Abstract
Global population growth, climate change, and the environmental impact of livestock production have accelerated the search for sustainable and efficient protein sources. Fruiting bodies (mushrooms) and mycelial biomass have emerged as promising alternatives due to their high nutritional quality, low ecological footprint, and compatibility with circular bioeconomy principles. This review highlights the nutritional, biotechnological, and environmental aspects of fungal proteins obtained from both fruiting bodies and mycelial biomass of Basidiomycetes. Emphasis is placed on amino acid composition, protein digestibility, and advances in cultivation and fermentation systems for large-scale production. Submerged and solid-state fermentation processes are analyzed in terms of scalability, resource efficiency, and integration with agro-industrial residues for sustainable bioprocessing. Comparative analyses reveal that mycelial biomass production achieves high protein yields with significantly reduced land, water, and energy requirements compared to conventional protein sources. Emerging fungal species such as Schizophyllum commune and Auricularia polytricha demonstrate strong potential for producing protein-rich mycelia applicable to functional and plant-based foods. Finally, the review discusses current technological innovations, regulatory frameworks, and market perspectives that position fungal biomass as a strategic component in the ongoing global protein transition.
1. Introduction
In recent decades, the world has faced increasing challenges regarding food security, driven by population growth, climate change, accelerated urbanization, and the growing demand for animal-derived foods. The global population is projected to reach nearly 10 billion by 2050, requiring a 70% increase in food production. Among essential nutrients, proteins play a central role in human health, being vital for growth, maintenance, and metabolic regulation. Traditionally, most dietary proteins have been obtained from animal sources such as meat, milk, eggs, and fish. However, conventional animal production systems are associated with significant environmental and ethical concerns. They are responsible for high greenhouse gas emissions, particularly methane and carbon dioxide, originating from intensive livestock operations [1]. These systems also demand excessive natural resources, including water and land, which compete with crops intended for human consumption. Moreover, pasture expansion and large-scale monocultures for animal feed contribute to biodiversity loss. Ethical and animal welfare concerns are increasingly influencing consumer preferences and driving demand for alternative proteins (APs). Finally, conventional livestock systems remain vulnerable to zoonotic outbreaks, such as avian influenza, swine fever, and COVID-19, which can disrupt food chains and threaten public health [2,3]. These limitations have driven global efforts to identify AP sources capable of sustainably meeting the growing demand. Emerging categories include plant-based proteins (legumes, cereals, oilseeds, and algae); insect proteins, noted for high efficiency and low environmental impact; cell-cultured meat, produced via animal cell cultivation; and microbial proteins, derived from yeasts, bacteria, and fungi, particularly mycelial biomass from mushrooms [4,5,6,7,8,9].
The transition toward APs is supported by combined environmental, social, and economic drivers: environmental sustainability, aiming to reduce the carbon footprint and ecological impact of food systems; shifting consumer behavior, with increasing vegetarian and vegan populations in both developed and emerging economies; technological advances, including precision fermentation and food biotechnology innovations; health and wellness awareness, as excessive consumption of red and processed meats is linked to chronic diseases; and public policies and incentives, encouraging sustainable production and deforestation reduction [10]. These factors have accelerated what is now referred to as the protein revolution, in which new ingredients and products emerge to meet the demand for sustainable, ethical, and nutritious food alternatives.
Basidiomycetes are a diverse class of higher fungi (phylum Basidiomycota) that includes most mushroom-forming species. They are distinguished by their ability to decompose lignin and other complex polymers, thereby converting lignin-rich agricultural residues into fungal biomass enriched in protein [11]. Many basidiomycetes are natural colonizers of wood, leaf litter, and other lignocellulosic materials, making them ideal candidates for valorizing side streams from forestry and agri-food industries. Because these fungi can be cultivated in enclosed or semi-controlled environments, their production does not require arable land, avoiding competition with crops or livestock systems traditionally used for protein supply.
Globally, approximately 2000 mushroom species are recognized as edible, and a subset of these has been granted Generally Recognized as Safe (GRAS) status for food use [12,13]. However, the majority of basidiomycete species have never entered the food chain, often due to their small fruiting bodies, unappealing taste or texture, or limited yield rather than toxicity [14]. The safety of non-traditional fungal species is regulated under frameworks such as GRAS in the U.S. and Novel Food in the E.U. These frameworks mandate comprehensive safety data, including compositional, allergenic, and toxicological evaluations, for market entry, thereby ensuring consumer safety [15]. Nutritionally, mushrooms are appreciated for their low fat and calorie content, high fiber, and richness in vitamins, minerals, and trace elements. Beyond their basic nutrient profile, several basidiomycetes also produce bioactive compounds associated with immunomodulatory, antioxidant, and metabolic health effects [12,16,17,18,19,20,21,22]. Nevertheless, large-scale mushroom cultivation is technically demanding and currently feasible for only about 80 species worldwide.
A promising alternative is to focus on mycelial biomass production instead of fruiting-body formation. To cultivate substrate mycelia of mushroom-forming fungi offers several advantages: (i) the vegetative (mycelial) phase of many species can grow efficiently on lignocellulosic feedstocks, whereas fruiting requires specific environmental triggers; (ii) bypassing the fruiting stage can significantly reduce production time, energy requirements, and infrastructure costs; (iii) species selection can emphasize protein yield and substrate conversion efficiency rather than mushroom morphology or sensory traits; and (iv) residual mycelial biomass remaining after mushroom cultivation can be recovered and processed as protein-rich material, enhancing resource circularity [11,23].
Despite these advantages, mycelial biomass from Basidiomycetes has received limited attention as a sustainable fungal protein source obtained from lignocellulosic substrates. Its classification as a “Novel Food” under current regulatory frameworks, together with the absence of standardized analytical protocols, has hindered broader adoption and comparative evaluation. Reported protein contents in basidiomycete mycelia vary considerably, largely due to inconsistent nitrogen-to-protein conversion factors, differences in cultivation media, and uncontrolled sampling conditions. Furthermore, knowledge gaps persist regarding optimal strategies for production, harvesting, and downstream processing of basidiomycete mycelia intended for food applications. Addressing these challenges is essential to fully accomplish the potential of Basidiomycetes as renewable and circular contributors to the emerging protein bioeconomy.
The main objective of this review is to provide a comprehensive overview of recent advances in the study of mycoproteins derived from Basidiomycetes, encompassing both fruiting bodies (mushrooms) and mycelial biomass. Special emphasis is placed on proteins produced by mycelial biomass through submerged and SSF systems, which represent promising, scalable, and resource-efficient biotechnological routes for sustainable protein production. By integrating nutritional, biotechnological, and environmental perspectives, this review aims to foster future research and industrial developments that leverage basidiomycete-derived mycoproteins as alternatives to conventional animal-based proteins. Industrial interest in fungal proteins is rapidly expanding, with pilot-scale initiatives and commercial ventures exploring basidiomycete biomass for food and nutraceutical applications. The success of mycoprotein products such as Quorn™ (https://www.quorn.co.uk/mycoprotein, accessed on 20 October 2025) [24], produced from the filamentous fungus Fusarium venenatum, demonstrates the market feasibility of fungal-derived proteins and stimulates the advancement of basidiomycete-based innovations.
2. Nutritional Quality and Biological Value of Proteins from Mushrooms
Proteins from mushrooms are increasingly recognized as valuable alternative sources of nutrition, thanks to their balanced amino acid composition and relatively good digestibility [25]. However, a detailed assessment of their nutritional quality requires evaluation not only of their amino acid profiles but also of their digestibility, protein efficiency, and comparison with conventional protein sources. A key metric for protein quality is the content and proportion of essential amino acids (EAAs) relative to the total amino acid pool [26]. Numerous studies show that many mushroom species contain all nine EAAs, although the relative abundances vary by species, substrate, and environmental conditions [27]. In the same work, environmental effects (soil type, pH, and tree diversity) had less influence on the amino acid profiles than species genetics. In cultivated mushrooms, Ionescu et al. report that Basidiomycota may contain protein levels ranging from 19% to 40% of their dry weight, with a complete and balanced amino acid composition [28]. In Pleurotus ostreatus, 13 amino acids were identified (5 essential, 8 non-essential), with aspartic acid being the most abundant (492.12 mg/100 g) and cysteine the least (9.32 mg/100 g). The ratio of EAAs to total amino acids was 0.11 [29]. In a broader review, Ayimbila et al. emphasize that many mushroom proteins, after in vitro digestion, meet the Food and Agriculture Organization of the United Nations (FAO) amino acid pattern for adults, reinforcing their suitability for human diets [25]. However, limitations are often observed in sulfur-containing amino acids (methionine and cysteine), which tend to be lower in fungal proteins compared to some animal sources [30]. To overcome the limitations of sulfur-containing amino acids, fungal proteins are often combined with complementary protein sources that provide a more balanced EAA profile. Cereal-based proteins such as wheat, rice, and corn are rich in methionine and cysteine, while legume proteins such as soy, lentils, peas, and chickpeas supply additional lysine and threonine, compensating for the limiting amino acids in fungi [31]. Such combinations not only improve the overall Protein Digestibility-Corrected Amino Acid Score (PDCAAS) and the Digestible Indispensable Amino Acid Score (DIAAS) but also enhance functional properties, including texture, water-holding capacity, and emulsification in food formulations. This synergistic approach has been successfully applied in hybrid or plant-forward food products, where fungal and plant proteins together yield complete amino acid composition and favorable sensory characteristics [24,31]. When comparing fungal proteins with more conventional sources (plant, animal), several advantages emerge (Table 1): (a) balanced amino acid profile, particularly good lysine content, helps to mitigate deficits often found in cereal proteins [30]; (b) high efficiency per unit area and lower environmental footprint, which will be discussed in later sections; and (c) lower levels of anti-nutritional factors relative to some plant proteins, although fungal cell wall polysaccharides (e.g., chitin, β-glucans) may affect digestibility unless treated or partially removed. Anti-nutritional factors are naturally occurring compounds, such as tannins, phytates, or trypsin inhibitors, that can reduce nutrient absorption or interfere with digestive enzymes, thereby limiting protein bioavailability [31,32]. Still, to assemble a fully “complete” protein with optimal levels of all EAAs, fungal proteins are often best combined with cereal proteins (which may supply extra methionine) to create a balanced matrix [31,32,33,34].

Table 1.
Essential amino acid composition (mg/g protein) in edible mushrooms compared with animal-derived proteins [25,33,34,35,36,37,38,39,40,41,42].
The data of Table 1 indicate that edible mushrooms provide a well-balanced amino acid profile, particularly rich in leucine, lysine, phenylalanine + tyrosine, and valine, which are often limiting in plant-based proteins such as cereals. Conversely, methionine and tryptophan are relatively lower, suggesting that combining fungal proteins with cereals (rich in sulfur-containing amino acids) could yield complementary nutritional profiles. According to Ayimbila et al., the protein digestibility of mushroom proteins can reach values between 70–85%, particularly after mild thermal or enzymatic processing, placing them in a similar range to legume proteins and approaching the digestibility of egg proteins [25]. Recent studies also confirm that mycelial proteins from submerged cultures can reach 40–45% crude protein content on a dry weight basis, with digestibility and EAA ratios comparable to conventional animal proteins [43,44,45]. The nutritional characteristics and biological value of these mycelial proteins are further elaborated in Section 6, which specifically addresses proteins derived from basidiomycete mycelial biomass.
3. Mushrooms in the Alternative Protein Landscape
Mushrooms and fungal mycelia (Figure 1) occupy a distinctive niche within the broader alternative-protein (AP) portfolio. Beyond their favorable nutritional profile and umami-rich sensorial qualities, they offer scalable bioprocess routes (SmF or SSF) and strong sustainability credentials that align with the goals of the circular bioeconomy. Recent syntheses position mycelium as a nutrient-dense ingredient capable of supporting health while enabling regenerative food systems, with promise for protein fortification and texture in plant-forward foods, that is, dietary patterns emphasizing plant-derived ingredients while still allowing limited inclusion of animal products [33,46].
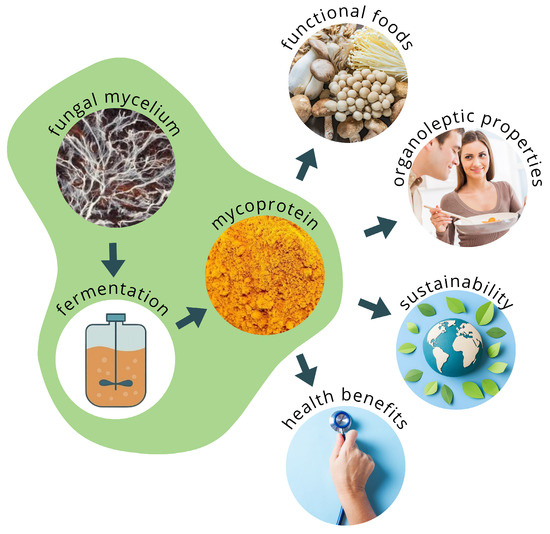
Figure 1.
Schematic representation of mycoprotein production and its potential applications.
Compared with most plant protein isolates and concentrates, fungal proteins generally exhibit a more balanced EAA profile, particularly due to their naturally high lysine levels, which makes them effective for complementing lysine-limited cereal proteins [47]. When subjected to appropriate processing (e.g., mild heat or enzymatic treatments), these proteins can also achieve high in vitro digestibility [47]. Contemporary reviews on mycoproteins (the best-studied form of food-grade fungal biomass) highlight evidence of favorable cardiometabolic effects, satiety responses, and muscle protein synthesis support, while detailing fermentation at scale and food development pathways. From a product-development standpoint, mushrooms contribute intrinsic fibrous structure and umami, enabling partial or full meat replacement with fewer additives and simpler labels. This has been emphasized in recent “mushrooms-as-meat-analogues” assessments and market-readiness overviews [48,49].
Environmental assessments consistently indicate that meals incorporating novel proteins (including fungal biomass) can reduce greenhouse gas (GHG) emissions, land use, and water footprints while maintaining nutritional value. A 2023 environmental and nutritional life-cycle assessment (LCA) reported up to 88% lower GWP, 83% lower land use, and 87% lower scarcity-weighted water use in meals featuring future/novel foods vs. animal source counterparts. A 2024 critical review of mycoprotein-based meat alternatives synthesized multiple LCAs and generally found impacts below those of beef and competitive with other plant-based analogues, while calling for more harmonized, product-level LCA reporting [50]. Earlier and broader syntheses also note lower climate impacts for mycoprotein, though legacy studies used older process assumptions.
On the demand side, recent analyses observe growing familiarity with APs and positive perceptions of mushroom-based products, driven by taste (umami), texture, and “naturalness.” Controlled studies comparing plant-based and mushroom-based meat alternatives report favorable consumer perceptions for shiitake/king oyster formats and highlight opportunities to optimize flavor/texture via processing. Contemporary market analyses (2024–2025) forecast steady expansion of the AP sector encompassing plant-, fungal-, algal-, and cell-cultivated sources. Within this landscape, fungal biomass is recognized for leveraging fermentation expertise and possessing clearer, near-term scalability prospects compared with cell-cultured meat [51].
Collectively, the evidence suggests that mushrooms and mycelia offer: (i) compelling nutrition-functionality, including EAA balance and techno-functional traits for binding, gelling, and texture; (ii) manufacturability through aerobic fermentation using low-cost carbon sources and agro-industrial side streams; and (iii) robust sustainability performance in LCAs relative to ruminant meat and competitive with most plant-based. Recent domain reviews synthesize these strengths and chart R&D priorities (strain improvement, substrate circularity, and product design) to accelerate adoption in staple foods and high-protein formats analogues [23,47].
4. Traditional Mushroom Cultivation as a Protein Source
Traditional mushroom cultivation remains one of the most established and accessible routes for obtaining fungal proteins for human consumption. This system focuses on the production of fruiting bodies (basidiomata), which are consumed fresh, dried, or processed into powders and extracts. Globally, the mushroom industry has evolved from small-scale artisanal production to a high-value agro-industrial sector supplying both domestic and export markets [52]. This cultivation strategy integrates bioconversion of lignocellulosic residues into high-protein food, aligning with circular bioeconomy principles. Agricultural by-products such as straw, sawdust, coffee husks, and fruit residues are transformed into nutrient-dense fungal biomass (Figure 2). Beyond its nutritional contribution, mushroom cultivation provides socioeconomic benefits, including job creation in rural communities and waste valorization from crop residues. The species most widely cultivated worldwide include Agaricus bisporus (button mushroom), Pleurotus spp. (oyster mushrooms), and Lentinula edodes (shiitake), which together account for more than 75% of the global production [53] (Figure 3). Table 2 summarizes their major cultivation features and nutritional relevance.
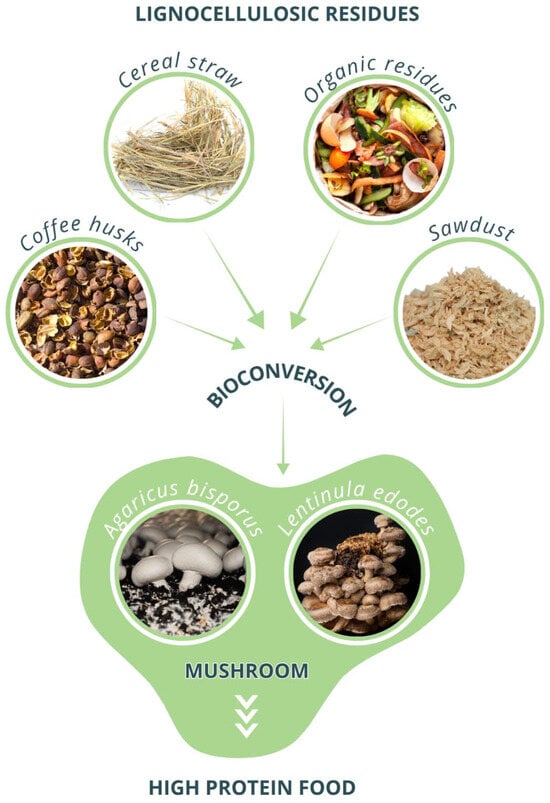
Figure 2.
Schematic representation of the bioconversion of agro-industrial residues into high-protein mushrooms. Agricultural and food by-products, such as coffee husks, cereal straw, fruit and vegetable residues, and sawdust, serve as lignocellulosic substrates for fungal growth. Through bioconversion processes mediated by basidiomycetes, these low-value residues are transformed into nutrient-rich biomass. The two images at the bottom illustrate the cultivation of edible mushrooms (Agaricus bisporus and Lentinula edodes), exemplifying the final stage of biotransformation into nutrient-dense, high-protein food products.
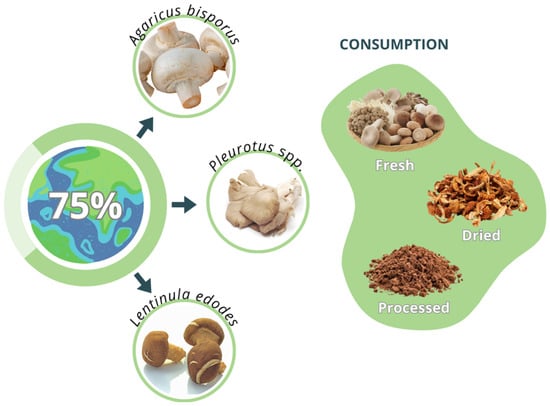
Figure 3.
The most widely cultivated mushroom species worldwide.

Table 2.
Main cultivated mushroom species and key characteristics [17,52,54,55].
The substrate composition is critical to mushroom productivity and protein yield. Substrates not only provide carbon, nitrogen, and minerals but also influence enzyme secretion and flavor profile. Increasingly, agricultural residues such as sugarcane bagasse, corn stover, wheat bran, coffee husks, cocoa shells, and brewery by-products are used to enhance sustainability. Successful cultivation depends on precise control of environmental parameters such as temperature, humidity, light, aeration, and pH (Table 3). These parameters vary by species and strongly determine the kinetics of mycelial growth and fruiting body formation.

Table 3.
Main environmental factors controlling mushroom growth and fruiting.
Large-scale mushroom farming contributes directly to food security and rural development. Rapid growth cycle and substrate versatility enable production in small or urban facilities, promoting distributed protein production with minimal land demand [45]. Moreover, the reuse of agro-industrial by-products reduces methane emissions from residue decomposition and supports sustainable waste management [60]. Nevertheless, the traditional fruiting body system has intrinsic constraints that limit scalability as a global protein source. These include long cultivation cycles, sensitivity to environmental fluctuations, and low protein yield per area and time due to high water content (80–90% in fresh biomass). Additionally, risk of contamination by competing fungi or bacteria remains a bottleneck requiring rigorous sanitation [25]. Hence, the integration of biotechnological production systems, notably mycelial biomass fermentation, offers a complementary and potentially superior pathway to achieve higher productivity, shorter cycles, and greater control, as discussed in the next section.
5. Mycelial Biomass Production: An Emerging Biotechnological Platform
The production of mycelial biomass, the vegetative filamentous network of fungi, represents a major innovation in the generation of fungal proteins and other functional biopolymers. Unlike traditional cultivation systems focused on fruiting bodies, this approach harnesses the continuous growth of mycelium under controlled bioreactor conditions, enabling scalable and standardized production. Recent reviews have emphasized that mycelium-based systems offer higher productivity, shorter growth cycles, and improved process control while aligning with circular economy principles using low-cost agricultural side streams as feedstocks (Table 4). Mycelial biomass can be used either as a whole protein ingredient or as a precursor for extraction of proteins, polysaccharides, and bioactive compounds [22,61,62,63]. The typical crude protein content ranges from 35–45% (dry weight), exceeding most edible mushrooms and comparable to soy protein isolates [45]. Although both derive from the same fungal organism, fruiting bodies (basidiomata) and mycelium differ markedly in morphology, composition, and production logistics [55]. These characteristics explain why the biotechnological route is increasingly considered the next-generation platform for fungal protein manufacturing, combining speed, safety, and flexibility [33].

Table 4.
Differences between fruiting bodies and mycelial biomass [64,65].
Mycelial biomass can be produced under either submerged fermentation (SmF) or solid-state fermentation (SSF), each offering distinct operational and economic characteristics that influence protein yield, composition, and process sustainability. In SmF, fungal cells grow in a fully immersed liquid medium under precisely controlled physicochemical conditions, enabling reproducible scale-up, continuous operation, and integration with automated monitoring systems [66,67,68]. This technology is well-established for industrial enzyme and metabolite production and has recently been adapted for the cultivation of edible and filamentous fungi to obtain uniform, food-grade mycelial biomass [69,70]. However, high energy input for aeration and agitation, water consumption exceeding 90%, and the need for soluble feedstock represent major constraints at large scale.
Conversely, SSF relies on fungal colonization of a moist solid matrix without free water, often using agro-industrial residues such as wheat bran, rice husk, bagasse, coffee husks, or fruit peels [66,71,72]. This system mimics natural fungal habitats and achieves high product concentration, low effluent generation, and superior circular bioeconomy potential [73,74]. Despite limitations in process control, temperature uniformity, and contamination risk, SSF is increasingly attractive for the sustainable production of mycoproteins and co-products such as enzymes, polysaccharides, and organic acids. The complementary features of SmF and SSF have also motivated the development of hybrid fermentation systems that combine efficient oxygen transfer with low resource demand, aligning with the broader goals of circular and low-carbon food production (as illustrated by Figure 1). Bioreactors for SSF, such as tray systems, packed beds, or rotating drums, are now being re-engineered to allow semi-continuous aeration, humidity control, and real-time monitoring of CO2 evolution and temperature to improve process consistency. Table 5 compares the key operational, economic, and environmental aspects of SmF and SSF, illustrating how each approach offers unique advantages for mycelial biomass cultivation and integration within circular bioprocessing frameworks.

Table 5.
Comparative overview of submerged (SmF) and solid-state (SSF) fermentation for mycoprotein production and other bioactives [68,73,75,76].
Table 5.
Comparative overview of submerged (SmF) and solid-state (SSF) fermentation for mycoprotein production and other bioactives [68,73,75,76].
| Aspect | Submerged Fermentation (SmF) | Solid-State Fermentation (SSF) |
|---|---|---|
| Medium | Liquid nutrient medium: biomass fully immersed | Moist solid matrix without free liquid |
| Typical bioreactors (see Figure 4) | Stirred-tank, airlift, or bubble-column reactors with controlled aeration and agitation | Trays, packed-bed, or rotating-drum reactors; rely on passive aeration |
| Installation cost | High. requires complex, stainless-steel, pressure-resistant systems | Low to moderate—simpler materials and modular configurations |
| Water consumption | High (90–95% moisture) | Very low (40–70% moisture) |
| Substrate flexibility | Requires soluble and clarified substrates (e.g., glucose, molasses, corn steep liquor) | Can directly use agro-industrial residues (bran, bagasse, coffee husks, fruit peels) |
| Oxygenation | Controlled by aeration and agitation; may be limited by viscosity | Passive diffusion through the solid matrix; generally efficient |
| Process control | Fully automated (pH, DO, temperature, agitation, aeration); supports continuous operation | Limited, but improving with sensor integration (humidity, temperature, CO2) |
| Scalability | Excellent (industrial fermenters 10–100 m3); high reproducibility | Moderate; scale-up limited by heat and mass transfer gradients |
| Production cycle | 2–10 days depending on strain and process mode | 5–15 days depending on substrate and aeration efficiency |
| Contamination risk | Very low due to sterile, closed operation | Moderate to high in open or semi-open systems |
| Biomass homogeneity | High; produces uniform, food-grade biomass | Heterogeneous; variable moisture and composition |
| Energy demand | Higher. Continuous agitation and aeration | Lower. Passive air circulation and minimal mixing |
| Downstream processing | Biomass easily recovered by filtration or centrifugation; compatible with drying | Complex separation from solid matrix; requires mechanical disruption |
| Protein yield and composition | 25–35% protein (dry weight); composition adjustable via C/N ratio and oxygenation | 20–30% protein; enriched in essential amino acids, depending on substrate |
| Environmental impact | Generates wastewater but can integrate with effluent treatment and biorefineries | Minimal wastewater; valorizes residues, reducing landfill burden |
| Circular bioeconomy potential | Moderate. Requires feedstock pre-treatment and liquid waste handling | High. Direct valorization of lignocellulosic residues and co-production of enzymes |
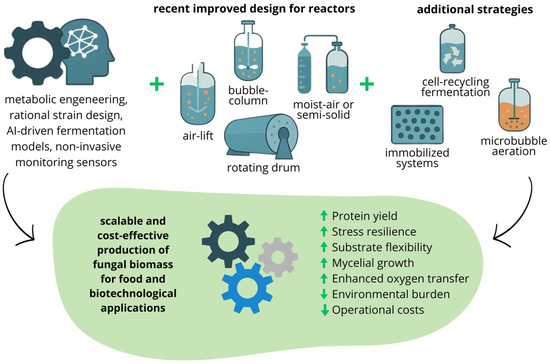
Figure 4.
Schematic overview of advanced bioreactor configurations and their advantages for fungal mycelial biomass and mycoprotein production.
Recent studies have explored hybrid fermentation systems designed to merge the operational precision of SmF with the resource efficiency of SSF. These configurations, such as rotating-drum, trickle-bed, and semi-solid bioreactors (partly illustrated by Figure 1), enable improved oxygen diffusion and moisture control while maintaining moderate energy inputs and low contamination risks [77]. Hybrid setups also facilitate cascading biorefinery concepts, where a single fermentation process yields multiple value-added products, including proteins, enzymes, and polysaccharides, thus enhancing process profitability and circularity [78]. The integration of sensor-based monitoring, AI-driven control, and digital twin modeling (Figure 1) further optimizes these hybrid systems, allowing precise regulation of dissolved oxygen, pH, and nutrient availability in real time [79]. Collectively, these innovations represent the next generation of precision fungal biomanufacturing, bridging the scalability of submerged systems with the sustainability and circularity of solid-state platforms [80,81]. Integration with digital technologies, such as soft-sensor monitoring, real-time control of dissolved oxygen and redox potential, and metabolic modeling, has transformed fungal protein production into a high-precision biomanufacturing platform. These hybrid systems enable cascading valorization, wherein a single process yields multiple products: proteins, enzymes, polysaccharides, and secondary metabolites. When coupled with biorefinery concepts and low-cost lignocellulosic substrates, they provide an efficient route toward sustainable food, feed, and biomaterial production aligned with circular economy goals.
In addition to the choice of fermentation mode (SmF or SSF), mycoprotein-oriented processes require carefully balanced media that provide fermentable carbon, assimilable nitrogen, minerals, vitamins and, in some cases, specific supplements (e.g., trace elements or organic acids) to support fast mycelial growth and high protein yields. For submerged cultivation of basidiomycetes, glucose, sucrose or lignocellulosic hydrolysates are typically combined with complex nitrogen sources such as yeast extract, peptone or corn steep liquor, often at C/N ratios between 10 and 20, together with phosphate and magnesium salts and trace minerals. Representative examples include Pleurotus ostreatus grown in glucose–yeast extract media optimized for protein-rich single-cell protein production, and Pleurotus albidus cultivated in sucrose–yeast extract medium for high mycelial biomass [61]. In SSF, the solid substrate itself provides most of the carbon (e.g., cereal straws, maize stalks or other agro-industrial residues) and is supplemented with cereal brans, simple sugars, urea or ammonium salts, plus mineral additives such as gypsum and lime to adjust structure and pH. Recent studies with Pleurotus and Lentinus spp. on highland barley straw or maize residues have shown that modest supplementation (1–3% glucose, 1–3% urea and 1–2% cereal meals) markedly enhances mycelial growth, crude protein content and digestibility of the fermented matrix, illustrating how nutrient formulation can be tuned to maximize the nutritional quality of the resulting mycoprotein-rich biomass [82]. Some examples of cultivation media are presented in Table 6.

Table 6.
Representative media and supplements used for mycelial biomass production of basidiomycetes in submerged (SmF) and solid-state fermentation (SSF) for mycoprotein applications.
6. Nutritional Quality and Biological Value of Proteins from Mycelial Mass
Edible basidiomycete mycelia (notably Pleurotus, Lentinula, Agaricus) provide complete proteins with all indispensable amino acids (IAA) and favorable digestibility, positioning them alongside high-quality plant and animal proteins by modern metrics of protein quality (PDCAAS/DIAAS). The FAO recommends DIAAS as the preferred regulatory metric and provides IAA reference patterns used to benchmark foods [26]; mycelial biomass typically meets or approaches these patterns, with lysine often abundant and sulfur amino acids supplied at adequate levels in mixed diets. In stirred-tank SmF, Pleurotus ostreatus produced mycelial biomass with high protein yield and a “rich amino acid profile”; mixed C6/C5 sugar feeds (glucose/xylose) improved both biomass and amino-acid composition, supporting use on lignocellulosic hydrolysates [61]. For Lentinula edodes, recent analyses of cultured mycelia report 17 amino acids with sufficient totals of the nine IAAs (≈358–398 mg g−1 protein), indicating high-quality, readily utilizable protein [87]. For Agaricus, classic SmF work scaled to commercial fermenters yielded protein-rich mycelium used to make meat analogues with superior texture/umami versus soy protein, corroborating a balanced amino-acid makeup in practice [88]. Comprehensive mushroom reviews also report protein ranges (≈23–40% DW in basidiomycetes) and EAA completeness relative to plant and animal foods [28].
For edible mycelial mass, SmF in stirred-tank or air-lift bioreactors is preferred due to tighter control and food-grade processability; recent work demonstrates 4-L STBR runs for Pleurotus on wood-hydrolysate and 100-L fed-batch edible mushroom cultures with >22 g L−1 biomass. Air-lift reactors reduce shear on filamentous mycelia and support prolonged/semi-continuous operation; recent reviews compare airlift to stirred-tank and bubble-column designs, and describe pelletization control for better mass transfer [61]. SSF is increasingly employed to cultivate structured, whole-cut mycelium on moist solid substrates, providing desirable texture when the goal is a fibrous mouthfeel rather than dispersed protein. Industrial examples demonstrate the use of SSF to produce whole-cut mycelium-based foods [45]. Under controlled SSF conditions, an edible aerial mycelium can be generated, yielding a cohesive and fibrous biomass suitable for use as a food ingredient. This structured mycelium forms the basis for mycelium-derived foods, including whole-cut products such as mycelium bacon, that reproduce the texture and sensory characteristics of conventional whole-muscle meats. Various Basidiomycetes, particularly Pleurotus and Lentinula species, have been successfully cultivated under these conditions to obtain high-protein, fiber-rich matrices suitable for meat analogues and other sustainable food formulations. Such processes exemplify the integration of fungal biomass engineering and circular bioprocessing for the creation of nutritious and environmentally responsible APs [89].
7. Environmental and Economic Sustainability of Fungal Biomass Production
Fungal biomass production, whether through fruiting-body cultivation or controlled fermentation, provides a low-impact route to high-quality proteins. Numerous LCA studies demonstrate that mycelial protein systems drastically reduce GHG emissions, land occupation, and freshwater consumption when compared with ruminant or monogastric livestock. The substation of 20% of global beef consumption with microbial or fungal proteins could cut agricultural GHG emissions by up to 50% by mid-century [90]. More granular LCAs show that mycoprotein-based foods emit 0.45–1.0 kg CO2-eq per kg product, compared with 27 kg CO2-eq per kg beef and 3.3 kg CO2-eq per kg soy [50]. Additionally, fermentative mycelium systems require more than 90% less land area and 80–90% less water than beef and pork supply chains [91]. Recent syntheses position mycelium as a nutrient-dense ingredient capable of supporting health while enabling regenerative food systems, with promise for protein fortification and texture in plant-forward foods. In particular, fungal fermentation of agro-residues (e.g., lignocellulosic biomass, fruit pomace, spent grains) demonstrates significant substrate flexibility and waste valorization potential [92,93,94]. LCA studies further show that processes based on fungal biomass grown on low-grade feedstocks can achieve lower eutrophication and acidification scores than many traditional protein systems [95]. This circular approach supports in full the multiple United Nations Sustainable Development Goals (SDGs 2, 9, 12, 13, and 15).
Table 7 summarizes comparative LCA indicators for beef, plant-based, and fungal/mycelial protein systems. The data highlights the substantial environmental advantages of fungal biomass production relative to conventional livestock and even to soy or pea protein isolates. Mycoprotein products typically emit between 0.5 and 1.2 kg CO2-eq kg−1, compared with 27–60 kg CO2-eq kg−1 for beef and 1–5 kg CO2-eq kg−1 for plant proteins. These fermentation-based systems also require more than 90% less land and 80–90% less water than beef or pork production chains, while maintaining moderate energy demands. In addition to the favorable LCA profile, fungal processes enable cascading residue valorization, converting low-value lignocellulosic materials into proteins, enzymes, and polysaccharides within a circular bioeconomy framework. Collectively, these metrics reinforce the potential of mycelial proteins as sustainable, resource-efficient alternatives to animal-derived proteins, contributing to climate-mitigation targets and several UN Sustainable Development Goals.

Table 7.
Comparative environmental indicators for beef, plant-based, and fungal/mycelial proteins.
The global alternative-protein market was valued at USD 15.3 billion in 2023 and is projected to reach USD 26.5 billion by 2030. Within this space, fungal and microbial proteins are among the fastest-growing segments [97]. Mycelial biomass production offers a compelling techno-economic balance, lower feedstock cost (due to waste-substrate utilization) and high yield per m3 reactor. Pilot techno-economic analyses suggest production costs of approximately USD 3.55 kg−1 of dry fungal biomass, comparable to plant-based protein isolates [85]. Reported benchmarks indicate that soy protein isolates are priced around USD 3 kg−1 [98], while protein isolates from legumes such as peas generally range between USD 4–5 kg−1 depending on purity and extraction method [99]. Modular bioreactor installations and co-location within existing food-processing plants further reduce logistics and energy overhead. From a policy standpoint, recent EU initiatives, especially the Commission Communication “Boosting Biotechnology and Biomanufacturing in the EU” (2024) and the CBE JU calls (2024–2025), explicitly support the deployment of bio-based and mycelial processes using residues and side-streams within regional agro-industrial clusters, strengthening circular value chains [100,101].
Integrating fungal fermentation within biorefinery frameworks maximizes resource efficiency. Residual solids from fermentation can serve as soil conditioners or feed additives, while process water and CO2 streams are recycled to subsequent cultivation batches. Hybrid bioprocesses co-producing proteins, enzymes, and polysaccharides (β-glucans, chitin derivatives) increase profitability while minimizing waste. Overall, the combination of low environmental footprint, competitive economics, and policy alignment positions mycelial biomass as a strategic enabler of circular food systems capable of decoupling protein supply from conventional agriculture [80,102].
8. Emerging Basidiomycetes Species for Protein-Rich Mycelial Biomass
While Pleurotus ostreatus, Lentinula edodes, and Ganoderma lucidum remain dominant in fungal biotechnology, several non-conventional basidiomycetes and ascomycetes have emerged as promising candidates for large-scale mycelial protein production. These species combine high protein content with functional properties such as emulsifying capacity, gelling ability, and antioxidant activity. Recent studies emphasize that expanding the taxonomic diversity of edible fungi is essential to ensure resilient and regionally adaptable protein sources, especially those compatible with low-cost agro-industrial residues as substrates.
Expanding the diversity of fungal species used for mycelial biomass production supports regionalized protein supply chains and stimulates innovation in functional foods. Each species exhibits distinctive biochemical and rheological traits, broadening potential applications that range from protein enrichment to natural emulsifiers and gelling agents. Recent technological developments have enabled dual-purpose fermentations, in which nutritional biomass and high-value metabolites (e.g., pleuromutilin, cordycepin, β-glucans) are co-produced, improving process economics and reinforcing the circular biorefinery model [65]. Table 8 exemplifies the growing potential of underexplored fungi in sustainable protein production. Collectively, these underexplored fungi demonstrate high nutritional quality, versatile bioprocess adaptability, and compatibility with agro-residue-based substrates, strengthening the role of fungal biotechnology in sustainable protein production and circular food systems.

Table 8.
Under-explored basidiomycetes that show promise as alternative mycoprotein sources via submerged fermentation (SmF) or solid-state fermentation (SSF).
9. Future Perspectives and Challenges
Modern strain-engineering strategies, supported by integrative omics, genomics, transcriptomics, proteomics, and metabolomics, are reshaping fungal biotechnology. Whole-genome sequencing and system-level analyses in key basidiomycetes have elucidated the molecular networks governing amino acid biosynthesis, carbon utilization, and environmental adaptation. In Pleurotus ostreatus, genome-wide and transcriptomic studies identified extensive gene families involved in lignocellulose breakdown, nitrogen metabolism, and oxidative defense [108]. The Ganoderma lucidum genome revealed central pathways for triterpenoid formation and stress tolerance [18], while comparative omics in Schizophyllum commune demonstrated coordinated regulation of carbohydrate and amino acid metabolism during development [109]. Insights from Hericium erinaceus expanded this perspective: its integrated genome–transcriptome map uncovered the molecular basis of polysaccharide and amino acid synthesis [110], and multi-omics coupling of transcriptome and metabolome profiling revealed carbon-flux reprogramming and glutamate-centered metabolic modules under varying substrates [111]. Collectively, these advances in omics and systems biology enable rational strain design and targeted metabolic engineering to boost protein productivity, enhance stress resilience, and broaden substrate adaptability, key attributes for sustainable mycoprotein and fungal biorefinery platforms.
Recent innovations in bioreactor engineering, such as air-lift, bubble-column, and stirred-tank configurations, have advanced scalable, cost-efficient fungal biomass production for food and industrial use. Airlift and bubble-column systems provide superior gas exchange and reduced shear relative to conventional stirred tanks, fostering robust mycelial growth in SmF [102]. Emerging designs, including rotating-drum, moist-air, and semi-solid bioreactors (Figure 1), aim to merge the aeration efficiency of liquid systems with the resource economy of solid-state processes [112]. Continuous, non-invasive monitoring of dissolved oxygen, pH, and CO2, coupled with feedback-control algorithms, ensures process stability and consistent yields.
The integration of digital-twin technology and AI-driven fermentation modeling has further optimized biomass kinetics, nutrient flow, and energy utilization, accelerating process intensification (Figure 1) [79]. Complementary approaches, such as immobilized fungal cultures, cell-recycle fermentation, and microbubble aeration, are increasingly adopted to improve oxygen delivery and substrate conversion while reducing operational costs and environmental impacts [45,113].
The global landscape for regulatory approval of fungal-based proteins is rapidly evolving. Several products derived from mycelial biomass have already achieved GRAS status in the United States [114], while the European Food Safety Authority (EFSA) has granted Novel Food authorization for Neurospora intermedia and Fusarium venenatum-based ingredients [115]. In 2025, the EFSA initiated a harmonized risk assessment framework for “novel microbial biomass foods,” covering compositional, allergenic, and toxicological evaluations [116]. These advances lower barriers for commercial adoption of mycelial biomass in functional foods, beverages, and nutraceuticals.
From the market perspective, consumer acceptance of mycelium-based products continues to improve, driven by sensory similarity to meat, clean-label perception, and sustainability narratives [49]. Nevertheless, communication strategies should emphasize safety, traceability, and local production to reinforce consumer trust.
The convergence of fungal and plant-protein technologies is driving a hybrid-protein paradigm. Recent work shows that edible mycelium can act as an inactivated or living scaffold for anchorage-dependent animal cells, offering both structural support and nutritional value. For example, heat-treated Aspergillus oryzae mycelium pellets successfully served as a scaffold, supporting the proliferation and differentiation of muscle precursor cells (C2C12) and bovine satellite cells in vitro [117]. Because the microcarrier remains edible and is incorporated into the final product, it removes the need for carrier-removal steps, thereby simplifying the bioprocess and improving sustainability [103].
Mycelial proteins can complement plant isolates to improve amino acid balance, texture, and binding functionality in food matrices. Hybrid systems that integrate mycelial scaffolding into plant-based meats or dairy analogues deliver superior mouthfeel and nutritional density, while reducing ingredient complexity [118]. This trend aligns with the principles of food system circularity, where multiple biological streams, plants, fungi, and microalgae, are integrated to minimize waste and maximize nutritional output. Emerging research also explores 3D food printing with fungal proteins, enabling customizable textures and nutrient profiles [111]. These technologies bridge sustainability and innovation, paving the way for next-generation functional foods.
Although remarkable progress has been achieved, several technical and socioeconomic barriers still limit the large-scale production and market adoption of mycoproteins. Differences in strain behavior, such as growth kinetics and metabolite profiles, make it difficult to ensure consistent process performance [67]. Rheological challenges, including high broth viscosity and oxygen transfer limitations, continue to affect bioreactor efficiency and downstream processing [102,119]. From a market perspective, many consumers still perceive fungal biomass as a “mushroom extract” rather than a genuine protein source, which slows public acceptance and demand [120]. Regulatory discrepancies, particularly across Novel Food approval systems in different regions, further delay product commercialization [121]. Moreover, additional in vivo research on digestibility, nutrient absorption, and long-term health outcomes is essential to support nutritional and safety claims [65]. Addressing these interconnected challenges will require collaboration across disciplines, linking mycology, bioprocess engineering, computational modeling, food science, and policy, to realize the full potential of mycelial proteins in future sustainable diets.
10. Conclusions
The transition toward sustainable and resilient food systems requires diversified protein sources that minimize environmental impact while ensuring nutritional adequacy. In this context, mushrooms and mycelial biomass from basidiomycetes represent one of the most promising biotechnological routes to produce APs at scale. This review demonstrates that fungal proteins provide balanced EAA profiles, high digestibility, and additional health-promoting compounds such as β-glucans and phenolic conjugates. Beyond nutritional aspects, fungal bioprocesses offer an exceptional environmental advantage: drastically lower greenhouse gas emissions, land use, and water consumption compared with conventional livestock, while valorizing agricultural residues and integrating into circular bioeconomy models. Traditional mushroom cultivation continues to play an important role in food security and rural income, yet bioreactor-based mycelial biomass production enables faster, controlled, and scalable systems capable of meeting industrial demand. Emerging fungal species, including Schizophyllum commune and Auricularia polytricha, expand the spectrum of high-protein, functional ingredients suited for various food matrices. Recent progress in omics-guided strain engineering, bioreactor automation, and hybrid protein formulations consolidates fungi as a central pillar of the ongoing protein transition. However, challenges remain in large-scale process optimization, regulatory harmonization, and consumer communication. Continued interdisciplinary research and policy support will be essential to translate the full potential of fungal biomanufacturing into commercially viable and nutritionally impactful solutions. Ultimately, integrating mycelial protein production within circular biorefineries and sustainable food systems can contribute substantially to global climate goals, resource efficiency, and health-oriented innovation, positioning fungi not merely as an alternative but as a cornerstone of the future food landscape.
Author Contributions
A.R.d.F.T.: Writing—original draft, Methodology, Investigation, Formal analysis. I.d.B.H.: Writing—original draft. E.A.G.d.R.: Writing—original draft. L.A.d.R.B.d.S.: Writing—original draft. C.G.M.d.S.: Writing—review & editing. B.M.R.F.: Conceptualization, Visualization. M.P.D.: Conceptualization, Visualization. R.C.G.C.: Writing—review & editing, Conceptualization, Visualization, Funding acquisition. N.U.Y.: Writing—review & editing, Conceptualization, Visualization, Funding acquisition. A.B.: Writing—review & editing, Funding acquisition. R.M.P.: Writing—review & editing, Validation, Conceptualization, Funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Council for Scientific and Technological Development (CNPq, Brazil) through the research grant contracts with A. Bracht, N.U. Yamaguchi [grant number 312892/2025-0]; R.M. Peralta, and R.C.G. Corrêa [grant number 313009/2025-2]. R.C.G. Corrêa is also a research grant recipient of Cesumar Institute of Science Technology and Innovation (ICETI).
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Verkuijl, C.; Smit, J.; Green, J.M.H.; Nordquist, R.E.; Sebo, J.; Hayek, M.N.; Hötzel, M.J. Climate Change, Public Health, and Animal Welfare: Towards a One Health Approach to Reducing Animal Agriculture’s Climate Footprint. Front. Anim. Sci. 2024, 5, 1281450. [Google Scholar] [CrossRef]
- Lima, M.; Costa, R.; Rodrigues, I.; Lameiras, J.; Botelho, G. A Narrative Review of Alternative Protein Sources: Highlights on Meat, Fish, Egg and Dairy Analogues. Foods 2022, 11, 2053. [Google Scholar] [CrossRef]
- Malila, Y.; Owolabi, I.O.; Chotanaphuti, T.; Sakdibhornssup, N.; Elliott, C.T.; Visessanguan, W.; Karoonuthaisiri, N.; Petchkongkaew, A. Current Challenges of Alternative Proteins as Future Foods. Npj Sci. Food 2024, 8, 53. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Chen, E.; Zhang, X.; Li, D.; Wang, Q.; Sun, Y. Nutritional Value and Physicochemical Characteristics of Alternative Protein for Meat and Dairy—A Review. Foods 2022, 11, 3326. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Nguyen, M.; Bu, Y. Consumer Alternative Protein Choice in Climate Change: Temporal Landmarks, Self-Transcendence, and Mindset Abstraction. Appetite 2025, 210, 107974. [Google Scholar] [CrossRef] [PubMed]
- Quintieri, L.; Nitride, C.; De Angelis, E.; Lamonaca, A.; Pilolli, R.; Russo, F.; Monaci, L. Alternative Protein Sources and Novel Foods: Benefits, Food Applications and Safety Issues. Nutrients 2023, 15, 1509. [Google Scholar] [CrossRef]
- Medeiros, F.; Aleman, R.S.; Gabríny, L.; You, S.W.; Hoskin, R.T.; Moncada, M. Current Status and Economic Prospects of Alternative Protein Sources for the Food Industry. Appl. Sci. 2024, 14, 3733. [Google Scholar] [CrossRef]
- Pastrana-Pastrana, Á.J.; Rodríguez-Herrera, R.; Solanilla-Duque, J.F.; Flores-Gallegos, A.C. Plant Proteins, Insects, Edible Mushrooms and Algae: More Sustainable Alternatives to Conventional Animal Protein. J. Future Foods 2025, 5, 248–256. [Google Scholar] [CrossRef]
- Hadi, J.; Brightwell, G. Safety of Alternative Proteins: Technological, Environmental and Regulatory Aspects of Cultured Meat, Plant-Based Meat, Insect Protein and Single-Cell Protein. Foods 2021, 10, 1226. [Google Scholar] [CrossRef]
- Augustin, M.A.; Hartley, C.J.; Maloney, G.; Tyndall, S. Innovation in Precision Fermentation for Food Ingredients. Crit. Rev. Food Sci. Nutr. 2024, 64, 6218–6238. [Google Scholar] [CrossRef]
- Scholtmeijer, K.; van den Broek, L.A.M.; Fischer, A.R.H.; van Peer, A. Potential Protein Production from Lignocellulosic Materials Using Edible Mushroom Forming Fungi. J. Agric. Food Chem. 2023, 71, 4450–4457. [Google Scholar] [CrossRef] [PubMed]
- Łysakowska, P.; Sobota, A.; Wirkijowska, A. Medicinal Mushrooms: Their Bioactive Components, Nutritional Value and Application in Functional Food Production—A Review. Molecules 2023, 28, 5393. [Google Scholar] [CrossRef] [PubMed]
- De Cianni, R.; Pippinato, L.; Mancuso, T. A Systematic Review on Drivers Influencing Consumption of Edible Mushrooms and Innovative Mushroom-Containing Products. Appetite 2023, 182, 106454. [Google Scholar] [CrossRef] [PubMed]
- Gardeli, C.; Mela, N.; Dedousi, M.; Kandyliari, A.; Kaparakou, E.; Diamantopoulou, P.; Pappas, C.; Mallouchos, A. The Influence of Substrate and Strain on Protein Quality of Pleurotus Ostreatus. Appl. Sci. 2024, 14, 4040. [Google Scholar] [CrossRef]
- Heo, S.; Lee, G.; Na, H.-E.; Park, J.-H.; Kim, T.; Oh, S.-E.; Jeong, D.-W. Current Status of the Novel Food Ingredient Safety Evaluation System. Food Sci. Biotechnol. 2024, 33, 1–11. [Google Scholar] [CrossRef]
- Angelova, G.; Brazkova, M.; Mihaylova, D.; Slavov, A.; Petkova, N.; Blazheva, D.; Deseva, I.; Gotova, I.; Dimitrov, Z.; Krastanov, A. Bioactivity of Biomass and Crude Exopolysaccharides Obtained by Controlled Submerged Cultivation of Medicinal Mushroom Trametes Versicolor. J. Fungi 2022, 8, 738. [Google Scholar] [CrossRef]
- Camilleri, E.; Blundell, R.; Baral, B.; Karpiński, T.M.; Aruci, E.; Atrooz, O.M. Unveiling the Full Spectrum of Maitake Mushrooms: A Comprehensive Review of Their Medicinal, Therapeutic, Nutraceutical, and Cosmetic Potential. Heliyon 2024, 10, e30254. [Google Scholar] [CrossRef]
- Chen, C.X.; Huang, B.; Li, T.; Wu, G.F. Preparation of Phosphoric Acid Activated Carbon from Sugarcane Bagasse by Mechanochemical Processing. BioResources 2012, 7, 5109–5116. [Google Scholar] [CrossRef]
- Chen, X.; Xu, B. Insights into Chemical Components, Health-Promoting Effects, and Processing Impact of Golden Chanterelle Mushroom Cantharellus Cibarius. Food Funct. 2024, 15, 7696–7732. [Google Scholar] [CrossRef]
- Malakar, S.; Sutaoney, P.; Singh, P.; Shah, K.; Chauhan, N.S.; Malakar, S.; Sutaoney, P.; Singh, P.; Shah, K.; Chauhan, N.S. Systems Biology for Mushroom Cultivation Promoting Quality Life. Circ. Agric. Syst. 2025, 5, e010. [Google Scholar] [CrossRef]
- Derbyshire, E.J.; Brameld, J.M.; Wall, B.T.; Thomas, P.; Arens, U.; Forde, C.G.; Hall, W.; Glenn, A.J.; Hill, T.R.; Paxman, J. Is There a Specific Role for Fungal Protein Within Food Based Dietary Guidelines? A Roundtable Discussion. Nutr. Bull. 2025, 50, 514–528. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Dong, S.; Luo, J.; Ma, F.; Jiang, W.; Han, C. Research Progress on the Function and Application of Proteins of Edible and Medicinal Mushrooms: A Review. Int. J. Med. Mushrooms 2022, 24, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.-J.; Ro, H.-S.; Kawauchi, M.; Honda, Y. Review on Mushroom Mycelium-Based Products and Their Production Process: From Upstream to Downstream. Bioresour. Bioprocess. 2025, 12, 3. [Google Scholar] [CrossRef] [PubMed]
- Finnigan, T.J.; Wall, B.T.; Wilde, P.J.; Stephens, F.B.; Taylor, S.L.; Freedman, M.R. Mycoprotein: The Future of Nutritious Nonmeat Protein, a Symposium Review. Curr. Dev. Nutr. 2019, 3, nzz021. [Google Scholar] [CrossRef]
- Ayimbila, F.; Keawsompong, S. Nutritional Quality and Biological Application of Mushroom Protein as a Novel Protein Alternative. Curr. Nutr. Rep. 2023, 12, 290–307. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (Ed.) Dietary Protein Quality Evaluation in Human Nutrition: Report of an FAO Expert Consultation, 31 March–2 April, 2011, Auckland, New Zealand; FAO Food and Nutrition Paper; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013; ISBN 978-92-5-107417-6. [Google Scholar]
- Stojek, K.; Bobrowska-Korczak, B.; Frączek, J.; Piotrowski, M.; Krośniak, M.; Jaroszewicz, B. Protein Content and Amino Acid Profile of Wild Mushrooms Depend on Environmental Conditions. Fungal Biol. 2025, 129, 101620. [Google Scholar] [CrossRef]
- Ionescu, M.; Dincă, M.-N.; Ferdeș, M.; Zăbavă, B.-Ș; Paraschiv, G.; Moiceanu, G. Proteins from Edible Mushrooms: Nutritional Role and Contribution to Well-Being. Foods 2025, 14, 3201. [Google Scholar] [CrossRef]
- Effiong, M.E.; Umeokwochi, C.P.; Afolabi, I.S.; Chinedu, S.N. Assessing the Nutritional Quality of Pleurotus Ostreatus (Oyster Mushroom). Front. Nutr. 2024, 10, 1279208. [Google Scholar] [CrossRef]
- West, S.; Monteyne, A.J.; Whelehan, G.; van der Heijden, I.; Abdelrahman, D.R.; Murton, A.J.; Finnigan, T.J.A.; Stephens, F.B.; Wall, B.T. Ingestion of Mycoprotein, Pea Protein, and Their Blend Support Comparable Postexercise Myofibrillar Protein Synthesis Rates in Resistance-Trained Individuals. Am. J. Physiol. Endocrinol. Metab. 2023, 325, E267–E279. [Google Scholar] [CrossRef]
- Derbyshire, E.J.; Theobald, H.; Wall, B.T.; Stephens, F. Food for Our Future: The Nutritional Science behind the Sustainable Fungal Protein–Mycoprotein. A Symposium Review. J. Nutr. Sci. 2023, 12, e44. [Google Scholar] [CrossRef]
- Salim, R.; Nehvi, I.B.; Mir, R.A.; Tyagi, A.; Ali, S.; Bhat, O.M. A Review on Anti-Nutritional Factors: Unraveling the Natural Gateways to Human Health. Front. Nutr. 2023, 10, 1215873. [Google Scholar] [CrossRef]
- Pashaei, K.H.A.; Irankhah, K.; Namkhah, Z.; Sobhani, S.R. Edible Mushrooms as an Alternative to Animal Proteins for Having a More Sustainable Diet: A Review. J. Health Popul. Nutr. 2024, 43, 205. [Google Scholar] [CrossRef] [PubMed]
- Mattila, P.; Salo-Väänänen, P.; Könkö, K.; Aro, H.; Jalava, T. Basic Composition and Amino Acid Contents of Mushrooms Cultivated in Finland. J. Agric. Food Chem. 2002, 50, 6419–6422. [Google Scholar] [CrossRef] [PubMed]
- Derbyshire, E. Food-Based Dietary Guidelines and Protein Quality Definitions—Time to Move Forward and Encompass Mycoprotein? Foods 2022, 11, 647. [Google Scholar] [CrossRef] [PubMed]
- Munks, B.; Robinson, A.; Beach, E.F.; Williams, H.H. Amino Acids in the Production of Chicken Egg and Muscle. Poult. Sci. 1945, 24, 459–464. [Google Scholar] [CrossRef]
- Cedeno, F.R.P.; Olubiyo, O.J.; Ferreira, S. From Microbial Proteins to Cultivated Meat for Alternative Meat-like Products: A Review on Sustainable Fermentation Approaches. J. Biol. Eng. 2025, 19, 44. [Google Scholar] [CrossRef]
- Dai, Z.; Zhao, Y.; Ke, Y.; Huang, J.; Zhu, J.; Wu, H.; Yang, Y.; Shang, H.; Xia, Z. Alternative Protein Sources and Healthy Skeletal Muscle Aging: A Narrative Review. J. Funct. Foods 2025, 133, 106990. [Google Scholar] [CrossRef]
- Attia, Y.A.; Al-Harthi, M.A.; Shafi, M.E.; Abdulsalam, N.M.; Nagadi, S.A.; Wang, J.; Kim, W.K. Amino Acids Supplementation Affects Sustainability of Productive and Meat Quality, Survivability and Nitrogen Pollution of Broiler Chickens during the Early Life. Life 2022, 12, 2100. [Google Scholar] [CrossRef]
- Gorissen, S.H.M.; Crombag, J.J.R.; Senden, J.M.G.; Waterval, W.A.H.; Bierau, J.; Verdijk, L.B.; van Loon, L.J.C. Protein Content and Amino Acid Composition of Commercially Available Plant-Based Protein Isolates. Amino Acids 2018, 50, 1685–1695. [Google Scholar] [CrossRef]
- Donadelli, R.A.; Jones, C.K.; Beyer, R.S. The Amino Acid Composition and Protein Quality of Various Egg, Poultry Meal by-Products, and Vegetable Proteins Used in the Production of Dog and Cat Diets. Poult. Sci. 2019, 98, 1371–1378. [Google Scholar] [CrossRef]
- Barr, B.; Levitt, D.E.; Gollahon, L. Red Meat Amino Acids for Beginners: A Narrative Review. Nutrients 2025, 17, 939. [Google Scholar] [CrossRef]
- Dudekula, U.T.; Doriya, K.; Devarai, S.K. A Critical Review on Submerged Production of Mushroom and Their Bioactive Metabolites. 3 Biotech 2020, 10, 337. [Google Scholar] [CrossRef] [PubMed]
- Maseko, K.H.; Regnier, T.; Bartels, P.; Meiring, B. Mushroom Mycelia as Sustainable Alternative Proteins for the Production of Hybrid Cell-Cultured Meat: A Review. J. Food Sci. 2025, 90, e70060. [Google Scholar] [CrossRef] [PubMed]
- Holt, R.R.; Munafo, J.P.J.; Salmen, J.; Keen, C.L.; Mistry, B.S.; Whiteley, J.M.; Schmitz, H.H. Mycelium: A Nutrient-Dense Food To Help Address World Hunger, Promote Health, and Support a Regenerative Food System. J. Agric. Food Chem. 2024, 72, 2697–2707. [Google Scholar] [CrossRef] [PubMed]
- Kent, G.; Kehoe, L.; Flynn, A.; Walton, J. Plant-Based Diets: A Review of the Definitions and Nutritional Role in the Adult Diet. Proc. Nutr. Soc. 2022, 81, 62–74. [Google Scholar] [CrossRef]
- Majumder, R.; Miatur, S.; Saha, A.; Hossain, S. Mycoprotein: Production and Nutritional Aspects: A Review. Sustain. Food Technol. 2024, 2, 81–91. [Google Scholar] [CrossRef]
- Boro, S.; Kambhampati, V.; Das, S.; Saikia, D. Edible Mushrooms as Meat Analogues: A Comprehensive Review of Nutritional, Therapeutic, and Market Potential. Food Res. Int. 2025, 214, 116632. [Google Scholar] [CrossRef]
- Wang, X.; Liu, H.; Li, J.; Wang, Y. Research Advances in Mushroom Umami: Substance Characteristics, Multidimensional Attributes, Umami Peptide Screening, and Umami Assessment. Curr. Res. Food Sci. 2025, 11, 101210. [Google Scholar] [CrossRef]
- Shahid, M.; Shah, P.; Mach, K.; Rodgers-Hunt, B.; Finnigan, T.; Frost, G.; Neal, B.; Hadjikakou, M. The Environmental Impact of Mycoprotein-Based Meat Alternatives Compared to Plant-Based Meat Alternatives: A Systematic Review. Future Foods 2024, 10, 100410. [Google Scholar] [CrossRef]
- Chaffee, O.; Ardoin, R. Consumer Perceptions of Plant-Based and Mushroom-Based Jerky: A Focus on Texture, Main Ingredient and Protein Information, and Willingness to Pay. Curr. Res. Food Sci. 2025, 10, 101058. [Google Scholar] [CrossRef]
- Wang, R.; Sar, T.; Mahboubi, A.; Fristedt, R.; Taherzadeh, M.J.; Undeland, I. In Vitro Protein Digestibility of Edible Filamentous Fungi Compared to Common Food Protein Sources. Food Biosci. 2023, 54, 102862. [Google Scholar] [CrossRef]
- FAO. Make Money by Growing Mushrooms; FAO: Rome, Italy, 2004. [Google Scholar]
- Lara-Parra, A.I.; Hernández-Hernández, A.A.; Jaguey-Hernández, Y.; Jiménez-Osorio, A.S.; Castañeda-Ovando, A.; Aguilar-Arteaga, K.; Añorve-Morga, J. Exploring Alternative Sources of Protein in Food: Trends in Nutrient and Functional Features. Food Res. Int. 2025, 208, 116224. [Google Scholar] [CrossRef] [PubMed]
- González, A.; Cruz, M.; Losoya, C.; Nobre, C.; Loredo, A.; Rodríguez, R.; Contreras, J.; Belmares, R. Edible Mushrooms as a Novel Protein Source for Functional Foods. Food Funct. 2020, 11, 7400–7414. [Google Scholar] [CrossRef] [PubMed]
- Chukwu, S.C.; Ibeji, C.A.; Ogbu, C.; Oselebe, H.O.; Okporie, E.O.; Rafii, M.Y.; Oladosu, Y. Primordial Initiation, Yield and Yield Component Traits of Two Genotypes of Oyster Mushroom (Pleurotus Spp.) as Affected by Various Rates of Lime. Sci. Rep. 2022, 12, 19054. [Google Scholar] [CrossRef]
- Meilleur, M.-A.; Bastien, D.; Monfet, D. Modeling Mushrooms’ Carbon Dioxide Emission and Heat Exchange Rates for Synergistic Cultivation with Leafy Greens. Sustainability 2023, 15, 16740. [Google Scholar] [CrossRef]
- De Bonis, M.; Locatelli, S.; Sambo, P.; Zanin, G.; Pecchia, J.A.; Nicoletto, C. Effect of Different LED Light Wavelengths on Production and Quality of Pleurotus Ostreatus Grown on Different Commercial Substrates. Horticulturae 2024, 10, 349. [Google Scholar] [CrossRef]
- Sultana, R.; Hossain, M.I.; Saifullah, A.R.; Chakraborty, R. Influence of Substrate pH and Watering Frequency on the Growth of Oyster Mushroom. Int. J. Plant Biol. Res. 2018, 6, 1097. [Google Scholar]
- Manea, E.E.; Bumbac, C.; Dinu, L.R.; Bumbac, M.; Nicolescu, C.M. Composting as a Sustainable Solution for Organic Solid Waste Management: Current Practices and Potential Improvements. Sustainability 2024, 16, 6329. [Google Scholar] [CrossRef]
- Bakratsas, G.; Polydera, A.; Nilson, O.; Chatzikonstantinou, A.V.; Xiros, C.; Katapodis, P.; Stamatis, H. Mycoprotein Production by Submerged Fermentation of the Edible Mushroom Pleurotus Ostreatus in a Batch Stirred Tank Bioreactor Using Agro-Industrial Hydrolysate. Foods 2023, 12, 2295. [Google Scholar] [CrossRef]
- Bakratsas, G.; Samiotaki, M.; Katapodis, P.; Stamatis, H. Proteomic Analysis of Pleurotus Ostreatus Grown on Glucose and Xylose Mixtures in Submerged Fermentation Provides Insights into Differentiated Mycelial Composition. Synth. Biol. Eng. 2024, 2, 10006. [Google Scholar] [CrossRef]
- Yang, M.; Qian, Z.; Zhan, Q.; Zhong, L.; Hu, Q.; Zhao, L. Application of Definitive Screening Design to Optimization of the Protein Extraction and Functional Properties of Proteins in Auricularia Auricula. J. Sci. Food Agric. 2023, 103, 1226–1236. [Google Scholar] [CrossRef]
- Parhizi, Z.; Dearnaley, J.; Kauter, K.; Mikkelsen, D.; Pal, P.; Shelley, T.; Burey, P. The Fungus Among Us: Innovations and Applications of Mycelium-Based Composites. J. Fungi 2025, 11, 549. [Google Scholar] [CrossRef] [PubMed]
- Finnigan, T.J.A.; Theobald, H.E.; Bajka, B. Mycoprotein: A Healthy and Sustainable Source of Alternative Protein-Based Foods. Annu. Rev. Food Sci. Technol. 2025, 16, 105–125. [Google Scholar] [CrossRef] [PubMed]
- Lizardi-Jiménez, M.A.; Hernández-Martínez, R. Solid State Fermentation (SSF): Diversity of Applications to Valorize Waste and Biomass. 3 Biotech 2017, 7, 44. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Chen, Z.; Liu, Y.; Hua, X.; Gao, C.; Liu, J. Morphological Engineering of Filamentous Fungi: Research Progress and Perspectives. J. Microbiol. Biotechnol. 2024, 34, 1197–1205. [Google Scholar] [CrossRef]
- Lee, S.Y.; Ra, C.H. Comparison of Liquid and Solid-State Fermentation Processes for the Production of Enzymes and Beta-Glucan from Hulled Barley. J. Microbiol. Biotechnol. 2022, 32, 317–323. [Google Scholar] [CrossRef]
- Martău, G.-A.; Unger, P.; Schneider, R.; Venus, J.; Vodnar, D.C.; López-Gómez, J.P. Integration of Solid State and Submerged Fermentations for the Valorization of Organic Municipal Solid Waste. J. Fungi 2021, 7, 766. [Google Scholar] [CrossRef]
- Šelo, G.; Planinić, M.; Tišma, M.; Tomas, S.; Koceva Komlenić, D.; Bucić-Kojić, A. A Comprehensive Review on Valorization of Agro-Food Industrial Residues by Solid-State Fermentation. Foods 2021, 10, 927. [Google Scholar] [CrossRef]
- Pérez-Contreras, S.; Avalos-de la Cruz, D.A.; Lizardi-Jiménez, M.A.; Herrera-Corredor, J.A.; Baltazar-Bernal, O.; Hernández-Martínez, R. Production of Ligninolytic and Cellulolytic Fungal Enzymes for Agro-Industrial Waste Valorization: Trends and Applicability. Catalysts 2025, 15, 30. [Google Scholar] [CrossRef]
- Mattedi, A.; Sabbi, E.; Farda, B.; Djebaili, R.; Mitra, D.; Ercole, C.; Cacchio, P.; Del Gallo, M.; Pellegrini, M. Solid-State Fermentation: Applications and Future Perspectives for Biostimulant and Biopesticides Production. Microorganisms 2023, 11, 1408. [Google Scholar] [CrossRef]
- Yafetto, L. Application of Solid-State Fermentation by Microbial Biotechnology for Bioprocessing of Agro-Industrial Wastes from 1970 to 2020: A Review and Bibliometric Analysis. Heliyon 2022, 8, e09173. [Google Scholar] [CrossRef]
- Li, Z.; Luo, R.; Zhang, Y.; Yan, X.; Pang, Q. Effective Protein Extraction from Mycelium and Fruiting Body of Auricularia Auricula for Proteomics Studies. Int. J. Food Prop. 2018, 21, 2156–2166. [Google Scholar] [CrossRef]
- Elhalis, H. Exploring Fungal Mycelium for Sustainable Food Solutions: From Biomass Utilization to Byproduct Innovation. Food Rev. Int. 2025, 1–33. [Google Scholar] [CrossRef]
- Ji, Z.; Ma, W.; Liang, P.; Wang, X.; Zhang, S.; Han, Y.; Guo, Y. Anti-Inflammatory Potential of Mycoprotein Peptides Obtained from Fermentation of Schizophyllum Commune DS1 with Young Apples. Int. J. Biol. Macromol. 2024, 281, 136638. [Google Scholar] [CrossRef]
- Mitchell, D.A.; Ruiz, H.A.; Krieger, N. A Critical Evaluation of Recent Studies on Packed-Bed Bioreactors for Solid-State Fermentation. Processes 2023, 11, 872. [Google Scholar] [CrossRef]
- Artola, A.; Font, X.; Moral-Vico, J.; Sánchez, A. The Role of Solid-State Fermentation to Transform Existing Waste Treatment Plants Based on Composting and Anaerobic Digestion into Modern Organic Waste-Based Biorefineries, in the Framework of Circular Bioeconomy. Front. Chem. Eng. 2024, 6, 1463785. [Google Scholar] [CrossRef]
- Moser, A.; Appl, C.; Pörtner, R.; Baganz, F.; Hass, V.C. A New Concept for the Rapid Development of Digital Twin Core Models for Bioprocesses in Various Reactor Designs. Fermentation 2024, 10, 463. [Google Scholar] [CrossRef]
- Albino, M.; Gargalo, C.L.; Nadal-Rey, G.; Albæk, M.O.; Krühne, U.; Gernaey, K.V. Hybrid Modeling for On-Line Fermentation Optimization and Scale-Up: A Review. Processes 2024, 12, 1635. [Google Scholar] [CrossRef]
- Wainaina, S.; Taherzadeh, M.J. Automation and Artificial Intelligence in Filamentous Fungi-Based Bioprocesses: A Review. Bioresour. Technol. 2023, 369, 128421. [Google Scholar] [CrossRef]
- Wang, Y.; Gou, C.; Chen, L.; Liao, Y.; Zhang, H.; Luo, L.; Ji, J.; Qi, Y. Solid-State Fermentation with White Rot Fungi (Pleurotus Species) Improves the Chemical Composition of Highland Barley Straw as a Ruminant Feed and Enhances In Vitro Rumen Digestibility. J. Fungi 2023, 9, 1156. [Google Scholar] [CrossRef]
- Bakratsas, G.; Polydera, A.; Nilson, O.; Kossatz, L.; Xiros, C.; Katapodis, P.; Stamatis, H. Single-Cell Protein Production by Pleurotus Ostreatus in Submerged Fermentation. Sustain. Food Technol. 2023, 1, 377–389. [Google Scholar] [CrossRef]
- Kirsch, L.D.S.; de Macedo, A.J.P.; Teixeira, M.F.S. Production of Mycelial Biomass by the Amazonian Edible Mushroom Pleurotus albidus. Braz. J. Microbiol. 2016, 47, 658–664. [Google Scholar] [CrossRef]
- Dulay, R.M.R.; Cabrera, E.C.; Kalaw, S.P.; Reyes, R.G. Optimization of Submerged Culture Conditions for Mycelial Biomass Production of Fourteen Lentinus Isolates from Luzon Island, Philippines. Biocatal. Agric. Biotechnol. 2021, 38, 102226. [Google Scholar] [CrossRef]
- Mkhize, S.S.; Zharare, G.E.; Basson, A.K.; Mthembu, M.S.; Cloete, J. Performance of Pleurotus pulmonarius Mushroom Grown on Maize Stalk Residues Supplemented with Various Levels of Maize Flour and Wheat Bran. Food Sci. Technol. 2017, 37, 570–577. [Google Scholar] [CrossRef]
- Yu, C.-X.; Zhang, Y.-R.; Ren, Y.-F.; Zhao, Y.; Song, X.-X.; Yang, H.-L.; Chen, M.-J. Composition and Contents of Fatty Acids and Amino Acids in the Mycelia of Lentinula Edodes. Food Sci. Nutr. 2023, 11, 4038–4046. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Choi, B.; Lee, I.; Lee, H.; Kwon, S.; Oh, K.; Kim, A.Y. Bioproduction of Mushroom Mycelium of Agaricus Bisporus by Commercial Submerged Fermentation for the Production of Meat Analogue. J. Sci. Food Agric. 2011, 91, 1561–1568. [Google Scholar] [CrossRef] [PubMed]
- Winiski, J.M.; Kaplan-Bie, J.H.; Mcintyre, G.R.; Mueller, P.; O’brien, M.; Carlton, A.; Bayer, E.; Hazen, R.; Lomnes, S.; Snyder, A.T. Edible Mycelia and Methods of Making the Same. U.S. Patent US20220333055A1, 20 October 2022. [Google Scholar]
- Humpenöder, F.; Bodirsky, B.L.; Weindl, I.; Lotze-Campen, H.; Linder, T.; Popp, A. Projected Environmental Benefits of Replacing Beef with Microbial Protein. Nature 2022, 605, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Nair, L.G.; Verma, P. Harnessing Carbon Potential of Lignocellulosic Biomass: Advances in Pretreatments, Applications, and the Transformative Role of Machine Learning in Biorefineries. Bioresour. Bioprocess. 2025, 12, 97. [Google Scholar] [CrossRef]
- dos Anjos, I.V.; Coelho, N.; Duarte, H.; Proença, D.N.; Duarte, M.F.; Barros, R.; Raposo, S.; Gonçalves, S.; Romano, A.; Medronho, B. From Lignocellulosic Residues to Protein Sources: Insights into Biomass Pre-Treatments and Conversion. Polymers 2025, 17, 2251. [Google Scholar] [CrossRef]
- Dhiman, S.; Kaur, P.; Narang, J.; Mukherjee, G.; Thakur, B.; Kaur, S.; Tripathi, M. Fungal Bioprocessing for Circular Bioeconomy: Exploring Lignocellulosic Waste Valorization. Mycology 2024, 15, 538–563. [Google Scholar] [CrossRef]
- Borkertas, S.; Viskelis, J.; Viskelis, P.; Streimikyte, P.; Gasiunaite, U.; Urbonaviciene, D. Fungal Biomass Fermentation: Valorizing the Food Industry’s Waste. Fermentation 2025, 11, 351. [Google Scholar] [CrossRef]
- Brancoli, P.; Gmoser, R.; Taherzadeh, M.J.; Bolton, K. The Use of Life Cycle Assessment in the Support of the Development of Fungal Food Products from Surplus Bread. Fermentation 2021, 7, 173. [Google Scholar] [CrossRef]
- Poore, J.; Nemecek, T. Reducing Food’s Environmental Impacts through Producers and Consumers. Science 2018, 360, 987–992. [Google Scholar] [CrossRef] [PubMed]
- Future Market Insights Alternative Protein Market Demand & Trends 2025 to 2035. Available online: https://www.futuremarketinsights.com/reports/alternative-protein-market (accessed on 31 October 2025).
- Brondi, M.G.; Elias, A.M.; Furlan, F.F.; Giordano, R.C.; Farinas, C.S. Performance Targets Defined by Retro-Techno-Economic Analysis for the Use of Soybean Protein as Saccharification Additive in an Integrated Biorefinery. Sci. Rep. 2020, 10, 7367. [Google Scholar] [CrossRef] [PubMed]
- Allotey, D.K.; Kwofie, E.M.; Asavajaru, P.; Samaranayaka, A.G.P. An Integrative Framework for Eco-Efficiency Assessment in Plant-Protein Extraction Processes: Hotspot Analysis, Protein Loss Tracking, and Uncertainty Analysis. J. Clean. Prod. 2025, 527, 146684. [Google Scholar] [CrossRef]
- European Commission. Building the Future with Nature: Boosting Biotechnology and Biomanufacturing in the EU; European Commission: Brussels, Belgium, 2024. [Google Scholar]
- Circular Bio-Based Europe Joint Undertaking Annual Work Programme 2025 (Second Amendment). Available online: https://www.cbe.europa.eu/?utm_source=chatgpt.com (accessed on 31 October 2025).
- Cerrone, F.; O’Connor, K.E. Cultivation of Filamentous Fungi in Airlift Bioreactors: Advantages and Disadvantages. Appl. Microbiol. Biotechnol. 2025, 109, 41. [Google Scholar] [CrossRef]
- Kachrimanidou, V.; Alexandri, M.; Papapostolou, H.; Papadaki, A.; Kopsahelis, N. Valorization of Grape Pomace for Trametes Versicolor Mycelial Mass and Polysaccharides Production. Sustainability 2023, 15, 15080. [Google Scholar] [CrossRef]
- Joshi, M.; Patel, H.; Gupte, S.; Gupte, A. Nutrient Improvement for Simultaneous Production of Exopolysaccharide and Mycelial Biomass by Submerged Cultivation of Schizophyllum Commune AGMJ-1 Using Statistical Optimization. 3 Biotech 2013, 3, 307–318. [Google Scholar] [CrossRef]
- Ahlborn, J.; Stephan, A.; Meckel, T.; Maheshwari, G.; Rühl, M.; Zorn, H. Upcycling of Food Industry Side Streams by Basidiomycetes for Production of a Vegan Protein Source. Int. J. Recycl. Org. Waste Agric. 2019, 8, 447–455. [Google Scholar] [CrossRef]
- Tešanović, K.; Pejin, B.; Šibul, F.; Matavulj, M.; Rašeta, M.; Janjušević, L.; Karaman, M. A Comparative Overview of Antioxidative Properties and Phenolic Profiles of Different Fungal Origins: Fruiting Bodies and Submerged Cultures of Coprinus Comatus and Coprinellus Truncorum. J. Food Sci. Technol. 2017, 54, 430–438. [Google Scholar] [CrossRef]
- Vieira, G.R.T.; Liebl, M.; Tavares, L.B.B.; Paulert, R.; Smânia Júnior, A. Submerged Culture Conditions for the Production of Mycelial Biomass and Antimicrobial Metabolites by Polyporus Tricholoma Mont. Braz. J. Microbiol. 2008, 39, 561–568. [Google Scholar] [CrossRef]
- Fernández-Fueyo, E.; Ruiz-Dueñas, F.J.; López-Lucendo, M.F.; Pérez-Boada, M.; Rencoret, J.; Gutiérrez, A.; Pisabarro, A.G.; Ramírez, L.; Martínez, A.T. A Secretomic View of Woody and Nonwoody Lignocellulose Degradation by Pleurotus Ostreatus. Biotechnol. Biofuels 2016, 9, 49. [Google Scholar] [CrossRef]
- Ohm, R.A.; de Jong, J.F.; Lugones, L.G.; Aerts, A.; Kothe, E.; Stajich, J.E.; de Vries, R.P.; Record, E.; Levasseur, A.; Baker, S.E.; et al. Genome Sequence of the Model Mushroom Schizophyllum Commune. Nat. Biotechnol. 2010, 28, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; Wang, Y.; Xie, C.; Zhou, Y.; Zhu, Z.; Peng, Y. Whole Genome Sequence of an Edible and Medicinal Mushroom, Hericium Erinaceus (Basidiomycota, Fungi). Genomics 2020, 112, 2393–2399. [Google Scholar] [CrossRef] [PubMed]
- Sekoai, P.T.; Roets-Dlamini, Y.; O’Brien, F.; Ramchuran, S.; Chunilall, V. Valorization of Food Waste into Single-Cell Protein: An Innovative Technological Strategy for Sustainable Protein Production. Microorganisms 2024, 12, 166. [Google Scholar] [CrossRef] [PubMed]
- Hardin, M.T.; Mitchell, D.A.; Howes, T. Approach to Designing Rotating Drum Bioreactors for Solid-State Fermentation on the Basis of Dimensionless Design Factors. Biotechnol. Bioeng. 2000, 67, 274–282. [Google Scholar] [CrossRef]
- Zhao, L.; Sun, G.; Jiang, J.; Chen, L.; Huang, J. Role of Microbubbles Coupling Fibrous-Bed Bioreactor in Butyric Acid Production by Clostridium Tyrobutyricum Using Brewer’s Spent Grain as Feedstock. Biochem. Eng. J. 2021, 172, 108051. [Google Scholar] [CrossRef]
- Food and Drug Administration. GRAS Notice for the Use of Phoenix Oyster Mushroom (Pleurotus pulmonarius) Mycelium as an Ingredient in Food; Food and Drug Administration: Silver Spring, MD, USA, 2024. [Google Scholar]
- European Food Safety Authority; Turck, D.; Bohn, T.; Castenmiller, J.; de Henauw, S.; Hirsch-Ernst, K.I.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Guidance on the Scientific Requirements for an Application for Authorisation of a Novel Food in the Context of Regulation (EU) 2015/2283. EFSA J. 2024, 22, e8961. [Google Scholar] [CrossRef]
- EFSA Panel; Turck, D.; Bohn, T.; Cámara, M.; Castenmiller, J.; De Henauw, S.; Jos, Á.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; et al. \Safety of the Fungal Biomass from Fusarium Species Strain Flavolapis as a Novel Food Pursuant to Article 10 of Regulation (EU) 2015/2283. EFSA J. 2025, 23, e9536. [Google Scholar] [CrossRef]
- Kawecki, N.S.; Chen, K.K.; Smith, C.S.; Xie, Q.; Cohen, J.M.; Rowat, A.C. Scalable Processes for Culturing Meat Using Edible Scaffolds. Annu. Rev. Food Sci. Technol. 2024, 15, 241–264. [Google Scholar] [CrossRef]
- Kaplan, D.L.; McClements, D.J. Hybrid Alternative Protein-Based Foods: Designing a Healthier and More Sustainable Food Supply. Front. Sci. 2025, 3, 1599300. [Google Scholar] [CrossRef]
- Meyer, V.; Cairns, T.; Barthel, L.; King, R.; Kunz, P.; Schmideder, S.; Müller, H.; Briesen, H.; Dinius, A.; Krull, R. Understanding and Controlling Filamentous Growth of Fungal Cell Factories: Novel Tools and Opportunities for Targeted Morphology Engineering. Fungal Biol. Biotechnol. 2021, 8, 8. [Google Scholar] [CrossRef]
- Chezan, D.; Flannery, O.; Patel, A. Factors Affecting Consumer Attitudes to Fungi-Based Protein: A Pilot Study. Appetite 2022, 175, 106043. [Google Scholar] [CrossRef]
- Lähteenmäki-Uutela, A.; Rahikainen, M.; Lonkila, A.; Yang, B. Alternative Proteins and EU Food Law. Food Control 2021, 130, 108336. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).