Multi-Objective Optimization of Supercritical Water Oxidation for Radioactive Organic Anion Exchange Resin Wastewater Using GPR–NSGA-II
Abstract
1. Introduction
2. Experimental Setup and Method
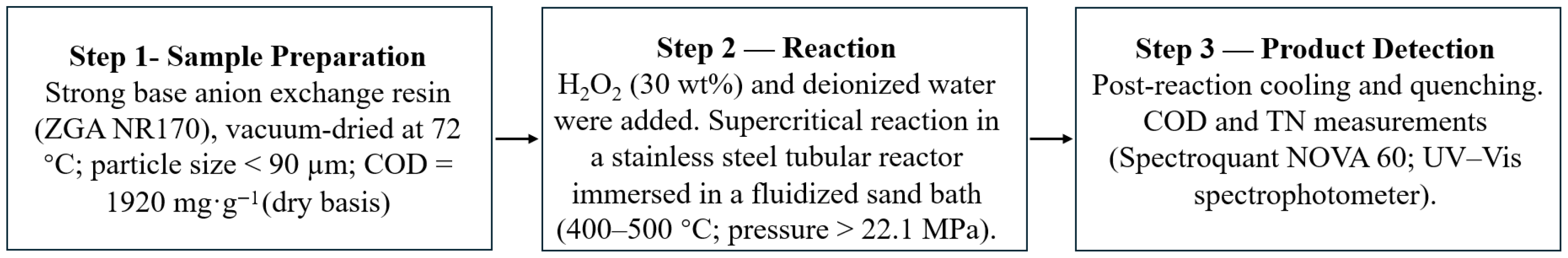
2.1. Materials and Experimental Procedure
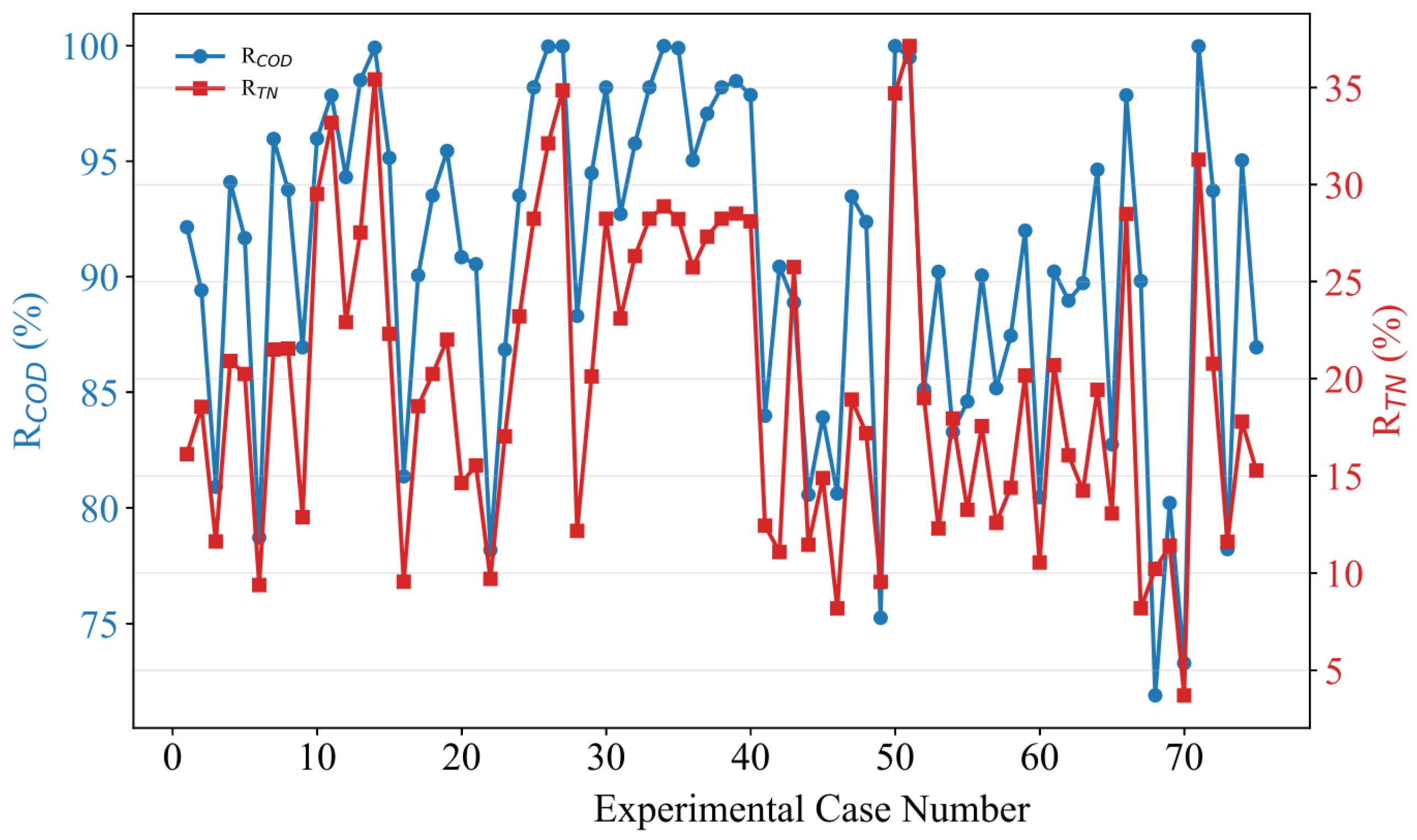
2.2. Experimental Data Preprocessing
2.3. Correlation and Sensitivity Analysis Methods
2.4. Predictive Modeling Methods
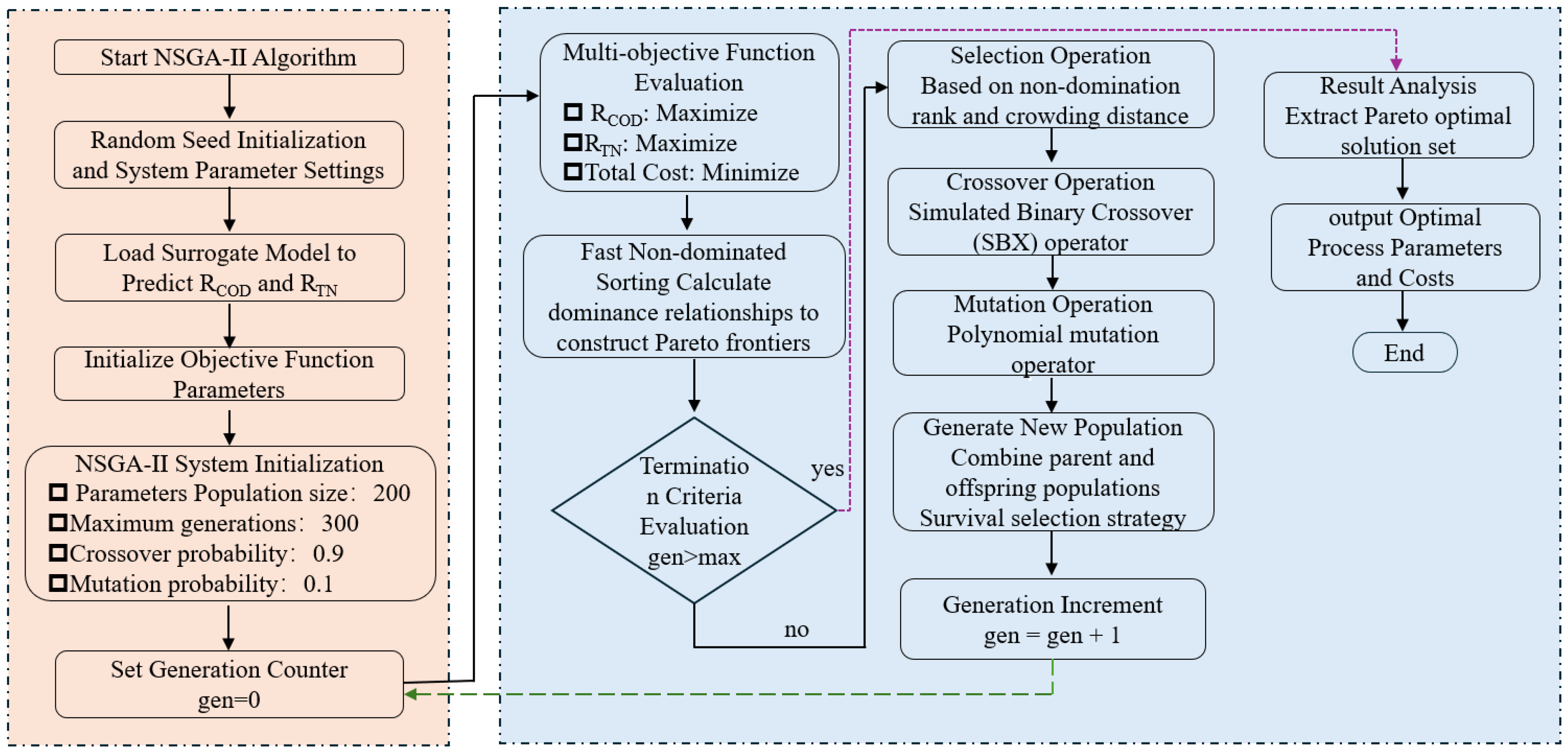
2.5. SCWO Process Optimization
3. Results and Discussion
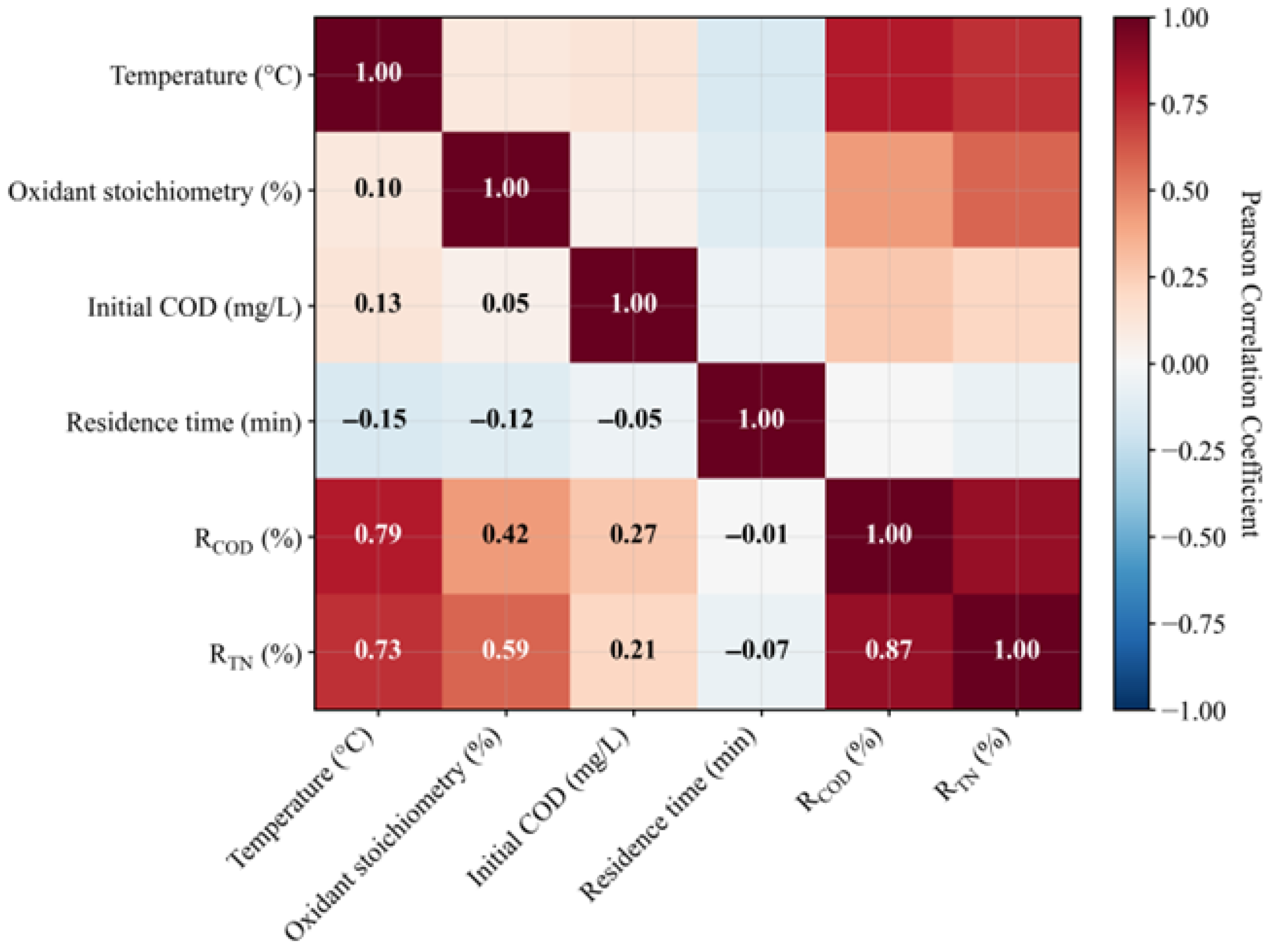
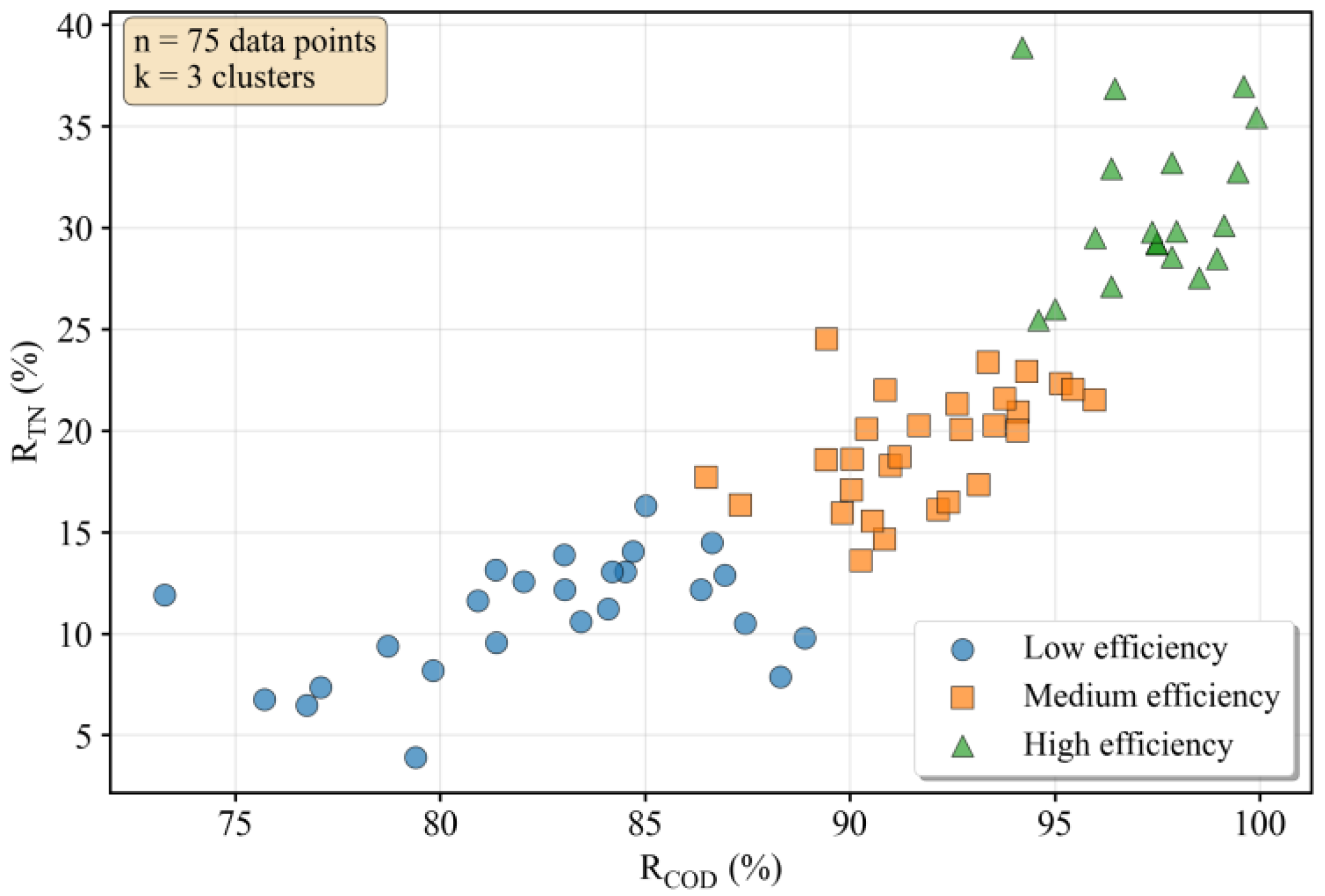
3.1. Correlation and Cluster Analysis
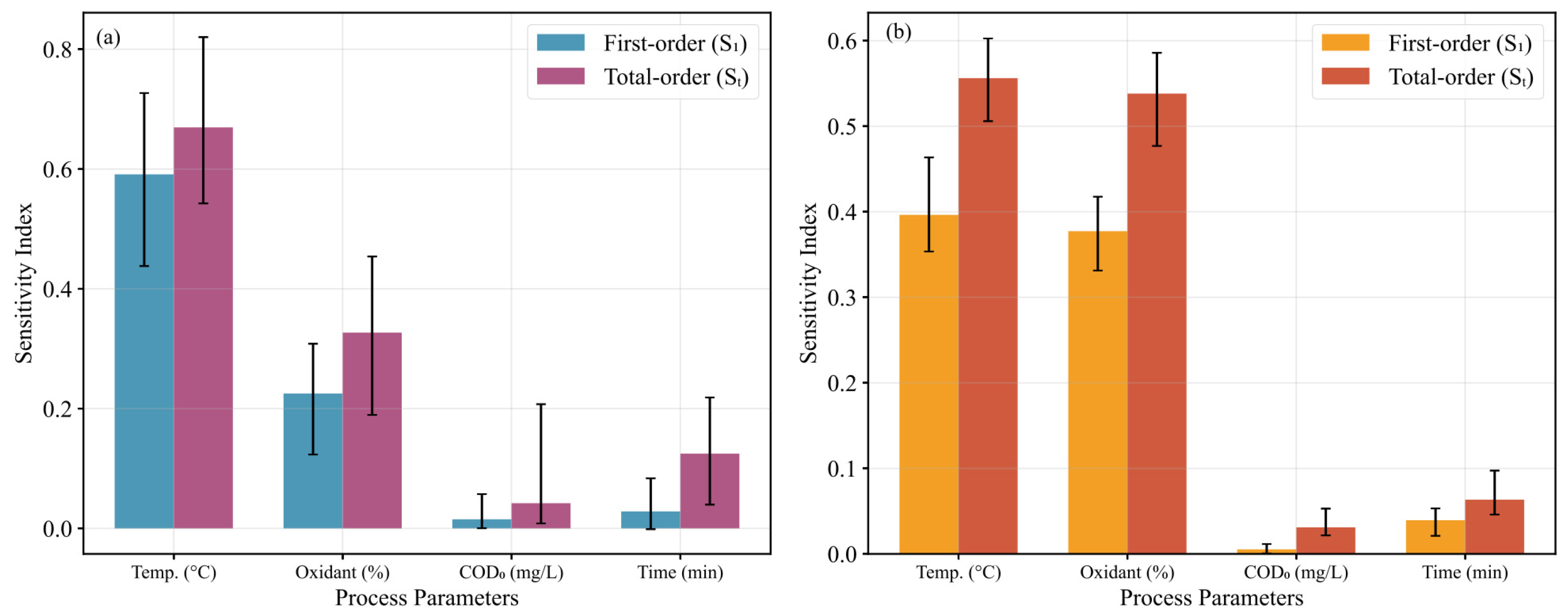
3.2. Parameter Sensitivity Analysis
3.3. Model Performance Analysis
3.3.1. Single-Target Prediction Performance
3.3.2. Multi-Objective Prediction Performance
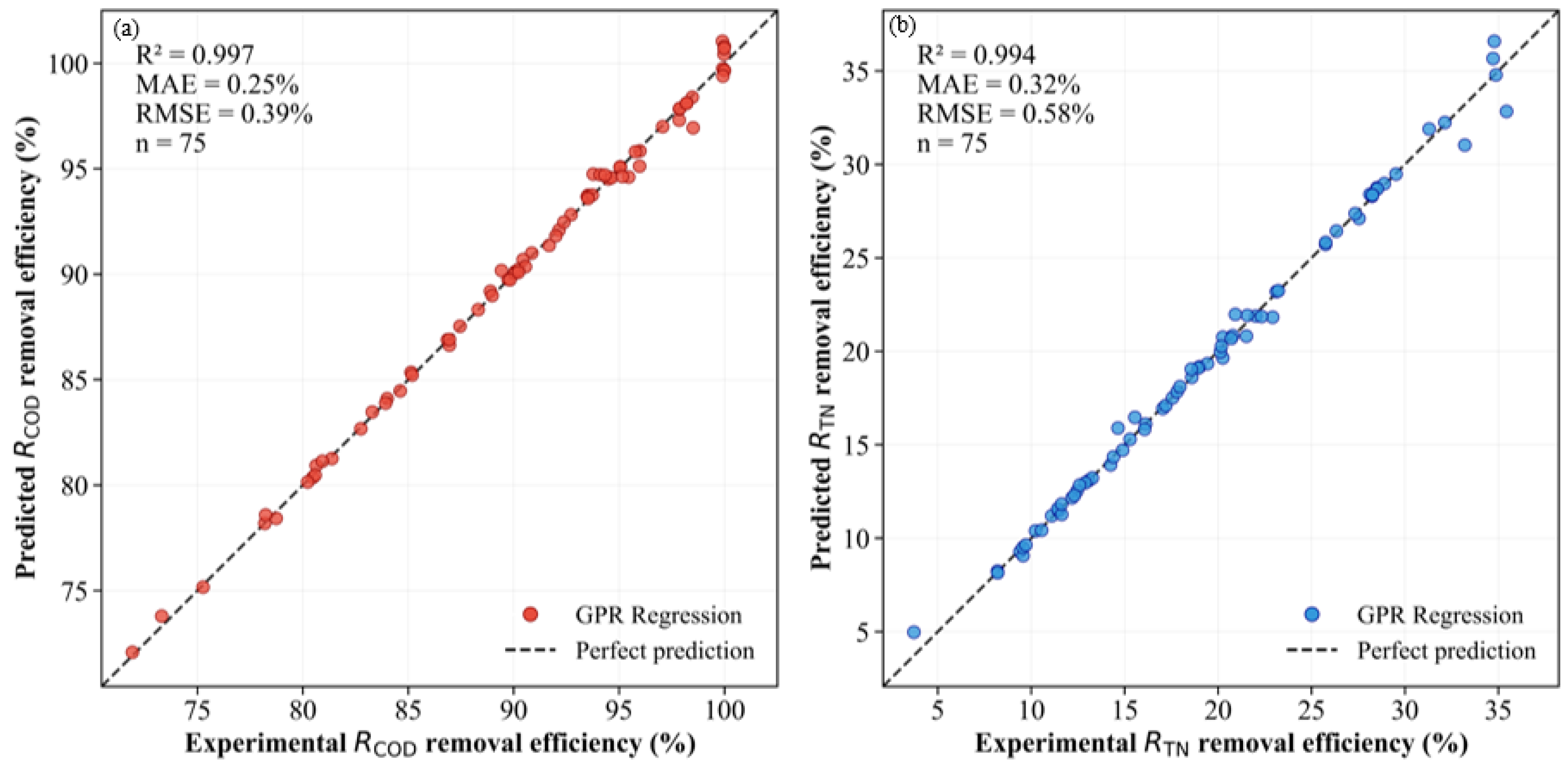
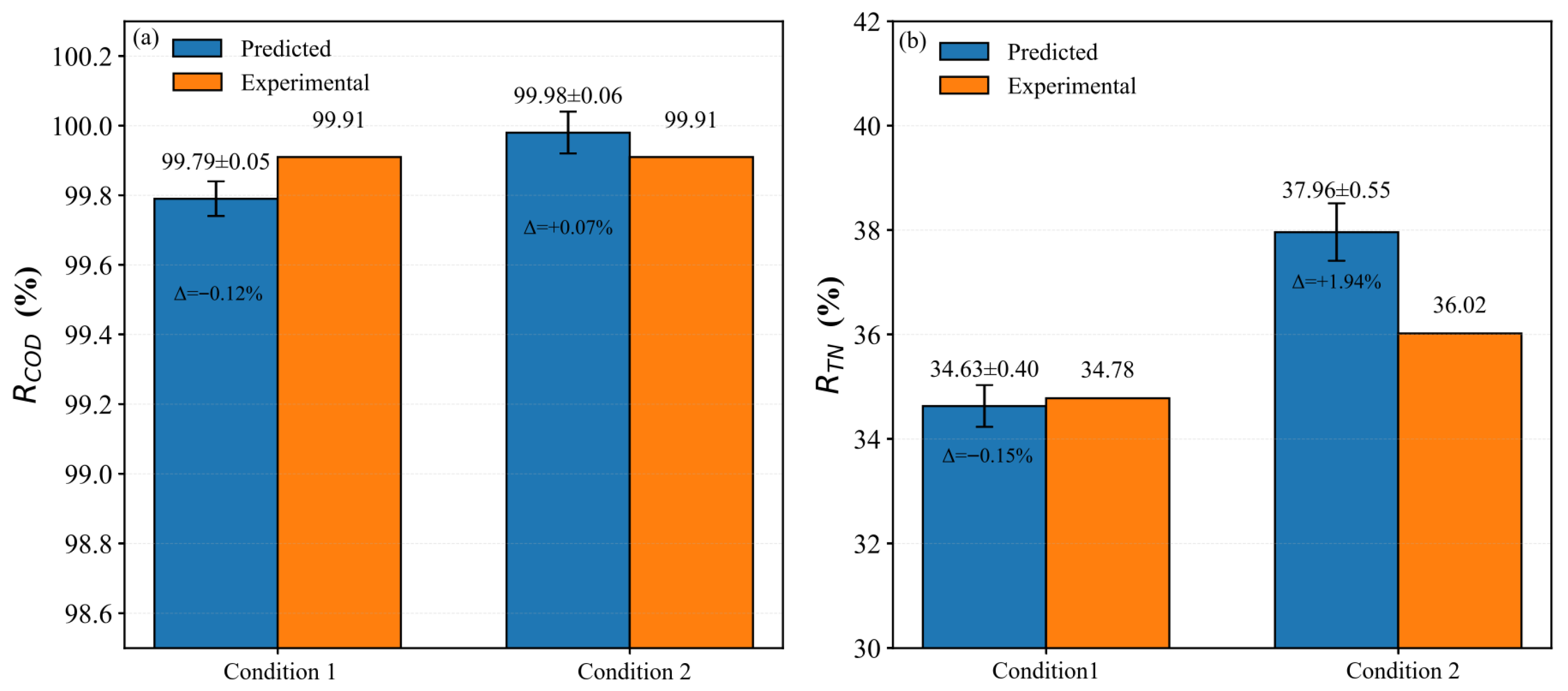
3.3.3. Experimental Validation of the GPR Model
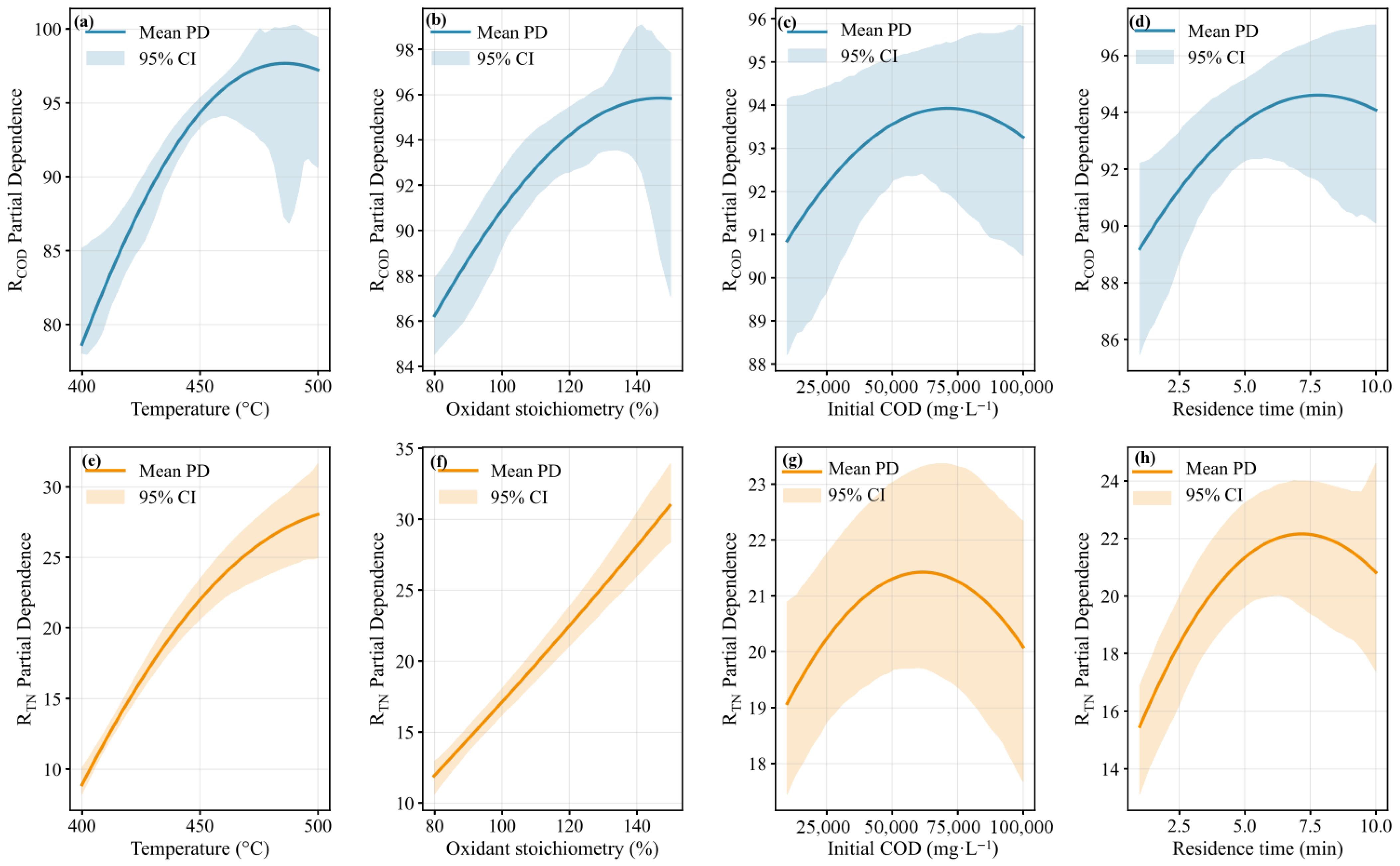
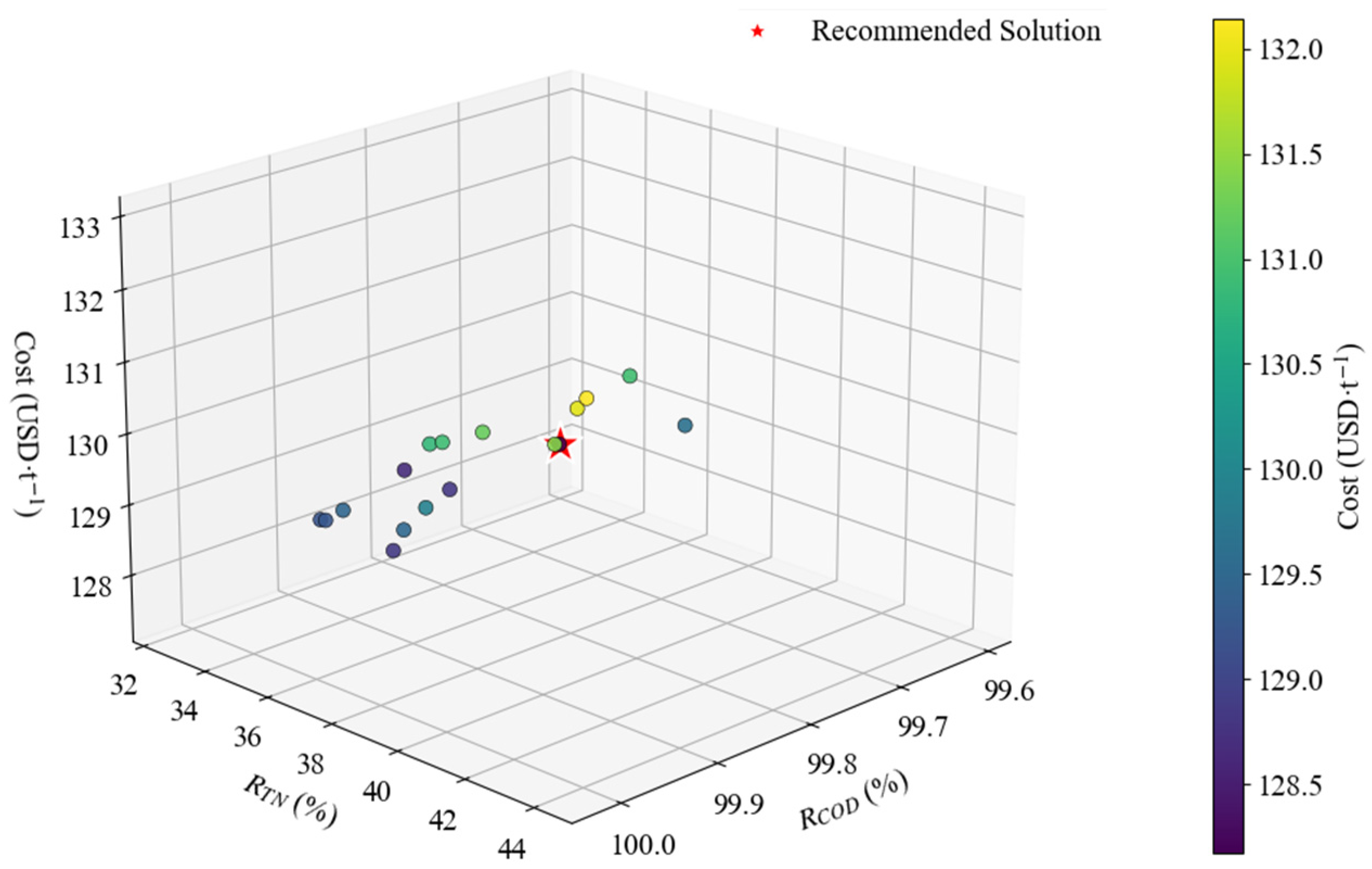
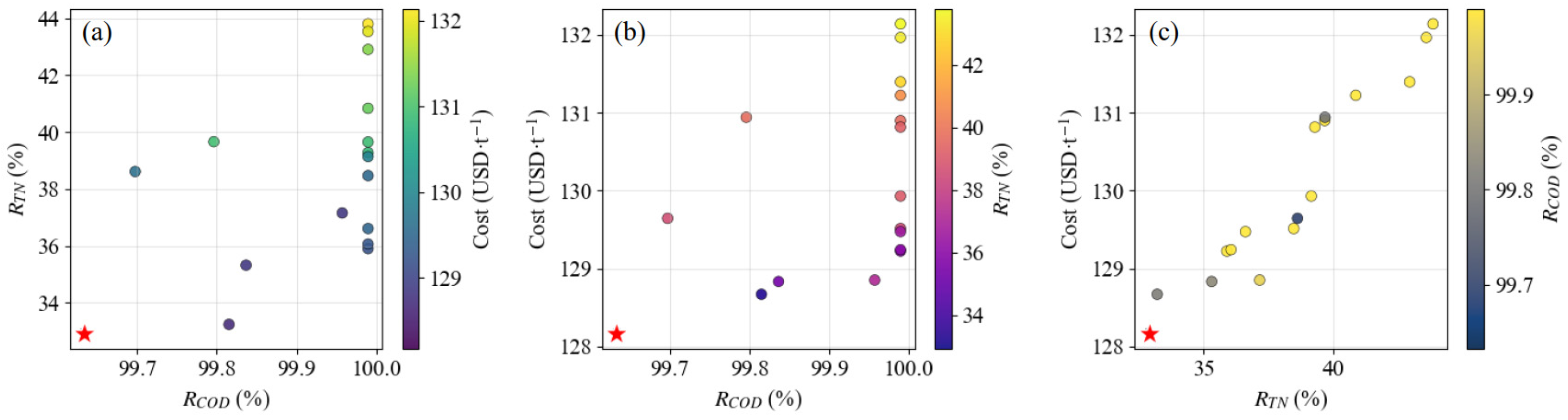
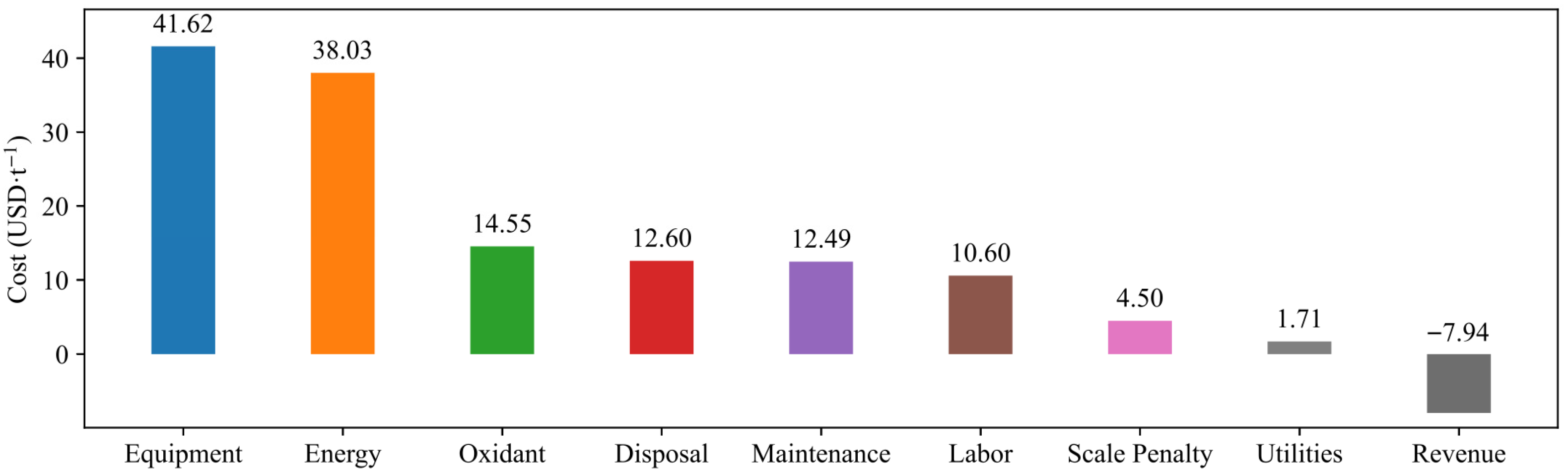
3.4. Process Parameter Optimization and Economic Analysis
4. Discussion
5. Conclusions
- (1)
- The oxidant stoichiometry plays a key synergistic role in the nitrogen conversion process during the degradation of nuclear anion resins by supercritical water oxidation. The initial COD concentration and residence time have little effect on COD and TN removal rates. The interaction effect between temperature and oxidation coefficient is significant, which is consistent with the coupled characteristics of free radical generation and reaction kinetics.
- (2)
- All six machine learning models can effectively fit the pollutant removal efficiency of the SCWO system. Among these, SVR and GPR performed well in single-objective prediction (R2 > 0.98), and the GPR model had extremely high accuracy in multi-objective prediction (R2 > 0.99).
- (3)
- Based on the multi-objective optimization of GPR–NSGA-II, it was shown that the COD removal rate and TN removal rate under the optimal conditions were 99.63% and 32.92%, respectively, and the treatment cost was 128.16 USD·t−1.
- (4)
- In practical engineering, improving the thermal management level of the SCWO system reactor and adopting a strategy of dynamically controlling the oxidant stoichiometry can simultaneously improve the removal rates of COD and TN. Reaction temperature and oxidant coefficient have the highest priority for control, while residence time and initial COD concentration are secondary control factors.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AI | Artificial intelligence |
| ANN | Artificial neural network |
| BOD | Biochemical oxygen demand |
| Cbase | Base equipment cost |
| CODi | Initial COD concentration of the feed (mg·L−1) |
| Ctotal | Total treatment cost (USD·t−1) |
| CCHP | Combined cooling, heating and power |
| CI | Confidence interval |
| COD | Chemical oxygen demand |
| D | Annual operating days (d·y−1) |
| DEAP | Distributed Evolutionary Algorithms in Python |
| Eheating | Heating demand (kWh) |
| GPR | Gaussian Process Regression |
| GPR–NSGA-II | Gaussian Process Regression–Non-Dominated Sorting Genetic Algorithm II framework |
| H | Daily operating hours (h·d−1) |
| K-means (k) | Clustering algorithm (number of clusters k) |
| L | Depreciation period (years) |
| MAE | Mean absolute error |
| NSGA-II | Non-Dominated Sorting Genetic Algorithm II |
| OS | Oxidant stoichiometric (%) |
| Pelec | Electricity price (USD·kWh−1) |
| Pgas | Natural gas price (USD·kWh−1 or USD·Nm3, as defined) |
| Qtpd | Processing scale (t·d−1) |
| LOOCV | Leave-One-Out Cross-Validation |
| R2 | Coefficient of determination |
| RR120 | Reactive Red 120 |
| RCOD | COD removal efficiency (%) |
| RTN | TN removal efficiency (%) |
| RBF | Radial basis function (kernel) |
| RMSE | Root mean square error |
| RSM | Response surface methodology |
| SCWO | Supercritical water oxidation |
| S1 | First-order Sobol sensitivity index |
| St | Total-effect Sobol sensitivity index |
| SVR | Support vector regression |
| TabPFN | Tabular Prior-Data Fitted Network |
| TN | Total nitrogen |
| TOC | Total organic carbon |
| T | Reaction temperature (°C) |
| t | Residence time (min) |
| TWR | Transpiring wall reactor |
| Z-score | Standard score (normalization) |
References
- Yang, H.-C.; Park, H.-O.; Park, K.-T.; Kim, S.-J.; Kim, H.-J.; Eun, H.-C.; Lee, K. Development of carbonization and a relatively high-temperature halogenation process for the removal of radionuclides from spent ion exchange resins. Processes 2021, 9, 96. [Google Scholar] [CrossRef]
- Benmakhlouf, N.; Outili, N.; García-Jarana, B.; Sánchez-Oneto, J.; Portela, J.R.; Jeguirim, M.; Meniai, A.-H. Applications of supercritical water in waste treatment and valorization: A review. Energies 2023, 16, 2081. [Google Scholar] [CrossRef]
- Palamarchuk, M.; Chervonetskiy, M.; Polkanova, N.; Bratskaya, S. Toward deep decontamination of intermediate-level-activity spent ion-exchange resins containing poorly soluble inorganic deposits. Sustainability 2023, 15, 3990. [Google Scholar] [CrossRef]
- Matskevich, A.; Tokar, E.; Markin, N.; Priimak, I.; Egorin, A. Effects of temperature and sulfuric acid and iron (II) concentrations on the efficacy of decontamination of spent ion-exchange resins containing hematite. Processes 2022, 10, 931. [Google Scholar] [CrossRef]
- Kim, K.; Kim, K.; Choi, M.; Son, S.H.; Han, J.H. Treatment of ion exchange resins used in nuclear power plants by super- and sub-critical water oxidation—A road to commercial plant from bench-scale facility. Chem. Eng. J. 2012, 189–190, 213–221. [Google Scholar] [CrossRef]
- Leybros, A.; Roubaud, A.; Guichardon, P.; Boutin, O. Supercritical water oxidation of ion exchange resins: Degradation mechanisms. Process Saf. Environ. Prot. 2010, 88, 213–222. [Google Scholar] [CrossRef]
- Jiang, Z.; Li, Y.; Wang, S.; Cui, C.; Yang, C.; Li, J. Review on mechanisms and kinetics for supercritical water oxidation processes. Appl. Sci. 2020, 10, 4937. [Google Scholar] [CrossRef]
- Erkonak, H.; Söğüt, O.Ö.; Akgün, M. Treatment of olive mill wastewater by supercritical water oxidation. J. Supercrit. Fluids 2008, 45, 150–155. [Google Scholar] [CrossRef]
- Li, G.; Lu, Y.; Glarborg, P. Oxidation kinetics of methane and methane/methanol mixtures in supercritical water. Ind. Eng. Chem. Res. 2022, 61, 3889–3899. [Google Scholar] [CrossRef]
- Li, J.; Wang, S.; Li, Y.; Wang, L.; Xu, T.; Zhang, Y.; Jiang, Z. Supercritical water oxidation of semi-coke wastewater: Effects of operating parameters, reaction mechanism and process enhancement. Sci. Total Environ. 2020, 710, 134396. [Google Scholar] [CrossRef] [PubMed]
- Cocero, M.J. Supercritical Water Oxidation (SCWO). Application to industrial wastewater treatment. Ind. Chem. Libr. 2001, 9, 509526. [Google Scholar] [CrossRef]
- Li, Y.; Qin, Q.; Zhang, Z.; Wang, S. Optimization and pathway study on destruction of the spent extraction solvent in supercritical water. RSC Adv. 2024, 14, 5167–5175. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Wang, S.; Huang, C.; Tang, X.; Guo, Y. Transpiring wall reactor in supercritical water oxidation. Chem. Eng. Res. Des. 2014, 92, 2626–2639. [Google Scholar] [CrossRef]
- Li, Y.; Duan, Y.; Wang, S.; Zhang, F.; Li, J.; Dai, Z.; Li, Z.; Zhang, Y.; Wang, Y. Supercritical water oxidation for the treatment and utilization of organic wastes: Factor effects, reaction enhancement, and novel process. Environ. Res. 2024, 251, 118571. [Google Scholar] [CrossRef]
- Bradford, E.; Schweidtmann, A.M.; Lapkin, A. Efficient multiobjective optimization employing Gaussian processes, spectral sampling and a genetic algorithm. J. Glob. Optim. 2018, 71, 407–438. [Google Scholar] [CrossRef]
- Yan, W.; Hu, S.; Yang, Y.; Gao, F.; Chen, T. Bayesian migration of Gaussian process regression for rapid process modeling and optimization. Chem. Eng. J. 2011, 166, 1043–1051. [Google Scholar] [CrossRef]
- Wolday, A.K.; Ramteke, M. Surrogate model-based optimization of methanol synthesis process for multiple objectives: A pathway towards achieving sustainable development goals. Chem. Eng. Res. Des. 2024, 204, 172–182. [Google Scholar] [CrossRef]
- Dantas, M.S.; Christofaro, C.; Oliveira, S.C. Artificial neural networks for performance prediction of full-scale wastewater treatment plants: A systematic review. Water Sci. Technol. 2023, 88, 1447–1470. [Google Scholar] [CrossRef]
- Lu, H.; Meng, Z.-H.; Zhang, B.; Song, S.; Zhan, S.-Y.; Li, Y.; Wu, Q.-L.; Wang, H.-Z.; Guo, W.-Q. Deep learning-based multiobjective optimization for balancing effluent quality, operational cost, and greenhouse gas emissions in wastewater treatment plant control. ACS ES&T Water 2024, 4, 2856–2867. [Google Scholar] [CrossRef]
- Şimşek, V.; Yarbay, R.Z.; Marttin, V.; Gül, Ü.D. Treatment of textile dye via economic fungi/MCM-41 bio-based adsorbent: Application of neural network approach. J. Clean. Prod. 2023, 421, 138448. [Google Scholar] [CrossRef]
- Wang, L.; Chen, J.; Cui, J.; Wang, G.; Jin, H.; Guo, L. Experimental study on treatment of mixed ion exchange resins by supercritical water gasification. J. Clean. Prod. 2023, 385, 135755. [Google Scholar] [CrossRef]
- Xu, T.; Wang, S.; Li, Y.; Zhang, J.; Li, J.; Zhang, Y.; Yang, C. Optimization and mechanism study on destruction of the simulated waste ion-exchange resin from the nuclear industry in supercritical water. Ind. Eng. Chem. Res. 2020, 59, 18269–18279. [Google Scholar] [CrossRef]
- Iwanaga, T.; Usher, W.; Herman, J. Toward SALib 2.0: Advancing the accessibility and interpretability of global sensitivity analyses. Socio-Environ. Syst. Model. 2022, 4, 18155. [Google Scholar] [CrossRef]
- Cuesta-Valero, F.J.; Beltrami, H.; Gruber, S.; García-García, A.; González-Rouco, J.F. A new bootstrap technique to quantify uncertainty in estimates of ground surface temperature and ground heat flux histories from geothermal data. Geosci. Model Dev. 2022, 15, 7913–7932. [Google Scholar] [CrossRef]
- Sobol′, I.M. Global sensitivity indices for nonlinear mathematical models and their Monte Carlo estimates. Math. Comput. Simul. 2001, 55, 271–280. [Google Scholar] [CrossRef]
- Saltelli, A. Making best use of model evaluations to compute sensitivity indices. Comput. Phys. Commun. 2002, 145, 280–297. [Google Scholar] [CrossRef]
- Song, X.; Bryan, B.A.; Paul, K.I.; Zhao, G. Variance-based sensitivity analysis of a forest growth model. Ecol. Model. 2012, 247, 135–143. [Google Scholar] [CrossRef]
- Arbelaitz, O.; Gurrutxaga, I.; Muguerza, J.; Pérez, J.M.; Perona, I. An Extensive Comparative Study of Cluster Validity Indices. Pattern Recognit. 2013, 46, 243–256. [Google Scholar] [CrossRef]
- Mahanna, H.; El-Rashidy, N.; Kaloop, M.R.; El-Sapakh, S.; Alluqmani, A.; Hassan, R. Prediction of wastewater treatment plant performance through machine learning techniques. Desalination Water Treat. 2024, 319, 100524. [Google Scholar] [CrossRef]
- Mutombo, N.M.-A.; Numbi, B.P. Development of a linear regression model based on the most influential predictors for a research office cooling load. Energies 2022, 15, 5097. [Google Scholar] [CrossRef]
- Yun, J.; Yu, Y.; Tao, C.; Zhai, M.; Zhang, H.; Chen, Y.; Li, H.; Zhang, B.; Ma, J. Machine learning-based optimization of enhanced nitrogen removal in a full-scale urban wastewater treatment plant with ecological combination ponds. Water Res. 2025, 285, 123976. [Google Scholar] [CrossRef]
- Manav-Demir, N.; Gelgor, H.B.; Oz, E.; Ilhan, F.; Ulucan-Altuntas, K.; Tiwary, A.; Debik, E. Effluent parameters prediction of a biological nutrient removal (BNR) process using different machine learning methods: A case study. J. Environ. Manag. 2024, 351, 119899. [Google Scholar] [CrossRef]
- Wan, X.; Li, X.; Wang, X.; Yi, X.; Zhao, Y.; He, X.; Wu, R.; Huang, M. Water quality prediction model using Gaussian process regression based on deep learning for carbon neutrality in papermaking wastewater treatment system. Environ. Res. 2022, 211, 112942. [Google Scholar] [CrossRef] [PubMed]
- Hollmann, N.; Müller, S.; Purucker, L.; Krishnakumar, A.; Körfer, M.; Bin Hoo, S.; Schirrmeister, R.T.; Hutter, F. Accurate predictions on small data with a tabular foundation model. Nature 2025, 637, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Deb, K.; Pratap, A.; Agarwal, S.; Meyarivan, T. A fast and elitist multiobjective genetic algorithm: NSGA-II. IEEE Trans. Evol. Comput. 2002, 6, 182–197. [Google Scholar] [CrossRef]
- Zhou, A.; Zhang, Q.; Zhang, G. A multiobjective evolutionary algorithm based on decomposition and probability model. In Proceedings of the 2012 IEEE Congress on Evolutionary Computation, Brisbane, Australia, 10–15 June 2012; pp. 1–8. [Google Scholar] [CrossRef]
- Cocero, M.; Alonso, E.; Sanz, M.; Fdz-Polanco, F. Supercritical water oxidation process under energetically self-sufficient operation. J. Supercrit. Fluids 2002, 24, 37–46. [Google Scholar] [CrossRef]
- Liao, W.; Zhao, Q.; Chen, H.; Liao, C.; Wang, Y.; Wang, X. Experimental investigation and simulation optimization of a pilot-scale supercritical water oxidation system. Energy Convers. Manag. 2019, 199, 111965. [Google Scholar] [CrossRef]
- Vadillo, V.; Sánchez-Oneto, J.; Portela, J.R.; de la Ossa, E.J.M. Problems in supercritical water oxidation process and proposed solutions. Ind. Eng. Chem. Res. 2013, 52, 7617–7629. [Google Scholar] [CrossRef]
- Marrone, P.A. Supercritical water oxidation—Current status of full-scale commercial activity for waste destruction. J. Supercrit. Fluids 2013, 79, 283–288. [Google Scholar] [CrossRef]
- Zhang, F.; Ding, Y.; Su, C.; Chen, Z. Energy self-sufficiency of a supercritical water oxidation system with an improved cooled-wall reactor for power generation. Appl. Therm. Eng. 2020, 172, 115158. [Google Scholar] [CrossRef]
- Bermejo, M.D.; Cocero, M.J. Supercritical water oxidation: A technical review. AIChE J. 2006, 52, 3933–3951. [Google Scholar] [CrossRef]
- Aki, S.N.V.K.; Abraham, M.A. An economic evaluation of catalytic supercritical water oxidation: Comparison with alternative waste treatment technologies. Environ. Prog. 1998, 17, 246–255. [Google Scholar] [CrossRef]
- Xu, T.; Li, Y.; Wang, S.; Xu, D.; Zhang, Q.; Jin, Y.; Song, W. Supercritical water oxidation of nuclear cation exchange resins: Process optimization and reaction mechanism. Processes 2025, 13, 3249. [Google Scholar] [CrossRef]
- Scheitlin, C.G.; Dasu, K.; Rosansky, S.; Dejarme, L.E.; Siriwardena, D.; Thorn, J.; Mullins, L.; Haggerty, I.; Shqau, K.; Stowe, J. Application of supercritical water oxidation to effectively destroy per- and polyfluoroalkyl substances in aqueous matrices. ACS ES&T Water 2023, 3, 2053–2062. [Google Scholar] [CrossRef]
- Chiang, S.-Y.D.; Saba, M.; Leighton, M.; Ballenghien, D.; Hatler, D.; Gal, J.; Deshusses, M.A. Supercritical water oxidation for the destruction of spent media wastes generated from PFAS treatment. J. Hazard. Mater. 2023, 460, 132264. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Chen, J.; Su, C.; Ma, C. Energy consumption and economic analyses of a supercritical water oxidation system with oxygen recovery. Processes 2018, 6, 224. [Google Scholar] [CrossRef]
- Li, Y.; Wang, S. Supercritical water oxidation for environmentally friendly treatment of organic wastes. In Advanced Supercritical Fluids Technologies; Pioro, I., Ed.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Zhang, F.; Li, Y.; Liang, Z.; Wu, T. Energy conversion and utilization in supercritical water oxidation systems: A review. Biomass Bioenergy 2022, 156, 106322. [Google Scholar] [CrossRef]
- Luo, C.; Teng, S.; Wang, J.; Xi, H. Energy yield from wastewater by supercritical water oxidation process: Experimental validation and simulation from the viewpoint of energy system. Energy Convers. Manag. 2024, 299, 117876. [Google Scholar] [CrossRef]
- Huang, X.; Wang, S.; Li, Y.; Sun, S.; Mi, Z.; Xing, L.; Duan, Y. Corrosion resistance and material optimization in supercritical water oxidation for radioactive waste treatment. J. Nucl. Mater. 2025, 616, 156063. [Google Scholar] [CrossRef]
- Luo, C.; Xi, H.; Feng, Y.-Q.; Hung, T.-C. Performance evaluation of a supercritical water oxidation cogeneration system using hydrogen peroxide as oxidant. Energy Convers. Manag. 2022, 269, 115914. [Google Scholar] [CrossRef]
- García-Rodríguez, Y.; Mato, F.A.; Martín, A.; Bermejo, M.D.; Cocero, M.J. Energy recovery from effluents of supercritical water oxidation reactors. J. Supercrit. Fluids 2015, 104, 1–9. [Google Scholar] [CrossRef]
- Fromonteil, C.; Bardelle, P.; Cansell, F. Hydrolysis and oxidation of an epoxy resin in sub-and supercritical water. Ind. Eng. Chem. Res. 2000, 39, 922–925. [Google Scholar] [CrossRef]
- Yang, B.; Cheng, Z.; Tang, Q.; Shen, Z. Nitrogen transformation of 41 organic compounds during SCWO: A study on TN degradation rate, N-containing species distribution and molecular characteristics. Water Res. 2018, 140, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Cheng, Z.; Tao, Y.; Gao, X.; Tan, Y.; Ma, Y.; Shen, Z. Temperature sensitivity of nitrogen-containing compounds decomposition during supercritical water oxidation (SCWO). J. Taiwan Inst. Chem. Eng. 2018, 93, 31–41. [Google Scholar] [CrossRef]
- Mitton, D.B.; Yoon, J.-H.; Cline, J.A.; Kim, H.-S.; Eliaz, N.; Latanision, R.M. Corrosion Behavior of Nickel-Based Alloys in Supercritical Water Oxidation Systems. Ind. Eng. Chem. Res. 2000, 39, 4689–4696. [Google Scholar] [CrossRef]
- Xu, T.; Wang, S.; Li, Y.; Li, J.; Cai, J.; Zhang, Y.; Xu, D.; Zhang, J. Review of the destruction of organic radioactive wastes by supercritical water oxidation. Sci. Total Environ. 2021, 799, 149396. [Google Scholar] [CrossRef]
| Model | R2 (COD) | MAE (%) | RMSE (%) | R2 (TN) | MAE (%) | RMSE (%) | Remarks |
|---|---|---|---|---|---|---|---|
| LR | 0.786 | 2.53 | 3.04 | 0.857 | 2.35 | 2.94 | performs poorly |
| RF | 0.962 | 0.86 | 1.29 | 0.859 | 0.89 | 1.57 | exhibits good stability |
| XGBoost | 0.980 | 0.37 | 0.94 | 0.972 | 0.46 | 1.30 | shows large prediction bias in the high-TN region |
| SVR | 0.985 | 0.43 | 0.80 | 0.990 | 0.36 | 0.78 | achieves the best performance on small samples |
| TabPFN | 0.964 | 0.92 | 1.24 | 0.995 | 0.33 | 0.56 | provides excellent fitting in the low-value region |
| GPR | 0.983 | 0.53 | 0.76 | 0.980 | 0.90 | 1.17 | possesses the capability of uncertainty quantification |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, Y.; Xu, T.; Zhang, L.; Zhang, Q.; Zhou, L.; Shen, Z.; Wan, Z. Multi-Objective Optimization of Supercritical Water Oxidation for Radioactive Organic Anion Exchange Resin Wastewater Using GPR–NSGA-II. Processes 2025, 13, 3759. https://doi.org/10.3390/pr13123759
Jin Y, Xu T, Zhang L, Zhang Q, Zhou L, Shen Z, Wan Z. Multi-Objective Optimization of Supercritical Water Oxidation for Radioactive Organic Anion Exchange Resin Wastewater Using GPR–NSGA-II. Processes. 2025; 13(12):3759. https://doi.org/10.3390/pr13123759
Chicago/Turabian StyleJin, Yabin, Tiantian Xu, Le Zhang, Qian Zhang, Liang Zhou, Zhe Shen, and Zhenjie Wan. 2025. "Multi-Objective Optimization of Supercritical Water Oxidation for Radioactive Organic Anion Exchange Resin Wastewater Using GPR–NSGA-II" Processes 13, no. 12: 3759. https://doi.org/10.3390/pr13123759
APA StyleJin, Y., Xu, T., Zhang, L., Zhang, Q., Zhou, L., Shen, Z., & Wan, Z. (2025). Multi-Objective Optimization of Supercritical Water Oxidation for Radioactive Organic Anion Exchange Resin Wastewater Using GPR–NSGA-II. Processes, 13(12), 3759. https://doi.org/10.3390/pr13123759






