Rapid Screening and Identification of Illegally Adulterated PDE-5 Inhibitors in Health Wines by UPLC-TOF-MS
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals, Reagents, and Instruments
2.2. Sample Preparation
2.3. Standard Solution Preparation
2.4. Sample Pretreatment
2.5. UPLC–TOF/MS Conditions
2.6. Establishment of the High-Resolution Screening Database
2.7. Method Validation Procedure
3. Results
3.1. Establishment and Application of the High-Resolution Screening Database
3.2. Optimization of Instrumental Conditions
Selection of the Chromatographic Column
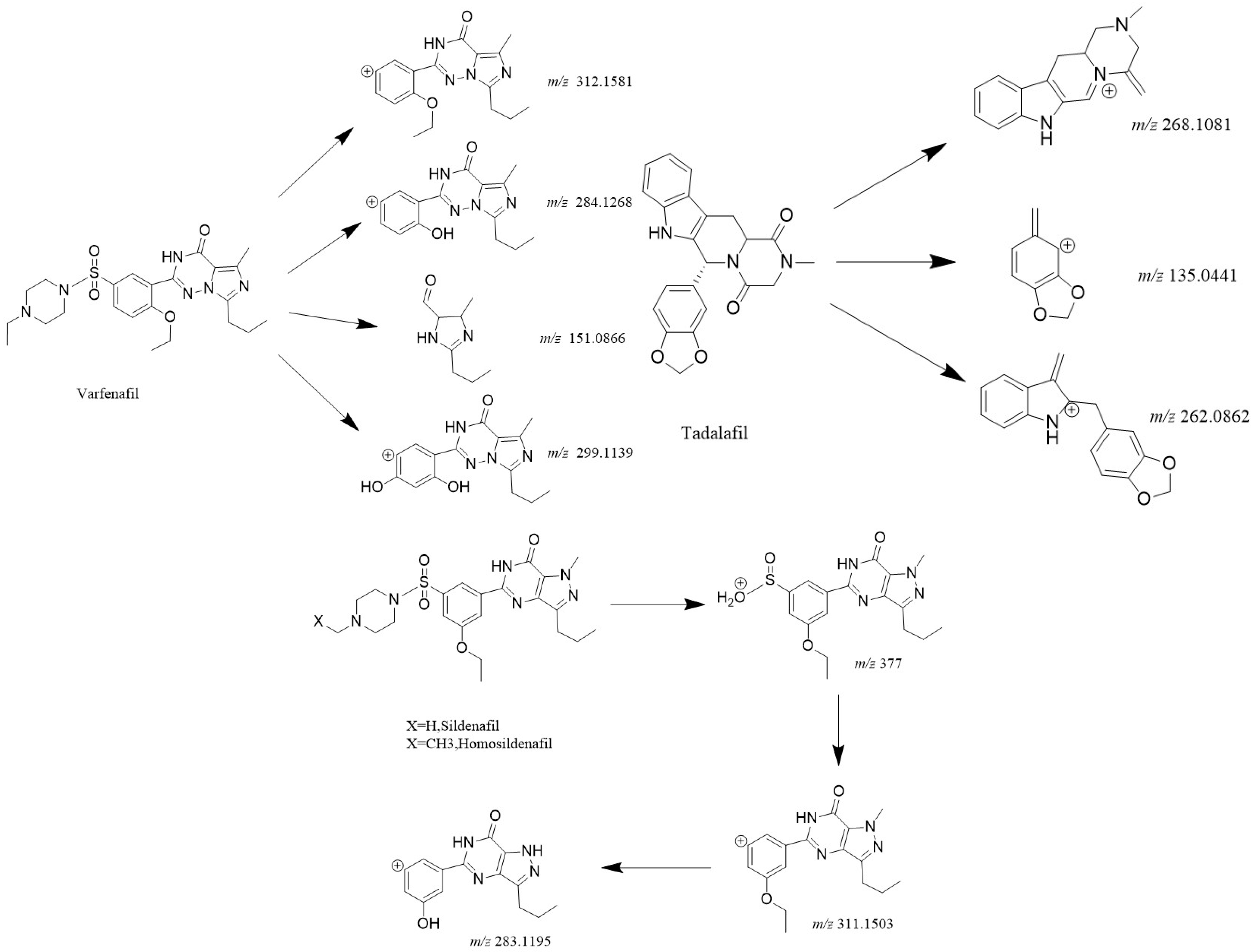
3.3. Mass Spectrometric Fragmentation Pathway Analysis of Representative PDE-5 Inhibitors
3.3.1. Sildenafil (Prototype Drug)
3.3.2. Tadalafil (Structural Isomer)
3.3.3. Vardenafil (N-Ethylpiperazine Variant)
3.4. Method Validation Results
3.5. Analysis of Real Samples and Discussion
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Song, K.X. “Healthy China” Under the Background of Far Wordbrand Guilingji Wine, Marketing Strategy Research. Master’s Thesis, Shanxi University of Finance and Economics, Taiyuan, China, 2023. [Google Scholar] [CrossRef]
- Luo, J. Research on the Marketing Strategy About the Health Wine of J Company. Master’s Thesis, Nanchang University, Nanchang, China, 2023. [Google Scholar]
- Guo, M. Extraction of Active Ingredients from Cistanche deserticola and Its Application in Health Wine. Master’s Thesis, Tianjin University of Science and Technology, Tianjin, China, 2023. [Google Scholar] [CrossRef]
- Wang, J.W.; Tang, X.; Li, S.C.; Zhang, Q.; Deng, J.; Wang, Y.J.; Li, B.C. Qualitative Analysis of Seven Components in Healthcare Liqueur by LC-MS. Liquor-Mak. Sci. Technol. 2022, 1, 112–119. [Google Scholar] [CrossRef]
- Chen, Y.; Yan, L.J.; Xu, D.M.; Li, C.Y.; Sun, T. Simultaneous Determination of 90 Phosphodiesterase-5 (PDE-5) Inhibitor Analogues in Distilled Liquors and Their Configured Alcoholic Products by High-Performance Liquid Chromatography-Tandem Mass Spectrometry. J. Instrum. Anal. 2023, 42, 519–530. [Google Scholar] [CrossRef]
- Huang, F.; Deng, X.; Zhang, Q.Y.; Zhou, X.; Liang, Z.Y.; Xu, C.F.; Lai, X.N.; Luo, H.T.; Wu, H.Q. Rapid Screening and Determination of 112 Illegally Adulterated Drugs in Anti-Fatigue Health Foods by Ultra-High Performance Liquid Chromatography-Quadrupole/Orbitrap High-Resolution Mass Spectrometry. J. Instrum. Anal. 2023, 42, 1221–1232. [Google Scholar] [CrossRef]
- Liu, Y.T.; Guo, Y.X.; Liu, Z.Q.; Liu, J.P.; Wang, H.; Liu, Y.; Dai, J.Y.; Wang, M.L. Screening and Quantitative Determination of a Novel Vardenafil Analogue in Food. J. Instrum. Anal. 2023, 42, 1488–1494+1502. [Google Scholar] [CrossRef]
- Gao, J.M.; Duan, Q.; Ma, C.Y.; Li, H.; Gao, W.H. Research Progress on Detection Technologies of Illegally Adulterated Substances in Health Foods. China Brew. 2021, 40, 19–24. Available online: https://manu61.magtech.com.cn/zgnz/EN/10.11882/j.issn.0254-5071.2021.09.004 (accessed on 21 November 2025).
- Ghofrani, H.; Osterloh, I.; Grimminger, F. Sildenafil: From angina to erectile dysfunction to pulmonary hypertension and beyond. Nat. Rev. Drug Discov. 2006, 5, 689–702. [Google Scholar] [CrossRef]
- Bertici, R.A.; Ridichie, A.; Bertici, N.S.; Ledeţi, A.; Ledeţi, I.; Văruţ, R.-M.; Sbârcea, L.; Albu, P.; Rădulescu, M.; Rusu, G.; et al. Compatibility Studies of Sildenafil-HPBCD Inclusion Complex with Pharmaceutical Excipients. Pharmaceutics 2025, 17, 1114. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Guo, C.C.; Shi, F.; Zeng, S.; Jiang, W. Research Progress on Detection Methods of Phosphodiesterase-5 (PDE-5) Inhibitors and Their Analogues Adulterated in Kidney-Tonifying and Yang-Invigorating Proprietary Chinese Medicines and Health Products. Chin. J. Pharm. Anal. 2018, 38, 566–574. [Google Scholar] [CrossRef]
- Liu, L.J. Clinical Study of Low-Intensity Extracorporeal Shock Wave Treatment of Erectile Dysfunction That Does Not Respond to PDESI. Master’s Thesis, Henan University, Kaifeng, China, 2021. [Google Scholar] [CrossRef]
- Zhang, T.J. The Clinical Analysis of EECP Combined with Low dosePDE5 Inhibitor in the Treatment of Diabetes Mellitus Erectile Dysfunction. Master’s Thesis, Shandong University, Jinan, China, 2020. [Google Scholar] [CrossRef]
- Lei, Y.; Huang, Y.T.; Luo, Z.Y. Determination of Four Thiosildenafil Analogs in Yang-Invigorating Health Products by High-Performance Liquid Chromatography. Phys. Test. Chem. Anal. Part B Chem. Anal. 2014, 50, 530–535. [Google Scholar]
- Wang, Z.L.; Zhang, J.L.; Zhang, Y.N. Determination of Four PDE-5 Inhibitor Drugs in Health Products by Gas Chromatography-Mass Spectrometry. J. Chin. Mass Spectrom. Soc. 2009, 30, 278–281. Available online: https://zpxb.xml-journal.net/en/article/id/zpxb-2124?viewType=citedby-info (accessed on 21 November 2025).
- Huang, Q.; Wu, H.; Qin, X. Extract of Pfaffia glomerata Ameliorates Paroxetine-Induced Sexual Dysfunction in Male Mice and the Characterization of Its Phytoconstituents by UPLC-MS. Foods 2023, 12, 3236. [Google Scholar] [CrossRef]
- Gumułka, P.; Żandarek, J.; Dąbrowska, M.; Starek, M. UPLC Technique in Pharmacy—An Important Tool of the Modern Analyst. Processes 2022, 10, 2498. [Google Scholar] [CrossRef]
- Wang, K.L.; Zhang, X.R.; Chen, F.Q.; Ni, Y.F.; Cai, Z.W.; Ding, J.F.; Chen, S.C.; Shu, C.; Ding, L. LC-MS/MS Methods for Simultaneous Determination of Youkenafil and Its Metabolite M1 in Human Seminal Plasma and Plasma: Application to Evaluate the Acute Effect of Youkenafil on Semen Quality and Its Pharmacokinetics in Human. J. Chromatogr. B 2024, 1237, 124105. [Google Scholar] [CrossRef]
- Zhou, Y.R.; Shan, M.; Li, Z.G.; Li, Y.; Zhang, L.; Wang, J. Determination of 11 Illegally Added Drugs in Typical Yang-Invigorating Chinese Herbal Medicines by Sin-QuEChERS Combined with LC-MS/MS. J. Pharm. Res. 2025, 44, 559–562+604. [Google Scholar] [CrossRef]
- Ma, J.M.; Wang, J.; Sun, W.Y.; Jiang, J.; Li, Q.; Fan, S.F. Determination of 90 nafils in health foods by ultra-highperformance liguid chromatography-tandem mass spectrometryyl. Food Sci. 2020, 41, 307–313. [Google Scholar] [CrossRef]
- Wang, J.; Ren, C.; Wang, J.; Fu, J.; Yin, Q.; Huang, Y.; He, Z. Liquid Chromatography Quadrupole Time-of-Flight Mass Spectrometry-Based Metabolic Characterization of Mango Ripened by Different Methods. Foods 2024, 13, 3548. [Google Scholar] [CrossRef]
- Eissa, M.A.; Hashim, Y.Z.H.-Y.; El-Kersh, D.M.; Abd-Azziz, S.S.S.; Salleh, H.M.; Isa, M.L.M.; Abd Warif, N.M. Metabolite Profiling of Aquilaria malaccensis Leaf Extract Using Liquid Chromatography-Q-TOF-Mass Spectrometry and Investigation of Its Potential Antilipoxygenase Activity In-Vitro. Processes 2020, 8, 202. [Google Scholar] [CrossRef]
- Darnal, A.; Poggesi, S.; Longo, E.; Arbore, A.; Boselli, E. Decoding the Identity of Pinot Gris and Pinot Noir Wines: A Comprehensive Chemometric Fusion of Sensory (from Dual Panel) and Chemical Analysis. Foods 2024, 13, 18. [Google Scholar] [CrossRef]
- Meng, Z.; Zheng, L.; Fang, H.; Yang, P.; Wang, B.; Li, L.; Wang, M.; Feng, W. Single Particle Inductively Coupled Plasma Time-of-Flight Mass Spectrometry—A Powerful Tool for the Analysis of Nanoparticles in the Environment. Processes 2023, 11, 1237. [Google Scholar] [CrossRef]
- Lin, C.C.; Zhu, X.L.; Zhang, X.X.; Zhu, X.N.; Liu, R.; Lu, Q. Authentic identification of Shennong multifloral honey based on metabolomics technology. J. Food Saf. Qual. 2023, 14, 213–221. [Google Scholar] [CrossRef]
- Liu, T.T.; Fang, F.; Luo, J.Y.; Cao, J.; Sun, S.S. Determination of A2 β-casein in Milk Powder by Liquid Chromatography High Resolution Tandem Mass Spectrometry and Study on The Relationship Between B-casein and Whey Protein. Food Nutr. China 2022, 28, 33–37. [Google Scholar] [CrossRef]
- Miao, Y.; Hu, G.; Sun, X.; Li, Y.; Huang, H.; Fu, Y. Comparing the Volatile and Soluble Profiles of Fermented and Integrated Chinese Bayberry Wine with HS-SPME GC–MS and UHPLC Q-TOF. Foods 2023, 12, 1546. [Google Scholar] [CrossRef]
- Xu, X.; Lu, D.; Huang, S.; Wang, F.; Min, Y.; Xu, Q. Multiscale Insights into Inorganic Filler Regulation, Ion Transport Mechanisms, and Characterization Advances in Composite Solid-State Electrolytes. Processes 2025, 13, 2795. [Google Scholar] [CrossRef]
- Fan, C.L.; Wang, H.B.; Jiang, P.; Shen, E.Y. UPLC-Q-TOF-MSE with UNIFl for High-Throughput Screening of 141 lllegal Additives in Facial Mask Cosmetics. J. Anal. Sci. 2025, 41, 405–414. [Google Scholar] [CrossRef]
- Diamantidou, D.; Tsochatzis, E.; Kalogiannis, S.; Alberto Lopes, J.; Theodoridis, G.; Gika, H. Analysis of Migrant Cyclic PET Oligomers in Olive Oil and Food Simulants Using UHPLC-qTOF-MS. Foods 2023, 12, 2739. [Google Scholar] [CrossRef] [PubMed]
- Niño-Ramírez, V.A.; Maldonado, M.; Cuero-Amu, K.J.; García-Castañeda, J.E.; Rivera-Monroy, Z.J. Challenges in the Characterization and Purification of (Peptide)n-Calix [4]Resorcinarene Conjugates Synthesized via Thiol-Maleimide Reaction Using Liquid Chromatography. Processes 2025, 13, 222. [Google Scholar] [CrossRef]
- Liu, M.; Su, F.; He, Y.; Sun, M.; Bai, C.; Zhang, W.; Li, B.; Sun, Y.; Wang, Q.; Kuang, H. An Extensive Analysis of Artemisia integrifolia Linn. on T2DM: Investigating Glycolipid Metabolism, Metabolic Profiling, and Molecular Docking for Potential Functional Food Applications. Foods 2025, 14, 2945. [Google Scholar] [CrossRef]
- Sagu, S.T.; Huschek, G.; Braga, T.W.; Rackiewicz, M.; Homann, T.; Rawel, H.M. Design of Experiment (DoE) for Optimization of HPLC Conditions for the Simultaneous Fractionation of Seven α-Amylase/Trypsin Inhibitors from Wheat (Triticum aestivum L.). Processes 2022, 10, 259. [Google Scholar] [CrossRef]
- Xue, L.; Bao, W.Y.B.L.G.; Li, G.R.L.; Li, T.L.G. Components analysis of the toxic and effective parts of the Mongolian medicine Euphorbiae Fischerianae Radix by UPLC-Q-TOF-MS/MS. Nat. Prod. Res. Dev. 2025, 37, 1671–1678+1705. [Google Scholar] [CrossRef]
- Shi, S. Construction of Sereening Database of Anti-Impotence Medicines Illegally Added to Dietary Supplements and Sex Hormones Illegally Added to Cosmetics based on UPLC-Q-TOF-MS. Master’s Thesis, Zhejiang Chinese Medical University, Hangzhou, China, 2020. [Google Scholar]
- Wang, Y.Q.; Wang, Y.C.; Chen, L.; Zhou, M.; Wang, Y.Q.; Gu, R.Z.; Ji, F.; Zheng, X. Screening for 26 illegally added chemicals in Chinesetraditional formulated medicine and health products by ultra-high performance liquid chromatography quadrupole-time-of-fight mass spectrometry. Food Ferment. Ind. 2020, 46, 263–269. [Google Scholar] [CrossRef]
- Poggesi, S.; Darnal, A.; Ceci, A.T.; Longo, E.; Vanzo, L.; Mimmo, T.; Boselli, E. Fusion of 2DGC-MS, HPLC-MS and Sensory Data to Assist Decision-Making in the Marketing of International Monovarietal Chardonnay and Sauvignon Blanc Wines. Foods 2022, 11, 3458. [Google Scholar] [CrossRef] [PubMed]
- Pollini, L.; Riccio, A.; Juan, C.; Tringaniello, C.; Ianni, F.; Blasi, F.; Mañes, J.; Macchiarulo, A.; Cossignani, L. Phenolic Acids from Lycium barbarum Leaves: In Vitro and In Silico Studies of the Inhibitory Activity against Porcine Pancreatic α-Amylase. Processes 2020, 8, 1388. [Google Scholar] [CrossRef]
- Yu, H.; Hu, Q.; Sun, J.; Feng, R.; Zhang, S.; Zhang, J.X.; Mao, X.H.; Ji, S. Qualitative analysis of illegally adulterated sildenafil and related compounds in dietary supplements by ultra-high performance liquid chromatography quadrupole-time-of-flight mass spectrometry. Chin. J. Chromatogr. 2018, 36, 1005–1017. [Google Scholar] [CrossRef]
- Bertrand, R. Proposed Confidence Scale and ID Score in the Identification of Known-Unknown Compounds Using High Resolution MS Data. J. Am. Soc. Mass Spectrom. 2017, 28, 709–723. [Google Scholar] [CrossRef]
- Zalidis, A.P.; Kalogiouri, N.P.; Mourtzinos, I.; Sarris, D.; Gkatzionis, K. Development and Validation of a LC-QTOF-MS/MS Method to Assess the Phenolic Profile of Pulse Flours. Molecules 2025, 30, 2730. [Google Scholar] [CrossRef]
- Dias, L.; Milheiro, J.; Ribeiro, M.; Fernandes, C.; Neves, N.; Filipe-Ribeiro, L.; Cosme, F.; Nunes, F.M. Fast and Simple UPLC–Q-TOF MS Method for Determination of Bitter Flavan-3-ols and Oligomeric Proanthocyanidins: Impact of Vegetable Protein Fining Agents on Red Wine Composition. Foods 2023, 12, 3313. [Google Scholar] [CrossRef]
- Hu, T.T.; Qu, X.Y.; Kang, M.Q.; Song, Q.L.; Yang, L.; Zhao, Y.H.; Zhang, D.H. Rapid screening and confirmation of illegally added antiimpotence preparations in health care products by high performance liquid chromatography-high resolution mass spectrometry. Chin. J. Chromatogr. 2015, 33, 897–901. [Google Scholar] [CrossRef]
- State Administration for Market Regulation. Announcement on Food Safety Supervision and Sampling Inspection Information (No. 25 of 2023). Available online: https://www.samr.gov.cn/ (accessed on 15 June 2023).
- Li, J.H.; Zhang, C.H.; Wang, L.; Han, S.; Cheng, J.; He, Y.; Cui, F.Y.; Zhao, S.Z.; Liu, W.H.; Li, X.L.; et al. Research progress of analysis of illegal additives in weight-reducing dietary supplements. J. Food Saf. Qual. 2017, 8, 1585–1595. [Google Scholar] [CrossRef]
- Kim, B.H.; Lee, W.; Kim, Y.L.; Lee, J.H.; Hong, J. Efficient Matrix Cleanup of Soft-Gel-Type Dietary Supplements for Rapid Screening of 92 Illegal Adulterants Using EMR-Lipid dSPE and UHPLC-Q/TOF-MS. Pharmaceuticals 2021, 14, 570. [Google Scholar] [CrossRef]
- GB/T 27404-2008; Criterion on Quality Control of Laboratories—Chemical Testing of Food. Standards Press of China: Beijing, China, 2008.
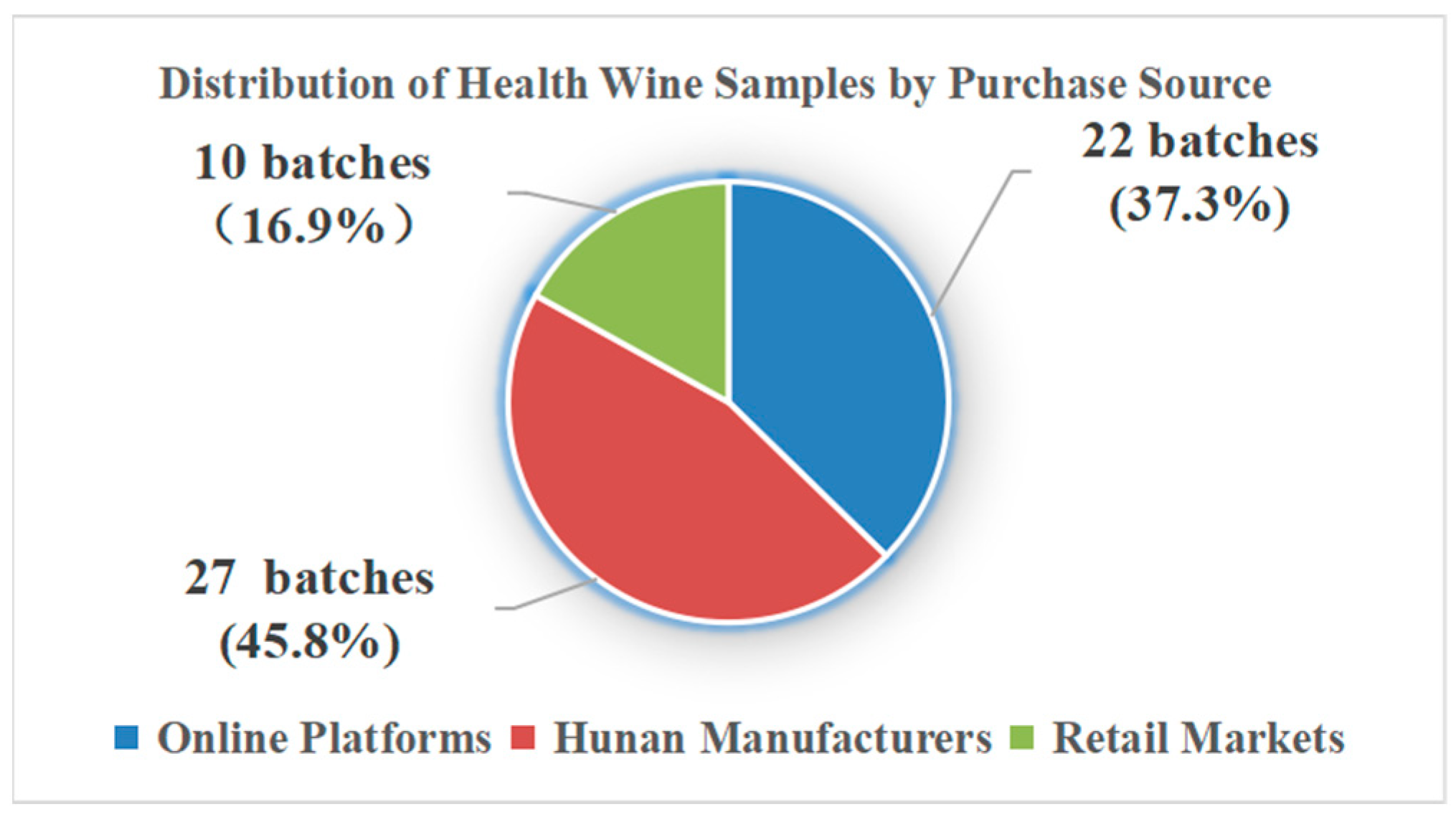
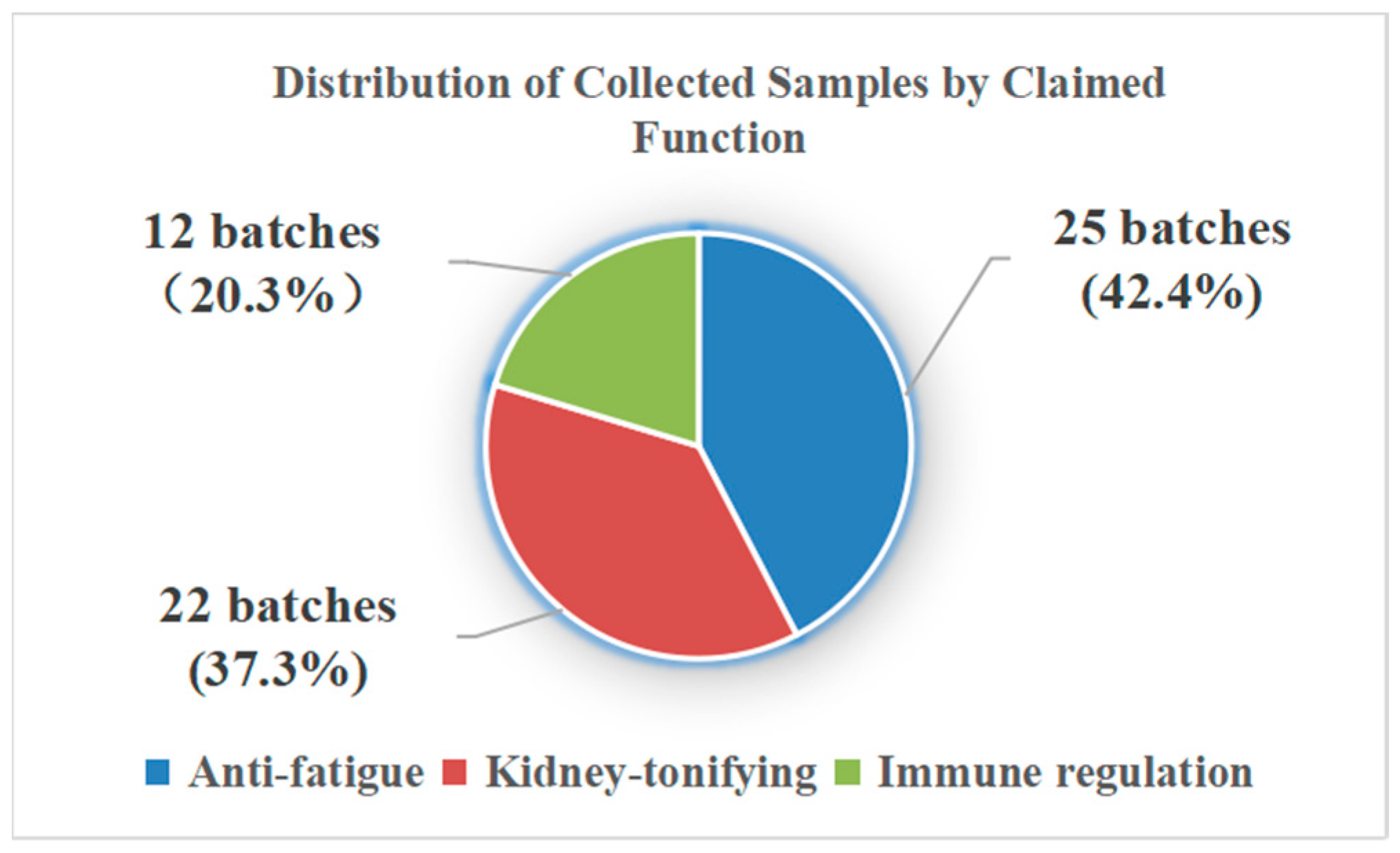
| Sample ID | Claimed Function | Purchase Source | Specification | Note | Sample ID | Claimed Function | Purchase Source | Specification | Note |
|---|---|---|---|---|---|---|---|---|---|
| S-01 | General Health | Retail Market | 500 mL, 35% vol. | ND | S-31 | Kidney-tonifying | Online Platform | 100 mL, 30% vol. | ND |
| S-02 | Kidney-tonifying | Online Platform | 135 mL, 32% vol. | ND | S-32 | Kidney-tonifying | Online Platform | 500 mL, 35% vol. | ND |
| S-03 | Kidney-tonifying | Online Platform | 125 mL, 35% vol. | ND | S-33 | General Health | Direct from Manufacturer | 500 mL, 35% vol. | ND |
| S-04 | Kidney-tonifying | Retail Market | 125 mL, 32% vol. | ND | S-34 | General Health | Direct from Manufacturer | 150 mL, 31% vol. | ND |
| S-05 | Kidney-tonifying | Retail Market | 1000 mL, 42% vol. | ND | S-35 | General Health | Direct from Manufacturer | 500 mL, 50% vol. | ND |
| S-06 | Kidney-tonifying | Retail Market | 500 mL, 35% vol. | ND | S-36 | General Health | Direct from Manufacturer | 750 mL, 8% vol. | ND |
| S-07 | Anti-fatigue | Retail Market | 600 mL, 35% vol. | ND | S-37 | General Health | Direct from Manufacturer | 500 mL, 50% vol. | ND |
| S-08 | Anti-fatigue | Retail Market | 125 mL, 36% vol. | ND | S-38 | General Health | Direct from Manufacturer | 500 mL, 50% vol. | ND |
| S-09 | Anti-fatigue | Retail Market | 500 mL, 38% vol. | ND | S-39 | General Health | Direct from Manufacturer | 500 mL, 42% vol. | ND |
| S-10 | General Health | Retail Market | 500 mL, 38% vol. | ND | S-40 | General Health | Direct from Manufacturer | 500 mL, 52% vol. | ND |
| S-11 | General Health | Retail Market | 500 mL, 42% vol. | ND | S-41 | Kidney-tonifying | Direct from Manufacturer | 1000 mL, 42% vol. | ND |
| S-12 | General Health | Retail Market | 2500 mL, 38% vol. | ND | S-42 | Kidney-tonifying | Direct from Manufacturer | 500 mL, 35% vol. | ND |
| S-13 | Kidney-tonifying | Online Platform | 500 mL, 35% vol. | ND | S-43 | General Health | Direct from Manufacturer | 500 mL, 52% vol. | ND |
| S-14 | General Health | Online Platform | 110 mL, 33% vol. | ND | S-44 | General Health | Direct from Manufacturer | 100 mL, 42% vol. | ND |
| S-15 | General Health | Online Platform | 110 mL, 33% vol. | ND | S-45 | General Health | Direct from Manufacturer | 500 mL, 52% vol. | ND |
| S-16 | General Health | Online Platform | 500 mL, 38% vol. | ND | S-46 | General Health | Direct from Manufacturer | 500 mL, 52% vol. | ND |
| S-17 | General Health | Online Platform | 75 mL, 35% vol. | ND | S-47 | Kidney-tonifying | Direct from Manufacturer | 500 mL, 53% vol. | Positive (Tadalafil) |
| S-18 | General Health | Online Platform | 500 mL, 35% vol. | ND | S-48 | General Health | Direct from Manufacturer | 500 mL, 52% vol. | ND |
| S-19 | Kidney-tonifying | Online Platform | 500 mL, 35% vol. | ND | S-49 | General Health | Direct from Manufacturer | 500 mL, 52% vol. | ND |
| S-20 | Kidney-tonifying | Online Platform | 125 mL, 38% vol. | ND | S-50 | General Health | Direct from Manufacturer | 500 mL, 45% vol. | ND |
| S-21 | General Health | Online Platform | 125 mL, 38% vol. | ND | S-51 | General Health | Direct from Manufacturer | 275 mL, 3.0% vol. | ND |
| S-22 | Kidney-tonifying | Online Platform | 110 mL, 38% vol. | ND | S-52 | General Health | Direct from Manufacturer | 275 mL, 3.0% vol. | ND |
| S-23 | Kidney-tonifying | Online Platform | 500 mL, 53% vol. | Positive (Tadalafil) | S-53 | General Health | Direct from Manufacturer | 500 mL, 26% vol. | ND |
| S-24 | Kidney-tonifying | Online Platform | 500 mL, 53% vol. | ND | S-54 | General Health | Direct from Manufacturer | 500 mL, 42% vol. | ND |
| S-25 | Kidney-tonifying | Online Platform | 100 mL, 38% vol. | ND | S-55 | General Health | Direct from Manufacturer | 500 mL, 42% vol. | ND |
| S-26 | General Health | Online Platform | 740 mL, 12% vol. | ND | S-56 | General Health | Direct from Manufacturer | 125 mL, 42% vol. | ND |
| S-27 | General Health | Online Platform | 500 mL, 30% vol. | ND | S-57 | General Health | Direct from Manufacturer | 125 mL, 42% vol. | ND |
| S-28 | General Health | Online Platform | 100 mL, 30% vol. | ND | S-58 | General Health | Direct from Manufacturer | 500 mL, 42% vol. | Positive (Sildenafil) |
| S-29 | Kidney-tonifying | Online Platform | 500 mL, 35% vol. | ND | S-59 | General Health | Direct from Manufacturer | 300 mL, 45% vol. | ND |
| S-30 | Kidney-tonifying | Online Platform | 500 mL, 38% vol. | ND |
| No. | Compound Name | Molecular Formula | Precursor Ions (m/z) | Mass Error (ppm) | Retention Time (min) | Major Product Ions (m/z) | CAS No. | Number of MS/MS Spectra | Ionization Mode |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Sildenafil | C22H30N6O4S | 475.2122 | 1.5 | 5.681 | 377.1272, 283.1191, 151.0862, 100.0996 | 139755-83-2 | 2 | ESI+ |
| 2 | Tadalafil | C22H19N3O4 | 390.1448 | −0.8 | 7.097 | 268.1082, 262.0860, 240.1126, 169.0755, 151.0754, 135.0437 | 171596-29-5 | 2 | ESI+ |
| 3 | Imidazosagatriazinone | C17H20N4O2 | 313.1659 | 2.1 | 9.734 | 285.1349, 256.0956, 241.0726, 201.0537, 166.0974, 136.0506, 120.0448 | 139756-21-1 | 2 | ESI+ |
| 4 | Gendenafil | C19H22N4O3 | 355.1765 | −1.2 | 8.334 | 327.1455, 311.1141, 298.1061, 285.1347, 256.0958, 216.0765, 166.0973 | 147676-66-2 | 2 | ESI+ |
| 5 | Acetil acid | C18H20N4O4 | 357.1557 | 0.5 | 7.075 | 329.1246, 300.0851, 285.1345, 268.1083, 256.0955, 166.0973 | 147676-78-6 | 2 | ESI+ |
| 6 | Xanthoanthrafil | C19H23N3O6 | 390.166 | −2.3 | 7.263 | 151.0755, 135.0437, 107.0491 | 1020251-53-9 | 2 | ESI+ |
| 7 | Aminotadalafil | C21H18N4O4 | 391.1401 | 3 | 6.433 | 269.1036, 262.0862, 233.0829, 205.0880, 169.0759, 135.0439 | 385769-84-6 | 2 | ESI+ |
| 8 | Chloropretadalafil | C22H19ClN2O5 | 427.1055 | −1.7 | 9.243 | 334.1077, 302.0811, 274.0864, 262.0864, 135.0440 | 171489-59-1 | 2 | ESI+ |
| 9 | Piperiacetildenafil | C24H31N5O3 | 438.25 | 0.9 | 5.576 | 341.1610, 325.1299, 313.1300, 297.1347, 166.0974 | 147676-50-4 | 1 | ESI+ |
| 10 | Carbodenafil | C24H32N6O3 | 453.2609 | −2.8 | 4.802 | 339.1452, 311.1141, 166.0973, 147.0076, 113.1076 | 944241-52-5 | 1 | ESI+ |
| 11 | Pseudovardenafil | C22H29N5O4S | 460.2013 | 1.1 | 8.332 | 312.1584, 301.1297, 299.1144, 284.1270, 151.0866 | 224788-34-5 | 2 | ESI+ |
| 12 | Norneosildenafil | C22H29N5O4S | 460.2013 | −0.4 | 10.199 | 432.1698, 329.1607, 312.1580, 299.1143, 283.1191, 256.0959 | 371959-09-0 | 2 | ESI+ |
| 13 | N-Desmethylsildenafil | C21H28N6O4S | 461.1966 | 2.5 | 4.725 | 377.1280, 361.1328, 311.1507, 299.1142, 283.1192 | 139755-82-1 | 1 | ESI+ |
| 14 | Acetildenafil | C25H34N6O3 | 467.2765 | −1.9 | 5.162 | 341.1610, 325.1657, 297.1347, 127.1230, 111.0916 | 831217-01-7 | 2 | ESI+ |
| 15 | Avanafil | C23H26ClN7O3 | 484.1858 | 0.7 | 5.633 | 375.1221, 357.1115, 233.1033, 155.0257 | 330784-47-9 | 2 | ESI+ |
| 16 | Vardenafil | C23H32N6O4S | 489.2279 | −2.1 | 4.853 | 376.1058, 312.1579, 285.1314, 198.0538, 151.0855, 133.1069 | 224785-90-4 | 2 | ESI+ |
| 17 | Thiosildenafil | C22H30N6O3S2 | 491.1894 | 1.8 | 7.557 | 341.1428, 327.1272, 313.1112, 299.0962, 100.0995 | 479073-79-5 | 2 | ESI+ |
| 18 | Hydroxyhomosildenafil | C23H32N6O5S | 505.2228 | −0.6 | 5.593 | 487.2119, 377.1277, 311.1505, 283.1191, 129.1020, 112.0995, 100.0977 | 139755-85-4 | 2 | ESI+ |
| 19 | Udenafil | C25H36N6O4S | 517.2592 | 2.8 | 6.201 | 474.2170, 325.1664, 299.1141, 283.1193, 191.0848, 112.1120 | 268203-93-6 | 1 | ESI+ |
| 20 | Hydroxythiohomosildenafil | C23H32N6O4S2 | 521.1999 | −1.4 | 7.407 | 503.1891, 327.1274, 315.0909, 299.0964, 129.1020, 112.0995 | 479073-82-0 | 2 | ESI+ |
| 21 | Norneovardenafil | C18H20N4O4 | 357.1557 | 0.3 | 5.416 | 329.1237, 300.0854, 151.0865, 123.0919 | 358390-39-3 | 2 | ESI+ |
| 22 | Nitrodenafil | C17H19N5O4 | 358.151 | −2.9 | 9.331 | 330.1199, 312.1582, 284.1269, 256.0956, 136.0506 | 147676-99-1 | 2 | ESI+ |
| 23 | Nortadalafil | C21H17N3O4 | 376.1292 | 1.6 | 6.561 | 302.0812, 274.0859, 263.0935, 262.0861, 254.0925, 169.0758, 135.0438 | 171596-36-4 | 2 | ESI+ |
| 24 | Hydroxychlorodenafil | C19H23ClN4O3 | 391.1531 | −0.9 | 8.114 | 363.1220, 313.1297, 285.1348, 256.0957, 166.0974 | 1391054-00-4 | 2 | ESI+ |
| 25 | N-Butyltadalafil | C25H25N3O4 | 432.1918 | 2.2 | 8.938 | 310.1553, 282.1601, 262.0861, 169.0762, 135.0440 | 171596-31-9 | 2 | ESI+ |
| 26 | Desmethylcarbodenafil | C23H30N6O3 | 439.2452 | −1.1 | 4.675 | 339.1454, 311.1141, 297.1345, 283.1188, 166.0974 | 147676-79-7 | 2 | ESI+ |
| 27 | Descarbonsildenafil | C21H30N6O4S | 463.2122 | 0.4 | 5.244 | 418.1544, 311.1505, 297.1343, 283.1192, 127.1229, 111.0914 | 1393816-99-3 | 2 | ESI+ |
| 28 | Oxohongdenafil | C25H32N6O4 | 481.2558 | −3.1 | 5.738 | 410.2191, 396.2030, 375.1223, 325.1298, 297.1348, 155.0259 | 1446144-70-2 | 2 | ESI+ |
| 29 | N-Octylnortadalafil | C29H33N3O4 | 488.2544 | 1.3 | 11.544 | 366.2177, 302.0812, 262.0862, 197.0708, 169.0759, 135.0440 | 1173706-35-8 | 2 | ESI+ |
| 30 | Dioxohongdenafil | C25H30N6O5 | 495.235 | −2 | 6.695 | 369.1383, 341.1070, 311.1139, 127.0865 | 1609405-33-5 | 2 | ESI+ |
| 31 | Hydroxythiovardenafil | C23H32N6O4S2 | 521.1999 | 2.7 | 6.212 | 393.1041, 328.1351, 315.0909, 299.0961, 167.0638 | 912576-30-8 | 1 | ESI+ |
| 32 | Cyclopentynafil | C26H36N6O4S | 529.2592 | −0.5 | 5.338 | 461.1966, 377.1268, 312.1581, 284.1263, 151.0866, 112.1118 | 1173706-34-7 | 2 | ESI+ |
| 33 | Propoxyphenyl thiohydroxyhomosildenafil | C24H34N6O4S2 | 535.2156 | 1.9 | 7.982 | 341.1430, 315.0911, 299.0964, 271.1010, 129.1020, 100.0979 | 479073-90-0 | 2 | ESI+ |
| 34 | Benzylsildenafil | C28H34N6O4S | 551.2435 | −1.8 | 7.053 | 459.1804, 377.1278, 355.1763, 312.1579, 134.0963, 117.0699 | 1446089-82-2 | 2 | ESI+ |
| 35 | Cinnamyldenafil | C32H38N6O3 | 555.3078 | 0.2 | 7.23 | 437.2299, 355.1767, 339.1455, 117.0696 | 1446089-83-3 | 2 | ESI+ |
| 36 | Lodenafil carbonate | C47H62N12O11S2 | 1035.4175 | −2.5 | 8.9 | 519.2206, 487.2120 | 398507-55-6 | 1 | ESI+ |
| 37 | Acetaminotadalafil | C23H20N4O5 | 433.1506 | 1.4 | 6.367 | 383.1717, 371.1717, 355.1395, 312.1580, 262.0861, 169.0758, 135.0439 | 1446144-71-3 | 2 | ESI+ |
| 38 | 2-Hydroxypropylnortadalafil | C24H23N3O5 | 434.171 | −0.7 | 6.804 | 312.1347, 284.1393, 262.0861, 197.0706, 169.0758, 135.0438 | 1353020-85-5 | 2 | ESI+ |
| 39 | Acetylvardenafil | C25H34N6O3 | 467.2765 | 2.9 | 4.105 | 396.2029, 355.1758, 341.1608, 297.1345, 151.0863, 127.1228, 111.0916 | 1261351-28-3 | 2 | ESI+ |
| 40 | Propoxyphenylhydroxyhomosildenafil | C24H34N6O5S | 519.2384 | −1.3 | 6.085 | 501.2274, 328.1351, 299.1071, 283.1190, 167.0639, 129.1020 | 139755-87-6 | 2 | ESI+ |
| 41 | Yohimbine | C21H26N2O3 | 355.2016 | 0.8 | 4.315 | 224.1280, 212.1281, 194.1176, 144.0807 | 146-48-5 | 2 | ESI+ |
| 42 | Dapoxetine | C21H23NO | 306.1852 | −2.2 | 7.086 | 261.1277, 233.0962, 183.0804, 157.0648, 117.0697 | 119356-77-3 | 2 | ESI+ |
| 43 | Desmethylthiosildenafil | C21H28N6O3S2 | 477.1737 | 1.7 | 7.436 | 393.1048, 327.1273, 315.0911, 299.0963, 271.1009 | 479073-86-4 | 2 | ESI+ |
| 44 | N-Boc-N-desethyl acetildenafil | C28H38N6O5 | 539.2976 | −0.9 | 6.898 | 439.2455, 353.1609, 339.1816, 297.1346, 100.0948 | 1246820-46-1 | 2 | ESI+ |
| 45 | N-Ethyltadalafil | C23H21N3O4 | 404.1605 | 2.4 | 7.622 | 364.1072, 282.1238, 262.0862, 169.0759, 135.0438 | 1609405-34-6 | 2 | ESI+ |
| 46 | O-Desethylsildenafil | C20H26N6O4S | 447.1809 | −1.6 | 5.869 | 415.1980, 371.1715, 347.0811, 283.1192, 101.1076 | 139755-91-2 | 2 | ESI+ |
| 47 | Vardenafil oxopiperazine | C21H26N6O5S | 475.1758 | 0.5 | 5.453 | 312.1578, 299.1137, 283.1190, 151.0864, 100.0997 | 448184-58-5 | 2 | ESI+ |
| 48 | Vardenafil N-oxide | C23H32N6O5S | 505.2228 | −2.7 | 4.974 | 477.1910, 377.1275, 335.1112, 312.1583, 151.0862, 113.1074 | 448184-48-3 | 2 | ESI+ |
| 49 | 2-Hydroxyethylnortadalafil | C23H21N3O5 | 420.1554 | 1.1 | 6.439 | 302.0808, 274.0863, 262.0863, 197.0707, 169.0758, 135.0440 | 385769-94-8 | 1 | ESI+ |
| 50 | Vardenafil acetyl analogue | C24H31N5O3 | 438.25 | −0.3 | 4.409 | 341.1610, 325.1299, 311.1144, 297.1347, 151.0866 | 224785-90-4 | 2 | ESI+ |
| 51 | Vardenafil dimer | C38H46N10O8S2 | 835.3014 | 2 | 8.618 | 312.1581, 151.0862 | 1255919-03-9 | 1 | ESI+ |
| 52 | Mirodenafil | C26H37N5O5S | 532.2588 | −1.8 | 7.025 | 514.2480, 404.1634, 339.1876, 312.1343, 296.1395, 129.1019 | 862189-95-5 | 2 | ESI+ |
| 53 | Mutaprodenafil | C27H35N9O5S2 | 630.2275 | 0.6 | 7.191 | 525.2225, 488.2167, 377.1279, 312.1560, 142.0068, 113.1072 | 1387577-30-1 | 2 | ESI+ |
| 54 | Thioquinapiperfil | C24H28N6OS | 449.2118 | −2.4 | 4.586 | 339.1450, 311.1142, 204.1382, 186.1280 | 220060-39-9 | 2 | ESI+ |
| 55 | Aminosildenafil | C18H23N5O4S | 406.1544 | 1.8 | 7.219 | 364.1073, 299.1141, 283.1191, 255.1243, 166.0974 | 319491-68-4 | 2 | ESI+ |
| 56 | Desethylcarbodenafil | C22H28N6O3 | 425.2296 | −0.5 | 4.559 | 339.1453, 311.1140, 166.0975, 147.0076 | 1027192-92-2 | 2 | ESI+ |
| 57 | Didescarbonsildenafil | C20H28N6O4S | 449.1966 | 2.3 | 5.117 | 312.1566, 311.1505, 283.1191, 204.1380, 166.0974, 121.0647 | 466684-88-8 | 2 | ESI+ |
| 58 | N-Phenylpropenyltadalafil | C30H24N4O4 | 505.187 | −1.9 | 9.664 | 383.1502, 299.0960, 262.0863, 169.0755, 135.0438, 113.1071 | 2064212-00-4 | 2 | ESI+ |
| 59 | N-Desethyl-N-methylvardenafil | C22H30N6O4S | 475.2122 | 0.7 | 4.754 | 376.1074, 312.1584, 299.1140, 151.0866 | 224785-87-9 | 2 | ESI+ |
| 60 | Dichlorodenafil | C19H20Cl2N4O2 | 407.1036 | 2.5 | 12.057 | 379.0726, 363.0415, 350.0336, 343.0957, 280.0953, 166.0973, 136.0505 | 1446089-84-4 | 2 | ESI+ |
| 61 | Propoxyphenyl thiosildenafil | C23H32N6O3S2 | 505.205 | −0.8 | 8.175 | 355.1590, 313.1118, 299.0964, 271.1012, 113.1074 | 479073-87-5 | 2 | ESI+ |
| 62 | Dithiodesethyl carbodenafil | C22H28N6OS2 | 457.1839 | 1.4 | 7.55 | 371.0997, 343.0685, 309.0802, 178.9620 | 1610830-81-3 | 2 | ESI+ |
| 63 | Hydroxythioacetildenafil | C25H34N6O3S | 499.2486 | −2.1 | 6.794 | 369.1385, 341.1069, 313.1127, 143.1178, 127.0864 | 1159977-47-5 | 2 | ESI+ |
| 64 | Tadalafil dichloro impurity | C22H18Cl2N2O5 | 461.0666 | 0.9 | 10.078 | 312.1559, 300.1173, 284.1236, 274.0862, 262.0860, 135.0438 | 1598416-08-0 | 2 | ESI+ |
| 65 | Sildenafil impurity 4 | C25H34N6OS2 | 499.2308 | −1.2 | 8.177 | 468.1886, 428.1575, 371.1000, 343.0687, 178.9622 | 2520113-03-3 | 2 | ESI+ |
| 66 | Demethylpiperaziny sildenafil sulfonic acid | C17H20N4O5S | 393.1227 | 2.8 | 4.586 | 365.0913, 336.0524, 285.1334, 256.0955, 136.0505 | 1357931-55-5 | 2 | ESI+ |
| 67 | Propoxyphenyl aildenafil | C24H34N6O4S | 503.2435 | −0.4 | 6.494 | 391.1432, 325.1660, 299.1143, 283.1190, 113.1074 | 1391053-82-9 | 1 | ESI+ |
| 68 | Sildenafil impurity 14 | C24H32N6OS2 | 485.2152 | 1.6 | 8.016 | 468.1881, 428.1573, 371.0999, 343.0682 | 2146091-79-2 | 2 | ESI+ |
| NO. | Compound | Linear Range (μg/L) | r | LOD (μg/L) | LOQ (μg/L) | Recovery (%) | RSD (%) |
|---|---|---|---|---|---|---|---|
| 1 | Nor-acetildenafil | 2.0–200 | 0.9998 | 0.5 | 2.0 | 83.5 | 6.5 |
| 2 | Acetildenafil | 4.0–400 | 0.9996 | 1.0 | 4.0 | 81.4 | 10.1 |
| 3 | Vardenafil | 4.0–400 | 0.9991 | 1.0 | 4.0 | 89.0 | 3.9 |
| 4 | Hydroxyhomosildenafil | 0.8–80 | 0.9990 | 0.2 | 0.8 | 86.5 | 4.2 |
| 5 | Sildenafil | 2.0–200 | 0.9998 | 0.5 | 2.0 | 87.6 | 5.8 |
| 6 | Homosildenafil | 2.0–200 | 0.9997 | 0.5 | 2.0 | 71.2 | 6.8 |
| 7 | AminoTadalafil | 2.0–200 | 0.9993 | 0.5 | 2.0 | 104.1 | 8.0 |
| 8 | Tadalafil | 4.0–400 | 0.9998 | 1.0 | 4.0 | 89.9 | 7.1 |
| 9 | Thioaildenafil | 4.0–400 | 0.9996 | 1.0 | 4.0 | 85.8 | 4.3 |
| 10 | PseudoVardenafil | 0.8–80 | 0.9991 | 0.2 | 0.8 | 96.0 | 6.4 |
| 11 | NorneoSildenafil | 2.0–200 | 0.9988 | 0.5 | 2.0 | 73.1 | 3.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Li, B.; Wang, H.; Yang, L.; Yi, Z.; Fu, Y.; Du, Y. Rapid Screening and Identification of Illegally Adulterated PDE-5 Inhibitors in Health Wines by UPLC-TOF-MS. Processes 2025, 13, 3800. https://doi.org/10.3390/pr13123800
Huang X, Li B, Wang H, Yang L, Yi Z, Fu Y, Du Y. Rapid Screening and Identification of Illegally Adulterated PDE-5 Inhibitors in Health Wines by UPLC-TOF-MS. Processes. 2025; 13(12):3800. https://doi.org/10.3390/pr13123800
Chicago/Turabian StyleHuang, Xiaobei, Ben Li, Hui Wang, Lixia Yang, Zi Yi, Yuli Fu, and Yun Du. 2025. "Rapid Screening and Identification of Illegally Adulterated PDE-5 Inhibitors in Health Wines by UPLC-TOF-MS" Processes 13, no. 12: 3800. https://doi.org/10.3390/pr13123800
APA StyleHuang, X., Li, B., Wang, H., Yang, L., Yi, Z., Fu, Y., & Du, Y. (2025). Rapid Screening and Identification of Illegally Adulterated PDE-5 Inhibitors in Health Wines by UPLC-TOF-MS. Processes, 13(12), 3800. https://doi.org/10.3390/pr13123800






