Recent Advances in the Application of Artificial Intelligence in Microalgal Cultivation
Abstract
1. Introduction
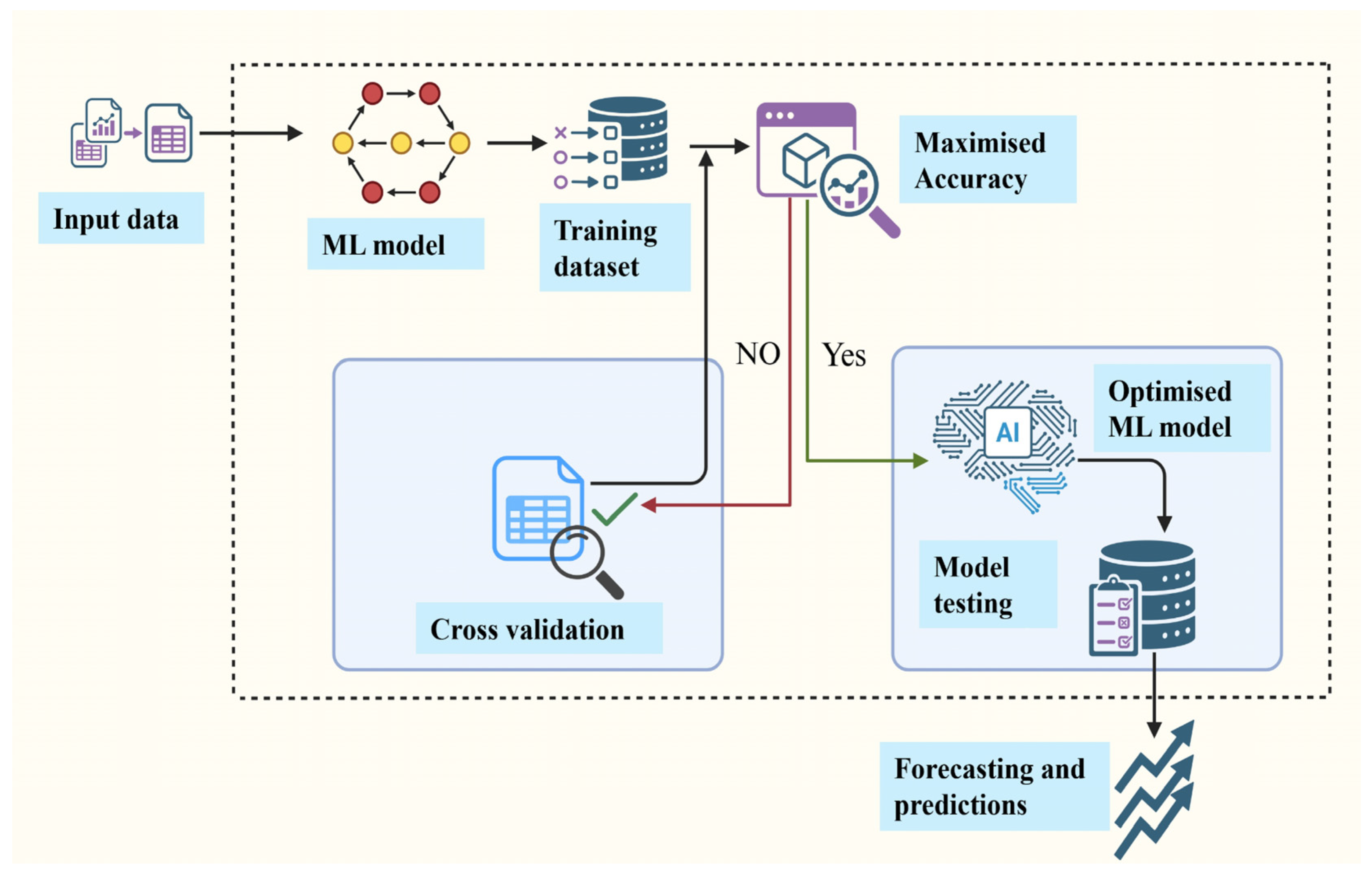
2. Methodology
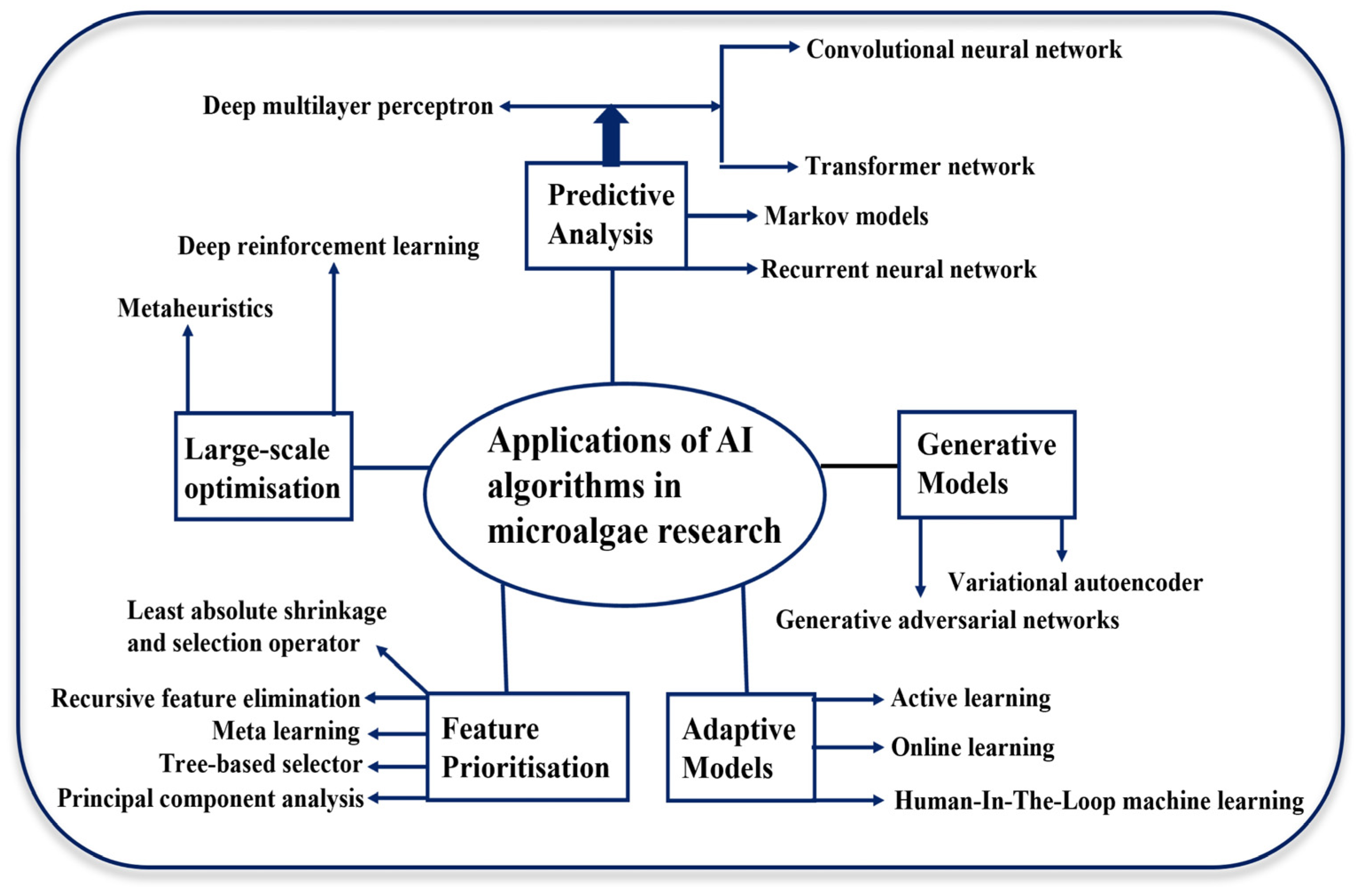
3. AI Models Used in Microalgae Cultivation
3.1. Artificial Neural Network (ANN)
3.2. Genetic Algorithm (GA)
3.3. Deep Learning (DL)
3.4. Decision Tree (DT)
3.5. Support Vector Machine (SVM)
| Species | Model | Input | Output | Efficiency | References |
|---|---|---|---|---|---|
| Kleibsormidium sp., Dictyosphaerium sp., Desmodesmus sp., Scenedesmus sp., and Micractinium sp. | ANN | Average solar irradiation, average water temperature, average pH, initial microalgae concentration, harvesting time, hydraulic retention time, addition of sodium acetate, and nitrate concentration | Concentration of microalgae throughout the cultivation phase | Coefficient of determination (R2) = 0.93 | [36] |
| Chlamydomonas reinhardtii | GA, ANN | Fluorescence emission spectra | Concentration of cell | R2 = 0.998 Mean square error (MSE) = 0.0000998 | [37] |
| Chlorella vulgaris | ANN | Initial biomass, phosphate, glucose, and nitrate concentrations; yield coefficients | Variation in the concentrations of biomass, phosphate, glucose, and nitrate | Not mentioned | [38] |
| Spirulina platensis | Multi-Layer perceptron (MLP) | Temperature, light intensity, pH, dissolved oxygen, rate of oxygen production, harvesting duration, nitrate, phosphate, bicarbonate, and initial biomass | Optical density, trichome size, and trichome concentration | R2 > 0.94 | [39] |
| C. vulgaris | Response surface methodology (RSM) and PLP | Cultivation time and pH | Concentrations of biomass, total fat, unsaturated fat, and oleic acid | R2 = 0.92 Root mean square error (RMSE) = 65.11 | [40] |
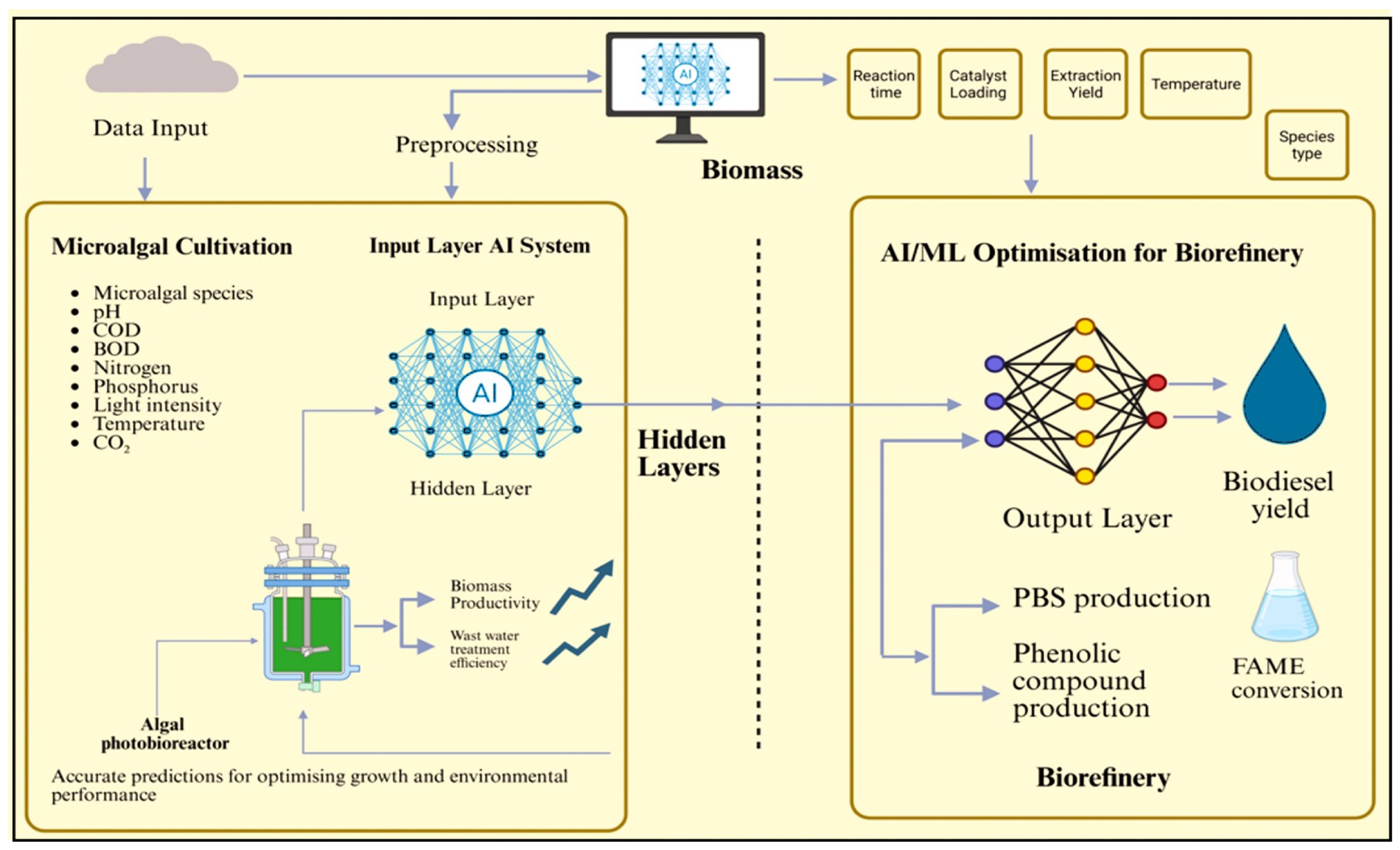
4. AI’s Applications in Microalgae Cultivation
4.1. AI Techniques for Optimising Biomass Production
| AI Technology | Overview | Applications |
|---|---|---|
| ML | Algorithms that improve their performance through repeated training | Predictions for the optimisation of growth conditions |
| Genetic algorithms | Algorithms for optimisation motivated by natural selection | Improvement for strain for enhanced productivity |
| Data Mining | Deriving valuable information from extensive datasets | Recognising trends in productivity and growth |
| Neural Networks | Computer models that simulate how the human brain works | Examining complicated relationships among variables |
4.2. Enhancing Lipid Accumulation for Biofuels
4.3. Optimising CO2 Sequestration and Carbon Capture
4.4. Computer Vision and Automated Monitoring
4.5. Cross-Species Comparisons of AI Applications in Microalgal Cultivation
| Aspect | Observation and Example | AI Technique Used | Species Involved | Key Insight and Outcome | References |
|---|---|---|---|---|---|
| Species-specific model performance | Due to physiological differences, AI models developed for one species frequently perform poorly when applied to another (light response, food uptake, and stress tolerance) | ANN | Synechocystis vs. Chlorella | Each strain requires correction in order to retain accuracy | [15] |
| Aggregated modelling | Merged datasets from several studies to produce forecasts that are broadly applicable | Decision tree | Mixed species (>100 studies) | Revealed broad trends in biomass and lipid optimisation | [15,77] |
| Multi-species CO2 fixation modelling | Predicts CO2 fixation across different algal species | Adaptive neuro-fuzzy inference system optimised by genetic algorithm (ANFIS–GA) | Multiple algae | Increased capacity for prediction, but limited by the variability of the data | [15] |
| Hybrid modelling approach | Combines mechanistic and data-driven models for adaptability | Hybrid ML–mechanistic | General application | Improved generalisation and interpretability | [76] |
| Interpretability in harvesting optimisation | Explains influence of species traits (cell size, morphology) on harvesting | Extreme gradient boosting + shapley additive explanations (SHAP) | Various microalgae | Achieved (R2 = 0.93); highlighted species-specific harvest efficiencies | [15] |
| Nutrient optimisation differences | Optimal N:P ratio differs even among close species | ANN/Regression | Chlorella kessleri vs. C. vulgaris | Demonstrated distinct nutrient needs despite phylogenetic similarity | [15,76] |
| Data scarcity solutions | Limited datasets hinder AI generalisation to new species. | Synthetic data generation/Transfer learning | Rare or new strains | Encourages dataset sharing and transfer learning for rapid adaptation | [7,76] |
4.6. AI-Based Bioinformatics for Genome Editing
| Species | Conversion Technology | AI Algorithm | Application Outcome | References |
|---|---|---|---|---|
| Algal Mat | Pyrolysis | Single-layer ANN | Predicted pyrolysis behaviour and improved understanding of thermal degradation characteristics | [77] |
| C. vulgaris, N. oceanica, Chlamydomonas sp. | Pyrolysis | Particle Swarm Optimisation (PSO) combined with independent parallel reaction model | Modelled microalgal pyrolysis kinetics by considering carbohydrates, proteins, and lipids as input parameters | [78] |
| C. vulgaris | Pyrolysis and Gasification | Neuro-evolution integrated with deep neural networks | Predicted thermal conversion efficiency and identified optimal operating conditions to minimise energy use | [2] |
| Spirulina sp. | Combustion | Single-layer ANN coupled with numerical methods | Predicted combustion efficiency, exhaust emissions, and blend performance for algal biodiesel formulations | [79] |
| Nannochloropsis oculata | Hydrothermal liquefaction (HTL) | Multiple linear component additivity model | Simulated HTL conversion behaviour for yield and bio-crude quality optimisation | [80] |
| Chlorella CG12 | Transesterification (supercritical methanol) | RSM, ANN, and GA | Optimised reaction conditions for biodiesel production under supercritical methanol | [81] |
| Jatropha–Algae | Transesterification (KOH-catalysed) | Neuro-Fuzzy inference system (NFIS) integrated with RSM | Predicted transesterification outcomes considering catalyst concentration, temperature, and reaction time | [82] |
| Chlorella sp. | Ultrasonic-Assisted transesterification | Single-layer ANN integrated with RSM | Modelled ultrasonic power, methanol ratio, and reaction time to enhance FAME content and exergy efficiency | [83] |
| Mixed Microalgal Biomass | Enzymatic hydrolysis | Single-layer ANN | Predicted sugar yield by correlating substrate concentration, temperature, pH, and retention time | [84] |
| Microalgal Species | Genetic Tool | Scientific Function/Application | References |
|---|---|---|---|
| Dunaliella salina | RNAi | RNAi was employed to generate gene knockouts and clone sequences, enabling regulation of specific metabolites and modulation of host cell physiology | [89] |
| Chlamydomonas reinhardtii | RNAi | Used to silence chlorophyllide and oxygenase genes, facilitating the functional characterisation of gene deactivation and its physiological consequences | [90] |
| C. reinhardtii | ZFNs | ZFNs were applied to target the COP3 gene, leading to altered phenotypic and physiological expression patterns | [91] |
| Nannochloropsis oceanica IMET1 | ZFNs | ZFN-mediated transformation enabled chloroplast mutagenesis to regulate uric acid biosynthesis and improve chloroplast engineering efficiency | [92] |
| Phaeodactylum tricornutum | TALENs | TALENs introduced targeted double-strand breaks at the PtAurea gene, a blue-light photoreceptor, allowing precise control over light response and colony formation | [93] |
| C. reinhardtii | TALENs | TALEN-based activation of ARS1 and ARS2 loci enhanced nutrient compound accumulation and promoted targeted genetic modifications in host cells | [94] |
| C. reinhardtii CC-124 | CRISPR–Cas9 | CRISPR–Cas9 enabled efficient, site-specific mutagenesis with greater precision and consistency than RNAi, improving strain stability | [95] |
| Nannochloropsis oceanica CCMP1779 | CRISPR–Cas9 | Utilised for high-lipid metabolism studies, the CRISPR–Cas9 system incorporating ribozyme-linked sgRNA enabled autonomous, targeted mutagenesis for lipid pathway optimisation | [96] |
4.7. Optimising Light, Temperature, Nutrients, Harvesting, and Extraction
| Optimisation | Technology | Description | Benefits | References |
|---|---|---|---|---|
| Light | Artificial neural network (ANN) | To optimise light conditions for Parachlorella kessleri’s production of polyphenols, ANN was combined with a genetic algorithm (ANN-GA) | Greater efficiency in computation; Time saving; exhibiting strong performance in a variety of light levels and photoperiods | [15,97] |
| Light | ML | A closed tubular photobioreactor was constructed with sensors to track temperature, light intensity, and other variables. ML models were then integrated to predict growth dynamics | Improvement of biomass productivity; Greater precision in predicting growth | [15,67] |
| Temperature | Deep neural network (DNN) and response surface methodology (RSM) | Temperature optimisation on C. vulgaris cultivation for carbon dioxide capture was performed using DNN and RSM | Increased biomass productivity and CO2 capture efficiency | [15,71] |
| Nutrients | Support vector regression (SVR) and GA | Utilising SVR together with GA to optimise Chlorella kessleri’s nitrogen–phosphorus ratio in municipal wastewater treatment | Improved nutrient removal efficiency | [15] |
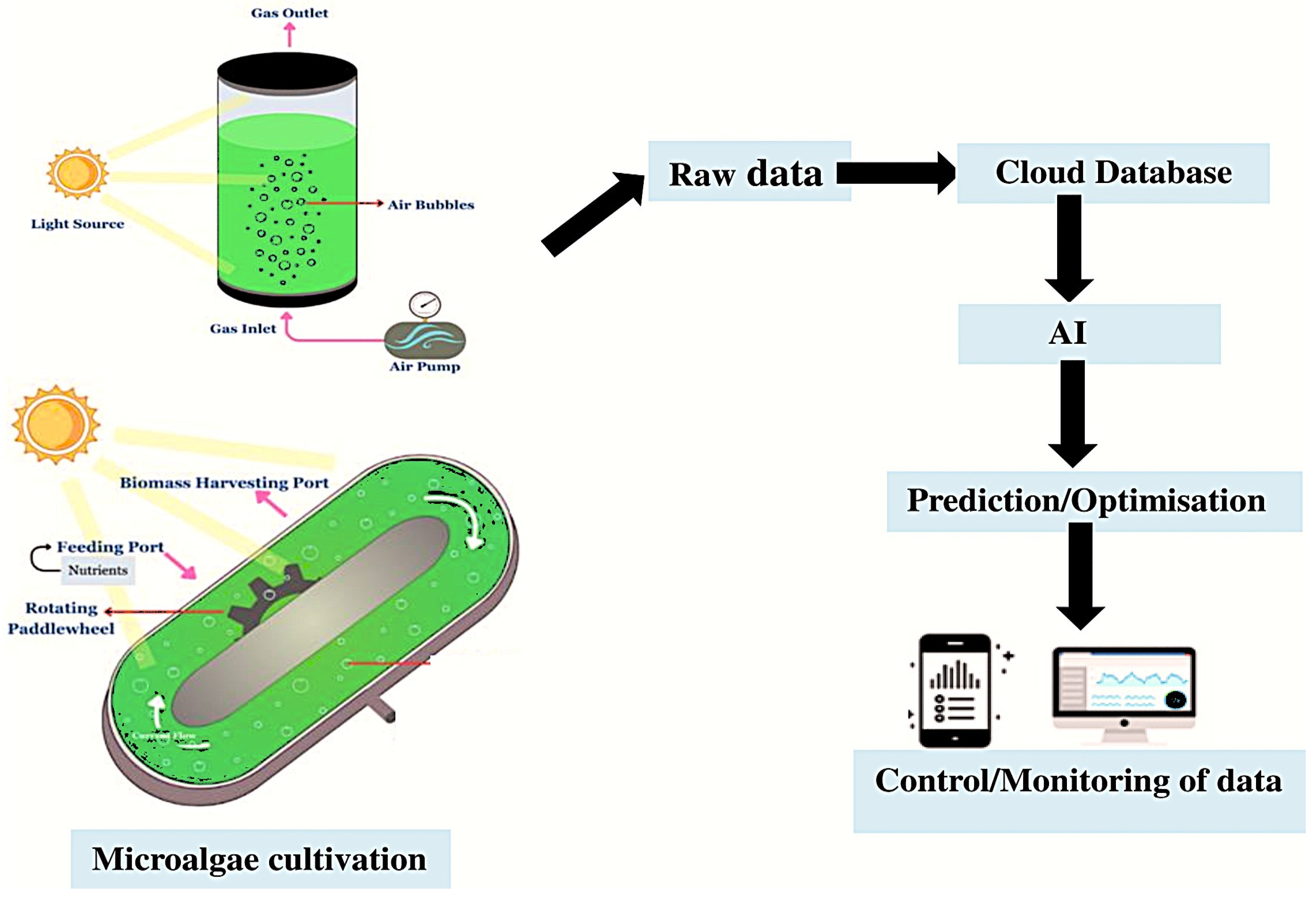
| Light and temperature | IoT | Development of an IoT-based system to maximise Arthrospira cultivation using sensors and Arduino microcontrollers for real-time monitoring of important factors like turbidity, light intensity, and water temperature | Maintenance of stable water temperature; Regulation of light intensity; Optimisation of turbidity level; Balance of nitrogen, oxygen, and CO2 supply | [15,66] |
| Harvesting and extraction | IoT | Development of an IoT-based system to optimise the growth and harvesting of Spirulina, employing real-time sensors to track important variables like water temperature, UV light intensity, and turbidity | Improved harvesting procedures; Increased production efficiency; Generated useful data for larger-scale applications and additional research | [15] |
5. Sustainability of AI/IoT in Microalgae Cultivation
6. Limitations and Possible Remedies for Microalgae Cultivation Using AI Models
7. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chew, K.W.; Yap, J.Y.; Show, P.L.; Suan, N.H.; Juan, J.C.; Ling, T.C.; Lee, D.J.; Chang, J.S. Microalgae biorefinery: High value products perspectives. Bioresour. Technol. 2017, 229, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Teng, S.Y.; Yew, G.Y.; Sukačová, K.; Show, P.L.; Máša, V.; Chang, J.-S. Microalgae with artificial intelligence: A digitalized perspective on genetics, systems and products. Biotechnol. Adv. 2020, 44, 107631. [Google Scholar] [CrossRef]
- Kumar, M.; Sun, Y.; Rathour, R.; Pandey, A.; Thakur, I.S.; Tsang, D.C.W. Algae as potential feedstock for the production of biofuels and value-added products: Opportunities and challenges. Sci. Total Environ. 2020, 716, 137116. [Google Scholar] [CrossRef]
- Acién, F.G.; Fernández, J.M.; Magán, J.J.; Molina, E. Production cost of a real microalgae production plant and strategies to reduce it. Biotechnol. Adv. 2012, 30, 1344–1353. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, J.; Chen, P.; Ji, C.; Kang, Q.; Lu, B.; Li, K.; Liu, J.; Ruan, R. Bio-mitigation of carbon dioxide using microalgal systems: Advances and perspectives. Renew. Sustain. Energy Rev. 2017, 76, 1163–1175. [Google Scholar] [CrossRef]
- Greulich, S.; Tran, N.; Kaldenhoff, R. Harnessing microalgae: From biology to innovation in sustainable solutions. Automatisierungstechnik 2024, 72, 606–615. [Google Scholar] [CrossRef]
- Imamoglu, E. Artificial Intelligence and/or Machine Learning Algorithms in Microalgae Bioprocesses. Bioengineering 2024, 11, 1143. [Google Scholar] [CrossRef] [PubMed]
- Igou, T.; Zhong, S.; Reid, E.; Chen, Y. Real-Time Sensor Data Profile-Based Deep Learning Method Applied to Open Raceway Pond Microalgal Productivity Prediction. Environ. Sci. Technol. 2023, 57, 17981–17989. [Google Scholar] [CrossRef]
- Chapman, R.L. Algae: The World’s Most Important “Plants”—An Introduction. Mitig. Adapt. Strateg. Glob. Change 2013, 18, 5–12. [Google Scholar] [CrossRef]
- Beal, C.M.; Gerber, L.N.; Thongrod, S.; Phromkunthong, W.; Kiron, V.; Granados, J.; Archibald, I.; Greene, C.H.; Huntley, M.E. Marine Microalgae Commercial Production Improves Sustainability of Global Fisheries and Aquaculture. Sci. Rep. 2018, 8, 15064. [Google Scholar] [CrossRef]
- Kavitha, S.; Ravi, Y.K.; Kumar, G.; Nandabalan, Y.K. Microalgal Biorefineries: Advancement in Machine Learning Tools for Sustainable Biofuel Production and Value-Added Products Recovery. J. Environ. Manag. 2024, 353, 120135. [Google Scholar] [CrossRef]
- Bisht, B.; Begum, J.P.S.; Dmitriev, A.A.; Kurbatova, A.; Singh, N.; Nishinari, K.; Nanda, M.; Kumar, S.; Vlaskin, M.S.; Kumar, V. Unlocking the Potential of Future Version 3D Food Products with next Generation Microalgae Blue Protein Integration: A Review. Trends Food Sci. Technol. 2024, 147, 104471. [Google Scholar] [CrossRef]
- Fu, Y.; Chen, T.; Chen, S.H.Y.; Liu, B.; Sun, P.; Sun, H.; Chen, F. The Potentials and Challenges of Using Microalgae as an Ingredient to Produce Meat Analogues. Trends Food Sci. Technol. 2021, 112, 188–200. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, G.; Chong, S.; Mak, N.K.; Chen, F.; Jiang, Y. Ultraviolet-B Radiation Improves Astaxanthin Accumulation in Green Microalga Haematococcus pluvialis. Biotechnol. Lett. 2010, 32, 1911–1914. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Shan, L.; Zhao, W.; Lu, X. Harnessing Artificial Intelligence to Revolutionize Microalgae Biotechnology: Unlocking Sustainable Solutions for High-Value Compounds and Carbon Neutrality. Mar. Drugs 2025, 23, 184. [Google Scholar] [CrossRef]
- Sajjadi, B.; Chen, W.-Y.; Raman, A.A.A.; Ibrahim, S. Microalgae lipid and biomass for biofuel production: A comprehensive review on lipid enhancement strategies and their effects on fatty acid composition. Renew. Sustain. Energy Rev. 2018, 97, 200–232. [Google Scholar] [CrossRef]
- Mohammady, N.G.E.; El-Khatib, K.M.; El-Galad, M.I.; Abo El-Enin, S.A.; Attia, N.K.; El-Araby, R.; El Diwani, G.; Manning, S.R. Preliminary study on the economic assessment of culturing Nannochloropsis sp. in Egypt for the production of biodiesel and high-value biochemicals. Biomass Convers. Biorefinery 2022, 12, 3319–3331. [Google Scholar] [CrossRef]
- Alzahmi, A.S.; Daakour, S.; Nelson, D.; Al-Khairy, D.; Twizere, J.C.; Salehi-Ashtiani, K. Enhancing Algal Production Strategies: Strain Selection, AI-Informed Cultivation, and Mutagenesis. Front. Sustain. Food Syst. 2024, 8, 1331251. [Google Scholar] [CrossRef]
- Rafa, N.; Ahmed, S.F.; Badruddin, I.A.; Mofijur, M.; Kamangar, S. Strategies to Produce Cost-Effective Third-Generation Biofuel from Microalgae. Front. Energy Res. 2021, 9, 749968. [Google Scholar] [CrossRef]
- Naeimi, S.M.; Darvish, S.; Salman, B.N.; Luchian, I. Artificial Intelligence in Adult and Pediatric Dentistry: A Narrative Review. Bioengineering 2024, 11, 431. [Google Scholar] [CrossRef]
- Reyes, L.T.; Knorst, J.K.; Ortiz, F.R.; Ardenghi, T.M. Scope and Challenges of Machine Learning-Based Diagnosis and Prognosis in Clinical Dentistry: A Literature Review. J. Clin. Transl. Res. 2021, 7, 523–539. [Google Scholar]
- Peter, A.P.; Chew, K.W.; Pandey, A.; Lau, S.Y.; Rajendran, S.; Ting, H.Y.; Munawaroh, H.S.H.; Van Phuong, N.; Show, P.L. Artificial Intelligence Model for Monitoring Biomass Growth in Semi-Batch Chlorella Vulgaris Cultivation. Fuel 2023, 333, 126438. [Google Scholar] [CrossRef]
- Singh, S.K.; Tiwari, A.K.; Paliwal, H.K. A state-of-the-art review on the utilization of machine learning in nanofluids, solar energy generation, and the prognosis of solar power. Eng. Anal. Bound. Elem. 2023, 155, 62–86. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, W.; Ngo, H.H.; Bui, X.T.; Tung, T.V.; Zhang, H. A Mini Review on Fundamentals and Practical Applications of Machine Learning in Algae-Based Wastewater Treatment. Algae Environ. 2025, 1, 2. [Google Scholar] [CrossRef]
- Fu, W.; Li, X.; Yang, Y.; Song, D. Enhanced degradation of bisphenol A: Influence of optimization of removal, kinetic model studies, application of machine learning and microalgae-bacteria consortia. Sci. Total Environ. 2023, 858, 159876. [Google Scholar] [CrossRef]
- de Sousa, N.F.S.; Perez, D.A.L.; Rosa, R.V.; Santos, M.A.S.; Rothenberg, C.E. Network Service Orchestration: A survey. Comput. Commun. 2019, 142–143, 69–94. [Google Scholar] [CrossRef]
- Khan, A.R.; Mahmood, A.; Safdar, A.; Khan, Z.A.; Khan, N.A. Load forecasting, dynamic pricing and DSM in smart grid: A review. Renew. Sustain. Energy Rev. 2016, 54, 1311–1322. [Google Scholar] [CrossRef]
- Otálora, P.; Guzmán, J.L.; Acién, F.G.; Berenguel, M.; Reul, A. An artificial intelligence approach for identification of microalgae cultures. New Biotechnol. 2023, 77, 58–67. [Google Scholar] [CrossRef]
- Khare, V.; Nema, S.; Baredar, P. Solar–wind hybrid renewable energy system: A review. Renew. Sustain. Energy Rev. 2016, 58, 23–33. [Google Scholar] [CrossRef]
- Camacho-Rodríguez, J.; Cerón-García, M.C.; Fernández-Sevilla, J.M.; Molina-Grima, E. Genetic algorithm for the medium optimization of the microalga Nannochloropsis gaditana cultured to aquaculture. Bioresour. Technol. 2015, 177, 102–109. [Google Scholar] [CrossRef]
- Antonopoulos, I.; Robu, V.; Couraud, B.; Kirli, D.; Norbu, S.; Kiprakis, A.; Flynn, D.; Elizondo-Gonzalez, S.; Wattam, S. Artificial intelligence and machine learning approaches to energy demand-side response: A systematic review. Renew. Sustain. Energy Rev. 2020, 130, 109899. [Google Scholar] [CrossRef]
- Ali, M.; Yaseen, M.; Ali, S.; Kim, H.-C. Deep Learning-Based Approach for Microscopic Algae Classification with Grad-CAM Interpretability. Electronics 2025, 14, 442. [Google Scholar] [CrossRef]
- Nweke, H.F.; Teh, Y.W.; Al-garadi, M.A.; Alo, U.R. Deep learning algorithms for human activity recognition using mobile and wearable sensor networks: State of the art and research challenges. Expert Syst. Appl. 2018, 105, 233–261. [Google Scholar] [CrossRef]
- Sonmez, M.E.; Eczacıoglu, N.; Gumuş, N.E.; Aslan, M.F.; Sabanci, K.; Aşikkutlu, B. Convolutional neural network—Support vector machine based approach for classification of cyanobacteria and chlorophyta microalgae groups. Algal Res. 2022, 61, 102568. [Google Scholar] [CrossRef]
- Li, L.; Liang, Z.; Liu, T.; Lu, C.; Yu, Q.; Qiao, Y. Transformer-Driven Algal Target Detection in Real Water Samples: From Dataset Construction and Augmentation to Model Optimization. Water 2025, 17, 430. [Google Scholar] [CrossRef]
- Supriyanto; Noguchi, R.; Ahamed, T.; Rani, D.S.; Sakurai, K.; Nasution, M.A.; Wibawa, D.S.; Demura, M.; Watanabe, M.M. Artificial neural networks model for estimating growth of polyculture microalgae in an open raceway pond. Biosyst. Eng. 2019, 177, 122–129. [Google Scholar] [CrossRef]
- Liu, J.-Y.; Zeng, L.-H.; Ren, Z.-H.; Du, T.-M.; Liu, X. Rapid in situ measurements of algal cell concentrations using an artificial neural network and single-excitation fluorescence spectrometry. Algal Res. 2020, 45, 101739. [Google Scholar] [CrossRef]
- Rio-Chanona, E.A.D.; Cong, X.; Bradford, E.; Zhang, D.; Jing, K. Review of advanced physical and data-driven models for dynamic bioprocess simulation: Case study of algae–bacteria consortium wastewater treatment. Biotechnol. Bioeng. 2019, 116, 342–353. [Google Scholar] [CrossRef]
- Susanna, D.; Dhanapal, R.; Mahalingam, R.; Ramamurthy, V. Increasing productivity of Spirulina platensis in photobioreactors using artificial neural network modeling. Biotechnol. Bioeng. 2019, 116, 2960–2970. [Google Scholar] [CrossRef]
- Liyanaarachchi, V.C.; Nishshanka, G.K.S.H.; Sakarika, M.; Nimarshana, P.H.V.; Ariyadasa, T.U.; Kornaros, M. Artificial neural network (ANN) approach to optimize cultivation conditions of microalga Chlorella vulgaris in view of biodiesel production. Biochem. Eng. J. 2021, 173, 108072. [Google Scholar] [CrossRef]
- Chong, J.W.R.; Tang, D.Y.Y.; Leong, H.Y.; Khoo, K.S.; Show, P.L.; Chew, K.W. Bridging Artificial Intelligence and Fucoxanthin for the Recovery and Quantification from Microalgae. Bioengineered 2023, 14, 2244232. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.R.; Khoo, K.S.; Chia, W.Y.; Chew, K.W.; Ho, S.H.; Show, P.L. Smart Microalgae Farming with Internet-of-Things for Sustainable Agriculture. Biotechnol. Adv. 2022, 57, 107931. [Google Scholar] [CrossRef]
- Pääkkönen, S.; Pölönen, I.; Raita-Hakola, A.-M.; Carneiro, M.; Cardoso, H.; Mauricio, D.; Rodrigues, A.M.C.; Salmi, P. Non-invasive monitoring of microalgae cultivations using hyperspectral imager. J. Appl. Phycol. 2024, 36, 1653–1665. [Google Scholar] [CrossRef]
- Hermann, L.; Kremling, A. A Hybrid Soft Sensor Approach Combining Partial Least-Squares Regression and an Unscented Kalman Filter for State Estimation in Bioprocesses. Bioengineering 2025, 12, 654. [Google Scholar] [CrossRef]
- Porras Reyes, L.; Havlik, I.; Beutel, S. Software sensors in the monitoring of microalgae cultivations. Rev. Environ. Sci. Bio/Technol. 2024, 23, 67–92. [Google Scholar] [CrossRef]
- Perera, Y.S.; Ratnaweera, D.A.A.C.; Dasanayaka, C.H.; Abeykoon, C. The role of artificial intelligence-driven soft sensors in advanced sustainable process industries: A critical review. Eng. Appl. Artif. Intell. 2023, 121, 105988. [Google Scholar] [CrossRef]
- Ohnuki, S.; Nogami, S.; Ota, S.; Watanabe, K.; Kawano, S.; Ohya, Y. Image-based monitoring system for green algal Haematococcus pluvialis (Chlorophyceae) cells during culture. Plant Cell Physiol. 2013, 54, 1917–1929. [Google Scholar] [CrossRef][Green Version]
- Stegemüller, L.; Caccavale, F.; Valverde-Pérez, B.; Angelidaki, I. Online monitoring of Haematococcus lacustris cell cycle using machine and deep learning techniques. Bioresour. Technol. 2025, 418, 131976. [Google Scholar] [CrossRef]
- Chong, J.W.R.; Khoo, K.S.; Chew, K.W.; Ting, H.-Y.; Iwamoto, K.; Showet, P.L. Digitalised prediction of blue pigment content from Spirulina platensis: Next-generation microalgae bio-molecule detection. Algal Res. 2024, 83, 103642. [Google Scholar] [CrossRef]
- Calderini, M.L.; Pääkkönen, S.; Yli-Tuomola, A.; Timilsina, H.; Pulkkinen, K.; Pölönen, I.; Salmiet, P. Accurate non-invasive quantification of astaxanthin content using hyperspectral images and machine learning. Algal Res. 2025, 87, 103979. [Google Scholar] [CrossRef]
- Sheik, A.G.; Kumar, A.; Ansari, F.A.; Raj, V.; Peleato, N.M.; Patan, A.K.; Kumari, S.; Bux, F. Reinvigorating algal cultivation for biomass production with digital twin technology—A smart sustainable infrastructure. Algal Res. 2024, 84, 103779. [Google Scholar] [CrossRef]
- Shahhoseyni, S.; Greco, L.; Sivaram, A.; Mansouri, S.S. A reduced-order hybrid model for photobioreactor performance and biomass prediction. Algal Res. 2024, 84, 103750. [Google Scholar] [CrossRef]
- Jia, L.; Wei, S.; Liu, J. A review of optimization approaches for controlling water-cooled central cooling systems. Build. Environ. 2021, 203, 108100. [Google Scholar] [CrossRef]
- Qiu, Y.; Liu, H.; Liu, J.; Li, D.; Liu, C.; Liu, W.; Wang, J.; Jiao, Y. A Digital Twin Lake Framework for Monitoring and Management of Harmful Algal Blooms. Toxins 2023, 15, 665. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Del Rio-Chanona, E.A.; Petsagkourakis, P.; Wagner, J. Hybrid physics-based and data-driven modeling for bioprocess online simulation and optimization. Biotechnol Bioeng 2019, 116, 2919–2930. [Google Scholar] [CrossRef]
- Franco Ortellado, B.M. Applications of Artificial Neural Networks in Three Agro-Environmental Systems: Microalgae Production, Nutritional Characterization of Soils and Meteorological Variables Management. Ph.D. Thesis, Universidad de Valladolid, Valladolid, Spain, 2019. [Google Scholar]
- Ayub, A.; Rahayu, F.; Khamidah, A.; Antarlina, S.S.; Iswari, K.; Supriyadi, K.; Mufidah, E.; Singh, A.; Chopra, C.; Wani, A.K. Harnessing microalgae as a bioresource for nutraceuticals: Advancing bioactive compound exploration and shaping the future of health and functional food innovation. Discov. Appl. Sci. 2025, 7, 389. [Google Scholar] [CrossRef]
- Oruganti, R.K.; Biji, A.P.; Lanuyanger, T.; Show, P.L.; Sriariyanun, M.; Upadhyayula, V.K.K.; Gadhamshetty, V.; Bhattacharyya, D. Artificial intelligence and machine learning tools for high-performance microalgal wastewater treatment and algal biorefinery: A critical review. Sci. Total Environ. 2023, 876, 162797. [Google Scholar] [CrossRef]
- Goswami, R.K.; Mehariya, S.; Verma, P. Advances in microalgae-based carbon sequestration: Current status and future perspectives. Environ. Res. 2024, 249, 118397. [Google Scholar] [CrossRef]
- Yu, T.; Fan, F.; Huang, L.; Wang, W.; Wan, M.; Li, Y. Artificial neural networks prediction and optimization based on four light regions for light utilization from Synechocystis sp. PCC 6803. Bioresour. Technol. 2024, 394, 130166. [Google Scholar] [CrossRef]
- Yeh, Y.-C.; Syed, T.; Brinitzer, G.; Frick, K.; Schmid-Staiger, U.; Haasdonk, B.; Tovar, G.E.M.; Krujatz, F.; Mädler, J.; Urbas, L. Improving microalgae growth modeling of outdoor cultivation with light history data using machine learning models: A comparative study. Bioresour. Technol. 2023, 390, 129882. [Google Scholar] [CrossRef]
- Szelag, B.; González-Camejo, J.; Eusebi, A.L.; Barat, R.; Kiczko, A.; Fatone, F. Multi-criteria analysis of the continuous operation of a membrane photobioreactor to treat sewage: Modeling and sensitivity analysis. Chem. Eng. J. 2024, 496, 154202. [Google Scholar] [CrossRef]
- Hossain, S.M.Z.; Sultana, N.; Razzak, S.A.; Hossain, M.M. Modeling and multi-objective optimization of microalgae biomass production and CO2 biofixation using hybrid intelligence approaches. Renew. Sustain. Energy Rev. 2022, 157, 112016. [Google Scholar] [CrossRef]
- Hossain, S.M.Z.; Hossain, M.M.; Razzak, S.A. Optimization of CO2 biofixation by Chlorella vulgaris using a tubular photobioreactor. Chem. Eng. Technol. 2018, 41, 1313–1323. [Google Scholar] [CrossRef]
- Pires, J.; Gonçalves, A.; Martins, F.; Alvim-Ferraz, M.; Simões, M. Effect of light supply on CO2 capture from atmosphere by Chlorella vulgaris and Pseudokirchneriella subcapitata. Mitig. Adapt. Strateg. Glob. Change 2014, 19, 1109–1117. [Google Scholar] [CrossRef]
- Ariawan, E.; Makalew, A.S. Smart micro farm: Sustainable algae spirulina growth monitoring system. In Proceedings of the 2018 10th International Conference on Information Technology and Electrical Engineering (ICITEE), Bali, Indonesia, 24–26 July 2018. [Google Scholar]
- Tummawai, T.; Rohitatisha, S.T.; Padungthon, S.; Sukpancharoen, S. Application of artificial intelligence and image processing for the cultivation of Chlorella sp. using tubular photobioreactors. ACS Omega 2024, 9, 46017–46029. [Google Scholar] [CrossRef]
- Saini, D.K.; Rai, A.; Devi, A.; Pabbi, S.; Chhabra, D.; Chang, J.S.; Shukla, P. A multi-objective hybrid machine learning approach-based optimization for enhanced biomass and bioactive phycobiliproteins production in Nostoc sp. CCC-403. Bioresour. Technol. 2021, 329, 124908. [Google Scholar] [CrossRef]
- Onay, A. Theoretical models constructed by artificial intelligence algorithms for enhanced lipid production: Decision support tools. Bitlis Eren Üniversitesi Fen Bilim. Derg. 2023, 12, 1195–1211. [Google Scholar] [CrossRef]
- Panahi, B.; Frahadian, M.; Dums, J.T.; Hejazi, M.A. Integration of cross species RNA-Seq meta-analysis and machine-learning models identifies the most important salt stress–responsive pathways in microalga Dunaliella. Front. Genet. 2019, 10, 752. [Google Scholar] [CrossRef]
- Janjua, M.Y.; Azfar, A.; Asghar, Z.; Shehzad Quraishi, K. Modeling and optimization of biomass productivity of Chlorella vulgaris using response surface methodology, analysis of variance and machine learning for carbon dioxide capture. Bioresour. Technol. 2024, 400, 130687. [Google Scholar] [CrossRef]
- Kushwaha, O.S.; Uthayakumar, H.; Kumaresan, K. Modeling of carbon dioxide fixation by microalgae using hybrid artificial intelligence (AI) and fuzzy logic (FL) methods and optimization by genetic algorithm (GA). Environ. Sci. Pollut. Res. 2023, 30, 24927–24948. [Google Scholar] [CrossRef]
- Kim, M.J.; Lee, J.; Song, J.M. Developing an algae culturing system using a microcontroller platform. Int. J. Biotechnol. Food Sci. 2016, 4, 1–9. [Google Scholar]
- Nayak, M.; Dhanarajan, G.; Dineshkumar, R.; Sen, R. Artificial intelligence driven process optimization for cleaner production of biomass with co-valorization of wastewater and flue gas in an algal biorefinery. J. Clean. Prod. 2018, 201, 1092–1100. [Google Scholar] [CrossRef]
- Xu, Z.; Jiang, Y.; Ji, J.; Forsberg, E.; Li, Y.; He, S. Classification, identification, and growth stage estimation of microalgae based on transmission hyperspectral microscopic imaging and machine learning. Opt. Express 2020, 28, 30686–30700. [Google Scholar] [CrossRef]
- Syed, T.; Krujatz, F.; Ihadjadene, Y.; Mühlstädt, G.; Hamedi, H.; Mädler, J.; Urbas, L. A review on machine learning approaches for microalgae cultivation systems. Comput. Biol. Med. 2024, 172, 108248. [Google Scholar] [CrossRef] [PubMed]
- Mayol, A.P.; Maningo, J.M.Z.; Chua-Unsu, A.G.A.Y.; Felix, C.B.; Rico, P.I.; Chua, G.S.; Manalili, E.V.; Fernandez, D.D.; Cuello, J.L.; Bandala, A.A.; et al. Application of artificial neural networks in prediction of pyrolysis behavior for algal mat (LABLAB) biomass. In Proceedings of the 2018 IEEE 10th International Conference on Humanoid, Nanotechnology, Information Technology, Communication and Control, Environment and Management, HNICEM, Baguio City, Philippines, 29 November–2 December 2018. [Google Scholar]
- Chen, W.H.; Chu, Y.S.; Liu, J.L.; Chang, J.S. Thermal degradation of carbohydrates, proteins and lipids in microalgae analyzed by evolutionary computation. Energy Convers. Manag. 2018, 160, 209–219. [Google Scholar] [CrossRef]
- Salam, S.; Verma, T.N. Appending empirical modelling to numerical solution for behaviour characterisation of microalgae biodiesel. Energy Convers. Manag. 2019, 180, 496–510. [Google Scholar] [CrossRef]
- Leow, S.; Witter, J.R.; Vardon, D.R.; Sharma, B.K.; Guest, J.S.; Strathmann, T.J. Prediction of microalgae hydrothermal liquefaction products from feedstock bio chemical composition. Green Chem. 2015, 17, 3584–3599. [Google Scholar] [CrossRef]
- Srivastava, G.; Paul, A.K.; Goud, V.V. Optimization of non-catalytic transesterification of microalgae oil to biodiesel under supercritical methanol condition. Energy Convers. Manag. 2018, 156, 269–278. [Google Scholar] [CrossRef]
- Kumar, S.; Jain, S.; Kumar, H. Performance evaluation of adaptive neuro-fuzzy inference system and response surface methodology in modeling biodiesel synthesis from jatropha–algae oil. Energy Sources Part A Recover. Util. Environ. Eff. 2018, 40, 3000–3008. [Google Scholar] [CrossRef]
- Karimi, M. Exergy-based optimization of direct conversion of microalgae biomass to biodiesel. J. Clean. Prod. 2017, 141, 50–55. [Google Scholar] [CrossRef]
- Shokrkar, H.; Ebrahimi, S.; Zamani, M. Extraction of sugars from mixed microalgae culture using enzymatic hydrolysis: Experimental study and modeling. Chem. Eng. Commun. 2017, 204, 1246–1257. [Google Scholar] [CrossRef]
- Coşgun, A.; Günay, M.E.; Yıldırım, R. Machine learning for algal biofuels: A critical review and perspective for the future. Green Chem. 2023, 25, 3354–3373. [Google Scholar] [CrossRef]
- Oey, M.; Ross, I.L.; Stephens, E.; Steinbeck, J.; Wolf, J.; Radzun, K.A.; Kügler, J.; Ringsmuth, A.K.; Kruse, O.; Hankamer, B. RNAi knock-down of LHCBM1, 2 and 3 increases photosynthetic H2 production efficiency of the green alga Chlamydomonas reinhardtii. PLoS ONE 2013, 8, e61375. [Google Scholar] [CrossRef] [PubMed]
- Sizova, I.; Greiner, A.; Awasthi, M.; Kateriya, S.; Hegemann, P. Nuclear gene targeting in Chlamydomonas using engineered zinc-finger nucleases. Plant J. Cell Mol. Biol. 2013, 73, 873–882. [Google Scholar] [CrossRef]
- Weyman, P.D.; Beeri, K.; Lefebvre, S.C.; Rivera, J.; McCarthy, J.K.; Heuberger, A.L.; Peers, G.; Allen, A.E.; Dupont, C.L. Inactivation of Phaeodactylum tricornutum urease gene using transcription activator-like effector nuclease-based targeted mutagenesis. Plant Biotechnol. J. 2015, 13, 460–470. [Google Scholar] [CrossRef]
- Jia, Y.; Xue, L.; Liu, H.; Li, J. Characterization of the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene from the halotolerant alga Dunaliella salina and inhibition of its expression by RNAi. Curr. Microbiol. 2009, 58, 426–431. [Google Scholar] [CrossRef]
- Perrine, Z.; Negi, S.; Sayre, R.T. Optimization of photosynthetic light energy utilization by microalgae. Algal Res. 2012, 1, 134–142. [Google Scholar] [CrossRef]
- Mussgnug, J.H. Genetic tools and techniques for Chlamydomonas reinhardtii. Appl. Microbiol. Biotechnol. 2015, 99, 5407–5418. [Google Scholar] [CrossRef]
- Kwon, Y.M.; Kim, K.W.; Choi, T.Y.; Kim, S.Y.; Kim, J.Y.H. Manipulation of the microalgal chloroplast by genetic engineering for biotechnological utilization as a green biofactory. World J. Microbiol. Biotechnol. 2018, 34, 183. [Google Scholar] [CrossRef] [PubMed]
- Serif, M.; Lepetit, B.; Weißert, K.; Kroth, P.G.; Rio Bartulos, C. A fast and reliable strategy to generate TALEN-mediated gene knockouts in the diatom Phaeodactylum tricornutum. Algal Res. 2017, 23, 186–195. [Google Scholar] [CrossRef]
- Gao, H.; Wright, D.A.; Li, T.; Wang, Y.; Horken, K.; Weeks, D.P.; Yang, B.; Spalding, M.H. TALE activation of endogenous genes in Chlamydomonas reinhardtii. Algal Res. 2014, 5, 52–60. [Google Scholar] [CrossRef]
- Shin, S.E.; Lim, J.M.; Koh, H.G.; Kim, E.K.; Kang, N.K.; Jeon, S.; Kwon, S.; Shin, W.S.; Lee, B.; Hwangbo, K.; et al. CRISPR/Cas9-induced knockout and knock-in mutations in Chlamydomonas reinhardtii. Sci. Rep. 2016, 6, 27810. [Google Scholar] [CrossRef] [PubMed]
- Poliner, E.; Takeuchi, T.; Du, Z.Y.; Benning, C.; Farré, E.M. Nontransgenic marker- free gene disruption by an Episomal CRISPR system in the oleaginous microalga, Nannochloropsis oceanica CCMP1779. ACS Synth. Biol. 2018, 7, 962–968. [Google Scholar] [CrossRef] [PubMed]
- Fernández Izquierdo, P.; Patiño Coral, M.; Ortiz Benavides, F. Application of an artificial neural network coupled to a genetic algorithm for the production of polyphenols in Parachlorella kessleri grown under mixotrophic conditions. Algal Res. 2024, 77, 103331. [Google Scholar] [CrossRef]
- Shamayleh, A.; Awad, M.; Farhat, J. IoT based predictive maintenance management of medical equipment. J. Med. Syst. 2020, 44, 72. [Google Scholar] [CrossRef]
- Paiva, E.M.; Hyttinen, E.; Dönsberg, T.; Barth, D. Biological contaminants analysis in microalgae culture by UV-vis spectroscopy and machine learning. Spectrochim. Acta. Part A Mol. Biomol. Spectrosc. 2025, 330, 125690. [Google Scholar] [CrossRef]
- Baughman, D.R.; Liu, Y.A. Neural Networks in Bioprocessing and Chemical Engineering; Academic Press: Cambridge, MA, USA, 2014. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rayamajhi, V.; Hussain, M.; Shin, H.; Jung, S. Recent Advances in the Application of Artificial Intelligence in Microalgal Cultivation. Processes 2025, 13, 3764. https://doi.org/10.3390/pr13123764
Rayamajhi V, Hussain M, Shin H, Jung S. Recent Advances in the Application of Artificial Intelligence in Microalgal Cultivation. Processes. 2025; 13(12):3764. https://doi.org/10.3390/pr13123764
Chicago/Turabian StyleRayamajhi, Vijay, Mudasir Hussain, Hyunwoung Shin, and Sangmok Jung. 2025. "Recent Advances in the Application of Artificial Intelligence in Microalgal Cultivation" Processes 13, no. 12: 3764. https://doi.org/10.3390/pr13123764
APA StyleRayamajhi, V., Hussain, M., Shin, H., & Jung, S. (2025). Recent Advances in the Application of Artificial Intelligence in Microalgal Cultivation. Processes, 13(12), 3764. https://doi.org/10.3390/pr13123764







