Sustainable Production of Alternative Proteins from Basidiomycetes: Valorization of Mycelial and Fruiting Body Biomass
Abstract
1. Introduction
2. Nutritional Quality and Biological Value of Proteins from Mushrooms
3. Mushrooms in the Alternative Protein Landscape
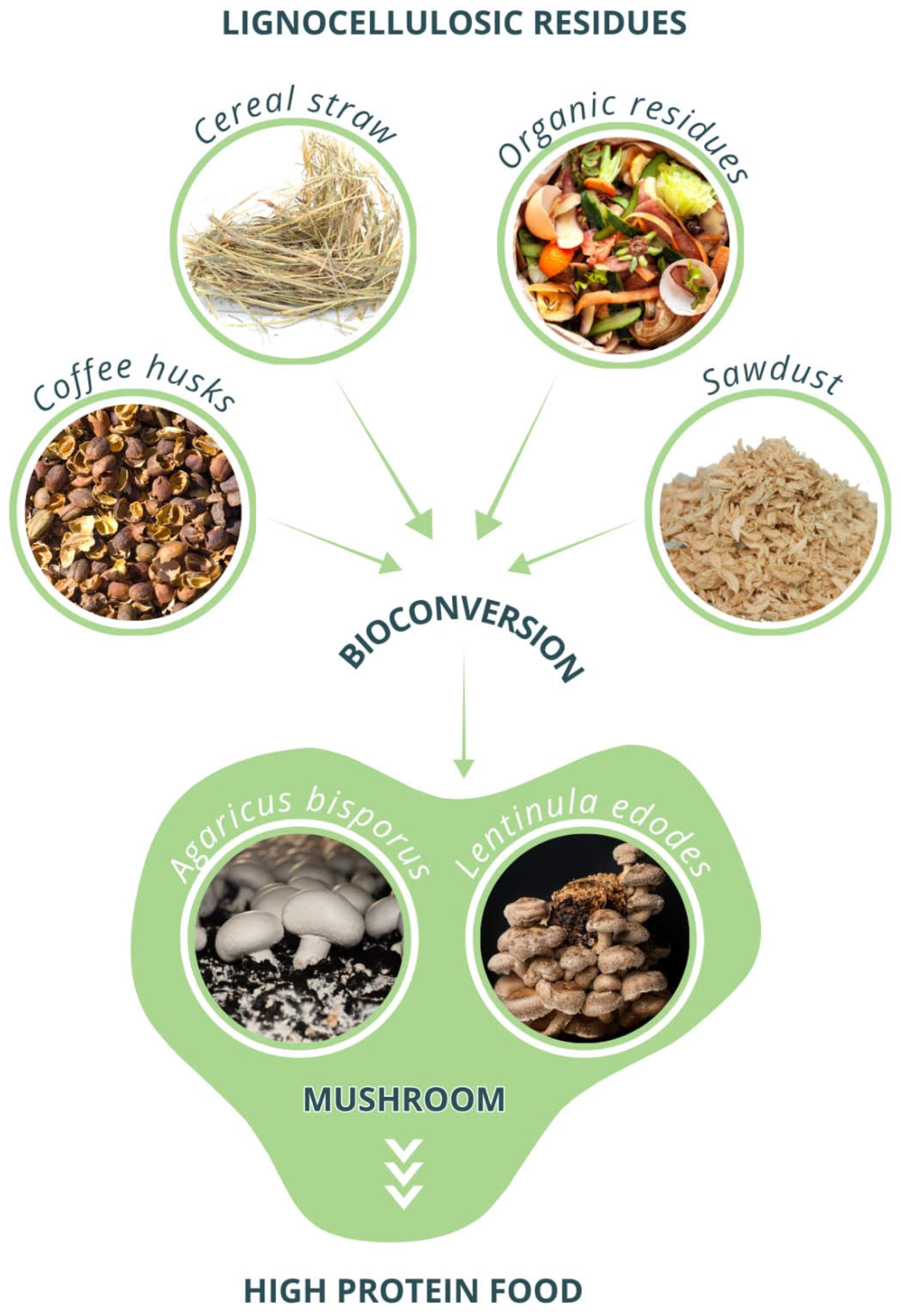
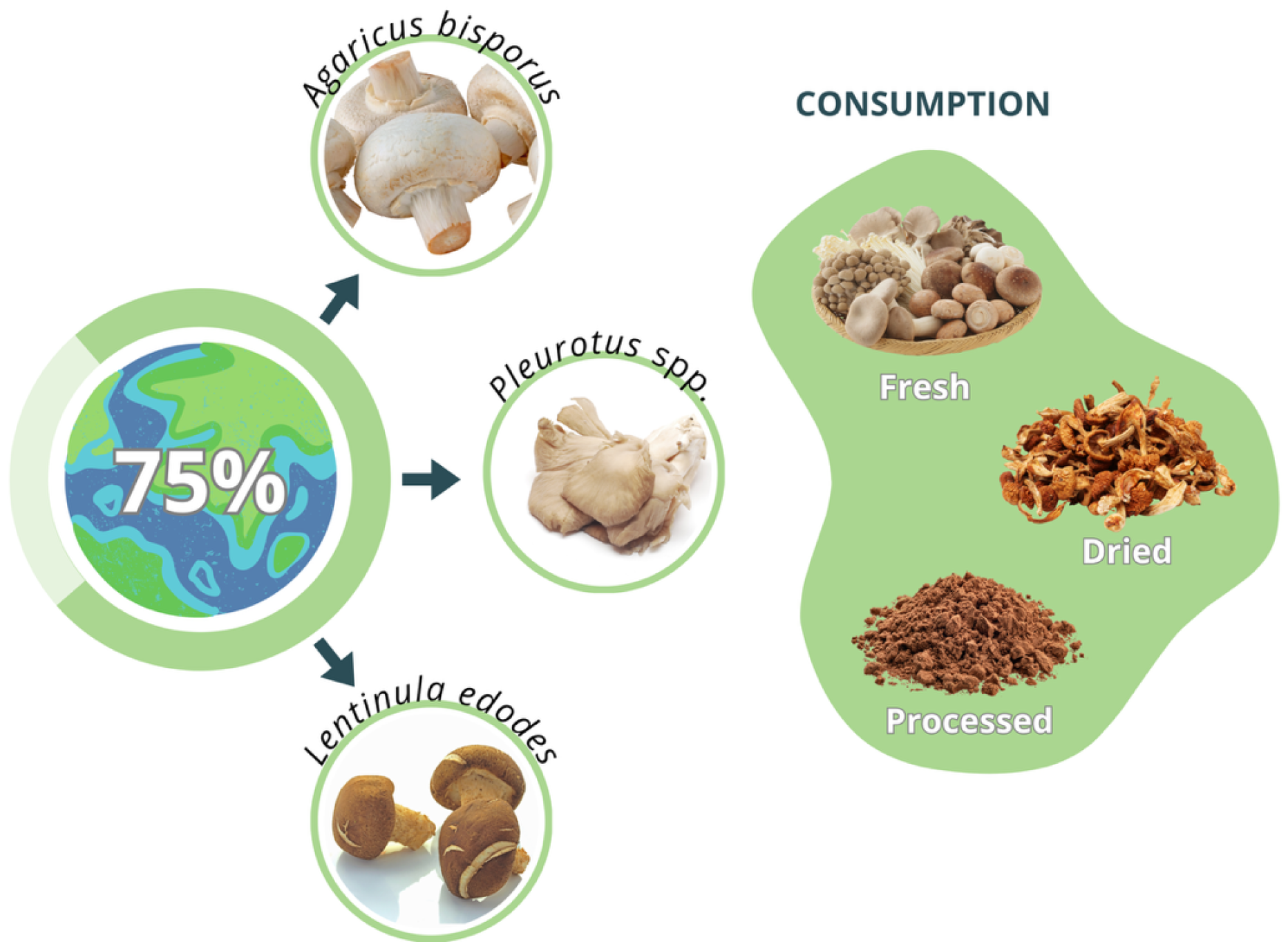
4. Traditional Mushroom Cultivation as a Protein Source
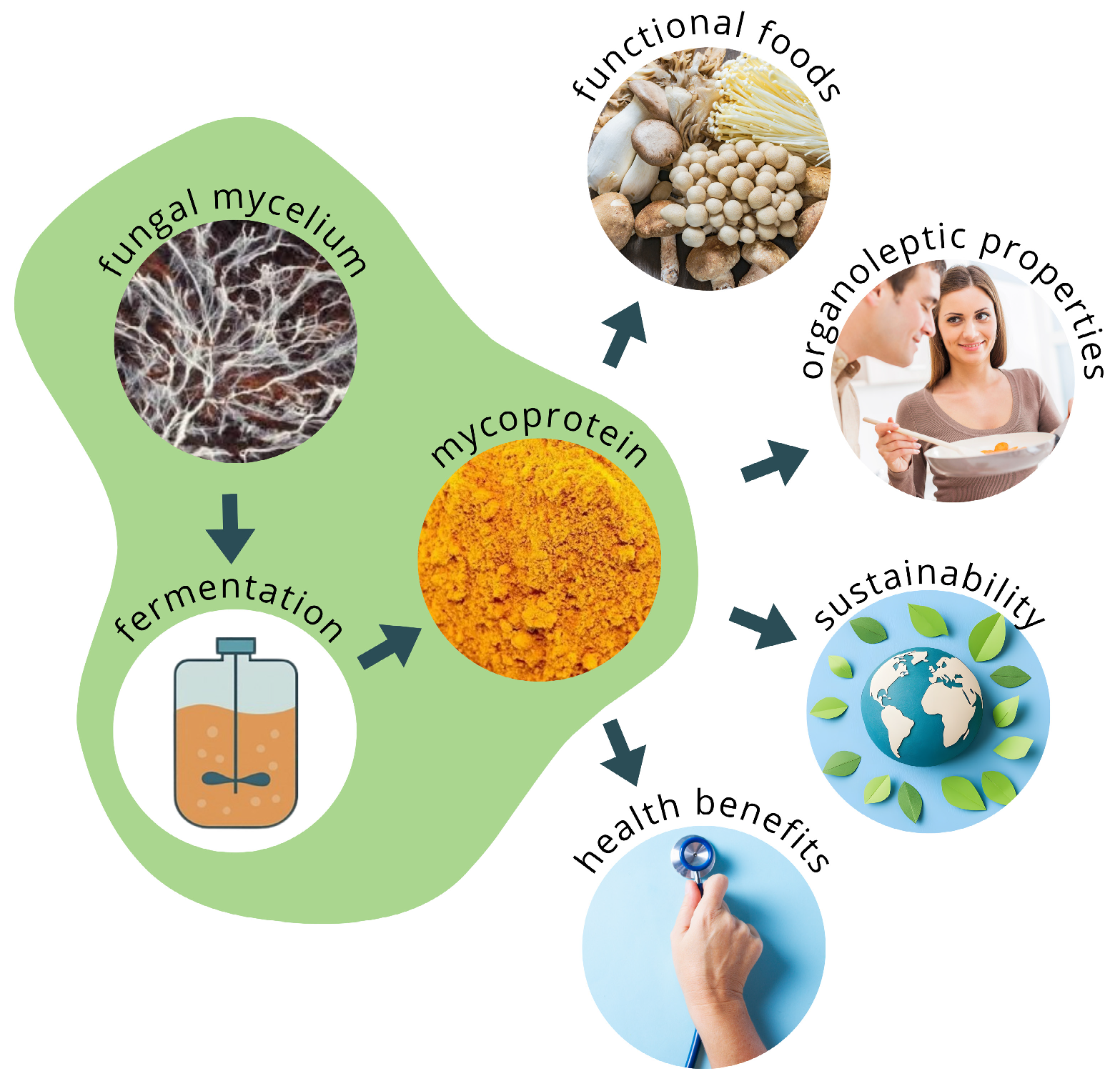
5. Mycelial Biomass Production: An Emerging Biotechnological Platform
| Aspect | Submerged Fermentation (SmF) | Solid-State Fermentation (SSF) |
|---|---|---|
| Medium | Liquid nutrient medium: biomass fully immersed | Moist solid matrix without free liquid |
| Typical bioreactors (see Figure 4) | Stirred-tank, airlift, or bubble-column reactors with controlled aeration and agitation | Trays, packed-bed, or rotating-drum reactors; rely on passive aeration |
| Installation cost | High. requires complex, stainless-steel, pressure-resistant systems | Low to moderate—simpler materials and modular configurations |
| Water consumption | High (90–95% moisture) | Very low (40–70% moisture) |
| Substrate flexibility | Requires soluble and clarified substrates (e.g., glucose, molasses, corn steep liquor) | Can directly use agro-industrial residues (bran, bagasse, coffee husks, fruit peels) |
| Oxygenation | Controlled by aeration and agitation; may be limited by viscosity | Passive diffusion through the solid matrix; generally efficient |
| Process control | Fully automated (pH, DO, temperature, agitation, aeration); supports continuous operation | Limited, but improving with sensor integration (humidity, temperature, CO2) |
| Scalability | Excellent (industrial fermenters 10–100 m3); high reproducibility | Moderate; scale-up limited by heat and mass transfer gradients |
| Production cycle | 2–10 days depending on strain and process mode | 5–15 days depending on substrate and aeration efficiency |
| Contamination risk | Very low due to sterile, closed operation | Moderate to high in open or semi-open systems |
| Biomass homogeneity | High; produces uniform, food-grade biomass | Heterogeneous; variable moisture and composition |
| Energy demand | Higher. Continuous agitation and aeration | Lower. Passive air circulation and minimal mixing |
| Downstream processing | Biomass easily recovered by filtration or centrifugation; compatible with drying | Complex separation from solid matrix; requires mechanical disruption |
| Protein yield and composition | 25–35% protein (dry weight); composition adjustable via C/N ratio and oxygenation | 20–30% protein; enriched in essential amino acids, depending on substrate |
| Environmental impact | Generates wastewater but can integrate with effluent treatment and biorefineries | Minimal wastewater; valorizes residues, reducing landfill burden |
| Circular bioeconomy potential | Moderate. Requires feedstock pre-treatment and liquid waste handling | High. Direct valorization of lignocellulosic residues and co-production of enzymes |
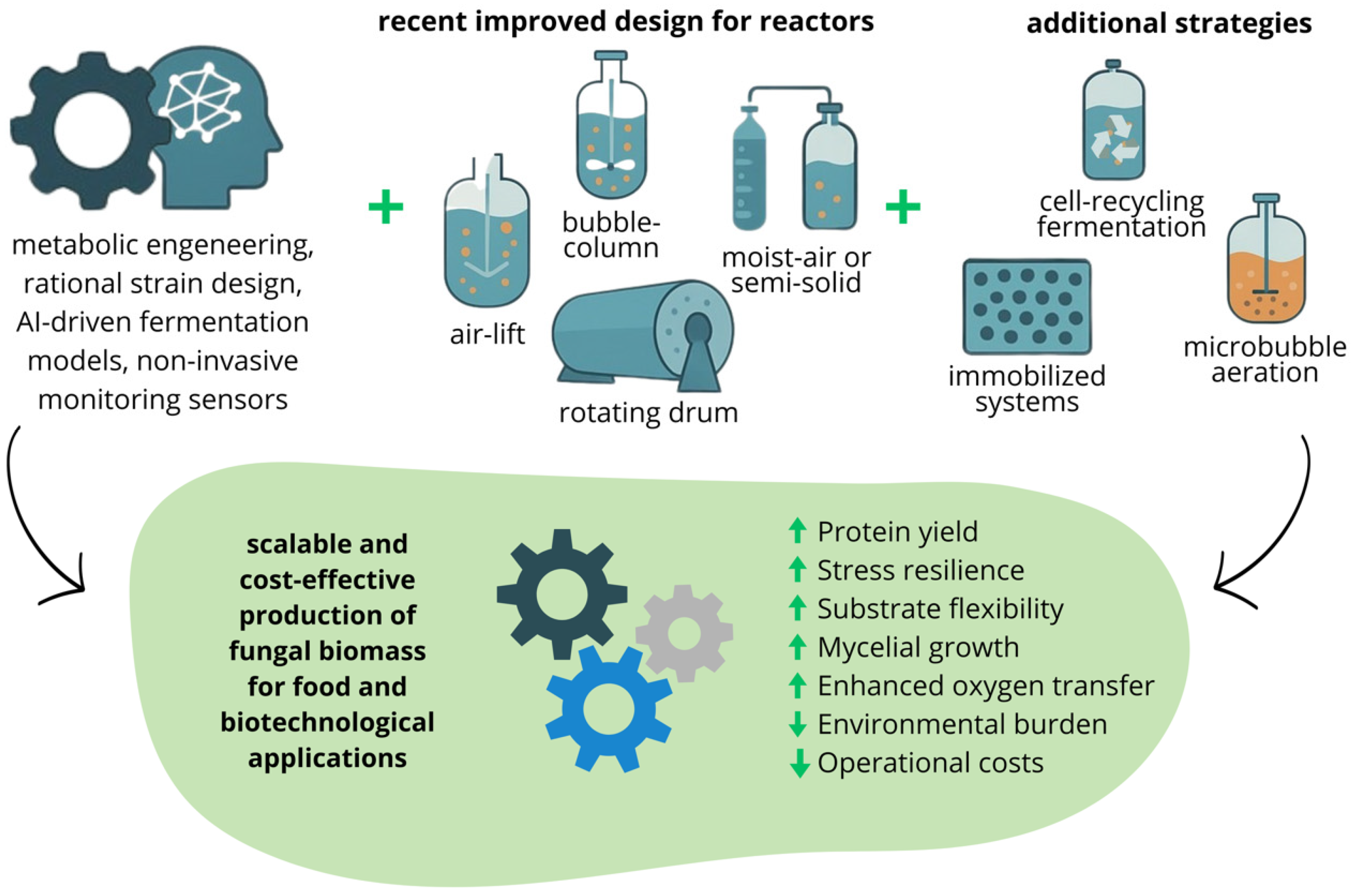
6. Nutritional Quality and Biological Value of Proteins from Mycelial Mass
7. Environmental and Economic Sustainability of Fungal Biomass Production
8. Emerging Basidiomycetes Species for Protein-Rich Mycelial Biomass
9. Future Perspectives and Challenges
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Verkuijl, C.; Smit, J.; Green, J.M.H.; Nordquist, R.E.; Sebo, J.; Hayek, M.N.; Hötzel, M.J. Climate Change, Public Health, and Animal Welfare: Towards a One Health Approach to Reducing Animal Agriculture’s Climate Footprint. Front. Anim. Sci. 2024, 5, 1281450. [Google Scholar] [CrossRef]
- Lima, M.; Costa, R.; Rodrigues, I.; Lameiras, J.; Botelho, G. A Narrative Review of Alternative Protein Sources: Highlights on Meat, Fish, Egg and Dairy Analogues. Foods 2022, 11, 2053. [Google Scholar] [CrossRef]
- Malila, Y.; Owolabi, I.O.; Chotanaphuti, T.; Sakdibhornssup, N.; Elliott, C.T.; Visessanguan, W.; Karoonuthaisiri, N.; Petchkongkaew, A. Current Challenges of Alternative Proteins as Future Foods. Npj Sci. Food 2024, 8, 53. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Chen, E.; Zhang, X.; Li, D.; Wang, Q.; Sun, Y. Nutritional Value and Physicochemical Characteristics of Alternative Protein for Meat and Dairy—A Review. Foods 2022, 11, 3326. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Nguyen, M.; Bu, Y. Consumer Alternative Protein Choice in Climate Change: Temporal Landmarks, Self-Transcendence, and Mindset Abstraction. Appetite 2025, 210, 107974. [Google Scholar] [CrossRef] [PubMed]
- Quintieri, L.; Nitride, C.; De Angelis, E.; Lamonaca, A.; Pilolli, R.; Russo, F.; Monaci, L. Alternative Protein Sources and Novel Foods: Benefits, Food Applications and Safety Issues. Nutrients 2023, 15, 1509. [Google Scholar] [CrossRef]
- Medeiros, F.; Aleman, R.S.; Gabríny, L.; You, S.W.; Hoskin, R.T.; Moncada, M. Current Status and Economic Prospects of Alternative Protein Sources for the Food Industry. Appl. Sci. 2024, 14, 3733. [Google Scholar] [CrossRef]
- Pastrana-Pastrana, Á.J.; Rodríguez-Herrera, R.; Solanilla-Duque, J.F.; Flores-Gallegos, A.C. Plant Proteins, Insects, Edible Mushrooms and Algae: More Sustainable Alternatives to Conventional Animal Protein. J. Future Foods 2025, 5, 248–256. [Google Scholar] [CrossRef]
- Hadi, J.; Brightwell, G. Safety of Alternative Proteins: Technological, Environmental and Regulatory Aspects of Cultured Meat, Plant-Based Meat, Insect Protein and Single-Cell Protein. Foods 2021, 10, 1226. [Google Scholar] [CrossRef]
- Augustin, M.A.; Hartley, C.J.; Maloney, G.; Tyndall, S. Innovation in Precision Fermentation for Food Ingredients. Crit. Rev. Food Sci. Nutr. 2024, 64, 6218–6238. [Google Scholar] [CrossRef]
- Scholtmeijer, K.; van den Broek, L.A.M.; Fischer, A.R.H.; van Peer, A. Potential Protein Production from Lignocellulosic Materials Using Edible Mushroom Forming Fungi. J. Agric. Food Chem. 2023, 71, 4450–4457. [Google Scholar] [CrossRef] [PubMed]
- Łysakowska, P.; Sobota, A.; Wirkijowska, A. Medicinal Mushrooms: Their Bioactive Components, Nutritional Value and Application in Functional Food Production—A Review. Molecules 2023, 28, 5393. [Google Scholar] [CrossRef] [PubMed]
- De Cianni, R.; Pippinato, L.; Mancuso, T. A Systematic Review on Drivers Influencing Consumption of Edible Mushrooms and Innovative Mushroom-Containing Products. Appetite 2023, 182, 106454. [Google Scholar] [CrossRef] [PubMed]
- Gardeli, C.; Mela, N.; Dedousi, M.; Kandyliari, A.; Kaparakou, E.; Diamantopoulou, P.; Pappas, C.; Mallouchos, A. The Influence of Substrate and Strain on Protein Quality of Pleurotus Ostreatus. Appl. Sci. 2024, 14, 4040. [Google Scholar] [CrossRef]
- Heo, S.; Lee, G.; Na, H.-E.; Park, J.-H.; Kim, T.; Oh, S.-E.; Jeong, D.-W. Current Status of the Novel Food Ingredient Safety Evaluation System. Food Sci. Biotechnol. 2024, 33, 1–11. [Google Scholar] [CrossRef]
- Angelova, G.; Brazkova, M.; Mihaylova, D.; Slavov, A.; Petkova, N.; Blazheva, D.; Deseva, I.; Gotova, I.; Dimitrov, Z.; Krastanov, A. Bioactivity of Biomass and Crude Exopolysaccharides Obtained by Controlled Submerged Cultivation of Medicinal Mushroom Trametes Versicolor. J. Fungi 2022, 8, 738. [Google Scholar] [CrossRef]
- Camilleri, E.; Blundell, R.; Baral, B.; Karpiński, T.M.; Aruci, E.; Atrooz, O.M. Unveiling the Full Spectrum of Maitake Mushrooms: A Comprehensive Review of Their Medicinal, Therapeutic, Nutraceutical, and Cosmetic Potential. Heliyon 2024, 10, e30254. [Google Scholar] [CrossRef]
- Chen, C.X.; Huang, B.; Li, T.; Wu, G.F. Preparation of Phosphoric Acid Activated Carbon from Sugarcane Bagasse by Mechanochemical Processing. BioResources 2012, 7, 5109–5116. [Google Scholar] [CrossRef]
- Chen, X.; Xu, B. Insights into Chemical Components, Health-Promoting Effects, and Processing Impact of Golden Chanterelle Mushroom Cantharellus Cibarius. Food Funct. 2024, 15, 7696–7732. [Google Scholar] [CrossRef]
- Malakar, S.; Sutaoney, P.; Singh, P.; Shah, K.; Chauhan, N.S.; Malakar, S.; Sutaoney, P.; Singh, P.; Shah, K.; Chauhan, N.S. Systems Biology for Mushroom Cultivation Promoting Quality Life. Circ. Agric. Syst. 2025, 5, e010. [Google Scholar] [CrossRef]
- Derbyshire, E.J.; Brameld, J.M.; Wall, B.T.; Thomas, P.; Arens, U.; Forde, C.G.; Hall, W.; Glenn, A.J.; Hill, T.R.; Paxman, J. Is There a Specific Role for Fungal Protein Within Food Based Dietary Guidelines? A Roundtable Discussion. Nutr. Bull. 2025, 50, 514–528. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Dong, S.; Luo, J.; Ma, F.; Jiang, W.; Han, C. Research Progress on the Function and Application of Proteins of Edible and Medicinal Mushrooms: A Review. Int. J. Med. Mushrooms 2022, 24, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.-J.; Ro, H.-S.; Kawauchi, M.; Honda, Y. Review on Mushroom Mycelium-Based Products and Their Production Process: From Upstream to Downstream. Bioresour. Bioprocess. 2025, 12, 3. [Google Scholar] [CrossRef] [PubMed]
- Finnigan, T.J.; Wall, B.T.; Wilde, P.J.; Stephens, F.B.; Taylor, S.L.; Freedman, M.R. Mycoprotein: The Future of Nutritious Nonmeat Protein, a Symposium Review. Curr. Dev. Nutr. 2019, 3, nzz021. [Google Scholar] [CrossRef]
- Ayimbila, F.; Keawsompong, S. Nutritional Quality and Biological Application of Mushroom Protein as a Novel Protein Alternative. Curr. Nutr. Rep. 2023, 12, 290–307. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (Ed.) Dietary Protein Quality Evaluation in Human Nutrition: Report of an FAO Expert Consultation, 31 March–2 April, 2011, Auckland, New Zealand; FAO Food and Nutrition Paper; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013; ISBN 978-92-5-107417-6. [Google Scholar]
- Stojek, K.; Bobrowska-Korczak, B.; Frączek, J.; Piotrowski, M.; Krośniak, M.; Jaroszewicz, B. Protein Content and Amino Acid Profile of Wild Mushrooms Depend on Environmental Conditions. Fungal Biol. 2025, 129, 101620. [Google Scholar] [CrossRef]
- Ionescu, M.; Dincă, M.-N.; Ferdeș, M.; Zăbavă, B.-Ș; Paraschiv, G.; Moiceanu, G. Proteins from Edible Mushrooms: Nutritional Role and Contribution to Well-Being. Foods 2025, 14, 3201. [Google Scholar] [CrossRef]
- Effiong, M.E.; Umeokwochi, C.P.; Afolabi, I.S.; Chinedu, S.N. Assessing the Nutritional Quality of Pleurotus Ostreatus (Oyster Mushroom). Front. Nutr. 2024, 10, 1279208. [Google Scholar] [CrossRef]
- West, S.; Monteyne, A.J.; Whelehan, G.; van der Heijden, I.; Abdelrahman, D.R.; Murton, A.J.; Finnigan, T.J.A.; Stephens, F.B.; Wall, B.T. Ingestion of Mycoprotein, Pea Protein, and Their Blend Support Comparable Postexercise Myofibrillar Protein Synthesis Rates in Resistance-Trained Individuals. Am. J. Physiol. Endocrinol. Metab. 2023, 325, E267–E279. [Google Scholar] [CrossRef]
- Derbyshire, E.J.; Theobald, H.; Wall, B.T.; Stephens, F. Food for Our Future: The Nutritional Science behind the Sustainable Fungal Protein–Mycoprotein. A Symposium Review. J. Nutr. Sci. 2023, 12, e44. [Google Scholar] [CrossRef]
- Salim, R.; Nehvi, I.B.; Mir, R.A.; Tyagi, A.; Ali, S.; Bhat, O.M. A Review on Anti-Nutritional Factors: Unraveling the Natural Gateways to Human Health. Front. Nutr. 2023, 10, 1215873. [Google Scholar] [CrossRef]
- Pashaei, K.H.A.; Irankhah, K.; Namkhah, Z.; Sobhani, S.R. Edible Mushrooms as an Alternative to Animal Proteins for Having a More Sustainable Diet: A Review. J. Health Popul. Nutr. 2024, 43, 205. [Google Scholar] [CrossRef] [PubMed]
- Mattila, P.; Salo-Väänänen, P.; Könkö, K.; Aro, H.; Jalava, T. Basic Composition and Amino Acid Contents of Mushrooms Cultivated in Finland. J. Agric. Food Chem. 2002, 50, 6419–6422. [Google Scholar] [CrossRef] [PubMed]
- Derbyshire, E. Food-Based Dietary Guidelines and Protein Quality Definitions—Time to Move Forward and Encompass Mycoprotein? Foods 2022, 11, 647. [Google Scholar] [CrossRef] [PubMed]
- Munks, B.; Robinson, A.; Beach, E.F.; Williams, H.H. Amino Acids in the Production of Chicken Egg and Muscle. Poult. Sci. 1945, 24, 459–464. [Google Scholar] [CrossRef]
- Cedeno, F.R.P.; Olubiyo, O.J.; Ferreira, S. From Microbial Proteins to Cultivated Meat for Alternative Meat-like Products: A Review on Sustainable Fermentation Approaches. J. Biol. Eng. 2025, 19, 44. [Google Scholar] [CrossRef]
- Dai, Z.; Zhao, Y.; Ke, Y.; Huang, J.; Zhu, J.; Wu, H.; Yang, Y.; Shang, H.; Xia, Z. Alternative Protein Sources and Healthy Skeletal Muscle Aging: A Narrative Review. J. Funct. Foods 2025, 133, 106990. [Google Scholar] [CrossRef]
- Attia, Y.A.; Al-Harthi, M.A.; Shafi, M.E.; Abdulsalam, N.M.; Nagadi, S.A.; Wang, J.; Kim, W.K. Amino Acids Supplementation Affects Sustainability of Productive and Meat Quality, Survivability and Nitrogen Pollution of Broiler Chickens during the Early Life. Life 2022, 12, 2100. [Google Scholar] [CrossRef]
- Gorissen, S.H.M.; Crombag, J.J.R.; Senden, J.M.G.; Waterval, W.A.H.; Bierau, J.; Verdijk, L.B.; van Loon, L.J.C. Protein Content and Amino Acid Composition of Commercially Available Plant-Based Protein Isolates. Amino Acids 2018, 50, 1685–1695. [Google Scholar] [CrossRef]
- Donadelli, R.A.; Jones, C.K.; Beyer, R.S. The Amino Acid Composition and Protein Quality of Various Egg, Poultry Meal by-Products, and Vegetable Proteins Used in the Production of Dog and Cat Diets. Poult. Sci. 2019, 98, 1371–1378. [Google Scholar] [CrossRef]
- Barr, B.; Levitt, D.E.; Gollahon, L. Red Meat Amino Acids for Beginners: A Narrative Review. Nutrients 2025, 17, 939. [Google Scholar] [CrossRef]
- Dudekula, U.T.; Doriya, K.; Devarai, S.K. A Critical Review on Submerged Production of Mushroom and Their Bioactive Metabolites. 3 Biotech 2020, 10, 337. [Google Scholar] [CrossRef] [PubMed]
- Maseko, K.H.; Regnier, T.; Bartels, P.; Meiring, B. Mushroom Mycelia as Sustainable Alternative Proteins for the Production of Hybrid Cell-Cultured Meat: A Review. J. Food Sci. 2025, 90, e70060. [Google Scholar] [CrossRef] [PubMed]
- Holt, R.R.; Munafo, J.P.J.; Salmen, J.; Keen, C.L.; Mistry, B.S.; Whiteley, J.M.; Schmitz, H.H. Mycelium: A Nutrient-Dense Food To Help Address World Hunger, Promote Health, and Support a Regenerative Food System. J. Agric. Food Chem. 2024, 72, 2697–2707. [Google Scholar] [CrossRef] [PubMed]
- Kent, G.; Kehoe, L.; Flynn, A.; Walton, J. Plant-Based Diets: A Review of the Definitions and Nutritional Role in the Adult Diet. Proc. Nutr. Soc. 2022, 81, 62–74. [Google Scholar] [CrossRef]
- Majumder, R.; Miatur, S.; Saha, A.; Hossain, S. Mycoprotein: Production and Nutritional Aspects: A Review. Sustain. Food Technol. 2024, 2, 81–91. [Google Scholar] [CrossRef]
- Boro, S.; Kambhampati, V.; Das, S.; Saikia, D. Edible Mushrooms as Meat Analogues: A Comprehensive Review of Nutritional, Therapeutic, and Market Potential. Food Res. Int. 2025, 214, 116632. [Google Scholar] [CrossRef]
- Wang, X.; Liu, H.; Li, J.; Wang, Y. Research Advances in Mushroom Umami: Substance Characteristics, Multidimensional Attributes, Umami Peptide Screening, and Umami Assessment. Curr. Res. Food Sci. 2025, 11, 101210. [Google Scholar] [CrossRef]
- Shahid, M.; Shah, P.; Mach, K.; Rodgers-Hunt, B.; Finnigan, T.; Frost, G.; Neal, B.; Hadjikakou, M. The Environmental Impact of Mycoprotein-Based Meat Alternatives Compared to Plant-Based Meat Alternatives: A Systematic Review. Future Foods 2024, 10, 100410. [Google Scholar] [CrossRef]
- Chaffee, O.; Ardoin, R. Consumer Perceptions of Plant-Based and Mushroom-Based Jerky: A Focus on Texture, Main Ingredient and Protein Information, and Willingness to Pay. Curr. Res. Food Sci. 2025, 10, 101058. [Google Scholar] [CrossRef]
- Wang, R.; Sar, T.; Mahboubi, A.; Fristedt, R.; Taherzadeh, M.J.; Undeland, I. In Vitro Protein Digestibility of Edible Filamentous Fungi Compared to Common Food Protein Sources. Food Biosci. 2023, 54, 102862. [Google Scholar] [CrossRef]
- FAO. Make Money by Growing Mushrooms; FAO: Rome, Italy, 2004. [Google Scholar]
- Lara-Parra, A.I.; Hernández-Hernández, A.A.; Jaguey-Hernández, Y.; Jiménez-Osorio, A.S.; Castañeda-Ovando, A.; Aguilar-Arteaga, K.; Añorve-Morga, J. Exploring Alternative Sources of Protein in Food: Trends in Nutrient and Functional Features. Food Res. Int. 2025, 208, 116224. [Google Scholar] [CrossRef] [PubMed]
- González, A.; Cruz, M.; Losoya, C.; Nobre, C.; Loredo, A.; Rodríguez, R.; Contreras, J.; Belmares, R. Edible Mushrooms as a Novel Protein Source for Functional Foods. Food Funct. 2020, 11, 7400–7414. [Google Scholar] [CrossRef] [PubMed]
- Chukwu, S.C.; Ibeji, C.A.; Ogbu, C.; Oselebe, H.O.; Okporie, E.O.; Rafii, M.Y.; Oladosu, Y. Primordial Initiation, Yield and Yield Component Traits of Two Genotypes of Oyster Mushroom (Pleurotus Spp.) as Affected by Various Rates of Lime. Sci. Rep. 2022, 12, 19054. [Google Scholar] [CrossRef]
- Meilleur, M.-A.; Bastien, D.; Monfet, D. Modeling Mushrooms’ Carbon Dioxide Emission and Heat Exchange Rates for Synergistic Cultivation with Leafy Greens. Sustainability 2023, 15, 16740. [Google Scholar] [CrossRef]
- De Bonis, M.; Locatelli, S.; Sambo, P.; Zanin, G.; Pecchia, J.A.; Nicoletto, C. Effect of Different LED Light Wavelengths on Production and Quality of Pleurotus Ostreatus Grown on Different Commercial Substrates. Horticulturae 2024, 10, 349. [Google Scholar] [CrossRef]
- Sultana, R.; Hossain, M.I.; Saifullah, A.R.; Chakraborty, R. Influence of Substrate pH and Watering Frequency on the Growth of Oyster Mushroom. Int. J. Plant Biol. Res. 2018, 6, 1097. [Google Scholar]
- Manea, E.E.; Bumbac, C.; Dinu, L.R.; Bumbac, M.; Nicolescu, C.M. Composting as a Sustainable Solution for Organic Solid Waste Management: Current Practices and Potential Improvements. Sustainability 2024, 16, 6329. [Google Scholar] [CrossRef]
- Bakratsas, G.; Polydera, A.; Nilson, O.; Chatzikonstantinou, A.V.; Xiros, C.; Katapodis, P.; Stamatis, H. Mycoprotein Production by Submerged Fermentation of the Edible Mushroom Pleurotus Ostreatus in a Batch Stirred Tank Bioreactor Using Agro-Industrial Hydrolysate. Foods 2023, 12, 2295. [Google Scholar] [CrossRef]
- Bakratsas, G.; Samiotaki, M.; Katapodis, P.; Stamatis, H. Proteomic Analysis of Pleurotus Ostreatus Grown on Glucose and Xylose Mixtures in Submerged Fermentation Provides Insights into Differentiated Mycelial Composition. Synth. Biol. Eng. 2024, 2, 10006. [Google Scholar] [CrossRef]
- Yang, M.; Qian, Z.; Zhan, Q.; Zhong, L.; Hu, Q.; Zhao, L. Application of Definitive Screening Design to Optimization of the Protein Extraction and Functional Properties of Proteins in Auricularia Auricula. J. Sci. Food Agric. 2023, 103, 1226–1236. [Google Scholar] [CrossRef]
- Parhizi, Z.; Dearnaley, J.; Kauter, K.; Mikkelsen, D.; Pal, P.; Shelley, T.; Burey, P. The Fungus Among Us: Innovations and Applications of Mycelium-Based Composites. J. Fungi 2025, 11, 549. [Google Scholar] [CrossRef] [PubMed]
- Finnigan, T.J.A.; Theobald, H.E.; Bajka, B. Mycoprotein: A Healthy and Sustainable Source of Alternative Protein-Based Foods. Annu. Rev. Food Sci. Technol. 2025, 16, 105–125. [Google Scholar] [CrossRef] [PubMed]
- Lizardi-Jiménez, M.A.; Hernández-Martínez, R. Solid State Fermentation (SSF): Diversity of Applications to Valorize Waste and Biomass. 3 Biotech 2017, 7, 44. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Chen, Z.; Liu, Y.; Hua, X.; Gao, C.; Liu, J. Morphological Engineering of Filamentous Fungi: Research Progress and Perspectives. J. Microbiol. Biotechnol. 2024, 34, 1197–1205. [Google Scholar] [CrossRef]
- Lee, S.Y.; Ra, C.H. Comparison of Liquid and Solid-State Fermentation Processes for the Production of Enzymes and Beta-Glucan from Hulled Barley. J. Microbiol. Biotechnol. 2022, 32, 317–323. [Google Scholar] [CrossRef]
- Martău, G.-A.; Unger, P.; Schneider, R.; Venus, J.; Vodnar, D.C.; López-Gómez, J.P. Integration of Solid State and Submerged Fermentations for the Valorization of Organic Municipal Solid Waste. J. Fungi 2021, 7, 766. [Google Scholar] [CrossRef]
- Šelo, G.; Planinić, M.; Tišma, M.; Tomas, S.; Koceva Komlenić, D.; Bucić-Kojić, A. A Comprehensive Review on Valorization of Agro-Food Industrial Residues by Solid-State Fermentation. Foods 2021, 10, 927. [Google Scholar] [CrossRef]
- Pérez-Contreras, S.; Avalos-de la Cruz, D.A.; Lizardi-Jiménez, M.A.; Herrera-Corredor, J.A.; Baltazar-Bernal, O.; Hernández-Martínez, R. Production of Ligninolytic and Cellulolytic Fungal Enzymes for Agro-Industrial Waste Valorization: Trends and Applicability. Catalysts 2025, 15, 30. [Google Scholar] [CrossRef]
- Mattedi, A.; Sabbi, E.; Farda, B.; Djebaili, R.; Mitra, D.; Ercole, C.; Cacchio, P.; Del Gallo, M.; Pellegrini, M. Solid-State Fermentation: Applications and Future Perspectives for Biostimulant and Biopesticides Production. Microorganisms 2023, 11, 1408. [Google Scholar] [CrossRef]
- Yafetto, L. Application of Solid-State Fermentation by Microbial Biotechnology for Bioprocessing of Agro-Industrial Wastes from 1970 to 2020: A Review and Bibliometric Analysis. Heliyon 2022, 8, e09173. [Google Scholar] [CrossRef]
- Li, Z.; Luo, R.; Zhang, Y.; Yan, X.; Pang, Q. Effective Protein Extraction from Mycelium and Fruiting Body of Auricularia Auricula for Proteomics Studies. Int. J. Food Prop. 2018, 21, 2156–2166. [Google Scholar] [CrossRef]
- Elhalis, H. Exploring Fungal Mycelium for Sustainable Food Solutions: From Biomass Utilization to Byproduct Innovation. Food Rev. Int. 2025, 1–33. [Google Scholar] [CrossRef]
- Ji, Z.; Ma, W.; Liang, P.; Wang, X.; Zhang, S.; Han, Y.; Guo, Y. Anti-Inflammatory Potential of Mycoprotein Peptides Obtained from Fermentation of Schizophyllum Commune DS1 with Young Apples. Int. J. Biol. Macromol. 2024, 281, 136638. [Google Scholar] [CrossRef]
- Mitchell, D.A.; Ruiz, H.A.; Krieger, N. A Critical Evaluation of Recent Studies on Packed-Bed Bioreactors for Solid-State Fermentation. Processes 2023, 11, 872. [Google Scholar] [CrossRef]
- Artola, A.; Font, X.; Moral-Vico, J.; Sánchez, A. The Role of Solid-State Fermentation to Transform Existing Waste Treatment Plants Based on Composting and Anaerobic Digestion into Modern Organic Waste-Based Biorefineries, in the Framework of Circular Bioeconomy. Front. Chem. Eng. 2024, 6, 1463785. [Google Scholar] [CrossRef]
- Moser, A.; Appl, C.; Pörtner, R.; Baganz, F.; Hass, V.C. A New Concept for the Rapid Development of Digital Twin Core Models for Bioprocesses in Various Reactor Designs. Fermentation 2024, 10, 463. [Google Scholar] [CrossRef]
- Albino, M.; Gargalo, C.L.; Nadal-Rey, G.; Albæk, M.O.; Krühne, U.; Gernaey, K.V. Hybrid Modeling for On-Line Fermentation Optimization and Scale-Up: A Review. Processes 2024, 12, 1635. [Google Scholar] [CrossRef]
- Wainaina, S.; Taherzadeh, M.J. Automation and Artificial Intelligence in Filamentous Fungi-Based Bioprocesses: A Review. Bioresour. Technol. 2023, 369, 128421. [Google Scholar] [CrossRef]
- Wang, Y.; Gou, C.; Chen, L.; Liao, Y.; Zhang, H.; Luo, L.; Ji, J.; Qi, Y. Solid-State Fermentation with White Rot Fungi (Pleurotus Species) Improves the Chemical Composition of Highland Barley Straw as a Ruminant Feed and Enhances In Vitro Rumen Digestibility. J. Fungi 2023, 9, 1156. [Google Scholar] [CrossRef]
- Bakratsas, G.; Polydera, A.; Nilson, O.; Kossatz, L.; Xiros, C.; Katapodis, P.; Stamatis, H. Single-Cell Protein Production by Pleurotus Ostreatus in Submerged Fermentation. Sustain. Food Technol. 2023, 1, 377–389. [Google Scholar] [CrossRef]
- Kirsch, L.D.S.; de Macedo, A.J.P.; Teixeira, M.F.S. Production of Mycelial Biomass by the Amazonian Edible Mushroom Pleurotus albidus. Braz. J. Microbiol. 2016, 47, 658–664. [Google Scholar] [CrossRef]
- Dulay, R.M.R.; Cabrera, E.C.; Kalaw, S.P.; Reyes, R.G. Optimization of Submerged Culture Conditions for Mycelial Biomass Production of Fourteen Lentinus Isolates from Luzon Island, Philippines. Biocatal. Agric. Biotechnol. 2021, 38, 102226. [Google Scholar] [CrossRef]
- Mkhize, S.S.; Zharare, G.E.; Basson, A.K.; Mthembu, M.S.; Cloete, J. Performance of Pleurotus pulmonarius Mushroom Grown on Maize Stalk Residues Supplemented with Various Levels of Maize Flour and Wheat Bran. Food Sci. Technol. 2017, 37, 570–577. [Google Scholar] [CrossRef]
- Yu, C.-X.; Zhang, Y.-R.; Ren, Y.-F.; Zhao, Y.; Song, X.-X.; Yang, H.-L.; Chen, M.-J. Composition and Contents of Fatty Acids and Amino Acids in the Mycelia of Lentinula Edodes. Food Sci. Nutr. 2023, 11, 4038–4046. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Choi, B.; Lee, I.; Lee, H.; Kwon, S.; Oh, K.; Kim, A.Y. Bioproduction of Mushroom Mycelium of Agaricus Bisporus by Commercial Submerged Fermentation for the Production of Meat Analogue. J. Sci. Food Agric. 2011, 91, 1561–1568. [Google Scholar] [CrossRef] [PubMed]
- Winiski, J.M.; Kaplan-Bie, J.H.; Mcintyre, G.R.; Mueller, P.; O’brien, M.; Carlton, A.; Bayer, E.; Hazen, R.; Lomnes, S.; Snyder, A.T. Edible Mycelia and Methods of Making the Same. U.S. Patent US20220333055A1, 20 October 2022. [Google Scholar]
- Humpenöder, F.; Bodirsky, B.L.; Weindl, I.; Lotze-Campen, H.; Linder, T.; Popp, A. Projected Environmental Benefits of Replacing Beef with Microbial Protein. Nature 2022, 605, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Nair, L.G.; Verma, P. Harnessing Carbon Potential of Lignocellulosic Biomass: Advances in Pretreatments, Applications, and the Transformative Role of Machine Learning in Biorefineries. Bioresour. Bioprocess. 2025, 12, 97. [Google Scholar] [CrossRef]
- dos Anjos, I.V.; Coelho, N.; Duarte, H.; Proença, D.N.; Duarte, M.F.; Barros, R.; Raposo, S.; Gonçalves, S.; Romano, A.; Medronho, B. From Lignocellulosic Residues to Protein Sources: Insights into Biomass Pre-Treatments and Conversion. Polymers 2025, 17, 2251. [Google Scholar] [CrossRef]
- Dhiman, S.; Kaur, P.; Narang, J.; Mukherjee, G.; Thakur, B.; Kaur, S.; Tripathi, M. Fungal Bioprocessing for Circular Bioeconomy: Exploring Lignocellulosic Waste Valorization. Mycology 2024, 15, 538–563. [Google Scholar] [CrossRef]
- Borkertas, S.; Viskelis, J.; Viskelis, P.; Streimikyte, P.; Gasiunaite, U.; Urbonaviciene, D. Fungal Biomass Fermentation: Valorizing the Food Industry’s Waste. Fermentation 2025, 11, 351. [Google Scholar] [CrossRef]
- Brancoli, P.; Gmoser, R.; Taherzadeh, M.J.; Bolton, K. The Use of Life Cycle Assessment in the Support of the Development of Fungal Food Products from Surplus Bread. Fermentation 2021, 7, 173. [Google Scholar] [CrossRef]
- Poore, J.; Nemecek, T. Reducing Food’s Environmental Impacts through Producers and Consumers. Science 2018, 360, 987–992. [Google Scholar] [CrossRef] [PubMed]
- Future Market Insights Alternative Protein Market Demand & Trends 2025 to 2035. Available online: https://www.futuremarketinsights.com/reports/alternative-protein-market (accessed on 31 October 2025).
- Brondi, M.G.; Elias, A.M.; Furlan, F.F.; Giordano, R.C.; Farinas, C.S. Performance Targets Defined by Retro-Techno-Economic Analysis for the Use of Soybean Protein as Saccharification Additive in an Integrated Biorefinery. Sci. Rep. 2020, 10, 7367. [Google Scholar] [CrossRef] [PubMed]
- Allotey, D.K.; Kwofie, E.M.; Asavajaru, P.; Samaranayaka, A.G.P. An Integrative Framework for Eco-Efficiency Assessment in Plant-Protein Extraction Processes: Hotspot Analysis, Protein Loss Tracking, and Uncertainty Analysis. J. Clean. Prod. 2025, 527, 146684. [Google Scholar] [CrossRef]
- European Commission. Building the Future with Nature: Boosting Biotechnology and Biomanufacturing in the EU; European Commission: Brussels, Belgium, 2024. [Google Scholar]
- Circular Bio-Based Europe Joint Undertaking Annual Work Programme 2025 (Second Amendment). Available online: https://www.cbe.europa.eu/?utm_source=chatgpt.com (accessed on 31 October 2025).
- Cerrone, F.; O’Connor, K.E. Cultivation of Filamentous Fungi in Airlift Bioreactors: Advantages and Disadvantages. Appl. Microbiol. Biotechnol. 2025, 109, 41. [Google Scholar] [CrossRef]
- Kachrimanidou, V.; Alexandri, M.; Papapostolou, H.; Papadaki, A.; Kopsahelis, N. Valorization of Grape Pomace for Trametes Versicolor Mycelial Mass and Polysaccharides Production. Sustainability 2023, 15, 15080. [Google Scholar] [CrossRef]
- Joshi, M.; Patel, H.; Gupte, S.; Gupte, A. Nutrient Improvement for Simultaneous Production of Exopolysaccharide and Mycelial Biomass by Submerged Cultivation of Schizophyllum Commune AGMJ-1 Using Statistical Optimization. 3 Biotech 2013, 3, 307–318. [Google Scholar] [CrossRef]
- Ahlborn, J.; Stephan, A.; Meckel, T.; Maheshwari, G.; Rühl, M.; Zorn, H. Upcycling of Food Industry Side Streams by Basidiomycetes for Production of a Vegan Protein Source. Int. J. Recycl. Org. Waste Agric. 2019, 8, 447–455. [Google Scholar] [CrossRef]
- Tešanović, K.; Pejin, B.; Šibul, F.; Matavulj, M.; Rašeta, M.; Janjušević, L.; Karaman, M. A Comparative Overview of Antioxidative Properties and Phenolic Profiles of Different Fungal Origins: Fruiting Bodies and Submerged Cultures of Coprinus Comatus and Coprinellus Truncorum. J. Food Sci. Technol. 2017, 54, 430–438. [Google Scholar] [CrossRef]
- Vieira, G.R.T.; Liebl, M.; Tavares, L.B.B.; Paulert, R.; Smânia Júnior, A. Submerged Culture Conditions for the Production of Mycelial Biomass and Antimicrobial Metabolites by Polyporus Tricholoma Mont. Braz. J. Microbiol. 2008, 39, 561–568. [Google Scholar] [CrossRef]
- Fernández-Fueyo, E.; Ruiz-Dueñas, F.J.; López-Lucendo, M.F.; Pérez-Boada, M.; Rencoret, J.; Gutiérrez, A.; Pisabarro, A.G.; Ramírez, L.; Martínez, A.T. A Secretomic View of Woody and Nonwoody Lignocellulose Degradation by Pleurotus Ostreatus. Biotechnol. Biofuels 2016, 9, 49. [Google Scholar] [CrossRef]
- Ohm, R.A.; de Jong, J.F.; Lugones, L.G.; Aerts, A.; Kothe, E.; Stajich, J.E.; de Vries, R.P.; Record, E.; Levasseur, A.; Baker, S.E.; et al. Genome Sequence of the Model Mushroom Schizophyllum Commune. Nat. Biotechnol. 2010, 28, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; Wang, Y.; Xie, C.; Zhou, Y.; Zhu, Z.; Peng, Y. Whole Genome Sequence of an Edible and Medicinal Mushroom, Hericium Erinaceus (Basidiomycota, Fungi). Genomics 2020, 112, 2393–2399. [Google Scholar] [CrossRef] [PubMed]
- Sekoai, P.T.; Roets-Dlamini, Y.; O’Brien, F.; Ramchuran, S.; Chunilall, V. Valorization of Food Waste into Single-Cell Protein: An Innovative Technological Strategy for Sustainable Protein Production. Microorganisms 2024, 12, 166. [Google Scholar] [CrossRef] [PubMed]
- Hardin, M.T.; Mitchell, D.A.; Howes, T. Approach to Designing Rotating Drum Bioreactors for Solid-State Fermentation on the Basis of Dimensionless Design Factors. Biotechnol. Bioeng. 2000, 67, 274–282. [Google Scholar] [CrossRef]
- Zhao, L.; Sun, G.; Jiang, J.; Chen, L.; Huang, J. Role of Microbubbles Coupling Fibrous-Bed Bioreactor in Butyric Acid Production by Clostridium Tyrobutyricum Using Brewer’s Spent Grain as Feedstock. Biochem. Eng. J. 2021, 172, 108051. [Google Scholar] [CrossRef]
- Food and Drug Administration. GRAS Notice for the Use of Phoenix Oyster Mushroom (Pleurotus pulmonarius) Mycelium as an Ingredient in Food; Food and Drug Administration: Silver Spring, MD, USA, 2024. [Google Scholar]
- European Food Safety Authority; Turck, D.; Bohn, T.; Castenmiller, J.; de Henauw, S.; Hirsch-Ernst, K.I.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Guidance on the Scientific Requirements for an Application for Authorisation of a Novel Food in the Context of Regulation (EU) 2015/2283. EFSA J. 2024, 22, e8961. [Google Scholar] [CrossRef]
- EFSA Panel; Turck, D.; Bohn, T.; Cámara, M.; Castenmiller, J.; De Henauw, S.; Jos, Á.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; et al. \Safety of the Fungal Biomass from Fusarium Species Strain Flavolapis as a Novel Food Pursuant to Article 10 of Regulation (EU) 2015/2283. EFSA J. 2025, 23, e9536. [Google Scholar] [CrossRef]
- Kawecki, N.S.; Chen, K.K.; Smith, C.S.; Xie, Q.; Cohen, J.M.; Rowat, A.C. Scalable Processes for Culturing Meat Using Edible Scaffolds. Annu. Rev. Food Sci. Technol. 2024, 15, 241–264. [Google Scholar] [CrossRef]
- Kaplan, D.L.; McClements, D.J. Hybrid Alternative Protein-Based Foods: Designing a Healthier and More Sustainable Food Supply. Front. Sci. 2025, 3, 1599300. [Google Scholar] [CrossRef]
- Meyer, V.; Cairns, T.; Barthel, L.; King, R.; Kunz, P.; Schmideder, S.; Müller, H.; Briesen, H.; Dinius, A.; Krull, R. Understanding and Controlling Filamentous Growth of Fungal Cell Factories: Novel Tools and Opportunities for Targeted Morphology Engineering. Fungal Biol. Biotechnol. 2021, 8, 8. [Google Scholar] [CrossRef]
- Chezan, D.; Flannery, O.; Patel, A. Factors Affecting Consumer Attitudes to Fungi-Based Protein: A Pilot Study. Appetite 2022, 175, 106043. [Google Scholar] [CrossRef]
- Lähteenmäki-Uutela, A.; Rahikainen, M.; Lonkila, A.; Yang, B. Alternative Proteins and EU Food Law. Food Control 2021, 130, 108336. [Google Scholar] [CrossRef]
| Essential Amino Acid | Pleurotus ostreatus | Lentinula edodes | Agaricus bisporus | Beef | Egg |
|---|---|---|---|---|---|
| Histidine | 23 | 19 | 18 | 33 | 24 |
| Isoleucine | 42 | 37 | 35 | 54 | 40 |
| Leucine | 71 | 67 | 64 | 86 | 70 |
| Lysine | 65 | 61 | 59 | 91 | 60 |
| Methionine + cysteine | 29 | 27 | 24 | 42 | 56 |
| Phenylalanine + tyrosine | 72 | 70 | 66 | 84 | 93 |
| Threonine | 43 | 39 | 38 | 46 | 47 |
| Tryptophan | 11 | 9 | 8 | 11 | 17 |
| Valine | 49 | 45 | 43 | 55 | 50 |
| Total EAAs | 405 | 374 | 355 | 502 | 457 |
| Species | Key Characteristics | Protein Content (% Dry Weight) | Typical Substrate | Main Production Regions |
|---|---|---|---|---|
| Agaricus bisporus (button mushroom) | Most widely produced mushroom (35% of world output); mild flavor, short cultivation cycle. | 20–25% | Compost of straw + manure | Europe, North America, China |
| Pleurotus spp. (oyster) | Fast-growing; degrades diverse lignocelluloses; low cost. | 25–30% | Wheat/rice straw, corn cobs, coffee husks | Asia, Latin America, Africa |
| Lentinula edodes (shiitake) | Distinct aroma; immune-modulatory β-glucans (lentinan). | 18–24% | Hardwood sawdust or logs | East Asia, Brazil |
| Flammulina velutipes (enoki) | Delicate, low-temperature species; mild taste. | 18–22% | Corncob + sawdust | Japan, China, Korea |
| Grifola frondosa (maitake) | “Dancing mushroom”; both food and medicinal value. | 20–25% | Hardwood sawdust | Japan, China |
| Volvariella volvacea (straw mushroom) | Short cycle (15–20 days); tropical species. | 18–21% | Rice straw, banana leaves | Southeast Asia |
| Parameter | Function | Optimal Range | Notes/Effects | References |
|---|---|---|---|---|
| Temperature | Regulates mycelial growth and fruiting body initiation | 15–25 °C | Pleurotus grows faster near 25 °C; Flammulina requires 10–15 °C | [55] |
| Relative humidity | Maintains moisture; prevents desiccation | 80–95% | Critical for primordia formation and cap expansion | [56] |
| Ventilation | Supplies O2, removes CO2 | Constant renewal | CO2 > 0.1% inhibits fruiting | [57] |
| Light intensity | Triggers fruiting in photophilic species | 200–500 lux (diffuse) | Pleurotus sp. and Lentinula sp. require light for morphogenesis | [58] |
| Substrate pH | Influences microbial competition and enzyme activity | 5.5–7.0 | pH < 5 inhibits growth; pH > 7 favors contaminants | [59] |
| Feature | Fruiting Body (Mushroom) | Mycelial Biomass |
|---|---|---|
| Structure | Reproductive organ, visible, formed under specific light and temperature conditions. | Filamentous vegetative network of hyphae, continuous growth. |
| Moisture content | 80–90% | 60–70% (dry biomass yield higher). |
| Production cycle | Weeks to months | Few days to 1–2 weeks. |
| Process control | Semi-open, environmental dependence | Fully closed, bioreactor controlled. |
| Main applications | Fresh or processed food | Protein ingredient, textured foods, nutraceuticals. |
| Contamination risk | Moderate to high | Low (sterile systems). |
| Fermentation Mode/Basidiomycete | Carbon Source/Nitrogen Source(s) and Supplements/Minerals/Other Additives | Ref. |
|---|---|---|
| SmF/Pleurotus ostreatus LGAM 1123 | Glucose 40–55 g L−1 (or fibre sludge hydrolysate adjusted to ~55 g L−1 glucose). Yeast extract 13–18 g L−1; C/N ≈ 10–15, optimized by RSM for maximal protein production. KH2PO4, MgSO4, trace-element solution; pH 5.0 | [83] |
| SmF/Pleurotus ostreatus (stirred-tank SCP production) | Mixture of glucose and xylose from agro-industrial hydrolysate (≈50–60 g L−1 total sugars). Corn steep liquor (~30–40 g L−1) supplying organic N, vitamins and minerals. Phosphate buffer; trace elements; pH 5.0 | [61] |
| SmF Pleurotus albidus | Sucrose 30 g L−1. Yeast extract 2.5 g L−1 (main N and vitamin source). pH 7.0; standard mineral salts (e.g., KH2PO4, MgSO4) | [84] |
| SmF Lentinula edodes (multiple isolates) | Coconut water (natural sugars), optionally supplemented with additional glucose (10–20 g L−1). Peptone or yeast extract 2–5 g L−1. Native K, Mg and micronutrients from coconut water; pH 4–7 | [85] |
| SSF Pleurotus spp., Lentinus sajor-caju | Highland barley straw (50 g, 1–2 cm pieces) at 65% moisture. 1% corn meal; 1–3% glucose; 1–3% urea (w/w of dry substrate). 0.5% gypsum; 1% lime; pH adjustment via lime | [82] |
| SSF Pleurotus pulmonarius | Maize stalk as base substrate. Wheat bran and maize flour (5–20% w/w) as supplementary carbon and nitrogen sources. Moisture adjustment to ~65%; CaCO3 or gypsum for pH and structure | [86] |
| Indicator | Beef | Plant Proteins (Soy/Pea) | Mycelial/Fungal Proteins | Ref. |
|---|---|---|---|---|
| GHG emissions (kg CO2-eq·kg−1 product) | 27–60 | 1–5 | 0.5–1.2 | [50,96] |
| Land use (m2·yr·kg−1 product) | 100–300 | 10–40 | 2–5 | [96] |
| Water use (L·kg−1 product) | 15,000–20,000 | 2000–4000 | 300–800 | [96] |
| Energy demand (MJ·kg−1 product) | 350–400 | 60–90 | 50–70 | [45,65] |
| Residue valorization potential | Minimal | Partial | Full (cascading reuse) | [78] |
| Scenario: 20% beef replacement by microbial protein | — | — | ↓ GHG and deforestation ≈ 50% by 2050 | [90] |
| Basidiomycete Species Type/Common Name | Key Findings in SmF or SSF | Protein/Biomass Yield Distinct Nutritional or Functional Traits | Reference |
|---|---|---|---|
| Trametes versicolor White-rot fungus (“turkey tail”) | Efficient growth in SmF on grape pomace extract; characterized amino acid profile; EPS and protein quantified. | 21 g/L biomass (grape pomace medium) Balanced essential amino acids; β-glucans; antioxidant mycelium. | [16,103] |
| Schizophyllum commune Split-gill mushroom | Statistical optimization of SmF for mycelial biomass and polysaccharides; robust nutrient conversion on agro-residues. | Up to 17 g/L dry biomass; 38% protein (dry basis) High β-glucan and hydrophobin content; strong umami potential. | [104] |
| Cyclocybe (Agrocybe) aegerita. Black poplar mushroom | Liquid culture and side-stream upcycling studies; nutrient-rich mycelium. | 15–20 g/L biomass in optimized media Edible, mild flavor, rich in lysine and leucine. | [105] |
| Coprinus comatus Shaggy mane mushroom | SmF versus fruit body comparison; biochemical composition and amino-acid profile analyzed. | 16 g/L biomass; 40% protein (dry basis). Rapid growth; savory profile; antioxidant enzymes. | [106] |
| Polyporus (Neolentinus) tricholoma. Wood-degrading basidiomycete | Stirred-tank SmF optimization for growth and metabolites. | 10–14 g/L biomass. Tolerant to low-cost lignocellulosic substrates; promising amino-acid profile. | [107] |
| Auricularia polytricha (“wood ear mushroom”) | SmF versus fruiting body comparison. Proteomic studies revealed that its protein, polysaccharide complexes possess superior gelation and emulsifying capacities, ideal for plant-based meats and soups. | Combines 15–25% protein with soluble fibers and bioactive polysaccharides | [74] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Figueiredo Trindade, A.R.; de Brito Hilario, I.; Gimenes da Rocha, E.A.; da Rosa Borges dos Santos, L.A.; Giatti Marques de Souza, C.; Proença Dantas, M.; Roldão Ferreira, B.M.; Carvalho Gomes Corrêa, R.; Yamaguchi, N.U.; Bracht, A.; et al. Sustainable Production of Alternative Proteins from Basidiomycetes: Valorization of Mycelial and Fruiting Body Biomass. Processes 2025, 13, 3746. https://doi.org/10.3390/pr13113746
de Figueiredo Trindade AR, de Brito Hilario I, Gimenes da Rocha EA, da Rosa Borges dos Santos LA, Giatti Marques de Souza C, Proença Dantas M, Roldão Ferreira BM, Carvalho Gomes Corrêa R, Yamaguchi NU, Bracht A, et al. Sustainable Production of Alternative Proteins from Basidiomycetes: Valorization of Mycelial and Fruiting Body Biomass. Processes. 2025; 13(11):3746. https://doi.org/10.3390/pr13113746
Chicago/Turabian Stylede Figueiredo Trindade, Amanda Rubia, Isadora de Brito Hilario, Ederson Aparecido Gimenes da Rocha, Leonardo Antônio da Rosa Borges dos Santos, Cristina Giatti Marques de Souza, Marina Proença Dantas, Bruna Mayara Roldão Ferreira, Rúbia Carvalho Gomes Corrêa, Natália Ueda Yamaguchi, Adelar Bracht, and et al. 2025. "Sustainable Production of Alternative Proteins from Basidiomycetes: Valorization of Mycelial and Fruiting Body Biomass" Processes 13, no. 11: 3746. https://doi.org/10.3390/pr13113746
APA Stylede Figueiredo Trindade, A. R., de Brito Hilario, I., Gimenes da Rocha, E. A., da Rosa Borges dos Santos, L. A., Giatti Marques de Souza, C., Proença Dantas, M., Roldão Ferreira, B. M., Carvalho Gomes Corrêa, R., Yamaguchi, N. U., Bracht, A., & Peralta, R. M. (2025). Sustainable Production of Alternative Proteins from Basidiomycetes: Valorization of Mycelial and Fruiting Body Biomass. Processes, 13(11), 3746. https://doi.org/10.3390/pr13113746











