Phytochemical Profile and Cosmeceutical Potential of Leaf Extracts of Two Species of the Anacardiaceae Family from the Mediterranean Scrubland: Pistacia lentiscus L. and Pistacia atlantica Desf.
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Sample Collection
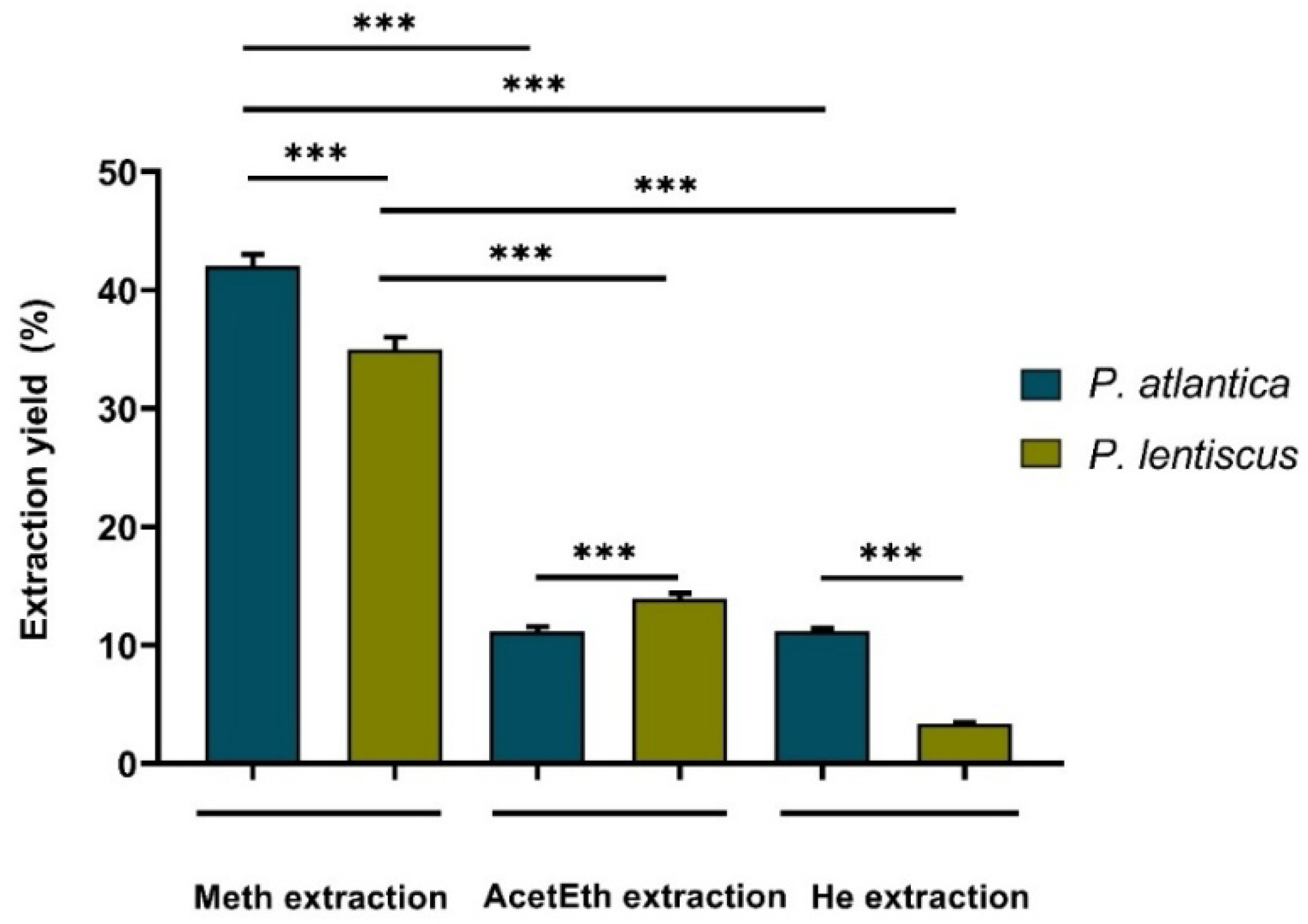
2.3. Extraction and Yield Assessment
2.4. Phytochemicals
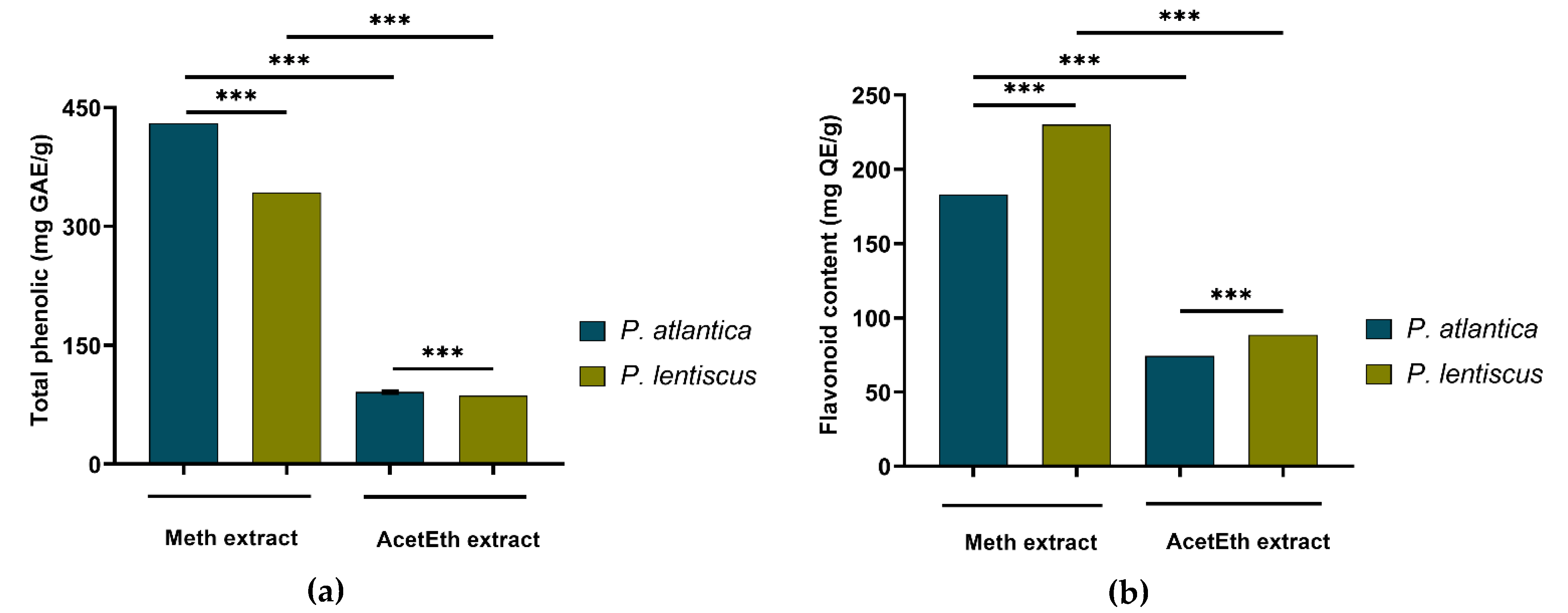
2.4.1. Quantification of Total Phenolic Constituents
2.4.2. Measurement of Total Flavonoid Contents
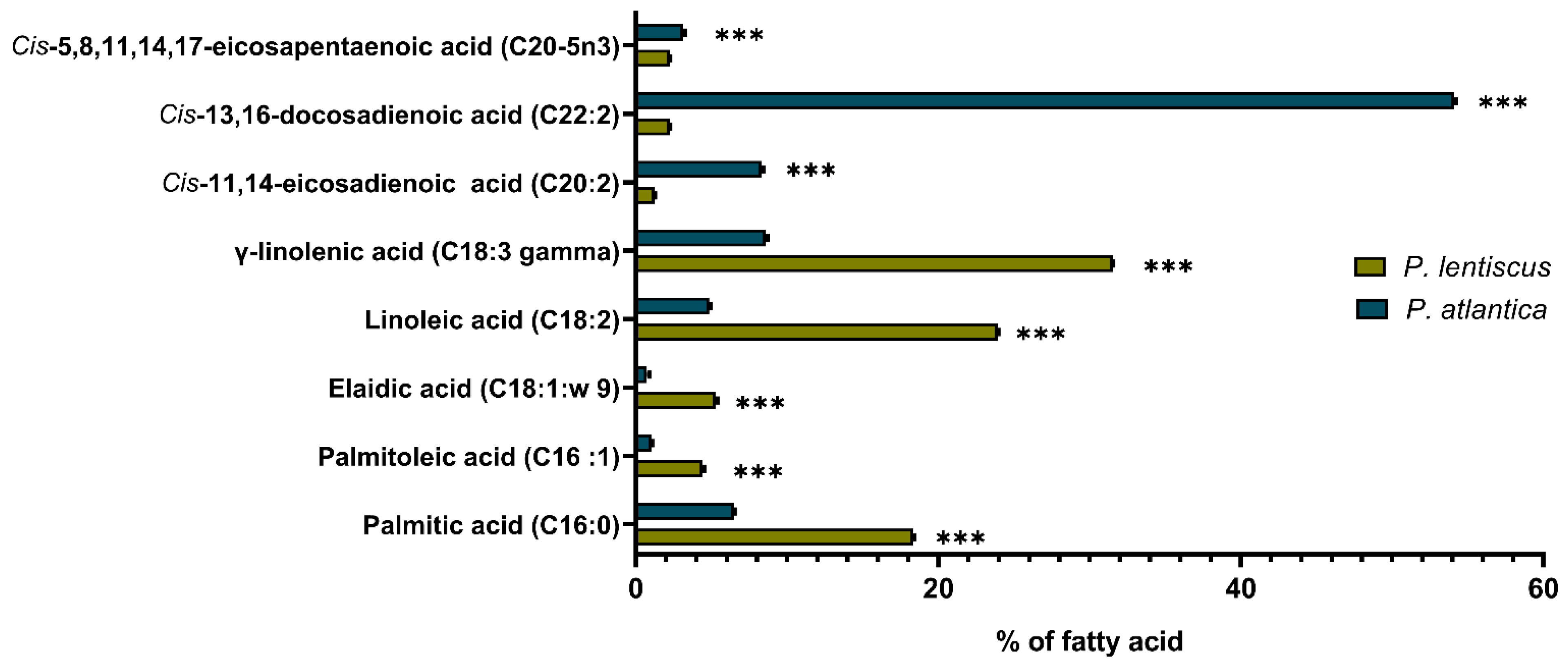
2.4.3. Fatty Acid GC-FID Analysis
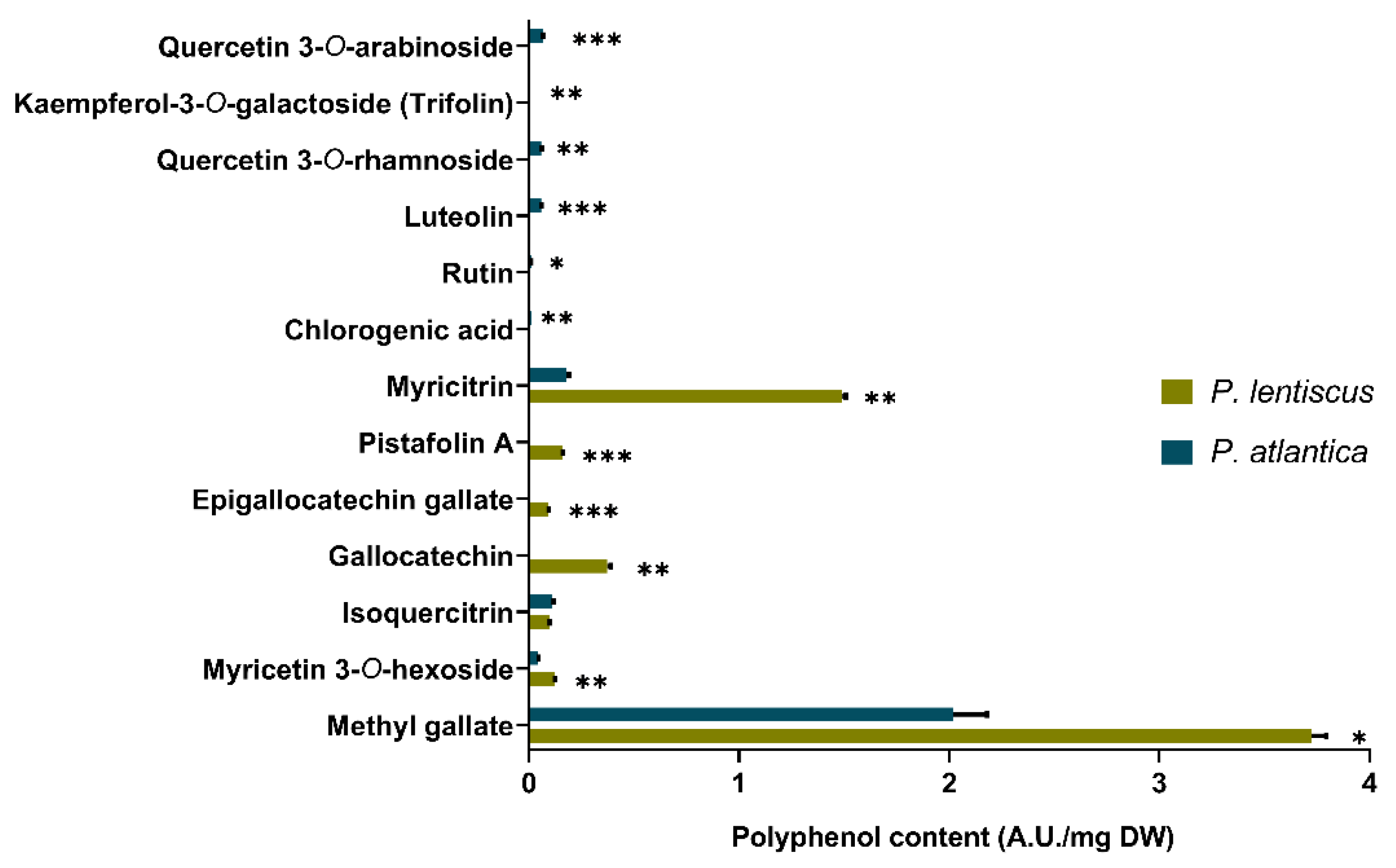
2.4.4. LC-MS/MS Analysis
2.5. Biological Activities
2.5.1. Antioxidant Assays
- DPPH assay (2,2-diphenyl-1-picrylhydrazyl radical scavenging test)
- Ferric reducing antioxidant power (FRAP) assay
2.5.2. Assessment of the Anti-Inflammatory Activities
- Bovine Serum Albumin Denaturation Assay
- Lipoxygenase inhibition assay
2.5.3. Anti-Tyrosinase Activity Evaluation
2.5.4. Data Analysis
3. Results and Discussion
3.1. Chemical Composition
3.1.1. Extraction Yields, Phenolic and Flavonoid Contents
3.1.2. Fatty Acid Composition
3.1.3. LC-MS/MS Screening and Identification of Polyphenolic Compounds
| N° | Compound | Formula | MRM (m/z) | MM | Predicted M-H | M-H Obs | Mass Error (ppm) | RT | Observed Fragments | Samples | References |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Isoquercetin | C21H20O12 | 463 → 301 | 464.0954 | 463.0876 | 463.0840 | 7.77 | 3.9 | 300.0290, 271.0265, 301.0370, 255.0320 | PL, PA | [74], PubChem |
| 2 | Chlorogenic acid | C16H18O9 | 353 → 191 | 354.0950 | 353.0872 | 353.0846 | 7.49 | 3.1 | 191.0561 | PA | [75], PubChem |
| 3 | Myricitrin | C21H20O12 | 463 → 317 | 464.0954 | 463.0876 | 463.0840 | 7.77 | 3.9 | 301.0357, 317.0309, 300.0280, 271.0262 | PL, PA | [76], MassBank |
| 4 | Methyl gallate | C8H8O5 | 183 → 124 | 184.0371 | 183.0293 | 183.0282 | 6.31 | 3.1 | 124.0163, 123.0089, 106.0057 | PL, PA | [77] |
| 5 | Myricetin 3-O-hexoside | C21H20O13 | 479 → 317 | 480.0903 | 479.0825 | 479.0784 | 8.59 | 3.6 | 316.0223, 271.0262, 287.0190, 317.0298, 179.0006, 151.0004 | PL, PA | [78] |
| 6 | Gallocatechin | C15H14O7 | 305 → 125 | 306.0739 | 305.0661 | 305.0661 | 7.72 | 2.6 | 125.0243, 137.0246, 109.0288, 139.0371, 167.0330 | PL | PubChem, MassBank |
| 7 | Epigallocatechin gallate | C22H18O11 | 457 → 169 | 458.0849 | 457.0771 | 457.0737 | 7.36 | 3.4 | 125.0248, 137.0247, 179.0355, 219.0669, 305.0671 | PL | [79], MassBank |
| 8 | Pistafolin A | C28H24O18 | 647 → 495 | 648.0962 | 647.0884 | 647.0828 | 8.72 | 3.5 | 343.0688, 169.0149, 495.0788 | PL | [80,81] |
| 9 | Rutin | C27H30O16 | 609 → 300 | 610.1533 | 609.1455 | 609.1551 | −15.73 | 3.8 | 300.0275, 301.0380, 272.0316, 178.3373, 151.0027 | PA | [82] |
| 10 | Luteolin | C15H10O6 | 285 → 133 | 286.0477 | 285.0399 | 285.0399 | −0.06 | 4.5 | 133.0399, 151.006, 270.2072, 107.0168, 175.0380, 121.0269 | PA | [83] |
| 11 | Quercetin 3-O-rhamnoside | C21H20O11 | 447 → 301 | 448.1005 | 447.0927 | 447.0959 | −7.15 | 4.2 | 300.0285, 301.0346, 271.0266, 255.0302, 178.9992 | PA | [84] |
| 12 | Kaempferol-3-O-galactoside (Trifolin) | C21H20O11 | 447 → 284 | 448.1005 | 447.0927 | 447.0959 | −7.15 | 4.2 | 325.0602, 285.0419, 284.0280, 255.0302 | PA | [85], PubChem |
| 13 | Quercetin 3-O-arabinoside | C20H18O11 | 433 → 301 | 434.0849 | 433.0771 | 433.0772 | −0.27 | 4.1 | 300.0268, 271.0256, 301.0344, 255.0271 | PA | [84] |
3.2. Biological Activities
3.2.1. Antioxidant Activities
3.2.2. Anti-Inflammatory Activities
3.2.3. Anti-Tyrosinase Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| FRAP | Ferric-reducing antioxidant power |
| LOX | Lipoxygenase |
| BSA | Bovine serum albumin |
| LC-MS/MS | Liquid Chromatography–Tandem Mass Spectrometry |
| GC-FID | Gas Chromatography–Flame Ionization Detection |
| LC-QTOF | Liquid Chromatography–Quadrupole Time-of-Flight |
| LC-TQ | Liquid Chromatography–Triple Quadrupole |
Appendix A
| Fatty Acid | Rt (min) | P. lentiscus (%) | P. atlantica (%) | p-Value | |
|---|---|---|---|---|---|
| 1 | Caproic acid (C6:0) | 3.59 | 0.13 ± 0.02 | 0.10 ± 0.06 | 0.54 |
| 2 | Undecanoic acid (C11:0) | 5.50 | 0.19 ± 0.07 | 0.15 ± 0.01 | 0.47 |
| 3 | Lauric acid (C12:0) | 7.81 | 0.38 ± 0.11 | 0.55 ± 0.31 | 0.42 |
| 4 | Tridecanoic acid (C13:0) | 8.96 | 0.40 ± 0.01 | 1.07 ± 0.13 | <0.001 |
| 5 | Myristic acid (C14:0) | 10.31 | 3.63 ± 0.07 | 1.52 ± 0.09 | <0.001 |
| 6 | Pentadecanoic acid (C15:0) | 11.74 | nd | 0.54 ± 0.23 | - |
| 7 | Palmitic acid (C16:0) | 13.34 | 18.35 ± 0.06 | 6.51 ± 0.02 | <0.001 |
| 8 | Palmitoleic acid (C16:1) | 13.72 | 4.42 ± 0.09 | 1.02 ±0.05 | <0.001 |
| 9 | Margaric acid (C17:0) | 14.88 | nd | nd | - |
| 10 | Stearic acid (C18:0) | 16.49 | 1.36 ± 0.04 | 0.91 ± 0.01 | <0.001 |
| 11 | Oleic acid (C18:1) | 16.50 | 1.36 ± 0.03 | nd | - |
| 12 | Elaidic acid (C18:1: ω9) | 16.83 | 5.30 ± 0.11 | 0.72 ± 0.17 | <0.001 |
| 13 | Vaccenic acid (C18:1: ω7) | 16.92 | 0.65 ± 0.01 | nd | - |
| 14 | Linoleic acid (C18:2) | 17.56 | 23.96 ± 0.04 | 4.86 ± 0.06 | <0.001 |
| 15 | γ-linolenic acid (C18:3 γ) | 18.60 | 31.54 ± 0.01 | 8.60 ± 0.09 | <0.001 |
| 16 | Arachidic acid (C20:0) | 19.63 | 0.61 ± 0.16 | 0.48 ± 0.01 | 0.21 |
| 17 | Cis-11,14-eicosadienoic acid (C20:2) | 20.76 | 1.24 ± 0.02 | 8.31 ± 0.11 | <0.001 |
| 18 | Cis-5,8,11,14,17-eicosapentaenoic acid (C20-5n3) | 22.22 | nd | 3.12 ± 0.12 | - |
| 19 | Behenic acid (C22:0) | 22.64 | 1.18 ± 0.05 | 0.45 ± 0.02 | <0.001 |
| 20 | Cis-13,16-docosadienoic acid (C22:2) | 23.73 | 2.24 ± 0.02 | 54.13 ± 0.09 | <0.001 |
| 21 | Lignoceric acid (C24:0) | 25.75 | 2.17 ± 0.02 | 0.63 ± 0.13 | <0.001 |
| 22 | Nervonic acid (C24:1n9) | 26.10 | nd | 4.90 ± 0.07 | - |
| Saturated fatty acids (SFA) [a] | 29.29 | 14.34 | |||
| Unsaturated fatty acids (UFA) [b] | 70.71 | 85.66 | |||
| Unsaturation ratio = UFA/SFA [c] | 2.41 | 5.97 | |||
References
- Papaccio, F.; D’Arino, A.; Caputo, S.; Bellei, B. Focus on the contribution of oxidative stress in skin aging. Antioxidants 2022, 11, 1121. [Google Scholar] [CrossRef]
- Davids, L.M.; Van Wyk, J.; Khumalo, N.P.; Jablonski, N.G. Phenomenon of skin lightening: Is it right to be light? S. Afr. J. Sci. 2016, 112, 5. [Google Scholar] [CrossRef]
- Villareal, M.O.; Kume, S.; Bourhim, T.; Bakhtaoui, F.Z.; Kashiwagi, K.; Han, J.; Gadhi, C.; Isoda, H. Activation of MITF by argan oil leads to the inhibition of the tyrosinase and dopachrome tautomerase expressions in B16 murine melanoma cells. Evid.-Based Complement. Altern. Med. 2013, 2013, 340107. [Google Scholar] [CrossRef] [PubMed]
- Mulholland, D.A.; Mwangi, E.M.; Dlova, N.C.; Plant, N.; Crouch, N.R.; Coombes, P.H. Non-toxic melanin production inhibitors from Garcinia livingstonei (Clusiaceae). J. Ethnopharmacol. 2013, 149, 570–575. [Google Scholar] [CrossRef] [PubMed]
- Opperman, L.; De Kock, M.; Klaasen, J.; Rahiman, F. Tyrosinase and melanogenesis inhibition by indigenous african plants: A review. Cosmetics 2020, 7, 60. [Google Scholar] [CrossRef]
- Chiocchio, I.; Mandrone, M.; Sanna, C.; Maxia, A.; Tacchini, M.; Poli, F. Screening of a hundred plant extracts as tyrosinase and elastase inhibitors, two enzymatic targets of cosmetic interest. Ind. Crops Prod. 2018, 122, 498–505. [Google Scholar] [CrossRef]
- Tian, L.; Zhao, Y.; Guo, C.; Yang, X. A comparative study on the antioxidant activities of an acidic polysaccharide and various solvent extracts derived from herbal Houttuynia cordata. Carbohydr. Polym. 2011, 83, 537–544. [Google Scholar] [CrossRef]
- Wang, Z.; Jiang, Y.; Ge, C.; Wang, Y.; He, J.; Chen, J.; Hou, X. Anti-inflammatory activity evaluation and molecular docking analysis of four new compounds isolated from M. oleifera seeds. J. Mol. Struct. 2024, 1318, 139269. [Google Scholar] [CrossRef]
- Shi, S.; Li, K.; Peng, J.; Li, J.; Luo, L.; Liu, M.; Chen, Y.; Xiang, Z.; Xiong, P.; Liu, L.; et al. Chemical characterization of extracts of leaves of Kadsua coccinea (Lem.) A.C. Sm. by UHPLC-Q-Exactive Orbitrap Mass spectrometry and assessment of their antioxidant and anti-inflammatory activities. Biomed. Pharmacother. 2022, 149, 112828. [Google Scholar] [CrossRef]
- Othman, S.O.K.; El-Hashash, M.A.; Hussein, S.; El-Mesallamy, A.M.D.; Rizk, S.A.; Elabbar, F.A. Phenolic content as antioxidant and antimicrobial activities Pistacia atlantica Desf. (Anacardiaceae) Extract from Libya. Egypt. J. Chem. 2018, 62, 21–28. [Google Scholar] [CrossRef]
- Yousfi, M.; Nedjmi, B.; Bellal, R.; Ben Bertal, D.; Palla, G. Fatty acids and sterols of Pistacia atlantica fruit oil. J. Am. Oil. Chem. Soc. 2002, 79, 1049–1050. [Google Scholar] [CrossRef]
- Benhassaini, H.; Benabderrahmane, M.; Chikhi, K. Contribution à l’évaluation de l’activité antiseptique de l’oléorésine et des huiles essentielles du Pistachier de l’Atlas sur certaines sources microbiennes: Candida albicans (ATC 20027), Candida albicans (ATCC 20032) et Saccharomyces cerevisiae. Ethnopharmacologia 2003, 30, 38–46. [Google Scholar]
- Farahpour, M.R.; Mirzakhani, N.; Doostmohammadi, J.; Ebrahimzadeh, M. Hydroethanolic Pistacia atlantica hulls extract improved wound healing process; evidence for mast cells infiltration, angiogenesis and RNA stability. Int. J. Surg. 2015, 17, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Pachi, V.K.; Mikropoulou, E.V.; Gkiouvetidis, P.; Siafakas, K.; Argyropoulou, A.; Angelis, A.; Mitakou, S.; Halabalaki, M. Traditional Uses, Phytochemistry and pharmacology of chios mastic gum (Pistacia lentiscus var. Chia, Anacardiaceae): A review. J. Ethnopharmacol. 2020, 254, 112485. [Google Scholar] [CrossRef] [PubMed]
- Mahjoub, F.; Rezayat, K.A.; Yousefi, M.; Mohebbi, M.; Salari, R. Pistacia atlantica Desf. A review of its traditional uses, phytochemicals and pharmacology. J. Med. Life 2018, 11, 180. [Google Scholar] [CrossRef] [PubMed]
- Bahmani, M.; Zargaran, A.; Rafieian-Kopaei, M.; Saki, K. Ethnobotanical study of medicinal plants used in the management of diabetes mellitus in the Urmia, Northwest Iran. Asian Pac. J. Trop. Med. 2014, 7, S348–S354. [Google Scholar] [CrossRef]
- Ellahi, H.; Khalili Sadrabad, E.; Hekmatimoghaddam, S.; Jebali, A.; Sarmast, E.; Akrami Mohajeri, F. Application of essential oil of Pistacia atlantica gum, polypropylene and silica nanoparticles as a new milk packaging. Food Sci. Nutr. 2020, 8, 4037–4043. [Google Scholar] [CrossRef]
- Belkessam, M.; Genva, M.; Souissi, N.; Dridi, M.; Dennemont, I.; Loiseau, P.M.; Fauconnier, M.-L.; Ben-Attia, M. Dioecy impacts the chemical profile and biological activities of essential oils from leaves of Pistacia lentiscus L. J. Essent. Oil Bear. Plants 2025, 28, 158–175. [Google Scholar] [CrossRef]
- Benmahieddine, A.; Belyagoubi-Benhammou, N.; Belyagoubi, L.; Amari, N.O.; Zerey-Belaskri, A.E.; Gismondi, A.; Di Marco, G.; Canini, A.; Habi, S.; Atik Bekkara, F. Leaf-buds of Pistacia atlantica: A novel source of bioactive molecules with high anti-inflammatory, antioxidant, anti-tyrosinase and antimicrobial properties. Physiol. Mol. Biol. Plants 2023, 29, 209–219. [Google Scholar] [CrossRef]
- Bullitta, S.; Piluzza, G.; Manunta, M.D.I. Cell-based and chemical assays of the ability to modulate the production of intracellular reactive oxygen species of eleven mediterranean plant species related to ethnobotanic traditions. Genet. Resour. Crop Evol. 2013, 60, 403–412. [Google Scholar] [CrossRef]
- Hashemnia, M.; Nikousefat, Z.; Yazdani-Rostam, M. Antidiabetic Effect of Pistacia Atlantica and Amygdalus Scoparia in Streptozotocin-Induced Diabetic Mice. Comp. Clin. Pathol. 2015, 24, 1301–1306. [Google Scholar] [CrossRef]
- Floris, S.; Di Petrillo, A.; Pintus, F.; Delogu, G.L. Pistacia Lentiscus: Phytochemistry and Antidiabetic Properties. Nutrients 2024, 16, 1638. [Google Scholar] [CrossRef]
- Benmohamed, M.; Guenane, H.; Messaoudi, M.; Zahnit, W.; Egbuna, C.; Sharifi-Rad, M.; Chouh, A.; Seghir, B.B.; Rebiai, A.; Boubekeur, S.; et al. Mineral Profile, Antioxidant, Anti-Inflammatory, Antibacterial, Anti-Urease and Anti-α-Amylase Activities of the Unripe Fruit Extracts of Pistacia Atlantica. Molecules 2023, 28, 349. [Google Scholar] [CrossRef]
- Quartu, M.; Serra, M.P.; Boi, M.; Pillolla, G.; Melis, T.; Poddighe, L.; Del Fiacco, M.; Falconieri, D.; Carta, G.; Murru, E. Effect of acute administration of Pistacia lentiscus L. essential oil on rat cerebral cortex following transient bilateral common carotid artery occlusion. Lipids Health Dis. 2012, 11, 8. [Google Scholar] [CrossRef] [PubMed]
- Remila, S.; Atmani-Kilani, D.; Delemasure, S.; Connat, J.-L.; Azib, L.; Richard, T.; Atmani, D. Antioxidant, cytoprotective, anti-inflammatory and anticancer activities of Pistacia lentiscus (Anacardiaceae) leaf and fruit extracts. Eur. J. Integr. Med. 2015, 7, 274–286. [Google Scholar] [CrossRef]
- Albalawi, A.E.; Althobaiti, N.A.; Alhasani, R.H.; Alnomasy, S.F. Anti-tumor effects and cellular mechanisms of Pistacia atlantica methanolic extract against Ehrlich solid tumor in mice. Asian Pac. J. Trop. Biomed. 2022, 12, 69–77. [Google Scholar] [CrossRef]
- Hosseini, S.; Nili-Ahmadabadi, A.; Nachvak, S.M.; Dastan, D.; Moradi, S.; Abdollahzad, H.; Mostafai, R. Antihyperlipidemic and antioxidative properties of Pistacia atlantica subsp. Kurdica in streptozotocin-induced diabetic mice. Diabetes Metab. Synd. Obes. 2020, 13, 1231–1236. [Google Scholar] [CrossRef] [PubMed]
- Moussaoui, S.; Mokrani, E.H.; Kabouche, Z.; Guendouze, A.; Laribi, A.; Bradai, N.; Bensouici, C.; Yilmaz, M.A.; Cakir, O.; Tarhan, A. Evaluation of polyphenolic profile, antioxidant, anti-cholinesterase, and anti-alpha-amylase activities of Pistacia lentiscus L. Leaves. Nat. Prod. Res. 2025, 39, 1–14. [Google Scholar] [CrossRef]
- Achili, I.; Amrani, A.; Bensouici, C.; Gül, F.; Altun, M.; Demirtas, I.; Zama, D.; Benayache, F.; Benayache, S. Chemical constituents, antioxidant, anticholinesterase and antiproliferative effects of Algerian Pistacia atlantica Desf. extracts. Recent Pat. Food Nutr. Agric. 2020, 11, 249–256. [Google Scholar] [CrossRef]
- Mehenni, C.; Atmani-Kilani, D.; Dumarçay, S.; Perrin, D.; Gérardin, P.; Atmani, D. Hepatoprotective and antidiabetic effects of Pistacia lentiscus leaf and fruit extracts. J. Food Drug Anal. 2016, 24, 653–669. [Google Scholar] [CrossRef]
- Hussein, S.A.A.; El-Mesallamy, A.; Othman, S.O.K.; Soliman, A.E.-M.M. Identification of novel polyphenolic secondary metabolites from Pistacia atlantica Desf. and demonstration of their cytotoxicity and CCL4 induced hepatotoxicity in rat. Egypt. J. Chem. 2020, 63, 117–130. [Google Scholar] [CrossRef]
- Elloumi, W.; Maalej, A.; Ortiz, S.; Michel, S.; Chamkha, M.; Boutefnouchet, S.; Sayadi, S. Pistacia lentiscus L. distilled leaves as a potential cosmeceutical ingredient: Phytochemical characterization, transdermal diffusion, and anti-elastase and anti-tyrosinase activities. Molecules 2022, 27, 855. [Google Scholar] [CrossRef] [PubMed]
- Tohidi, M.; Khayami, M.; Nejati, V.; Meftahizade, H. Evaluation of antibacterial activity and wound healing of Pistacia atlantica and Pistacia khinjuk. J. Med. Plants Res. 2011, 5, 4310–4314. [Google Scholar]
- Guerine, L.; Hadjadj, K. Ecodendrometric characterization of Atlas pistachio (Pistacia atlantica desf.) stands in the Ain Ben Khelil region (southwestern Algeria). Indian Four 2019, 145, 1053–1061. [Google Scholar]
- Hamelian, M.; Hemmati, S.; Varmira, K.; Veisi, H. Green synthesis, antibacterial, antioxidant and cytotoxic effect of gold nanoparticles using Pistacia atlantica extract. J. Taiwan Inst. Chem. Eng. 2018, 93, 21–30. [Google Scholar] [CrossRef]
- Belyagoubi-Benhammou, N.; Belyagoubi, L.; Benmahieddine, A.; El Zerey-Belaskri, A.; Di Marco, G.; D’Agostino, A.; Canini, A.; Gismondi, A. Nutraceutical content and biological properties of lipophilic and hydrophilic fractions of the phytocomplex from Pistacia atlantica Desf. buds, roots, and fruits. Plants 2024, 13, 611. [Google Scholar] [CrossRef]
- Ouahabi, S.; Loukili, E.H.; Daoudi, N.E.; Chebaibi, M.; Ramdani, M.; Rahhou, I.; Bnouham, M.; Fauconnier, M.-L.; Hammouti, B.; Rhazi, L. Study of the phytochemical composition, antioxidant properties, and in-vitro anti-diabetic efficacy of Gracilaria bursa-pastoris extracts. Mar. Drugs 2023, 21, 372. [Google Scholar] [CrossRef]
- Nea, F.; Bitchi, M.B.; Genva, M.; Ledoux, A.; Tchinda, A.T.; Damblon, C.; Frederich, M.; Tonzibo, Z.F.; Fauconnier, M.-L. Phytochemical investigation and biological activities of Lantana rhodesiensis. Molecules 2021, 26, 846. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Hbika, A.; Daoudi, N.E.; Bouyanzer, A.; Bouhrim, M.; Mohti, H.; Loukili, E.H.; Mechchate, H.; Al-Salahi, R.; Nasr, F.A.; Bnouham, M. Artemisia absinthium L. aqueous and ethyl acetate extracts: Antioxidant effect and potential activity in vitro and in-vivo against pancreatic α-amylase and intestinal α-glucosidase. Pharmaceutics 2022, 14, 481. [Google Scholar] [CrossRef]
- Nair, S.S.; Kavrekar, V.; Mishra, A. In-vitro studies on alpha amylase and alpha glucosidase inhibitory activities of selected plant extracts. Eur. J. Exp. Biol. 2013, 3, 128–132. [Google Scholar]
- Kim, J.-H.; Park, S.-M.; Ha, H.-J.; Moon, C.-J.; Shin, T.-K.; Kim, J.-M.; Lee, N.-H.; Kim, H.-C.; Jang, K.-J.; Wie, M.-B. Opuntia ficus-indica attenuates neuronal injury in in-vitro and in-vivo models of cerebral ischemia. J. Ethnopharmacol. 2006, 104, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Bourgou, S.; Bettaieb Rebey, I.; Ben Kaab, S.; Hammami, M.; Dakhlaoui, S.; Sawsen, S.; Msaada, K.; Isoda, H.; Ksouri, R.; Fauconnier, M.-L. Green solvent to substitute hexane for bioactive lipids extraction from black cumin and basil seeds. Foods 2021, 10, 1493. [Google Scholar] [CrossRef] [PubMed]
- Saha Tchinda, J.; Mbitnkeu Fetngna Tchebe, T.; Tchoukoua, A.; Cheumani Yona, A.M.; Fauconnier, M.L.; Ndikontar Kor, M.; Richel, A. Fatty acid profiles, antioxidant, and phenolic contents of oils extracted from Acacia polyacantha and Azadirachta indica (neem) seeds using green solvents. J. Food Process. Preserv. 2021, 45, e15115. [Google Scholar] [CrossRef]
- Wojdyło, A.; Jáuregui, P.N.N.; Carbonell-Barrachina, Á.A.; Oszmiański, J.; Golis, T. Variability of phytochemical properties and content of bioactive compounds in Lonicera caerulea L. var. Kamtschatica Berries. J. Agric. Food Chem. 2013, 61, 12072–12084. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Tanoh, A.E.; Blanchard Boué, G.; Nea, F.; Genva, M.; Wognin, E.L.; Ledoux, A.; Martin, H.; Tonzibo, Z.F.; Frederich, M.; Fauconnier, M.-L. Seasonal effect on the chemical composition, insecticidal properties and other biological activities of Zanthoxylum leprieurii Guill. & Perr. essential oils. Foods 2020, 9, 550. [Google Scholar] [CrossRef]
- Kar, B.; Kumar, R.B.S.; Karmakar, I.; Dola, N.; Bala, A.; Mazumder, U.K.; Hadar, P.K. Antioxidant and in-vitro anti-inflammatory activities of Mimusops elengi leaves. Asian Pac. J. Trop. Biomed. 2012, 2, S976–S980. [Google Scholar] [CrossRef]
- Nea, F.; Tanoh, E.A.; Wognin, E.L.; Kemene, T.K.; Genva, M.; Saive, M.; Tonzibo, Z.F.; Fauconnier, M.-L. A new chemotype of Lantana rhodesiensis Moldenke essential oil from Côte d’Ivoire: Chemical composition and biological activities. Ind. Crops Prod. 2019, 141, 111766. [Google Scholar] [CrossRef]
- Nikhila, G.S.; Sangeetha, G. Anti inflammatory properties of the root tubers of Gloriosa superba and its conservation through micropropagation. J. Med. Plants Res. 2015, 9, 1–7. [Google Scholar] [CrossRef]
- Rangkadilok, N.; Sitthimonchai, S.; Worasuttayangkurn, L.; Mahidol, C.; Ruchirawat, M.; Satayavivad, J. Evaluation of free radical scavenging and antityrosinase activities of standardized longan fruit extract. Food Chem. Toxicol. 2007, 45, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Genva, M.; Lheureux, L.; Saive, M.; Maes, C.; Fauconnier, M.-L. Study of the cosmetic potential uses of plants from Mayotte as skin care agents through the screening of their biological activities. Nutraceuticals 2022, 2, 420–440. [Google Scholar] [CrossRef]
- Belyagoubi, L.; Belyagoubi-Benhammou, N.; Atik-Bekkara, F.; Coustard, J.M. Effects of extraction solvents on phenolic content and antioxidant properties of Pistacia atlantica Desf fruits from Algeria. Int. Food Res. J. 2016, 23, 948. [Google Scholar]
- Chaabani, E.; Abert Vian, M.; Bott, R.; Ginies, C.; Defoort, C.; Ksouri, R.; Chemat, F. Extraction of aromas from Pistacia lentiscus L. leaves using alternative solvents: COSMO-RS-Assisted solvent screening and GC-MS metabolites profiling. Sep. Sci. Technol. 2020, 55, 716–727. [Google Scholar] [CrossRef]
- Amel, Z.; Nabila, B.-B.; Nacéra, G.; Fethi, T.; Fawzia, A.-B. Assessment of phytochemical composition and antioxidant properties of extracts from the leaf, stem, fruit and root of Pistacia lentiscus L. Int. J. Pharmacogn. Phytochem. Res. 2016, 8, 627–633. [Google Scholar]
- Seddoqi, S.; Aouinti, F.; Fatnassi, H.; Nouioura, G.; Conte, R.; Abdullah Alsahli, A.; Bourhia, M.; Mahamat, O.B.; Kandsi, F.; Gseyra, N. phytochemical composition analysis, antioxidant, antimitotic, and anti-inflammatory effects of leaf and stem extracts of Pistacia lentiscus L. Int. J. Food Prop. 2024, 27, 1415–1433. [Google Scholar] [CrossRef]
- Peksel, A.; Arisan-Atac, I.; Yanardag, R. Evaluation of antioxidant and antiacetylcholinesterase activities of the extracts of Pistacia atlantica Desf. Leaves. J. Food Biochem. 2010, 34, 451–476. [Google Scholar] [CrossRef]
- Terouzi, W.; Yacine, Z.A.; Hanine, H.; Boulli, A.; Oussama, A. Comparative study of physical and chemical propriety of the oil of some varieties of olive trees. Int. J. Innov. Appl. Stud. 2014, 6, 1096. [Google Scholar]
- Brahmi, F.; Haddad, S.; Bouamara, K.; Yalaoui-Guellal, D.; Prost-Camus, E.; De Barros, J.-P.P.; Prost, M.; Atanasov, A.G.; Madani, K.; Boulekbache-Makhlouf, L. Comparison of chemical composition and biological activities of algerian seed oils of Pistacia lentiscus L., Opuntia ficus indica (L.) Mill. and Argania spinosa L. skeels. Ind. Crops Prod. 2020, 151, 112456. [Google Scholar] [CrossRef]
- Šamec, D.; Karalija, E.; Šola, I.; Vujčić Bok, V.; Salopek-Sondi, B. The role of polyphenols in abiotic stress response: The influence of molecular structure. Plants 2021, 10, 118. [Google Scholar] [CrossRef]
- Kähkönen, M.P.; Hopia, A.I.; Vuorela, H.; Rauha, J.P.; Pihlaja, K.; Kujala, T.S.; Heinonen, M. Antioxidant activity of plant extracts containing phenolic compounds. J. Agric. Food Chem. 1999, 47, 3954–3962. [Google Scholar] [CrossRef]
- Naczk, M.; Shahidi, F. Extraction and analysis of phenolics in food. J. Chromatogr. A 2004, 1054, 95–111. [Google Scholar] [CrossRef]
- Boschin, G.; D’Agostina, A.; Annicchiarico, P.; Arnoldi, A. The fatty acid composition of the oil from Lupinus albus cv. luxe as affected by environmental and agricultural factors. Eur. Food Res. Technol. 2007, 225, 769–776. [Google Scholar] [CrossRef]
- Akremi, I.; Kabtni, S.; Ammar, H.B.; Genva, M.; Hejazi, S.; Elbok, S.; Rouz, S.; Marghali, S.; Fauconnier, M.-L. Comparative highlights of morphological, phytochemical and nutritional key characteristics of mediterranean Lupinus species. Food Chem. 2025, 480, 143962. [Google Scholar] [CrossRef]
- Nejib, M. GC/MS chemical analysis of Pistacia lentiscus fatty oil from the north of Tunisia. Int. J. Pharm. Tech Res. 2011, 3, 2245–2248. [Google Scholar]
- Trabelsi, H.; Cherif, O.A.; Sakouhi, F.; Villeneuve, P.; Renaud, J.; Barouh, N.; Boukhchina, S.; Mayer, P. Total lipid content, fatty acids and 4-desmethylsterols accumulation in developing fruit of Pistacia lentiscus L. growing wild in Tunisia. Food Chem. 2012, 131, 434–440. [Google Scholar] [CrossRef]
- Mezni, F.; Khaldi, A.; Maaroufi, A.; Hamrouni, L.; Msallem, M.; Boussaid, M.; Khouja, M.L. Fatty acid composition and biological properties of the fixed oil fruits of Pistacia lentiscus L. Acta Hortic. 2013, 997, 219–224. [Google Scholar] [CrossRef]
- Haouli, A.; Seridi, R.; Djemli, S.; Bourdjiba, O.; Frih, H. Contribution to the analysis of Pistacia lentiscus extracted oil. Am.-Eur. J. Agric. Environ. Sci. 2015, 15, 1075–1081. [Google Scholar]
- Boke Sarikahya, N.; Sumer Okkali, G.; Coven, F.O.; Isen, F.; Goren, A.C.; Nalbantsoy, A. Chemical characteristics and biological activity screening of Pistacia lentiscus mastic gum and leaves from Türkiye. J. Sci. Food Agric. 2024, 104, 1691–1701. [Google Scholar] [CrossRef]
- Kohl, E.; Steinbauer, J.; Landthaler, M.; Szeimies, R. Skin Ageing. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 873–884. [Google Scholar] [CrossRef]
- Ashawat, M.; Banchhor, M.; Saraf, S.; Saraf, S. Herbal cosmetics:” Trends in skin care formulation”. Pharmacogn. Rev. 2009, 3, 82. [Google Scholar]
- Ahmed, I.A.; Mikail, M.A. Anti-aging skincare: The natural and organic way. In Anti-Aging. Pharmacology; Academic Press: New York, NY, USA, 2023; pp. 269–284. [Google Scholar] [CrossRef]
- Soimee, W.; Nakyai, W.; Charoensit, P.; Grandmottet, F.; Worasakwutiphong, S.; Phimnuan, P.; Viyoch, J. Evaluation of moisturizing and irritation potential of Sacha inchi oil. J. Cosmet. Dermatol. 2020, 19, 915–924. [Google Scholar] [CrossRef]
- Lim, D.J.; Song, J.-S.; Lee, B.-H.; Son, Y.K.; Kim, Y. Qualitative and quantitative analysis of the major bioactive components of Juniperus chinensis L. using LC-QTOF-MS and LC-MSMS and investigation of antibacterial activity against pathogenic bacteria. Molecules 2023, 28, 3937. [Google Scholar] [CrossRef]
- Pearson, J.L.; Lee, S.; Suresh, H.; Low, M.; Nang, M.; Singh, S.; Lamin, F.; Kazzem, M.; Sullivan, S.; Khoo, C.S. The liquid chromatographic determination of chlorogenic and caffeic acids in Xu duan (Dipsacus asperoides) raw herb. Int. Sch. Res. Not. 2014, 2014, 968314. [Google Scholar] [CrossRef]
- Zhang, C.; Ma, Y.; Zhao, Y.; Hong, Y.; Cai, S.; Pang, M. Phenolic composition, antioxidant and pancreatic lipase inhibitory activities of Chinese sumac (Rhus chinensis Mill.) fruits extracted by different solvents and interaction between myricetin-3-o-rhamnoside and quercetin-3-o-rhamnoside. Int. J. Food Sci. Technol. 2018, 53, 1045–1053. [Google Scholar] [CrossRef]
- Shahzad, M.N.; Ahmad, S.; Tousif, M.I.; Ahmad, I.; Rao, H.; Ahmad, B.; Basit, A. Profiling of phytochemicals from aerial parts of Terminalia neotaliala using LC-ESI-MS2 and determination of antioxidant and enzyme inhibition activities. PLoS ONE 2022, 17, e0266094. [Google Scholar] [CrossRef]
- Wojdyło, A.; Nowicka, P.; Grimalt, M.; Legua, P.; Almansa, M.S.; Amorós, A.; Carbonell-Barrachina, Á.A.; Hernández, F. Polyphenol compounds and biological activity of Caper (Capparis spinosa L.) flowers buds. Plants 2019, 8, 539. [Google Scholar] [CrossRef]
- Aissat, A.K.; Chaher-Bazizi, N.; Richard, T.; Kilani-Atmani, D.; Pedrot, E.; Renouf, E.; Atmani, D.; Fonayet, J.V. Analysis of individual anthocyanins, flavanols, flavonols and other polyphenols in Pistacia lentiscus L. fruits during ripening. J. Food Compos. Anal. 2022, 106, 104286. [Google Scholar] [CrossRef]
- Rodríguez-Pérez, C.; Quirantes-Piné, R.; Amessis-Ouchemoukh, N.; Madani, K.; Segura-Carretero, A.; Fernández-Gutierrez, A. A metabolite-profiling approach allows the identification of new compounds from Pistacia lentiscus leaves. J. Pharm. Biomed. Anal. 2013, 77, 167–174. [Google Scholar] [CrossRef]
- Boucheffa, S.; Sobhi, W.; Attoui, A.; Selli, S.; Kelebek, H.; Semmeq, A.; Benguerba, Y. Effect of the main constituents of Pistacia lentiscus leaves against the DPPH radical and xanthine oxidase: Experimental and theoretical study. J. Biomol. Struct. Dyn. 2022, 40, 9870–9884. [Google Scholar] [CrossRef]
- Mashitoa, F.M.; Shoko, T.; Shai, J.L.; Slabbert, R.M.; Sivakumar, D. Changes in phenolic metabolites and biological activities of Pumpkin Leaves (Cucurbita moschata Duchesne ex Poir.) during Blanching. Front. Nutr. 2021, 8, 641939. [Google Scholar] [CrossRef]
- Abdl Aziz, F.T.; Temraz, A.S.; Hassan, M.A. Metabolites profiling by LC-ESI-MS/MS technique and in-vitro antioxidant activity of Bauhinia madagascariensis Desv. and Bauhinia purpurea L. aerial parts cultivated in Egypt: A comparative study. Azhar Int. J. Pharm. Med. Sci. 2024, 4, 169–188. [Google Scholar] [CrossRef]
- Li, A.; Hou, X.; Wei, Y. Fast screening of flavonoids from switchgrass and Mikania micrantha by liquid chromatography hybrid-ion trap time-of-flight mass spectrometry. Anal. Methods 2018, 10, 109–122. [Google Scholar] [CrossRef]
- An, H.; Wang, H.; Lan, Y.; Hashi, Y.; Chen, S. Simultaneous qualitative and quantitative analysis of phenolic acids and flavonoids for the quality control of Apocynum venetum L. leaves by HPLC–DAD–ESI–IT–TOF–MS and HPLC–DAD. J. Pharm. Biomed. Anal. 2013, 85, 295–304. [Google Scholar] [CrossRef]
- Lee, J.-E.; Jayakody, J.; Kim, J.-I.; Jeong, J.-W.; Choi, K.-M.; Kim, T.-S.; Seo, C.; Azimi, I.; Hyun, J.; Ryu, B. The influence of solvent choice on the extraction of bioactive compounds from Asteraceae: A comparative review. Foods 2024, 13, 3151. [Google Scholar] [CrossRef]
- Pekacar, S.; Deliorman Orhan, D. Investigation of antidiabetic effect of Pistacia atlantica leaves by activity-guided fractionation and phytochemical content analysis by LC-QTOF-MS. Front. Pharmacol. 2022, 13, 826261. [Google Scholar] [CrossRef]
- Huang, W.-Y.; Cai, Y.-Z.; Zhang, Y. Natural phenolic compounds from medicinal herbs and dietary plants: Potential use for cancer prevention. Nutr. Cancer 2009, 62, 1–20. [Google Scholar] [CrossRef]
- Achakzai, A.K.K.; Achakzai, P.; Masood, A.; Kayani, S.A.; Tareen, R.B. Response of plant parts and age on the distribution of secondary metabolites on plants found in Quetta. Pak. J. Bot. 2009, 41, 2129–2135. [Google Scholar]
- Naghiloo, S.; Movafeghi, A.; Delazar, A.; Nazemiyeh, H.; Asnaashari, S.; Dadpour, M.R. Ontogenetic variation of total phenolics and antioxidant activity in roots, leaves and flowers of Astragalus compactus Lam. (Fabaceae). Bioimpacts 2012, 2, 105. [Google Scholar] [CrossRef]
- Harborne, J.B.; Williams, C.A. Advances in flavonoid research since 1992. Phytochemistry 2000, 55, 481–504. [Google Scholar] [CrossRef]
- Havsteen, B.H. The biochemistry and medical significance of the flavonoids. Pharmacol. Ther. 2002, 96, 67–202. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Lee, J.; Yoon, S.H.; Park, D.; Hwang, J.S.; Jung, E. Anti-glycation activities of methyl gallate in-vitro and in human explants. J. Cosmet. Dermatol. 2022, 21, 2602–2609. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.W.; Jeong, H.S.; Kim, J.K.; Lee, J.K.; Kim, H.R.; Yun, H.Y.; Baek, K.J.; Kwon, N.S.; Park, K.C.; Kim, D.S. Methyl gallate from Acer barbinerve decreases melanin synthesis in Mel-Ab cells. Pharmazie 2015, 70, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Saeed, F.; Hussain, G.; Imran, A.; Mehmood, Z.; Gondal, T.A.; El-Ghorab, A.; Ahmad, I.; Pezzani, R.; Arshad, M.U. Myricetin: A comprehensive review on its biological potentials. Food Sci. Nutr. 2021, 9, 5854–5868. [Google Scholar] [CrossRef]
- Irshad, M.; Zafaryab, M.D.; Singh, M.; Rizvi, M.M.A. Comparative analysis of the antioxidant activity of Cassia fistula extracts. Int. J. Med. Chem. 2012, 2012, 157125. [Google Scholar] [CrossRef]
- Rice-Evans, C.; Miller, N.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Leopoldini, M.; Marino, T.; Russo, N.; Toscano, M. Antioxidant properties of phenolic compounds: H-atom versus electron transfer mechanism. J. Phys. Chem. A 2004, 108, 4916–4922. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Ekaprasada, M.T.; Nurdin, H.; Ibrahim, S.; Dachriyanus, D. Antioxidant activity of methyl gallate isolated from the leaves of Toona sureni. Indones. J. Chem. 2009, 9, 457–460. [Google Scholar] [CrossRef]
- Liang, H.; Huang, Q.; Zou, L.; Wei, P.; Lu, J.; Zhang, Y. Methyl gallate: Review of pharmacological activity. Pharmacol. Res. 2023, 194, 106849. [Google Scholar] [CrossRef]
- Milenković, D.; Đorović, J.; Petrović, V.; Avdović, E.; Marković, Z. Hydrogen Atom Transfer versus Proton Coupled Electron Transfer Mechanism of Gallic Acid with Different Peroxy Radicals. React. Kinet. Mech. Catal. 2018, 123, 215–230. [Google Scholar] [CrossRef]
- Shojaee, M.S.; Moeenfard, M.; Farhoosh, R. Kinetics and stoichiometry of gallic acid and methyl gallate in scavenging dpph radical as affected by the reaction solvent. Sci. Rep. 2022, 12, 8765. [Google Scholar] [CrossRef]
- Li, X.; Mai, W.; Chen, D. Chemical study on protective effect against hydroxyl-induced DNA damage and antioxidant mechanism of myricitrin. J. Chin. Chem. Soc. 2014, 61, 383–390. [Google Scholar] [CrossRef]
- Amessis-Ouchemoukh, N.; Madani, K.; Falé, P.L.V.; Serralheiro, M.L.; Araújo, M.E.M. Antioxidant capacity and phenolic contents of some mediterranean medicinal plants and their potential role in the inhibition of cyclooxygenase-1 and acetylcholinesterase activities. Ind. Crops Prod. 2014, 53, 6–15. [Google Scholar] [CrossRef]
- Bourroubey, B.; Chelli, N.; Touil, A.T.; Meddah, B. Ethnobotanical survey, phytochemical screening and antioxidant activity of methanolic extracts of Pistacia lentiscus L. growing in northwestern Algeria. Fr.-Ukr. J. Chem. 2023, 11, 1–16. [Google Scholar] [CrossRef]
- Hemma, R.; Belhadj, S.; Ouahchia, C.; Saidi, F. Antioxidant activity of Pistacia lentiscus methanolic extracts. Agrobiologia 2018, 8, 845–852. [Google Scholar]
- Zerkani, H.; Tagnaout, I.; Khiya, Z.; Boutahiri, S.; Amalich, S.; Fadili, K.; Cherrat, A.; Mouradi, A.; Benhlima, N.; Zair, T. Comparative study of the antioxidant power of polyphenols of leaves, fruits, and bark of Pistacia atlantica Desf. from Morocco. J. Chem. 2022, 2022, 7432169. [Google Scholar] [CrossRef]
- Benabdallah, F.Z.; Zellagui, A. HPLC/UV analysis and in vitro, promising antioxidant, antidiabetic, anti-alzheimer, and anti-tyrosinase potentials of the Algerian Pistacia atlantica Desf. methanolic extract. Phytothérapie 2023, 21, 214–224. [Google Scholar] [CrossRef]
- Rawat, P.; Dasila, K.; Singh, M.; Kuniyal, J.C. Influence of environmental factors on phytochemical compositions and antioxidant activity of Juniperus communis L. Discov. Environ. 2025, 3, 1–17. [Google Scholar] [CrossRef]
- Gardeli, A.; Papageorgiou, V.; Mallouchos, A.; Kibouris, T.; Komaitis, M. Essential Oil Composition of Pistacia lentiscus L. and Myrtus communis L.: Evaluation of antioxidant capacity of methanolic extracts. Food Chem. 2008, 107, 1120–1130. [Google Scholar] [CrossRef]
- Salhi, A.; Bellaouchi, R.; El Barkany, S.; Rokni, Y.; Bouyanzer, A.; Asehraou, A.; Amhamdi, H.; Zarrouk, A.; Hammouti, B. Total phenolic content, antioxidant and antimicrobial activities of extracts from Pistacia lentiscus leaves. Caspian J. Environ. Sci. 2019, 17, 189–198. [Google Scholar] [CrossRef]
- Ouedraogo, R.A.; Koala, M.; Dabire, C.; Hema, A.; Bazie, V.; Outtara, L.P.; Gnoula, C.; Pale, E.; Nebie, R.H.C. Teneur en phénols totaux et activité antioxydante des extraits des trois principales variétés d’oignons (Allium cepa L.) cultivées dans la région du centre-nord du Burkina Faso. Int. J. Biol. Chem. Sci. 2015, 9, 281–291. [Google Scholar] [CrossRef]
- Bozorgi, M.; Memariani, Z.; Mobli, M.; Salehi Surmaghi, M.H.; Shams-Ardekani, M.R.; Rahimi, R. Five Pistacia species (P. vera, P. atlantica, P. terebinthus, P. khinjuk and P. lentiscus): A review of their traditional uses, phytochemistry, and pharmacology. Sci. World J. 2013, 2013, 219815. [Google Scholar] [CrossRef]
- Bourroubey, B.; Chelli, N.; Touil, A.T.; Meddah, B.; Bettouati, A.; Berkane, I. Evaluation of the in-vitro toxicity and anti-inflammatory activity of the methanolic extract of the leaves of Pistacia lentiscus L. harvested from northwestern Algeria. Acta. Biol. Slov. 2024, 67, 50–60. [Google Scholar] [CrossRef]
- Bakka, C.; Smara, O.; Hadjadj, M.; Dendougui, H.; Mahdjar, S.; Benzid, A. In vitro anti-inflammatory activity of Pistacia atlantica Desf. extracts. Asian J. Res. Chem. 2019, 12, 322. [Google Scholar] [CrossRef]
- Labhar, A.; Benamari, O.; El-Mernissi, Y.; Salhi, A.; Ahari, M.; El Barkany, S.; Amhamdi, H. Phytochemical, anti-inflammatory and antioxidant activities of Pistacia lentiscus L. leaves from Ajdir, Al Hoceima province, Morocco. Ecol. Eng. Environ. Technol. 2023, 24, 172–177. [Google Scholar] [CrossRef]
- Zam, W.; Ali, A.; Hasan, R. Determination of phenolic compounds’ extraction conditions from Pistacia palaestina leaves at two different stages of maturity. Curr. Nutr. Food Sci. 2020, 16, 808–814. [Google Scholar] [CrossRef]
- Tebbi, S.O.; Trapali, M.; Letsiou, S. Exploring the anti-diabetic, antioxidant and anti-microbial properties of Clematis flammula L. leaves and Pistacia lentiscus L. fruits using choline chloride-based deep eutectic solvent. Waste Biomass Valoriz. 2024, 15, 2869–2879. [Google Scholar] [CrossRef]
- Michalak, M.; Zagórska-Dziok, M.; Klimek-Szczykutowicz, M.; Szopa, A. Phenolic profile and comparison of the antioxidant, anti-ageing, anti-inflammatory, and protective activities of Borago officinalis extracts on skin cells. Molecules 2023, 28, 868. [Google Scholar] [CrossRef]
- Bakhouche, I.; Aliat, T.; Boubellouta, T.; Gali, L.; Şen, A.; Bellik, Y. Phenolic contents and in vitro antioxidant, anti-tyrosinase, and anti-inflammatory effects of leaves and roots extracts of the halophyte Limonium delicatulum. S. Afr. J. Bot. 2021, 139, 42–49. [Google Scholar] [CrossRef]
- Kim, S.J.; Jin, M.; Lee, E.; Moon, T.C.; Quan, Z.; Yang, J.H.; Son, K.H.; Kim, K.-U.; Son, J.K.; Chang, H.W. Effects of methyl gallate on arachidonic acid metabolizing enzymes: Cyclooxygenase-2 and 5-lipoxygenase in mouse bone marrow-derived mast cells. Arch. Pharm. Res. 2006, 29, 874–878. [Google Scholar] [CrossRef]
- Kutil, Z.; Temml, V.; Maghradze, D.; Pribylova, M.; Dvorakova, M.; Schuster, D.; Vanek, T.; Landa, P. Impact of wines and wine constituents on cyclooxygenase-1, cyclooxygenase-2, and 5-lipoxygenase catalytic activity. Mediat. Inflamm. 2014, 2014, 406–413. [Google Scholar] [CrossRef]
- Sadik, C.D.; Sies, H.; Schewe, T. Inhibition of 15-Lipoxygenases by flavonoids: Structure–activity relations and mode of action. Biochem. Pharmacol. 2003, 65, 773–781. [Google Scholar] [CrossRef]
- Hu, C.; Ma, S. Recent development of lipoxygenase inhibitors as anti-inflammatory agents. Med. Chem. Commun. 2018, 9, 212–225. [Google Scholar] [CrossRef] [PubMed]
- Ijaz, S.; Shoaib Khan, H.M.; Anwar, Z.; Talbot, B.; Walsh, J.J. HPLC profiling of Mimosa pudica polyphenols and their non-invasive biophysical investigations for anti-dermatoheliotic and skin reinstating potential. Biomed. Pharmacother. 2019, 109, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Ghafari, S.; Sahranavard, S.; Sarkhail, P.; Keramatian, B. Tyrosinase inhibitory activity of selected plants based on iranian traditional medicine: Detection of mushroom tyrosinase inhibitors based on ITM. Int. Pharm. Acta 2022, 5, e4. [Google Scholar] [CrossRef]
- Eghbali-Feriz, S.; Taleghani, A.; Al-Najjar, H.; Emami, S.A.; Rahimi, H.; Asili, J.; Hasanzadeh, S.; Tayarani-Najaran, Z. Anti-melanogenesis and anti-tyrosinase properties of Pistacia atlantica subsp. mutica extracts on B16F10 murine melanoma cells. Res. Pharm. Sci. 2018, 13, 533–545. [Google Scholar] [CrossRef]
- Espín, J.C.; Wichers, H.J. Effect of captopril on mushroom tyrosinase activity in vitro. Biochim. Biophys. Acta BBA 2001, 1544, 289–300. [Google Scholar] [CrossRef]
- Lee, O.-S.; Kim, E.-J. Skin Lightening: A review of melamin formation and the isolation of a new ingredient for products that minimize skin discolorations due to excessive melamin production. Cosmet. Toilet. 1995, 110, 51–56. [Google Scholar]
- Slominski, A.; Zbytek, B.; Slominski, R. Inhibitors of melanogenesis increase toxicity of cyclophosphamide and lymphocytes against melanoma cells. Int. J. Cancer 2009, 124, 1470–1477. [Google Scholar] [CrossRef]
- Chuang, S.-Y.; Lin, Y.-K.; Lin, C.-F.; Wang, P.-W.; Chen, E.-L.; Fang, J.-Y. Elucidating the skin delivery of aglycone and glycoside flavonoids: How the structures affect cutaneous absorption. Nutrients 2017, 9, 1304. [Google Scholar] [CrossRef] [PubMed]
- Kubo, I.; Kinst-Hori, I.; Kubo, Y.; Yamagiwa, Y.; Kamikawa, T.; Haraguchi, H. Molecular design of antibrowning agents. J. Agric. Food Chem. 2000, 48, 1393–1399. [Google Scholar] [CrossRef]
- Meda, A.; Lamien, C.E.; Romito, M.; Millogo, J.; Nacoulma, O.G. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005, 91, 571–577. [Google Scholar] [CrossRef]
- Kishore, N.; Twilley, D.; Blom van Staden, A.; Verma, P.; Singh, B.; Cardinali, G.; Kovacs, D.; Picardo, M.; Kumar, V.; Lall, N. Isolation of flavonoids and flavonoid glycosides from Myrsine africana and their inhibitory activities against mushroom tyrosinase. J. Nat. Prod. 2018, 81, 49–56. [Google Scholar] [CrossRef]
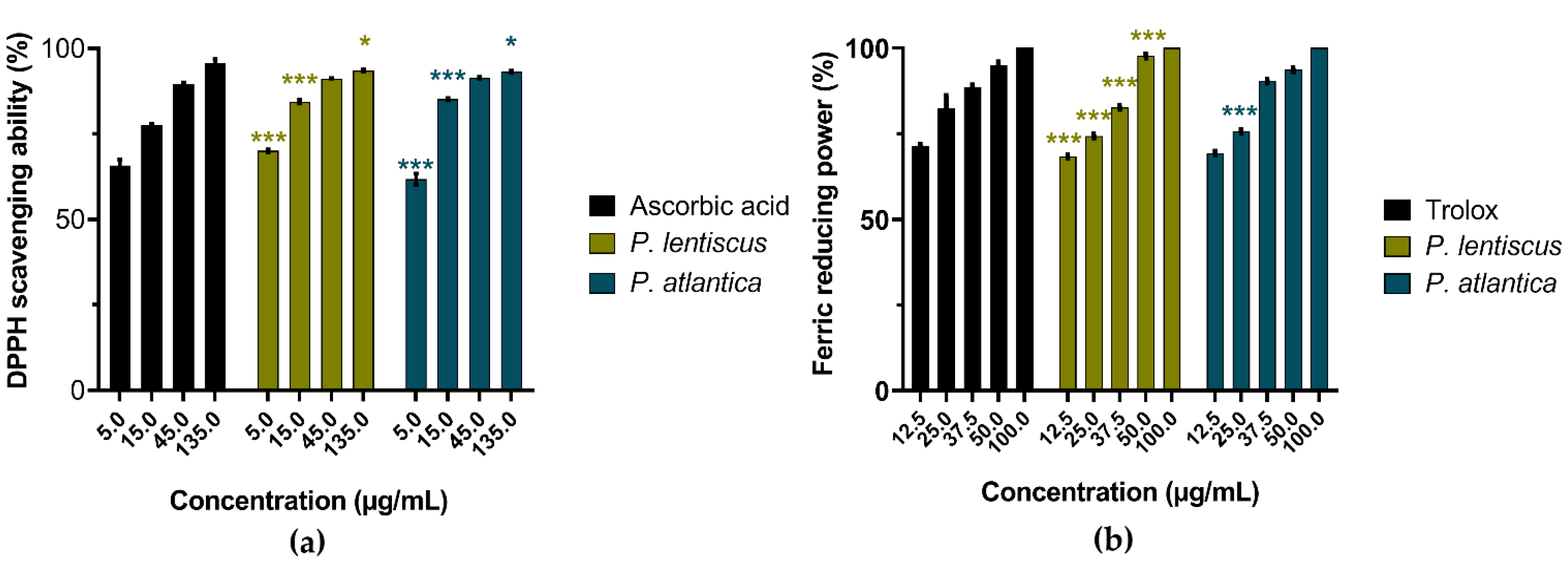
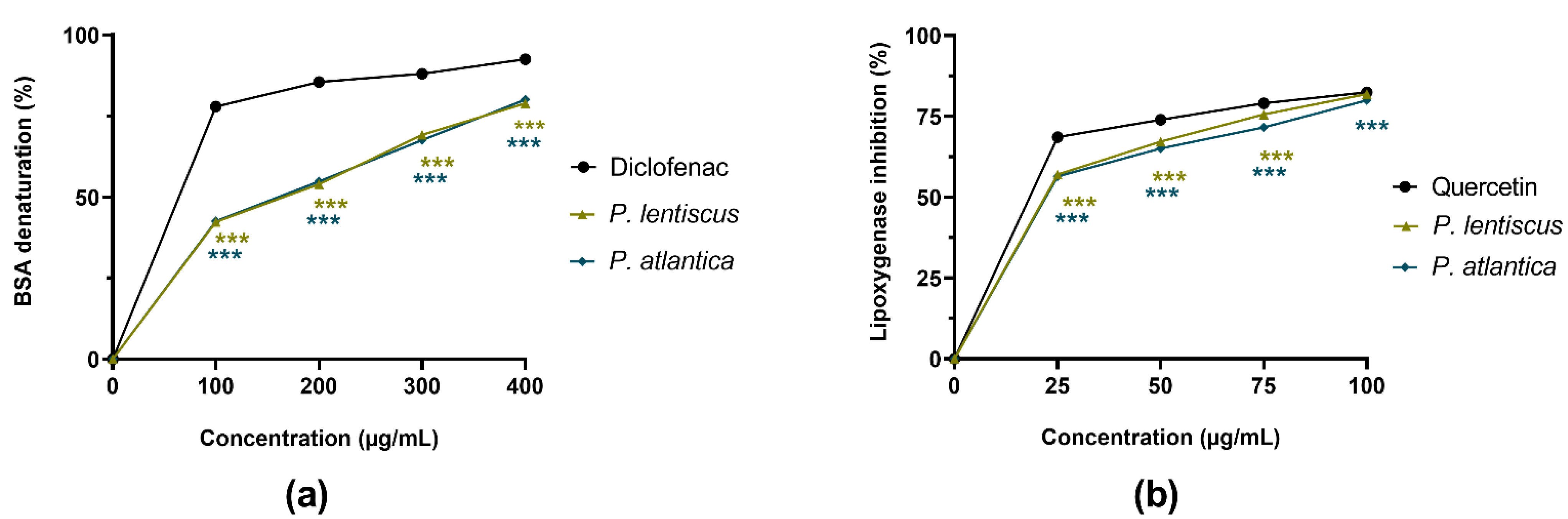
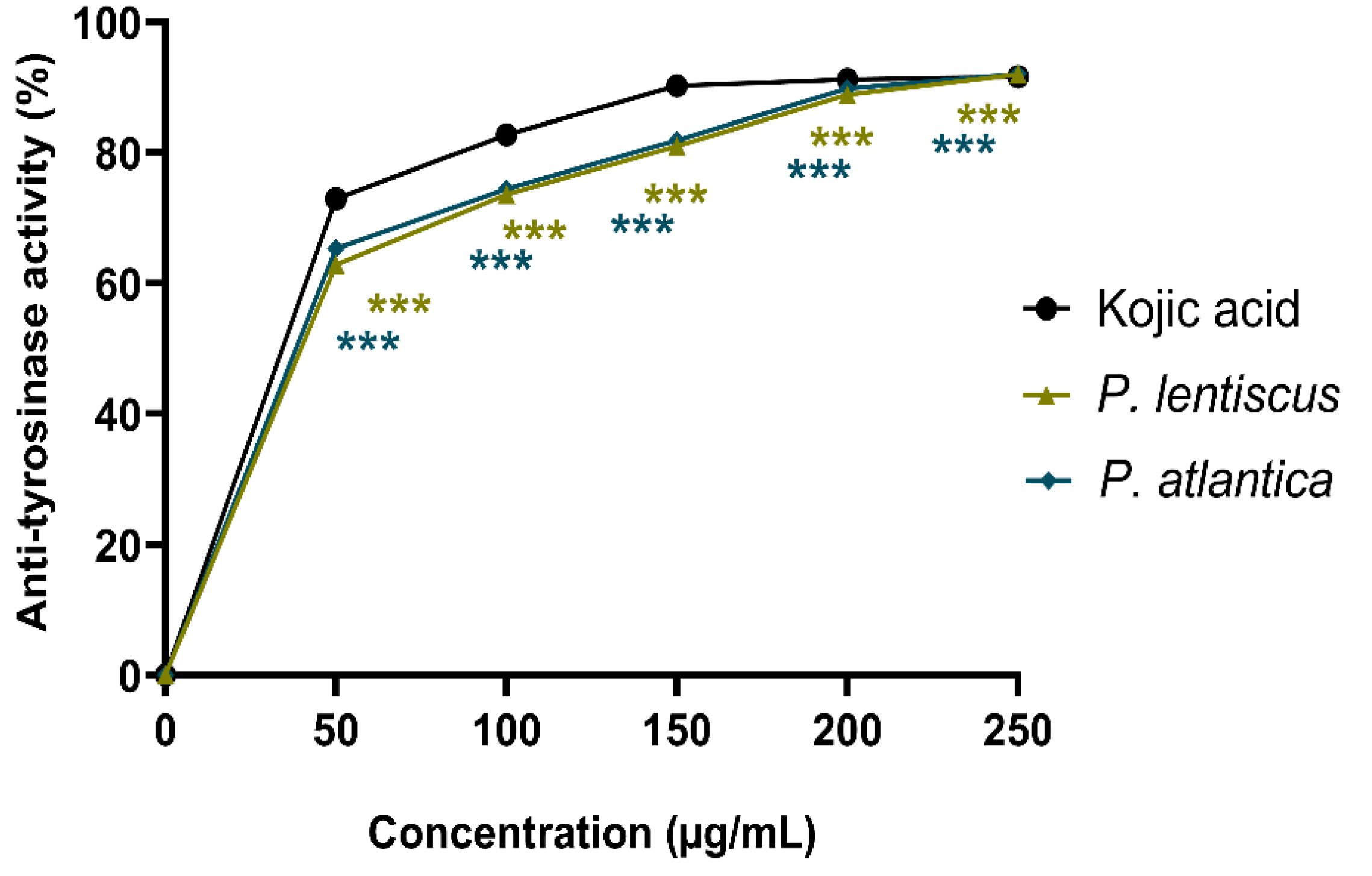
| Samples and Standards | Biological Activities IC50 (µg/mL) [a] | |||
|---|---|---|---|---|
| DPPH | BSA Denaturation | LOX Denaturation | Anti-Tyrosinase | |
| P. lentiscus | 5.19 ± 0.01 *** | 143.00 ± 0.70 *** | 22.53 ± 0.05 *** | 39.80 ± 0.08 *** |
| P. atlantica | 5.61 ± 0.04 *### | 139.10 ± 0.55 ***## | 22.67 ± 0.04 ***ns | 38.25 ± 0.02 ***### |
| Ascorbic acid | 5.84 ± 0.05 | - | - | - |
| Diclofenac | - | 60.88 ± 0.03 | - | - |
| Quercetin | - | - | 17.91 ± 0.03 | |
| Kojic acid | - | - | - | 34.28 ± 0.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belkessam, M.; Genva, M.; Kouki, A.; Vilain, L.; Ahmed-Alem, M.; Mohaddab, M.; Taguimjeu, P.L.K.T.; El-Bok, S.; Ben-Attia, M.; Fauconnier, M.-L. Phytochemical Profile and Cosmeceutical Potential of Leaf Extracts of Two Species of the Anacardiaceae Family from the Mediterranean Scrubland: Pistacia lentiscus L. and Pistacia atlantica Desf. Processes 2025, 13, 3712. https://doi.org/10.3390/pr13113712
Belkessam M, Genva M, Kouki A, Vilain L, Ahmed-Alem M, Mohaddab M, Taguimjeu PLKT, El-Bok S, Ben-Attia M, Fauconnier M-L. Phytochemical Profile and Cosmeceutical Potential of Leaf Extracts of Two Species of the Anacardiaceae Family from the Mediterranean Scrubland: Pistacia lentiscus L. and Pistacia atlantica Desf. Processes. 2025; 13(11):3712. https://doi.org/10.3390/pr13113712
Chicago/Turabian StyleBelkessam, Mouna, Manon Genva, Ahmed Kouki, Louise Vilain, Moussa Ahmed-Alem, Marouane Mohaddab, Pierre Leonel K. Tafokeu Taguimjeu, Safia El-Bok, Mossadok Ben-Attia, and Marie-Laure Fauconnier. 2025. "Phytochemical Profile and Cosmeceutical Potential of Leaf Extracts of Two Species of the Anacardiaceae Family from the Mediterranean Scrubland: Pistacia lentiscus L. and Pistacia atlantica Desf." Processes 13, no. 11: 3712. https://doi.org/10.3390/pr13113712
APA StyleBelkessam, M., Genva, M., Kouki, A., Vilain, L., Ahmed-Alem, M., Mohaddab, M., Taguimjeu, P. L. K. T., El-Bok, S., Ben-Attia, M., & Fauconnier, M.-L. (2025). Phytochemical Profile and Cosmeceutical Potential of Leaf Extracts of Two Species of the Anacardiaceae Family from the Mediterranean Scrubland: Pistacia lentiscus L. and Pistacia atlantica Desf. Processes, 13(11), 3712. https://doi.org/10.3390/pr13113712










