Effects of Organic Acid Catalysts on the Ethanol Organosolv Treatment of Wheat Bran to Produce Ferulate-Enriched Extracts
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Wheat Bran Procurement
2.3. Treatments
2.4. Examination of Extraction Kinetics
2.5. Treatment Severity Determination
2.6. Experimental Design and Response Surface Methodology
2.7. Determination of Total Polyphenol Yield and Antioxidant Activity
2.8. Liquid Chromatography–Tandem Mass Spectrometry (LC-MS/MS)
2.9. Data Elaboration and Statistics
3. Results and Discussion
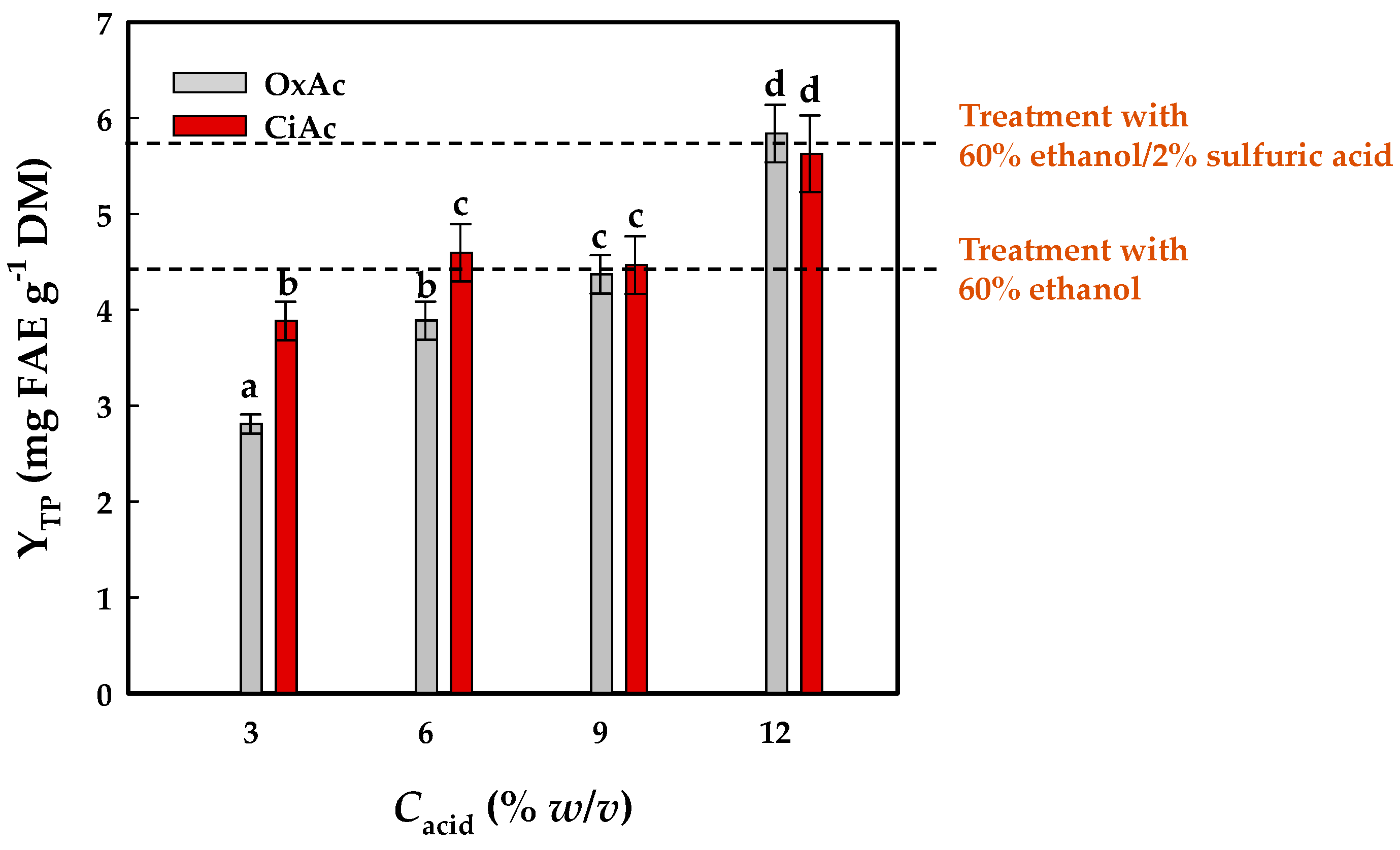
3.1. The Effects of the Acid Type and Concentration
3.2. Treatment Severity-Based Trials
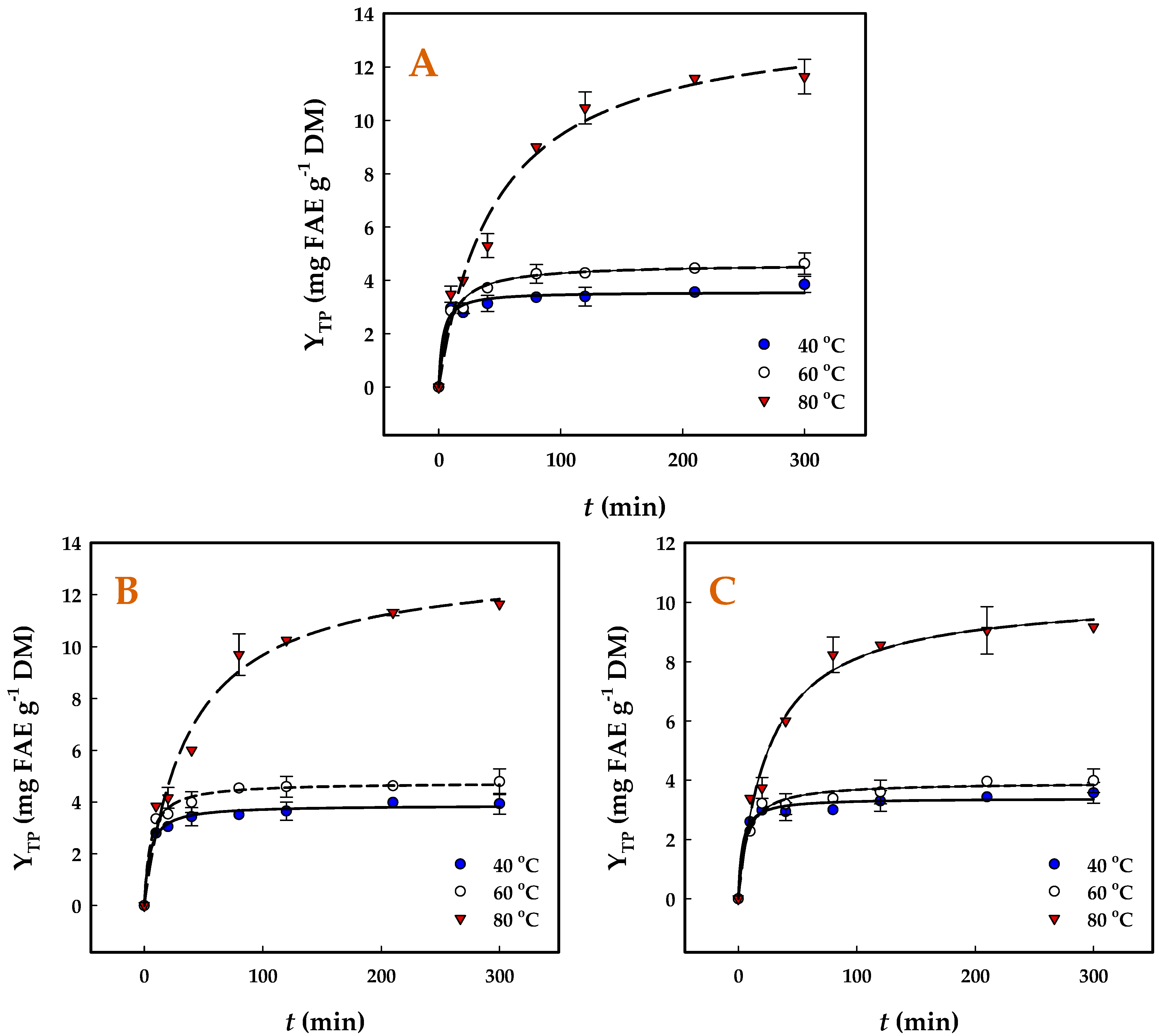
3.3. Polyphenol Release Kinetics
3.4. Design of Experiments and Treatment Optimization
3.5. Polyphenolic Profile and Antioxidant Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ouro-Salim, O.; Guarnieri, P. Circular economy of food waste: A literature review. Environ. Qual. Manag. 2022, 32, 225–242. [Google Scholar] [CrossRef]
- García-González, A.G.; Rivas-García, P.; Escamilla-Alvarado, C.; Ramírez-Cabrera, M.A.; Paniagua-Vega, D.; Galván-Arzola, U.; Cano-Gómez, J.J.; Escárcega-González, C.E. Fruit and vegetable waste as a raw material for obtaining functional antioxidants and their applications: A review of a sustainable strategy. Biofuels Bioprod. Biorefining 2025, 19, 231–249. [Google Scholar] [CrossRef]
- Lizárraga-Velázquez, C.E.; Leyva-López, N.; Hernández, C.; Gutiérrez-Grijalva, E.P.; Salazar-Leyva, J.A.; Osuna-Ruíz, I.; Martínez-Montaño, E.; Arrizon, J.; Guerrero, A.; Benitez-Hernández, A.; et al. Antioxidant molecules from plant waste: Extraction techniques and biological properties. Processes 2020, 8, 1566. [Google Scholar] [CrossRef]
- Osorio, L.L.D.R.; Flórez-López, E.; Grande-Tovar, C.D. The potential of selected agri-food loss and waste to contribute to a circular economy: Applications in the food, cosmetic and pharmaceutical industries. Molecules 2021, 26, 515. [Google Scholar] [CrossRef] [PubMed]
- De Camargo, A.C.; Schwember, A.R.; Parada, R.; Garcia, S.; Marostica Junior, M.R.; Franchin, M.; Regitano-d’Arce, M.A.B.; Shahidi, F. Opinion on the hurdles and potential health benefits in value-added use of plant food processing by-products as sources of phenolic compounds. Int. J. Mol. Sci. 2018, 19, 3498. [Google Scholar] [CrossRef]
- Shahidi, F.; Varatharajan, V.; Oh, W.Y.; Peng, H. Phenolic compounds in agri-food by-products, their bioavailability and health effects. Food Bioact. 2019, 5, 57–119. [Google Scholar] [CrossRef]
- Rudjito, R.C.; Matute, A.C.; Jiménez-Quero, A.; Olsson, L.; Stringer, M.A.; Krogh, K.B.R.M.; Eklöf, J.; Vilaplana, F. Integration of subcritical water extraction and treatment with xylanases and feruloyl esterases maximises release of feruloylated arabinoxylans from wheat bran. Bioresour. Technol. 2024, 395, 130387. [Google Scholar] [CrossRef]
- Apprich, S.; Tirpanalan, Ö.; Hell, J.; Reisinger, M.; Böhmdorfer, S.; Siebenhandl-Ehn, S.; Novalin, S.; Kneifel, W. Wheat bran-based biorefinery 2: Valorization of products. LWT-Food Sci. Technol. 2014, 56, 222–231. [Google Scholar] [CrossRef]
- Katileviciute, A.; Plakys, G.; Budreviciute, A.; Onder, K.; Damiati, S.; Kodzius, R. A sight to wheat bran: High value-added products. Biomolecules 2019, 9, 887. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.; Abraham, T.E. Ferulic acid: An antioxidant found naturally in plant cell walls and feruloyl esterases involved in its release and their applications. Crit. Rev. Biotech. 2004, 24, 59–83. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, A.; Razavi, S.H. The role of bioconversion processes to enhance bioaccessibility of polyphenols in rice. Food Biosci. 2020, 35, 100605. [Google Scholar] [CrossRef]
- Bento-Silva, A.; Patto, M.C.V.; do Rosário Bronze, M. Relevance, structure and analysis of ferulic acid in maize cell walls. Food Chem. 2018, 246, 360–378. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, R.; Liu, C.; Zheng, X.; Liu, B. Enhancing antioxidant activity and antiproliferation of wheat bran through steam flash explosion. J. Food Sci. Technol. 2016, 53, 3028–3034. [Google Scholar] [CrossRef] [PubMed]
- Pazo-Cepeda, M.V.; Aspromonte, S.G.; Alonso, E. Extraction of ferulic acid and feruloylated arabinoxylo-oligosaccharides from wheat bran using pressurized hot water. Food Biosci. 2021, 44, 101374. [Google Scholar] [CrossRef]
- Janiak, M.; Renzetti, S.; Noort, M.; Amarowicz, R. Effect of heat treatment on the antioxidant capacity of dry wheat bran. Bulgar. Chem. Com. 2019, 51, 79–82. [Google Scholar]
- Papadaki, E.S.; Palaiogiannis, D.; Lalas, S.I.; Mitlianga, P.; Makris, D.P. Polyphenol release from wheat bran using ethanol-based organosolv treatment and acid/alkaline catalysis: Process modeling based on severity and response surface optimization. Antioxidants 2022, 11, 2457. [Google Scholar] [CrossRef]
- Papadaki, E.; Grigorakis, S.; Palaiogiannis, D.; Lalas, S.I.; Mitlianga, P. Hydrothermal treatment of wheat bran under mild acidic or alkaline conditions for enhanced polyphenol recovery and antioxidant activity. Molecules 2024, 29, 1193. [Google Scholar] [CrossRef]
- Naik, S.; Lentz, H.; Maheshwari, R. Extraction of perfumes and flavours from plant materials with liquid carbon dioxide under liquid—Vapor equilibrium conditions. Fluid Phase Equilibria 1989, 49, 115–126. [Google Scholar] [CrossRef]
- Karageorgou, I.; Grigorakis, S.; Lalas, S.; Mourtzinos, I.; Makris, D.P. Incorporation of 2-hydroxypropyl β-cyclodextrin in a biomolecule-based low-transition temperature mixture (LTTM) boosts efficiency of polyphenol extraction from Moringa oleifera Lam leaves. J. Appl. Res. Med. Aromat. Plants 2018, 9, 62–69. [Google Scholar] [CrossRef]
- Overend, R.P.; Chornet, E. Fractionation of lignocellulosics by steam-aqueous pretreatments. Philos. Trans. R. Soc. Lond. Ser. A Math. Phys. Sci. 1987, 321, 523–536. [Google Scholar]
- Pedersen, M.; Meyer, A.S. Lignocellulose pretreatment severity–relating pH to biomatrix opening. New Biotech. 2010, 27, 739–750. [Google Scholar] [CrossRef]
- Bezerra, M.A.; Ferreira, S.L.C.; Novaes, C.G.; Dos Santos, A.M.P.; Valasques, G.S.; da Mata Cerqueira, U.M.F.; dos Santos Alves, J.P. Simultaneous optimization of multiple responses and its application in Analytical Chemistry–A review. Talanta 2019, 194, 941–959. [Google Scholar] [CrossRef]
- Cicco, N.; Lanorte, M.T.; Paraggio, M.; Viggiano, M.; Lattanzio, V. A reproducible, rapid and inexpensive Folin–Ciocalteu micro-method in determining phenolics of plant methanol extracts. Microchem. J. 2009, 91, 107–110. [Google Scholar] [CrossRef]
- Lakka, A.; Karageorgou, I.; Kaltsa, O.; Batra, G.; Bozinou, E.; Lalas, S.; Makris, D. Polyphenol extraction from Humulus lupulus (hop) using a neoteric glycerol/L-alanine deep eutectic solvent: Optimisation, kinetics and the effect of ultrasound-assisted pretreatment. AgriEngineering 2019, 1, 403–417. [Google Scholar] [CrossRef]
- Smyrnakis, G.; Stamoulis, G.; Palaiogiannis, D.; Chatzimitakos, T.; Athanasiadis, V.; Lalas, S.I.; Makris, D.P. Recovery of polyphenolic antioxidants from coffee silverskin using acid-catalyzed ethanol organosolv treatment. ChemEngineering 2023, 7, 72. [Google Scholar] [CrossRef]
- Pazo-Cepeda, V.; Benito-Román, Ó.; Navarrete, A.; Alonso, E. Valorization of wheat bran: Ferulic acid recovery using pressurized aqueous ethanol solutions. Waste Biomass Valorization 2020, 11, 4701–4710. [Google Scholar] [CrossRef]
- Casasni, S.; Guenaoui, A.; Grigorakis, S.; Makris, D.P. Acid-Catalyzed organosolv treatment of potato peels to boost release of polyphenolic compounds using 1-and 2-Propanol. Appl. Sci. 2023, 13, 9484. [Google Scholar] [CrossRef]
- Jacquet, N.; Richel, A. Adaptation of severity factor model according to the operating parameter variations which occur during steam explosion process. In Hydrothermal Processing in Biorefineries: Production of Bioethanol and High Added-Value Compounds of second and Third Generation Biomass; Springer: Berlin/Heidelberg, Germany, 2017; pp. 333–351. [Google Scholar]
- Svärd, A.; Brännvall, E.; Edlund, U. Rapeseed straw polymeric hemicelluloses obtained by extraction methods based on severity factor. Ind. Crops Prod. 2017, 95, 305–315. [Google Scholar] [CrossRef]
- Peleg, M.; Engel, R.; Gonzalez-Martinez, C.; Corradini, M.G. Non-Arrhenius and non-WLF kinetics in food systems. J. Sci. Food Agric. 2002, 82, 1346–1355. [Google Scholar] [CrossRef]
- Chan, C.-H.; Yusoff, R.; Ngoh, G.-C. Modeling and kinetics study of conventional and assisted batch solvent extraction. Chem. Eng. Res. Des. 2014, 92, 1169–1186. [Google Scholar] [CrossRef]
- Jancheva, M.; Grigorakis, S.; Loupassaki, S.; Makris, D.P. Optimised extraction of antioxidant polyphenols from Satureja thymbra using newly designed glycerol-based natural low-transition temperature mixtures (LTTMs). J. Appl. Res. Med. Aromat. Plants 2017, 6, 31–40. [Google Scholar] [CrossRef]
- de Oliveira, D.M.; Finger-Teixeira, A.; Rodrigues Mota, T.; Salvador, V.H.; Moreira-Vilar, F.C.; Correa Molinari, H.B.; Craig Mitchell, R.A.; Marchiosi, R.; Ferrarese-Filho, O.; Dantas dos Santos, W. Ferulic acid: A key component in grass lignocellulose recalcitrance to hydrolysis. Plant Biotech. J. 2015, 13, 1224–1232. [Google Scholar] [CrossRef] [PubMed]
- Buranov, A.U.; Mazza, G. Lignin in straw of herbaceous crops. Ind. Crops Prod. 2008, 28, 237–259. [Google Scholar] [CrossRef]
- Linh, T.N.; Fujita, H.; Sakoda, A. Release kinetics of esterified p-coumaric acid and ferulic acid from rice straw in mild alkaline solution. Bioresour. Techol. 2017, 232, 192–203. [Google Scholar] [CrossRef]
- Abou Samra, M.; Chedea, V.S.; Economou, A.; Calokerinos, A.; Kefalas, P. Antioxidant/prooxidant properties of model phenolic compounds: Part I. Studies on equimolar mixtures by chemiluminescence and cyclic voltammetry. Food Chem. 2011, 125, 622–629. [Google Scholar] [CrossRef]
- Choueiri, L.; Chedea, V.S.; Calokerinos, A.; Kefalas, P. Antioxidant/pro-oxidant properties of model phenolic compounds. Part II: Studies on mixtures of polyphenols at different molar ratios by chemiluminescence and LC–MS. Food Chem. 2012, 133, 1039–1044. [Google Scholar] [CrossRef]
- Verma, B.; Hucl, P.; Chibbar, R. Phenolic acid composition and antioxidant capacity of acid and alkali hydrolysed wheat bran fractions. Food Chem. 2009, 116, 947–954. [Google Scholar] [CrossRef]
- Zhang, L.; Gao, W.; Chen, X.; Wang, H. The effect of bioprocessing on the phenolic acid composition and antioxidant activity of wheat bran. Cereal Chem. 2014, 91, 255–261. [Google Scholar] [CrossRef]
- Povilaitis, D.; Šulniūtė, V.; Venskutonis, P.R.; Kraujalienė, V. Antioxidant properties of wheat and rye bran extracts obtained by pressurized liquid extraction with different solvents. J. Cereal Sci. 2015, 62, 117–123. [Google Scholar] [CrossRef]
- Kim, K.-H.; Tsao, R.; Yang, R.; Cui, S.W. Phenolic acid profiles and antioxidant activities of wheat bran extracts and the effect of hydrolysis conditions. Food Chem. 2006, 95, 466–473. [Google Scholar] [CrossRef]
- Chalas, J.; Claise, C.; Edeas, M.; Messaoudi, C.; Vergnes, L.; Abella, A.; Lindenbaum, A. Effect of ethyl esterification of phenolic acids on low-density lipoprotein oxidation. Biomed. Pharmacother. 2001, 55, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Kylli, P.; Nousiainen, P.; Biely, P.; Sipilä, J.; Tenkanen, M.; Heinonen, M. Antioxidant potential of hydroxycinnamic acid glycoside esters. J. Agric. Food Chem. 2008, 56, 4797–4805. [Google Scholar] [CrossRef] [PubMed]
- Ohta, T.; Nakano, T.; Egashira, Y.; Sanada, H. Antioxidant activity of ferulic acid β-glucuronide in the LDL oxidation system. Biosci. Biotechnol. Biochem. 1997, 61, 1942–1943. [Google Scholar] [CrossRef] [PubMed]




| Treatment Variable | Code | Coded and Actual Variable Level | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| T (°C) | X2 | 40 | 60 | 80 |
| t (min) | X1 | 120 | 210 | 300 |
| T (°C) | t (min) | CSF | CSF′ | YTP (mg FAE g−1 DM) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Catalyst | Catalyst | Catalyst | ||||||||
| 2% SuAc | 12% OxAc | 12% CiAc | 2% SuAc | 12% OxAc | 12% CiAc | 2% SuAc | 12% OxAc | 12% CiAc | ||
| 40 | 120 | −0.60 | −0.39 | −1.81 | 6.40 | 6.61 | 5.19 | 3.4 ± 0.2 a | 3.6 ± 0.2 a | 3.3 ± 0.1 a |
| 210 | −0.35 | −0.14 | −1.56 | 6.65 | 6.86 | 5.44 | 3.6 ± 0.3 a,b | 3.9 ± 0.3 a | 3.4 ± 0.2 a | |
| 300 | −0.20 | 0.01 | −1.41 | 6.80 | 7.01 | 5.59 | 3.9 ± 0.3 b | 3.9 ± 0.2 a | 3.6 ± 0.2 a | |
| 60 | 120 | −0.01 | 0.20 | −1.22 | 6.99 | 7.20 | 5.78 | 4.3 ± 0.3 b | 4.6 ± 0.3 b | 3.6 ± 0.2 a |
| 210 | 0.23 | 0.44 | −0.98 | 7.23 | 7.44 | 6.02 | 4.5 ± 0.3 b | 4.6 ± 0.3 b | 4.0 ± 0.1 b | |
| 300 | 0.39 | 0.60 | −0.82 | 7.39 | 7.60 | 6.18 | 4.6 ± 0.4 b | 4.8 ± 0.4 b | 4.0 ± 0.1 b | |
| 80 | 120 | 0.58 | 0.79 | −0.63 | 7.58 | 7.79 | 6.37 | 10.5 ± 0.4 c | 10.2 ± 0.5 c | 8.6 ± 0.3 c |
| 210 | 0.82 | 1.03 | −0.39 | 7.82 | 8.03 | 6.61 | 11.4 ± 0.4 d | 11.3 ± 0.5 d | 9.1 ± 0.3 c,d | |
| 300 | 0.98 | 1.19 | −0.23 | 7.98 | 8.19 | 6.77 | 11.6 ± 0.6 d | 11.6 ± 0.7 d | 9.2 ± 0.2 d | |
| Catalyst | T (°C) | k (×10−3) (g mg−1 min−1) | t0.5 (min) | h (mg g−1 min−1) | YTP(s) (mg FAE g−1 DM) |
|---|---|---|---|---|---|
| SuAc | 40 | 87.1 | 3.19 | 1.1 | 3.6 ± 0.2 a |
| 60 | 26.8 | 8.12 | 0.6 | 4.6 ± 0.3 b | |
| 80 | 1.5 | 47.29 | 0.3 | 13.9 ± 1.0 c | |
| OxAc | 40 | 56.6 | 4.53 | 0.9 | 3.9 ± 0.4 a |
| 60 | 40.8 | 5.22 | 0.9 | 4.7 ± 0.3 b | |
| 80 | 2.0 | 37.64 | 0.4 | 13.3 ± 0.9 c | |
| CiAc | 40 | 89.1 | 3.30 | 1.0 | 3.4 ± 0.2 a |
| 60 | 38.3 | 6.70 | 0.6 | 3.9 ± 0.2 a | |
| 80 | 3.7 | 26.36 | 0.4 | 10.3 ± 0.8 d |
| Design Point | Variable | Response (YTP—mg FAE g−1 DM) | ||||||
|---|---|---|---|---|---|---|---|---|
| X1 (T, °C) | X2 (t, min) | SuAc | OxAc | CiAc | ||||
| Measured | Predicted | Measured | Predicted | Measured | Predicted | |||
| 1 | −1 (40) | −1 (120) | 3.4 | 3.4 | 3.6 | 3.8 | 3.3 | 3.3 |
| 2 | −1 (40) | 1 (300) | 3.9 | 3.7 | 3.9 | 3.9 | 3.6 | 3.5 |
| 3 | 1 (80) | −1 (120) | 10.5 | 10.6 | 10.3 | 10.5 | 8.6 | 8.6 |
| 4 | 1 (80) | 1 (300) | 11.6 | 11.6 | 11.7 | 11.6 | 9.2 | 9.2 |
| 5 | −1 (40) | 0 (210) | 3.6 | 3.6 | 4.0 | 3.9 | 3.4 | 3.5 |
| 6 | 1 (80) | 0 (210) | 11.4 | 11.2 | 11.3 | 11.1 | 9.1 | 9.0 |
| 7 | 0 (60) | −1 (120) | 4.3 | 4.1 | 4.6 | 4.2 | 3.6 | 3.6 |
| 8 | 0 (60) | 1 (300) | 4.6 | 4.7 | 4.8 | 4.9 | 4.0 | 4.0 |
| 9 | 0 (60) | 0 (210) | 4.5 | 4.5 | 4.6 | 4.6 | 4.0 | 3.9 |
| 10 | 0 (60) | 0 (210) | 4.4 | 4.5 | 4.8 | 4.6 | 4.0 | 3.9 |
| 11 | 0 (60) | 0 (210) | 4.5 | 4.5 | 4.1 | 4.6 | 3.8 | 3.9 |
| Catalyst | Equation (Model) | R2 | p |
|---|---|---|---|
| 2% SuAc | YTP = 4.5 + 3.8X1 + 0.3X2 + 2.9X12 | 1.00 | <0.0001 |
| 12% OxAc | YTP = 4.7 + 3.6X1 + 0.3X2 + 2.9X12 | 1.00 | <0.0001 |
| 12% CiAc | YTP = 3.9 + 2.8X1 + 0.2X2 + 2.3X12 | 1.00 | <0.0001 |
| Catalyst | Maximum Predicted Response (mg GAE g−1 DM) | Optimal Conditions | |
|---|---|---|---|
| t (min) | T (°C) | ||
| 2% SuAc | 11.6 ± 0.4 a | 300 | 80 |
| 12% OxAc | 11.6 ± 0.8 a | 300 | 80 |
| 12% CiAc | 9.2 ± 0.2 b | 300 | 80 |
| Peak Number | Retention Time (min) | UV–Vis Maxima (nm) | [M − H]− (m/z) | Other Ions | Tentative Identity |
|---|---|---|---|---|---|
| 1 | 13.79 | 327 | 325 | - | Feruloyl–pentose |
| 2 | 13.84 | 325 | 325 | - | Feruloyl–pentose |
| 3 | 14.21 | 272, 334 | 247 | 219 | Unknown |
| 4 | 14.33 | 323 | 193 | Ferulic acid | |
| 5 | 14.60 | 291, 323 | 193 | - | Ferulic acid derivative |
| 6 | 17.57 | 326 | 353 | 325, 249, 194 | Ferulic acid derivative |
| 7 | 17.81 | 326 | 355 | 194 | Ferulic acid derivative |
| Peak Number | Compound | Yield (μg g−1 DM) | |||
|---|---|---|---|---|---|
| No Catalyst | 2% SuAc | 12% OxAc | 12% CiAc | ||
| 1 | Feruloyl–pentose | 8.0 ± 0.5 b | 210.6 ± 12.3 e | 179.4 ± 10.5 d | - a |
| 2 | Feruloyl–pentose | 24.4 ± 1.8 a | 212.8 ± 10.0 e | 170.8 ± 9.8 d | 74.8 ± 3.3 b |
| 3 | Unknown | 141.8 ± 9.0 c | 130.7 ± 8.4 c | 113.1 ± 6.3 b | 89.5 ± 4.4 a |
| 4 | Ferulic acid | 15.6 ± 1.0 b | 127.8 ± 9.6 e | 59.7 ± 3.3 c | 10.9 ± 0.4 a |
| 5 | Ferulic acid derivative | 13.7 ± 0.7 c | 21.5 ± 1.3 e | 16.4 ± 0.6 d | 9.4 ± 0.1 a |
| 6 | Ferulic acid derivative | - a | 358.2 ± 12.5 e | 281.2 ± 10.8 d | 11.8 ± 0.7 b |
| 7 | Ferulic acid derivative | - a | 416.7 ± 19.8 e | 252.0 ± 11.4 d | 2.5 ± 0.1 b |
| Total | 203.5 | 1475.9 | 1081.0 | 198.8 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahouche, Z.; Refai, H.; Grigorakis, S.; Makris, D.P. Effects of Organic Acid Catalysts on the Ethanol Organosolv Treatment of Wheat Bran to Produce Ferulate-Enriched Extracts. Processes 2025, 13, 3794. https://doi.org/10.3390/pr13123794
Mahouche Z, Refai H, Grigorakis S, Makris DP. Effects of Organic Acid Catalysts on the Ethanol Organosolv Treatment of Wheat Bran to Produce Ferulate-Enriched Extracts. Processes. 2025; 13(12):3794. https://doi.org/10.3390/pr13123794
Chicago/Turabian StyleMahouche, Zahida, Hela Refai, Spyros Grigorakis, and Dimitris P. Makris. 2025. "Effects of Organic Acid Catalysts on the Ethanol Organosolv Treatment of Wheat Bran to Produce Ferulate-Enriched Extracts" Processes 13, no. 12: 3794. https://doi.org/10.3390/pr13123794
APA StyleMahouche, Z., Refai, H., Grigorakis, S., & Makris, D. P. (2025). Effects of Organic Acid Catalysts on the Ethanol Organosolv Treatment of Wheat Bran to Produce Ferulate-Enriched Extracts. Processes, 13(12), 3794. https://doi.org/10.3390/pr13123794










