Abstract
Blackthorn (Prunus spinosa L.) is a wild, understudied plant rich in bioactive compounds such as polyphenols with designated antioxidant potential. The main objective of this research was to optimize ultrasound-assisted extraction of blackthorn pomace using natural deep eutectic solvents (NADES). To obtain the highest yield of polyphenols and improved in vitro antioxidant activity, response surface methodology (RSM) and central composite experimental design were used. The screening step of the study included ten different NADESs using a one-factor-at-a-time approach. Two NADES mixtures (N12, containing proline and lactic acid in a molar ratio of 1:2, and N14, containing choline chloride and glycerol in a molar ratio of 1:1) were chosen for the second step of the study, which aimed to select the most influential process parameters. A fractional factorial 25−1 design was used, varying five different parameters at two levels: extraction time (30 and 60 min), extraction temperature (40 and 50 °C), and liquid-to-solid ratio (10 and 20 mL/g), water content in NADES (15 and 20%), and NADES type (N12 and N14). After the second step, N12 containing 20% water was chosen as the most potent solvent for the optimization study. For the final step, the other three parameters were varied on three levels, and thus optimal conditions were obtained (extraction time 90 min, extraction temperature 65 °C, and liquid-to-solid ratio 22.65 mL/g). Blackthorn juice was also tested in the first step, as well as under optimal conditions established for pomace, in order to evaluate whether these conditions are suitable for juice and to determine the percentage of improvement in extraction efficiency.
1. Introduction
Prunus spinosa L. belongs to the Prunus genus under the Rosaceae family, which comprises more than 400–430 species distributed worldwide [1]. Blackthorn is one of many species of the Prunus genus that are found in the wild. Blackthorn is widespread at altitudes ranging from 0 to 1700 m in Western Asia, Europe, and Northwestern Africa. Although blackthorn can be eaten raw, it is most frequently processed into jam, marmalade, compote, or jelly. Due to the presence of bioactive compounds, blackthorn is also known for a broad spectrum of biological activities [2]. Blackthorn is often found along roadsides, as it was once planted as a hedge to protect livestock holdings from pollution caused by nearby industries. It is extremely resilient to harsh weather conditions and grows as a prickly shrub [3]. Bioactive compounds found in blackthorn include phenolic acids and flavonoids, such as anthocyanins, flavonols, and flavones, with 3-O-caffeoyl-quinic acid and quercetin 3-O-rutinoside being particularly abundant [4]. In the study by Nistor et al. [5], various technological approaches were applied to valorize blackthorn fruits in the production of marmalade, jam, jelly, and nutraceutical products. These different products were used as raw materials for extraction with 70% ethanol acidified with citric acid, assisted by ultrasound, and the resulting extracts were evaluated for their total phenolic, flavonoid, and anthocyanin contents. Additionally, the inhibitory activities against α-glucosidase, α-amylase, and tyrosinase were assessed in the extracts obtained from the blackthorn skins. Katanić Stanković et al. [6] stated that the blackthorn fruit extract exhibited the highest contents of total phenols, flavonoids, and flavonols compared to extracts of medlar and hawthorn. Moreover, the contents of phenolic acids, galantamine, and anthocyanins in the blackthorn fruit extract were also higher than extracts obtained from hawthorn and medlar fruits [6]. All extracts were prepared under the same conditions, using 75% ethanol as the solvent, and ultrasound-assisted extraction was applied. The antioxidant activity, as well as the inhibitory effects on α-glucosidase, was also analyzed.
Green chemistry continuously seeks to identify new solvents that could replace conventional organic solvents. Natural deep eutectic solvents (NADES) are composed exclusively of naturally derived compounds, such as primary metabolites including organic acids, amino acids, and sugars [7]. NADESs are mixtures formed by combining two or more compounds, either in liquid or in solid state, in specific ratios. Their eutectic point is significantly lower than the melting points of each component due to the formation of specific interactions between constituents, such as intramolecular hydrogen bonds and van der Waals interactions (Fourmentin et al., 2020) [8]. Once formed, NADESs are viscous, transparent, and stable liquids. In addition to their natural constituents, NADESs may also contain a certain molar fraction of water. Depending on the type and position of hydrogen bond donors and acceptors involved in their composition, solvents of varying stability can be obtained [9]. In recent years, NADES-based solvents have gained considerable attention as green extraction media, as they combine environmental sustainability with practical efficiency. They are biodegradable, non-toxic, and suitable for applications in food and pharmaceuticals. Their preparation is simple and cost-effective, while their physicochemical properties (e.g., viscosity, polarity, conductivity, and melting point) can be tuned by varying the components and water content. Through extensive hydrogen-bonding networks, NADESs significantly enhance the solubility and stability of bioactive compounds, including poorly water-soluble molecules, while providing protection against degradation. In addition, they are compatible with a wide range of analytical techniques, and their low volatility and recyclability further minimize environmental impact. These features establish NADESs as efficient and sustainable solvents for the extraction of natural products [10]. In the study conducted by Koraqi et al. [11], the simplex lattice mixture design method was employed to formulate an NADES composed of lactic acid, maltose, and water for the extraction of phenolic compounds, including anthocyanins, as well as for the assessment of their antioxidant activity from blackthorn fruits. Optimization revealed that the NADES mixture with the ratio lactic acid:maltose:water = 66:16:16 (v/v/v) was the most suitable for the simultaneous maximization of total polyphenols content (TPC) and antioxidant activity as measured by the 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay. For achieving the highest total anthocyanin content (TAC), the NADES mixture with an equal proportion of lactic acid, maltose, and water (33:33:33, v/v/v) was identified as optimal.
Considering that the fruit processing industry generates substantial amounts of by-products, which often retain bioactive compounds naturally present in the raw fruit, the aim of this study was to optimize the extraction of blackthorn pomace to obtain the highest possible yield of polyphenols and antioxidants. The study consisted of three main steps. In the first step, a screening of ten different NADES systems was performed to select the two most efficient solvents for the extraction of bioactive compounds from blackthorn pomace, based on the determination of TPC and DPPH radical-scavenging activity. The second step involved the identification of the most influential extraction parameters using a 25−1 fractional factorial design. Five factors were analyzed: extraction time, extraction temperature, solid-to-liquid ratio, water content in the NADES, and the type of NADES. After identifying the three most significant factors, the third step focused on process optimization using response surface methodology (RSM) to determine the optimal conditions for the recovery of polyphenols. The extracts obtained under these conditions were evaluated for TPC, total flavonoid content (TFC), and antioxidant activity assessed by DPPH, FRAP, and ABTS assays. Finally, the optimal extraction conditions predicted by the RSM model were validated experimentally to confirm their effectiveness in the recovery of polyphenols from blackthorn pomace using the selected NADES system.
2. Materials and Methods
2.1. Chemicals and Standards
Hydrochloric acid, lactic acid, fructose, and potassium persulfate were purchased from Lach:ner (Neratovice, Czech Republic). Gallic acid, 2,2-diphenyl-1-picrylhydrazyl (DPPH), Trolox, and quercetin were purchased from Sigma Alrdrich (Steinhem, Germany). From Tokyo Chemical Industry (Tokyo, Japan), choline chloride (98%), 1,2-propanediol (99%), and 2,4,6-tris(2-pyridyl)-s-triazine (TPTZ) were supplied. Centrohem (Stara Pazova, Serbia) was the supplier for urea, citric acid (80%), and aluminum chloride hexahydrate. Sodium nitrite and iron(III) chloride were purchased from Alkaloid (Skopje, North Macedonia), and sodium acetate trihydrate and acetic acid were purchased from Zorka Pharma (Šabac, Serbia). Ethanol (96%) was obtained from Sani Hem (Novi Sad, Serbia), glycerol (99.7%) from Reahem (Novi Sad, Serbia), L-proline from Thermo Scientific Alfa Aesar (Ward Hill, MA, USA), Folin–Ciocalteu reagent from Biochem Chemopharma (Cosne-Cours-sur-Loire, France), sodium carbonate from Betahem (Belgrade, Serbia), sodium hydroxide from NRK Inženjering (Belgrade, Serbia), methanol (p.a.) from T.T.T. d.o.o. Sveta Nedjelja (Novaki, Croatia), iron(II) sulfate from Zdravlje Leskovac (Leskovac, Serbia), and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) from J&K Scientific Ltd. (Beijing, China).
2.2. Sample Preparation
Blackthorn fruit was harvested during the fruiting stage in October 2024 from the southern slopes of Fruška Gora Mountain. Blackthorn pomace used in this research was obtained by blending pitted fruit in a laboratory blender (Waring products, 8010EG, McConnellsburg, PA, USA) and subsequently draining it through sterile gauze. The leftover juice was analyzed in the final step. After that, samples were frozen in an ultra-freezer on −86 °C (Snijders Lab, Evo-Safe series Ultra Low Freezer −86 °C VF360-86, Tilburg, The Netherlands). Frozen samples were then lyophilized (Christ, Alpha 1-2-LD, Osterode am Harz, Germany) under the following conditions: temperature: −20 °C; pressure: 1 bar; time: 48 h; vacuum: 1 mbar; and condenser temperature: −53 °C. Samples were vacuum-sealed and kept at 4 °C.
2.3. NADES Preparation
In total, 10 different hydrophilic NADESs were prepared using an ultrasound water bath (Elmasonic, S100K, Singen Germany) at 50 °C for 30 min until a stable, transparent liquid was formed. All used NADESs were stable at room temperature for at least 7 days. The water content in all NADESs used in the first optimization step was adjusted to 20%. Initial water content in chemicals used for NADES preparation was considered in the calculation. Table 1 contains the list of all 10 NADESs prepared and used in this research. The sample codes do not follow a consecutive order (from 1 to 10) based on preliminary results regarding viscosity and extraction yield [12].

Table 1.
List of 10 NADESs prepared and used in this research.
2.4. Preparation of Extracts
All extracts used in this research were prepared in an ultrasonic water bath (Elmasonic, S100K, Singen, Germany). For the screening step of optimization, extraction conditions were as follows: temperature: 40 °C; time: 30 min; solid-to-liquid ratio: 1:10 m/v; and water content in NADES: 20%.
Control extracts were prepared under the same conditions using 50% ethanol as a solvent, as well as 50% ethanol with 1% hydrochloric acid. Immediately after the extraction, distilled water was added to each extract in a 1:1 volumetric ratio to lower extract viscosity. Extracts were then mixed using a vortex mixer (IKA-Werke GmbH & Co., KG, Vortex shaker Genius 3 Orbital, Staufen, Germany) and centrifuged (Boeco, U320R, Hamburg, Germany) at 5000 rpm for 10 min. Added water made extracts less stable, so they were kept at −18 °C and used within the following 3 days.
2.5. Total Phenol Content
Total phenol content (TPC) was determined using a spectrophotometric method [13]. To each test tube, 0.1 mL of an appropriate dilution of extract was added, followed by 7.9 mL of distilled water, as well as 0.5 mL of Folin–Ciocalteu reagent and 1.5 mL of 20% sodium carbonate solution. Blank was prepared using 0.1 mL of distilled water instead of the sample. Tubes were incubated at room temperature for 1 h in the dark. Absorbance was measured using a UV-Vis spectrophotometer (Shimadzu, UV-1900 i, Kyoto, Japan) at a wavelength of 750 nm. The calibration curve was obtained using different concentrations of gallic acid solutions. Results were expressed as mg gallic acid equivalent/g dry weight. All measurements were performed 4 times.
2.6. Total Flavonoid Content
Determination of total flavonoid content (TFC) was obtained using a spectrophotometric method [14]. Briefly, 1 mL of appropriately diluted extract was added to each test tube, followed by 4 mL of distilled water and 0.3 mL of 5% sodium nitrite solution. Test tubes were incubated for 5 min, and 0.3 mL of 10% aluminum chloride hexahydrate solution was added to each tube, and they were incubated for 6 min. That was followed by adding 1 mL of 1 M sodium hydroxide. Blank was prepared the same way, but instead of the extract, 1 mL of distilled water was added. All measurements were performed 4 times. Absorbance was measured using a UV-Vis spectrophotometer (Shimadzu, UV-1900 i, Kyoto, Japan) at a wavelength of 510 nm. Different concentrations of quercetin standard were used to obtain a calibration curve, and the results were expressed as mg quercetin equivalent/g dry weight.
2.7. Antioxidant Activity Assays
2.7.1. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Assay
The ability of extracts to neutralize 2,2-diphenyl-1-picrylhydrazyl (DPPH) radicals and show their antioxidant activity was measured using a spectrophotometric method [15]. Extracts were properly diluted, and 0.1 mL of each extract was added to a different test tube, followed by 2.9 mL of the methanol dilution of DPPH reagent whose absorbance was adjusted to 0.70 ± 0.02. Blank was prepared using 0.1 mL of distilled water instead of the sample. Test tubes were incubated at room temperature for 1 h, protected from light. Absorbance was measured using a UV-Vis spectrophotometer (Shimadzu, UV-1900 i, Kyoto, Japan) at a wavelength of 517 nm. Different concentrations of Trolox standard were used to obtain a calibration curve, and the results were expressed as mg Trolox equivalent/g dry weight. All measurements were performed 4 times.
2.7.2. Ferric Reducing Antioxidant Power (FRAP) Assay
The ability of extracts to reduce Fe3+ ions to Fe2+ was determined using the Ferric Reducing Antioxidant Power (FRAP) assay [16]. To each test tube, 0.1 mL of properly diluted extract was added, followed by 2.9 mL of freshly made FRAP reagent. FRAP reagent contains 10 M 2,4,6-tris(2-pyridyl)-s-tirazine diluted in 40 mM hydrochloric acid, 20 mM solution of iron(III) chloride solution in 300 mM acetate buffer solution (pH 3.6), and 300 mM acetate buffer solution (pH 3.6) in a 1:1:10 volumetric ratio. Blank was prepared the same way, but instead of the extract, 0.1 mL of distilled water was added. Test tubes were protected from light and incubated at 37 °C for 10 min. Absorbance was measured using a UV-Vis spectrophotometer (Shimadzu, UV-1900 i, Kyoto, Japan) at a wavelength of 593 nm. All measurements were performed 4 times. Fe2+ was used to obtain a calibration curve, and results were expressed as mg Fe2+/g dry weight.
2.7.3. 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) Assay
The ability of extracts to neutralize 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS+) radicals was determined using a spectrophotometric method [17]. To each test tube, 0.1 mL of properly diluted extract was added, followed by 2.9 mL of working ABTS solution. Blank was prepared the same way, but instead of the extract, 0.1 mL of distilled water was added. A stock solution of 7 mM ABTS reagent was prepared by diluting ABTS in a 2.45 mM potassium persulfate solution and mixing it with distilled water in a 1:1 volumetric ratio. Stock solution of ABTS was incubated at room temperature for 16 h, protected from light. After incubation, the stock solution of ABTS was diluted with 300 mM acetate buffer solution (pH 3.6) in a 1:39 volumetric ratio to obtain a working ABTS solution whose absorbance was adjusted to 0.70 ± 0.02. Different concentrations of Trolox solutions were used to obtain a calibration curve. Absorbance was measured using a UV-Vis spectrophotometer (Shimadzu, UV-1900 i, Kyoto, Japan) at a wavelength of 734 nm. All measurements were performed 4 times. Results were expressed as mg gallic acid equivalent/g dry weight.
2.8. Experimental Design and Statistical Analysis
The initial set of experiments focused only on varying the solvent type, while other extraction parameters, such as temperature, extraction time, and solid-to-liquid ratio, were kept constant. This approach is known as the one-factor-at-a-time (OFAT) method. The OFAT strategy enabled the identification of the most effective NADES system for the extraction of bioactive compounds under the given conditions. To evaluate extraction efficiency, the total polyphenol content and the DPPH radical scavenging capacity of the obtained extracts were determined spectrophotometrically. Preliminary screening was obtained using a factorial design 25−1 with five factors (extraction time, extraction temperature, liquid-to-solid ratio, water content in NADES, and type of NADES). TPC and antioxidant activity using the DPPH assay were analyzed during this stage of research. N12 and N14 were the two chosen solvents for the experimental design’s 16 sample runs. To achieve the best extraction conditions, two distinct responses were employed: TPC (mg GAE/g DW) and DPPH (mg TE/g DW). Equation (1) states that the impact of several input parameters on the responses was noted and optimized.
Here, Xi and Xj are the independent variables that influenced the response, while Y stands for the response variable (TPC and DPPH). In this instance, there were five independent parameters (n = 5). In Equation (1), the intercept is denoted by β0, and the regression coefficients for the linear, quadratic, and interaction terms are βi, βii, and βij, respectively. Design-Expert v.11 software (Stat-Ease, Minneapolis, MN, USA) was utilized in this study to assess experimentally acquired results, generate 2D plots, and determine the projected NADES extraction values for responses. All responses were presented with the mean ± standard deviation (SD) of four replicates for each analysis. Using Statistica 13 Software (Statsoft Inc., Tulsa, OK, USA), the one-way analysis of variance (ANOVA) (p ≤ 0.05) and post hoc Tukey test were used to screen for NADES in the first experimental stage. The statistical significance of the impact of the independent variables and their interactions on the applied responses (TPC and DPPH) was examined in the second stage using an ANOVA. The desirability function was used to perform simultaneous optimization. Additionally, using Design-Expert v.11 software (Stat-Ease, Minneapolis, MN, USA), the coefficient of determination (R2) and coefficient of variation (CV) were examined to assess the model’s sufficiency, the p-value for the model’s significance, the lack of fit, and the residuals’ normal probability plot. Face-centered central composite experimental design (CCD) and response surface methodology (RSM) were used in tandem to achieve the maximum phenolic component content and antioxidant activity. Factor A (extraction time at 30, 60, and 90 min), Factor B (temperature at 45, 55, and 65 °C), and Factor C (liquid-to-solid ratio at 15, 20, and 25 mL/g) were the three independent variables that were changed during NADES extraction. By contrasting the predicted values with the N12 sample extracts produced under ideal extraction conditions, the suggested model was verified.
3. Results and Discussion
The initial step of the present study was the selection of the most suitable NADES mixtures among ten investigated different hydrophilic NADES systems for the extraction of polyphenols from blackthorn pomace. In addition to the NADES-based extracts, two conventional solvent systems, 50% ethanol and acidified 50% ethanol containing 1% HCl, were prepared to serve as reference points for comparison with the novel green NADES.
The first step of the study involved a screening of the selected NADES systems for their efficiency in extracting bioactive compounds from blackthorn pomace, which was determined by TPC and DPPH. TPC in blackthorn pomace ranged from 11.45 to 21.51 mg GAE/g DW (Figure 1a). The lowest TPC value was obtained using 50% ethanol as a solvent, while the highest values were recorded for the extracts obtained with solvents N6, N8, and N14, with no statistically significant differences observed among them (p ≤ 0.05). N6 and N8 are ternary systems, in contrast to N14, which contains two components. The TPC values of defatted blackthorn fruit obtained in the study by Drăghici-Popa et al. [18] ranged from 4.50 to 37.40 mg GAE/g DM, with the highest value obtained under the following optimal extraction conditions: 50% ethanol acidified with lactic acid, an extraction time of 30 min, and an extraction temperature of 60 °C. Katanić Stanković et al. [6] used ultrasound-assisted extraction and reported a TPC value of 25.9 mg GAE/g for blackthorn fruit. Additionally, Nistor et al. [5] determined TPC value of 23.34, 7.61, 5.32, and 5.76 mg GAE/g DW for unprocessed pulp, marmalade, jam, and jelly, respectively, in their study on various blackthorn-based products. In the study by Milošević et al. [19], the NADES that yielded the highest total polyphenol content from strawberry tree fruit was composed of choline chloride and glycerol in a molar ratio of 1:2, whereas in our study, one of the most effective NADESs was made from the same components, but in a 1:1 molar ratio. In the study conducted by Bertolo et al. [20], the NADES that demonstrated the most effective performance for the recovery of phenolic compounds from pomegranate peel, used as agro-industrial waste, was composed of choline chloride and lactic acid in a 1:1 molar ratio, with 20% water content. This composition is similar to the one applied in our study, although our system also included a third component. These results demonstrate that choline chloride-based NADES systems provided the highest TPC values, consistent with previous studies using food waste and by-products. Therefore, these solvent mixtures should be used in further steps of this study.
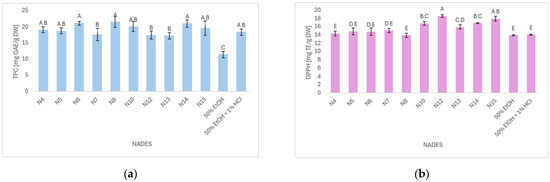
Figure 1.
(a) Total phenol content; (b) DPPH radical-scavenging activity of Blackthorn pomace extracts. Results are expressed as mean ± standard deviation, and different letters represent statistically significant differences (p ≤ 0.05) according to Tukey’s test.
The DPPH radical scavenging capacity of the extracts ranged from 13.96 to 18.59 mg TE/g DW (Figure 1b). The lowest DPPH value was observed for the extract obtained with solvent N8, while the highest activity was recorded for the extract prepared using N12. The antioxidant activity of blackthorn fruit extract, evaluated by DPPH radical scavenging, was expressed as IC50 (µg ascorbic acid equivalents/mL) and reported to be 610.7 µg/mL in the study by Katanić Stanković et al. [6]. The DPPH radical scavenging potential reported by Popović et al. [21] ranged from 0.62 to 3.46 mg DW/mL, also expressed as IC50. In this study, the extracts were obtained by maceration of different blackthorn genotypes in 50% ethanol, with the extraction process assisted by ultrasound at 40 °C. In the study conducted by Pavlić et al. [22] on the optimization of phenolic compound extraction and antioxidant activity assessment from wild thyme herbal dust, twenty NADES systems were evaluated. Among them, the second most effective solvent in the initial screening was composed of L-proline and lactic acid (1:2), while the most efficient one was a NADES based on L-proline and glycerol (1:2) with 20% water content. Pojić et al. [23] performed optimization of the extraction of phenolic compounds from hemp hulls using a NADES system. The optimal solvent for the recovery of phenolic compounds and antioxidant properties of the extract was found to be L-proline and lactic acid, in the same molar ratio as applied in our study. Mansinhos et al. [24] evaluated ten different NADES formulations for the extraction of bioactive compounds from Lavandula pedunculata subsp. lusitanica, and reported that the combination of L-proline and lactic acid in a 1:1 molar ratio yielded the highest total polyphenol content and antioxidant capacity. Similarly, in the study on Salicornia europaea extracts obtained using NADES-UAE extraction, the solvent composed of proline and malic acid (Pro:MA) showed remarkable antioxidant activity with a DPPH IC50 value of 0.09 mg GAE/g, confirming the high radical scavenging potential of proline-based NADES systems [25]. These findings are consistent with other studies reporting that natural compounds, such as levan-type exopolysaccharides from Bacillus subtilis, also exhibit notable DPPH radical scavenging activity (IC50 = 1.42 mg/mL), highlighting the broad potential of biobased molecules as effective antioxidants [26].
The Pearson correlation analysis revealed a weak positive correlation between TPC and DPPH radical scavenging activity (r = 0.176), which was not statistically significant (p = 0.584). These results suggest that polyphenols were not the primary contributors to the antioxidant activity of the extracts under the tested conditions. Based on the obtained results and taking into account data from the literature, N12 and N14 were selected for further analysis, as they demonstrated the highest DPPH radical scavenging capacity and potential for polyphenol recovery.
3.1. Preliminary Study
In the second step of the study, in addition to the selected NADES systems, N12 and N14, various factors influencing the extraction of bioactive compounds were investigated. Based on literature reports and previous experience in optimization studies [22], we designed preliminary screening experiments using a 25−1 factorial design with five factors. Among these, there were four numerical variables: temperature, extraction time, water content in NADES, and solid-to-liquid ratio, while the type of NADES was treated as a categorical variable. This design was applied to evaluate the main effects of individual factors as well as their potential interactions that could significantly affect the TPC yield and antioxidant activity. The experimental response values for TPC and DPPH obtained in the second step of the study are presented in Table 2, together with the factor levels for the 16 runs. TPC ranged from 21.18 to 29.81 mg GAE/g DW, indicating that TPC values can be further enhanced through the adjustment and combination of extraction parameters. When comparing the results between the first and second steps of the study, a pronounced increase in TPC was observed, with extracts richest in polyphenols showing approximately a 38% improvement. In the case of DPPH, the experimental values ranged from 16.05 to 48.32 mg TE/g DW, which corresponds to an even greater improvement, with radical scavenging capacity increasing by a factor of 2.5 (approximately 160%) compared to the first step of the study. The lowest and highest TPC and DPPH values were not observed in the same experimental runs. The highest TPC values were obtained in run 12, whereas the lowest were observed in run 16. Conversely, the highest DPPH radical scavenging capacity was recorded in run 8, while the lowest was found in run 2. Pearson correlation analysis revealed a moderate positive correlation between TPC and DPPH (r = 0.408), suggesting that an increase in TPC tends to be accompanied by an increase in DPPH. However, the relationship was not strong. The obtained p-value was 0.117, which exceeds the 0.05 threshold. Therefore, the correlation was not statistically significant. These findings further confirm that polyphenols are not the primary compounds contributing to the antioxidant activity of the extracts under the tested experimental conditions.

Table 2.
Fractional factorial 25−1 design with coded extraction parameters of NADESs N12 and N14 and experimentally observed values of target responses: total phenol content and antioxidant activity (DPPH).
ANOVA revealed that the fitted models for both TPC and DPPH were highly significant, with the values given in Table S1. The models showed excellent predictive ability, as indicated by high coefficients of determination (R2 = 0.9692 for TPC and R2 = 0.9815 for DPPH), and low coefficients of variation (CV = 2.71% and 6.49%), confirming the good reliability of the experimental data. Furthermore, the Pareto chart (Figure 2) illustrates the impact of individual effects and the interaction between them. The L/S ratio had the greatest influence on the TPC values, followed by extraction temperature and time, both of which exerted significant positive effects according to their t-values. In contrast, the type of NADES and the water content in the NADES systems showed insignificant effects on TPC values. Regarding the DPPH values, the L/S ratio again exhibited a positive effect, whereas the differences between the tested NADESs were significant, with N12 performing better than N14. Interestingly, the effects of extraction temperature and time on DPPH values were nonsignificant factors, as indicated by t-values below the statistical threshold. Since the type of NADES demonstrated a strong influence on DPPH, while its impact on TPC was negligible, and considering that the highest DPPH scavenging capacity was obtained with the N12 system, this solvent was selected for further optimization. Previous studies have shown that carboxylic acids, especially in combination with amino acids such as L-proline, are suitable components for the formation of NADES with high efficiency in polyphenol extraction [27,28]. These properties are particularly evident in the lactic acid–L-proline system, which has already been identified as the optimal solvent for polyphenol extraction from Michelia alba [29]. Water content in the NADES systems showed insignificant effects on both TPC and DPPH values. For this reason, the water content was fixed at 20% and applied in further optimization experiments. By amending two factors—the type of NADES and the water content in the NADES systems—the number of examined variables was reduced from five to three to decrease the number of required experiments for further optimization. At the same time, the selected variables were studied at three different levels to allow more reliable evaluation of their influence on the extraction process.
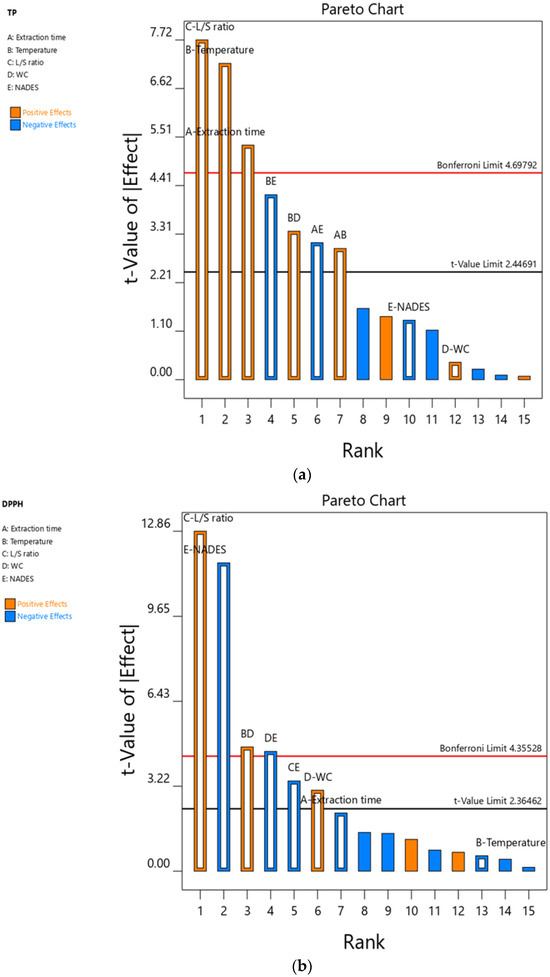
Figure 2.
Pareto chart exhibiting effects of extraction time (A), temperature (B), L/S ratio (C), water content (D), and NADES (E) on (a) TPC and (b) DPPH.
3.2. Optimization Study
After selecting the three most significant factors in the preliminary study, optimization of these factors was conducted. This optimization aimed to identify the best values of the factors that could provide the highest responses of the targeted outputs. For this purpose, the response surface methodology (RSM) was applied using a Central Composite Design (CCD). Several studies have employed statistical experimental designs similar to ours for the optimization of extraction processes. For example, Dabetić et al. [30] applied a Box–Behnken design to optimize the extraction of phenolic compounds from red grape seeds using NADES and conventional solvents. Likewise, in the study by Çolak et al. [31], the extraction of phenolic acids from Origanum species with novel DES solvents was optimized using a Box–Behnken design within the RSM framework. Alpat et al. [32] investigated the potential of NADES for the extraction of phenolic compounds from propolis and optimized the extraction process using CCD. Similarly, Bouallegue et al. [33] applied a comparable RSM approach to optimize the production of levan, evaluating the influence of parameters on levan structure and its angiotensin-I-converting enzyme inhibitory activity. The subsequent phenolic profiling confirmed the hypothesis that NADES can serve as promising alternatives to conventional solvents for propolis extraction. These studies solidify the methodological basis for employing RSM and CCD in optimizing NADES extraction parameters, similarly to our approach.
The three key factors selected for optimization, extraction time (A), extraction temperature (B), and liquid-to-solid ratio (C), were varied to examine their influence on the experimental responses (TPC, TFC, DPPH, FRAP, ABTS). In the CCD, different combinations of these factors were tested at three levels, namely the lowest (−1), medium (0), and highest (1), and the results of these combinations are presented in Table 3.

Table 3.
Central composite experimental design with coded extraction parameters of NADES with water content of 20% and experimentally observed values of target responses: total phenol content, total flavonoid content, and antioxidant activity (DPPH, FRAP, ABTS).
3.3. Assessment of Model Fitness and Factor Influence
A quadratic polynomial model (Equation (1)) was used to fit the data, whereas ANOVA and statistical parameters (R2 and CV) were employed to verify the reliability and accuracy of the fit (Table 4). The quadratic polynomial model provided an excellent fit for all evaluated responses, as confirmed by high R2 values (0.95–0.99) and acceptable coefficients of variation (CV < 10%), which indicates good reproducibility of the tested values. TPC (R2 = 0.99, CV = 4.12%) and FRAP (R2 = 0.97, CV = 5.58%) were best described by the model, with highly significant regression (p ≤ 0.001) and insignificant lack of fit, confirming model adequacy. Similarly, DPPH (R2 = 0.96, CV = 5.35%) and ABTS (R2 = 0.95, CV = 6.86%) were reliably predicted, with insignificant lack of fit values supporting the validity of the model. In contrast, although the TFC model showed a strong correlation (R2 = 0.98, CV = 8.22%), its significant lack of fit (p = 0.001086) indicated some limitations in explaining the variability of the data. Overall, the results demonstrate that the quadratic model adequately describes the experimental data, with the best fit achieved for TPC and FRAP. Detailed statistical significance of the linear, interaction, and quadratic model terms for all responses (TPC, TFC, DPPH, FRAP, and ABTS) is provided in Table S2. Recent studies have successfully applied RSM combined with NADES and ultrasound-assisted extraction for optimizing polyphenol recovery. For instance, Li et al. [34] investigated a NADES system composed of L-proline and lactic acid, combined with ultrasound-assisted extraction, for the recovery of polyphenolic compounds from celtuce leaves, and performed a comparative study using conventional solvents. By applying RSM and the Box–Behnken design, the extraction parameters were successfully optimized, thereby providing a theoretical foundation and guidelines for industrial-scale production. A similar approach was employed in the extraction from Vitis davidii pomace, where Zhang et al. [35] also applied RSM to optimize the total anthocyanin content. Balan et al. [36] studied two types of solvents, ethanol and NADES, using ultrasound-assisted extraction techniques for the isolation of polyphenolic compounds from red grape pomace. Optimization and validation of the extraction conditions leading to maximization of the desired responses were performed using RSM with CCD.

Table 4.
Analysis of variance (ANOVA) and descriptive statistics (R2 and CV) of the fitted model for TPC, TFC, DPPH, FRAP, and ABTS.
By applying the least squares approach, the regression coefficients of the polynomial models were calculated, yielding the predictive equations presented below:
The TPC values ranged from 23.95 (run 17) to 55.81 mg GAE/g DW (run 3), indicating that different extraction conditions, i.e., their respective levels, have a significant effect on the recovery of bioactive compounds. The lowest TPC yield was observed at the lower level of extraction time (30 min), the lower level of extraction temperature (45 °C), and the middle level of L/S ratio (20 mL/g). It was noted that a low TPC yield was obtained at the lower temperature level (45 °C), whereas the highest TPC values were recorded at the highest extraction temperature (65 °C). Temperature thus exerts a strong positive influence on the polyphenol content in blackthorn pomace, and it can be concluded that all higher TPC values in this experimental design were obtained at the maximum applied temperature. The TFC values ranged from 10.92 (run 17) to 35.97 mg QE/g DW (run 3), showing that both the lowest and the highest values were obtained under the same experimental runs as TPC. Temperature was also the most influential factor for higher TFC yields, since all lower yields were recorded at the lower temperature level (45 °C), whereas higher yields were consistently obtained at 65 °C. Extraction time also exhibited a positive effect on both TPC and TFC, although to a lesser extent than temperature, while the L/S ratio showed almost no significant influence in either case.
The results of the in vitro antioxidant assays (DPPH, FRAP, and ABTS) ranged from 33.17 to 65.69 mg TE/g DW, 15.84 to 33.10 mg Fe2+/g DW, and 71.28 to 142.93 mg TE/g DW, respectively. In all antioxidant assays, the highest activity was observed in run 7, while the lowest was recorded in run 10. The experimental conditions corresponding to the highest antioxidant activity across all three tests were the highest level of temperature (65 °C), the highest L/S ratio (25 mL/g), and the middle level of extraction time (60 min). Conversely, the lowest antioxidant activity was obtained at the lowest level of temperature (45 °C) and L/S ratio (15 mL/g), while the extraction time remained the same as in the experiment with the highest activity.
Based on the obtained results, it can be concluded that temperature exerted the most pronounced influence on all analyzed responses at this stage of the study. This finding can be associated with the fact that the viscosity and surface tension of solvents are properties strongly dependent on temperature [37]. Alongside viscosity, the conductivity of NADES is also considered, since it is governed by similar physicochemical factors, with temperature being one of the key determinants. As temperature increases, the kinetic energy of the system rises, which in turn leads to a significant enhancement of conductivity in binary NADES systems. Viscosity is highly sensitive to kinetic energy; higher temperatures provide greater kinetic energy, which can overcome the strength of intermolecular forces and consequently decrease the viscosity of the NADES system [38]. These physicochemical changes induced by increasing temperature enhance mass transfer and thereby intensify the extraction process, ultimately resulting in higher yields of target compounds. In the study conducted by Yang et al. [39], optimization was performed to determine the optimal conditions for the extraction of ellagic acid from Geum japonicum using NADES and based on ultrasound-assisted extraction. Temperature was identified as the most influential factor affecting extraction efficiency. The content of ellagic acid increased with rising temperature from 40 to 60 °C, while further heating from 60 to 80 °C had only a limited effect. Using RSM for process optimization, the optimal conditions were identified as an extraction temperature of 70 °C, extraction time of 31 min, solid–liquid ratio of 10:1, water content of 47%, and extraction power of 300 W. The decline in extraction yield at 80 °C was attributed to the thermal degradation of components at elevated temperatures [40]. Another study by Cabrera et al. [41] also highlighted the major influence of temperature, as well as the L/S ratio, on the optimization of phenolic extraction from Uruguayan olive pomace using NADES. RSM was applied to evaluate the effects of extraction temperature, water content in NADES systems, and solid-to-liquid ratio on the TPC and antioxidant activity. The optimal conditions maximizing both TPC and antioxidant activity were 80 °C, 68% water, and a solid-to-liquid ratio of 0.014 g/mL. The authors concluded that higher temperatures enhance mass transfer within the extraction system, primarily due to increased diffusivity and decreased viscosity and surface tension of NADES [42]. However, caution is required during extraction at elevated temperatures, as excessive heating may cause the degradation of phenolic compounds [42], as well as the possible thermal degradation of NADES themselves [43].
After the optimization study in our research, the next step was the application of the optimized extraction conditions and their validation in order to achieve the maximum yield of the desired responses. To determine the best parameters for NADES extraction, it was necessary that these parameters simultaneously maximize the contents of polyphenols and flavonoids, as well as the antioxidant activity. Since five different responses were considered in this study (TPC, TFC, DPPH, FRAP, and ABTS), the desirability function was employed to obtain a single optimal solution that accounts for all responses (Table 5). The overall desirability value reached 0.979. Based on the software analysis, the following optimal conditions were determined: extraction time of 90 min, temperature of 65 °C, and liquid-to-solid ratio of 22.65 mL NADES/g DW.

Table 5.
Optimized NADES parameters of polyphenols, flavonoids, and antioxidant activity by the RSM approach.
To validate the predicted values of all responses, an additional set of experiments was performed, and the predicted and experimental results are presented in Table 5. A good agreement between the predicted and experimental data was observed, except for TFC, where the experimental value (23.27 mg QE/g DW) was considerably lower than the predicted one (33.50 mg QE/g DW). The other responses showed a high level of agreement with the predicted values, thus confirming the adequacy of the applied quadratic model. These findings indicate that successful optimization and maximization of the desired responses were achieved within the prediction domain. Therefore, it can be concluded that the targeted optimization of polyphenol recovery and antioxidant activity from blackthorn pomace using NADES was accomplished after three steps of optimization and one validation step.
In addition to the values obtained for blackthorn pomace (Table 5), the results of all five responses were also determined when blackthorn juice was used as the raw material. Furthermore, the juice fraction was evaluated under the optimized extraction conditions in the final stage of the study, to compare its extraction efficiency and bioactive compound content with those of pomace. Given the significance of blackthorn pomace, which is a readily available industrial by-product that generally remains underutilized despite its potential as a source of bioactive compounds with antioxidant activity, the entire optimization study was conducted on this raw material.
As shown in Table 5, the extraction efficiency improved for both pomace and juice. When comparing the yields of total phenols and the responses for antioxidant activity measured using the DPPH assay, it can be concluded that the total phenol yield exhibited a 3.27-fold increase when pomace was extracted under optimal parameters compared to the initial step. Antioxidant activity was also significantly enhanced, showing a 3.86-fold increase. Furthermore, comparison of the values obtained from juice extraction under initial and optimal conditions confirmed the expected outcome, with a 3.50-fold increase in total phenol yield and an 11.14-fold increase in antioxidant activity.
In the literature, similar approaches to optimization and validation have also been applied to other plant materials. For example, Milošević et al. [19] applied a three-step optimization study focused on the maximization of polyphenol yield and antioxidant activity obtained by NADES extraction from strawberry tree fruit. Optimization was further confirmed by experimental validation, suggesting that RSM could be used as an effective tool for optimization of extraction systems based on NADES mixtures. After experimental validation, it was shown that both sets of optimal parameters resulted in extracts with higher polyphenol content and stronger antioxidant activity compared to 80% ethanol extraction. Nevertheless, slight deviations between predicted and obtained values were observed, which the authors attributed to incomplete hydrolysis of certain compounds. Overall, the adequacy of the model was confirmed, as well as the potential of NADES extraction as a sustainable technique. In a study conducted by Vo et al. [44], the validation of the regression models was studied and carried out under the optimal NADES8-based ultrasound-assisted extraction conditions, demonstrating a good agreement between the predicted and experimentally obtained values of TPC, TFC, and antioxidant activities (ABTS, OH-hydroxyl radical quenching capacity, DPPH). The prediction errors were low (≤7.17%), indicating that the models reliably predict the influence of extraction parameters on the quantitative and functional characteristics of the extract. The largest deviation was observed for the ABTS assay, which may be attributed to its higher sensitivity to minor variations in experimental conditions. Overall, the results confirm that the regression model is adequate for the optimization of the ultrasound-assisted extraction using NADESs.
4. Conclusions
Using three-step optimization and validation of obtained parameters for ultrasound-assisted NADES extraction of blackthorn pomace, as well as blackthorn juice, it can be concluded that N12, with 20% of water containing L-proline and lactic acid in a molar ratio of 1:2, was the best solvent for the recovery of polyphenols. Furthermore, the best antioxidant activity was obtained using the same solvent mixture, considering that polyphenols are not the main compounds that affect antioxidant activity. Based on RSM and CCD, optimal extraction parameters were obtained: extraction time (90 min), extraction temperature (65 °C), and liquid-to-solid ratio (22.65 mL/g). In the final stage, predicted variables were validated for blackthorn pomace. A good agreement was observed between predicted and experimental results, confirming the adequacy of the applied model. These findings demonstrate that targeted optimization of polyphenol recovery and antioxidant activity from blackthorn pomace using NADES was successfully achieved. Blackthorn pomace extracts showed 3.27-fold higher TPC yield and 3.86-fold improvement of antioxidant activity compared to the initial step of optimization. Considering that blackthorn juice was not the main objective of this research, and that optimal parameters for pomace were applied to juice to evaluate potential improvement, the extract showed significant enhancement. TPC yielded 3.50 times more, and antioxidant activity was 11.14 times higher than in the initial step of the optimization.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pr13113737/s1, Table S1. Analysis of variance (ANOVA) and descriptive statistics (R2 and CV) of the fitted model; Table S2. Statistical significance of linear, interaction and quadratic model terms for TPC, TFC, DPPH, FRAP, ABTS.
Author Contributions
Conceptualization, S.H., B.M.P. and B.P.; methodology, S.H., J.V., N.T. and R.Ž.P.; software, S.H. and B.P.; validation, S.H. and J.V.; formal analysis, S.H., J.V. and R.Ž.P.; investigation, S.H. and J.V.; resources, N.T., B.M.P. and B.P.; data curation, S.H. and J.V.; writing—original draft preparation, S.H. and J.V.; writing—review and editing, N.T., R.Ž.P., B.M.P. and B.P.; visualization, S.H. and J.V.; supervision, B.M.P. and B.P.; funding acquisition, B.M.P. and B.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by the Ministry of Science, Technological Development, and Innovation of the Republic of Serbia, Grant No. 451-994/2024-03/3412, No. 451-03-136/2025-03/200134, and No. 451-03-137/2025-03/ 200117.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to thank Sanja Gavrić and Davod Jalali (native English speakers) for the English editing in the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Popović, B.M.; Blagojević, B.; Kucharska, A.Z.; Agić, D.; Magazin, N.; Milović, M.; Serra, A.T. Exploring fruits from genus Prunus as a source of potential pharmaceutical agents–In vitro and in silico study. Food Chem. 2021, 358, 129812. [Google Scholar] [CrossRef] [PubMed]
- Ozzengin, B.; Zannou, O.; Koca, I. Quality attributes and antioxidant activity of three wild plums from Prunus spinosa and Prunus domestica species. Meas. Food 2023, 10, 100079. [Google Scholar] [CrossRef]
- Sallustio, V.; Rossi, M.; Marto, J.; Coelho, T.; Chinnici, F.; Mandrone, M.; Chiocchio, I.; Cappadone, C.; Luppi, B.; Bigucci, F. Green extraction of Rosa canina L. and Prunus spinosa L. by NaDES and their encapsulation in chitosan nanoparticles for cosmetic industry. Ind. Crops Prod. 2024, 218, 119042. [Google Scholar] [CrossRef]
- Popović, B.M.; Blagojević, B.; Pavlović, R.Ž.; Mićić, N.; Bijelić, S.; Bogdanović, B.; Mišan, A.; Duarte, C.M.M.; Serra, A.T. Comparison between polyphenol profile and bioactive response in blackthorn (Prunus spinosa L.) genotypes from north Serbia-from raw data to PCA analysis. Food Chem. 2020, 302, 125373. [Google Scholar] [CrossRef] [PubMed]
- Nistor, O.V.; Milea, Ș.A.; Păcularu-Burada, B.; Andronoiu, D.G.; Râpeanu, G.; Stănciuc, N. Technologically driven approaches for the integrative use of wild blackthorn (Prunus spinosa L.) fruits in foods and nutraceuticals. Antioxidants 2023, 12, 1637. [Google Scholar] [CrossRef]
- Katanić Stanković, J.S.; Mićanović, N.; Grozdanić, N.; Kostić, A.Ž.; Gašić, U.; Stanojković, T.; Popović-Djordjević, J.B. Polyphenolic profile, antioxidant and antidiabetic potential of medlar (Mespilus germanica L.), blackthorn (Prunus spinosa L.) and common hawthorn (Crataegus monogyna Jacq.) fruit extracts from Serbia. Horticulturae 2022, 8, 1053. [Google Scholar] [CrossRef]
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural deep eutectic solvents–solvents for the 21st century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071. [Google Scholar] [CrossRef]
- Fourmentin, S.; Gomes, M.C.; Lichtfouse, E. (Eds.) Deep Eutectic Solvents for Medicine, Gas Solubilization and Extraction of Natural Substances; Springer Nature: London, UK, 2020; Volume 56. [Google Scholar]
- Wu, K.; Ren, J.; Wang, Q.; Nuerjiang, M.; Xia, X.; Bian, C. Research progress on the preparation and action mechanism of natural deep eutectic solvents and their application in food. Foods 2022, 11, 3528. [Google Scholar] [CrossRef]
- de los Ángeles Fernández, M.; Boiteux, J.; Espino, M.; Gomez, F.J.V.; Silva, M.F. Natural deep eutectic solvents-mediated extractions: The way forward for sustainable analytical developments. Anal. Chim. Acta 2018, 1038, 1–10. [Google Scholar] [CrossRef]
- Koraqi, H.; Aydar, A.Y.; Pandiselvam, R.; Qazimi, B.; Khalid, W.; Petkoska, A.T.; Çesko, C.; Ramniwas, S.; Asdaq, S.M.B.; Rustagi, S. Optimization of extraction condition to improve blackthorn (Prunus spinosa L.) polyphenols, anthocyanins and antioxidant activity by natural deep eutectic solvent (NADES) using the simplex lattice mixture design method. Microchem. J. 2024, 200, 110497. [Google Scholar] [CrossRef]
- Jurić, T.; Hourani, S.; Petković, M.; Vukosavljević, J.; Ždero Pavlović, R.; Pavlić, B.; Popović, B.M. Exploring Natural Deep Eutectic Solvents for Extraction and Stabilization of Grape Pomace Phytochemicals. Food Bioprocess Technol. 2025, 18, 10968–10985. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Harborne, J.B. Methods of Plant Analysis; Springer: Dordrecht, The Netherlands, 1984. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity appliying an improved ABTS radical cation decolorizatio assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Drăghici-Popa, A.-M.; Boscornea, A.C.; Brezoiu, A.-M.; Tomas, Ș.T.; Pârvulescu, O.C.; Stan, R. Effects of extraction process factors on the composition and antioxidant activity of blackthorn (Prunus spinosa L.) fruit extracts. Antioxidants 2023, 12, 1897. [Google Scholar] [CrossRef] [PubMed]
- Milošević, S.; Markovinović, A.B.; Teslić, N.; Mišan, A.; Pojić, M.; Karačonji, I.B.; Jurica, K.; Lasić, D.; Putnik, P.; Kovačević, D.B. Use of natural deep eutectic solvent (NADES) as a green extraction of antioxidant polyphenols from strawberry tree fruit (Arbutus unedo L.): An optimization study. Microchem. J. 2024, 200, 110284. [Google Scholar] [CrossRef]
- Bertolo, M.R.V.; Martins, V.C.A.; Plepis, A.M.G.; Junior, S.B. Utilization of pomegranate peel waste: Natural deep eutectic solvents as a green strategy to recover valuable phenolic compounds. J. Clean. Prod. 2021, 327, 129471. [Google Scholar] [CrossRef]
- Popovic, B.M.; Micic, N.; Potkonjak, A.; Blagojevic, B.; Pavlovic, K.; Milanov, D.; Juric, T. Novel extraction of polyphenols from sour cherry pomace using natural deep eutectic solvents—Ultrafast microwave-assisted NADES preparation and extraction. Food Chem. 2022, 366, 130562. [Google Scholar] [CrossRef]
- Pavlić, B.; Mrkonjić, Ž.; Teslić, N.; Cvetanović Kljakić, A.; Pojić, M.; Mandić, A.; Stupar, A.; Santos, F.; Rita, A.; Duarte, C.; et al. Natural Deep Eutectic Solvent (NADES) Extraction Improves Polyphenol Yield and Antioxidant Activity of Wild Thyme (Thymus serpyllum L.) Extracts. Molecules 2022, 27, 1508. [Google Scholar] [CrossRef]
- Pojić, M.; Teslić, N.; Banjac, V.; Mrkonjić, Ž.; Stupar, A.; Mandić, A.; Mišan, A.; Pavlić, B. Natural deep eutectic solvents for efficient recovery of bioactive compounds from by-product of industrial hemp processing: Pretreatment, modeling and optimization. Ind. Crops Prod. 2024, 222, 119617. [Google Scholar] [CrossRef]
- Mansinhos, I.; Gonçalves, S.; Rodriguez-Solana, R.; Ordóñez-Díaz, J.L.; Moreno-Rojas, J.M.; Romano, A.; Rodríguez-Solana, R.; Ordóñez-Díaz, J.L.; Moreno-Rojas, J.M.; Romano, A. Ultrasonic-assisted extraction and natural deep eutectic solvents combination: A green strategy to improve the recovery of phenolic compounds from Lavandula pedunculata subsp. lusitanica (chaytor) franco. Antioxidants 2021, 10, 582. [Google Scholar] [CrossRef] [PubMed]
- Limongelli, F.; Aresta, A.M.; Tardugno, R.; Clodoveo, M.L.; Barbarossa, A.; Carocci, A.; Zambonin, C.; Crupi, P.; Panić, M.; Corbo, F. Exploitation of Apulian Salicornia europaea L. via NADES-UAE: Extraction, Antioxidant Activity and Antimicrobial Potential. Molecules 2025, 30, 3367. [Google Scholar] [CrossRef]
- Bouallegue, A.; Casillo, A.; Chaari, F.; La Gatta, A.; Lanzetta, R.; Corsaro, M.M.; Bachoual, R.; Ellouz-Chaabouni, S. Levan from a new isolated Bacillus subtilis AF17: Purification, structural analysis and antioxidant activities. Int. J. Biol. Macromol. 2020, 144, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Cablé, P.-A.; Le Brech, Y.; Mutelet, F. Liquid-liquid extraction of phenolic compounds from aqueous solution using hydrophobic deep eutectic solvents. J. Mol. Liq. 2022, 366, 120266. [Google Scholar] [CrossRef]
- Rahman, M.S.; Roy, R.; Jadhav, B.; Hossain, M.N.; Halim, M.A.; Raynie, D.E. Formulation, structure, and applications of therapeutic and amino acid-based deep eutectic solvents: An overview. J. Mol. Liq. 2021, 321, 114745. [Google Scholar] [CrossRef]
- Wang, W.; Pan, Y.; Zhao, J.; Wang, Y.; Yao, Q.; Li, S. Development and optimization of green extraction of polyphenols in Michelia alba using natural deep eutectic solvents (NADES) and evaluation of bioactivity. Sustain. Chem. Pharm. 2024, 37, 101425. [Google Scholar] [CrossRef]
- Dabetic, N.; Todorovic, V.; Malenovic, A.; Sobajic, S.; Markovic, B. Optimization of Extraction and HPLC–MS/MS Profiling of Phenolic Compounds from Red Grape Seed Extracts Using Conventional and Deep Eutectic Solvents. Antioxidants 2022, 11, 1595. [Google Scholar] [CrossRef] [PubMed]
- Çolak, N.U.; Badem, M.; Şener, S.Ö.; Kanbolat, Ş.; Yaşar, A.; Özgen, U.; Değirmenci, A. Optimization of phenolic acid extraction from Origanum species via novel deep eutectic solvents using response surface methodology. J. Food Meas. Charact. 2025, 19, 6528–6539. [Google Scholar] [CrossRef]
- Alpat, U.; Nar, T.; Karasu, S.; Sagdic, O. Optimization of propolis extraction with natural deep eutectic solvents using central composite design. Phytochem. Lett. 2023, 58, 49–59. [Google Scholar] [CrossRef]
- Bouallegue, A.; Casillo, A.; Chaari, F.; Cimini, D.; Corsaro, M.M.; Bachoual, R.; Ellouz-Chaabouni, S. Statistical optimization of levan: Influence of the parameter on levan structure and angiotensin I-converting enzyme inhibitory. Int. J. Biol. Macromol. 2020, 158, 945–952. [Google Scholar] [CrossRef]
- Li, S.; Wang, G.; Zhao, J.; Ou, P.; Yao, Q.; Wang, W. Ultrasound-assisted extraction of phenolic compounds from celtuce (Lactuca sativa var. augustana) leaves using natural deep eutectic solvents (NADES): Process optimization and extraction mechanism research. Molecules 2024, 29, 2385. [Google Scholar]
- Zhang, S.; Lin, S.; Zhang, J.; Liu, W. Ultrasound-assisted natural deep eutectic solvent extraction of anthocyanin from Vitis davidii Foex. pomace: Optimization, identification, antioxidant activity and stability. Heliyon 2024, 10, e33066. [Google Scholar] [CrossRef] [PubMed]
- Balan, N.; Măntăilă, S.; Râpeanu, G.; Stănciuc, N. Enhanced Extraction of Bioactive Compounds from Red Grape Pomace: Optimizing Ultrasound-Assisted Extraction with Ethanol and NaDES as Solvents. Antioxidants 2025, 14, 526. [Google Scholar] [CrossRef]
- Savi, L.K.; Carpiné, D.; Waszczynskyj, N.; Ribani, R.H.; Haminiuk, C.W.I. Influence of temperature, water content and type of organic acid on the formation, stability and properties of functional natural deep eutectic solvents. Fluid Phase Equilibria 2019, 488, 40–47. [Google Scholar] [CrossRef]
- Liu, Y.; Friesen, J.B.; McAlpine, J.B.; Lankin, D.C.; Chen, S.-N.; Pauli, G.F. Natural deep eutectic solvents: Properties, applications, and perspectives. J. Nat. Prod. 2018, 81, 679–690. [Google Scholar] [CrossRef]
- Yang, Z.; Yue, S.-J.; Gao, H.; Zhang, Q.; Xu, D.-Q.; Zhou, J.; Li, J.-J.; Tang, Y.-P. Natural deep eutectic solvent-ultrasound assisted extraction: A green approach for ellagic acid extraction from Geum japonicum. Front. Nutr. 2023, 9, 1079767. [Google Scholar] [CrossRef]
- Qi, X.-L.; Peng, X.; Huang, Y.-Y.; Li, L.; Wei, Z.-F.; Zu, Y.-G.; Fu, Y.-J. Green and efficient extraction of bioactive flavonoids from Equisetum palustre L. by deep eutectic solvents-based negative pressure cavitation method combined with macroporous resin enrichment. Ind. Crops Prod. 2015, 70, 142–148. [Google Scholar] [CrossRef]
- Cabrera, L.; Xavier, L.; Zecchi, B. Extraction of phenolic compounds with antioxidant activity from olive pomace using natural deep eutectic solvents: Modelling and optimization by response surface methodology. Discov. Food 2024, 4, 29. [Google Scholar] [CrossRef]
- Ivanović, M.; Alañón, M.E.; Arráez-Román, D.; Segura-Carretero, A. Enhanced and green extraction of bioactive compounds from Lippia citriodora by tailor-made natural deep eutectic solvents. Food Res. Int. 2018, 111, 67–76. [Google Scholar] [CrossRef]
- Craveiro, R.; Aroso, I.; Flammia, V.; Carvalho, T.; Viciosa, M.T.; Dionísio, M.; Barreiros, S.; Reis, R.L.; Duarte, A.R.C.; Paiva, A. Properties and thermal behavior of natural deep eutectic solvents. J. Mol. Liq. 2016, 215, 534–540. [Google Scholar] [CrossRef]
- Vo, T.P.; Pham, T.V.; Weina, K.; Tran, T.N.H.; Vo, L.T.V.; Nguyen, P.T.; Bui, T.L.H.; Phan, T.H.; Nguyen, D.Q. Green extraction of phenolics and flavonoids from black mulberry fruit using natural deep eutectic solvents: Optimization and surface morphology. BMC Chem. 2023, 17, 119. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).