaka): Molecular Cloning, Expression, Identification, and Characterization. Food Biosci. 2023, 55, 103093. [Google Scholar] [CrossRef]
- BahramParvar, M.; Tehrani, M.M. Application and functions of stabilizers in ice cream. Food Rev. Int. 2011, 27, 389–407. [Google Scholar] [CrossRef]
- Carvalho, A.S.; Silva, J.; Ho, P.; Teixeira, P.; Malcata, F.X.; Gibbs, P. Protective Effect of Sorbitol and Monosodium Glutamate during Storage of Freeze-Dried Lactic Acid Bacteria. Lait 2003, 83, 203–210. [Google Scholar] [CrossRef]
- Afzal, A.; Afzaal, M.; Saeed, F.; Shah, Y.A.; Raza, M.A.; Khan, M.H.; Asres, D.T. Milk Protein Based Encapsulation of Probiotics and Other Food Material: Comprehensive Review. Int. J. Food Prop. 2024, 27, 245–262. [Google Scholar] [CrossRef]
- Chang, L.L.; Shepherd, D.; Sun, J.; Tang, X.; Pikal, M.J. Effect of Sorbitol and Residual Moisture on the Stability of Lyophilized Antibodies: Implications for the Mechanism of Protein Stabilization in the Solid State. J. Pharm. Sci. 2005, 94, 1445–1455. [Google Scholar] [CrossRef] [PubMed]
- Clarke, C. The Science of Ice Cream, 2nd ed.; Royal Society of Chemistry: Cambridge, UK, 2012; 213p. [Google Scholar] [CrossRef]
- Cotrell, J.I.L.; Pass, G.; Philips, G.O. Assessment of polysaccharides as ice cream stabilizers. J. Sci. Food Agric. 1979, 30, 1085–1088. [Google Scholar] [CrossRef]
- Ding, W.K.; Shah, N.P. Effect of various encapsulating materials on the stability of probiotic bacteria. J. Food Sci. 2009, 74, M100–M107. [Google Scholar] [CrossRef]
- El-Salam, M.H.A.; El-Shibiny, S.; Salem, A. Effects of milk powder addition on the quality of frozen dairy desserts. Dairy Sci. Technol. 2018, 98, 355–371. [Google Scholar]
- El-Salam, M.H.A.; El-Shibiny, S.; Mehanna, N. Iron Fortification of Milk and Dairy Products. Egypt. J. Dairy Sci. 2017, 45, 15–28. Available online: https://www.researchgate.net/profile/Mohamed-Abd-El-Salam 4/publication/330999278_iron_fortification/links/5c6053a9a6fdccb608b75998/ironfortification? Origin=scientificContributions&__cf_chl_tk=JXaO61.I6v81OgoKu7EeEFQFN_q985cmxOHuNjt9GeY-1763729693-1.0.1.1-J6qbf.Z99TmG2wi2gC5ExtDZa.BxyXZK6zC8HQi39Hs (accessed on 27 October 2025).
- FAO/WHO. Joint FAO/WHO Working Group Report on Drafting Guidelines for the Evaluation of Probiotics in Food, London, Ontario, Canada, 30 April–1 May 2002; Food and Agriculture Organization of the United Nations & World Health Organization. Available online: https://www.fao.org/3/a-a0512e.pdf (accessed on 27 October 2025).
- Fonseca, F.; Cenard, S.; Passot, S. Freeze-Drying of Lactic Acid Bacteria. In Cryopreservation and Freeze-Drying Protocols; Wolkers, W.F., Oldenhof, H., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2015; Volume 1257, pp. 477–488. [Google Scholar] [CrossRef]
- Gbassi, G.K.; Vandamme, T.; Ennahar, S.; Marchioni, E. Microencapsulation of Lactobacillus plantarum spp. in an alginate matrix coated with whey proteins. Int. J. Food Microbiol. 2011, 129, 103–105. [Google Scholar] [CrossRef]
- Goff, H.D. Formation and stabilisation of structure in ice-cream and related products. Curr. Opin. Colloid Interface Sci. 2002, 7, 432–437. [Google Scholar] [CrossRef]
- Goff, H.D.; Hartel, R.W. Ice Cream, 7th ed.; Springer: New York, NY, USA, 2013; ISBN 978-1-4614-6097-3. [Google Scholar] [CrossRef]
- Sahagian, M.E.; Goff, H.D. Thermal, Mechanical and Molecular Relaxation Properties of Stabilized Sucrose Solutions at Sub-Zero Temperatures. Food Res. Int. 1995, 28, 1–8. [Google Scholar] [CrossRef]
- Kavaz Yüksel, A.; Yüksel, M.; Şat, İ.G. Determination of certain physicochemical characteristics and sensory properties of green tea powder (matcha) added ice creams and detection of their organic acid and mineral contents. Gıda 2017, 42, 116–126. [Google Scholar] [CrossRef]
- Güven, M.; Karaca, O.B. Effects of varying sugar content and fruit concentration on the physical properties of vanilla and fruit-flavored frozen yogurts. Int. J. Dairy Technol. 2002, 55, 27–31. [Google Scholar] [CrossRef]
- Heidebach, T.; Först, P.; Kulozik, U. Influence of casein-based microencapsulation on freeze-thaw and storage stability of Bifidobacterium lactis Bb-12. J. Food Eng. 2010, 98, 309–316. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term “probiotic”. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Homayouni, A.; Azizi, A.; Ehsani, M.R.; Yarmand, M.S.; Razavi, S.H. Effect of microencapsulation and resistant starch on the probiotic survival and sensory properties of symbiotic ice cream. Food Chem. 2008, 111, 50–55. [Google Scholar] [CrossRef]
- Soukoulis, C.; Lebesi, D.; Tzia, C. Enrichment of ice cream with dietary fibre: Effects on rheological properties, ice crystallisation and glass transition phenomena. Food Chem. 2009, 115, 665–671. [Google Scholar] [CrossRef]
- Jimenez-Florez, R.; Klipfel, N.J.; Tobias, J. Ice creams and frozen desserts. In Dairy Science and Technology Handbook, Volume 2: Product Manufacturing; Hui, Y.H., Ed.; VCH Publishers: New York, NY, USA, 1993; pp. 57–102. ISBN 978-1560812653. [Google Scholar]
- Kailasapathy, K. Encapsulation technologies for functional foods and nutraceutical product development. CAB Rev. 2009, 4, 1–19. [Google Scholar] [CrossRef]
- Karaman, S.; Toker, O.S.; Yüksel, F.; Çam, M.; Kayacier, A.; Dogan, M. Physicochemical, bioactive, and sensory properties of persimmon-based ice cream: Technique for Order Preference by Similarity to Ideal Solution to determine optimum concentration. J. Dairy Sci. 2011, 97, 97–110. [Google Scholar] [CrossRef]
- Kurt, A. Guide to the Examination and Analysis Methods of Milk and Dairy Products; Atatürk University Publications: Erzurum, Turkey, 1993; ISBN 975-401-384-1. Available online: https://www.atauni.edu.tr/ (accessed on 27 October 2025).
- Leslie, S.B.; Israeli, E.; Lighthart, B.; Crowe, J.H.; Crowe, L.M. Trehalose and sucrose protect both membranes and proteins in intact bacteria during drying. Appl. Environ. Microbiol. 1995, 61, 3592–3597. [Google Scholar] [CrossRef]
- Toker, O.S.; Doğan, M.; Canıyılmaz, E.; Ersöz, N.B.; Kaya, Y. The Effects of Different Gums and Their Interactions on the Rheological Properties of a Dairy Dessert: A Mixture Design Approach. Food Bioprocess Technol. 2013, 6, 896–908. [Google Scholar] [CrossRef]
- Mousavi, M.; Heshmati, A.; Garmakhany, A.D.; Vahidinia, A.; Taheri, M. Optimization of the Viability of Lactobacillus acidophilus and Physico-Chemical, Textural and Sensorial Characteristics of Flaxseed-Enriched Stirred Probiotic Yogurt by Using Response Surface Methodology. LWT—Food Sci. Technol. 2019, 102, 80–88. [Google Scholar] [CrossRef]
- Muse, M.R.; Hartel, R.W. Ice cream structural elements that affect melting rate and hardness. J. Dairy Sci. 2004, 87, 1–10. [Google Scholar] [CrossRef]
- Mandal, S.; Puniya, A.K.; Singh, K. Effect of alginate concentrations on survival of microencapsulated Lactobacillus casei NCDC-298. Int. Dairy J. 2006, 16, 1190–1195. [Google Scholar] [CrossRef]
- Marshall, R.T.; Goff, H.D.; Hartel, R.W. Ice Cream, 6th ed.; Springer: New York, NY, USA, 2003; ISBN 978-0-387-95471-1. [Google Scholar] [CrossRef]
- Patist, A.; Zoerb, H. Preservation mechanisms of trehalose in food and biosystems. Colloids Surf. B Biointerfaces 2005, 40, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Diaz, J.; Ruiz-Ojeda, F.J.; Gil-Campos, M.; Gil, A. Mechanisms of action of probiotics. Adv. Nutr. 2019, 10 (Suppl. S1), S49–S66. [Google Scholar] [CrossRef] [PubMed]
- Regand, A.; Goff, H.D. Effect of biopolymers on structure and ice recrystallization in dynamically frozen ice cream model systems. J. Dairy Sci. 2002, 85, 2722–2732. [Google Scholar] [CrossRef]
- Rodrigues, D.; Sousa, S.; Soares, J.C. Microencapsulation of Probiotics for Food Application. In Encapsulation of Active Molecules and Their Delivery System; Sonawane, S.H., Bhanvase, B.A., Sivakumar, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Sert, D.; Mercan, E.; Dertli, E. Characterization of lactic acid bacteria from yogurt-like product fermented with pine cone and determination of their role on physicochemical, textural and microbiological properties of product. LWT 2017, 78, 70–76. [Google Scholar] [CrossRef]
- Shah, N.P.; Ravula, R.R. Microencapsulation of Probiotic Bacteria and Their Survival in Frozen Fermented Dairy Desserts. Aust. J. Dairy Technol. 2000, 55, 139–144. Available online: https://www.proquest.com/scholarly-journals/microencapsulation-probiotic-bacteria-their/docview/199496413/se-2?accountid=15333 (accessed on 27 October 2025).
- Sofjan, R.P.; Hartel, R.W. Effects of overrun on structural and physical characteristics of ice cream. Int. Dairy J. 2004, 14, 255–262. [Google Scholar] [CrossRef]
- Soukoulis, C.; Chandrinos, I.; Tzia, C. Study of the Functionality of Selected Hydrocolloids and Their Blends with κ-Carrageenan on Storage Quality of Vanilla Ice Cream. LWT—Food Sci. Technol. 2008, 41, 1816–1827. [Google Scholar] [CrossRef]
- Talwalkar, A.; Kailasapathy, K. The role of oxygen in the viability of probiotic bacteria with reference to Lactobacillus acidophilus and Bifidobacterium spp. Curr. Issues Intest. Microbiol. 2004, 5, 1–8. [Google Scholar]
- Terpou, A.; Papadaki, A.; Lappa, I.K.; Kachrimanidou, V.; Bosnea, L.A.; Kopsahelis, N. Probiotics in food systems: Significance and emerging strategies for encapsulation and delivery. J. Funct. Foods 2019, 56, 1591. [Google Scholar] [CrossRef]
- Üçok, G. Determination of Cryoprotective Roles of Different Dairy By-Products During Freeze-Drying of Lactobacillus plantarum Strains Isolated from Sourdoughs. Ph.D. Thesis, Necmettin Erbakan University, Konya, Turkey, 2020. Available online: https://www.proquest.com/dissertations-theses/ekşï-hamurlardan-ïzole-edïlen-i-lactobacillus/docview/2600230218/se-2?accountid=15333 (accessed on 27 October 2025).
- Yılmaz, T.M. Effects of Exopolysaccharide (EPS)-Producing Lactic Acid Bacteria on Textural, Rheological and Microstructural Properties of Certain Foods; TÜBİTAK Project Report No. 12O169; TÜBİTAK: Istanbul, Turkey, 2014; 175p. Available online: https://www.tubitak.gov.tr/ (accessed on 27 October 2025).
| Firmness (g) | Consistency (g.s) | Cohesiveness (g) | Index of Viscosity (g.s) | |
|---|---|---|---|---|
| Control | 21.98 ± 1.16 e | 32.57 ± 2.26 d | −18.34 ± 2.80 bc | −17.72 ± 1.37 a |
| Whey Powder | 27.88 ± 0.32 c | 40.85 ± 2.99 c | −19.99 ± 0.24 a | −15.77 ± 0.52 b |
| Sorbitol | 25.34 ± 1.36 d | 27.13 ± 1.10 e | −15.73 ± 1.49 bc | −14.05 ± 0.57 c |
| Whole Milk Powder | 36.30 ± 1.73 b | 48.07 ± 3.80 b | −15.11 ± 1.55 c | −19.65 ± 0.95 a |
| Skim Milk Powder | 41.96 ± 1.98 a | 58.65 ± 2.09 a | −18.69 ± 2.31 a | −18.30 ± 2.81 a |
| Protein (%) | Drymatter (%) | |
|---|---|---|
| Control | 3.79 ± 0.03 a | 40.58 ± 0.79 a |
| Whey Powder | 3.79 ± 0.02 a | 40.32 ± 1.99 a |
| Sorbitol | 3.78 ± 0.03 a | 40.67 ± 2.00 a |
| Whole Milk Powder | 3.80 ± 0.04 a | 40.80 ± 1.84 a |
| Skim Milk Powder | 3.81 ± 0.03 a | 39.99 ± 1.78 a |
| Day | TA (% as Lactic Acid) | pH | L* | a* | b* | First Drip (min.) | Complete Melting (min.) | Overrun (%) | Hardnes (N) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Control | Day 1 | 0.260 ± 0.003 | 6.30 ± 0.03 | 87.39 ± 4.38 | 1.65 ± 0.12 | 8.72 ± 0.31 | 13.97 ± 1.45 | 75.10 ± 2.00 | 38.67 ± 1.97 | 10.43 ± 0.58 |
| Day 30 | 0.262 ± 0.002 | 6.30 ± 0.02 | 87.07 ± 5.33 | 1.63 ± 0.13 | 8.69 ± 0.46 | 16.22 ± 1.80 | 76.50 ± 1.50 | 36.99 ± 1.66 | 10.35 ± 1.38 | |
| Day 60 | 0.263 ± 0.004 | 6.28 ± 0.02 | 89.82 ± 1.33 | 1.08 ± 0.08 | 10.09 ± 0.62 | 17.63 ± 1.40 | 78.73 ± 1.78 | 31.52 ± 1.15 | 12.22 ± 1.42 | |
| Day 90 | 0.264 ± 0.003 | 6.30 ± 0.01 | 90.53 ± 1.28 | 1.41 ± 0.25 | 8.74 ± 0.51 | 18.40 ± 1.60 | 79.58 ± 1.98 | 30.16 ± 0.75 | 19.37 ± 8.25 | |
| Whey Powder | Day 1 | 0.261 ± 0.006 | 6.31 ± 0.02 | 87.51 ± 1.39 | 1.67 ± 0.14 | 8.22 ± 0.72 | 15.25 ± 1.88 | 75.68 ± 1.45 | 38.15 ± 0.90 | 13.53 ± 4.79 |
| Day 30 | 0.264 ± 0.005 | 6.31 ± 0.03 | 87.78 ± 2.26 | 1.64 ± 0.17 | 8.44 ± 0.59 | 16.48 ± 1.30 | 76.94 ± 1.47 | 36.47 ± 0.89 | 18.89 ± 1.23 | |
| Day 60 | 0.264 ± 0.003 | 6.30 ± 0.02 | 88.84 ± 1.08 | 1.32 ± 0.14 | 8.89 ± 0.68 | 19.42 ± 1.02 | 82.03 ± 2.67 | 30.30 ± 2.11 | 30.60 ± 1.99 | |
| Day 90 | 0.267 ± 0.007 | 6.31 ± 0.01 | 90.17 ± 0.10 | 1.54 ± 0.12 | 8.41 ± 0.52 | 22.37 ± 0.80 | 83.67 ± 1.46 | 26.52 ± 0.78 | 35.58 ± 2.97 | |
| Sorbitol | Day 1 | 0.262 ± 0.004 | 6.31 ± 0.02 | 87.12 ± 2.59 | 1.65 ± 0.12 | 8.60 ± 0.40 | 13.31 ± 1.17 | 71.72 ± 1.81 | 41.98 ± 1.62 | 9.95 ± 1.53 |
| Day 30 | 0.262 ± 0.006 | 6.30 ± 0.02 | 87.28 ± 1.85 | 1.66 ± 0.11 | 8.69 ± 0.38 | 15.34 ± 0.86 | 74.47 ± 0.67 | 39.55 ± 0.71 | 12.84 ± 1.81 | |
| Day 60 | 0.261 ± 0.004 | 6.30 ± 0.02 | 89.38 ± 2.08 | 0.98 ± 0.19 | 10.08 ± 1.45 | 16.46 ± 0.99 | 77.23 ± 1.63 | 35.71 ± 0.76 | 19.88 ± 5.73 | |
| Day 90 | 0.262 ± 0.005 | 6.32 ± 0.03 | 89.46 ± 3.43 | 1.48 ± 0.09 | 8.42 ± 0.92 | 18.39 ± 0.88 | 78.40 ± 0.92 | 31.52 ± 0.89 | 21.97 ± 6.23 | |
| Whole Milk Powder | Day 1 | 0.262 ± 0.005 | 6.30 ± 0.02 | 88.80 ± 1.05 | 1.56 ± 0.04 | 9.02 ± 0.45 | 15.80 ± 1.61 | 76.17 ± 1.15 | 37.30 ± 1.18 | 14.93 ± 5.14 |
| Day 30 | 0.263 ± 0.004 | 6.30 ± 0.02 | 88.96 ± 1.21 | 1.61 ± 0.11 | 8.70 ± 0.82 | 16.60 ± 1.11 | 77.57 ± 1.06 | 35.95 ± 1.14 | 16.74 ± 2.96 | |
| Day 60 | 0.261 ± 0.003 | 6.29 ± 0.03 | 87.38 ± 3.46 | 1.21 ± 0.22 | 9.28 ± 0.63 | 19.04 ± 1.42 | 79.30 ± 0.88 | 34.20 ± 0.54 | 19.41 ± 4.74 | |
| Day 90 | 0.262 ± 0.004 | 6.31 ± 0.01 | 88.07 ± 1.30 | 1.83 ± 0.31 | 7.33 ± 1.37 | 22.15 ± 1.61 | 81.84 ± 1.68 | 31.82 ± 1.21 | 25.27 ± 2.44 | |
| Skim Milk Powder | Day 1 | 0.261 ± 0.004 | 6.30 ± 0.03 | 88.37 ± 3.66 | 1.52 ± 0.14 | 9.24 ± 0.37 | 17.74 ± 1.04 | 79.82 ± 0.45 | 34.54 ± 0.87 | 19.62 ± 1.14 |
| Day 30 | 0.262 ± 0.005 | 6.29 ± 0.02 | 88.27 ± 2.85 | 1.65 ± 0.19 | 8.46 ± 0.48 | 20.39 ± 0.98 | 81.34 ± 0.83 | 30.57 ± 0.57 | 25.69 ± 2.44 | |
| Day 60 | 0.262 ± 0.003 | 6.31 ± 0.02 | 88.68 ± 1.55 | 1.27 ± 0.21 | 9.04 ± 0.54 | 23.57 ± 1.07 | 84.43 ± 0.88 | 27.04 ± 1.37 | 29.55 ± 1.25 | |
| Day 90 | 0.261 ± 0.005 | 6.31 ± 0.01 | 89.53 ± 1.48 | 1.61 ± 0.25 | 8.11 ± 0.39 | 25.18 ± 1.57 | 87.35 ± 0.83 | 23.94 ± 0.78 | 39.22 ± 1.13 |
| TA | pH | L* | a* | b* | First Drip (min.) | Complete Melting (min.) | Overrun (%) | Hardnes (N) | |
|---|---|---|---|---|---|---|---|---|---|
| Control | 0.263 a | 6.30 a | 88.70 a | 1.44 a | 9.06 a | 16.55 ab | 77.48 ab | 34.33 b | 13.09 a |
| Whey Powder | 0.263 a | 6.31 a | 88.58 a | 1.54 a | 8.49 a | 18.37 b | 79.58 b | 32.86 b | 24.65 bc |
| Sorbitol | 0.262 a | 6.30 a | 88.31 a | 1.44 a | 8.95 a | 15.88 a | 75.46 a | 37.19 c | 16.16 a |
| Whole Milk Powder | 0.262 a | 6.31 a | 88.30 a | 1.55 a | 8.58 a | 18.40 b | 78.72 b | 34.82 b | 19.09 ab |
| Skim Milk Powder | 0.262 a | 6.31 a | 88.71 a | 1.51 a | 8.71 a | 21.72 c | 83.23 c | 29.02 a | 28.52 c |
| Day | Tonset (°C) | Tend (°C) | Enthalpy (J/g) | |
|---|---|---|---|---|
| Control | Day 1 | −16.02 ± 1.36 | 9.89 ± 0.44 | 127.70 ± 3.54 |
| Day 90 | −12.99 ± 0.76 | 10.99 ± 0.42 | 124.76 ± 3.39 | |
| Whey Powder | Day 1 | −15.25 ± 1.34 | 12.31 ± 2.19 | 140.57 ± 7.98 |
| Day 90 | −15.00 ± 1.08 | 12.00 ± 1.02 | 141.68 ± 3.15 | |
| Sorbitol | Day 1 | −13.71 ± 0.77 | 9.91 ± 1.82 | 131.60 ± 0.69 |
| Day 90 | −14.25 ± 1.06 | 10.62 ± 0.59 | 127.28 ± 9.79 | |
| Whole Milk Powder | Day 1 | −14.02 ± 1.31 | 11.29 ± 0.89 | 139.18 ± 4.29 |
| Day 90 | −14.23 ± 1.37 | 11.96 ± 2.41 | 136.18 ± 5.86 | |
| Skim Milk Powder | Day 1 | −13.29 ± 1.29 | 9.31 ± 1.05 | 139.13 ± 3.86 |
| Day 90 | −12.12 ± 2.64 | 9.54 ± 0.69 | 134.41 ± 5.81 |
| Tonset | Tend | Enthalpy | |
|---|---|---|---|
| Control | −14.50 a | 10.44 a | 126.23 a |
| Whey Powder | −15.12 a | 12.00 a | 140.00 b |
| Sorbitol | −13.98 a | 10.26 a | 129.44 ab |
| Whole Milk Powder | −14.12 a | 11.62 a | 137.68 ab |
| Skim Milk Powder | −12.71 a | 9.42 a | 136.77 ab |
| Day | G′ (Elastic) (Pa) | G″ (Viscos) (Pa) | G* (Complex) (Pa) | Viscosity (Pa.s) | |
|---|---|---|---|---|---|
| Control | Day 1 | 21.97 ± 2.19 | 11.28 ± 1.45 | 25.87 ± 0.66 | 20.92 ± 3.21 |
| Day 90 | 20.50 ± 2.13 | 15.34 ± 1.22 | 29.28 ± 1.59 | 24.50 ± 1.84 | |
| Whey Powder | Day 1 | 16.07 ± 0.64 | 11.58 ± 1.02 | 30.40 ± 2.41 | 21.18 ± 3.17 |
| Day 90 | 17.30 ± 1.70 | 13.53 ± 3.95 | 23.68 ± 3.85 | 24.73 ± 4.82 | |
| Sorbitol | Day 1 | 14.64 ± 1.10 | 9.43 ± 1.10 | 22.35 ± 2.76 | 19.95 ± 0.42 |
| Day 90 | 19.04 ± 1.81 | 14.38 ± 1.24 | 30.87 ± 4.79 | 22.88 ± 3.42 | |
| Whole Milk Powder | Day 1 | 29.51 ± 1.34 | 19.96 ± 0.57 | 39.30 ± 1.27 | 29.27 ± 2.93 |
| Day 90 | 26.75 ± 2.05 | 17.35 ± 1.20 | 36.37 ± 5.98 | 23.25 ± 4.17 | |
| Skim Milk Powder | Day 1 | 23.38 ± 4.21 | 16.85 ± 2.12 | 38.35 ± 5.44 | 26.68 ± 2.32 |
| Day 90 | 28.93 ± 4.99 | 20.57 ± 0.64 | 31.53 ± 10.00 | 27.33 ± 4.20 |
| G′ | G″ | G* | Viscosity | |
|---|---|---|---|---|
| Control | 21.05 a | 13.31 a | 27.57 ab | 22.71 a |
| Whey Powder | 16.69 b | 12.55 a | 27.04 a | 22.95 a |
| Sorbitol | 16.84 b | 11.90 a | 26.61 a | 21.41 a |
| Whole Milk Powder | 26.15 c | 18.66 b | 37.84 b | 26.26 a |
| Skim Milk Powder | 28.13 c | 18.71 b | 34.94 ab | 27.01 a |
| Day | L. acidophilus (log CFU/g) | Viability Rate (%) | |
|---|---|---|---|
| Control | Day 1 | 5.04 ± 0.02 | |
| Day 30 | 4.07 ± 0.06 | ||
| Day 60 | 3.23 ± 0.06 | ||
| Day 90 | 3.00 ± 0.03 | ||
| Day 150 | 2.18 ± 0.06 | ||
| Whey Powder | Day 1 | 9.95 ± 0.14 | 96.79 |
| Day 30 | 8.73 ± 0.06 | 84.92 | |
| Day 60 | 8.00 ± 0.03 | 77.82 | |
| Day 90 | 7.22 ± 0.09 | 70.23 | |
| Day 150 | 6.88 ± 0.02 | 66.93 | |
| Sorbitol | Day 1 | 9.99 ± 0.07 | 92.67 |
| Day 30 | 8.70 ± 0.03 | 80.71 | |
| Day 60 | 8.35 ± 0.08 | 77.46 | |
| Day 90 | 8.08 ± 0.08 | 74.95 | |
| Day 150 | 7.23 ± 0.08 | 67.07 | |
| Whole Milk Powder | Day 1 | 10.05 ± 0.09 | 90.30 |
| Day 30 | 9.27 ± 0.05 | 83.29 | |
| Day 60 | 8.47 ± 0.03 | 76.10 | |
| Day 90 | 8.10 ± 0.03 | 72.78 | |
| Day 150 | 7.69 ± 0.09 | 69.10 | |
| Skim Milk Powder | Day 1 | 9.91 ± 0.12 | 93.14 |
| Day 30 | 9.14 ± 0.07 | 85.90 | |
| Day 60 | 8.80 ± 0.03 | 82.71 | |
| Day 90 | 7.76 ± 0.06 | 72.93 | |
| Day 150 | 7.32 ± 0.05 | 68.80 |
| L. acidophilus | |
|---|---|
| Control | 3.51 a |
| Whey Powder | 8.16 b |
| Sorbitol | 8.47 c |
| Whole Milk Powder | 8.71 e |
| Skim Milk Powder | 8.59 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kilinç, M.; Sevik, R. Effect of Dairy Powders and Sorbitol-Based Encapsulation Systems on Functional, Thermal, and Microstructural Quality of Probiotic Ice Cream. Processes 2025, 13, 3803. https://doi.org/10.3390/pr13123803
Kilinç M, Sevik R. Effect of Dairy Powders and Sorbitol-Based Encapsulation Systems on Functional, Thermal, and Microstructural Quality of Probiotic Ice Cream. Processes. 2025; 13(12):3803. https://doi.org/10.3390/pr13123803
Chicago/Turabian StyleKilinç, Mehmet, and Ramazan Sevik. 2025. "Effect of Dairy Powders and Sorbitol-Based Encapsulation Systems on Functional, Thermal, and Microstructural Quality of Probiotic Ice Cream" Processes 13, no. 12: 3803. https://doi.org/10.3390/pr13123803
APA StyleKilinç, M., & Sevik, R. (2025). Effect of Dairy Powders and Sorbitol-Based Encapsulation Systems on Functional, Thermal, and Microstructural Quality of Probiotic Ice Cream. Processes, 13(12), 3803. https://doi.org/10.3390/pr13123803





