Energy Performance Evaluation of an Ammonia–Water Absorption Chiller for Varying Operating Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Basic Absorption Cooling System
2.2. Absorption Cooling System with Internal Heat Recovery
2.3. Assumptions and Development of the Thermodynamic Model
- Thermodynamic equilibrium and steady-state condition exist throughout the system.
- There is no heat exchange with the environment.
- Pressure drops in components and pipes are negligible.
- There are saturated conditions at the outlet of the condenser and evaporator.
- The solutions at the generator and absorber outlet are in phase balance.
- The steam leaving the rectifier is pure ammonia (0.999 wt.)
- The expansion process in the throttle valves is isenthalpic.
- Kinetic and potential energy are negligible.
- The software was configured to operate and calculate the thermodynamic and transport properties of the ammonia–water mixture:The configuration was based on an exercise with known thermal data, where the 12 available Aspen-plus methods of activity coefficients were evaluated. The selected method was UNIFAC (Universal Functional Activity Coefficient) because it presented among the methods the minimum deviation of 1.53% with respect to the results of known temperatures of the exercise.
- The absorber, generator, and rectifier are represented by a FLASH2 block. This block is used for single-stage separation processes, with sufficient space for the vapor to be released from the liquid. FLASH2 performs rigorous calculations on phase equilibrium (vapor–liquid), produces one output flow in vapor phase and another in liquid state.
- The valve and pump are selected by the same name in the software menu.
- For the first heat recovery carried out in the rectifier, the unitary HEATER operation was used. This block allows dew point or bubble calculations to be performed. Add or remove any amount of heat specified by the user, establish the level of overheating or undercooling of a flow, and determine the heat or cooling load required to reach a certain fraction of vapor.
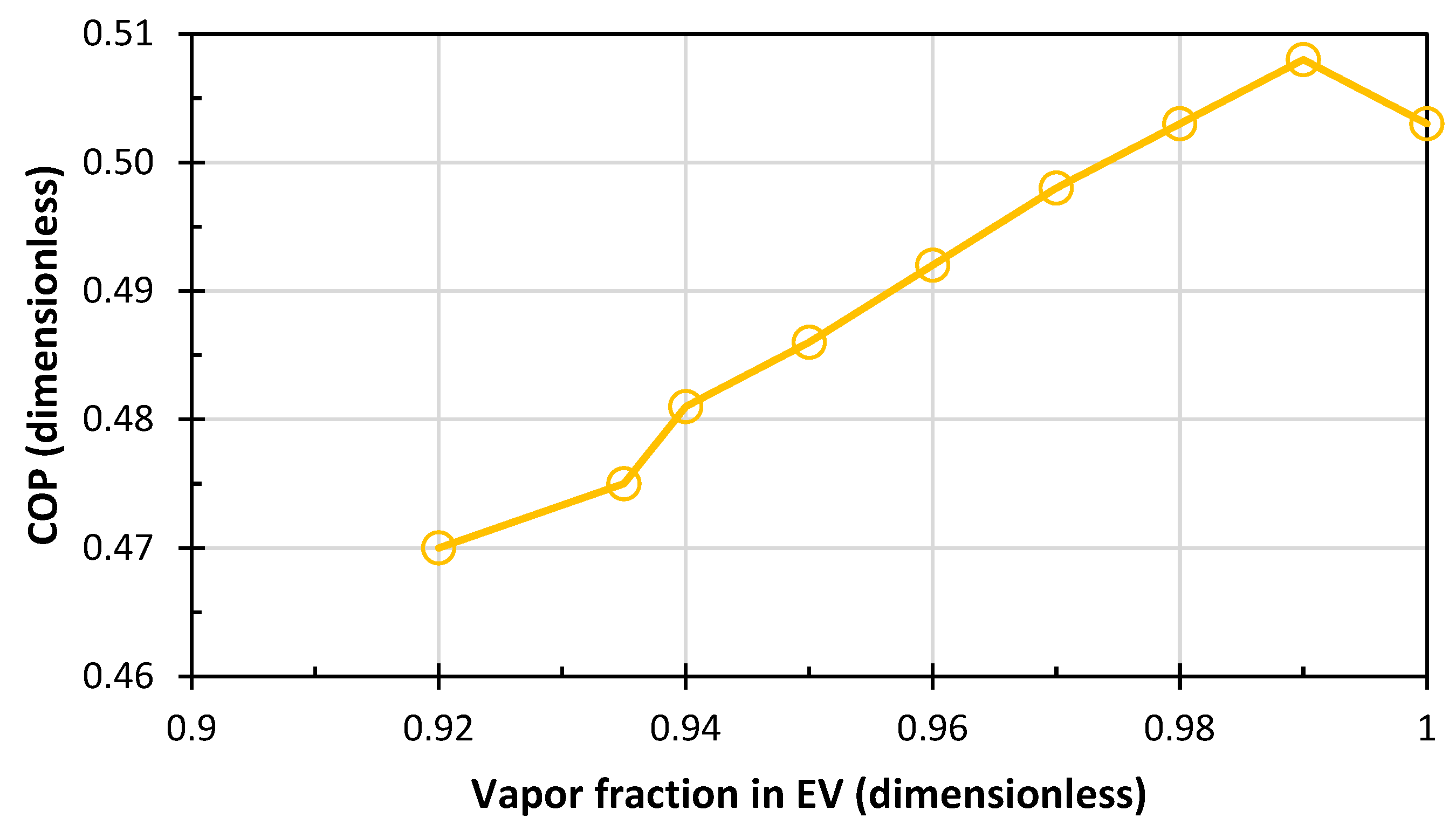
- The condenser and evaporator are also represented by a HEATER block, in which the vapor fraction of 0 and 1 is assumed for the condenser and evaporator, respectively.
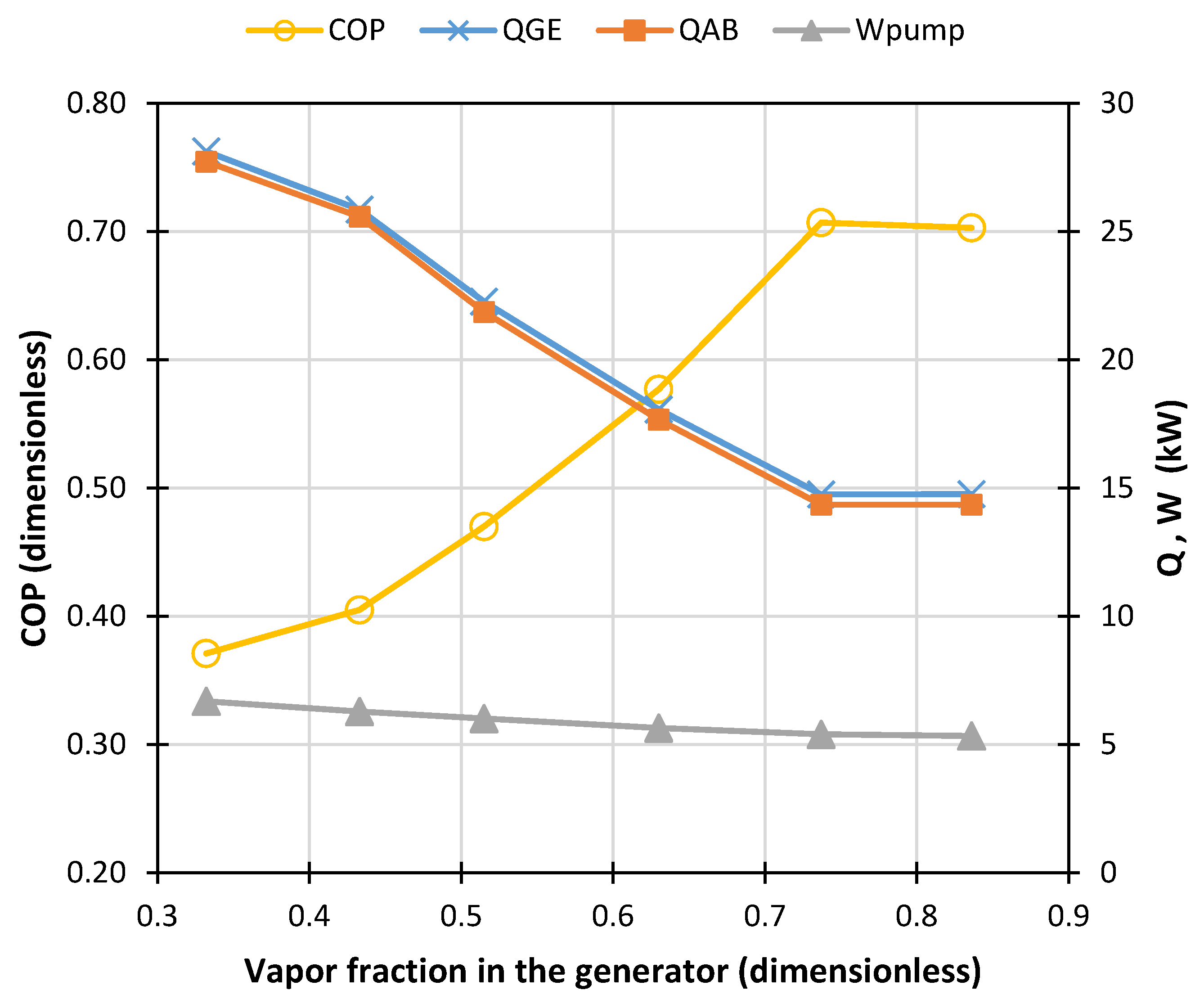
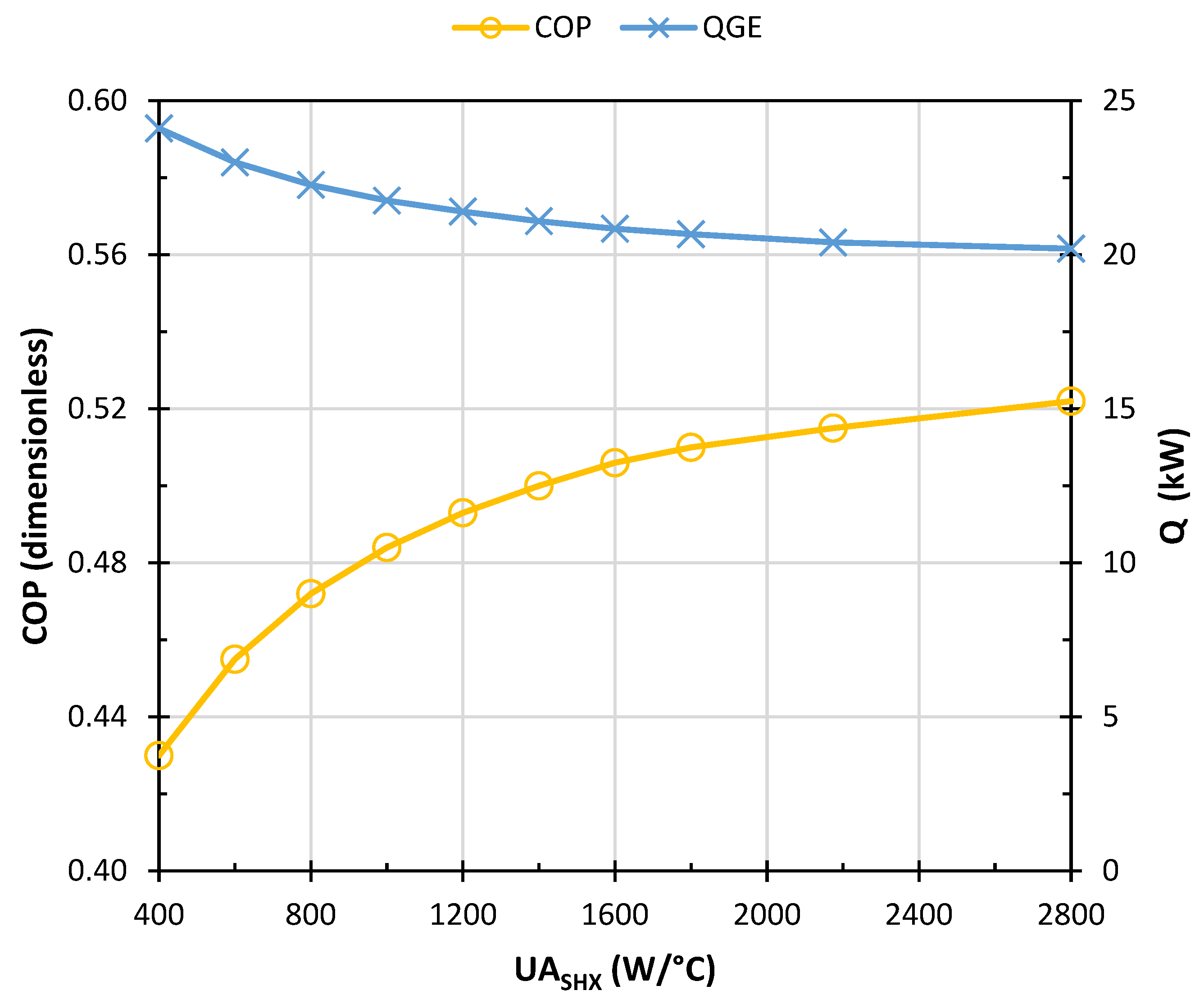
- The solution heat exchanger (SHX) was developed by the unitary HEATX operation. For the input data of this module, the direct method and countercurrent configuration for fluid heat exchange was chosen. In the HEATX module a temperature difference of 9.5 °C (conventional design criterion) is assumed between the output flows of the SHX. The suitability of this first assumption is discussed below.
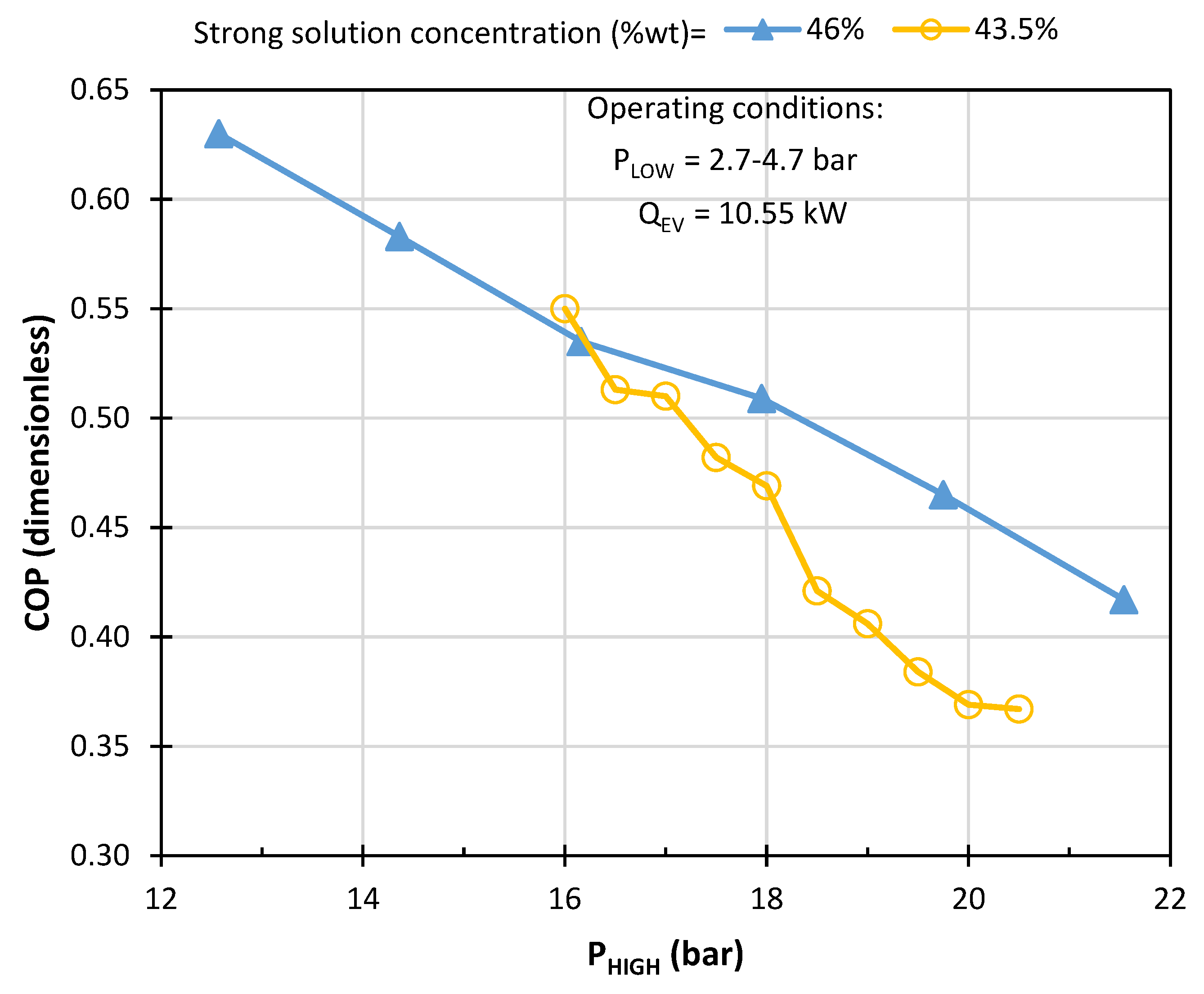
- According to each device in the system, high and low pressure were considered as initial conditions of 19.75 and 3.94 bar, respectively. This data was obtained by Manrique [44] in an experimental way from a refrigeration machine with thermodynamic cycle similar to this study.
- The flows connect the unit operation models or blocks, carrying material or energy flow between them. Because the program requires an initial assumption from which to start, the following initial assumptions were made for the flow leaving the solution pump (SOLFRT2): water mass fraction of 0.62, ammonia mass fraction of 0.38, pressure of 17.95 bar, solution mass flow of 200 kg/h, and solution temperature of 43.28 °C. These assumptions only serve as a starting point for the ASPEN numerical procedure, then it calculates these conditions to meet the various considerations of the model.
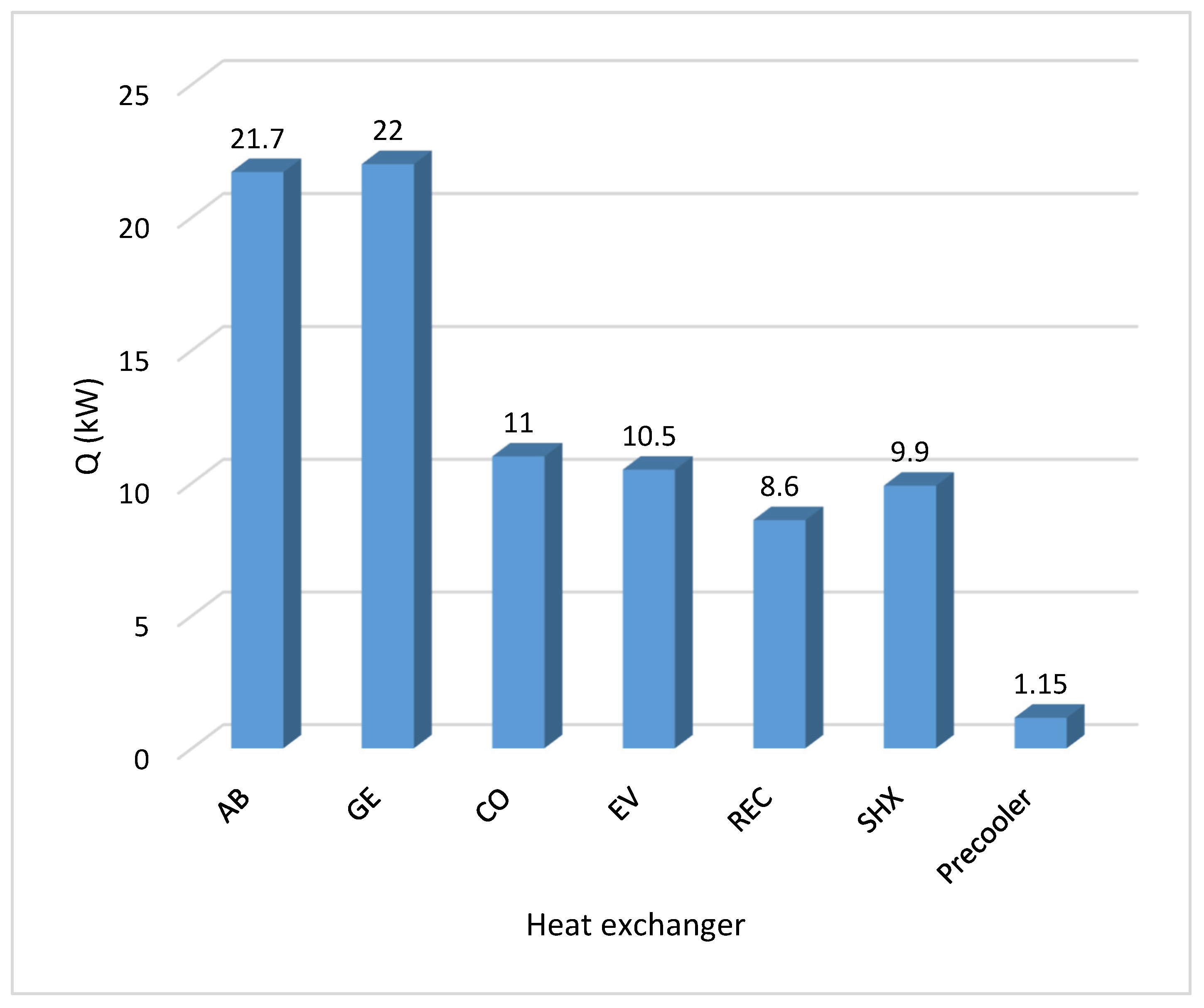
- The Design Spec submenu was used to define operating conditions from known operating conditions of a commercial absorption cooling machine. The generator specification was 22.1 kW heat flow to calculate the vapor fraction in this device. A design specification procedure was applied to the rectifier to know the vapor fraction with which it must operate to obtain an output flow with an ammonia mass fraction close to 0.999. In the ASPEN Plus environment, the validity of this ammonia concentration value is ensured by calculating the heat that must be removed in the rectifier using a FLASH2 block which performs strict thermodynamic equilibrium calculations and rigorous mass and energy balances. The resulting heat duty of 8.63 kW in the rectifier will then guarantee that the ammonia concentration would be achieved, within the assumptions in the model. As Klein et al. [34] mention, a lower concentration value would negatively impact the pressure of the evaporator and the absorber, which in turn would affect the refrigeration capacity of the system. To ensure the refrigeration load of the commercial machine, the design specification was assigned to the 10.551 kW evaporator to know the mass flow of the solution leaving the pump.
- To determine if the results from our simulation runs may be accepted, convergence criteria were established in the Convergence menu. This last section also allows us to indicate the maximum number of iterations that the program will use to find the solution of the developed model; for the single-effect absorption equipment that was worked on in this section, a maximum of 1400 iterations were reached.
2.4. Thermodynamic Analysis
- Mass balance
- Species balance
- Energy balance
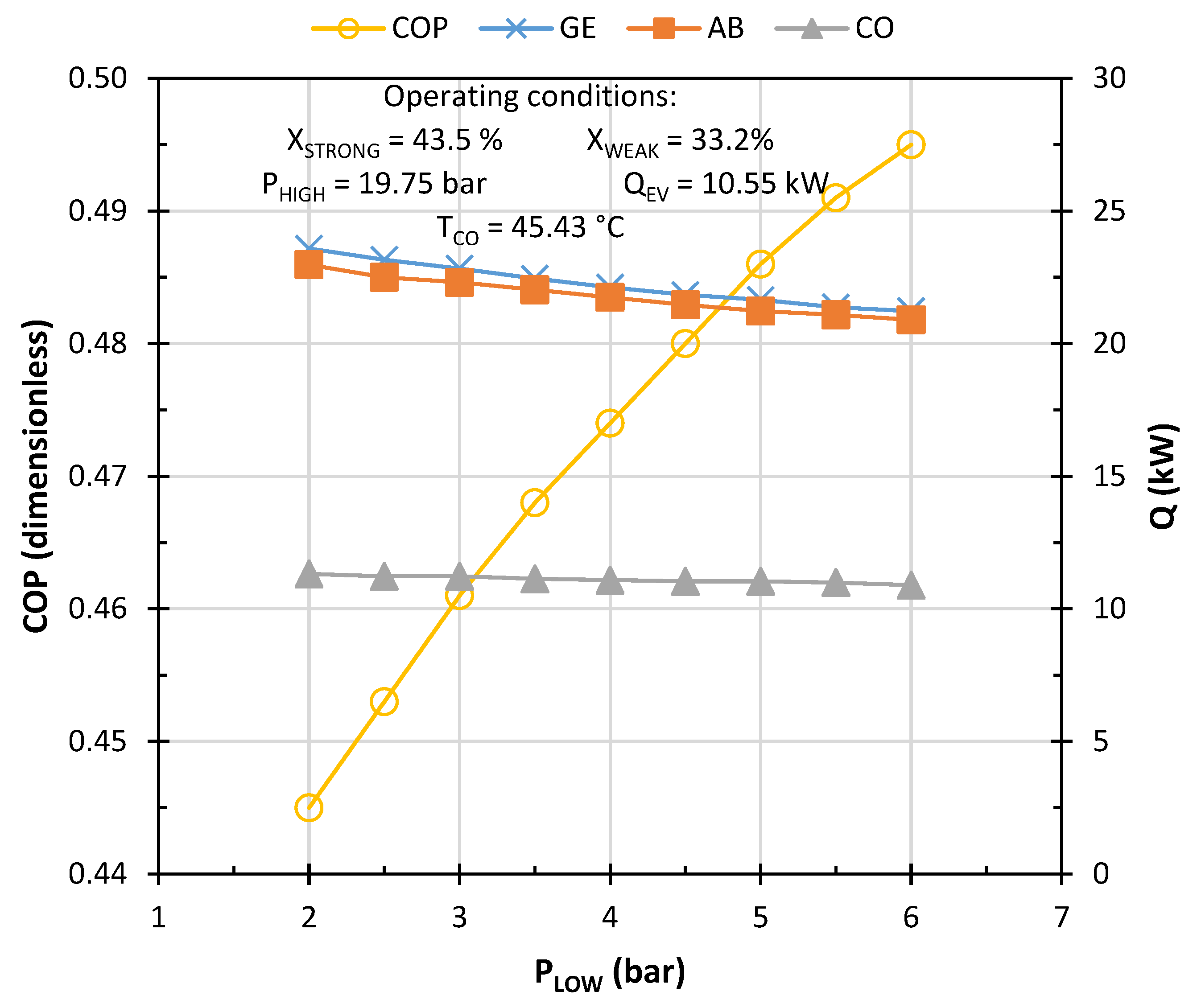
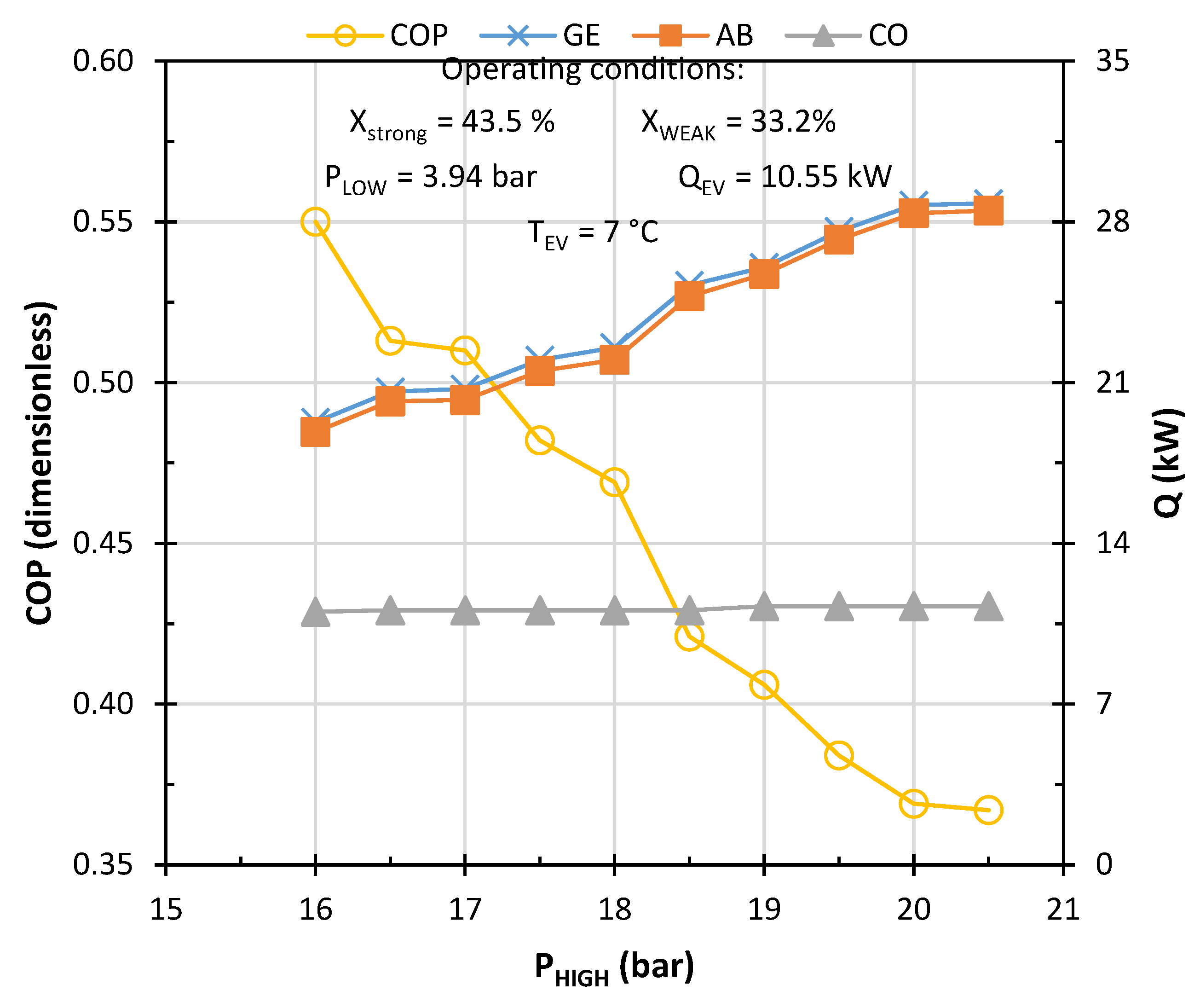
3. Results and Discussion
Characterization of the Model to Operate Under Weather Conditions in Monterrey, NL, Mexico
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| A | Heat transfer area (m2) |
| COP | Coefficient of performance (dimensionless) |
| h | Specific enthalpy (kJ/kg) |
| Mass flow rate (kg/s) | |
| NH3 | Ammonia |
| P | Pressure (bar) |
| Q | Heat load (kW) |
| SHX | Solution heat exchanger |
| T | Temperature (°C) |
| U | Overall heat transfer coefficient (W/°C m2) |
| W | Mechanical work (kW) |
| X | Solution concentration (% wt) |
| Subscripts | |
| AB | Absorber |
| CO | Condenser |
| EV | Evaporator |
| GE | Generator |
| REC | Rectifier |
| 1–15 | Stream lines |
References
- Brückner, S.; Liu, S.; Miró, L.; Radspieler, M.; Cabeza, L.F.; Lävemann, E. Industrial waste heat recovery technologies: An eco-nomic analysis of heat transformation technologies. Appl. Therm. Eng. 2015, 151, 157–167. [Google Scholar] [CrossRef]
- Xu, Z.Y.; Wang, R.Z. Absorption heat pump for waste heat reuse: Current states and future development. Front. Energy 2017, 11, 414–436. [Google Scholar] [CrossRef]
- Garimella, S.; Ponkala, M.J.; Goyal, A.; Staedter, A. Waste-heat driven ammonia-water absorption chiller for severe ambient operation. Appl. Therm. Eng. 2019, 154, 442–449. [Google Scholar] [CrossRef]
- Yan, C.; Abed, A.; Chaturvedi, R.; Dahari, M.; Abdullaev, S.; Zhou, X.; Mahariq, I.; Elmasry, Y. Sustainable commercially-scaled greenhouse building cooling solution: Integrating PCM storage, desiccant wheels, and absorption chillers powered by dual-source solar/biomass energy. J. Energy Storage 2024, 101, 113871. [Google Scholar] [CrossRef]
- Alhuyi-Nazari, M.; Mukhtar, A.; Md Yasir, A.; Ahmadi, M.; Kumar, R.; Luong, T. Applications of geothermal sources for absorption chillers as efficient and clean cooling technologies for buildings: A comprehensive review. J. Build. Eng. 2024, 82, 108340. [Google Scholar] [CrossRef]
- Xu, Z.; Mao, H.; Liu, D.; Wang, R. Waste heat recovery of power plant with large scale serial absorption heat pumps. Energy 2018, 165, 1097–1105. [Google Scholar] [CrossRef]
- Du, S.; Wang, R.; Lin, P.; Xu, Z.; Pan, Q.; Xu, S. Experimental studies on an air-cooled two-stage NH3-H2O solar absorption air-conditioning prototype. Energy 2012, 45, 581–587. [Google Scholar] [CrossRef]
- Izquierdo, M.; Marcos, J.; Palacios, M.; González-Gil, A. Experimental evaluation of a low-power direct air-cooled double-effect LiBr–H2O absorption prototype. Energy 2012, 37, 737–748. [Google Scholar] [CrossRef]
- Yin, H.; Qu, M.; Archer, D. Model based experimental performance analysis of a microscale LiBr–H2O steam-driven double-effect absorption chiller. Appl. Therm. Eng. 2010, 30, 1741–1750. [Google Scholar] [CrossRef]
- Matsushima, H.; Fujii, T.; Komatsu, T.; Nishiguchi, A. Dynamic simulation program with object-oriented formulation for absorption chillers (modelling, verification, and application to triple-effect absorption chiller). Int. J. Refrig. 2010, 33, 259–268. [Google Scholar] [CrossRef]
- Gómez, V.; Vidal, A.; Best, R.; García-Valladares, O.; Velázquez, N. Theoretical and experimental evaluation of an indirect-fired GAX cycle cooling system. Appl. Therm. Eng. 2008, 28, 975–987. [Google Scholar] [CrossRef]
- Jawahar, C.; Saravanan, R. Experimental studies on aircooled NH3–H2O based modified gax absorption cooling system. Int. J. Refrig. 2011, 34, 658–666. [Google Scholar] [CrossRef]
- Priedeman, D.; Garrabrant, M.; Mathias, J.; Stout, R.; Christensen, R. Performance of a Residential Sized GAX Absorption Chiller. Am. Soc. Mech. Eng. Adv. Energy Syst. Div. AES 1998, 277–279. [Google Scholar]
- Eames, I.; Wu, S. Experimental proof-of-concept testing of an innovative heat-powered vapour recompression–absorption refrigerator cycle. Appl. Therm. Eng. 2000, 20, 721–736. [Google Scholar] [CrossRef]
- Kang, Y.; Kunugi, Y.; Kashiwagi, T. Review of advanced absorption cycles: Performance improvement and temperature lift enhancement. Int. J. Refrig. 2000, 23, 388–401. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, H.; You, T.; Li, X. Performance comparison of absorption heating cycles using various low-GWP and natural refrigerants. Int. J. Refrig. 2017, 82, 56–70. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, R. Absorption refrigeration cycles: Categorized based on the cycle construction. Int. J. Refrig. 2016, 62, 114–136. [Google Scholar] [CrossRef]
- Boer, D.; Gebreslassie, B.; Medrano, M.; Nogués, M. Effect of internal heat recovery in ammonia-water absorption cooling cycles: Exergy and structural analysis. Int. J. Thermodyn. 2009, 12, 17–27. [Google Scholar]
- Du, S.; Wang, R.; Xia, Z. Optimal ammonia water absorption refrigeration cycle with maximum internal heat recovery derived from pinch technology. Energy 2014, 68, 862–869. [Google Scholar] [CrossRef]
- Kang, Y.; Akisawa, A.; Kashiwagi, T. Analytical investigation of two different absorption modes: Falling film and bubble types. Int. J. Refrig. 2000, 23, 430–443. [Google Scholar] [CrossRef]
- Mendes, L.; Collares-Pereira, M.; Ziegler, F. A rich solution spray as a refining method in a small capacity, single effect, solar assisted absorption machine with the pair NH3/H2O: Experimental results. Energy Convers. Manag. 2007, 48, 2996–3000. [Google Scholar] [CrossRef]
- Kim, J.; Jung, J.; Kang, Y. Absorption performance enhancement by nanoparticles and chemical surfactants in binary nanofluids. Int. J. Refrig. 2007, 30, 50–57. [Google Scholar] [CrossRef]
- Garimella, S.; Determan, M.; Meacham, J.; Lee, S.; Emst, T. Microchannel component technology for system-wide application in ammonia/water absorption heat pumps. Int. J. Refrig. 2011, 34, 1184–1196. [Google Scholar] [CrossRef]
- Du, S.; Wang, R.; Xia, Z. Graphical analysis on internal heat recovery of a single stage ammonia-water absorption refrigeration system. Energy 2015, 80, 687–694. [Google Scholar] [CrossRef]
- Du, S.; Wang, R.; Chen, X. Analysis on maximum internal heat recovery of a mass-coupled two-stage ammonia water absorption refrigeration system. Energy 2017, 133, 822–831. [Google Scholar] [CrossRef]
- Klein, S. A Model of the Steady-State Performance of an Absorption Heat Pump; National Bureau of Standards: Gaithersburg, MD, USA, 1982; Volume 83, p. 29471. [Google Scholar]
- Lazzarin, R.; Gasparella, A.; Longo, G. Ammonia-water absorption machines for refrigeration: Theoretical and real performances. Int. J. Refrig. 1996, 19, 239–246. [Google Scholar] [CrossRef]
- Darwish, N.; Al-Hashimi, S.; Al-Mansoori, A. Performance analysis and evaluation of a commercial absorption–refrigeration water–ammonia (ARWA) system. Int. J. Refrig. 2008, 31, 1214–1223. [Google Scholar] [CrossRef]
- Mansouri, R.; Boukholda, I.; Bourouis, M.; Bellagi, A. Modelling and testing the performance of a commercial ammonia/water absorption chiller using Aspen-Plus platform. Energy 2015, 93, 2374–2383. [Google Scholar] [CrossRef]
- El May, S.; Boukholda, I.; Bellagi, A. Energetic and exergetic analysis of a commercial ammonia-water absorption chiller. Int. J. Exergy 2011, 8, 33–50. [Google Scholar] [CrossRef]
- Shena, Y.; Tang, Z.; Guo, D.; Wu, C.; Wang, D.; Jiang, A.; Wen, Y. A simulation and experimental study on primary cooler for COG heat pump. Appl. Therm. Eng. 2019, 160, 113983. [Google Scholar] [CrossRef]
- Overney, R.; Bernards, M. Aspen Plus 12.1 Instructional Tutorials; Department of Chemical Engineering, University of Washington: Washington, DC, USA, 2004. [Google Scholar]
- ASPEN Technology, Inc. ASPEN Plus® User Guide; ASPEN Technology, Inc.: Cambridge, MA, USA, 2000. [Google Scholar]
- Klein, S.; Herold, K.; Radermacher, R. Absorption Chillers and Heat Pumps; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Constantino, M.; Kanizawa, F. Evaluation of pressure drop effect on COP of single-stage vapor compression refrigeration cycles. Therm. Sci. Eng. Prog. 2022, 28, 101048. [Google Scholar] [CrossRef]
- Minh, N.; Hewitt, N.; Eames, P. Improved Vapour Compression Refrigeration Cycles: Literature Review and Their Application to Heat Pumps. In Proceedings of the International Refrigeration and Air Conditioning Conference, West Lafayette, Indiana, 17–20 July 2006; p. 795. Available online: http://docs.lib.purdue.edu/iracc/795 (accessed on 14 November 2025).
- Shovon, M.; Kim, H.; Lee, J. Study on Thermo-Fluid Dynamics of the Throttling Loss in a Refrigeration System. Master’s Thesis, Andong National University, Andong, Republic of Korea, 2020. [Google Scholar] [CrossRef]
- Adamson, K.; Gordon, T.; Carson, J.; Chen, Q.; Schlosser, F.; Kong, L.; Cleland, D. High-temperature and transcritical heat pump cycles and advancements: A review. Renew. Sustain. Energy Rev. 2022, 167, 112798. [Google Scholar] [CrossRef]
- Kaynakli, O.; Kilic, M. Theoretical study on the effect of operating conditions on performance of absorption refrigeration system. Energy Convers. Manag. 2007, 48, 599–607. [Google Scholar] [CrossRef]
- Hernández-Magallanes, J.A.; Ibarra-Bahena, J.; Rivera, W.; Romero, R.J.; Gómez-Arias, E.; Dehesa-Carrasco, U.; Espinoza-Ojeda, O.M.; Kozhiparambil Chandran, S. Thermodynamic Analysis of a Half-Effect Absorption Cooling System Powered by a Low-Enthalpy Geothermal Source. Appl. Sci. 2019, 9, 1220. [Google Scholar] [CrossRef]
- Wang, M.; Infante, C. Performance analysis of double-effect absorption heat pump cycle using NH3/ILs pairs. In Proceedings of the 12th IEA Heat Pump Conference, Rotterdam, The Netherlands, 15–18 May 2017. [Google Scholar]
- Saka, K.; Fatih, M. Parametric study and thermal sensitivity analysis of a triple effect absorption refrigeration system. Case Stud. Therm. Eng. 2025, 69, 106030. [Google Scholar] [CrossRef]
- Li, K.; Wu, W.; Liang, K.; Zhang, H. Simulation on key influencing factors of CO2-ionic liquid mixture compression-absorption refrigeration cycle based on Aspen Plus. Int. Commun. Heat Mass Transf. 2025, 161, 108506. [Google Scholar] [CrossRef]
- Manrique, J. Solar Driven Ammonia-Absorption Cooling Machine. U.S. Patent No. 5,666,818, 16 September 1997. [Google Scholar]
- Sistema Integral de Monitoreo Ambiental (SIMA). Meteorología—Historial; Gobierno del Estado de Nuevo León: Monterrey, México, 2009. [Google Scholar]
- Standard 55-1992; Thermal Environmental Conditions for Human Occupancy (ANSI Approved). American Society of Heating; Refrigerating and Air-Conditioning Engineers, Inc.: Atlanta, GA, USA, 1995.
- Vargas-Bautista, J.; García-Cuéllar, A.; Rivera-Solorio, C. Design and economic analysis of a solar air-conditioning system: Case of study in Monterrey, Mexico. In Proceedings of the 30th ISES Biennial Solar World Congress 2011, Kassel, Germany, 28 August–2 September 2011; Volume 4, pp. 2798–2809. [Google Scholar]
- Al-Alili, A.; Islam, M.; Kubo, I.; Hwang, Y.; Radermacher, R. Modeling of a solar powered absorption cycle for Abu Dhabi. Appl. Energy 2012, 93, 160–167. [Google Scholar] [CrossRef]
- Al-Falahi, A.; Alobaid, F.; Epple, B. Thermo-Economic Evaluation of Aqua-Ammonia Solar Absorption Air Conditioning System Integrated with Various Collector Types. Entropy 2020, 22, 1165. [Google Scholar] [CrossRef]
- Horuz, I.; Callander, T. Experimental investigation of a vapor absorption refrigeration system. Int. J. Refrig. 2004, 27, 10–16. [Google Scholar] [CrossRef]
- Park, Y.; Kim, J.-S.; Lee, H. Physical properties of the lithium bromide + 1,3-propanediol + water system. Int. J. Refrig. 1997, 20, 319–325. [Google Scholar] [CrossRef]
- Hernández-Magallanes, J.; Domínguez-Inzunza, L.; Gutiérrez-Urueta, G.; Soto, P.; Jiménez, C.; Rivera, W. Experimental assessment of an absorption cooling system operating with the ammonia/lithium nitrate mixture. Energy 2014, 78, 685–692. [Google Scholar] [CrossRef]
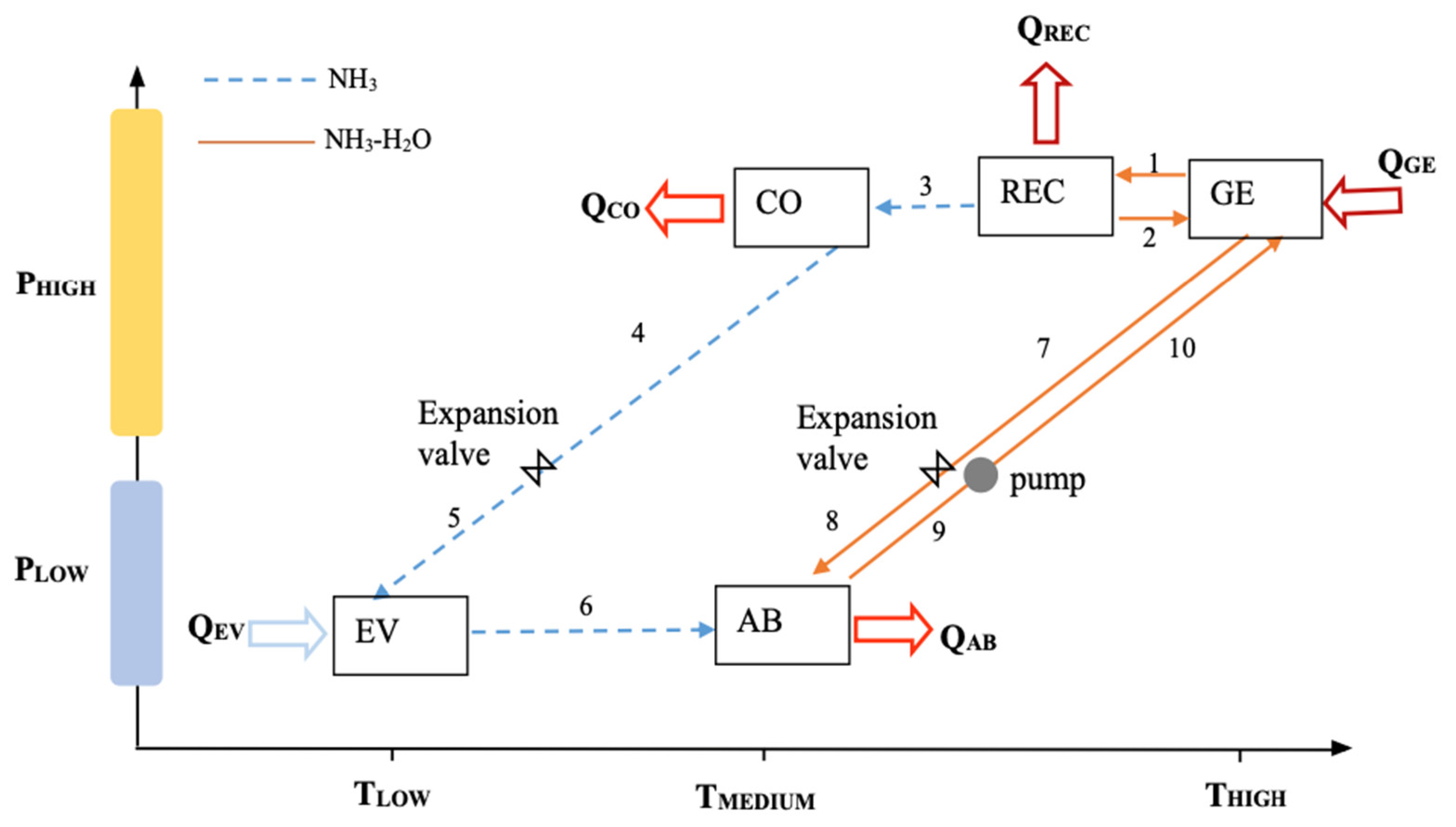
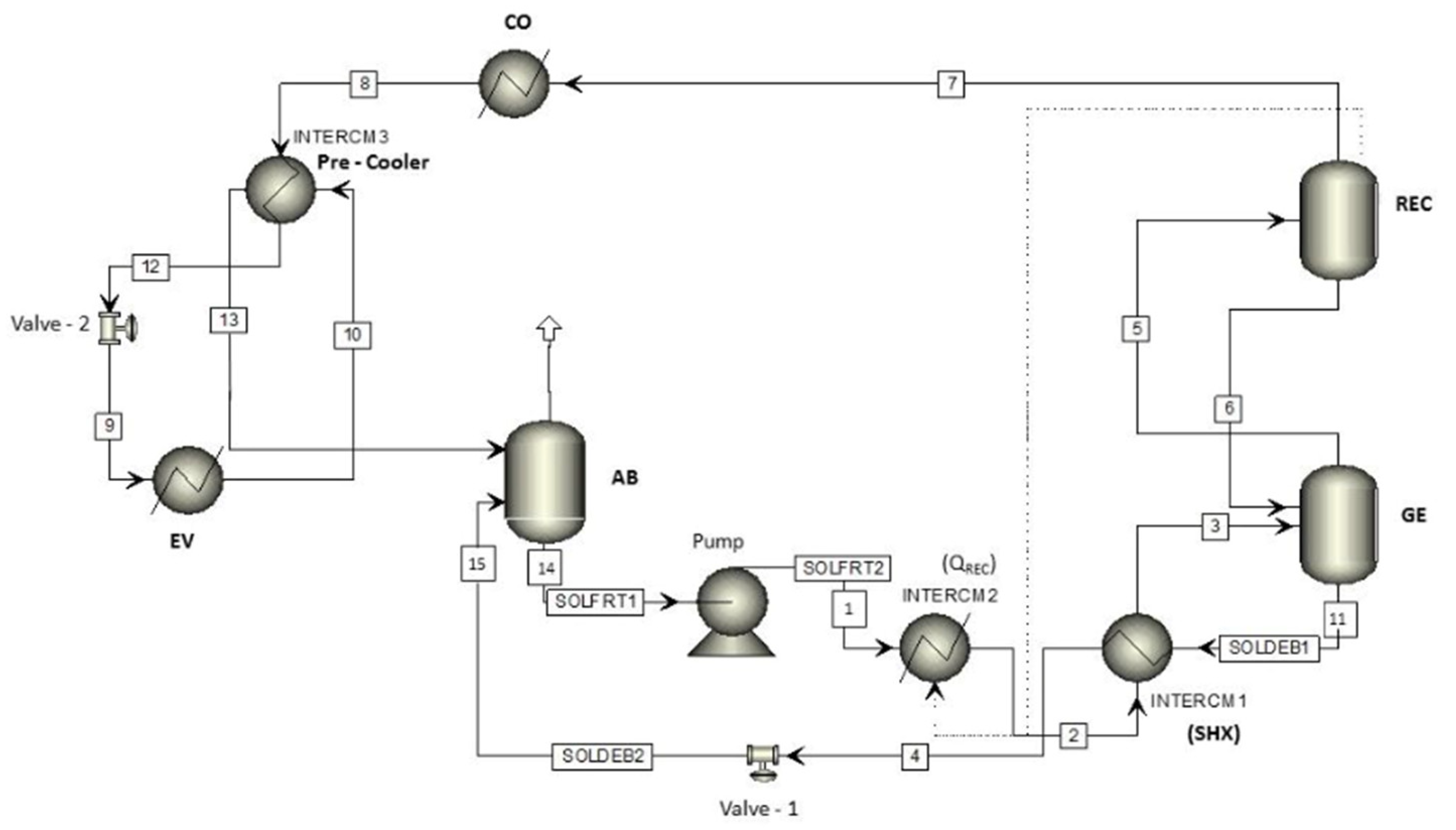
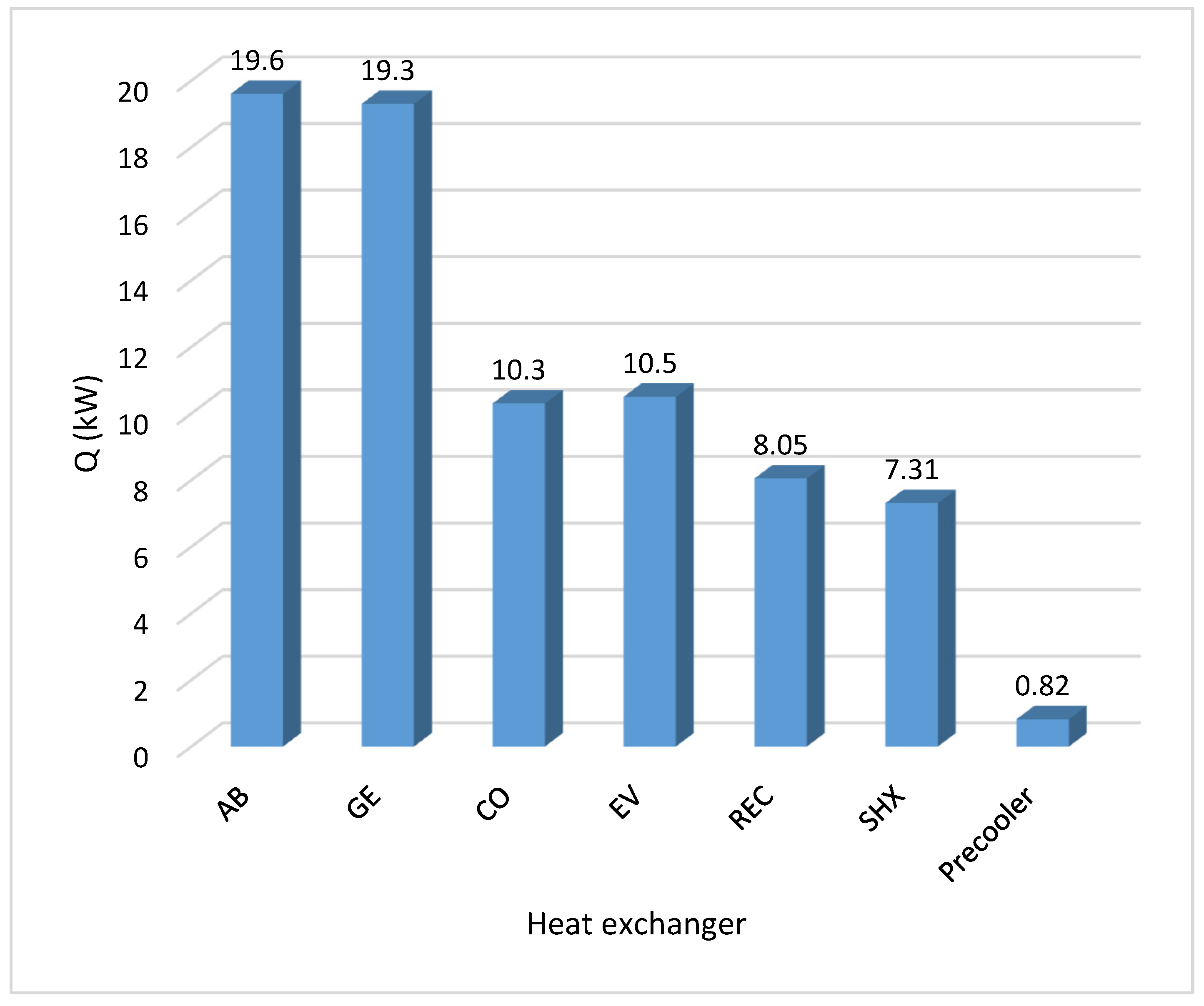
| Model Element | Code Name | Aspen Plus Block Type |
|---|---|---|
| Generator | GE | FLASH2 |
| Absorber | AB | FLASH2 |
| Condenser | CO | HEATER |
| Evaporator | EV | HEATER |
| Rectifier | REC | FLASH2 |
| Pump | Pump | PUMP |
| First Heat Recovery | INTERCM2 | HEATER |
| Solution heat exchanger | INTERCM1 or SHX | HEATX |
| Expansion Valves | Valve-1, Valve-2 | VALVE |
| Pre-cooler | INTERCM3 | HEATX |
| Heat Exchanger | Energy Balance |
|---|---|
| Generator | |
| Absorber | |
| Condenser | |
| Evaporator | |
| Rectifier | |
| Solution heat exchanger | |
| Pre-cooler |
| Parameter | Darwish et al. [28] | Model Result |
|---|---|---|
| Evaporator heat transfer (kW) | 9.60 | 10.55 |
| Absorber heat transfer (kW) | 21.60 | 21.76 |
| Condenser heat transfer (kW) | 9.70 | 11.07 |
| Generator heat transfer (kW) | 20.80 | 22.12 |
| Condenser temperature (°C) | 40.24 | 45.43 |
| Ammonia mass concentration (strong solution) | 0.389 | 0.435 |
| Ammonia mass concentration (weak solution) | 0.150 | 0.332 |
| COP | 0.450 | 0.473 |
| Material Stream | T (°C) | P (bar) | (kg/s) | NH3 (Mass%) |
|---|---|---|---|---|
| 1 | 18.3 | 17.9 | 0.07 | 43 |
| 2 | 46.6 | 17.9 | 0.07 | 43 |
| 3 | 76.5 | 17.9 | 0.07 | 43 |
| 4 | 56.1 | 17.9 | 0.06 | 33 |
| 5 | 91.4 | 17.9 | 0.02 | 97 |
| 6 | 49.0 | 17.9 | 0.01 | 91 |
| 7 | 49.0 | 17.9 | 0.01 | 100 |
| 8 | 45.4 | 17.9 | 0.01 | 100 |
| 9 | −2.2 | 3.9 | 0.01 | 100 |
| 10a 1 | 6.85 | 3.9 | 0.01 | 100 |
| 10b 1 | −1.8 | 3.9 | 0.01 | 100 |
| 11 | 91.4 | 17.9 | 0.06 | 33 |
| 12 | 25.1 | 17.9 | 0.01 | 100 |
| 13 | 9.2 | 3.9 | 0.01 | 100 |
| 14 | 18.0 | 3.9 | 0.07 | 43 |
| 15 | 32.7 | 3.9 | 0.06 | 33 |
| Material Stream | T (°C) | P (bar) | (kg/s) | NH3 (Mass%) |
|---|---|---|---|---|
| 1 | 18.3 | 17.9 | 0.07 | 43 |
| 2 | 57.3 | 17.9 | 0.06 | 43 |
| 3 | 78.7 | 17.9 | 0.06 | 43 |
| 4 | 63.8 | 17.9 | 0.06 | 33 |
| 5 | 91.3 | 17.9 | 0.02 | 97 |
| 6 | 49.0 | 17.9 | 0.01 | 91 |
| 7 | 49.0 | 17.9 | 0.01 | 100 |
| 8 | 45.4 | 17.9 | 0.01 | 100 |
| 9 | 7.42 | 5.6 | 0.01 | 100 |
| 10b 1 | 9.57 | 5.6 | 0.01 | 100 |
| 11 | 91.3 | 17.9 | 0.05 | 33 |
| 12 | 30.0 | 17.9 | 0.01 | 100 |
| 13 | 43.2 | 5.6 | 0.01 | 100 |
| 14 | 29.6 | 5.6 | 0.06 | 43 |
| 15 | 44.6 | 5.6 | 0.05 | 33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Márquez-Nolasco, A.; Pérez-García, S.L.; García-Cuéllar, A.J. Energy Performance Evaluation of an Ammonia–Water Absorption Chiller for Varying Operating Conditions. Processes 2025, 13, 3790. https://doi.org/10.3390/pr13123790
Márquez-Nolasco A, Pérez-García SL, García-Cuéllar AJ. Energy Performance Evaluation of an Ammonia–Water Absorption Chiller for Varying Operating Conditions. Processes. 2025; 13(12):3790. https://doi.org/10.3390/pr13123790
Chicago/Turabian StyleMárquez-Nolasco, A., Santiago L. Pérez-García, and Alejandro J. García-Cuéllar. 2025. "Energy Performance Evaluation of an Ammonia–Water Absorption Chiller for Varying Operating Conditions" Processes 13, no. 12: 3790. https://doi.org/10.3390/pr13123790
APA StyleMárquez-Nolasco, A., Pérez-García, S. L., & García-Cuéllar, A. J. (2025). Energy Performance Evaluation of an Ammonia–Water Absorption Chiller for Varying Operating Conditions. Processes, 13(12), 3790. https://doi.org/10.3390/pr13123790






