Valorization of Pistachio Green Hull: Advances in Extraction and Characterization of Phenolic Compounds
Abstract
1. Introduction
2. Review Methodology
3. Pistachio Fruit
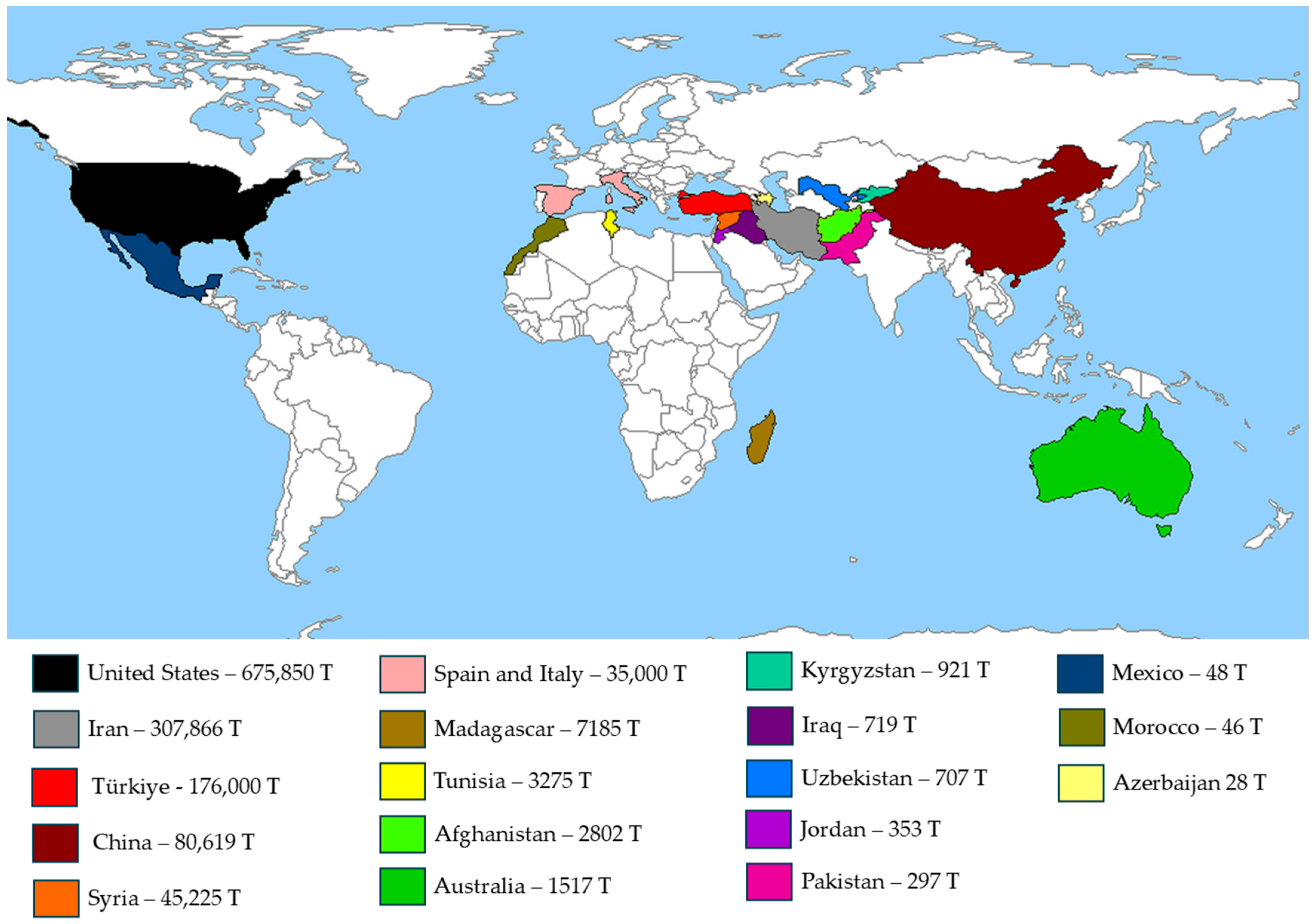
4. Pistachio Green Hull By-Products
Composition of Pistachio Green Hull
| Country | Carbohydrates (%) | Fat (%) | Protein (%) | Ash (%) | Crude Fiber (%) | Moisture (%) | Reference |
|---|---|---|---|---|---|---|---|
| Tunisia | 39.70 | 20.41 | 11.23 | 14.74 | ND | 10.46 (DB) | Hamed et al. [34] |
| Iran | ND | ND | 0.24* | ND | ND | 97.33 * | Azhdari et al. [35] |
| Iran | ND | 9.67 | 13.1 | 13.1 | ND | ND | Mohammadi-Moghaddam et al. [36] |
| Iran | ND | ND | 11.30 | 15.08 | ND | DB | Bakhshizadeh et al. [30] |
| Iran | ND | 5.8 | 12.15 | 11.98 | 15.17 | DB | Noruzi et al. [37] |
| Iran | 40 | 5.7 | 16.6 | 12.7 | 25 | DB | Bohluli et al. [31] |
| Mexico | 63.37–67.70 | 3.67–4.89 | 8.78–10.22 | 10.79–11.98 | ND | DB | Martínez-Ruíz et al. [13] |
| Türkiye | 13.8 | 9.5 | 8.26 | 12.56 | ND | 71.05 (WB) | Özbek et al. [29] |
| Türkiye | ND | ND | 7.27–14.99 | 8.50–19.86 | 18.25–22.49 | DB | Boğa et al. [38] |
5. Phenolic Compounds
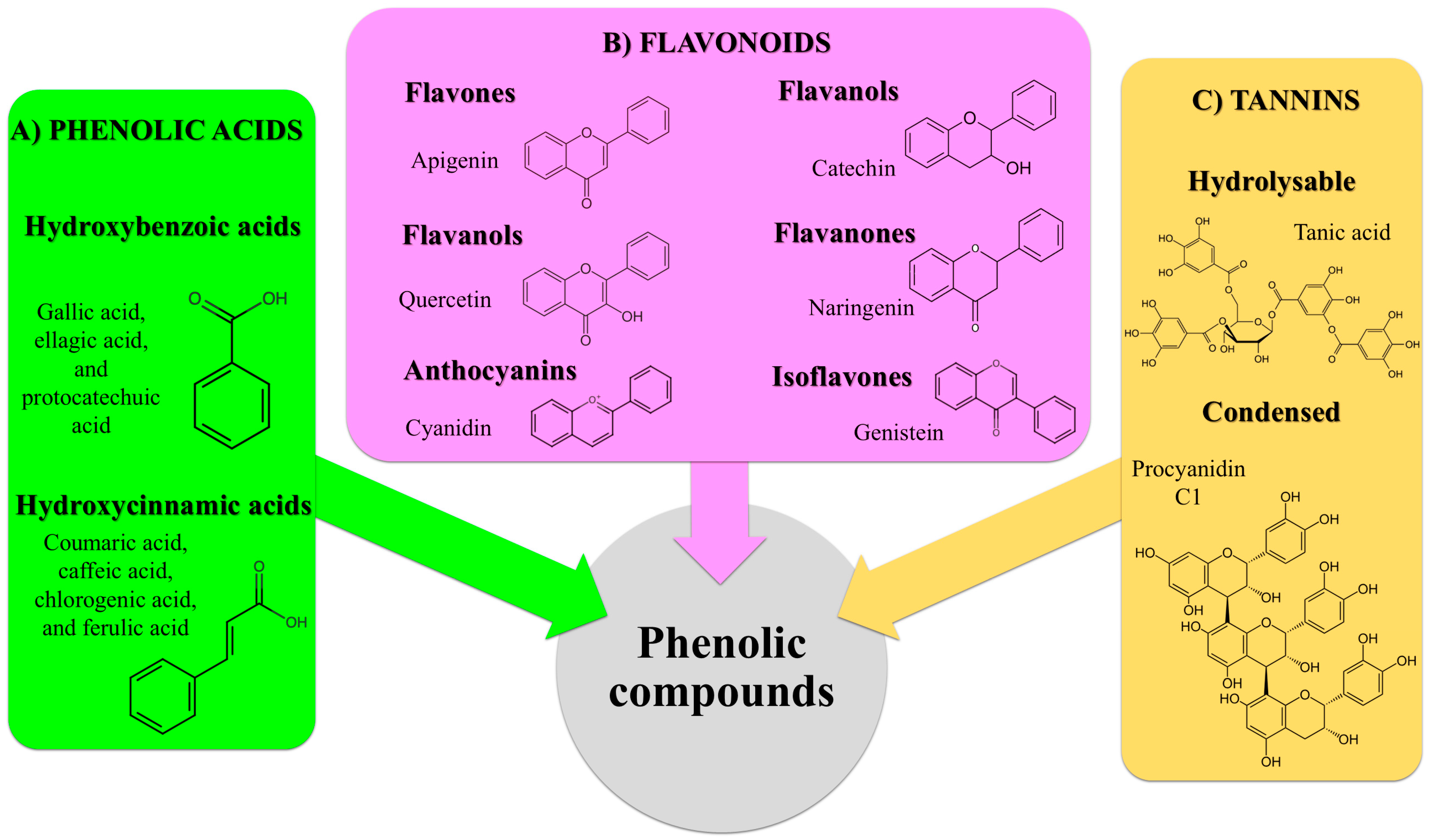
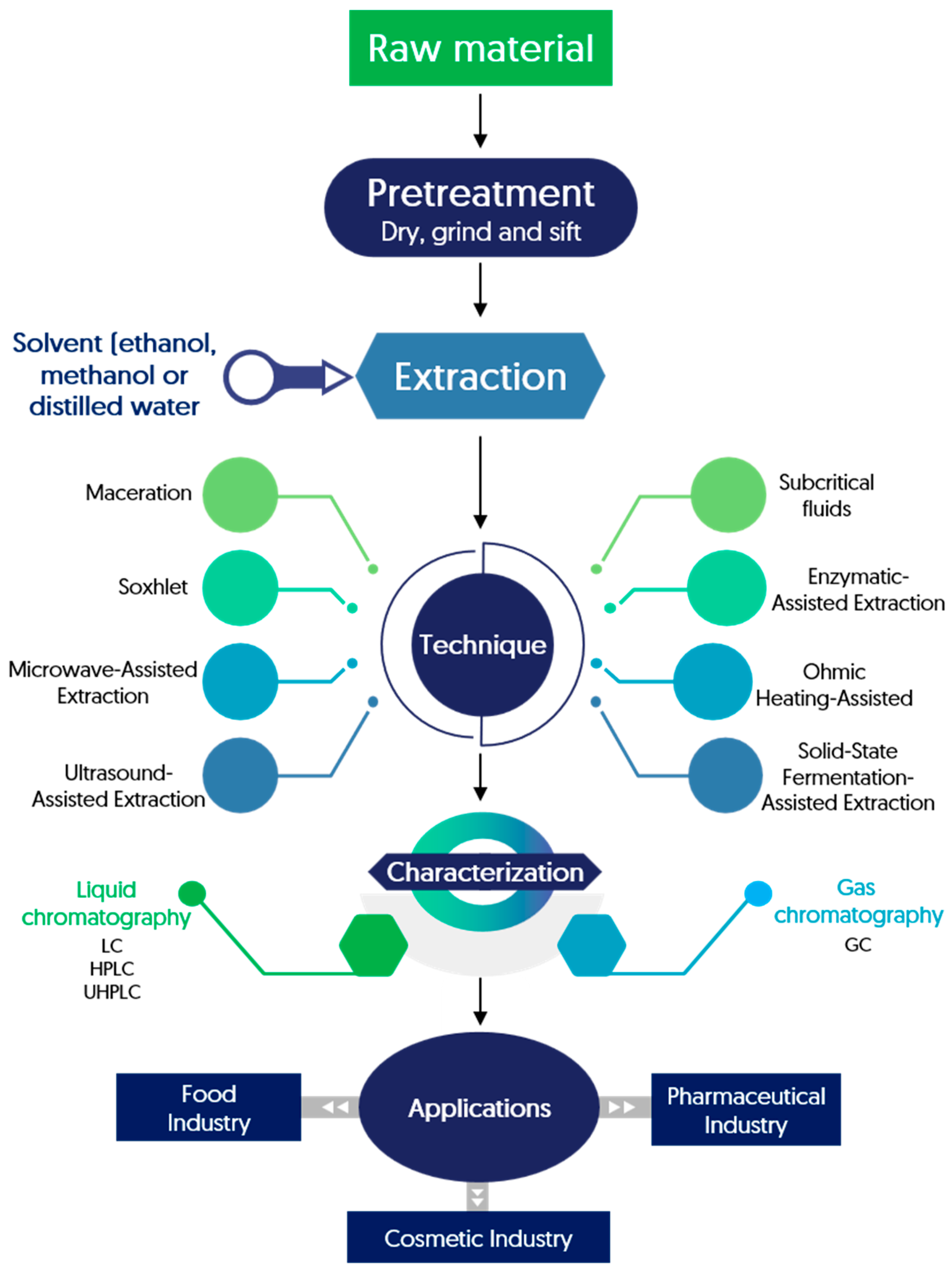
5.1. Extraction of Phenolic Compounds from Pistachio Green Hull
| Extraction Technique | Yield | Cost/ Effectiveness | Environmental Impact | Scaling-Up | References |
|---|---|---|---|---|---|
| Decoction | 24.54% (TPC) | Low cost/ Moderate efficiency | Moderate-high energy consumption | Highly scalable with low maintenance and instrumentation cost | Shahdadi et al. [39] |
| Maceration | 0.0001–81% (TPC) 0.03–32.66% (FLA) 2.31–7.91% (TAN) 0.001–0.71% (CT) 1.82–11.86% (ANT) 0.99% (NTAN) 0.009–0.018% (PRAN) | Low cost/ Low-Very high efficiency | Large volume of solvent required | Scale-up easily controlled | Farrokhi et al. [48] Roudbari et al. [5] Karaogul and Ugurtay [49] Shakerardekani et al. [50] Noruzi et al. [37] Moreno-Rojas et al. [51] Elhadef et al. [52] Ozay et al. [53] Pakdaman et al. [54] Noorolahi et al. [55] Rafiee et al. [56] Barreca et al. [21] Grace et al. [33] Tabaraki and Ghadiri [57] Rajaei et al. [58] Goli et al. [59] |
| Soxhlet | 0.024% (TPC) | Moderate cost/ Low efficiency | High energy consumption | Scale-up involves a high solvent consumption and large waste production | Kepekci et al. [60] |
| Microwave-Assisted Extraction | 1.47–20% (TPC) 0.29–7.5% (FLA) | High maintenance cost/ Moderate-high Efficiency | Reduced energy consumption | Highly scalable by reducing energy consumption and solvent use | Seker and Akbas [61] Özbek et al. [29] Özbek et al. [10] Garavand et al. [4] Tabaraki and Ghadiri [57] |
| Ultrasound-Assisted Extraction | 0.5–22% (TPC) 0.34–11.98% (FLA) 1.06% (GLT) 6.75% (ANA) | Moderate-high maintenance cost/ Moderate-high Efficiency | Reduced energy consumption | Highly scalable by reducing energy consumption and solvent use | Elakremi et al. [62] Erşan et al. [63] Garavand et al. [4] Tabaraki and Ghadiri [57] Goli et al. [59] |
| Atmospheric Cold-Plasma-Assisted | 1.8% (TPC) 39.09% (FLA) | High initial setup and operating cost/ High efficiency | Low energy consumption | Expensive scale-up by initial investment and maintenance cost | Farrokhi et al. [48] |
| Ohmic Heating- Assisted | 2.05% (TPC) 48.41% (FLA) | Low maintenance cost/ High efficiency | Environmentally friendly process | Scale-up involves adaptations for efficient energy Utilization | Farrokhi et al. [48] |
| Subcritical fluids | 0.66–3.95% (TPC) 0.07–0.57% (FLA) 2.04–3.31% (GLT) 0.11–0.28% (ANA) | High cost/ Low efficiency | Environmental and safety issues caused by high pressure | Expensive scale-up by equipment and conditions | Erşan et al. [63] Goli et al. [59] |
| Enzymatic- Assisted Extraction | 3.21–10.1% (TPC) 3.66–3.69% (FLA) 2.23–2.91% (TAN) 5.51–6.24% (HT) 0.47–12.11% (GA) 2.88–3.57% (PHG) 0.22% (NRG) | High cost/ Moderate Efficiency | Environmentally friendly process | Expensive scale-up by cost and availability of enzymes | Ghandehari-Yazdi et al. [64] Ghandahari-Yazdi et al. [65] Azhdari et al. [35] |
| Solid-State Fermentation- Assisted Extraction | 0.054–6.3% (TPC) 0.019–0.18% (FLA) 3.47% (HT) 0.68% (CT) | Low cost/ Low-moderate Efficiency | Environmentally friendly process | Scale-up involves adaptations for reactors and operating conditions | Ordoñez-Cano et al. [66] Karimi et al. [67] Abbasi et al. [68] |
5.2. Quantification of Phenolic Compounds in Pistachio Green Hull
| Component | Content | Unit | References |
|---|---|---|---|
| TPC | 0.0002–810 | mg GAE g dm−1 | Karaogul and Ugurtay [49]; Noruzi et al. [37]; Roudbari et al. [5]; Seker and Akbas [61]; Ghandehari-Yazdi et al. [64]; Pakdaman et al. [54]; Noorolahi et al. [55]; Özbek et al. [29]; Garavand et al. [4]; Tabaraki and Ghadiri [57]; Karimi et al. [67]; Rajaei et al. [58]; Kepekci et al. [60]; Farrokhi et al. [48]; Azhdari et al. [35]; Elhadef et al. [52]; Özbek et al. [10]; Grace et al. [33] |
| 0.1–41.48 | mg PCs g dm−1 | Ordoñez-Cano et al. [66]; Shakerardekani et al. [50]; Ozay et al. [53] | |
| 245.43 | mg GAE mL of extract−1 | Shahdadi et al. [39] | |
| 1.46–5.92 | mmol GAE 100 g fm−1 | Moreno-Rojas et al. [51] | |
| 218.97 | mg GAE g de−1 | Elakremi et al. [62] | |
| 22.2–81.8 | g PCs kg dm−1 | Erşan et al. [63] | |
| 163.3–614.9 | mg GAE g fe−1 | Rafiee et al. [56] | |
| 6.74–11.7 | µM GAE g fw−1 | Barreca et al. [21] | |
| ~49–63 | mg CAE g dm−1 | Abbasi et al. [68] | |
| 5.02–34.7 | mg TAE g dw−1 | Goli et al. [59] | |
| FLA | 2.22–484.1 | mg QE g dm−1 | Karaogul and Ugurtay [49]; Seker and Akbas [61]; Farrokhi et al. [48]; Elhadef et al. [52]; Grace et al. [33] |
| 30.46–85 | mg CE g dm−1 | Noruzi et al. [37]; Noorolahi et al. [55]; Garavand et al. [4]; Azhdari et al. [35] | |
| 0.34–0.688 | mg QE g fm−1 | Shakerardekani et al. [50]; Barreca et al. [21] | |
| 119.75 | mg CE g de−1 | Elakremi et al. [62] | |
| 0.7–5.65 | g FLA kg dm−1 | Erşan et al. [63] | |
| 27.4–73.3 | mg CE g fe−1 | Rafiee et al. [56] | |
| 0.186–1.855 | mg RE g dm−1 | Karimi et al. [67] | |
| CT | 7.07 | mg CT g dm−1 | Karaogul and Ugurtay [49] |
| 0.32–6.77 | mg CE g dm−1 | Ordoñez-Cano et al. [66]; Elhadef et al. [52] | |
| 0.013–0.071 | mg CE g fm−1 | Barreca et al. [21] | |
| HT | 34.71–62.35 | mg GAE g dm−1 | Azhdari et al. [35]; Ordoñez-Cano et al. [66] |
| ANT | 18.21–40.98 | µg C-3-O-glu g dm−1 | Elhadef et al. [52] |
| 35.5–118.6 | µg Cy-3-g g fe−1 | Rafiee et al. [56] | |
| PRAN | 0.088–0.177 | mg CE g fm−1 | Barreca et al. [21] |
| TAN | 23.14–32.03 | mg GAE g dm−1 | Noruzi et al. [37]; Noorolahi et al. [55] |
| 22.29–29.09 | mg TAE g dm−1 | Azhdari et al. [35] | |
| NTAN | 9.93 | mg GAE g dm−1 | Noorolahi et al. [55] |
| ANA | 1.13–67.5 | g ANA kg dm−1 | Erşan et al. [63] |
| GLT | 10.6–33.1 | g GLT kg dm−1 | Erşan et al. [63] |
| Gallic Acid | 4.69–121.10 | mg g de−1 | Ghandahari-Yazdi et al. [65] |
| Phloroglucinol | 28.82–35.71 | mg g de−1 | Ghandahari-Yazdi et al. [65] |
| Naringin | 2.21 | mg g de−1 | Ghandahari-Yazdi et al. [65] |
5.3. Identification of Phenolic Compounds in Pistachio Green Hull
5.4. Biological Activities of Pistachio Green Hull
5.4.1. Antioxidant Activity
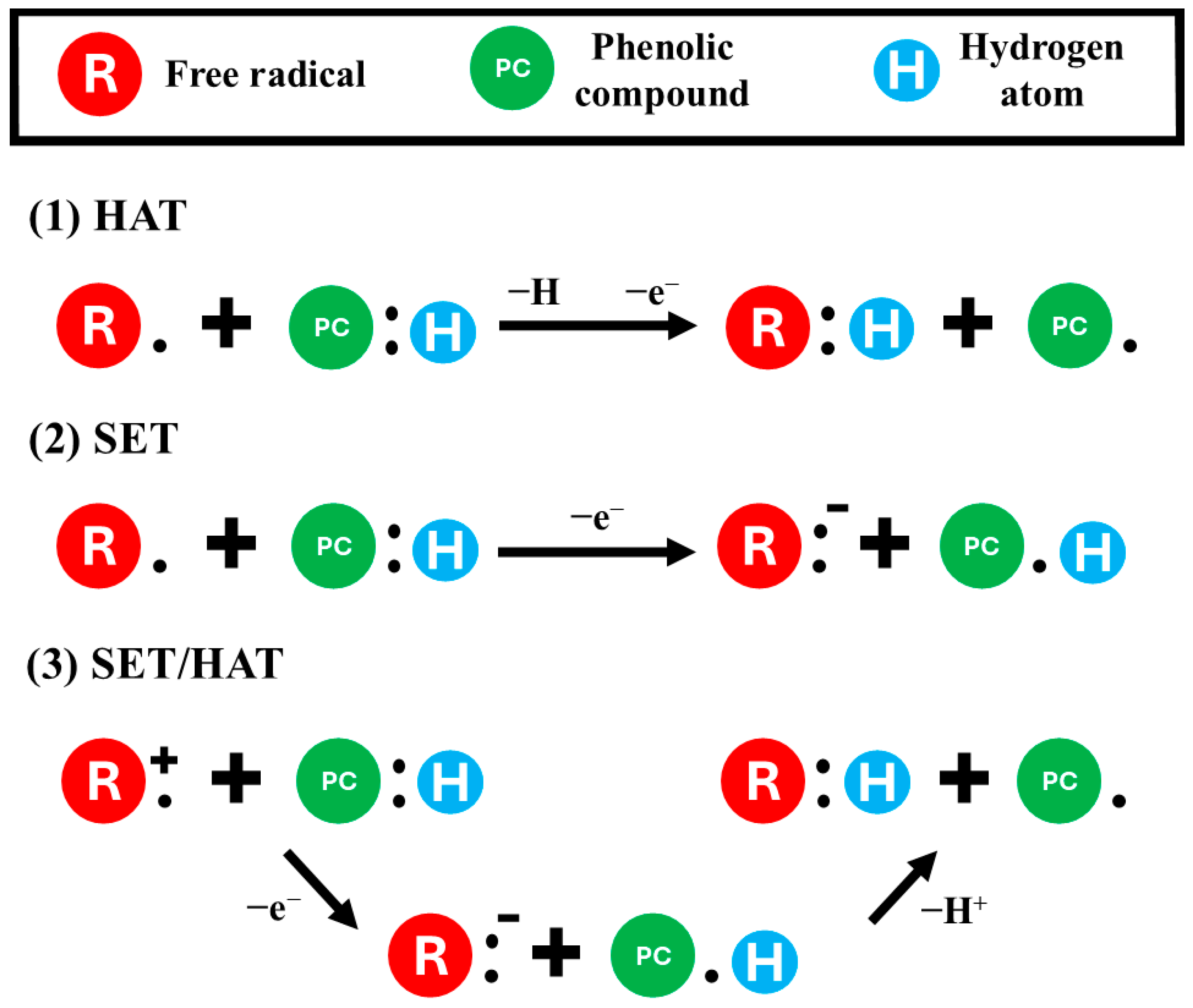
Efficiency of Extraction of Phenolic Compounds on Antioxidant Activity
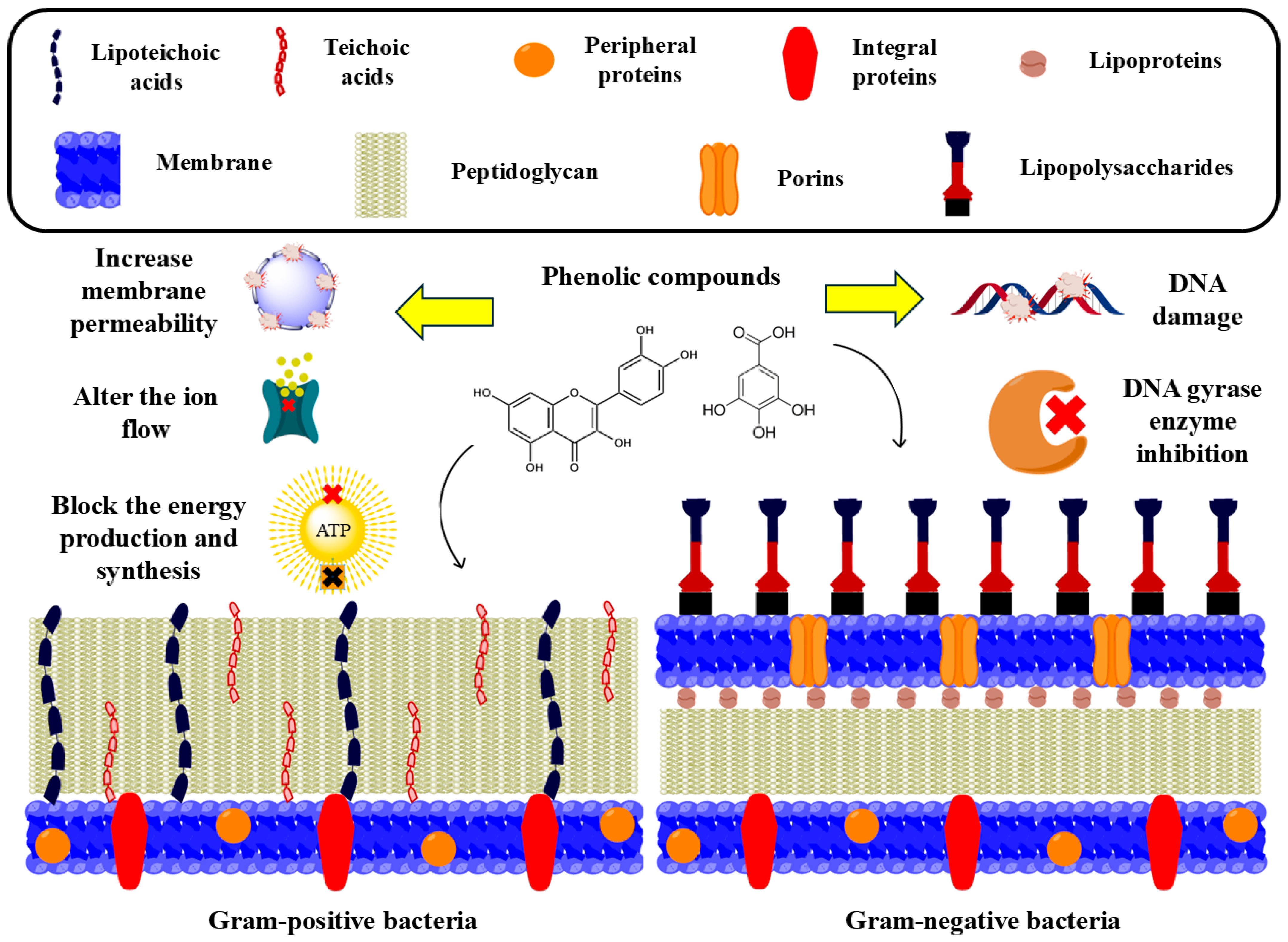
5.4.2. Antibacterial Activity
Effectiveness of Phenolic Compound Extraction on Antibacterial Activity
6. Future Perspectives
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cárdenas-Hernández, E.; Torres-León, C.; Chavez González, M.L.; Ximenes, R.M.; Gonçalves-Silva, T.; Ascacio-Valdés, J.A.; Martínez-Hernández, J.L.; Aguilar, C.N. From Agroindustrial Waste to Nutraceuticals: Potential of Mango Seed for Sustainable Product Development. Trends Food Sci. Technol. 2024, 154, 104754. [Google Scholar] [CrossRef]
- Arjeh, E.; Akhavan, H.-R.; Barzegar, M.; Carbonell-Barrachina, Á.A. Bio-active compounds and functional properties of pistachio hull: A review. Trends Food Sci. Technol. 2020, 97, 55–64. [Google Scholar] [CrossRef]
- Kazemi, M.; Aboutalebzadeh, S.; Mojaverian, S.P.; Samani, S.A.; Kouhsari, F.; PourvatanDoust, S.; Salimi, A.; Savarolyia, M.; Najafi, A.; Hosseini, S.S.; et al. Valorization of pistachio industrial waste: Simultaneous recovery of pectin and phenolics, and their application in low-phenylalanine cookies for phenylketonuria. Int. J. Biol. Macromol. 2023, 249, 126086. [Google Scholar] [CrossRef]
- Garavand, F.; Madadlou, A.; Moini, S. Determination of phenolic profile and antioxidant activity of pistachio hull using high-performance liquid chromatography–diode array detector–electro-spray ionization–mass spectrometry as affected by ultrasound and microwave. Int. J. Food Prop. 2017, 20, 19–29. [Google Scholar] [CrossRef]
- Roudbari, M.; Barzegar, M.; Sahari, M.A. Pistachio green hull and pomegranate peel extracts as two natural antiglycation agents. Food Sci. Nutr. 2024, 12, 3688–3695. [Google Scholar] [CrossRef]
- USDA. Tree Nuts: World Markets and Trade; United States Department of Agriculture: Washington, DC, USA, 2025; p. 3. [Google Scholar]
- Demirer, G.N. Biogas production from pistachio (Pistacia vera L.) de-hulling waste. Int. J. Green Energy 2016, 13, 1320–1324. [Google Scholar] [CrossRef]
- Fatemi, A.; Najafi, A.; Razavi, R.; Jafarzadeh, S. Characterizing the antioxidant and antifungal properties of nano-encapsulated pistachio hull extract in fenugreek seed gum to maintain the quality and safety of fresh pistachio. Food Sci. Nutr. 2024, 12, 5561–5571. [Google Scholar] [CrossRef] [PubMed]
- Shakerardekani, A.; Molaei, M. Post-Harvest Pistachio Waste: Methods of Its Reduction and Conversion. Pist. Health J. 2020, 3, 40–51. [Google Scholar] [CrossRef]
- Özbek, H.N.; Halahlih, F.; Göğüş, F.; Koçak Yanık, D.; Azaizeh, H. Pistachio (Pistacia vera L.) Hull as a Potential Source of Phenolic Compounds: Evaluation of Ethanol–Water Binary Solvent Extraction on Antioxidant Activity and Phenolic Content of Pistachio Hull Extracts. Waste Biomass Valorization 2018, 11, 2101–2110. [Google Scholar] [CrossRef]
- Taghipour, S.; Ehtesham Nia, A.; Hokmabadi, H.; Martínez-Gómez, P. Physicochemical and quality characters of fresh pistachio (Pistacia vera L.) cultivars in response to chitosan/ZnO Nanocomposite coating. Food Chem. 2024, 435, 137136. [Google Scholar] [CrossRef]
- Cardullo, N.; Leanza, M.; Muccilli, V.; Tringali, C. Valorization of Agri-Food Waste from Pistachio Hard Shells: Extraction of Polyphenols as Natural Antioxidants. Resources 2021, 10, 45. [Google Scholar] [CrossRef]
- Martínez-Ruíz, N.R.; Rodrigo-García, J.; Corral-Díaz, B. Efecto del Secado Controlado Sobre la Calidad Nutrimental del Pistache (Pistacia vera L.) y Subproductos Producido en el Valle de Juárez, Chihuahua, México; Universidad Autónoma de Ciudad Juárez: Ciudad Juárez, Mexico, 2019; p. 22. [Google Scholar]
- Ripari-Garrido, J.; Patrignani, M.; Puppo, M.C.; Salinas, M.V. Nutritional and bioactive characterization of pistachio—A review with special focus on health. Explor. Foods Foodomics 2024, 2, 363–390. [Google Scholar] [CrossRef]
- De León-Delgado, M.M.; Legarreta González, M.A.; Olivas García, J.M.; Guerrero Morales, S.; Baray Guerrero, M.R. Análisis financiero y económico del cultivo del pistache en el Municipio de López, Chihuahua. Rev. Biológico Agropecu. Tuxpan 2020, 8, 14–22. [Google Scholar] [CrossRef]
- Garcia-Moreno, P.J.; de la Rosa, L.A.; Stevens-Barron, J.C.; Rodríguez-Ramirez, R.; Corral-Diaz, B.; Alvarez-Parrilla, E.; Olivas-Aguirre, F.J.; Wall-Medrano, A. Dehiscence and prolonged storage of ‘Kerman’ Pistachios: Effects on morphometry and nutraceutical value. J. Food Sci. Technol. 2021, 58, 1958–1968. [Google Scholar] [CrossRef] [PubMed]
- Mateos, R.; Salvador, M.D.; Fregapane, G.; Goya, L. Why Should Pistachio Be a Regular Food in Our Diet? Nutrients 2022, 14, 3207. [Google Scholar] [CrossRef]
- Ahmadi, R.; Honarvar, M.; Ghavami, M.; Daali, Y. Optimization of Lutein Extraction from Pistachio Waste Using Experimental Design and Ultrasonic Method. Waste Biomass Valorization 2024, 15, 3077–3091. [Google Scholar] [CrossRef]
- Sá, A.G.A.; Franczyk, A.; Neufeld, J.; House, J.D. In vivo protein quality of pistachios (Pistacia vera L.). J. Food Compos. Anal. 2024, 132, 106351. [Google Scholar] [CrossRef]
- Schulze-Kaysers, N.; Feuereisen, M.M.; Schieber, A. Phenolic compounds in edible species of the Anacardiaceae family—A review. R. Soc. Chem. Adv. 2015, 5, 73301–73314. [Google Scholar] [CrossRef]
- Barreca, D.; Laganà, G.; Leuzzi, U.; Smeriglio, A.; Trombetta, D.; Bellocco, E. Evaluation of the nutraceutical, antioxidant and cytoprotective properties of ripe pistachio (Pistacia vera L., variety Bronte) hulls. Food Chem. 2016, 196, 493–502. [Google Scholar] [CrossRef]
- FAO. Crops and Livestock Products. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 25 October 2025).
- Fereidooni, L.; Morais, A.R.C.; Shiflett, M.B. Environmental applications of pistachio waste: A review. J. Environ. Manag. 2025, 380, 124927. [Google Scholar] [CrossRef]
- Toghiani, J.; Fallah, N.; Nasernejad, B.; Mahboubi, A.; Taherzadeh, M.J.; Afsham, N. Sustainable Pistachio Dehulling Waste Management and Its Valorization Approaches: A Review. Curr. Pollut. Rep. 2023, 9, 60–72. [Google Scholar] [CrossRef]
- Noorolahi, Z.; Sahari, M.A.; Ahmadi Gavlighi, H.; Barzegar, M. Pistachio green hull extract as natural antioxidant incorporated to omega-3 rich kappa-carrageenan oleogel in dry fermented sausage. Food Biosci. 2022, 50, 101986. [Google Scholar] [CrossRef]
- Karimi, A.; Ghandehari Yazdi, A.P.; Barzegar, M.; Rahmati, M.; Bazsefidpar, N.; Soltani, A.; Jafari, S.M. Utilizing pistachio green hull extract to produce sugar-free muffins with antioxidant and antidiabetic potential. Appl. Food Res. 2024, 4, 100510. [Google Scholar] [CrossRef]
- Tavakoli, M.; Barzegar, M.; Sahari, M.A.; Hosseinmardi, N. Development of a novel sugar-free functional beverage containing pistachio green hull extract. Appl. Food Res. 2025, 5, 100926. [Google Scholar] [CrossRef]
- Koyuncu, I.; Temiz, E.; Yüksekdag, O.; Egi, K.; Cakmak, Y.; Zengin, G. From Waste to Wealth: Pistachio Green Hulls as a Novel Source of Antioxidants and Dietary Supplements. Waste Biomass Valorization 2025, 16, 6741–6759. [Google Scholar] [CrossRef]
- Özbek, H.N.; Yanık, D.K.; Fadıloğlu, S.; Göğüş, F. Optimization of microwave-assisted extraction of bioactive compounds from pistachio (Pistacia vera L.) hull. Sep. Sci. Technol. 2019, 55, 289–299. [Google Scholar] [CrossRef]
- Bakhshizadeh, S.; Taghizadeh, A.; Janmohammadi, H.; Alijani, S. Chemical composition and the nutritive value of pistachio epicarp (in situ degradation and in vitro gas production techniques). Vet. Res. Forum 2014, 5, 43–47. [Google Scholar]
- Bohluli, A.; Naserian, A.; Valizadeh, R. The chemical composition and ruminal disappearance of pistachio by-product. Proc. Br. Soc. Anim. Sci. 2007, 2007, 224. [Google Scholar] [CrossRef]
- Akbari-Alavijeh, S.; Soleimanian-Zad, S.; Sheikh-Zeinoddin, M.; Hashmi, S. Pistachio hull water-soluble polysaccharides as a novel prebiotic agent. Int. J. Biol. Macromol. 2018, 107, 808–816. [Google Scholar] [CrossRef]
- Grace, M.H.; Esposito, D.; Timmers, M.A.; Xiong, J.; Yousef, G.; Komarnytsky, S.; Lila, M.A. Chemical composition, antioxidant and anti-inflammatory properties of pistachio hull extracts. Food Chem. 2016, 210, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Hamed, M.; Bougatef, H.; Karoud, W.; Krichen, F.; Haddar, A.; Bougatef, A.; Sila, A. Polysaccharides extracted from pistachio external hull: Characterization, antioxidant activity and potential application on meat as preservative. Ind. Crops Prod. 2020, 148, 112315. [Google Scholar] [CrossRef]
- Azhdari, P.; Seif Zadeh, N.; Sahari, M.A.; Ahmadi Gavlighi, H. Application of enzymatic treatments in concentration of pistachio hull extract by ultrafiltration. Food Chem. Adv. 2023, 3, 100471. [Google Scholar] [CrossRef]
- Mohammadi-Moghaddam, T.; Razavi, S.M.A.; Malekzadegan, F.; Shaker Ardekani, A. Chemical Composition and Rheological Characterization of Pistachio Green Hull’s marmalade. J. Texture Stud. 2009, 40, 390–405. [Google Scholar] [CrossRef]
- Noruzi, H.; Aziz-Aliabadi, F.; Imari, Z.K. Effects of different levels of pistachio (Pistacia vera) green hull aqueous extract on performance, intestinal morphology and antioxidant capacity in Eimeria challenged broilers. Poult. Sci. 2024, 103, 103667. [Google Scholar] [CrossRef]
- Boğa, M.; Guven, I.; Atalay, A.İ.; Kaya, E. Effect of varieties on potential nutritive value of pistachio hulls. Kafkas Univ. Vet. Fak. Derg. 2013, 19, 699–703. [Google Scholar] [CrossRef]
- Shahdadi, F.; Khorasani, S.; Salehi-Sardoei, A.; Fallahnajmabadi, F.; Fazeli-Nasab, B.; Sayyed, R.Z. GC-MS profiling of Pistachio vera L., and effect of antioxidant and antimicrobial compounds of it’s essential oil compared to chemical counterparts. Sci. Rep. 2023, 13, 21694. [Google Scholar] [CrossRef]
- Smeriglio, A.; Denaro, M.; Barreca, D.; Calderaro, A.; Bisignano, C.; Ginestra, G.; Bellocco, E.; Trombetta, D. in vitro Evaluation of the Antioxidant, Cytoprotective, and Antimicrobial Properties of Essential Oil from Pistacia vera L. Variety Bronte Hull. Int. J. Mol. Sci. 2017, 18, 1212. [Google Scholar] [CrossRef] [PubMed]
- Martín-Gordo, D.A. Los Compuestos Fenólicos, Un Acercamiento A Su Biosíntesis, Síntesis Y Actividad Biológica. Rev. Investig. Agrar. Ambient. 2018, 9, 81–104. [Google Scholar] [CrossRef]
- Yusoff, I.M.; Mat Taher, Z.; Rahmat, Z.; Chua, L.S. A review of ultrasound-assisted extraction for plant bioactive compounds: Phenolics, flavonoids, thymols, saponins and proteins. Food Res. Int. 2022, 157, 111268. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of phenolic compounds: A review. Curr. Res. Food Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef] [PubMed]
- Edo, G.I.; Nwachukwu, S.C.; Ali, A.B.M.; Yousif, E.; Jikah, A.N.; Zainulabdeen, K.; Ekokotu, H.A.; Isoje, E.F.; Igbuku, U.A.; Opiti, R.A.; et al. A review on the composition, extraction and applications of phenolic compounds. Ecol. Front. 2024, 45, 7–23. [Google Scholar] [CrossRef]
- Saini, N.; Anmol, A.; Kumar, S.; Wani, A.W.; Bakshi, M.; Dhiman, Z. Exploring phenolic compounds as natural stress alleviators in plants- a comprehensive review. Physiol. Mol. Plant Pathol. 2024, 133, 102383. [Google Scholar] [CrossRef]
- Ramirez-Esparza, U.; De La Rosa-Esteban, A.K.; Baeza-Jiménez, R.; Martínez-Ávila, G.; Ascacio-Valdés, J.A.; Buenrostro Figueroa, J.J. Recent advances in the extraction of phenolic compounds using biotechnological processes. In Enzymatic Processes for Food Valorization; González, M.L.C., Buenrostro Figueroa, J.J., Verma, D.K., Aguilar, C.N., Eds.; Academic Press: New York, NY, USA, 2024; Chapter 11; pp. 157–172. [Google Scholar]
- Osorio-Tobón, J.F. Recent advances and comparisons of conventional and alternative extraction techniques of phenolic compounds. J. Food Sci. Technol. 2020, 57, 4299–4315. [Google Scholar] [CrossRef]
- Farrokhi, H.; Eskandari, M.H.; Shakerardekani, A.; Golmakani, M.T.; Niakousari, M. Pistachio hull extract as a natural antioxidant incorporated into pistachio paste: Antioxidant activity and oxidative stability. Appl. Food Res. 2025, 5, 100907. [Google Scholar] [CrossRef]
- Karaogul, E.; Ugurtay, A. Unveiling modeling and SEM/XRD insights into enhanced antibacterial, antioxidant, and bioactive potentials of Micro-encapsulated Pistacia vera hull extract. Food Chem. 2025, 477, 143510. [Google Scholar] [CrossRef]
- Shakerardekani, A.; Banihashemi, S.; Taghavi, E.; Morshedi, A. The Effect of Pistachio Green Hull Extract on the Phenolic Compounds, Peroxide Value and Carbonyl Value of Salmon Oil Emulsion in Water. J. Food Chem. Nanotechology 2024, 10, 129–133. [Google Scholar] [CrossRef]
- Moreno-Rojas, J.M.; Velasco-Ruiz, I.; Lovera, M.; Ordoñez-Díaz, J.L.; Ortiz-Somovilla, V.; De Santiago, E.; Arquero, O.; Pereira-Caro, G. Evaluation of Phenolic Profile and Antioxidant Activity of Eleven Pistachio Cultivars (Pistacia vera L.) Cultivated in Andalusia. Antioxid. 2022, 11, 609. [Google Scholar] [CrossRef]
- Elhadef, K.; Akermi, S.; Ben Hlima, H.; Ennouri, K.; Fourati, M.; Ben Braïek, O.; Mellouli, L.; Smaoui, S. Tunisian Pistachio Hull Extracts: Phytochemical Content, Antioxidant Activity, and Foodborne Pathogen Inhibition. J. Food Qual. 2021, 2021, 9953545. [Google Scholar] [CrossRef]
- Ozay, Y.; Ozdemir, S.; Gonca, S.; Canli, O.; Dizge, N. Phenolic compounds recovery from pistachio hull using pressure-driven membrane process and a cleaner production of biopesticide. Environ. Technol. Innov. 2021, 24, 101993. [Google Scholar] [CrossRef]
- Pakdaman, N.; Dargahi, R.; Nadi, M.; Javanshah, A.; Shakerardekani, A.; Saberi, N. Optimizing the Extraction of Phenolic Compounds from Pistachio Hulls. J. Nuts 2021, 4, 361–370. [Google Scholar] [CrossRef]
- Noorolahi, Z.; Sahari, M.A.; Barzegar, M.; Ahmadi Gavlighi, H. Tannin fraction of pistachio green hull extract with pancreatic lipase inhibitory and antioxidant activity. J. Food Biochem. 2020, 44, e13208. [Google Scholar] [CrossRef] [PubMed]
- Rafiee, Z.; Barzegar, M.; Sahari, M.A.; Maherani, B. Nanoliposomal carriers for improvement the bioavailability of high-valued phenolic compounds of pistachio green hull extract. Food Chem. 2017, 220, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Tabaraki, R.; Ghadiri, F. Comparative study of extraction methods for pistachio hull antioxidants by multiple assays. Appl. Chem. Today 2016, 10, 19–30. [Google Scholar] [CrossRef]
- Rajaei, A.; Barzegar, M.; Mobarez, A.M.; Sahari, M.A.; Esfahani, Z.H. Antioxidant, anti-microbial and antimutagenicity activities of pistachio (Pistachia vera) green hull extract. Food Chem. Toxicol. 2010, 48, 107–112. [Google Scholar] [CrossRef]
- Goli, A.H.; Barzegar, M.; Sahari, M.A. Antioxidant activity and total phenolic compounds of pistachio (Pistachia vera) hull extracts. Food Chem. 2005, 92, 521–525. [Google Scholar] [CrossRef]
- Kepekci, R.A.; Şekeroğlu, G.; Alhveis, I. Development of bioactive and environmentally friendly chitosan-based film using waste of pistachio dehulling process as a novel promising food packaging material. Int. J. Biol. Macromol. 2024, 272, 132866. [Google Scholar] [CrossRef]
- Seker, G.; Akbas, M.Y. Evaluation of bioactivities of Pistacia vera L. hull extracts as a potential antimicrobial and antioxidant natural source. Food Sci. Technol. Int. 2023, 30, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Elakremi, M.; Sillero, L.; Ayed, L.; Labidi, J.; Moussaoui, Y. Chemical Composition of Leaves and Hull from Pistacia Vera, L. an Evaluation of Phenolic Content and Antioxidant Properties of their Extracts. Res. Sq. 2020, 1–17. [Google Scholar] [CrossRef]
- Erşan, S.; Güçlü Üstündağ, Ö.; Carle, R.; Schweiggert, R.M. Subcritical water extraction of phenolic and antioxidant constituents from pistachio (Pistacia vera L.) hulls. Food Chem. 2018, 253, 46–54. [Google Scholar] [CrossRef]
- Ghandehari-Yazdi, A.P.; Barzegar, M.; Sahari, M.A.; Ahmadi Gavlighi, H. Encapsulation of Pistachio Green Hull Phenolic Compounds by Spray Drying. Rev. Cienc. Tecnol. Agrícola 2021, 23, 51–64. [Google Scholar]
- Ghandahari-Yazdi, A.P.; Barzegar, M.; Sahari, M.A.; Ahmadi Gavlighi, H. Optimization of the enzyme-assisted aqueous extraction of phenolic compounds from pistachio green hull. Food Sci. Nutr. 2019, 7, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Ordoñez-Cano, A.J.; Ramírez-Esparza, U.; Méndez-González, F.; Alvarado-González, M.; Baeza-Jiménez, R.; Sepúlveda-Torre, L.; Prado-Barragán, L.A.; Buenrostro-Figueroa, J.J. Recovery of Phenolic Compounds with Antioxidant Capacity Through Solid-State Fermentation of Pistachio Green Hull. Microorganisms 2025, 13, 35. [Google Scholar] [CrossRef]
- Karimi, E.; Oskoueian, E.; Hendra, R.; Hze, J. Solid state fermentation effects on pistachio hulls antioxidant activities. Asia-Pac. J. Sci. Technol. 2010, 15, 360–366. [Google Scholar]
- Abbasi, S.; Vahabzadeh, F.; Mehranian, M. Profiles of Phenolics and Antioxidant Activity of Pistachio Hulls During Solid-State Fermentation by Phanerochaete chrysosporium—Involvement of Lignin Peroxidase and Manganese Peroxidase. Sci. Iran. 2007, 14, 373–378. [Google Scholar]
- Bastos, K.V.; de Souza, A.B.; Tomé, A.C.; Souza, F.D. New Strategies for the Extraction of Antioxidants from Fruits and Their By-Products: A Systematic Review. Plants 2025, 14, 755. [Google Scholar] [CrossRef]
- da Costa Maia, I.; Thomaz dos Santos D’Almeida, C.; Guimarães Freire, D.M.; d’Avila Costa Cavalcanti, E.; Cameron, L.C.; Furtado Dias, J.; Simões Larraz Ferreira, M. Effect of solid-state fermentation over the release of phenolic compounds from brewer’s spent grain revealed by UPLC-MSE. LWT 2020, 133, 110136. [Google Scholar] [CrossRef]
- Khoddami, A.; Wilkes, M.A.; Roberts, T.H. Techniques for Analysis of Plant Phenolic Compounds. Molecules 2013, 18, 2328–2375. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Nicolescu, A.; Bunea, C.I.; Mocan, A. Total flavonoid content revised: An overview of past, present, and future determinations in phytochemical analysis. Anal. Biochem. 2025, 700, 115794. [Google Scholar] [CrossRef]
- Erşan, S.; Güçlü Üstündağ, Ö.; Carle, R.; Schweiggert, R.M. Determination of pistachio (Pistacia vera L.) hull (exo- and mesocarp) phenolics by HPLC-DAD-ESI/MSn and UHPLC-DAD-ELSD after ultrasound-assisted extraction. J. Food Compos. Anal. 2017, 62, 103–114. [Google Scholar] [CrossRef]
- Shirzadi-Ahodashti, M.; Mizwari, Z.M.; Mohammadi-Aghdam, S.; Ahmadi, S.; Ali Ebrahimzadeh, M.; Mortazavi-Derazkola, S. Optimization and evaluation of anticancer, antifungal, catalytic, and antibacterial activities: Biosynthesis of spherical-shaped gold nanoparticles using Pistacia vera hull extract (AuNPs@PV). Arab. J. Chem. 2023, 16, 104423. [Google Scholar] [CrossRef]
- Fattahifar, E.; Barzegar, M.; Ahmadi Gavlighi, H.; Sahari, M.A. Evaluation of the inhibitory effect of pistachio (Pistacia vera L.) green hull aqueous extract on mushroom tyrosinase activity and its application as a button mushroom postharvest anti-browning agent. Postharvest Biol. Technol. 2018, 145, 157–165. [Google Scholar] [CrossRef]
- Erşan, S.; Güçlü Üstündağ, Ö.; Carle, R.; Schweiggert, R.M. Identification of Phenolic Compounds in Red and Green Pistachio (Pistacia vera L.) Hulls (Exo- and Mesocarp) by HPLC-DAD-ESI-(HR)-MSn. J. Agric. Food Chem. 2016, 64, 5334–5344. [Google Scholar] [CrossRef]
- Ventura, G.; Calvano, C.D.; Blasi, D.; Coniglio, D.; Losito, I.; Cataldi, T.R.I. Uncovering heterogeneity of anacardic acids from pistachio shells: A novel approach for structural characterization. Food Chem. 2023, 426, 136636. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.S.; Ton, S.H.; Abdul Kadir, K. Ellagitannin geraniin: A review of the natural sources, biosynthesis, pharmacokinetics and biological effects. Phytochem. Rev. 2017, 16, 159–193. [Google Scholar] [CrossRef]
- Vázquez-Flores, A.A.; Álvarez-Parrilla, E.; López-Díaz, J.A.; Wall-Medrano, A.; De la Rosa, L.A. Taninos hidrolizables y condensados: Naturaleza química, ventajas y desventajas de su consumo: Hydrolyzable and condensed tannins: Chemistry, advantages and disadvantages of their intake. TECNOCIENCIA Chihuah. 2012, 6, 84–93. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Yang, T.; Saad, A.M.; Alkafaas, S.S.; Elkafas, S.S.; Eldeeb, G.S.; Mohammed, D.M.; Salem, H.M.; Korma, S.A.; Loutfy, S.A.; et al. Polyphenols: Chemistry, bioavailability, bioactivity, nutritional aspects and human health benefits: A review. Int. J. Biol. Macromol. 2024, 277, 134223. [Google Scholar] [CrossRef]
- Benli, H.; Bahtiyari, M.İ. An approach for the valorization of bio-waste pistachio shells (Pistacia vera L.): Dyeing of cellulose-based fabrics. J. Clean. Prod. 2024, 445, 141213. [Google Scholar] [CrossRef]
- Christodoulou, M.C.; Orellana Palacios, J.C.; Hesami, G.; Jafarzadeh, S.; Lorenzo, J.M.; Domínguez, R.; Moreno, A.; Hadidi, M. Spectrophotometric Methods for Measurement of Antioxidant Activity in Food and Pharmaceuticals. Antioxidants 2022, 11, 2213. [Google Scholar] [CrossRef]
- Shi, L.; Zhao, W.; Yang, Z.; Subbiah, V.; Suleria, H.A.R. Extraction and characterization of phenolic compounds and their potential antioxidant activities. Environ. Sci. Pollut. Res. 2022, 29, 81112–81129. [Google Scholar] [CrossRef]
- Gerasimova, E.; Gazizullina, E.; Kolbaczkaya, S.; Ivanova, A. The Novel Potentiometric Approach to Antioxidant Capacity Assay Based on the Reaction with Stable Radical 2,2′-diphenyl-1-picrylhydrazyl. Antioxidants 2022, 11, 1974. [Google Scholar] [CrossRef] [PubMed]
- Ordoñez-Cano, A.J. Extracción Biotecnológica de Compuestos Fenólicos de Ruezno de Pistache (Pistacia vera L.); Centro de Investigación en Alimentación y Desarrollo: Hermosillo, Mexico, 2024. [Google Scholar]
- Hasheminya, S.-M.; Dehghannya, J. Composition, phenolic content, antioxidant and antimicrobial activity of Pistacia atlantica subsp. kurdica hulls’ essential oil. Food Biosci. 2020, 34, 100510. [Google Scholar] [CrossRef]
- Todorov, S.D.; de Almeida, B.M.; Lima, E.M.F.; Fabi, J.P.; Lajolo, F.M.; Hassimotto, N.M.A. Phenolic Compounds and Bacteriocins: Mechanisms, Interactions, and Applications in Food Preservation and Safety. Mol. Nutr. Food Res. 2025, 69, e202400723. [Google Scholar] [CrossRef] [PubMed]
- Cuervo-Parra, J.A.; Aparicio-Burgos, J.E.; Pérez-España, V.H.; Morales-Ovando, M.A.; Peralta-Gil, M.; Romero-Cortes, T. Bioquímica de la pared celular de Gram positivas y Gram negativas. Pädi Boletín Científico Cienc. Básicas E Ing. Del ICBI 2024, 12, 1–8. [Google Scholar] [CrossRef]
| Technique | Main Compounds | Reference |
|---|---|---|
| LC-DAD-ESI-MS/MS | Gallic acid, protocatechuic acid, catechin, rutin, eriodictol-7-O-glucoside, quercetin and naringenin | Farrokhi et al. [48] |
| RP-HPLC-ESI-MS | Gallic acid 4-O-glucoside and geraniin | Ordoñez-Cano et al. [66] |
| LC-MS/MS | Tannic acid, gallic acid, protocatechuic acid, isoquercetin, miquelianin, ellagic acid, p-coumaric acid, rutin, hesperidin, quercetin, naringenin, luteolin, kaempferol and apigenin | Kepekci et al. [60] |
| GC | Gallic acid, protocatechuic acid, vanillic acid, chlorogenic acid, naringenin, naringin, quercetin, quercetin-3-galacatoside, luteolin, apigenin, catechin and epicatechin | Shakerardekani et al. [50] |
| HPLC | Catechin and gallic acid | Shirzadi-Ahodashti et al. [75] |
| LC-MS/MS | Gallic acid, caffeic acid, cinnamic acid, ferulic acid, gentisic acid, coumaric acid, vanillic acid, syringic acid and succinic acid | Ozay et al. [53] |
| HPLC/DAD | Phloroglucinol, gallic acid, protocatechuic acid, catechin, vanillic acid and naringin | Ghandahari-Yazdi et al. [65] |
| HPLC-DAD-ESI/MSn | Gallic acid, digallic acid, protocatechuic acid, galloyl- shikimic acid, penta-O-galloyl-β-D-glucose, quercetin 3-O-glucoside, anacardic acids and myricetin hexoxide | Erşan et al. [63] |
| RP-HPLC-DAD | Phloroglucinol, gallic acid, naringenin, vanillic acid, catechin and protocatechuic acid | Fattahifar et al. [76] |
| HPLC-DAD-ESI/MSn and UHPLC-DAD-ELSD | Gallic acid, galloyl-shikimic acid, protocatechuic acid, digallic acid, cyanidin-3-O-β-D-galactopyranoside, luteic acid, myricetin 3-O-galactoside, quercetin 3-O-glucoside, kaempferol hexoxide and anacardic acids. | Erşan et al. [74] |
| HPLC-DAD-FLD-MS | Gallic acid, phloroglucinol, vanillic acid, p-coumaric acid, sinapic acid, naringenin and catechin | Garavand et al. [4] |
| RP-HPLC-DAD-FLU | Gallic acid, protocatechuic acid, vanillic acid, chlorogenic acid, eriodictyol-7-O-glucoside, naringenin, quercetin, kaempferol, luteolin, apigenin, catechin and epicatechin | Barreca et al. [21] |
| HPLC-DAD-ESI-MSn and HPLC-ESI-HR-MS | Gallic acid, protocatechuic acid, cyanidin 3-O-β-D- galactopyranoside, luteolin, quercetin 3-O-glucuronide, kaempferol hexoside, anacardic acids, methyl digallate and myricetin galloyl hexoxide | Erşan et al. [77] |
| GC–MS and HPLC-MS-IT-TOF | Gallic acid, luteolin, quercetin, catechin gallate, myricetin gallate, digallic acid, myricetin-3-glucoside, galloylshikimic acid, quercetin-3-glucoronide, tetragalloyl hexoxide and anacardic acids. | Grace et al. [33] |
| Identification Technique | Sensitivity | Cost | Suitability |
|---|---|---|---|
| LC | High with MS detector | Low-moderate by solvents used | Phenolic compounds |
| HPLC | High-very high with detectors | Moderate-high by solvents used and maintenance cost | Large, volatile and non-volatile compounds |
| UHPLC | Very high with detectors | High initial and maintenance cost | Highly complex samples |
| GC | High with detectors | Low cost by inert gases and small volumes | Volatile compounds |
| Type of Assay | Antioxidant Activity | Unit | Reference |
|---|---|---|---|
| ABTS | ~72–92 | % | Farrokhi et al. [48] |
| 132.75–466.73 | mg TE g dm−1 | Ordoñez-Cano et al. [66]; Noorolahi et al. [55] | |
| 427.06 | mg TE g de−1 | Elakremi et al. [62] | |
| 3.32 | mg AAE mg PCs−1 | Rajaei et al. [58] | |
| ~37–50 | % free radical inhibition 80 mg/L−1 | Ghandahari-Yazdi et al. [65] | |
| 0.47–1.18 | mmol TE g dm−1 | Erşan et al. [61] | |
| 1.28–3.53 | mmol TE 100 g fm−1 | Moreno-Rojas et al. [51] | |
| ABTS (IC50) | 1.2278 | mg mL−1 | Karaogul and Ugurtay [49] |
| ABTS (EC50) | 0.09–0.91 | mg mL−1 | Elhadef et al. [52] |
| DPPH | ~50–90 | % | Farrokhi et al. [48]; Pakdaman et al. [54]; Tabaraki and Ghadiri [57]; Abbasi et al. [68] |
| 131.68–411.98 | mg TE g dm−1 | Ordoñez-Cano et al. [66]; Noorolahi et al. [55] | |
| 332.92 | mg TE g de−1 | Elakremi et al. [62] | |
| ~87–95 | % free radical inhibition 80 mg/L−1 | Ghandahari-Yazdi et al. [65] | |
| 31.2–50.4 | % free radical inhibition 300 µg dm−1 | Karimi et al. [67] | |
| 0.51–0.84 | mmol TE g dm−1 | Erşan et al. [63] | |
| 1.28–3.53 | mmol TE 100 g fm−1 | Moreno-Rojas et al. [51] | |
| DPPH (IC50) | 0.003–4.49 | mg mL−1 | Karaogul and Ugurtay [49]; Kepekci et al. [60] Roudbari et al. [5]; Azhdari et al. [35]; Özbek et al. [29]; Özbek et al. [10] |
| 206.32 | µL L−1 | Shahdadi et al. [39] | |
| DPPH (SC50) | 0.022 | mg mL−1 | Benli and Bahtiyari [82] |
| DPPH (EC50) | 2.53 | µg PCs mL DPPH−1 | Rajaei et al. [58] |
| 0.025–1.5 | mg mL−1 | Elhadef et al. [52]; Ghandehari-Yazdi et al. [64]; Garavand et al. [4] | |
| FRAP | 504.59–2230.8 | mg Fe+2 g dm−1 | Ordoñez-Cano et al. [66]; Noorolahi et al. [55] |
| 517.96 | mg TE g de−1 | Elakremi et al. [62] | |
| ~130–350 | µM Fe+2 80 mg/L−1 | Ghandahari-Yazdi et al. [65] | |
| 0.49–1.20 | mmol TE g dm−1 | Erşan et al. [63] | |
| 1.111 | µmol TE mg ext−1 | Benli and Bahtiyari [82] | |
| 380.4–508.8 | µmol Fe+2 g dm−1 | Tabaraki and Ghadiri [57] | |
| 2.36–6.04 | µM TE 100 g fm−1 | Barreca et al. [21] | |
| ORAC | 3.5–15.8 | mmol TE 100 g fm−1 | Moreno-Rojas et al. [51] |
| ~50–250 | µmol TE g dm−1 | Özbek et al. [10] | |
| 1.79–3.48 | µmol TE 100 g fm−1 | Barreca et al. [21] |
| Assay | Bacterial Strains | Result | Reference |
|---|---|---|---|
| MIC | Escherichia coli Salmonella subsp. enterica serovar Typhimurium Staphylococcus aureus Pseudomonas aeruginosa Bacillus subtilis Bacillus cereus Enterococcus faecalis Enterococcus hirae Enterobacter aerogenes Klebsiella pneumonia Acinetobacter baumannii Proteus mirabilis Legionella pneumophila Streptococcus faecalis | 0.42–857.12 mg mL−1 0.26 mg mL−1 0.16–807.5 mg mL−1 0.31–1615 mg mL−1 0.25–121.25 mg mL−1 0.21–1 mg mL−1 4–100.9 mg mL−1 50.7–428.56 mg mL−1 485.00 mg mL−1 4–14.55 mg mL−1 4 mg mL−1 4 mg mL−1 202.95–857.12 mg mL−1 0.21 mg mL−1 | Ordoñez-Cano [86] Karaogul and Ugurtay [49] Shahdadi et al. [39] Shirzadi-Ahodashti et al. [75] Ozay et al. [53] Hasheminya and Dehghannya [87] Smeriglio et al. [40] Rajaei et al. [58] |
| Disk diffusion method | Escherichia coli Salmonella subsp. enterica serovar Typhimurium Salmonella enterica Staphylococcus aureus Pseudomonas aeruginosa Bacillus subtilis Bacillus cereus Enterococcus faecalis Enterobacter aerogenes Klebsiella pneumonia Streptococcus faecalis Clostridioides difficile Listeria monocytogenes | 11.3–20.8 mm 21.5 mm 13.83–16 mm 7.36–21.8 mm 9.31–21.8 mm 12.96 mm 12–22.5 mm 3.30–9 mm 15.18 mm 13.80 mm 22.3 mm 18 mm 14.23–19 mm | Ordoñez-Cano [86] Karaogul and Ugurtay [49] Kepekci et al. [60] Elhadef et al. [52] Hasheminya and Dehghannya [87] Rajaei et al. [58] |
| MBC | Staphylococcus aureus Bacillus subtilis | ~500 µg mL−1 ~500 µg mL−1 | Shahdadi et al. [39] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ordoñez-Cano, A.J.; Ramírez-Esparza, U.; Alvarado-González, M.; Baeza-Jiménez, R.; Espinoza-Hicks, J.C.; Prado-Barragán, L.A.; Buenrostro-Figueroa, J.J. Valorization of Pistachio Green Hull: Advances in Extraction and Characterization of Phenolic Compounds. Processes 2025, 13, 3761. https://doi.org/10.3390/pr13123761
Ordoñez-Cano AJ, Ramírez-Esparza U, Alvarado-González M, Baeza-Jiménez R, Espinoza-Hicks JC, Prado-Barragán LA, Buenrostro-Figueroa JJ. Valorization of Pistachio Green Hull: Advances in Extraction and Characterization of Phenolic Compounds. Processes. 2025; 13(12):3761. https://doi.org/10.3390/pr13123761
Chicago/Turabian StyleOrdoñez-Cano, Andrés Javier, Ulises Ramírez-Esparza, Mónica Alvarado-González, Ramiro Baeza-Jiménez, José Carlos Espinoza-Hicks, Lilia Arely Prado-Barragán, and José Juan Buenrostro-Figueroa. 2025. "Valorization of Pistachio Green Hull: Advances in Extraction and Characterization of Phenolic Compounds" Processes 13, no. 12: 3761. https://doi.org/10.3390/pr13123761
APA StyleOrdoñez-Cano, A. J., Ramírez-Esparza, U., Alvarado-González, M., Baeza-Jiménez, R., Espinoza-Hicks, J. C., Prado-Barragán, L. A., & Buenrostro-Figueroa, J. J. (2025). Valorization of Pistachio Green Hull: Advances in Extraction and Characterization of Phenolic Compounds. Processes, 13(12), 3761. https://doi.org/10.3390/pr13123761








