Development of a Polyurethane Lost Circulation Material Suitable for Malignant Leakage of Drilling Fluid
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Polyurethane Leak Sealing Agent
2.3. Performance Test and Mechanism Analysis
3. Results and Discussion
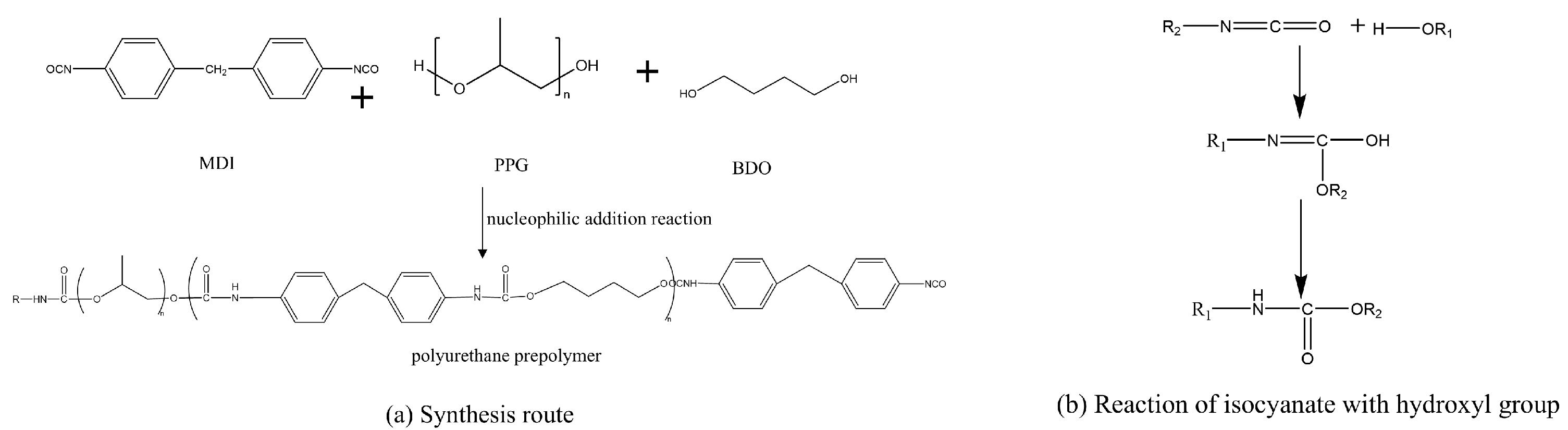
3.1. Synthesis of the Plugging Agent
3.2. Effect of Catalysts on Polyurethane Sealants
3.3. Effect of Water on Polyurethane Leak Sealing Agents
3.3.1. Effect of Water Content on Setting Time and Setting Strength
3.3.2. Effect of Saline Water on Leak-Sealing Agents
3.4. Evaluation of Pressure-Bearing Performance of Polyurethane Sealant
3.4.1. Pressure-Bearing Performance of Polyurethane Leak Sealing Agent
3.4.2. Salinity Resistance of Polyurethane Sealant
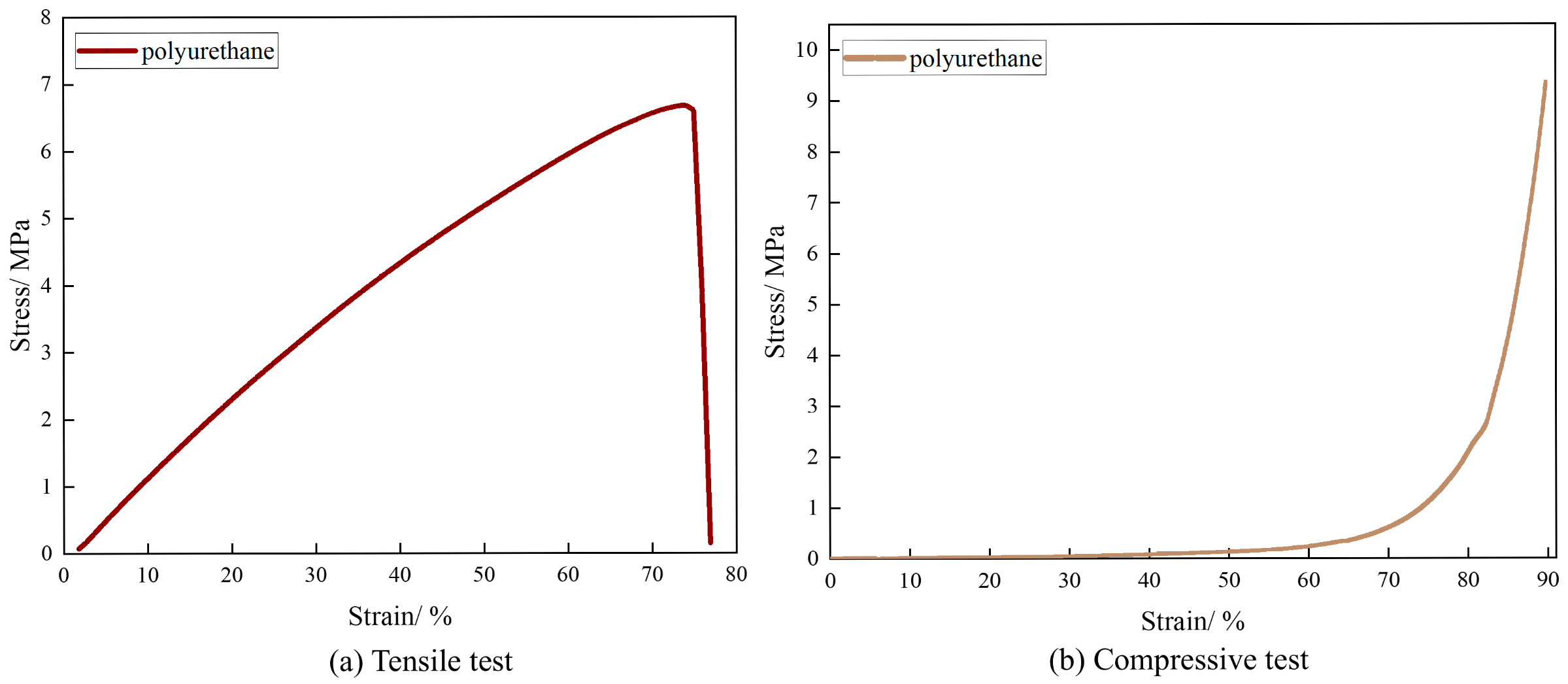
3.5. Evaluation of Mechanical Properties of Polyurethane Leak-Sealing Agent
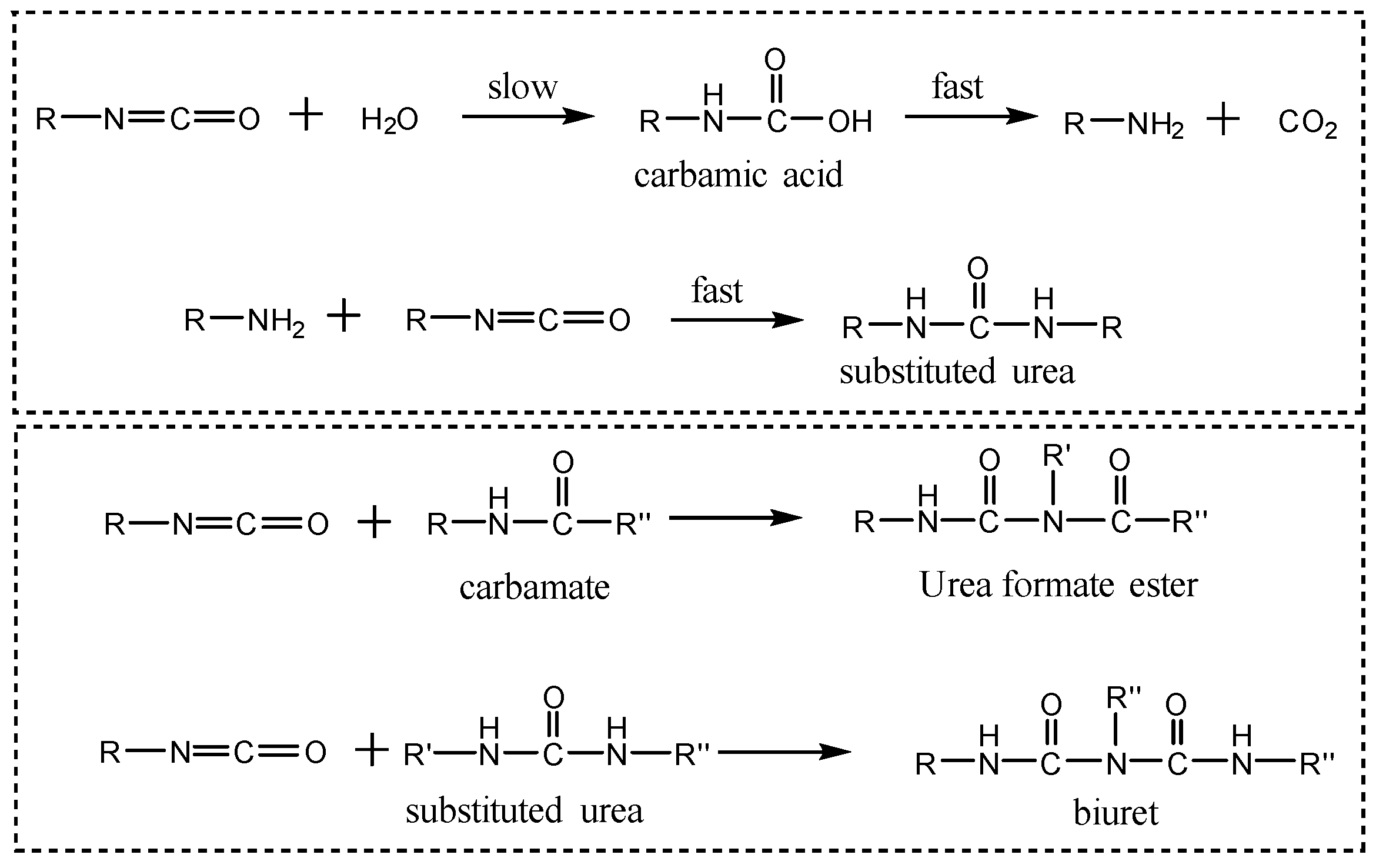
3.5.1. Leak-Sealing Mechanism of Polyurethane Sealant
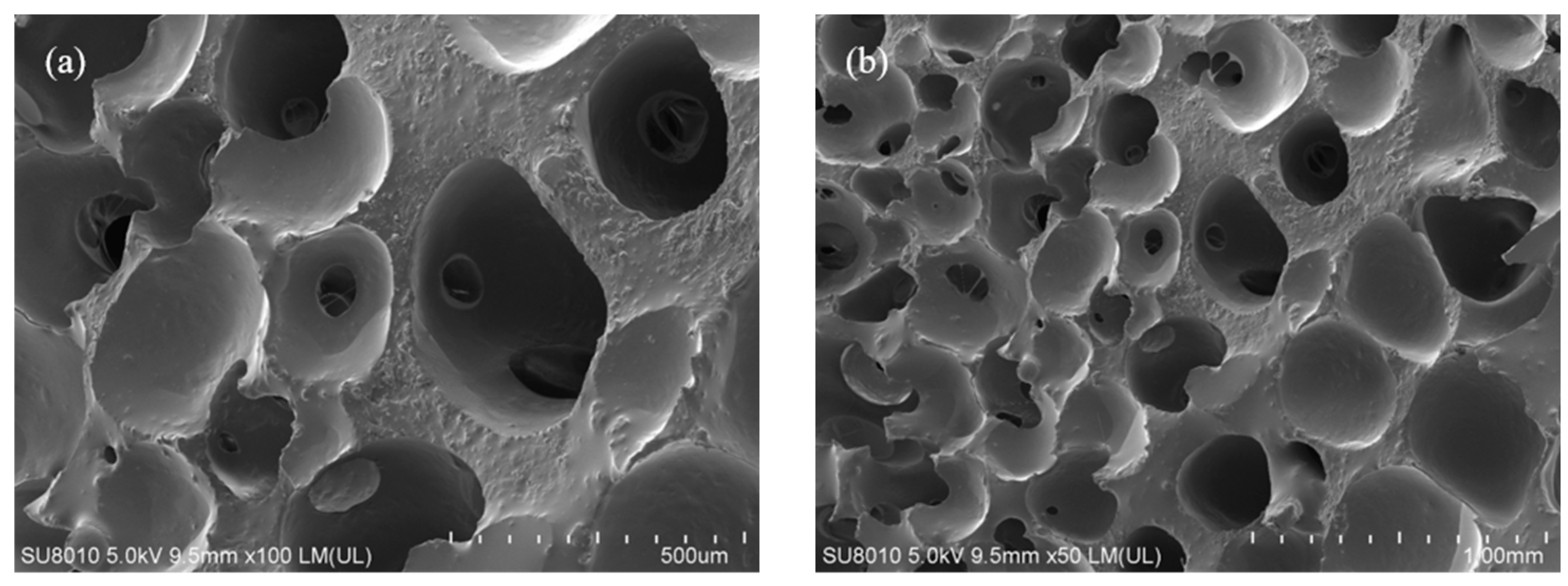
3.5.2. SEM Analysis
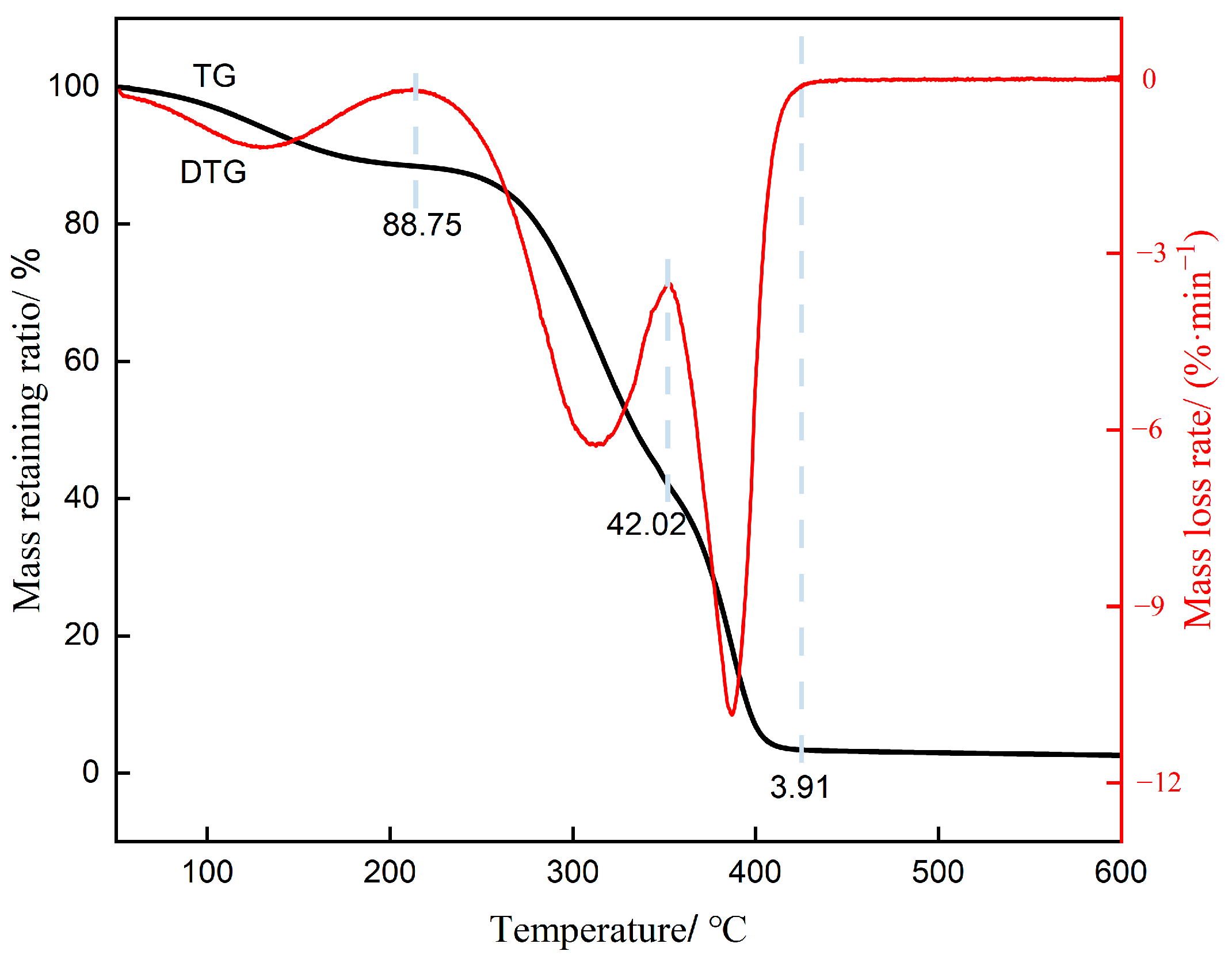
3.5.3. Thermogravimetric Analysis
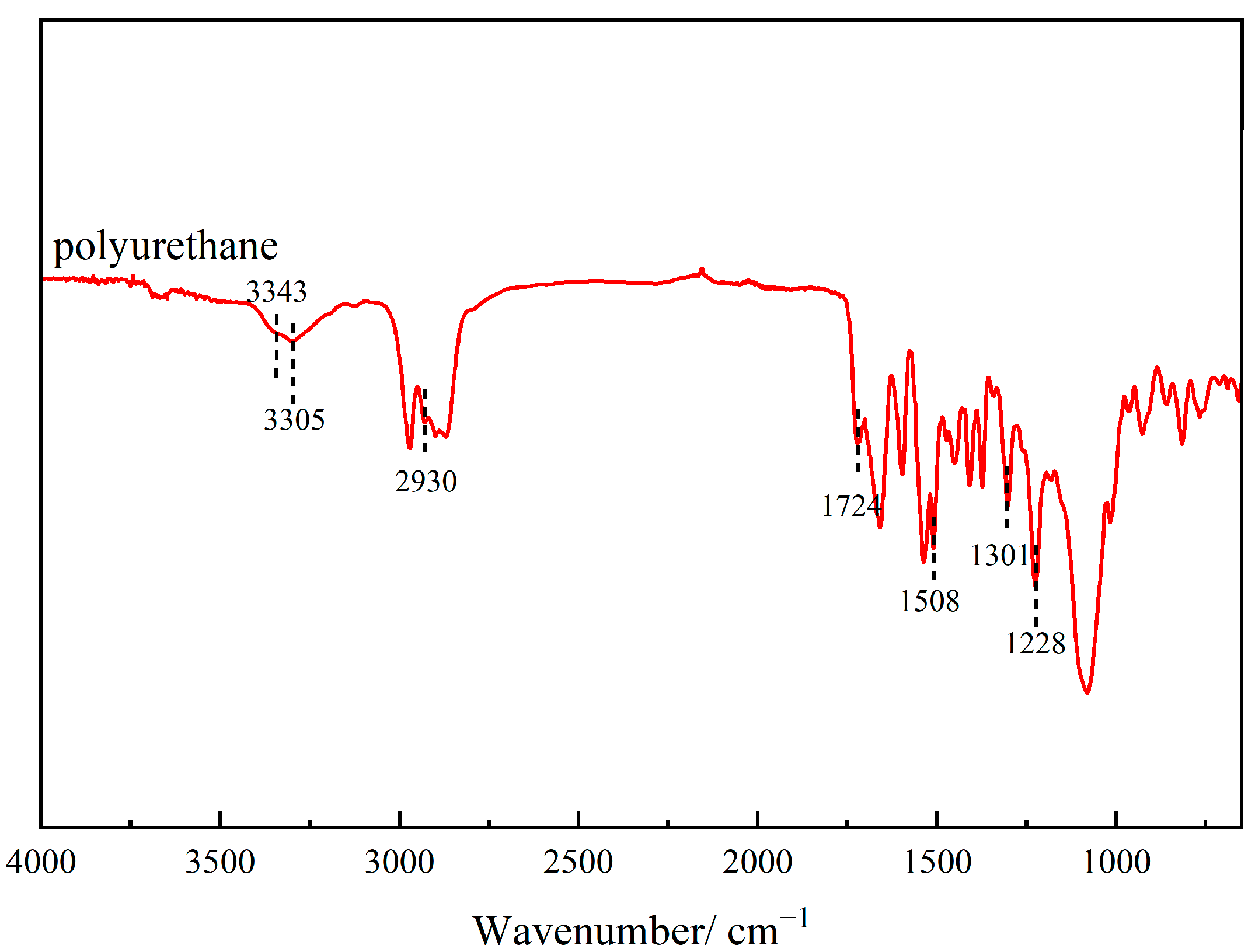
3.5.4. FTIR Analysis
3.6. Application Process of Polyurethane Sealant
3.6.1. Influence of Water on Application Process
3.6.2. Application Process Design
4. Conclusions
- (1)
- A water-reactive polyurethane grouting material was synthesized using PPG and BDO as soft segments, MDI as the hard segment, and a composite catalyst system composed of PC-8 and T-12. The polyurethane was synthesized at an –NCO/–OH molar ratio of 1.8, 1 wt% BDO, and a 3 wt% composite catalyst, a polyurethane sealant exhibiting controllable gelation time and high mechanical strength, was obtained.
- (2)
- FTIR spectroscopy confirmed the formation of urethane linkages, verifying the successful polymerization reaction. SEM revealed that the cured polyurethane exhibited a dense and continuous microstructure, while TGA demonstrated excellent thermal stability.
- (3)
- The polyurethane lost circulation material was capable of setting and hardening in aqueous environments of various concentrations without alteration of its gelation behavior. It effectively sealed 10~20-mesh sand beds and 5 mm crack plates under pressures exceeding 3 MPa, thereby controlling severe fluid losses. Moreover, the cured polyurethane could maintain integrity and pressure resistance in saturated NaCl and CaCl2 solutions, exhibiting outstanding resistance to salinity interference.
- (4)
- The primary sealing mechanism of the polyurethane lost circulation material lies in its interaction with formation water. Upon contact with water, the -NCO groups in the polyurethane react with water, carbamates, and the substituted urea group, leading to rapid crosslinking and curing. This process forms a high-strength cemented layer that effectively seals leakage channels. Moreover, during the curing process, the material undergoes volumetric expansion, filling voids within the loss zone. Consequently, dual sealing is achieved through both cementation and expansion mechanisms.
- (5)
- Considering the strong water reactivity of the polyurethane lost circulation material, its sensitivity to aqueous environments during field application was thoroughly investigated to optimize operational techniques. In practical leak-sealing operations, synthesized base oil was employed as an isolating medium. The downhole injection sequence consisted of an isolation fluid, a polyurethane sealant, an isolation fluid, a catalyst solution, and subsequently a flushing fluid. Throughout this process, exposure of the sealant to water was minimized to ensure controlled gelation and optimal sealing performance.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| PPG | Polypropylene glycol |
| BDO | 1,4-butanediol |
| MDI | Diphenylmethane diisocyanate |
| PC-8 | N, N- dimethylcyclohexylamine |
| T-12 | Dibutyltin dilaurate |
| R | The ratio of isocyanate group to hydroxyl group |
| FL | Filter loss |
| SEM | Scanning electron microscopy |
| TGA | Thermogravimetric analysis |
| FTIR | Fourier-transform infrared |
References
- Zhang, D.Q. The Technology of Interval Push Clay-Cement Slurry to Lost Circulation in Water Bearing Formation. Drill. Fluid Complet. Fluid 2022, 39, 87–91. [Google Scholar] [CrossRef]
- Hou, G.Z.; Xu, J.; Tao, L.; Zhu, G.W.; Su, J.; Chen, K. Research on Combined Chemical and Physical Leakage Plugging Technology in Complex Leakage Formations in Bozhong Oilfield. Contemp. Chem. Ind. 2024, 53, 1959–1965. [Google Scholar] [CrossRef]
- Wang, Z.H. The Status and Development Direction of Plugging Technology for Complex Formation Lost Circulation. Sino-Glob. Energy 2014, 19, 39–48. [Google Scholar]
- Sum, J.S.; Zang, X.W. Situations, Challenges, Demands and Trends of Drilling Fluid Technology. Drill. Fluid Complet. Fluid 2011, 28, 67–76. [Google Scholar]
- Xu, C.Y.; Yan, X.P.; Kang, Y.L.; You, L.J.; Zhang, J.Y. Structural failure mechanism and strengthening method of plugging zone in deep naturally fractured reservoirs. Pet. Explor. Dev. 2020, 47, 399–408. [Google Scholar] [CrossRef]
- Li, J.F. The status and development direction of plugging technology for severe circulation loss formation. Explor. Eng. Rock Soil Drill. Tunneling 2019, 46, 19–27. [Google Scholar]
- Liu, F.B.; Yin, D.; Wei, T.X.; Wu, H.Y.; Wang, Z.W. Settling Plugging Technology for Severe Lost Circulation at the Salt Bottom of Tarim Piedmont Zone. Xinjiang Oil Gas 2024, 20, 1–7. [Google Scholar]
- Feng, Y.; Gray, K.E. Review of Fundamental Studies on Lost Circulation and Wellbore Strengthening; Springer: Cham, Switzerland, 2018; pp. 15–18. [Google Scholar] [CrossRef]
- Sun, J.S.; Bai, Y.R.; Cheng, R.C.; Lu, K.H.; Liu, F.; Feng, J.; Lei, S.F.; Zhang, J.; Hao, H.J. Research progress and prospect of plugging technologies for fractured formation with severe lost circulation. Pet. Explor. Dev. 2021, 48, 630–638. [Google Scholar] [CrossRef]
- Wang, K. Application Study of Magnesium Aluminum Silicate and Graphene Oxide Layered Nano-Materials in Water-Based Drilling Fluids; China University of Petroleum, Beijing: Beijing, China, 2021. [Google Scholar]
- Wang, H.H. Research and Application of Plugging Drilling Fluid System in Zhenjing and Hangjinqi Oil and Gas Field; China University of Petroleum, Huadong: Qingdao, China, 2020. [Google Scholar]
- Zhang, P.Y. Progressive Bridge Plugging Technology for Lost Circulation. Drill. Fluid Complet. Fluid 2010, 27, 67–69+92–93. [Google Scholar]
- Wang, B. Development of Temperature Sensitive Gel and Study on Plugging Mechanism in Fractures; China University of Petroleum, Huadong: Qingdao, China, 2022. [Google Scholar]
- Hu, P.B.; Li, C.K.; Qiao, Y.S.; Jing, R.L.; Wang, Y.D. Research Progress on Application of Polymer Gel Plugging Materials in Petroleum Engineering. Guangzhou Chem. Ind. 2024, 52, 14–16+59. [Google Scholar]
- Lin, M.; Zhang, G.; Hua, Z.; Zhao, Q.; Sun, F. Conformation and plugging properties of crosslinked polymer microspheres for profile control. Colloids Surf. A Physicochem. Eng. Asp. 2015, 477, 49–54. [Google Scholar] [CrossRef]
- Shi, X.; Yue, X. Migration and plugging mechanisms of self-aggregated microspheres as a novel profile control. J. Pet. Sci. Eng. 2020, 184, 106458. [Google Scholar] [CrossRef]
- Yuan, X.Q. Study on the Formula of Rapid Filtration Solidification Plugging; China University of Petroleum, Beijing: Beijing, China, 2023. [Google Scholar]
- Chen, N.; Deng, K.; Chen, X.R.; Zhu, M.M.; Yang, Y.; Liu, Z.X. Research and application of curable plugging technology in Changqing Oilfield. China Pet. Chem. Stand. Qual. 2021, 41, 157–159. [Google Scholar] [CrossRef]
- Liu, J.H.; Liu, S.H.; Long, D.Q.; Chen, Z.W.; Jin, R.H. Strengthening Plugging Operations by Combining Cross-Linked Film and Chemical Consolidation in Well Ming-1. Pet. Drill. Tech. 2017, 45, 54–60. [Google Scholar] [CrossRef]
- Han, C.; Luo, M.; Yang, Y.H.; Liu, X.Y.; Li, W.T. Key drilling technologies for HTHP wells with narrow safety density window in the Yingqiong Basin. Oil Drill. Prod. Technol. 2019, 41, 568–572. [Google Scholar]
- Liu, Y.T.; Sun, D.W.; Ran, Q.P.; Lu, L.Q.; Li, B.; Yin, H. Preparation and Performance Study on High Performance Water Reactive Polyurethane Grouting Material. China Build. Waterproofing 2015, 11–14. [Google Scholar] [CrossRef]
- Gai, B.W.; Duan, H.T.; Jiang, Y.; Lu, Y.; Jiang, Z.G.; Yao, M.; Dun, Y.C. Development of environmentally friendly hydrophilic polyurethane sealant. New Chem. Mater. 2024, 52, 224–227. [Google Scholar]
- Li, X.L.; Peng, C.; Ao, Y.N.; Hao, M.M.; Zhong, Y.H.; Zhang, B. Impact of Composition Ratio on the Expansion Behavior of Polyurethane Grout. Materials 2024, 17, 1835. [Google Scholar] [CrossRef] [PubMed]
- Feng, L. Synthesis of Water Soluble Polyether Polyols and Preparation of Polyurethane Grouting Materials; Lanzhou University: Lanzhou, China, 2023. [Google Scholar]
- Li, L.; Li, W.; Ou, Y.W. Application of a Fast-swelling Gel Lost Circulation Material in Shale Gas Drilling in Block Changning. Drill. Fluid Complet. Fluid 2019, 36, 181–188. [Google Scholar]
- Duan, H.T. Synthesis and Application of Environmentally Friendly Water-Based Sealing Agent; Beijing University of Chemical Technology: Beijing, China, 2022. [Google Scholar]
- Zhu, M.M.; Sun, H.; Sun, Y.; Cong, C.; Shi, D.Y.; Jia, J.G. Loss Circulation Control Technology for Malignant Water Leakage Layer in Longdong Tight Oil Region. Pet. Drill. Tech. 2023, 51, 50–56. [Google Scholar] [CrossRef]
| Number | BDO (%) | R | Viscosity (mPa·s) | Compressive Strength/(MPa) |
|---|---|---|---|---|
| 1 | 0 | 1.6:1 | 35,692 | 6.88 |
| 2 | 1 | 1.6:1 | 13,518 | 6.55 |
| 3 | 2 | 1.6:1 | 11,634 | 6.50 |
| 4 | 1 | 1.8:1 | 21,731 | 9.35 |
| 5 | 1 | 2.0:1 | 43,910 | 10.32 |
| Number | T-12 (%) | PC-8 (%) | Synthesis Time (h) | Gel Time (min) |
|---|---|---|---|---|
| 1 | 10 | 1 | - | - |
| 2 | 0.1 | 1 | 5 | 30 ± 0.6 |
| 3 | 1 | 1 | 1 | 25 ± 0.6 |
| 4 | 1 | 2 | 1 | 15 ± 0.3 |
| 5 | 1 | 3 | 1 | 5 ± 0.1 |
| 6 | 1 | 5 | 1 | 3 ± 0.05 |
| Test Conditions | Type | Temperature (°C) | FL1 MPa (mL) | FL2 MPa (mL) | FL3 MPa (mL) |
|---|---|---|---|---|---|
| sand bed (20~40) | Polyurethane Sealant | 93 | 0 | 0 | 0 |
| sand bed (10~20) | Polyurethane Sealant | 93 | 0 | 0 | 0 |
| crack plate (3 mm) | Polyurethane Sealant | 93 | 0 | 0 | 0 |
| crack plate (5 mm) | Polyurethane Sealant | 93 | 0 | 0 | 0 |
| sand bed (10~20) | PF-SEAL | 93 | 50 | Complete leakage | Complete leakage |
| crack plate (3 mm) | PF-SEAL | 93 | Complete leakage | Complete leakage | Complete leakage |
| sand bed (10~20) | PF-SZDL | 93 | 60 | Complete leakage | Complete leakage |
| crack plate (3 mm) | PF-SZDL | 93 | 132 | Complete leakage | Complete leakage |
| sand bed (10~20) | XZ-APA | 93 | Complete leakage | Complete leakage | Complete leakage |
| crack plate (3 mm) | XZ-APA | 93 | 96 | Complete leakage | Complete leakage |
| Triggering Agent | Temperature (°C) | Height of Consolidation Body (cm) | FL1 MPa (mL) | FL2 MPa (mL) | FL3 MPa (mL) |
|---|---|---|---|---|---|
| Saturated NaCl | 93 | 10 | 0 | 0 | 3 |
| Saturated CaCl2 | 0 | 0 | 0 |
| Temperature (°C) | Water Addition Percentage (%) | Gel Time (h) |
|---|---|---|
| 70 | 0 | 7 |
| 70 | 20 | 3 |
| 70 | 50 | 2 |
| 80 | 0 | 6 |
| 80 | 20 | 1.5 |
| 80 | 50 | 1 |
| 100 | 0 | 3 |
| 100 | 20 | 0.5 |
| 100 | 50 | 0.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Wu, J.; Hu, J.; He, G.; Huang, S.; Yang, L. Development of a Polyurethane Lost Circulation Material Suitable for Malignant Leakage of Drilling Fluid. Processes 2025, 13, 3707. https://doi.org/10.3390/pr13113707
Liu X, Wu J, Hu J, He G, Huang S, Yang L. Development of a Polyurethane Lost Circulation Material Suitable for Malignant Leakage of Drilling Fluid. Processes. 2025; 13(11):3707. https://doi.org/10.3390/pr13113707
Chicago/Turabian StyleLiu, Xiaodong, Jiale Wu, Jinjun Hu, Guoxin He, Sanpeng Huang, and Lili Yang. 2025. "Development of a Polyurethane Lost Circulation Material Suitable for Malignant Leakage of Drilling Fluid" Processes 13, no. 11: 3707. https://doi.org/10.3390/pr13113707
APA StyleLiu, X., Wu, J., Hu, J., He, G., Huang, S., & Yang, L. (2025). Development of a Polyurethane Lost Circulation Material Suitable for Malignant Leakage of Drilling Fluid. Processes, 13(11), 3707. https://doi.org/10.3390/pr13113707






