Layered Double Hydroxide-Based Materials for Wastewater Treatment: Recent Progress in Multifunctional Environmental Applications
Abstract
1. Introduction
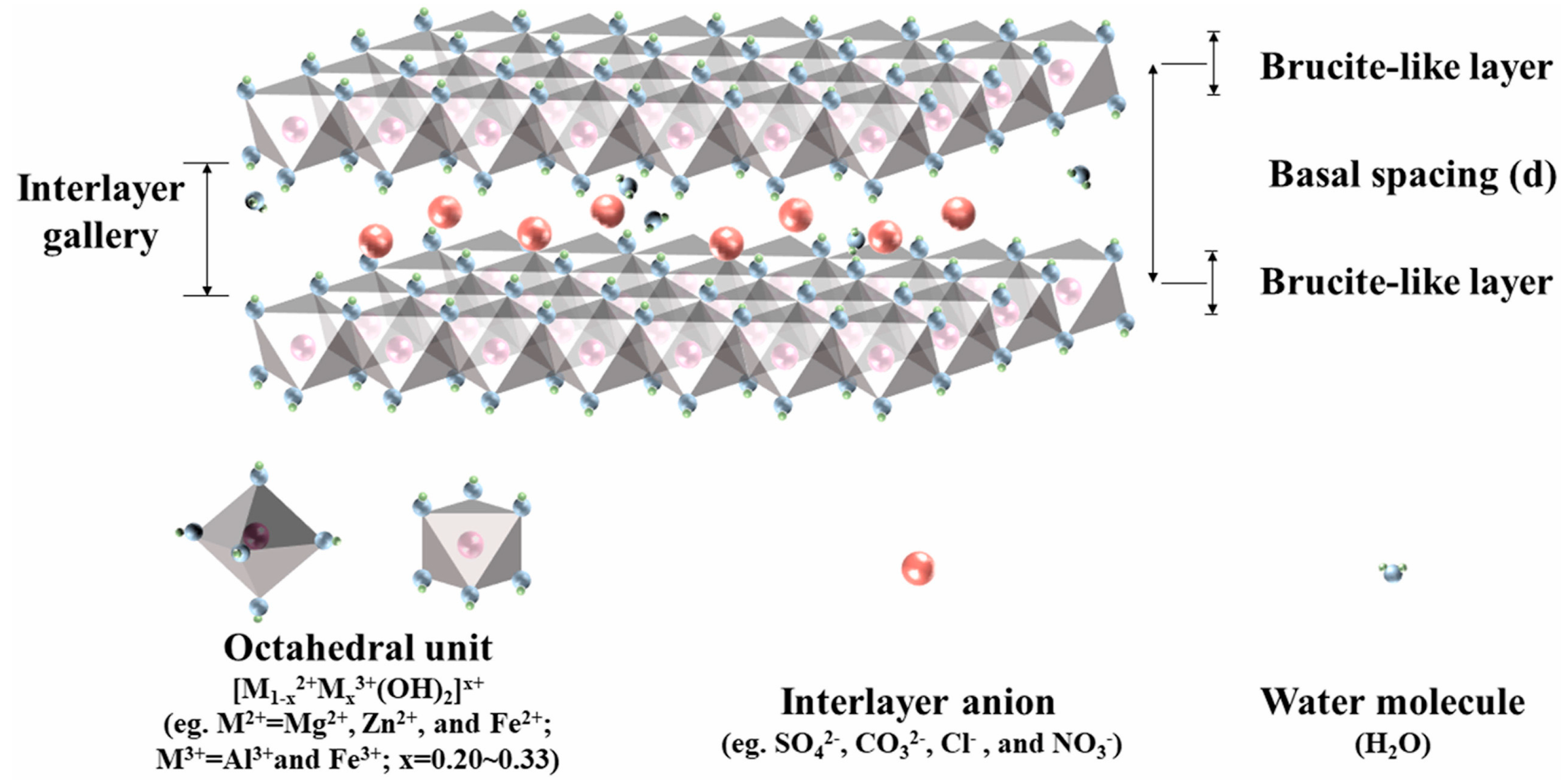
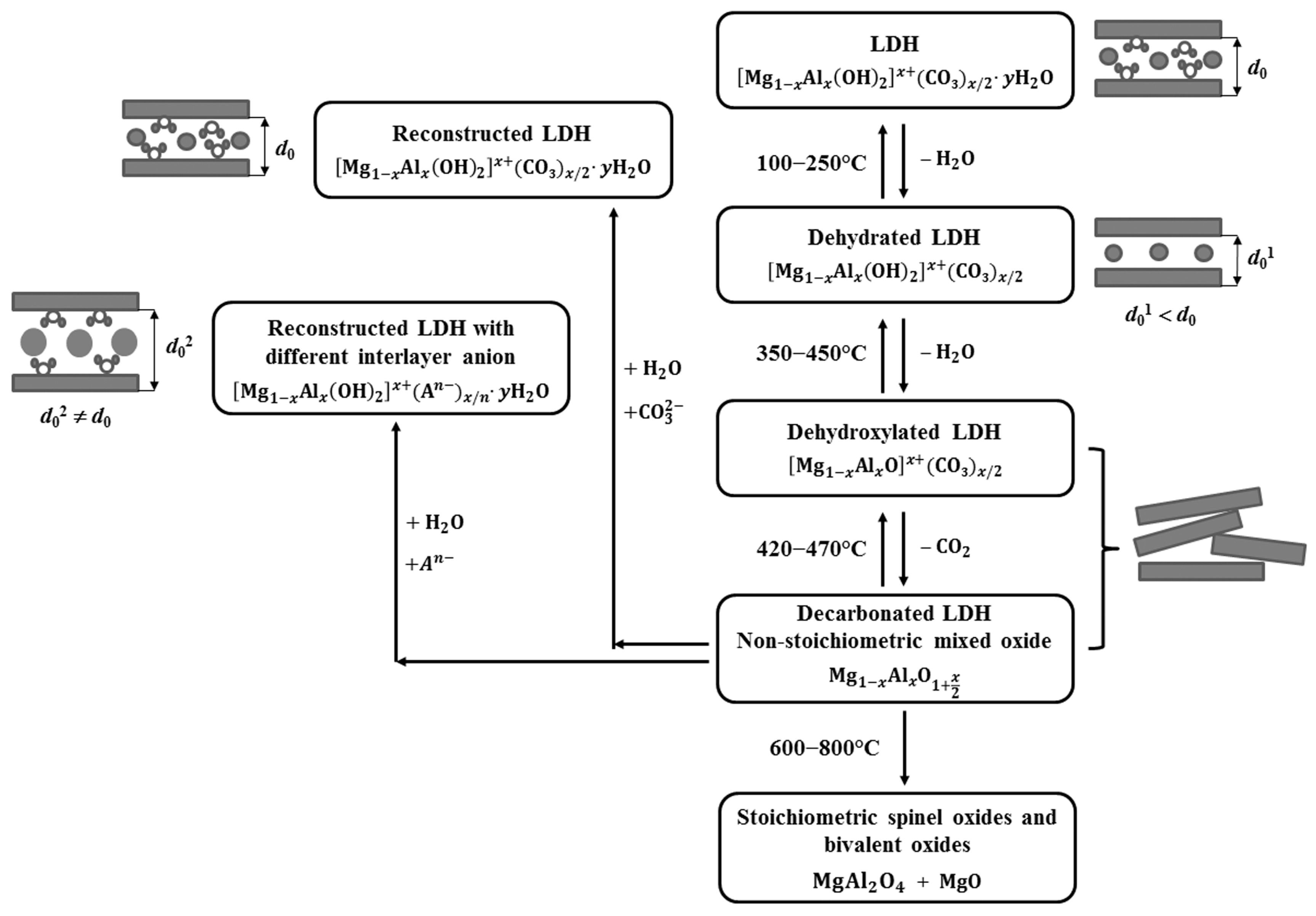
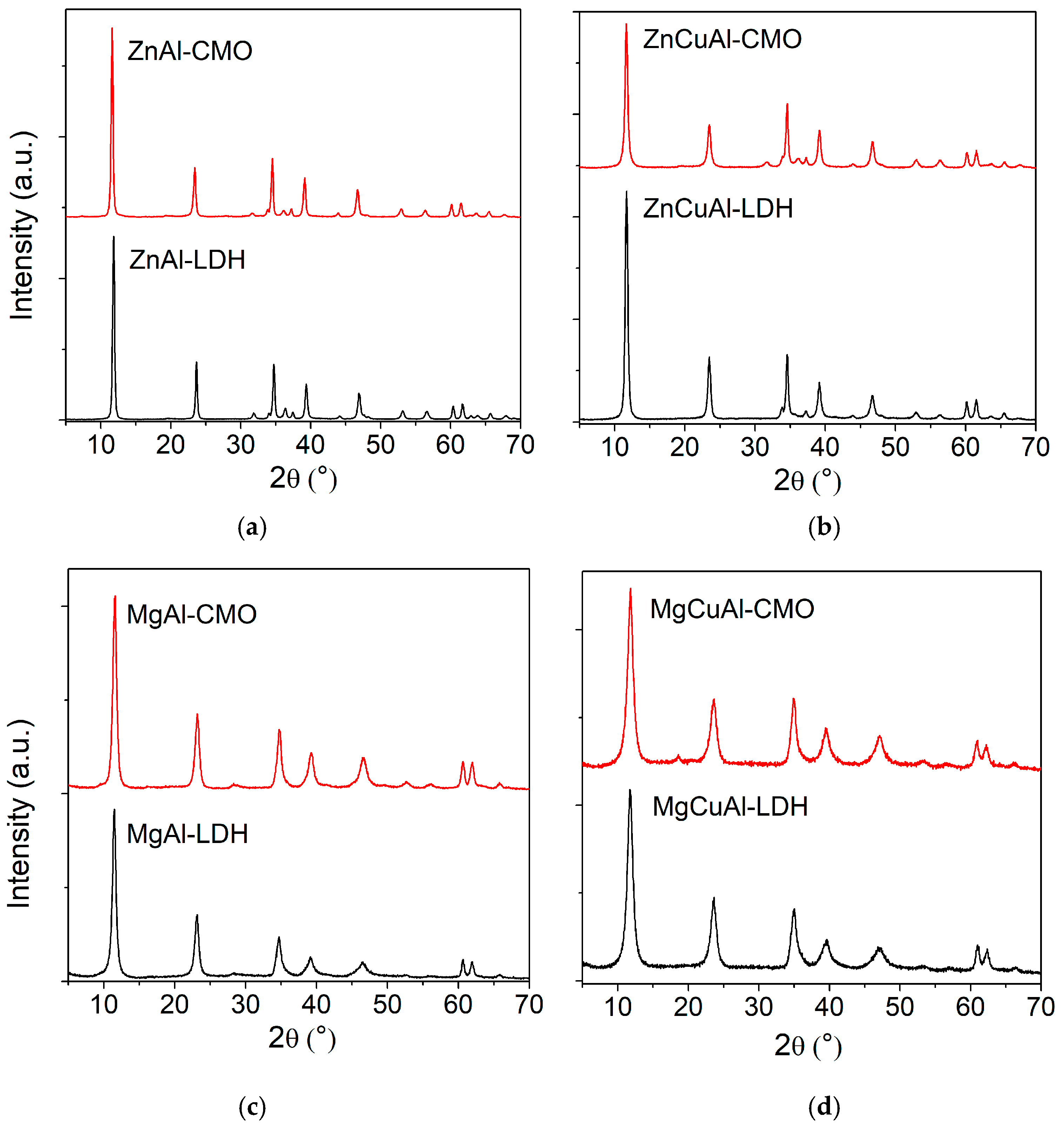
2. General Properties of Layered Double Hydroxides and Derived Mixed Oxides
3. Synthesis of LDHs
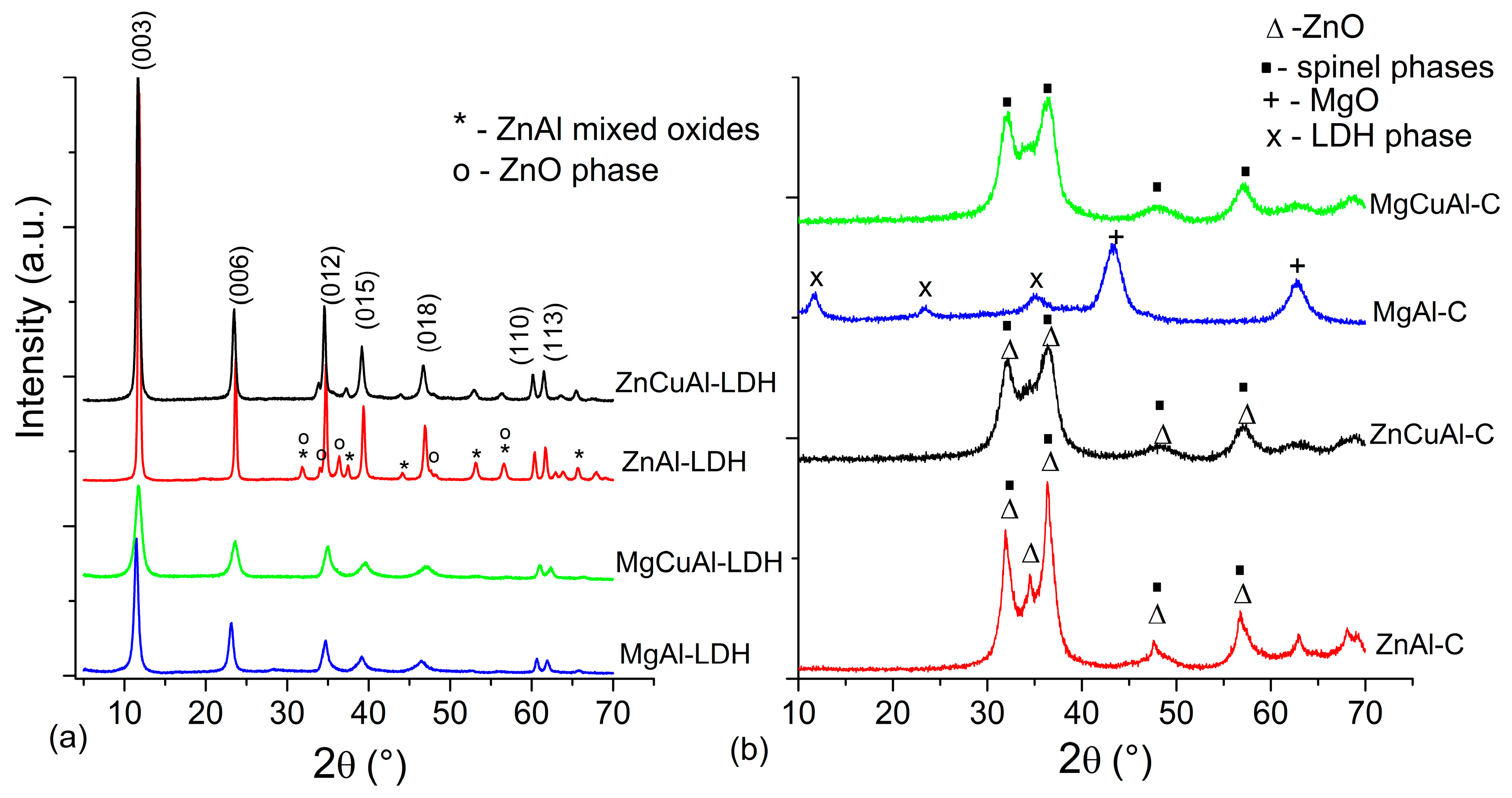
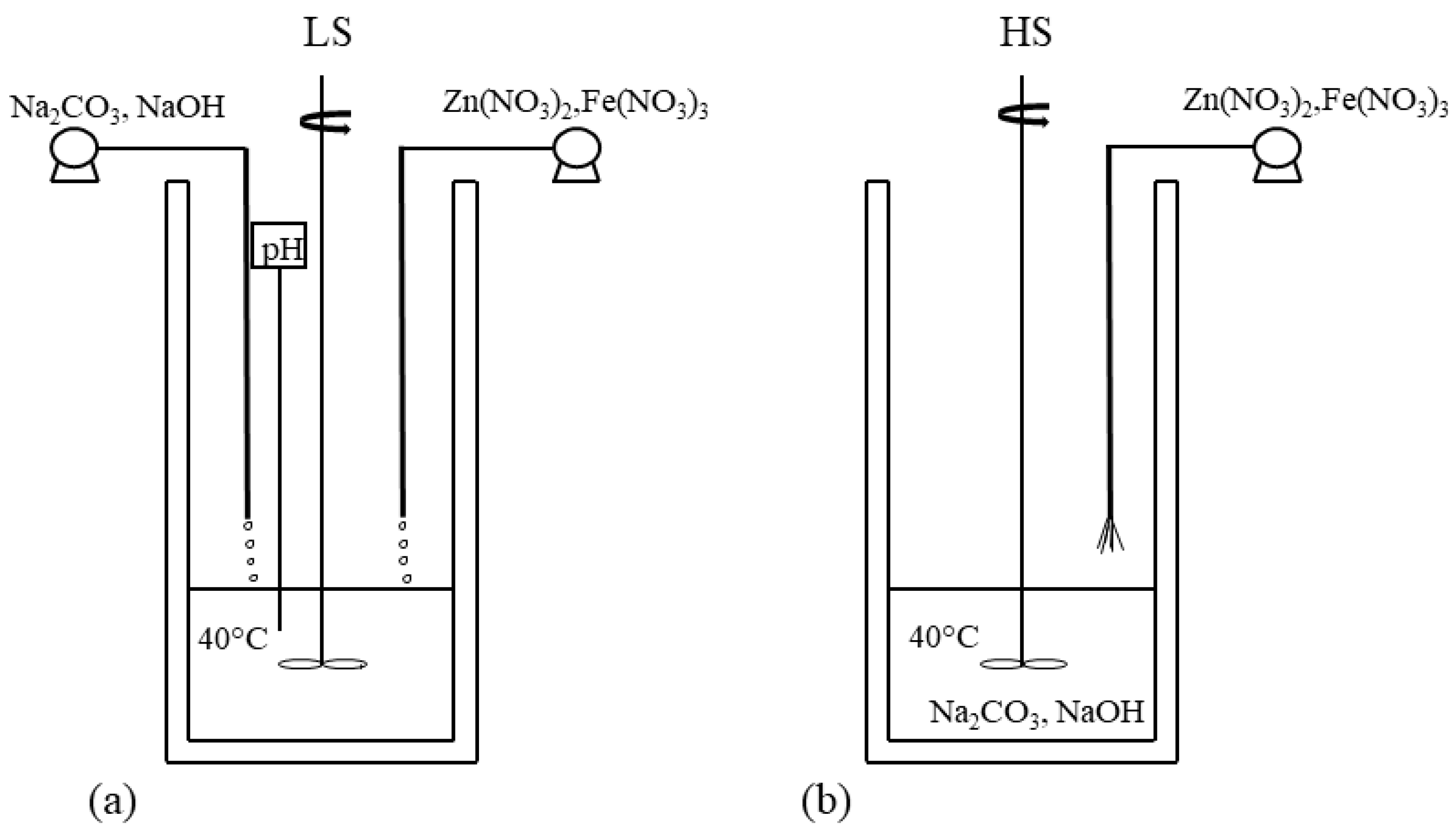
3.1. Co-Precipitation Method
3.2. Urea Method
3.3. Hydrothermal Method
3.4. Functionalisation/Composites
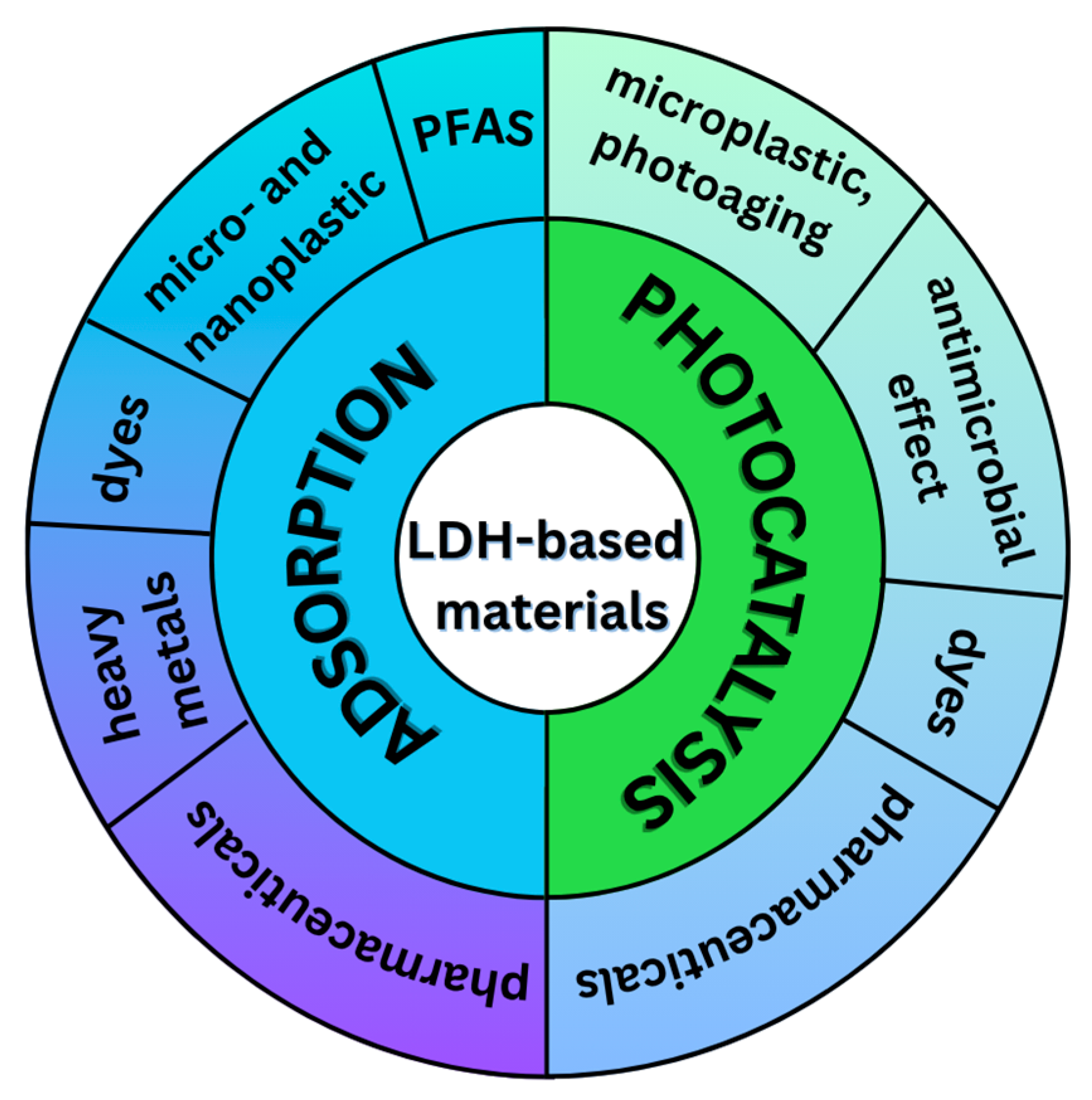
4. Application of LDHs in Wastewater Treatments
4.1. Adsorption Processes
4.1.1. Adsorption of Dyes
4.1.2. Adsorption of Pharmaceuticals
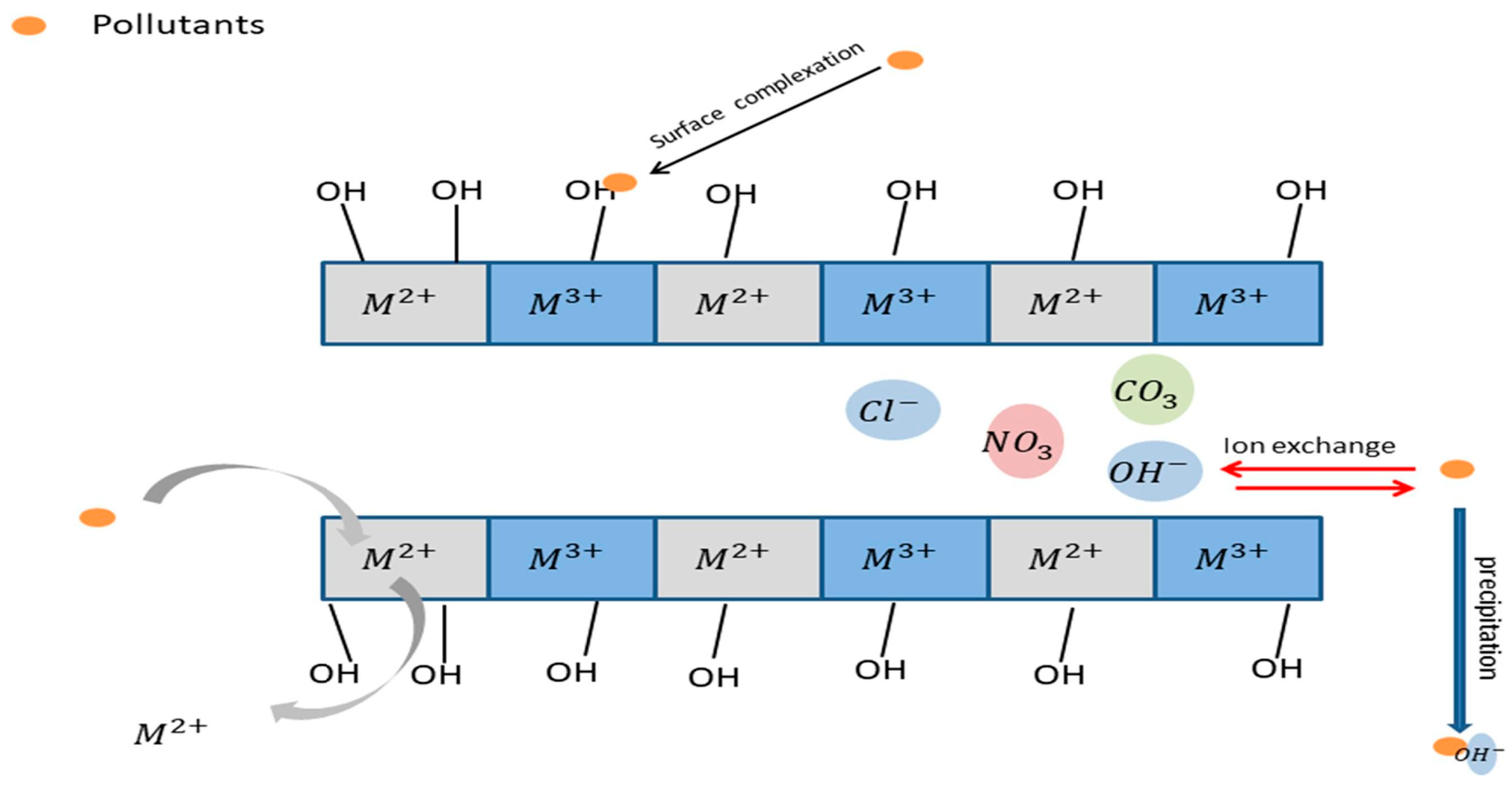
4.1.3. Adsorption of Heavy Metals
4.1.4. Adsorption of Per- and Polyfluoroalkyl Substances
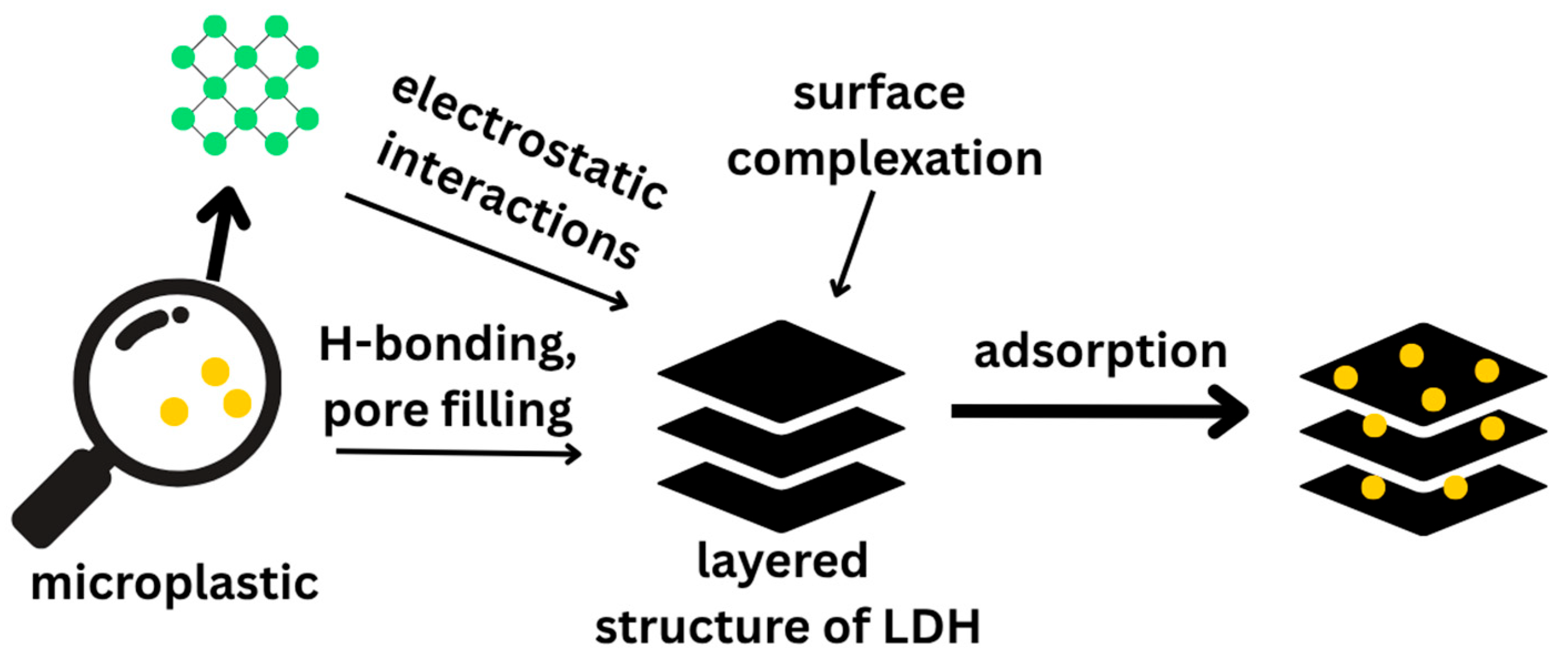
4.1.5. Adsorption of Micro- and Nanoplastics
4.2. Photocatalytic Processes
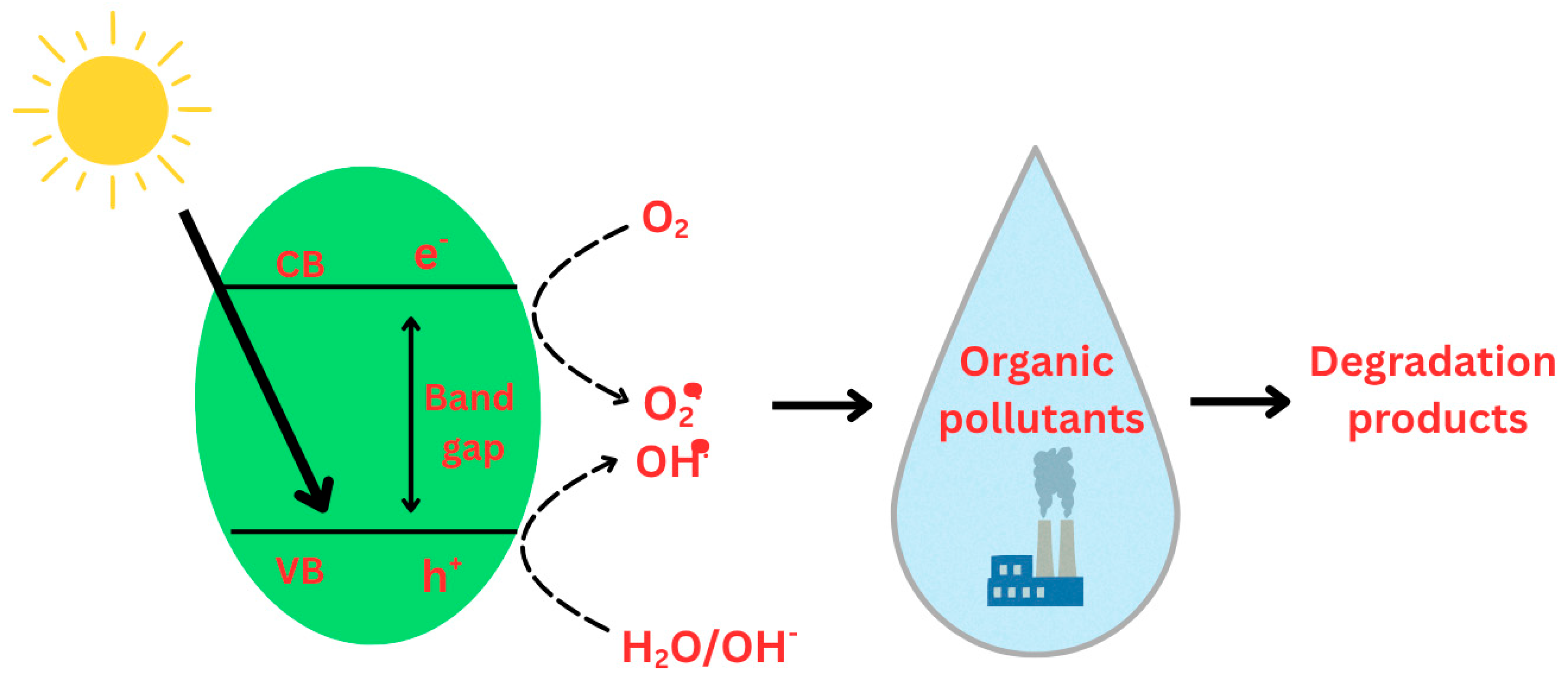
- Adsorption of pollutant molecules from the medium on photocatalyst active sites through physisorption or chemisorption.
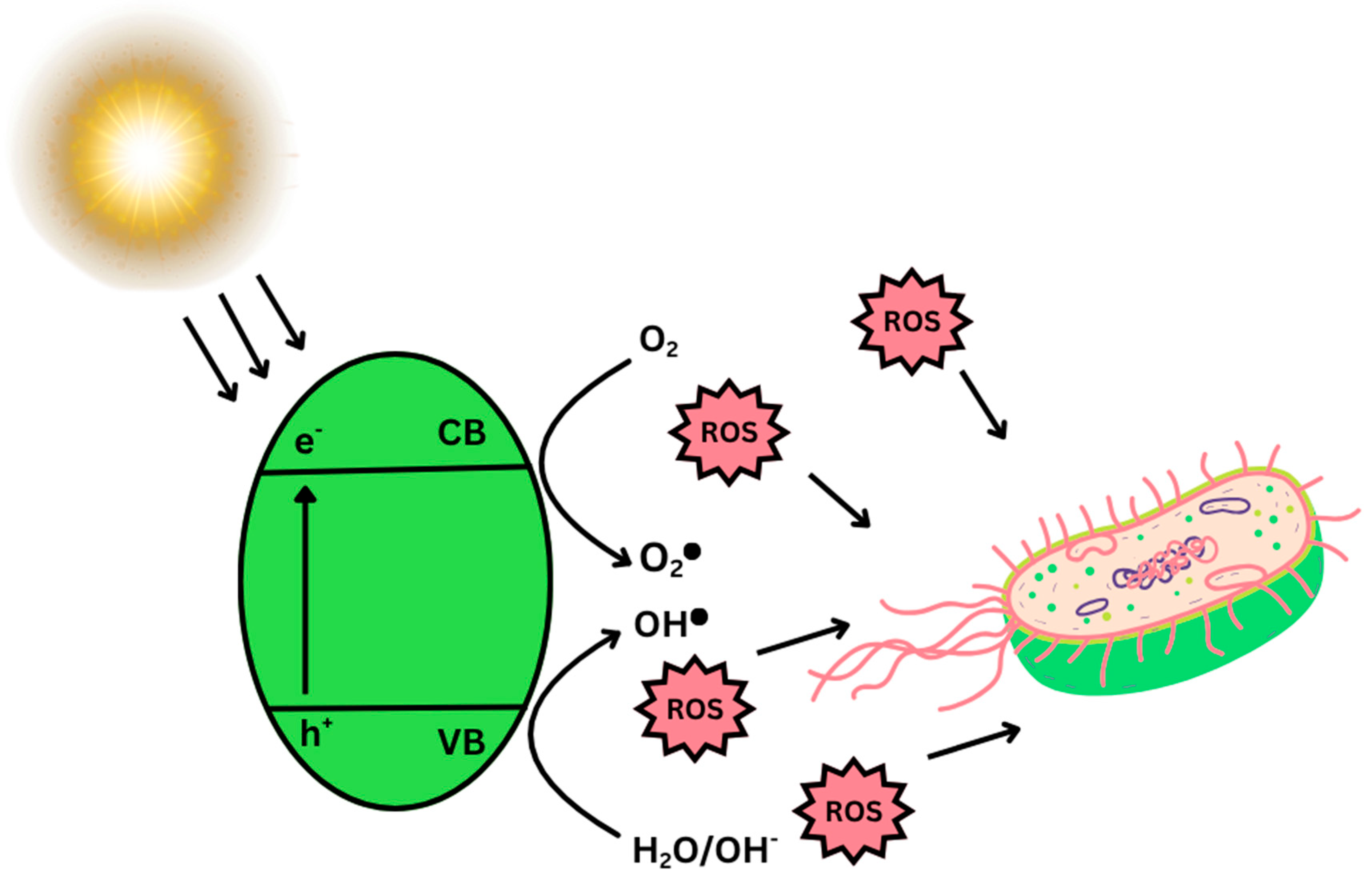
- Photon absorption of suitable wavelength, with energy equal to or higher than the band gap energy. Electrons are promoted from the valence band (VB) to the conduction band (CB), triggering positively charged holes (h+) in the VB. This process generates electron–hole (e−/h+) pairs, which are the primary charge carriers in photocatalytic reactions (Figure 12).
- Migration of generated e−/h+ pairs to the surface of the photocatalyst, where reactant molecules are adsorbed.
- At the surface, simultaneous oxidation and reduction processes occur. The photogenerated electrons in the CB are typically transferred to molecular oxygen, yielding superoxide radical anions (•O2−) as the first reactive oxygen species (ROS). Simultaneously, photogenerated holes in the VB oxidize hydroxide ions or water molecules to produce hydroxyl radicals (•OH), which are among the most powerful oxidizing agents. These ROS, together with the direct redox activity of electrons and holes, initiate the degradation of adsorbed pollutants. The organic contaminants are gradually converted into intermediate species and, ultimately, mineralized to harmless products such as carbon dioxide (CO2) and water (H2O).
- Desorption of the final products from the photocatalyst surface, thereby regenerating the active sites and allowing subsequent catalytic cycles.
4.2.1. Photocatalytic Degradation of Dyes
4.2.2. Photocatalytic Degradation of Pharmaceuticals
4.2.3. Photocatalytic Degradation and Photoaging of Microplastic
4.3. Photocatalytically Induced Antimicrobial Activity
- Oxidative stress: Free radicals produced by photocatalysis initiate the oxidation of membrane lipids causing the damage to proteins and mRNA [121,124]. The ultimate result is lipid peroxidation and protein denaturation due to permanent damage to nucleic acids. The crystallinity and the phase composition of photocatalysts can significantly affect their photocatalytic properties, such as the ability to generate higher amounts of ROS in the presence of sunlight, leading to oxidative stress in the cell [121,122,123]. When exposed to UV or visible light, LDH-based composites coupled with semiconductors such as TiO2, ZnO, g-C3N4, or Ag nanoparticles are capable of generating ROS that inflict oxidative damage on microbial membranes, nucleic acids, and intracellular proteins, leading to rapid inactivation of pathogens [4,122]. In such systems, the LDH structure is crucial for separating photogenerated charge carriers, prolonging their lifetimes and increasing ROS yield.
- Release of metal ions from photocatalysts: Metal ions penetrate the cell membrane and react with -SH, -NH, and -COOH groups of nucleic acids and proteins, causing damage. In systems based on Zn, Cu, Co, or Fe, divalent or trivalent metal ions are gradually released into the aqueous environment, disrupting essential microbial processes such as membrane transport, enzyme activity and oxidative balance [125]. Zn2+ ions are recognized for their antibacterial properties, which include disrupting the integrity of the cell membrane and enzyme functions [126,127]. However, this mechanism is insufficiently studied and is mostly mentioned in the literature as an additional reason for cell death. Elrafey et al. synthesized a NiFe LDH and evaluated its bactericidal activity against Proteus mirabilis, Salmonella Typhimurium, Escherichia coli, Pseudomonas aeruginosa and Klebsiella pneumoniae. They reported that Fe- and Ni-containing LDHs strongly interact with bacterial proteins, disrupting regulated transport across the plasma membrane by altering its permeability, which triggers a stress response in the transport system and ultimately leads to bacterial death [128]. ZnFe-LDHs synthesized via co-precipitation and intercalated with nitrate have been reported to eliminate both Escherichia coli and Staphylococcus aureus effectively, while simultaneously removing heavy metals and organic dyes from solution [129]. Similarly, CoFe-LDHs treated with gamma irradiation showed notable antibacterial activity coupled with significant removal of Malachite green and Methylene blue dyes [130].
| LDH | Light Source | Microorganisms | Antimicrobial Mechanism | Performance | Ref. |
|---|---|---|---|---|---|
| Zn-Fe LDH | Ambient light (field conditions) | E. coli, S. aureus, total coliforms | Bactericidal surface interactions, possible ROS activity under ambient light | High disinfection efficiency (>90%) of real wastewater; performance robust in complex matrix | [129] |
| Co-Fe LDH nanosheets | Visible light | E. coli, S. aureus | Synergistic effect of gamma-induced structural changes and ROS generation; enhanced surface reactivity | Gamma irradiation increased antibacterial activity and dye removal; improved magnetization and dielectric properties | [130] |
| ZnAl-SO4 LDH | Solar-simulated light | E. coli, S. aureus | ROS generation (•OH, •O2−) from photoactivated ZnO-like phases; surface interactions | Thermal annealing improved both antimicrobial and photocatalytic activity; post-treatment materials showed higher bacterial growth inhibition | [14] |
| Ag–LDH embedded in polymeric hydrogel | Visible light (simulated daylight) | E. coli, S. aureus, P. aeruginosa, Candida albicans | Sustained Ag+ ion release from LDH matrix; ROS generation under visible light; enhanced contact killing via hydrogel network | Strong antimicrobial activity against Gram-positive, Gram-negative, and fungal strains; hydrogel improved dispersion and release control | [131] |
| ZnCr LDH mixed oxides | Simulated solar irradiation | E. coli, S. aureus | ROS-mediated cell damage | ZnCr900 achieved ~100% bacterial growth inhibition and complete dye removal; activity enhanced by high crystallinity and optimal band structure | [4] |
| ZnO nanoparticles (various sizes) | Ambient visible light (no UV lamp) | S. aureus, E. coli, other clinical isolates | ROS production (H2O2, •OH, 1O2); direct contact cell wall/membrane disruption; | Smaller particles led to higher antibacterial activity; broad-spectrum effect without UV pre-activation | [136] |
| ZnO nanomaterials (different morphologies) | Light conditions not specified for antimicrobial tests; photocatalysis under visible light | B. manliponensis, M. luteus, S. aureus, E. coli | •O2− radical generation > •OH; ROS-induced oxidative stress | ZnO-4M had strongest antibacterial and photocatalytic activity; morphology less critical than ROS generation profile | [137] |
| NiAl-LDH/Cu-MOF | Solar light | S. aureus, E. faecalis, E. coli, P. fluorescens | ROS-mediated cell damage | Strong antimicrobial activity against bacteria, inhibition zone between 12 and 14 mm, very efficient ROS generation, high surface area, and stability | [134] |
5. Future Perspectives and Conclusions
- The development of LDH materials with tailored properties requires a more detailed insight into the relationship between composition, microstructure and performance. Future research should prioritize controlled synthesis at the nanoscale, enabling fine-tuning of metal cation ratios, interlayer structure, and defect sites. Coupling LDHs with semiconductors, carbon-based materials, or other metals of interest could enhance textural properties and charge separation and extend light absorption into the visible and near-infrared regions. This would improve not only the photocatalytic efficiency under natural sunlight, but also adsorption capacity for targeted pollutants. Therefore, by controlled functional modification of pure-phase LDHs through interlayer variation, surface compounding, and calcination LDH-based materials can effectively remove emerging contaminants from water, owing to their obtained excellent electronic structures and crystal structures. Also, complex synthesis of hybrid materials such as metal–organic frameworks (MOFs) and LDHs should be thoroughly investigated and promoted considering that this combination of systems highly influences structural and functional properties, favorable particularly in catalysis and environmental remediation. Future study should also be directed to research on the relationship between structural properties and functional capabilities of these hybrid materials, forwarding the design of more efficient and versatile materials for desired applications. Additionally, 2D/2D heterojunctions and core–shell nanostructures represent promising systems for achieving synergistic adsorption, photocatalytic and antimicrobial effects. Understanding the correlation between structure, electronic properties and performance will be essential for optimizing multifunctional efficiency.
- In order to fully elucidate the multifunctional behavior of LDH-based materials in pollutant removal, there is a general consensus that a more comprehensive understanding of their adsorption and photocatalytic mechanisms is required. Despite extensive studies, the processes governing adsorption dynamics, electron transfer, radical formation and active-site evolution remain only partially understood, highlighting the need for advanced and integrated characterization approaches.
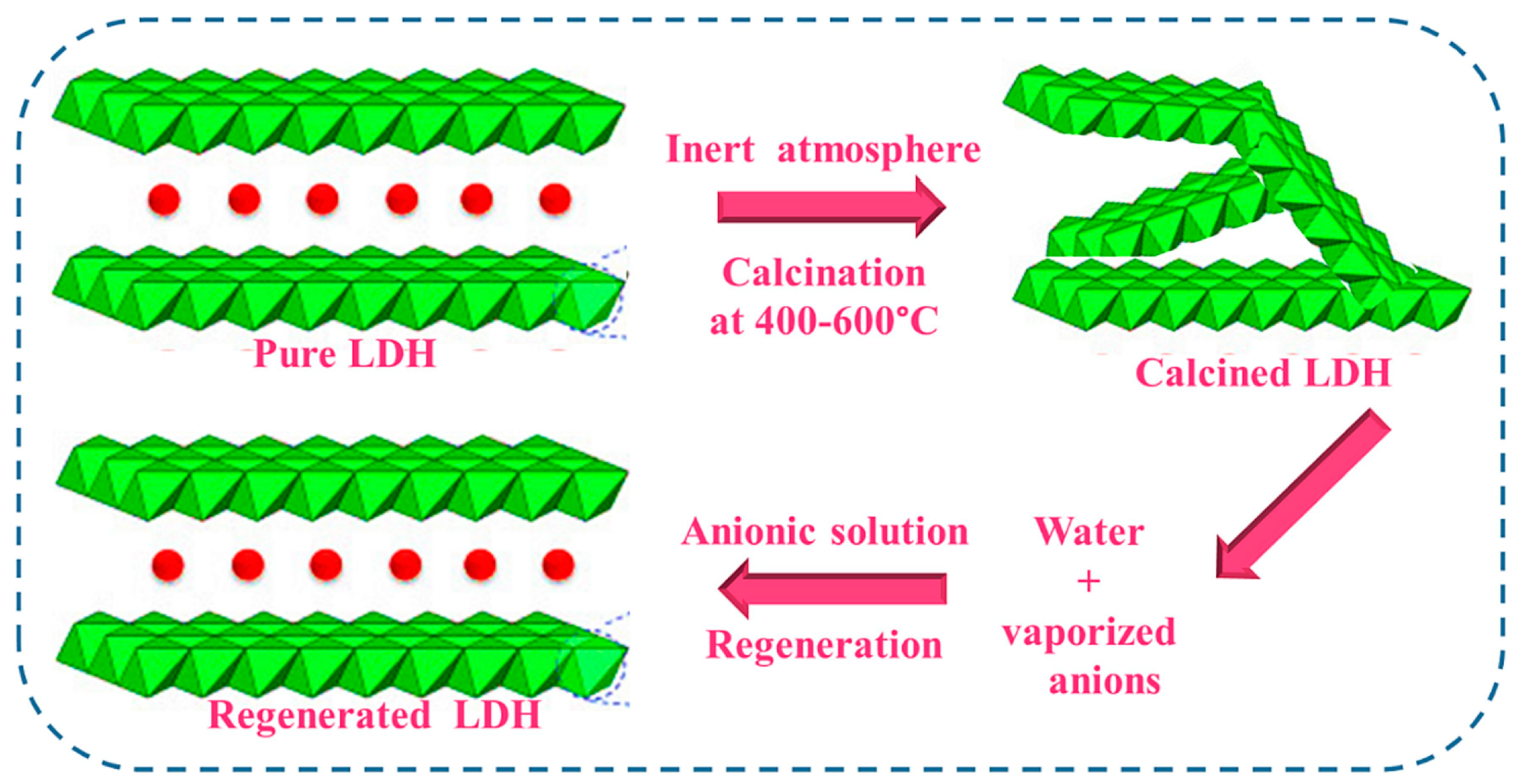
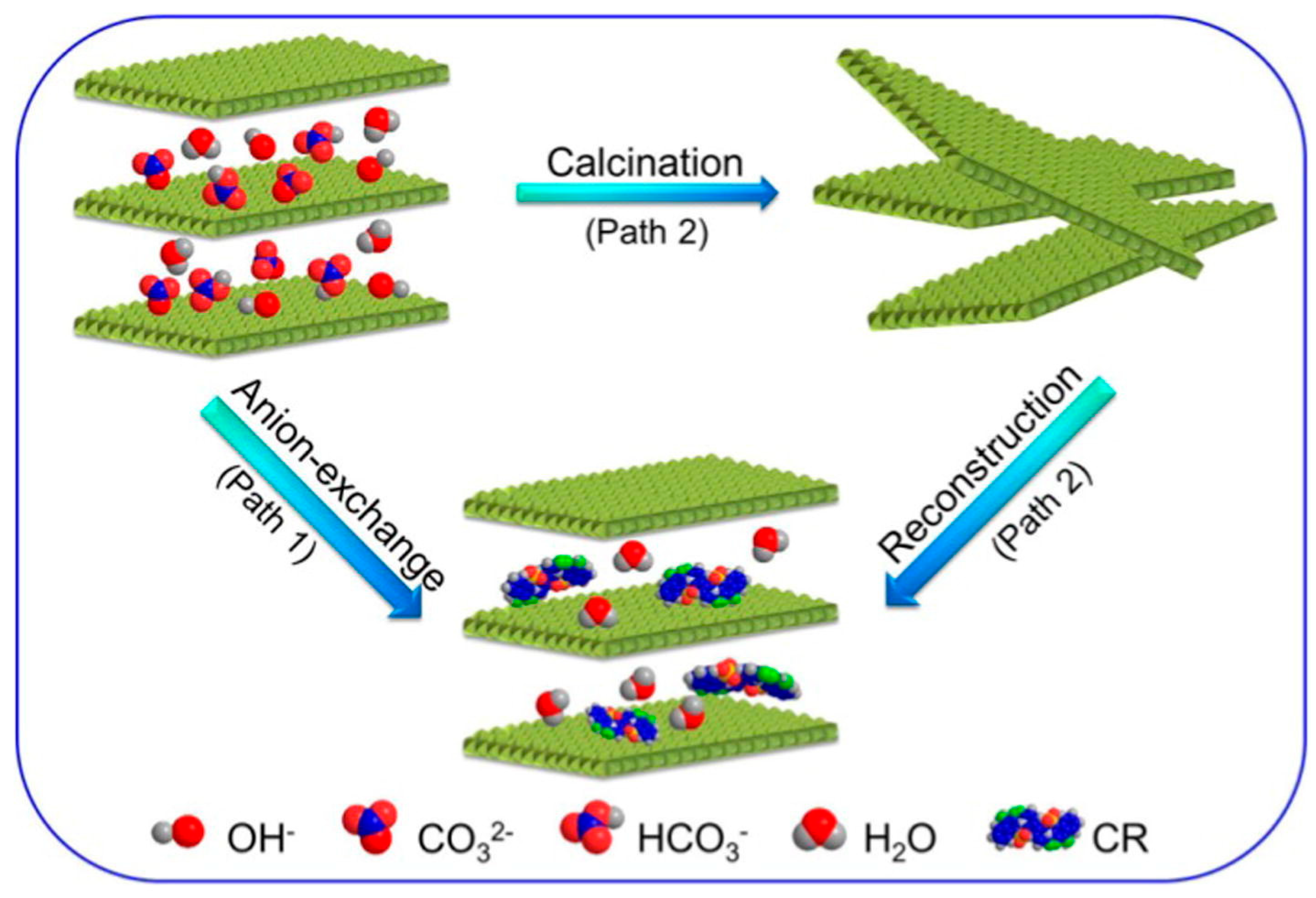
- The reversible transformation between LDHs, their mixed oxides, and the rehydrated forms, defined as the memory effect, is a unique feature that allows fine-tuning of surface area, porosity and the distribution of active sites, while also enabling material regeneration. However, as mentioned in the review, this behavior is sensitive to calcination temperature, as excessive heating can cause irreversible phase transitions that diminish the material’s ability to recover its layered structure. To ensure long-term stability and consistent performance, future studies should focus on mapping out the temperature and compositional reconstruction windows across different metal systems and correlate them to catalytic and adsorptive durability. The effect of incorporating structure-stabilizing dopants, promoters, or protective surface layers should be further investigated because it may widen the thermal tolerance and prevent degradation during repeated use. This in-depth research would contribute to mild regeneration protocols that could lead to activity resilience and stability over multiple operational cycles. A deeper understanding of the memory effect in mixed oxides will be essential for designing LDH-based materials with improved resilience, recyclability, and sustained efficiency in practical applications.
- A promising route for future research should also be directed to the development of multifunctional LDH-based materials efficient in pollutant degradation, antimicrobial protection, and resource recovery. As emphasized in the review, because of their structural versatility, LDHs can effectively couple adsorption, photocatalysis, and light-driven antimicrobial action through ion release and reactive oxygen species generation. Therefore, further work should be focused on future materials engineered for targeted multifunctionality, such as heterojunction LDH/semiconductor/carbon hybrids that enhance charge separation, offer efficient anion-exchange sites for contaminant capture, and allow controlled metal release for microbial control. Combining these effects would increase their environmental performance and versatility.
- Emerging multifunctional LDH-based systems that combine pollutant degradation, antimicrobial protection and resource recovery represent a particularly promising research direction. Recent studies have shown their capability to degrade persistent contaminants such as pharmaceuticals, per- and polyfluoroalkyl substances (PFAS), and even micro- and nanoplastic. These systems can also provide photo-induced antibacterial activity through ROS generation and controlled ion release, offering integrated approaches for chemical and biological pollutant removal. The next generation of LDH-based materials should thus be engineered for targeted multifunctionality that would be capable not only of removing pollutants, but also contributing to circular resource flows.
- Considering the environmental motivation of wastewater remediation, the synthesis of LDH-based materials should highly consider sustainable, low-waste, and resource-efficient approaches. Upcoming research should emphasize on developing green synthesis routes that would replace hazardous reagents with eco-friendly solvents, while also incorporating waste-derived precursors such as industrial residues, slags, and biomass ashes. These strategies not only reduce production costs and the environmental footprint but also align LDH manufacturing with circular economy principles. Recent studies have shown that high-performance LDHs can be successfully obtained from industrial by-products, demonstrating both feasibility and efficiency. However, to ensure true sustainability, future work must include assessments concerning economic aspects and life-cycle analyses (LCA) to evaluate scalability, energy demand, and environmental impact, thereby guiding the transition from laboratory-scale synthesis to practical, green production of LDH materials.
- One of the main obstacles to advancing LDH-based technologies is the lack of standardized testing and reporting practices. Numerous studies report results with differences in experimental procedure, such as light sources, pollutant concentrations, solution composition, and regeneration protocols, limiting the possibility to compare results among published studies. In order to enable meaningful evaluation and accelerate progress, the research community should establish unified testing standards that include experiments with real wastewater effluents and consistent reporting of key parameters that influence the efficiency of LDH-based materials. This would lead to more reliable cross-study comparisons and faster innovation.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Mohamed, A.A. A systematic review of layered double hydroxide-based materials for environmental remediation of heavy metals and dye pollutants. Inorg. Chem. Commun. 2023, 148, 110325. [Google Scholar] [CrossRef]
- Hadnađev-Kostić, M.; Vulic, T.; Karanovic, D.; Milanovic, M. Advanced dye removal by multifunctional layered double hydroxide-based materials: Adsorption and kinetic studies. J. Serb. Chem. Soc. 2022, 87, 1011–1024. [Google Scholar] [CrossRef]
- Karanovic, D.; Hadnadjev-Kostic, M.; Vulic, T.; Markov, S.; Tomic, A.; Miljevic, B.; Rajakovic-Ognjanovic, V. Thermal treatment impact on the evolution of active phases in layered double hydroxide-based ZnCr photocatalysts: Photodegradation and antibacterial performance. Green. Process Synth. 2024, 13, 20230269. [Google Scholar] [CrossRef]
- Farhan, A.; Khalid, A.; Maqsood, N.; Iftekhar, S.; Sharif, H.M.A.; Qi, F.; Sillanpaa, M.; Asif, M.B. Progress in layered double hydroxides (LDHs): Synthesis and application in adsorption, catalysis and photoreduction. Sci. Total Environ. 2024, 912, 169160. [Google Scholar] [CrossRef] [PubMed]
- Yagub, M.T.; Sen, T.K.; Afroze, S.; Ang, H.M. Dye and its removal from aqueous solution by adsorption: A review. Adv. Colloid Interface Sci. 2014, 209, 172–184. [Google Scholar] [CrossRef]
- Arrabito, G.; Bonasera, A.; Prestopino, G.; Orsini, A.; Mattoccia, A.; Martinelli, E.; Pignataro, B.; Medaglia, P.G. Layered double hydroxides: A toolbox for chemistry and biology. Crystals 2019, 9, 361. [Google Scholar] [CrossRef]
- Sadeghi Rad, T.; Khataee, A.; Arefi-Oskoui, S.; Sadeghi Rad, S.; Orooji, Y.; Gengec, E.; Kobya, M. Graphene-based ZnCr layered double hydroxide nanocomposites as bactericidal agents with high sonophotocatalytic performances for degradation of rifampicin. Chemosphere 2022, 286, 131740. [Google Scholar] [CrossRef]
- Mishra, G.; Dash, B.; Pandey, S. Layered double hydroxides: A brief review from fundamentals to application as evolving biomaterials. Appl. Clay Sci. 2018, 153, 172–186. [Google Scholar] [CrossRef]
- Dong, Y.; Kong, X.; Luo, X.; Wang, H. Adsorptive removal of heavy metal anions from water by layered double hydroxide: A review. Chemosphere 2022, 303, 134685. [Google Scholar] [CrossRef]
- Li, F.; Duan, X. Applications of layered double hydroxides. In Layered Double Hydroxides. Structure and Bonding; Duan, X., Evans, D.G., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; Volume 119, pp. 193–223. [Google Scholar] [CrossRef]
- Morcos, C.; Seron, A.; Maubec, N.; Ignatiadis, I.; Betelu, S. Comprehension of the Route for the Synthesis of Co/Fe LDHs via the Method of Coprecipitation with Varying pH. Nanomaterials 2022, 12, 1570. [Google Scholar] [CrossRef]
- Jiang, Y.; Shen, Z.; Tang, C.S.; Shi, B. Synthesis and application of waste-based layered double hydroxide: A review. Sci. Total Environ. 2023, 903, 166245. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, A.M.; Alberti, S.; Reverberi, A.P.; Catauro, M.; Ghibaudo, N.; Fortunato, M. Antibacterial and Photocatalytic Activities of LDH-Based Sorbents of Different Compositions. Microorganisms 2023, 11, 1045. [Google Scholar] [CrossRef] [PubMed]
- Omonmhenle, S.I.; Ifijen, I.H. Assessment of the Antibacterial Potential of Layered Double Hydroxides. Dutse J. Pure Appl. Sci. 2023, 9, 58–69. [Google Scholar] [CrossRef]
- Vaccari, A. Preparation and catalytic properties of cationic and anionic clays. Catal. Today 1998, 41, 53–71. [Google Scholar] [CrossRef]
- Vaccari, A. Clays and catalysis: A promising future. Appl. Clay Sci. 1999, 14, 161–198. [Google Scholar] [CrossRef]
- Hadnadjev-Kostic, M.; Vulic, T.; Marinkovic-Neducin, R. Thermal Activation of Layered Hydroxide-Based Catalysts. In Reactions and Mechanisms in Thermal Analysis of Advanced Materials; Tiwari, A., Raj, B., Eds.; Wiley-Scrivener: Hoboken, NJ, USA, 2015; pp. 483–513. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, X.; Zhou, B.; Meng, F.; Wang, Y.; Wen, G. Recent advance of layered double hydroxides materials: Structure, properties, synthesis, modification and applications of wastewater treatment. J. Environ. Chem. Eng. 2023, 11, 111191. [Google Scholar] [CrossRef]
- Smalenskaite, A.; Kaba, M.M.; Grigoraviciute-Puroniene, I.; Mikoliunaite, L.; Zarkov, A.; Ramanauskas, R.; Morkan, I.A.; Kareiva, A. Sol-gel synthesis and characterization of coatings of Mg-Al layered double hydroxides. Materials 2019, 12, 3738. [Google Scholar] [CrossRef]
- Yang, C.; Wang, L.; Yu, Y.; Wu, P.; Wang, F.; Liu, S.; Luo, X. Highly efficient removal of amoxicillin from water by Mg-Al layered double hydroxide/cellulose nanocomposite beads synthesized through in-situ coprecipitation method. Int. J. Biol. Macromol. 2020, 149, 93–100. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, X.; Li, C.; Wang, H.; Wang, L. The role of soft colloidal templates in the shape evolution of flower-like MgAl-LDH hierarchical microstructures. RSC Adv. 2015, 5, 29757–29765. [Google Scholar] [CrossRef]
- Hadnadjev-Kostic, M.; Karanovic, D.; Vulic, T.; Dostanić, J.; Lončarević, D. Photocatalytic properties of ZnFe-mixed oxides synthesized via a simple route for water remediation. Green Process. Synth. 2023, 12, 20228153. [Google Scholar] [CrossRef]
- Paušová, Š.; Krýsa, J.; Jirkovský, J.; Forano, C.; Mailhot, G.; Prevot, V. Insight into the photocatalytic activity of ZnCr-CO3 LDH and derived mixed oxides. Appl. Catal. B 2015, 170–171, 25–33. [Google Scholar] [CrossRef]
- Guan, T.; Fang, L.; Wu, F.; Yang, Y. High-Performance La-, Mo-, and W-Doped NiFe-Layered Double Hydroxide for Methyl Orange Dye and Cr(VI) Adsorption. Processes 2025, 13, 156. [Google Scholar] [CrossRef]
- Xu, Z.P.; Zhang, J.; Adebajo, M.O.; Zhang, H.; Zhou, C. Catalytic applications of layered double hydroxides and derivatives. Appl. Clay Sci. 2011, 53, 139–150. [Google Scholar] [CrossRef]
- Ahmed, A.A.A.; Talib, Z.A.; Hussein, M.Z.; Zakaria, A. Improvement of the crystallinity and photocatalytic property of zinc oxide as calcination product of Zn-Al layered double hydroxide. J. Alloys Compd. 2012, 539, 154–160. [Google Scholar] [CrossRef]
- Asif, M.B.; Kang, H.; Zhang, Z. Assembling CoAl-layered metal oxide into the gravity-driven catalytic membrane for Fenton-like catalytic degradation of pharmaceuticals and personal care products. Chem. Eng. J. 2023, 463, 142340. [Google Scholar] [CrossRef]
- Kowalik, P.; Konkol, M.; Kondracka, M.; Próchniak, W.; Bicki, R.; Wiercioch, P. Memory effect of the CuZnAl-LDH derived catalyst precursor-In situ XRD studies. Appl. Catal. A 2013, 464–465, 339–347. [Google Scholar] [CrossRef]
- Mascolo, G.; Mascolo, M.C. On the synthesis of layered double hydroxides (LDHs) by reconstruction method based on the “memory effect”. Microporous Mesoporous Mater. 2015, 214, 246–248. [Google Scholar] [CrossRef]
- Nascimento, R.S.; Corrêa, J.A.M.; Figueira, B.A.M.; Paris, E.C.; Quaranta, S. High-performance, layered double hydroxides-derived mixed metal oxides from bauxite tailings as effective adsorbers for water remediation-ponceau 4R study. J. Water Process Eng. 2025, 69, 106754. [Google Scholar] [CrossRef]
- Altalhi, A.A.; Mohamed, E.A.; Negm, N.A. Recent advances in layered double hydroxide (LDH)-based materials: Fabrication, modification strategies, characterization, promising environmental catalytic applications, and prospective aspects. Energy Adv. 2024, 3, 2136–2151. [Google Scholar] [CrossRef]
- Musella, E.; Gualandi, I.; Giorgetti, M.; Scavetta, E.; Basile, F.; Rivalta, A.; Venuti, E.; Corticelli, F.; Christian, M.; Morandi, V.; et al. Electrosynthesis and characterization of Layered Double Hydroxides on different supports. Appl. Clay Sci. 2021, 202, 105949. [Google Scholar] [CrossRef]
- Naseem, S.; Gevers, B.; Boldt, R.; Labuschagné, F.J.W.J.; Leuteritz, A. Comparison of transition metal (Fe, Co, Ni, Cu, and Zn) containing tri-metal layered double hydroxides (LDHs) prepared by urea hydrolysis. RSC Adv. 2019, 9, 3030–3040. [Google Scholar] [CrossRef]
- Li, J.; Xu, Y.; Ding, Z.; Mahadi, A.H.; Zhao, Y.; Song, Y.F. Photocatalytic selective oxidation of benzene to phenol in water over layered double hydroxide: A thermodynamic and kinetic perspective. Chem. Eng. J. 2020, 388, 124248. [Google Scholar] [CrossRef]
- Di Michele, A.; Boccalon, E.; Costantino, F.; Bastianini, M.; Vivani, R.; Nocchetti, M. Insight into the synthesis of LDH using the urea method: Morphology and intercalated anion control. Dalton Trans. 2024, 53, 12543–15253. [Google Scholar] [CrossRef] [PubMed]
- Bukhtiyarova, M.V. A review on effect of synthesis conditions on the formation of layered double hydroxides. J. Solid. State Chem. 2019, 269, 494–506. [Google Scholar] [CrossRef]
- Cavani, F.; Trifirb, F.; Vaccari, A. Hydrotalcite-type anionic clays: Preparation, properties and applications. Catal. Today 1999, 11, 173–301. [Google Scholar] [CrossRef]
- Bencherif, S.D.; Gallardo, J.J.; Carrillo-Berdugo, I.; Bahmani, A.; Navas, J. Synthesis, characterization and photocatalytic performance of calcined ZnCr-layered double hydroxides. Nanomaterials 2021, 11, 3051. [Google Scholar] [CrossRef]
- Lee, J.Y.; Gwak, G.H.; Kim, H.M.; Kim, T.-I.; Lee, G.J.; Oh, J.M. Synthesis of hydrotalcite type layered double hydroxide with various Mg/Al ratio and surface charge under controlled reaction condition. Appl. Clay Sci. 2016, 134, 44–49. [Google Scholar] [CrossRef]
- Belskaya, O.B.; Terekhova, E.N.; Gorbunova, O.V.; Muromtsev, I.V.; Trenikhin, M.V.; Salanov, A.N.; Likholobov, V. Synthesis of CuAl-LDHs by Co-Precipitation and Mechanochemical Methods and Selective Hydrogenation Catalysts Based on Them. Inorganics 2023, 11, 247. [Google Scholar] [CrossRef]
- Wei, C.; Yan, X.; Zhou, Y.; Xu, W.; Gan, Y.; Zhang, Y.; Zhang, N. Morphological Control of Layered Double Hydroxides Pre-pared by Co-Precipitation Method. Crystals 2022, 12, 1713. [Google Scholar] [CrossRef]
- Crepaldi, E.L.; Pavan, P.C.; Valim, J.B. Comparative Study of the Coprecipitation Methods for the Preparation of Layered Double Hydroxides. J. Braz. Chem. Soc. 2000, 11, 64–70. [Google Scholar] [CrossRef]
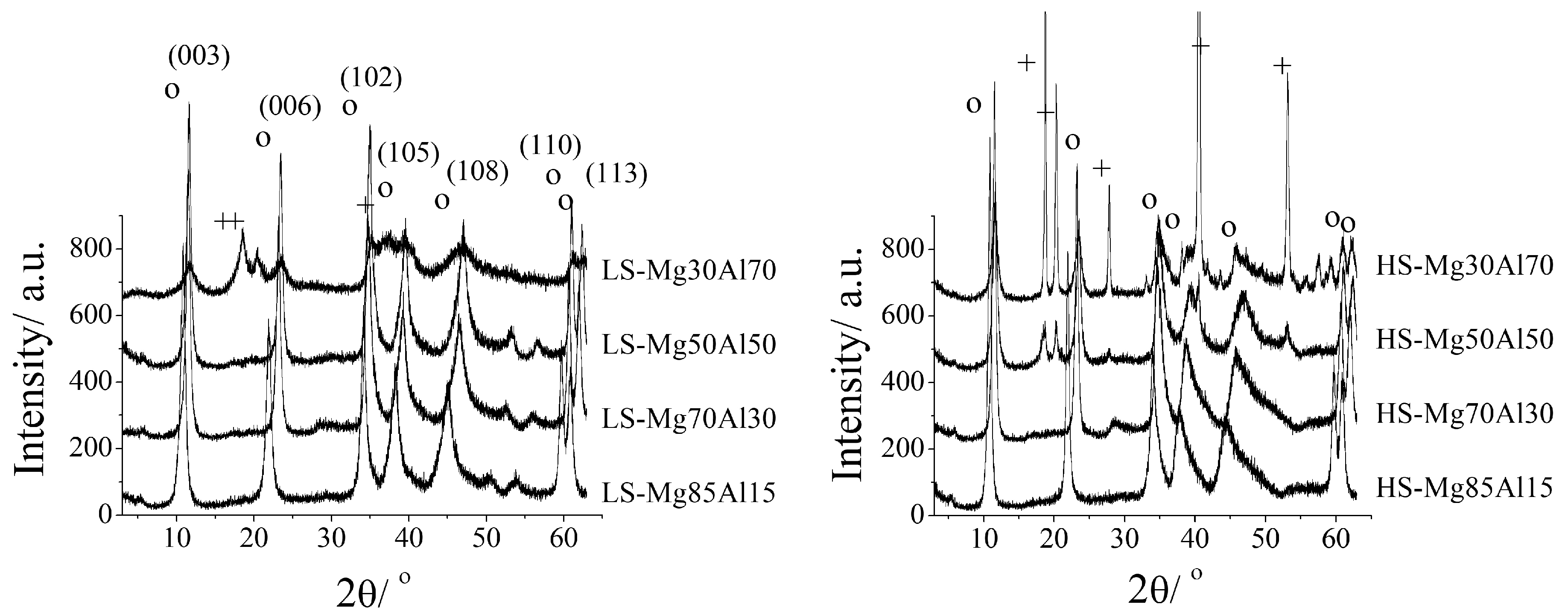
- Karanović, Đ.M.; Hadnađev-Kostić, M.S.; Vulić, T.J.; Milanović, M.M.; Rajaković-Ognjanović, V.N.; Marinković-Nedučin, R.P. The influence of the coprecipitation synthesis methods on photodegradation efficiency of ZnFe based photo-catalysts. J. Serb. Chem. Soc. 2024, 89, 667–678. [Google Scholar] [CrossRef]
- Vulic, T.; Reitzmann, A.; Ranogajec, J.; Marinkovic-Neducin, R. The influence of synthesis method and Mg-Al-Fe content on the thermal stability of layered double hydroxides. J. Therm. Anal. Calorim. 2012, 110, 227–233. [Google Scholar] [CrossRef]
- Mittal, J. Recent progress in the synthesis of Layered Double Hydroxides and their application for the adsorptive removal of dyes: A review. J. Environ. Manag. 2021, 295, 113017. [Google Scholar] [CrossRef] [PubMed]
- Chagas, L.H.; De Carvalho, G.S.G.; Do Carmo, W.R.; San Gil, R.A.S.; Chiaro, S.S.X.; Leitão, A.A.; Diniz, R.; De Sena, L.A.; Achete, C.A. MgCoAl and NiCoAl LDHs synthesized by the hydrothermal urea hydrolysis method: Structural characterization and thermal de-composition. Mater. Res. Bull. 2015, 64, 207–215. [Google Scholar] [CrossRef]
- Chaillot, D.; Bennici, S.; Brendlé, J. Layered double hydroxides and LDH-derived materials in chosen environmental applications: A review. Environ. Sci. Pollut. 2021, 28, 24375–24405. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Liang, H.; Zhang, Z.; Zhang, Q.; Han, Q.; Wang, J.; Gao, M.; Fan, H.; Yang, J.; Lang, J. The photocatalytic degradation and mechanism of rhodamine B by Zn–Al layered double hydroxide. Opt. Mater. 2022, 131, 112636. [Google Scholar] [CrossRef]
- Bao, S.; Shi, Y.; Zhang, Y.; He, L.; Yu, W.; Chen, Z.; Wu, Y.; Li, L. Study on the efficient removal of azo dyes by heterogeneous photo-Fenton process with 3D flower-like layered double hydroxide. Water Sci. Technol. 2020, 81, 2368–2380. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, Y.; Li, J.; Song, W.; Li, X.; Yan, L.; Yu, H. Highly efficient removal of aqueous Cu(II) and Cd(II) by hydrothermal synthesized CaAl-layered double hydroxide. Colloids Surf. A Physicochem. Eng. Asp. 2022, 641, 128584. [Google Scholar] [CrossRef]
- Hadnadjev-Kostic, M.; Vulic, T.; Marinkovic-Neducin, R. Solar light induced rhodamine B degradation assisted by TiO2-Zn-Al LDH based photocatalysts. Adv. Powder Technol. 2014, 25, 1624–1633. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, C.; Rad, S.; He, H.; Qin, L. A Comprehensive Review of Layered Double Hydroxide-Based Carbon Composites as an Environmental Multifunctional Material for Wastewater Treatment. Processes 2022, 10, 617. [Google Scholar] [CrossRef]
- Mutahir, S.; Khan, M.A.; Noor, W.; Butt, R.; Elkholi, S.M.; Bououdina, M.; Alsuhaibani, A.M.; Munawar, K.S.; Humayun, M. Oxygen doped g-C3N4/LDH composite as highly efficient photocatalyst for wastewater treatment. Z. Phys. Chem. 2024, 238, 2183–2197. [Google Scholar] [CrossRef]
- Niknam, M.; Vandchali, M.B.; Ghasemi, E.; Kazemi, A.; Yousefi-Limaee, N. Synthesis and characterization of a novel g-C3N4/NiAl-LDH/CeO2 photocatalyst for degradation of rhodamine B. Int. J. Environ. Sci. Technol. 2025, 22, 4215–4228. [Google Scholar] [CrossRef]
- Aadil, M.; Safira, A.R.; Alkaseem, M.; Choi, T.; Kaseem, M. Transformative chelation pathways unveiling NiMOF-LDH hybrids on MgO for high-efficiency photocatalysis. J. Mater. Chem. A 2025, 13, 8526–8540. [Google Scholar] [CrossRef]
- Luo, Y.; Shi, G.; Yu, S.; Liu, Z.; Yin, J.; Xue, M.; Sun, Q.; Shen, F.-F.; Li, X.; Yin, Z.; et al. Novel MIL-88B(Fe)/ZnTi-LDH high-low junctions for adsorption and photodegradation of tetracycline: Characteristics, performance, and mechanisms. Chem. Eng. J. 2023, 473, 145198. [Google Scholar] [CrossRef]
- Soltani, R.; Pelalak, R.; Pishnamazi, M.; Marjani, A.; Albadarin, A.B.; Sarkar, S.M.; Shirazian, S. A novel and facile green synthesis method to prepare LDH/MOF nanocomposite for removal of Cd(II) and Pb(II). Sci. Rep. 2021, 11, 1609. [Google Scholar] [CrossRef]
- Abo El-Reesh, G.Y.; Farghali, A.A.; Taha, M.; Mahmoud, R.K. Novel synthesis of Ni/Fe layered double hydroxides us-ing urea and glycerol and their enhanced adsorption behavior for Cr(VI) removal. Sci. Rep. 2020, 10, 587. [Google Scholar] [CrossRef]
- Morimoto, K.; Tamura, K.; Iyi, N.; Ye, J.; Yamada, H. Adsorption and photodegradation properties of anionic dyes by layered double hydroxides. J. Phys. Chem. Solids 2011, 72, 1037–1045. [Google Scholar] [CrossRef]
- Satyam, S.; Patra, S. Innovations and challenges in adsorption-based wastewater remediation: A comprehensive review. Heliyon 2024, 10, e29573. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.; Chen, B.; Xu, Y.; Wang, H.; Jin, F.; Shen, Z.; Hou, D. Green synthesis of layered double hydroxides (LDH) for the re-mediation of As and Cd in water and soil. Appl. Clay Sci. 2024, 249, 107262. [Google Scholar] [CrossRef]
- Benhiti, R.; Ait Ichou, A.; Aboussabek, A.; Carja, G.; Zerbet, M.; Sinan, F.; Chiban, M. Efficient removal of Cr (VI) from aqueous solution using memory effect property of layered double hydroxide material. Chemosphere 2023, 341, 140127. [Google Scholar] [CrossRef]
- Mudhoo, A.; Ramasamy, D.L.; Bhatnagar, A.; Usman, M.; Sillanpää, M. An analysis of the versatility and effectiveness of composts for sequestering heavy metal ions, dyes and xenobiotics from soils and aqueous milieus. Ecotoxicol. Environ. Saf. 2020, 197, 110587. [Google Scholar] [CrossRef] [PubMed]
- Bharali, D.; Deka, R.C. Adsorptive removal of congo red from aqueous solution by sonochemically synthesized NiAl layered double hydroxide. J. Environ. Chem. Eng. 2017, 5, 2056–2067. [Google Scholar] [CrossRef]
- Farghali, M.A.; Selim, A.M.; Khater, H.F.; Bagato, N.; Alharbi, W.; Alharbi, K.H.; Radwan, I.T. Optimized adsorption and effective disposal of Congo red dye from wastewater: Hydrothermal fabrication of MgAl-LDH nanohydrotalcite-like materials. Arab. J. Chem. 2022, 15, 104171. [Google Scholar] [CrossRef]
- Abdelkawy, A.M.; Elantabli, F.M.; Mahmoud, R.; Mahgoub, S.M.; El-Ela, F.I.A.; Mohamed, H.A.; Abdel-Moaty, S.A. Enhanced Zn-Co-Fe Layered Double Hydroxides for Effective Levofloxacin Removal: Innovation in Reuse of Waste Adsorbent. J. Pharm. Innov. 2025, 20, 86. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, L.; Zhang, C.; Shang, D.; Wu, L. Preparation of ZnAl layered double hydroxides supported by silica for the treatment of Cr(VI) and Cu(II) in aqueous solution. Sci. Rep. 2025, 15, 2522. [Google Scholar] [CrossRef]
- Adim, S.; Eddahmi, M.; Boussetta, A.; Mansori, M.; Essoumhi, A.; Bouissane, L. Green four-component reaction and dye adsorption studies of new ZnCoAl-LDH and ZnCoCr-LDH materials. J. Organomet. Chem. 2025, 1036, 123719. [Google Scholar] [CrossRef]
- Dai, X.; Jing, C.; Li, K.; Zhang, X.; Song, D.; Feng, L.; Liu, X.; Ding, H.; Ran, H.; Zhu, K.; et al. Enhanced bifunctional adsorption of anionic and cationic pollutants by MgAl LDH nanosheets modified montmorillonite via acid-salt activation. Appl. Clay Sci. 2023, 233, 106815. [Google Scholar] [CrossRef]
- Mahmoud, R.K.; Taha, M.; Zaher, A.; Amin, R.M. Understanding the physicochemical properties of Zn–Fe LDH nanostructure as sorbent material for removing of anionic and cationic dyes mixture. Sci. Rep. 2021, 11, 21365. [Google Scholar] [CrossRef]
- Al-Furhud, S.F.; Mohamed, R.M.K.; Alsohaimi, I.; Kamel, M.M.; El-sayed, M.Y.; Youssef, H.M.; Aldawsari, A.M.; Hassan, H.M.A. Adsorption of light green dye using Mg–Al-layered double hydroxides (LDHs) with carbonate and nitrate anions. Int. J. Environ. Sci. Technol. 2025, 22, 14015–14032. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Brick, A.A.; Mohamed, A.A. An efficient adsorption of indigo carmine dye from aqueous solution on mesoporous Mg/Fe layered double hydroxide nanoparticles prepared by controlled sol-gel route. Chemosphere 2017, 174, 280–288. [Google Scholar] [CrossRef]
- Missau, J.; Bertuol, D.A.; Tanabe, E.H. Highly efficient adsorbent for removal of Crystal Violet Dye from Aqueous Solution by CaAl/LDH supported on Biochar. Appl. Clay Sci. 2021, 214, 106297. [Google Scholar] [CrossRef]
- Abdel-Hady, E.E.; Mohamed, H.F.M.; Hafez, S.H.M.; Fahmy, A.M.M.; Magdy, A.; Mohamed, A.S.; Ali, E.O.; Abdelhamed, H.R.; Mahmoud, O.M. Textural properties and adsorption behavior of Zn–Mg–Al layered double hydroxide upon crystal violet dye removal as a low cost, effective, and recyclable adsorbent. Sci. Rep. 2023, 13, 6435. [Google Scholar] [CrossRef]
- Ramezani, A.M.; Amiri, P.F.; Heydari, D.M.; Adnan, A.E.; Ahmadi, R.; Nazari, S. Zn/Ce-layered double hydroxide for adsorptive removal of doxycycline from water. Mater. Chem. Phys. 2024, 318, 139223. [Google Scholar] [CrossRef]
- Manzar, M.S.; Palaniandy, P.; Georgin, J.; Franco, D.S.P.; Zubair, M.; Muazu, N.D.; Faisal, W.; El Messaoudi, N. Synthesis of LDH-MgAl and LDH-MgFe composites for the efficient removal of the antibiotic from water. Environ. Sci. Pollut. Res. 2024, 31, 55577–55596. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Guang, Z.; Wang, Z.; Liu, Y.; Cheng, X.; Liu, Y. Robust Adsorption of Pb(II) and Cd(II) by GLDA-Intercalated ZnAl-LDH: Structural Engineering, Mechanistic Insights, and Environmental Applications. Coatings 2025, 15, 613. [Google Scholar] [CrossRef]
- Aita, S.A.; Mahmoud, R.; Hafez, S.H.M.; Zaher, A. Investigating adsorption of aqueous heavy metals through isotherms and kinetics with Zn-Co-Fe/LDH for remarkable removal efficiency. Appl. Water Sci. 2025, 15, 75. [Google Scholar] [CrossRef]
- Huo, J.; Min, X.; Dong, Q.; Xu, S.; Wang, Y. Comparison of Zn–Al and Mg–Al layered double hydroxides for adsorption of perfluorooctanoic acid. Chemosphere 2022, 287, 133297. [Google Scholar] [CrossRef]
- Chen, Y.; Georgi, A.; Zhang, W.; Kopinke, F.D.; Yan, J.; Saeidi, N.; Li, J.; Gu, M.; Chen, M. Mechanistic insights into fast adsorption of per-fluoroalkyl substances on carbonate-layered double hydroxides. J. Hazard. Mater. 2021, 408, 124815. [Google Scholar] [CrossRef]
- Tiwari, E.; Singh, N.; Khandelwal, N.; Monikh, F.A.; Darbha, G.K. Application of Zn/Al layered double hydroxides for the removal of nano-scale plastic debris from aqueous systems. J. Hazard. Mater. 2020, 397, 122769. [Google Scholar] [CrossRef]
- Zhang, P.; Zeng, B.; Zhang, J.; Wang, T.; Zou, Y.; Xie, B.; Song, Z.; Mao, Y. Enhanced removal of polystyrene microplastics by three-dimensional flower-like layered double oxide: Behavior and mechanism insight. J. Environ. Chem. Eng. 2024, 12, 114237. [Google Scholar] [CrossRef]
- Salleh, M.A.M.; Mahmoud, D.K.; Karim, W.A.W.A.; Idris, A. Cationic and anionic dye adsorption by agricultural solid wastes: A comprehensive review. Desalination 2011, 280, 1–13. [Google Scholar] [CrossRef]
- Starukh, H.; Levytska, S. The simultaneous anionic and cationic dyes removal with Zn–Al layered double hydroxides. Appl. Clay Sci. 2019, 180, 105183. [Google Scholar] [CrossRef]
- dos Santos, R.M.M.; Gonçalves, R.G.L.; Constantino, V.R.L.; Santilli, C.V.; Borges, P.D.; Tronto, J.; Pinto, F.G. Adsorption of Acid Yellow 42 dye on calcined layered double hydroxide: Effect of time, concentration, pH and temperature. Appl. Clay Sci. 2017, 140, 132–139. [Google Scholar] [CrossRef]
- Lei, C.; Pi, M.; Kuang, P.; Guo, Y.; Zhang, F. Organic dye removal from aqueous solutions by hierarchical calcined Ni-Fe layered double hydroxide: Isotherm, kinetic and mechanism studies. J. Colloid Interface Sci. 2017, 496, 158–166. [Google Scholar] [CrossRef]
- Gidado, S.M.; Akanyeti, İ. Comparison of Remazol Brilliant Blue Reactive Adsorption on Pristine and Calcined ZnAl, MgAl, ZnMgAl Layered Double Hydroxides. Water Air Soil. Pollut. 2020, 231, 146. [Google Scholar] [CrossRef]
- Santamaría, L.; López-Aizpún, M.; García-Padial, M.; Vicente, M.A.; Korili, S.A.; Gil, A. Zn-Ti-Al layered double hydroxides synthesized from aluminum saline slag wastes as efficient drug adsorbents. Appl. Clay Sci. 2020, 187, 105486. [Google Scholar] [CrossRef]
- Gaskell, E.E.; Ha, T.; Hamilton, A.R. Ibuprofen intercalation and release from different layered double hydroxides. Ther. Deliv. 2018, 9, 653–666. [Google Scholar] [CrossRef]
- Zhao, S.; Meng, Z.; Fan, X.; Jing, R.; Yang, J.; Shao, Y.; Liu, X.; Wu, M.; Zhang, Q.; Liu, A. Removal of heavy metals from soil by vermiculite supported layered double hydroxides with three-dimensional hierarchical structure. Chem. Eng. J. 2020, 390, 124554. [Google Scholar] [CrossRef]
- Lyu, F.; Yu, H.; Hou, T.; Yan, L.; Zhang, X.; Du, B. Efficient and fast removal of Pb2+ and Cd2+ from an aqueous solution using a chitosan/Mg-Al-layered double hydroxide nanocomposite. J. Colloid Interface Sci. 2019, 539, 184–193. [Google Scholar] [CrossRef]
- Shadreck, M.; Mugadza, T. Chromium, an essential nutrient and pollutant: A review. Afr. J. Pure Appl. Chem. 2013, 7, 310–317. [Google Scholar]
- Yan, H.; Chen, Q.; Lin, Y.; Zu, Y.; Zhang, J.; Feng, T.; He, S.; Liao, X. New insights into removal efficacy of perfluoroalkyl substances by layered double hydroxide and its composite materials. Chem. Eng. J. Adv. 2024, 19, 100636. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, C.; Song, X.; Tang, Z.; Li, Y.; Zhang, M. Rapid and effective removal of PFOS from water using ultrathin MgAl-LDH nanosheets with defects. Chem. Eng. J. 2025, 505, 158998. [Google Scholar] [CrossRef]
- Yang, C.; Xiong, R.; Zeng, B.; Wang, T.; Zhang, P.; Zhang, W.; You, L.; Zeng, B.; Song, Z. Three-dimensional superparamagnetic layered double metal oxides for efficient adsorption of polystyrene microplastics. Sep. Purif. Technol. 2025, 371, 133357. [Google Scholar] [CrossRef]
- Ramalingam, G.; Perumal, N.; Priya, A.K.; Rajendran, S. A review of graphene-based semiconductors for photocatalytic degradation of pollutants in wastewater. Chemosphere 2022, 300, 134391. [Google Scholar] [CrossRef] [PubMed]
- Nemiwal, M.; Zhang, T.C.; Kumar, D. Recent progress in g-C3N4, TiO2 and ZnO based photocatalysts for dye degradation: Strategies to improve photocatalytic activity. Sci. Total Environ. 2021, 767, 144896. [Google Scholar] [CrossRef]
- Djurišić, A.B.; Leung, Y.H.; Ching, A.M. Strategies for improving the efficiency of semiconductor metal oxide photocatalysis. Mater. Horiz. 2014, 1, 400–410. [Google Scholar] [CrossRef]
- Keyikoğlu, R.; Doğan, I.N.; Khataee, A.; Orooji, Y.; Kobya, M.; Yoon, Y. Synthesis of visible light responsive ZnCoFe lay-ered double hydroxide towards enhanced photocatalytic activity in water treatment. Chemosphere 2022, 309, 136534. [Google Scholar] [CrossRef]
- Dostanić, J.; Lončarević, D.; Hadnađev-Kostić, M.; Vulić, T. Recent Advances in the Strategies for Developing and Modifying Photocatalytic Materials for Wastewater Treatment. Processes 2024, 12, 1914. [Google Scholar] [CrossRef]
- Luo, L.; Hou, C.; Wang, L.; Zhang, W.; Wang, C.; Liu, J.; Wu, Y.; Wang, C. Layered Double Hydroxide-Based Photocatalysts for the Removal of Emerging Contaminants: Progress in Past Ten Years. Catalysts 2024, 14, 252. [Google Scholar] [CrossRef]
- Sahoo, D.P.; Patnaik, S.; Parida, K. An amine functionalized ZnCr LDH/MCM-41 nanocomposite as efficient visible light induced photocatalyst for Cr(VI) reduction. Mater. Today Proc. 2021, 35, 252–257. [Google Scholar] [CrossRef]
- Karim, A.V.; Hassani, A.; Eghbali, P.; Nidheesh, P.V. Nanostructured modified layered double hydroxides (LDHs)-based catalysts: A review on synthesis, characterization, and applications in water remediation by advanced oxidation processes. Curr. Opin. Solid State Mater. Sci. 2022, 26, 100965. [Google Scholar] [CrossRef]
- Sun, H.; Heo, Y.J.; Park, J.H.; Rhee, K.Y.; Park, S.J. Advances in layered double hydroxide-based ternary nanocomposites for photocatalysis of contaminants in water. Nanotechnol. Rev. 2021, 9, 1381–1396. [Google Scholar] [CrossRef]
- Armenise, S.; García-Bordejé, E.; Valverde, J.L.; Romeo, E.; Monzón, A. A Langmuir-Hinshelwood approach to the kinetic modelling of catalytic ammonia decomposition in an integral reactor. Phys. Chem. Chem. Phys. 2013, 15, 12104–12117. [Google Scholar] [CrossRef] [PubMed]
- Salehi, G.; Bagherzadeh, M.; Abazari, R.; Hajilo, M.; Taherinia, D. Visible Light-Driven Photocatalytic Degradation of Methylene Blue Dye Using a Highly Efficient Mg-Al LDH@g-C3N4@Ag3PO4 Nanocomposite. ACS Omega 2024, 9, 4581–4593. [Google Scholar] [CrossRef] [PubMed]
- Castro, L.V.; Alcántar-Vázquez, B.; Manríquez, M.E.; Albiter, E.; Ortiz-Islas, E. Photodegradation of Emerging Pollutants Using a Quaternary Mixed Oxide Catalyst Derived from Its Corresponding Hydrotalcite. Catalysts 2025, 15, 173. [Google Scholar] [CrossRef]
- Hanifah, Y.; Mohadi, R.; Mardiyanto, M.; Lesbani, A. Photocatalytic Degradation of Malachite Green by NiAl-LDH Intercalated Polyoxometalate Compound. Bull. Chem. React. Eng. Catal. 2022, 17, 627–637. [Google Scholar] [CrossRef]
- Di, G.; Zhu, Z.; Zhang, H.; Zhu, J.; Lu, H.; Zhang, W.; Qiu, Y.; Zhu, L.; Kuppers, S. Simultaneous removal of several pharmaceuticals and arsenic on Zn-Fe mixed metal oxides: Combination of photocatalysis and adsorption. Chem. Eng. J. 2017, 328, 141–151. [Google Scholar] [CrossRef]
- Despotović, V.; Hadnađev-Kostić, M.; Vulić, T.; Bognár, S.; Karanović, Đ.; Tot, N.; Šojić-Merkulov, D. Utilizing Zn(Cu/Cr)Al-Layered Double Hydroxide-Based Photocatalysts for Effective Photodegradation of Environmental Pollutants. Separations 2024, 11, 308. [Google Scholar] [CrossRef]
- Wang, J.H.; Kong, F.; Liu, B.F.; Zhuo, S.N.; Ren, N.Q.; Ren, H.Y. Enhanced photocatalytic degradation of diclofenac by UiO-66/MgAl-LDH: Excellent performances and mechanisms. Environ. Sci. Nano 2024, 11, 3286–3293. [Google Scholar] [CrossRef]
- Jiang, S.; Yin, M.; Ren, H.; Qin, Y.; Wang, W.; Wang, Q.; Li, X. Novel CuMgAlTi-LDH Photocatalyst for Efficient Degradation of Microplastics under Visible Light Irradiation. Polymers 2023, 15, 2347. [Google Scholar] [CrossRef]
- Chen, S.; Yang, F.; Cao, Z.; Yu, C.; Wang, S.; Zhong, H. Enhanced photocatalytic activity of molybdenum disulfide by compositing ZnAl–LDH. Colloids Surf. A Physicochem. Eng. Asp. 2020, 586, 124140. [Google Scholar] [CrossRef]
- Hu, Z.; Chen, C.; O’Hare, D.; Lei, J.; Liu, J. Recent Advances in Photocatalysis of Layered Double Hydroxide-Biochar Composites. ACS Mater. Lett. 2025, 7, 2732–2748. [Google Scholar] [CrossRef]
- Kishore, G.; Pritha, P.; Xavier, S.; Bhakiaraj, D.; Paularokiadoss, F.; Celaya, C.A.; Khan, M.M. Synergistic effects of Bi-metal oxide–graphene oxide nanocomposites in photodegradation applications. Next Nanotechnol. 2025, 8, 100208. [Google Scholar] [CrossRef]
- Al-Ammari, R.H.; Al-Malwi, S.D.; Abdel-Fadeel, M.A.; Bawaked, S.M.; Mostafa, M.M.M. Zn-Layered Double Hydroxide Intercalated with Graphene Oxide for Methylene Blue Photodegradation and Acid Red Adsorption Studies. Catalysts 2024, 14, 897. [Google Scholar] [CrossRef]
- Su, W.; Zhong, Y.; Ye, Q.; Wu, J.; He, D.; Wu, W.; Wu, P. Photoaging process of polyethylene microplastics mediated by layered double hydroxide: Experiments and density functional theory calculations. Colloids Surf. A 2025, 726, 137883. [Google Scholar] [CrossRef]
- Wu, F.; Qin, J.; Yin, B.; Zhang, Y.; Li, C.; Dou, Y.; Helix-Nielsen, C.; Zhang, W. In-and-out of inert sites on high-entropy layered double hydroxide to facilitate peroxymonosulfate-assisted photocatalytic removal of microplastics. Appl. Catal. B 2025, 365, 124853. [Google Scholar] [CrossRef]
- Sarkar, S.; Upadhyay, C. Layered double hydroxides for industrial wastewater remediation: A review. Catal. Today 2025, 445, 115101. [Google Scholar] [CrossRef]
- Dadakhani, S.; Dehghan, G.; Khataee, A.; Erfanparast, A. Design and application of histidine-functionalized ZnCr-LDH nanozyme for promoting bacteria-infected wound healing. RSC Adv. 2024, 14, 1195–1206. [Google Scholar] [CrossRef]
- Markov, S.L.; Vidaković, A.M. Testing methods for antimicrobial activity of TiO2 photocatalyst. Acta Period. Technol. 2014, 45, 141–152. [Google Scholar] [CrossRef]
- Janani, F.Z.; Taoufik, N.; Khiar, H.; Boumya, W.; Elhalil, A.; Sadiq, M.; Puga, A.V.; Barka, N. Nanostructured layered double hydroxides based photocatalysts: Insight on synthesis methods, application in water decontamination/splitting and antibacterial activity. Surf. Interfaces 2021, 25, 101263. [Google Scholar] [CrossRef]
- Wang, W.; Huang, G.; Yu, J.C.; Wong, P.K. Advances in photocatalytic disinfection of bacteria: Development of photocatalysts and mechanisms. J. Environ. Sci. 2015, 34, 232–247. [Google Scholar] [CrossRef] [PubMed]
- Gautam, R.K.; Sharma, S.K.; Mahiya, S.; Chattopadhyaya, M.C. Contamination of Heavy Metals in Aquatic Media: Transport, Toxicity and Technologies for Remediation. In Heavy Metals in Water: Presence, Removal and Safety; Sharma, S., Ed.; The Royal Society of Chemistry: London, UK, 2014; pp. 1–366. [Google Scholar] [CrossRef]
- Pasquet, J.; Chevalier, Y.; Pelletier, J.; Couval, E.; Bouvier, D.; Bolzinger, M.A. The contribution of zinc ions to the antimicrobial activity of zinc oxide. Colloids Surf. A 2014, 457, 263–274. [Google Scholar] [CrossRef]
- Almoudi, M.M.; Hussein, A.S.; Abu Hassan, M.I.; Mohamad, Z.N. A systematic review on antibacterial activity of zinc against Streptococcus mutans. Saudi Dent. J. 2018, 30, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Elrafey, A.; Abdel Moaty, S.A.; Arafa, E.G.; Abdel Gawad, O.F.; Kamel, M.M.; Abdel Aziz, S.A.A.; Allam, A.A.; Alfassam, H.E.; Mahmoud, R. Removal of ciprofloxacin from water: Antimicrobial activity and desalination potential using synthetic Ni/Fe clay. Int. J. Environ. Sci. Techol. 2025, 22, 14451–14478. [Google Scholar] [CrossRef]
- Abdel Aziz, S.A.A.; GadelHak, Y.; Mohamed, M.B.E.D.; Mahmoud, R. Antimicrobial properties of promising Zn–Fe based layered double hydroxides for the disinfection of real dairy wastewater effluents. Sci. Rep. 2023, 13, 7601. [Google Scholar] [CrossRef]
- Amin, R.M.; Taha, M.; Abdel Moaty, S.A.; Abo El-Ela, F.I.; Nassar, H.F.; Gadelhak, Y.; Mahmoud, R.K. Gamma radiation as a green method to enhance the dielectric behaviour, magnetization, antibacterial activity and dye removal capacity of Co-Fe LDH nanosheets. RSC Adv. 2019, 9, 32544–32561. [Google Scholar] [CrossRef]
- Kotta, S.; Aldawsari, H.M.; Alhakamy, N.A.; Elfaky, M.A.; Badr-Eldin, S.M. Synthesis, Structural Characterization, and Antimicrobial Evaluation of Silver Nanoparticle-embedded Layered Double Hydroxides for Delivery in Polymeric Hydrogel Matrices. J. Pharm. Innov. 2024, 19, 80. [Google Scholar] [CrossRef]
- Balcik, C.; Ozbey-Unal, B.; Sahin, B.; Buse Aydın, E.; Cifcioglu-Gozuacik, B.; Keyikoglu, R.; Khataee, A. Development of ZnFeCe Layered Double Hydroxide Incorporated Thin Film Nanocomposite Membrane with Enhanced Separation Performance and Antibacterial Properties. Water 2023, 15, 264. [Google Scholar] [CrossRef]
- Wang, Z.; Fang, P.; Kumar, P.; Wang, W.; Liu, B.; Li, J. Controlled growth of LDH films with enhanced photocatalytic activity in a mixed wastewater treatment. Nanomaterials 2019, 9, 807. [Google Scholar] [CrossRef]
- Batool, I.; Aroob, S.; Anwar, F.; Taj, M.B.; Baamer, D.F.; Almasoudi, A.; Ali, O.M.; Aldahiri, R.H.; Alsulami, F.M.H.; Khan, M.I. Synergistic Effect of NiAl-Layered Double Hydroxide and Cu-MOF for the Enhanced Photocatalytic Degradation of Methyl Orange and Antibacterial Properties. Catalysts 2024, 14, 719. [Google Scholar] [CrossRef]
- Gao, R.; Gao, S.H.; Li, J.; Su, Y.; Huang, F.; Liang, B.; Fan, L.; Guo, J.; Wang, A. Emerging Technologies for the Control of Biological Contaminants in Water Treatment: A Critical Review. Engineering 2025, 48, 185–204. [Google Scholar] [CrossRef]
- Raghupathi, K.R.; Koodali, R.T.; Manna, A.C. Size-dependent bacterial growth inhibition and mechanism of antibacterial activity of zinc oxide nanoparticles. Langmuir 2011, 27, 4020–4028. [Google Scholar] [CrossRef]
- Kim, S.; Park, H.; Pandey, S.; Jeong, D.; Lee, C.T.; Do, J.Y.; Park, S.M.; Kang, M. Effective Antibacterial/Photocatalytic Activity of ZnO Nanomaterials Synthesized under Low Temperature and Alkaline Conditions. Nanomaterials 2022, 12, 4417. [Google Scholar] [CrossRef]
| Synthesis Method | Advantages | Disadvantages |
|---|---|---|
| Co-precipitation |
|
|
| Urea method |
|
|
| Hydrothermal method |
|
|
| Functionalization/Composites |
|
|
| LDH | Pollutant | Adsorption Kinetics | Adsorption Mechanism | Ref. |
|---|---|---|---|---|
| NiAl LDH—S | Congo red | PSO | H-bonding and electrostatic attraction | [65] |
| ZnCoCr LDH | Congo red | PSO | Electrostatic attractions | [69] |
| MgAl LDH nanosheet-modified montomorillonite | Methyl orange, Methylene blue | PSO | Intercalation-split mechanism, ion exchange | [70] |
| MgAl LDH nanosorbent | Congo red | PSO | Electrostatic interactions, surface complexation | [67] |
| ZnFe LDH | Methyl orange | Fits well into several models | Electrostatic interactions, H-bonding | [71] |
| MgAl-NO3 LDH MgAl-CO3 LDH | Light green dye | PSO | Electrostatic interactions, H-bonding | [72] |
| MgFe LDH nanoparticles | Indigo carmine | PSO | Interactions between solid and dye molecules | [73] |
| CaAl LDH/biochar | Crystal violet | PSO | Electrostatic interactions | [74] |
| ZnMgAl LDH | Crystal violet | PSO | Hydrogen bonding, electrostatic attraction, n–π interaction, π–π interaction, mesoporous filling | [75] |
| La-, Mo-, W-doped NiFe LDH | Methyl orange | PSO | Anion exchange and the attraction of layer charge | [25] |
| MIL 88B (Fe)/ZnTi LDH (MZ 30%) | Tetracycline | PSO | External mass transfer and internal particle diffusion | [57] |
| ZnCoFe LDH | Levofloxacin | Fits well into several models | Electrostatic interactions, hydrogen bonding, surface complexation, ion exchange, π–π stacking, and redox activity | [67] |
| Zn/Ce LDH | Doxycyline | PSO | Interactions of doxycycline molecules with Zn cations | [76] |
| LDH MgAl/MgFe composites | Tetracycline | PSO | Chemical and π–π interactions | [77] |
| MgAl LDH/cellulose nanocomposite | Amoxicillin | PSO | Electrostatic interactions | [21] |
| GLDA-ZnAl LDH | Pb(II), Cd(II) | PSO | Chemisorption, metal-LDH interactions | [78] |
| NiFe LDH/glycerol | Cr (VI) | Fits well into several models | Electrostatic attraction, ion exchange, complexation, hydrogen bond formation | [59] |
| ZnAl LDH/silica | Cr (VI) | PSO | Intercalation of Cr2O72− and exchange between Cr2O72− and OH− | [68] |
| NiCo LDH/MOF nanocomposite | Pb(II), Cd(II) | Combination of PFO and PSO | Surface complexation, cation–π interactions | [58] |
| ZnCoFe LDH | As3+, Pb2+, Hg2+ | PSO | Non-electrostatic attraction, ion exchange, surface complexation | [79] |
| MgAl LDH calcined MgAl LDH | Cr (VI) | PSO | Electrostatic attractions, intercalation | [63] |
| ZnAl LDH, MgAl LDH | PFOA | PSO | Electrostatic interactions | [80] |
| CuMgFe-LDH | PFOA, PFOS | - | Electrostatic interactions, hydrophobic interactions | [81] |
| nanostructured ZnAl LDHs | Polystyrene nanoplastics | - | Electrostatic interactions | [82] |
| calcined ZnAl LDH | Polystyrene nanoplastics | Intraparticle diffusion model | Electrostatic interactions, hydrogen bonds, pore filling and complexation | [83] |
| LDH | Pollutant | Reaction Kinetics | Light Source | Energy Band Gap (eV) | Ref. |
|---|---|---|---|---|---|
| ZnAl LDH | Rhodamine B | PFO | UV light | - | [49] |
| ZnCoFe LDH | Methylene blue | PFO | Visible light | 2.14 | [100] |
| g-C3N4/NiAl-LDH/CeO2 NC | Rhodamine B | PFO | UV light | 2.54 | [55] |
| Mg–Al LDH@g-C3N4@Ag3PO4 NC | Methylene blue | - | Visible light | 2.69 | [107] |
| ZnCr mixed oxides | Methylene blue, Brilliant cresyl blue | PFO | Simulated solar irradiation | - | [4] |
| ZnCr 500 | Crystal violet | PFO | Simulated solar irradiation | 1.98 | [39] |
| ZnFe mixed oxides | Methylene blue | PFO | Simulated solar irradiation | - | [44] |
| mixed oxides ZnO/TiO2/CeO2/Al2O3 | Methylene blue, Methyl orange | PFO | UV light | 3.19 | [108] |
| TiO2—ZnAl LDH | Rhodamine B | PFO | Solar light | - | [52] |
| NiAl-LDH/Polyoxometalate | Malachite green | PFO | UV light | 3.22 | [109] |
| ZnCr LDH/rGO | Rifampicin | - | Visible light | - | [8] |
| ZnFe mixed metal oxides | Ibuprofen | PFO | Simulated solar irradiation | - | [110] |
| mixed oxides ZnO/TiO2/CeO2/Al2O3 | Paracetamol | PFO | UV light | 3.19 | [108] |
| ZnAl LDH, ZnAl mixed oxide | Tolperisone hydrochloride | - | UV light | - | [111] |
| MgAl LDH/MOF | Diclofenac | PFO | Visible light | 4.02 | [112] |
| CuMgAlTi—LDH | Polyethylene and polystyrene microplastics | - | Visible light | 2.82 | [113] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hadnadjev-Kostic, M.; Vulic, T.; Karanovic, D.; Tomic, A.; Cvetkovic, D. Layered Double Hydroxide-Based Materials for Wastewater Treatment: Recent Progress in Multifunctional Environmental Applications. Processes 2025, 13, 3757. https://doi.org/10.3390/pr13123757
Hadnadjev-Kostic M, Vulic T, Karanovic D, Tomic A, Cvetkovic D. Layered Double Hydroxide-Based Materials for Wastewater Treatment: Recent Progress in Multifunctional Environmental Applications. Processes. 2025; 13(12):3757. https://doi.org/10.3390/pr13123757
Chicago/Turabian StyleHadnadjev-Kostic, Milica, Tatjana Vulic, Djurdjica Karanovic, Ana Tomic, and Dragoljub Cvetkovic. 2025. "Layered Double Hydroxide-Based Materials for Wastewater Treatment: Recent Progress in Multifunctional Environmental Applications" Processes 13, no. 12: 3757. https://doi.org/10.3390/pr13123757
APA StyleHadnadjev-Kostic, M., Vulic, T., Karanovic, D., Tomic, A., & Cvetkovic, D. (2025). Layered Double Hydroxide-Based Materials for Wastewater Treatment: Recent Progress in Multifunctional Environmental Applications. Processes, 13(12), 3757. https://doi.org/10.3390/pr13123757







