Thermal Cycling Stability of NiO/YSZ Anode-Supported SOFC Button Cells: An Experimental Study
Abstract
1. Introduction
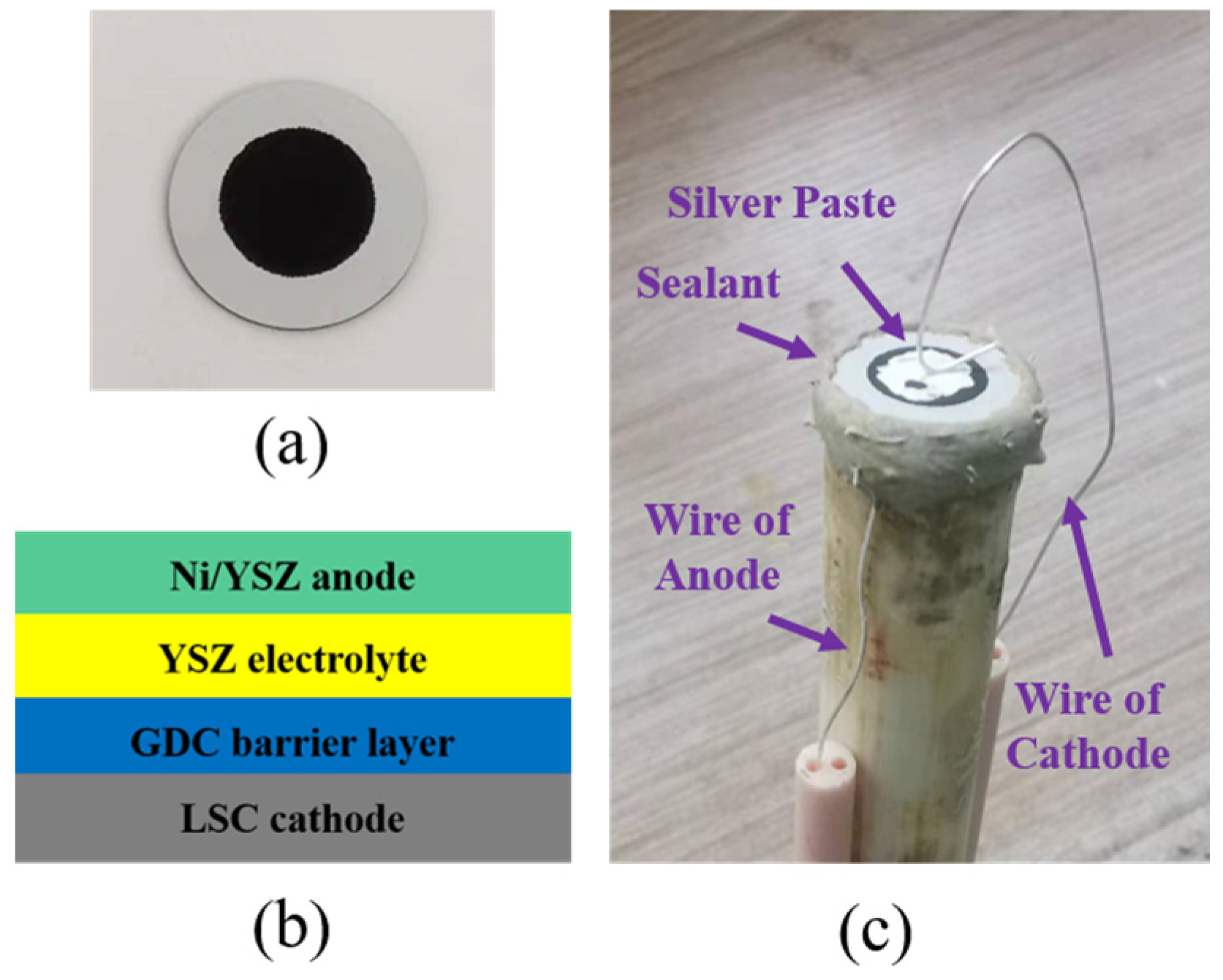
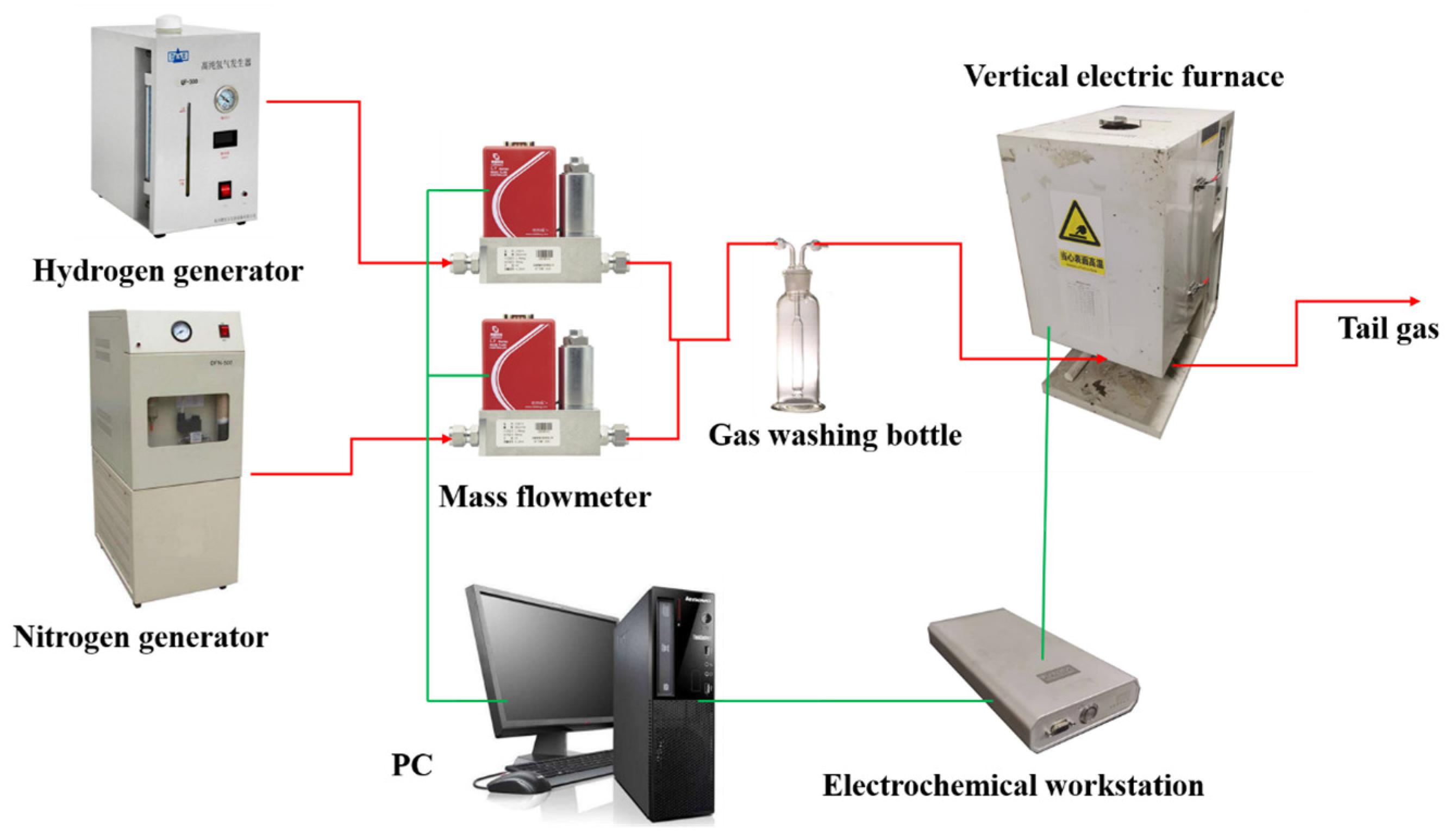
2. Experimental Setup and Methods
3. Results and Analysis
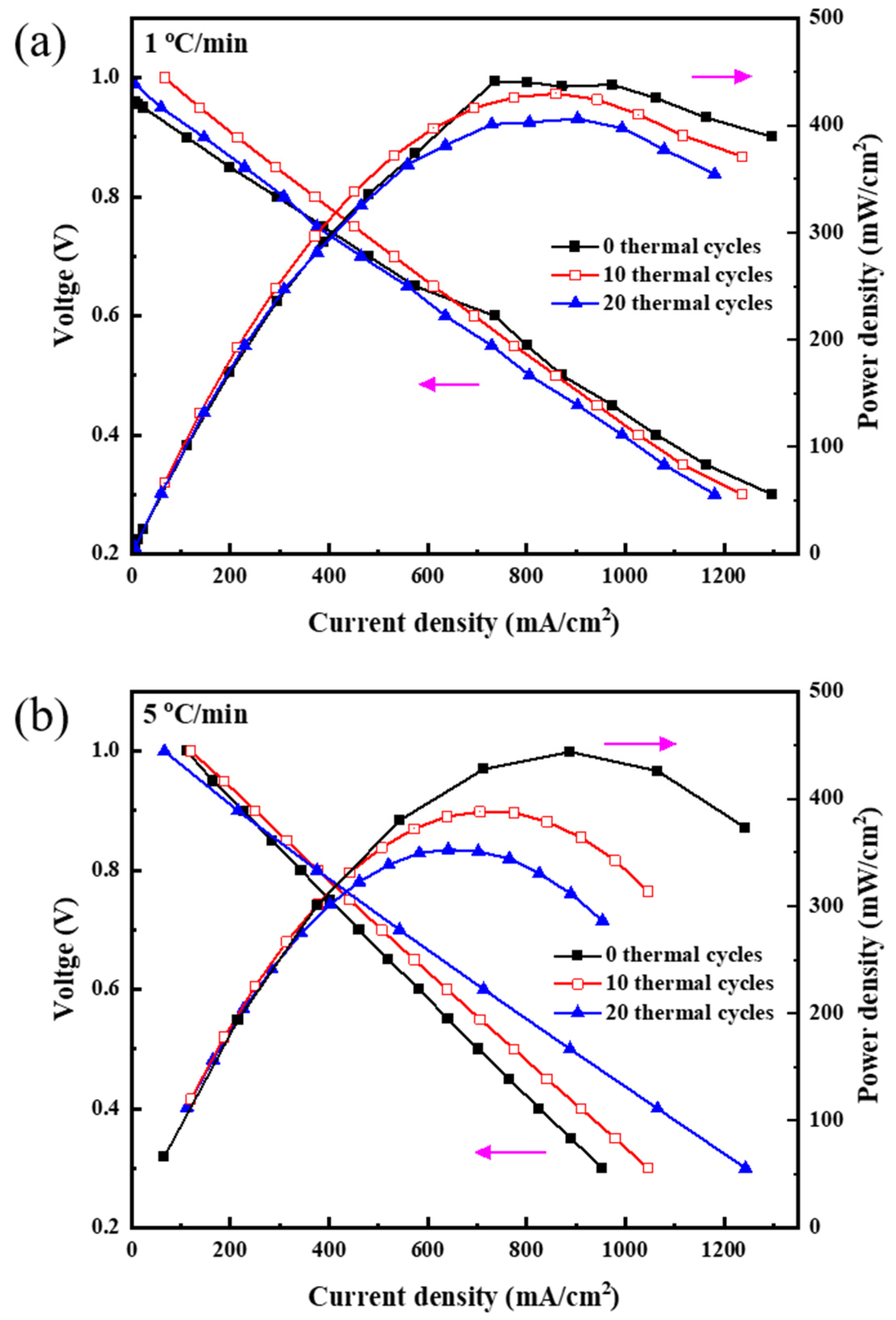
3.1. Electrochemical Performance
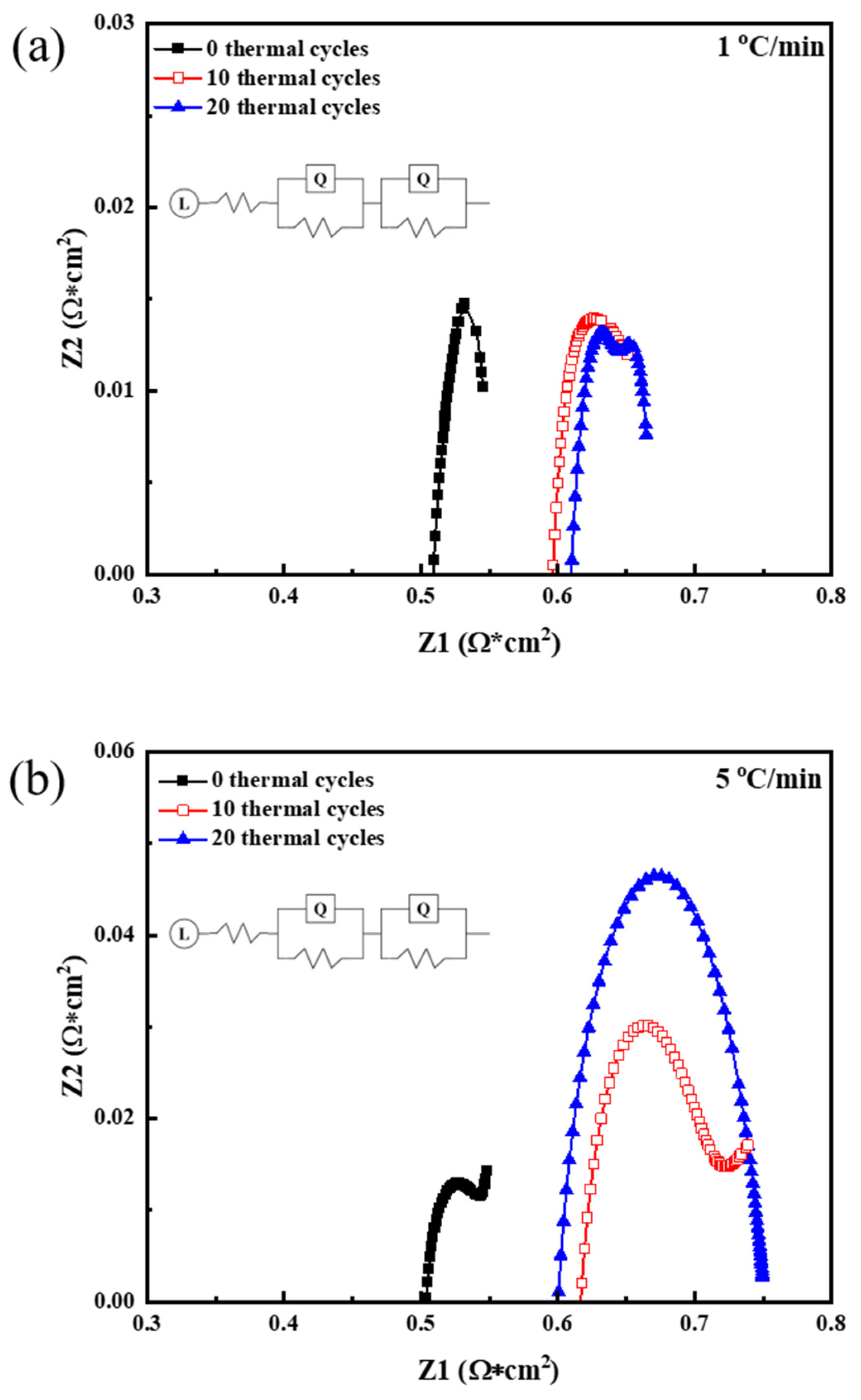
3.2. EIS Performance
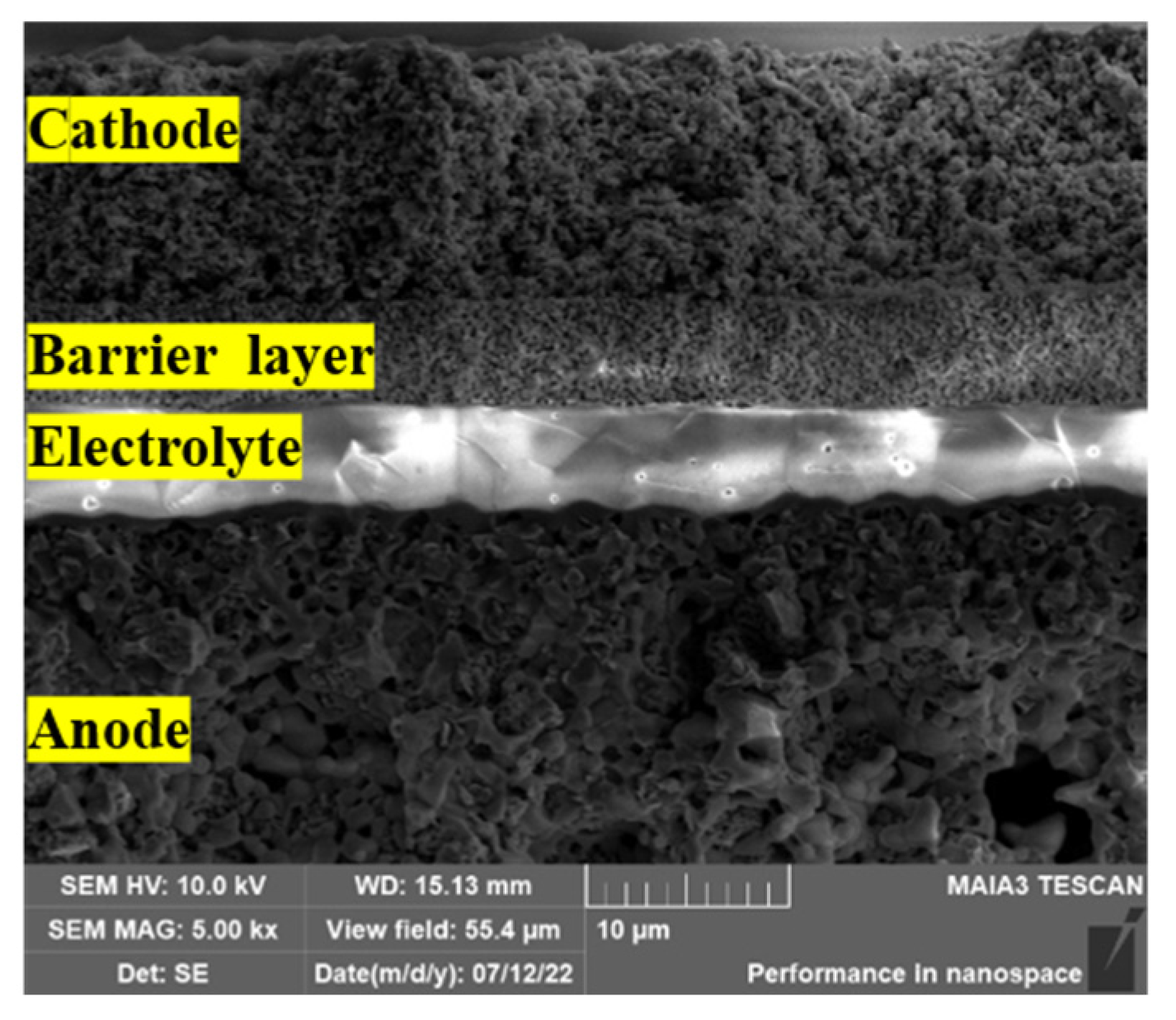
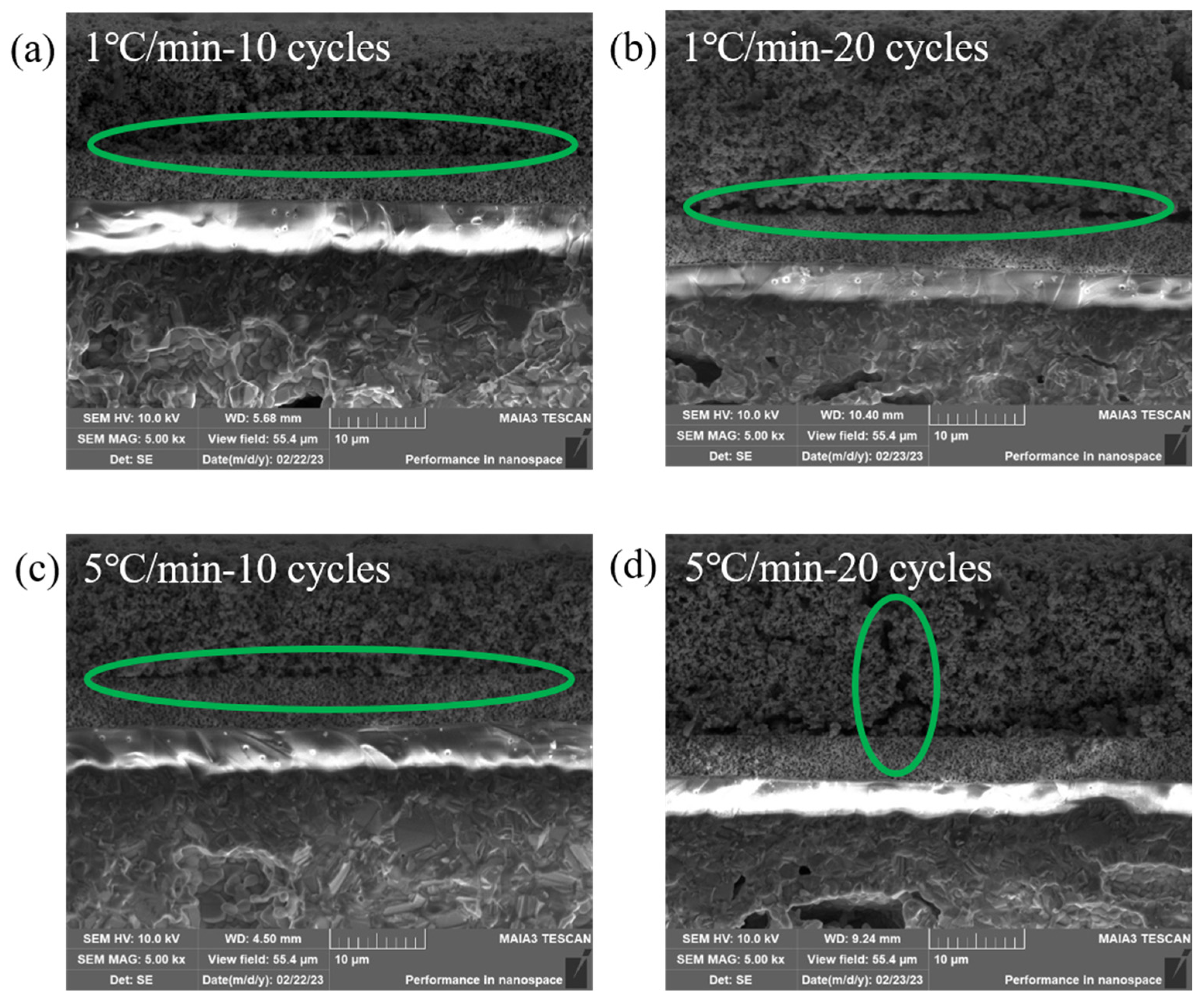
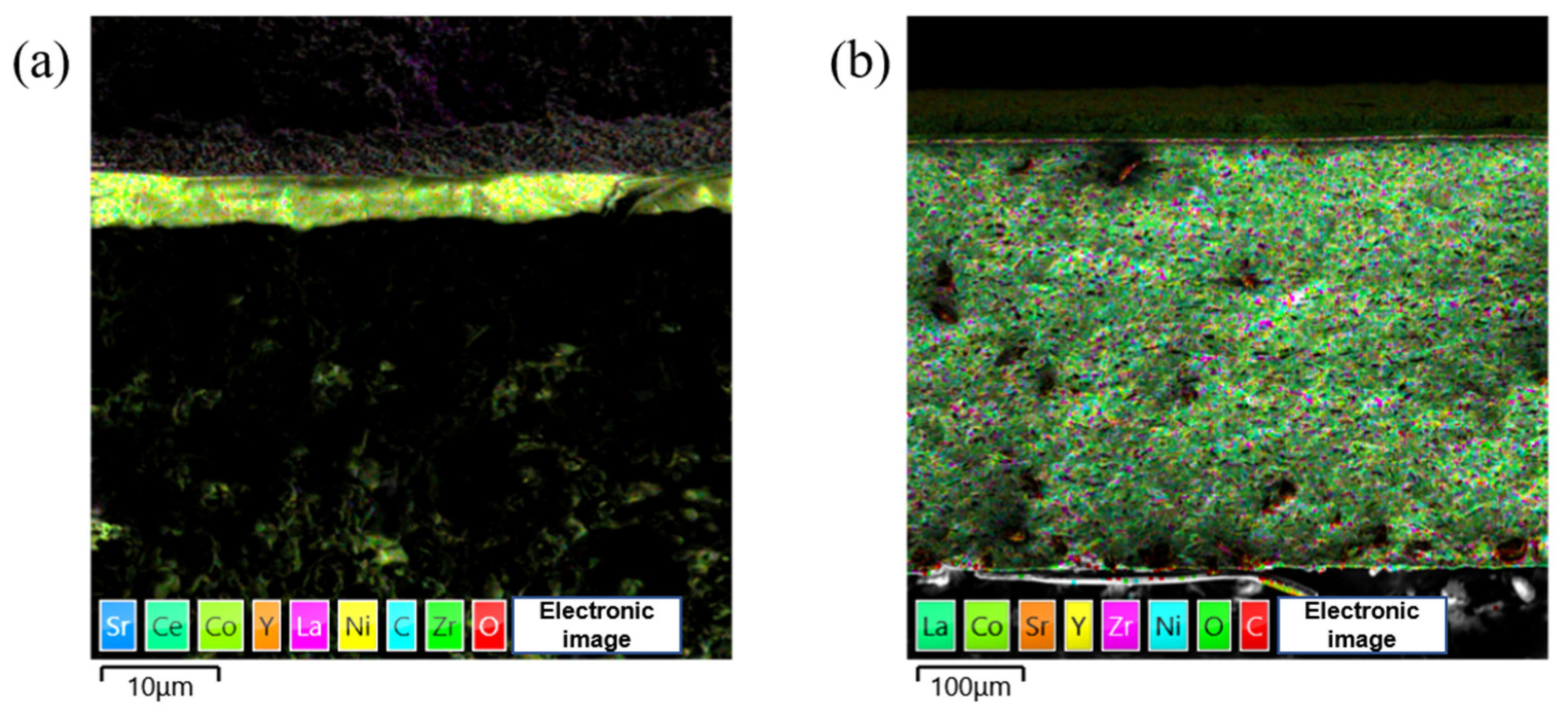
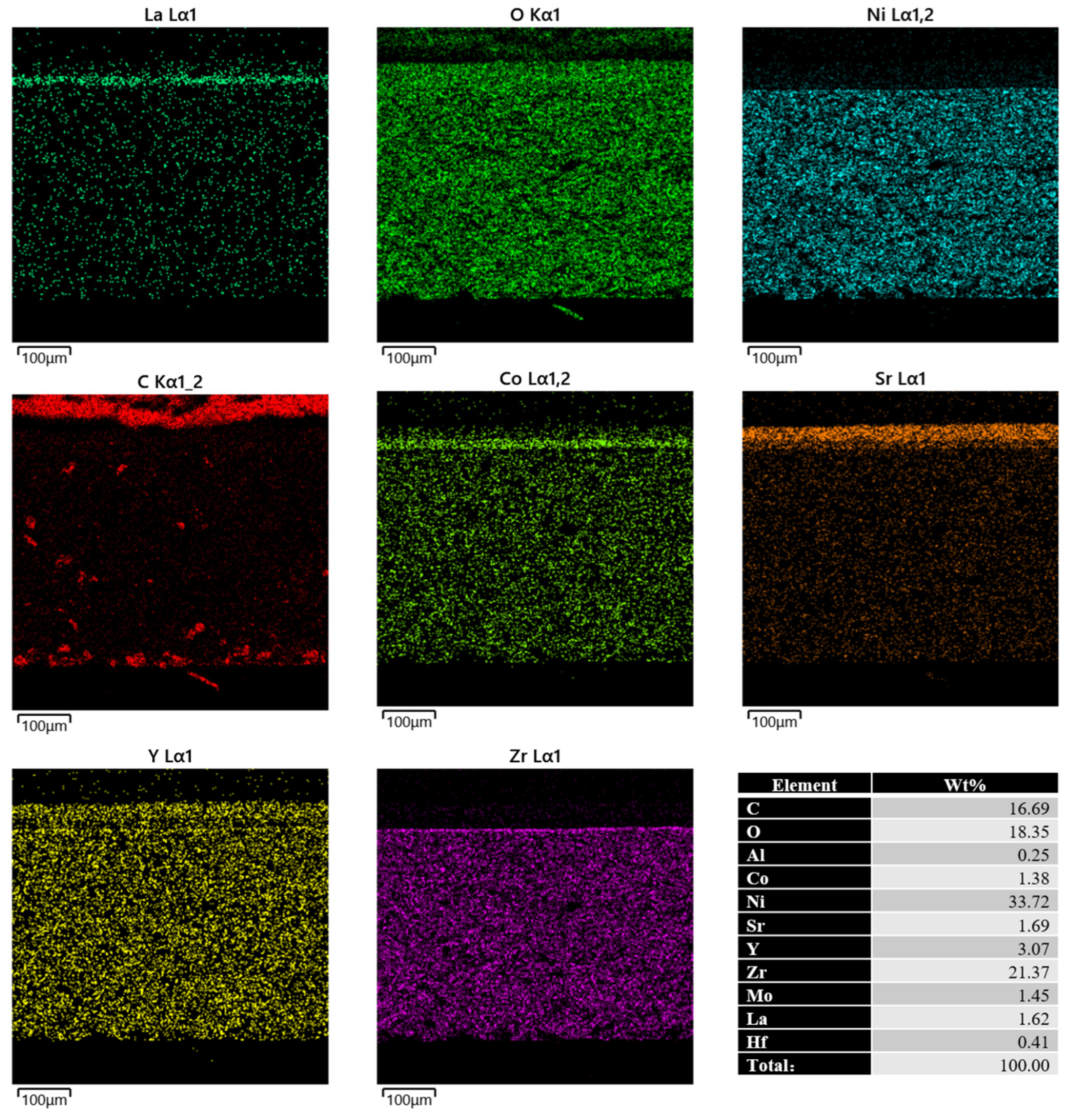
3.3. Microstructural Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zheng, K.; Gao, H.; Zhu, M.; Yan, K.; Li, L.; Lin, X.M.; Wang, Y.; Ni, M. A methane-fueled two-stage SOFC power generation system with carbon capture. Appl. Therm. Eng. 2025, 277, 127066. [Google Scholar] [CrossRef]
- Wachsman, E.D.; Lee, K.T. Lowering the Temperature of Solid Oxide Fuel Cells. Science 2011, 334, 935–939. [Google Scholar] [CrossRef]
- Li, Z.; Yu, J.; Wang, C.; Bello, I.T.; Yu, N.; Chen, X.; Zheng, K.; Han, M.; Ni, M. Multi-objective optimization of protonic ceramic electrolysis cells based on a deep neural network surrogate model. Appl. Energy 2024, 365, 13. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, B.; Guan, D.; Xu, M.; Ran, R.; Ni, M.; Zhou, W.; O’Hayre, R.; Shao, Z. Thermal-expansion offset for high-performance fuel cell cathodes. Nature 2021, 591, 246–251. [Google Scholar] [CrossRef]
- Lee, H.W.; Kim, S.M.; Kim, H.; Jung, H.Y.; Jung, H.G.; Lee, J.H.; Song, H.; Kim, H.R.; Son, J.W. Advanced planar SOFC stack with improved thermo-mechanical reliability and electrochemical performance. Solid State Ion. 2008, 179, 1454–1458. [Google Scholar] [CrossRef]
- Yang, J.; Yan, D.; Huang, W.; Li, J.; Pu, J.; Chi, B.; Jian, L. Improvement on durability and thermal cycle performance for solid oxide fuel cell stack with external manifold structure. Energy 2018, 149, 903–913. [Google Scholar] [CrossRef]
- Pan, J.; Yang, J.; Yan, D.; Pu, J.; Chi, B.; Li, J. Effect of thermal cycling on durability of a solid oxide fuel cell stack with external manifold structure. Int. J. Hydrogen Energy 2020, 45, 17927–17934. [Google Scholar] [CrossRef]
- Shin, J.S.; Saqib, M.; Jo, M.; Park, K.; Park, K.M.; Ahn, J.S.; Lim, H.-T.; Park, J.Y. Degradation Mechanisms of Solid Oxide Fuel Cells under Various Thermal Cycling Conditions. ACS Appl. Mater. Interfaces 2021, 13, 49868–49878. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Jiang, W.; Luo, Y.; Song, M.; Zhang, X.; Tu, S.T. Coupled degradation mechanism of electrochemical and mechanical performance of solid oxide fuel cells under thermal cycling. Appl. Energy 2025, 381, 125187. [Google Scholar] [CrossRef]
- Wu, A.; Yang, J.; Zhang, Y.; Han, B.; Guan, W. High stability of flat-tube solid oxide short stack over 100 thermal cycles. J. Power Sources 2025, 643, 237066. [Google Scholar] [CrossRef]
- Kim, Y.J.; Hwang, S.C.; Lee, J.G.; Lim, H.T. Thermal cycling of anode supported solid oxide fuel cells under various conditions: Electrical anode protection. Int. J. Hydrogen Energy 2016, 41, 23173–23182. [Google Scholar] [CrossRef]
- Hamayun, M.A.; Gong, M.; Park, K.; Jo, M.; Bae, Y.; Kim, M.; Na, Y.; Lim, H.-T.; Park, J.-Y. Degradation patterns and mechanisms of solid oxide fuel cells under rapid thermal cycling at various current densities. J. Power Sources 2025, 658, 238273. [Google Scholar] [CrossRef]
- Xu, Q.; Guo, Z.; Xia, L.; He, Q.; Li, Z.; Bello, I.T.; Zheng, K.; Ni, M. A comprehensive review of solid oxide fuel cells operating on various promising alternative fuels. Energy Convers. Manag. 2022, 253, 115175. [Google Scholar] [CrossRef]
- Zewei, L.; Minfang, H.; Zaihong, S.; Kaihua, S. Evolution of Electrochemical Characteristics of Solid Oxide Fuel Cells During Initial-Stage Operation. Acta Chim. Sin. 2021, 79, 763. [Google Scholar] [CrossRef]
- Zhang, Y.; Xia, C. A durability model for solid oxide fuel cell electrodes in thermal cycle processes. J. Power Sources 2010, 195, 6611–6618. [Google Scholar] [CrossRef]
- Liu, L.; Kim, G.Y.; Chandra, A. Modeling of thermal stresses and lifetime prediction of planar solid oxide fuel cell under thermal cycling conditions. J. Power Sources 2010, 195, 2310–2318. [Google Scholar] [CrossRef]
- Cai, P.Z.; Green, D.J.; Messing, G.L. Constrained Densification of Alumina/Zirconia Hybrid Laminates, I: Experimental Observations of Processing Defects. J. Am. Ceram. Soc. 1997, 80, 1929–1939. [Google Scholar] [CrossRef]
- Joo, J.H.; Jeong, J.; Kim, S.Y.; Yoo, C.Y.; Jung, D.W.; Park, H.J.; Kwak, C.; Yu, J.H. Mosaic-shaped cathode for highly durable solid oxide fuel cell under thermal stress. J. Power Sources 2014, 247, 534–538. [Google Scholar] [CrossRef]
- Chen, K.; Jiang, S.P. Surface segregation in solid oxide cell oxygen electrodes: Phenomena, mitigation strategies and electrochemical properties. Electrochem. Energy Rev. 2020, 3, 730–765. [Google Scholar] [CrossRef]
- Hanif, M.B. Tailoring thermal expansion for next-generation energy systems: Integrating the potential of NTE materials in SOFCs and beyond. J. Power Sources 2025, 633, 236460. [Google Scholar] [CrossRef]
- Lin, C.; Kerscher, F.; Spliethoff, H. Thermal gradient management in solid oxide fuel cells: Mechanisms, strategies, and future directions. J. Power Sources 2025, 656, 238017. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, M.; Cai, B.; Yan, Y.; Zheng, K. Thermal Cycling Stability of NiO/YSZ Anode-Supported SOFC Button Cells: An Experimental Study. Processes 2025, 13, 3747. https://doi.org/10.3390/pr13113747
Zhu M, Cai B, Yan Y, Zheng K. Thermal Cycling Stability of NiO/YSZ Anode-Supported SOFC Button Cells: An Experimental Study. Processes. 2025; 13(11):3747. https://doi.org/10.3390/pr13113747
Chicago/Turabian StyleZhu, Meng, Bowen Cai, Yangtian Yan, and Keqing Zheng. 2025. "Thermal Cycling Stability of NiO/YSZ Anode-Supported SOFC Button Cells: An Experimental Study" Processes 13, no. 11: 3747. https://doi.org/10.3390/pr13113747
APA StyleZhu, M., Cai, B., Yan, Y., & Zheng, K. (2025). Thermal Cycling Stability of NiO/YSZ Anode-Supported SOFC Button Cells: An Experimental Study. Processes, 13(11), 3747. https://doi.org/10.3390/pr13113747





