1. Introduction
Energy consumption and carbon dioxide (CO
2) emissions worldwide continue to increase yearly [
1]. Among the different forms of energy produced globally, electricity is the most consumed, as it drives both productive and service activities. One of the most energy-intensive technologies worldwide is vapor-compression refrigeration, which is estimated to account for approximately 30% of total electricity consumption [
2]. Although this technology is the most widely used for cooling, it poses two major environmental issues: first, it indirectly emits CO
2 into the atmosphere by relying on electricity; second, it employs toxic refrigerant gases that directly harm the ozone layer, reducing its capacity to absorb shortwave ultraviolet solar radiation and thereby contributing to global warming through the greenhouse effect [
3].
For this reason, it is urgent to seek alternatives to vapor-compression technology to reduce or eliminate electricity consumption while using refrigerants with minimal environmental impact, such as water, ammonia, amines, and alcohols. A promising alternative is sorption refrigeration (absorption, adsorption, and thermochemical refrigeration). These technologies operate using thermal energy instead of electricity. The required thermal energy can be sourced from solar, geothermal, biomass, or industrial waste heat. Sorption refrigeration and solar energy complement each other well, as the highest cooling demand coincides with periods of high solar irradiance (I).
Thermal refrigeration encompasses absorption (liquid–gas), adsorption (solid–gas), and chemical reactions between solids and refrigerant vapors (solid–gas absorption). In adsorption refrigeration, the adsorbent is a compact or granular solid, while the adsorbate is the refrigerant, with adsorption being characterized by physical bonding through Van der Waals forces. Commonly used working pairs include silica gel–water, zeolite–water, activated carbon–methanol, activated carbon–ethanol, and activated carbon–ammonia [
4,
5].
In absorption refrigeration, liquid or solid absorbents are used, and the absorbate is the refrigerant, interacting exothermically with the absorbent to form a new compound, typically named after the gaseous refrigerant used (e.g., ammoniates, aminates, hydrates, and alcoholates).
For absorption cooling systems using liquids, the H
2O–NH
3 and LiBr–NH
3 working pairs are the most commonly employed [
6,
7]. In contrast, for solid absorption, alkali and alkaline earth metal halide salts with ammonia have been widely studied in recent decades, including CaCl
2–NH
3 [
8,
9,
10], SrCl
2–NH
3 [
11], and BaCl
2–NH
3 [
12,
13]. In the generation process (i.e., separation of the refrigerant from the solid), thermal energy is supplied, making the thermal conductivity of the solid absorbent a critical factor. However, alkali metal salts have low thermal conductivity, in the range of 0.1 to 0.2 W/m·K [
14], and tend to agglomerate during absorption, which can hinder performance. Matrix additives have been introduced to prevent agglomeration while enhancing thermal conductivity to mitigate this issue. Various materials have been proposed for this purpose, including expanded graphite [
15], vermiculite [
16,
17], sibunit carbon, and Al
2O
3 [
17].
For many years, thermal refrigeration systems have been developed using conventional and renewable energy sources, ranging from prototypes to fully functional refrigeration systems. These technologies have primarily been applied to space cooling, ice production, and food preservation [
18].
Several theoretical and laboratory-scale studies have investigated the operation of the BaCl
2–NH
3 pair, with some using pure salt [
12,
13] while others combine it with materials such as expanded graphite, vermiculite, etc. [
19,
20,
21]. However, few BaCl
2–NH
3 system prototypes operate directly or indirectly with solar thermal technology. Previous studies have been conducted with interesting results, which are mentioned below.
Driss Stitou [
22] developed a pilot solar air-conditioning plant with a capacity of 20 kWh, providing a temperature of 4 °C for residential buildings in France. He used BaCl
2 mixed with natural expanded graphite and flat-plate solar collectors, achieving cold production of 0.8 to 1.2 kWh per square meter of collector area. The annual efficiency of the solar collectors was between 40% and 50%, while the process COP ranged from 40% to 50%. On the other hand, N. Le Pierrès (2008) designed a thermochemical process to cool 560 L to −30 °C using solar energy at 70 °C, obtaining a COP between 0.07 and 0.05 [
23]. Later, he demonstrated the feasibility of achieving −30 °C with a COP of 0.031 [
24] and modelled a freezing process for a 500 L container at −20 °C using flat-plate solar collectors at 70 °C. The system required a collector area of 5.8 m
2 and 39 kg of reactive salt, achieving a COP of 0.1 and a solar COP of 0.05 [
25]. C. Rivera (2007) demonstrated the feasibility of operating a thermochemical system with flat-plate solar collectors at the laboratory level [
12]. He established heating fluid conditions between 70 and 95 °C, generation at 53 °C, condensation at 23 °C, and evaporation–absorption temperatures between −10 and 0 °C. Dueñas (2001) presented theoretical results for a BaCl
2–NH
3 thermochemical system, showing that COP values remain relatively stable across different operating temperatures [
13]. Additionally, the generation temperatures ranged from 50 to 60 °C, making the system compatible with simple heating methods. Other studies have investigated BaCl
2–NH
3 for heat pump applications [
26].
Regarding studying different compounds and other forms of refrigeration, Veselovskaya et al. analyzed the viability of novel sorbent materials for heat transformation. The BaCl
2/vermiculite compound was shown to have a COP suitable for ice production and air-conditioning cycles, reaching up to 0.6 [
27]. M. Pons evaluated six solar-powered air-conditioning systems based on four principles: LiBr + H
2O (liquid sorption), silica gel + water (solid sorption), BaCl
2 + NH
3 (thermochemical reaction), and desiccant + humid air (open cycles). The results demonstrated the potential of these technologies for building cooling, with COP values around 0.1 [
28].
Other swarm intelligence-based cooling systems promise to maximize cooling through environmental adaptation, although they rely on multiple physical and software components, increasing their complexity and maintenance costs. Methods such as those of Wang et al. (2025) successfully optimize cooling channels with promising results [
29].
On the other hand, passive waste heat removal systems, such as the one proposed by Bi et al. (2025) for nuclear reactors, operate without additional electrical components through natural heat exchange [
30]. However, their design is sensitive to fluid circulation disturbances.
1.1. Justification and Limitations of the Industrial-Grade BaCl2–NH3 Compound Without Additives
As can be seen, there are few studies on thermochemical solar refrigeration related to ice production via solid–gas reactions, since most focus on air-conditioning applications, despite the significant demand for ice in developing countries [
31,
32,
33]. Furthermore, while several studies address ice production, most incorporate additives into the absorbent to improve thermal conductivity [
34,
35]. These compounds present some disadvantages: (a) an increase in the solid mass to be heated, (b) the possibility of chemical interactions with the gas, and (c) the limited availability of additives, which increases equipment costs [
34,
35]. Meanwhile, compounds without any additives offer the following advantages: (1) chemical and processing simplicity, (2) less inactive material mass, (3) reduced initial material costs, (4) less manufacturing complexity, and (5) compatibility with low generation temperatures.
While these compounds do have several advantages, such as low thermal conductivity and agglomeration, intermittent operation and scalability complexity, the need for pre-dehydration, and the quality and impurities of the industrial-grade BaCl
2–NH
3 compound, these disadvantages can be mitigated by improving mechanical/geometric heat transfer through controlled pretreatment, a multi-layer (quasi-continuous) architecture, controlling impurities through load analysis and adjustment, heat recovery and reuse, a safety protocol, modeling, and sensitivity testing [
18,
36].
This study highlights the importance of using additive-free industrial-grade barium chloride, as it offers the advantage of simplicity and compatibility for refrigeration systems operating at low generation temperatures, ranging from 53 to 66 °C [
12,
18]. Furthermore, as mentioned previously, it is possible to increase the temperature differential and improve heat transfer in each component, eliminating the need for special materials that would complicate and increase the manufacturing cost of the absorbent/adsorbent [
37]. Considering these limitations, a simple system for ice production was developed, featuring low-cost construction and operation while utilizing a solar heating system for its functionality, demonstrating technical feasibility for ice production (−7 to −3, with COP ~0.24–0.31) [
13,
18].
Therefore, we can say that the BaCl
2–NH
3 working pair offers an optimal balance between thermodynamic efficiency, material stability, and compatibility with low-temperature solar heat sources. Its high ammonia capacity, suitable equilibrium pressure, and extensive validation in previous studies justify its selection for this research [
12,
18]. While other pairs, such as CaCl
2–NH
3 and SrCl
2–NH
3, are viable, their higher temperature requirements, cost, and structural instabilities make BaCl
2–NH
3 the most practical and technically sound option for solar thermochemical cooling systems.
1.2. Thermodynamic Cycle Description
The principle of thermochemical reaction processes is based on the thermal effect of a reversible reaction between a solid and a reactive gas, as shown in Equation (1) [
24,
38,
39]:
The mixture used for the experimental prototype consisted of barium chloride (a salt) and ammonia (a refrigerant), which undergoes the following reaction, shown in Equation (2) [
13,
18,
36,
40]:
The equilibrium relationship for solid–gas reactions (chemisorption) is monovariant, meaning there is a one-to-one correspondence between the reaction temperature and the equilibrium vapor pressure. This relationship can be expressed by the Clausius–Clapeyron equation, as shown in Equation (3) [
23,
41]:
1.3. Thermochemical Process
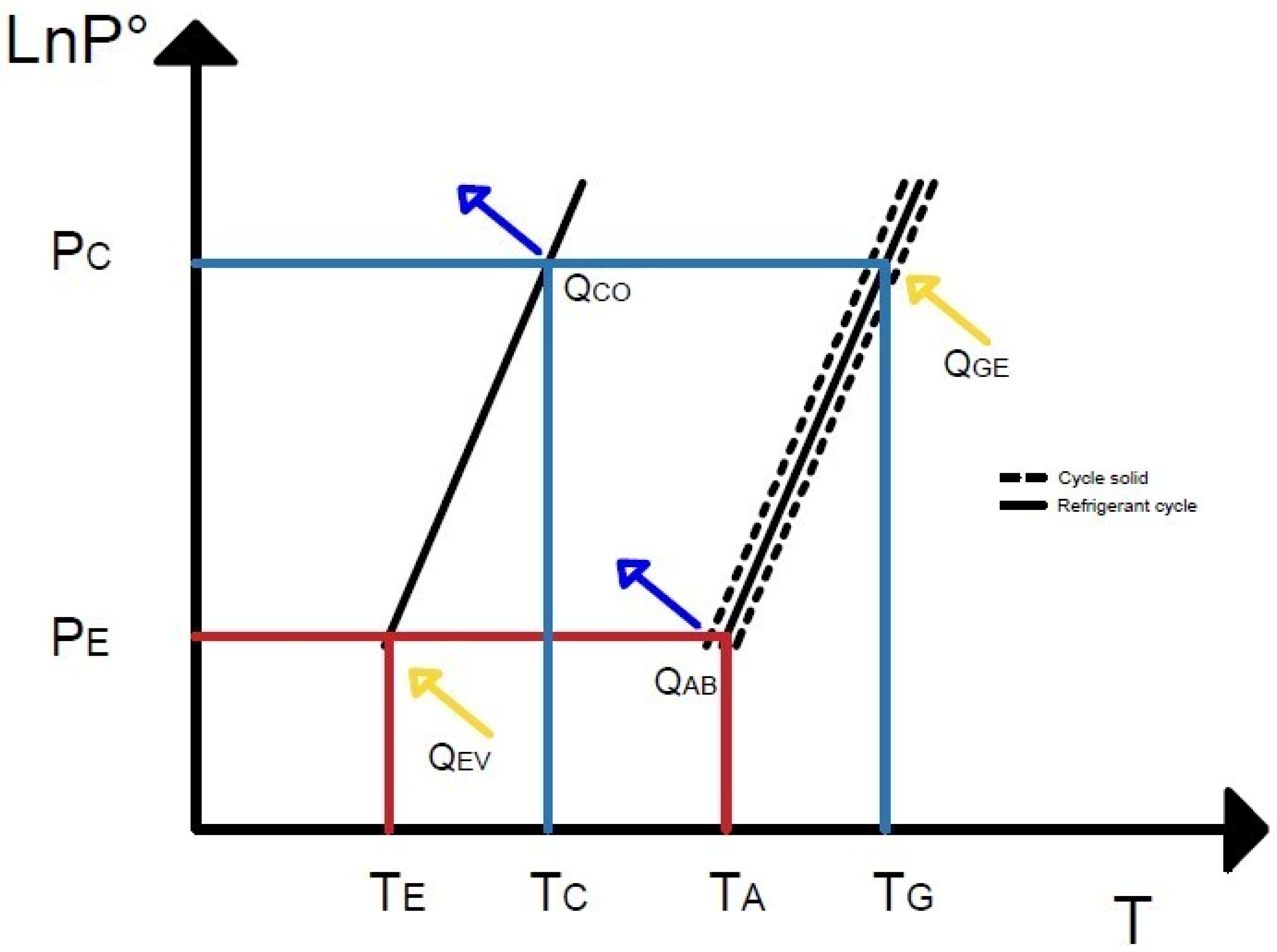
In refrigeration systems using solid absorbents, the operating cycle is intermittent or discontinuous due to the difficulty of establishing continuous circulation of the solid absorbent. Consequently, the generator (G) also functions as the absorber (A) during the thermodynamic cycle. The generator contains the solid–gas compound in its absorbed phase. The refrigerant gas is generated at a specific pressure (PGE) and temperature (TGE) by supplying thermal energy. The refrigerant vapor is transferred to the condenser (C), liquefied by chilled water at a condensation temperature (TCO), dissipating this heat. Once in the liquid phase, the refrigerant is stored at high pressure (PCO). This simultaneous process of generation, condensation, and storage occurs in the first phase of the cycle.
The stored liquid refrigerant expands to the evaporation pressure (P
EV) for cooling, reaching the desired refrigeration temperature (T
EV). Simultaneously, the solid absorbent reabsorbs the refrigerant vapor at low pressure (P
AB) and absorption temperature (T
AB), reforming the compound and initiating a new thermochemical absorption cycle (see
Figure 1).
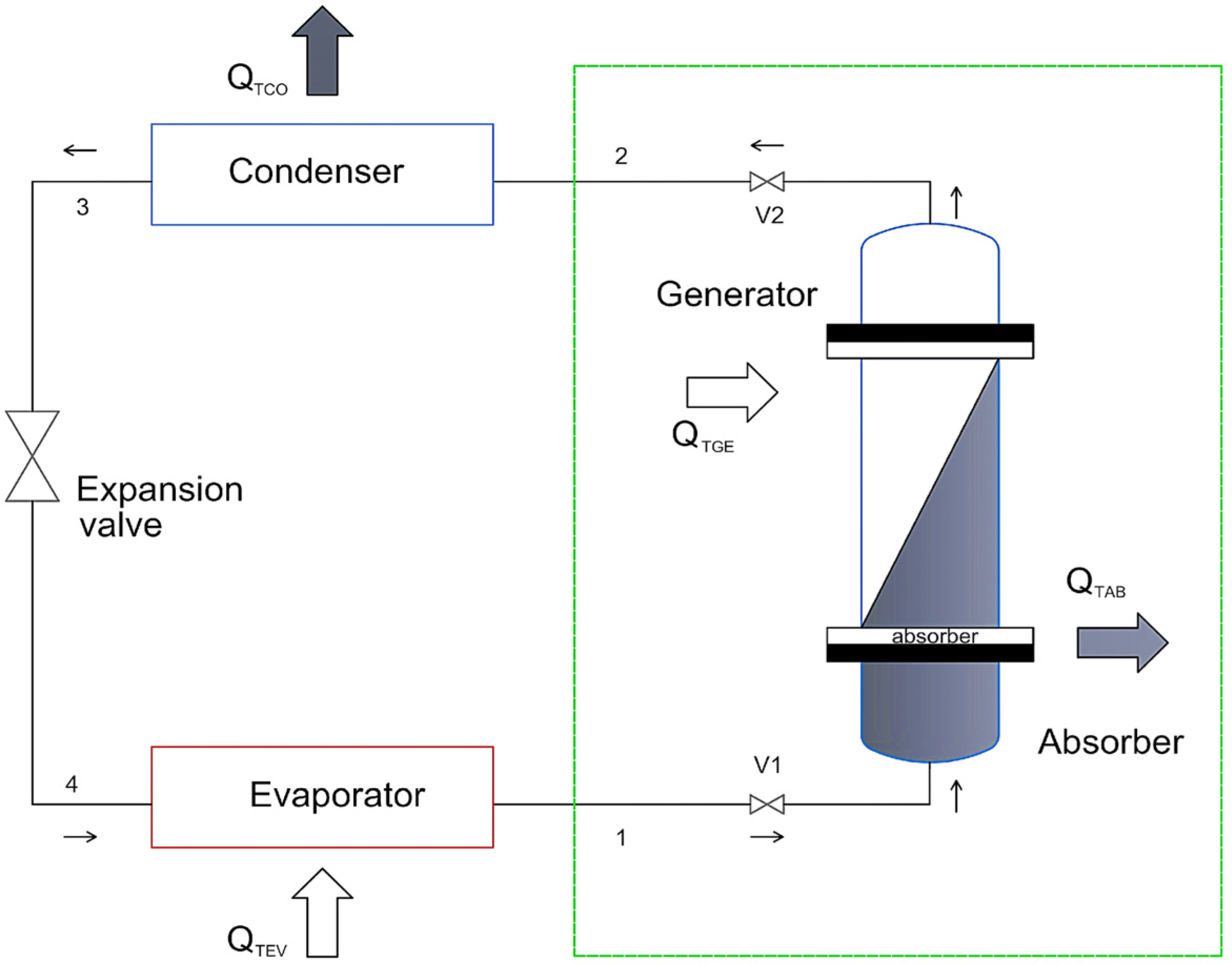
Now, according to
Figure 2, the performance (COPₜ) and overall efficiency (η
g) can be obtained through an energy balance, as shown in Equation (4) [
13,
36]:
where
QTGE,
QTEV,
QTCO, and
QTAB represent the total heat at the different stages of generation, evaporation, condensation, and absorption, respectively. These values can be determined using Equations (5)–(17) [
36]:
- (b)
Total Condensation Heat
- (c)
Total Evaporation Heat
- (e)
Coefficient of Performance (COPt)
- (f)
Overall Efficiency (ηg)
2. Materials and Methods
2.1. Selection of the BaCl2–NH3 Compound
The BaCl2–NH3 compound was selected for this application for the following reasons: it combines high ammonia absorption capacity, an equilibrium pressure suitable for producing cold from −5 to 0 °C, good chemical stability and acceptable reversibility, and is readily available, cost-effective, and safe compared to other metal halides. Its thermal behavior allows for direct coupling to low- and medium-temperature solar collectors (55–70 °C), without the need for concentrators, which is essential for rural or off-grid systems.
In short, it represents an optimal balance between thermodynamic performance, economic viability, and operational efficiency, justifying its preferential use over other options.
2.2. Synthesis of the BaCl2–NH3 Compound
The working mixture used for the thermochemical refrigeration cycle was industrial-grade barium chloride dihydrate, BaCl
2 · 2H
2O (98% X-HUMATE, Tianjin, China), which required pre-dehydration to absorb the 8 moles of NH
3 that BaCl
2 can accept in anhydrous form [
42]. It is essential to highlight that the refrigerant used in this working pair is considered natural, with a simple inorganic molecule, without carbon, so it does not contribute to the depletion of the ozone layer or to global warming significantly (global warming potential~0 and ozone depletion potential = 0) [
43]. The quantities used were 17.55 kg of BaCl
2 and 4.2 kg of NH
3. For this study, empirical equilibrium pressure–temperature relationships for ammonia and the compound were obtained by analyzing experimental data obtained in this study.
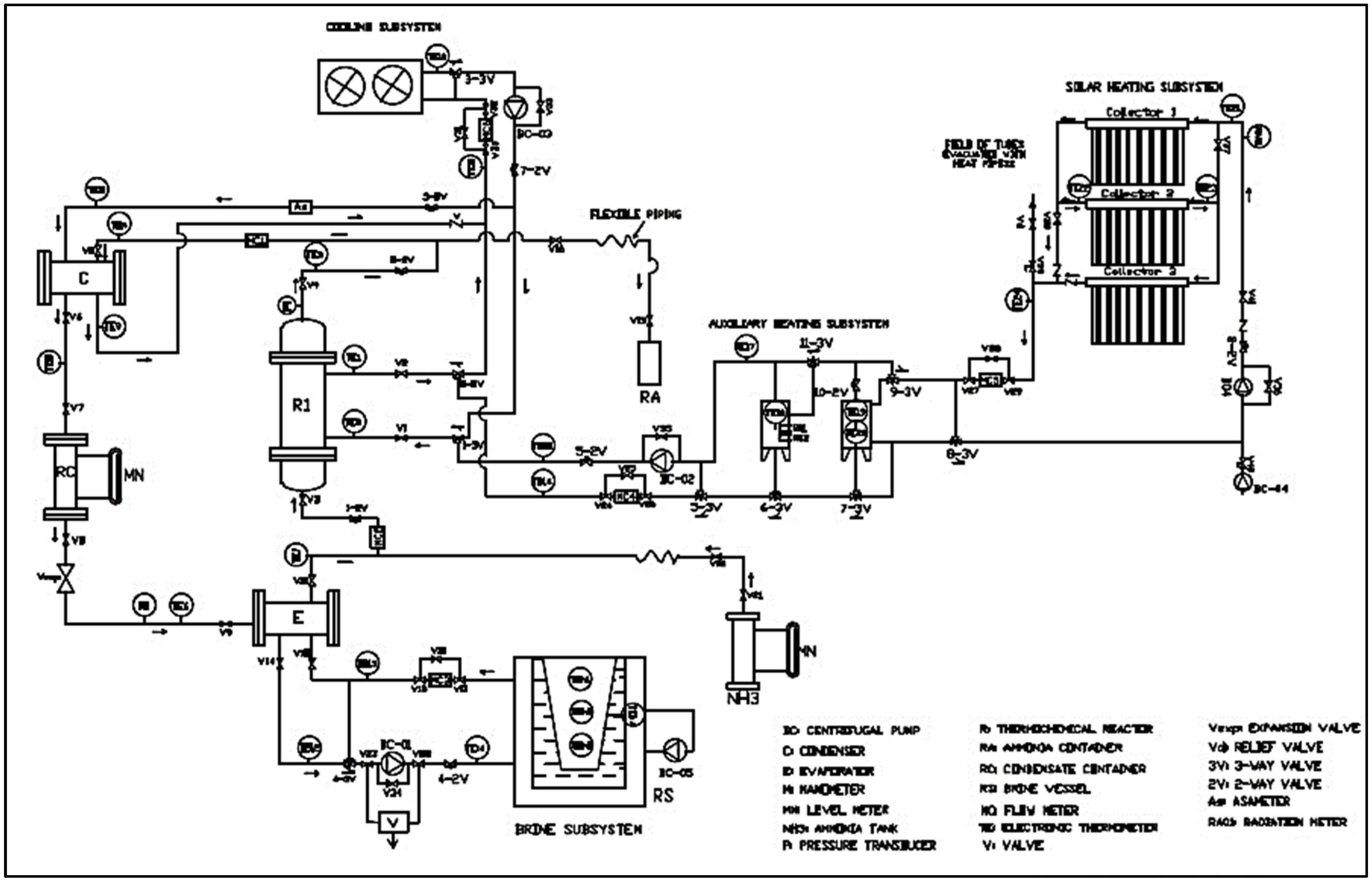
2.3. Description of the Thermochemical Refrigeration System
The solar thermochemical refrigeration system (STRS) consists of the following elements: Firstly, there is a thermochemical reactor (TR), formed of a shell-and-tube heat exchanger made of carbon steel and stainless-steel tubes. Inside the internal tubes is a stainless-steel distributor that supplies vapor to each tube after being filled with dehydrated (anhydrous) industrial barium chloride. The reactor has an outer casing where water circulates as a heating and cooling thermal fluid to dissociate the refrigerant and absorb the heat from the reaction. There is also a condenser (C), formed of a corrugated plate heat exchanger made of 316 stainless steel with a 6 kW capacity, and a condensate reservoir (CR), made up of a 304 stainless-steel container with a 7 L ammonia storage capacity. An expansion valve (Vexp.) separates the system’s high- and low-pressure zones and regulates the refrigerant mass flow rate (ṁ) up to 0.035 kg/s. It also contains an evaporator (E), a corrugated plate heat exchanger made of 316 stainless steel with a 6 kW capacity.
The STRS includes various subsystems: (a) A cooling subsystem, which is composed of a water chiller controlled at temperatures between 7 and 30 °C and a 1100 L water storage tank. (b) A cold production subsystem, which includes a 50 L thermotank with two internal storage containers for ice production that operate indirectly using a secondary thermal fluid after ammonia evaporation. (c) A solar heating subsystem, which consists of three concentric evacuated tube solar collectors with hermetic heat exchangers (heat pipes). Each collector contains 30 borosilicate glass tubes, 47 mm in diameter and 150 mm in length, with a total solar collection area of 6.99 m
2. The inner surface of the glass tubes is coated with aluminum nitride (Al-N/Al), which has an absorption coefficient of 92%. The tubes are evacuated to a pressure of 5 mPa, with a linear heat loss coefficient of 0.8 W/m
2 °C. The solar collector field is oriented southward, with an inclination corresponding to the latitude of Temixco, Morelos, Mexico (18.85° N, −99.23° W), and a spacing of 29.3 cm between them.
Figure 3 and
Figure 4 show a schematic and real representation of the STRS and its operational subsystems, respectively [
39]. The solar heating system operates under forced convection, and its thermal efficiency is given by Equation (18) [
44]:
where
Note: Safety and environment for ammonia.
It is important to mention that, as a safety measure, the thermochemical cooling cycle (BaCl
2–NH
3) has storage tanks to recover ammonia in case of a leak. This is because ammonia is corrosive, irritating, and toxic, so it can cause severe burns to the skin, eyes, and respiratory tract. Due to its high volatility, characteristic odor (which serves as a warning), and potential to form flammable and explosive mixtures at specific concentrations, these additional measures should be necessary; see
Figure 3.
2.4. Experimental Study
The solar thermochemical refrigeration system (STRS) is located on the solar platform of the Institute of Renewable Energies of the National Autonomous University of Mexico (IER-UNAM) in Temixco, Morelos, at approximately 18°46′ to 18°55′ north latitude and 99°12′ to 99°21′ west longitude. According to data from the IER’s meteorological and solarimetric station, the climate is considered warm and humid. The ambient temperature ranges from 18 to 35 °C annually, and solar irradiance ranges from 14 to 15 MJ/m2. The thermochemical cooling system was evaluated between February and December 2024.
The STRS was evaluated using a single-stage, intermittent operating system. The system operated with evacuated tube solar collectors with heat pipes. The operating temperatures were established within the following ranges: 55 to 66 °C for generation, 20 to 30 °C for condensation, 24 to 31 °C for absorption, and −7 to −3 °C for evaporation.
It is important to mention that the efficiency of the solar heating subsystem was obtained from Equation (18) and the measurements obtained of temperatures and solar irradiance of the collectors.
Acquisition of Experimental Data
For the analysis and obtaining of the experimental data of the STRS, it was necessary to develop a program for the acquisition and automatic management of data for which the instrumentation of the following sensors was used: (a) temperature (type T class 1 thermocouple and thermistor with an accuracy of ± 0.5 and ±0.1 °C, respectively), (b) pressure (differential pressure sensors with an accuracy of ±0.15%), (c) liquid and vapor flow (Coriolis type with an accuracy of ±0.1%), and (d) spectral pyranometer class B (accuracy < 2%) [
18].
3. Results and Discussion
In order to carry out the experimental evaluation of the solar thermochemical refrigeration system, several thermodynamic cycles were carried out, such as the desorption–condensation and absorption–evaporation processes, to determine the practical viability of the system, quantifying parameters such as the rate of production and absorption of ammonia, the internal thermal performance coefficients, and the overall efficiency, as well as the temperature–pressure equilibrium equation from the experimental data of the barium chloride–industrial ammonia working pair. These indicators allowed the validation of the viability of the solar thermochemical refrigeration system in real operating conditions, and it was possible to establish the bases for optimizing the design and construction of this equipment.
The operating conditions for the system evaluation were determined using various operating parameters, as shown in
Table 1.
3.1. System Operation
Different thermodynamic cycles were tested for the experimental evaluation of the STRS, graphically presenting the characteristic behavior of each process. The STRS operates intermittently, and two fundamental processes occur: (a) Generation–condensation–storage: This process was carried out during the day, harnessing the thermal energy from the evacuated tube solar collectors with heat pipes. The operating temperatures were between 55 and 66 °C for generation in the TR and between 20 and 30 °C for condensation, with a solar irradiance of 750 to 900 W/m2. (b) Evaporation–absorption: This process was carried out at night, reaching evaporation temperatures between −7 and −3 °C and absorption temperatures in the TR between 24 and 31 °C. Under these operating conditions, optimal cooling temperatures were achieved.
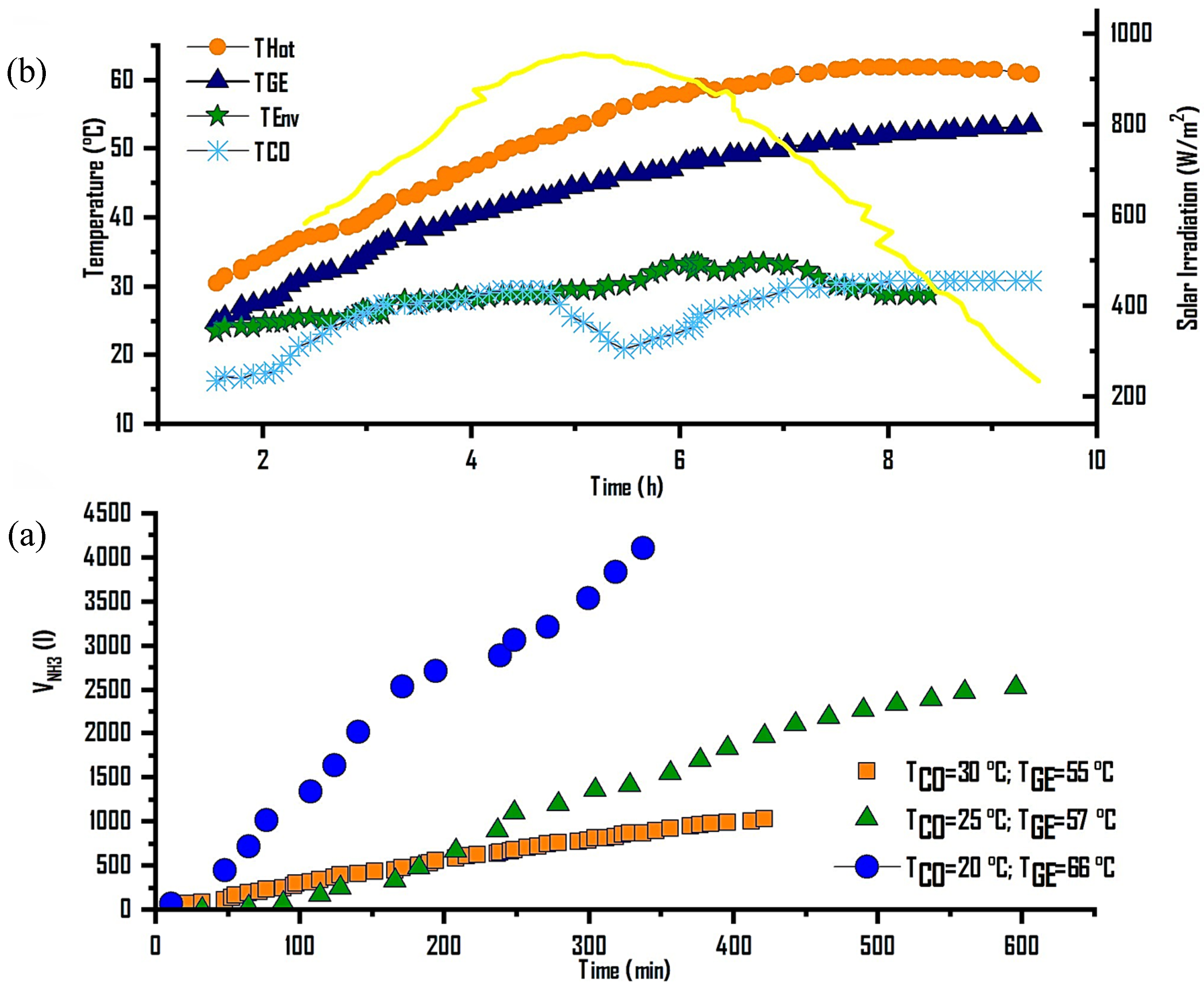
3.1.1. Desorption–Condensation–Storage
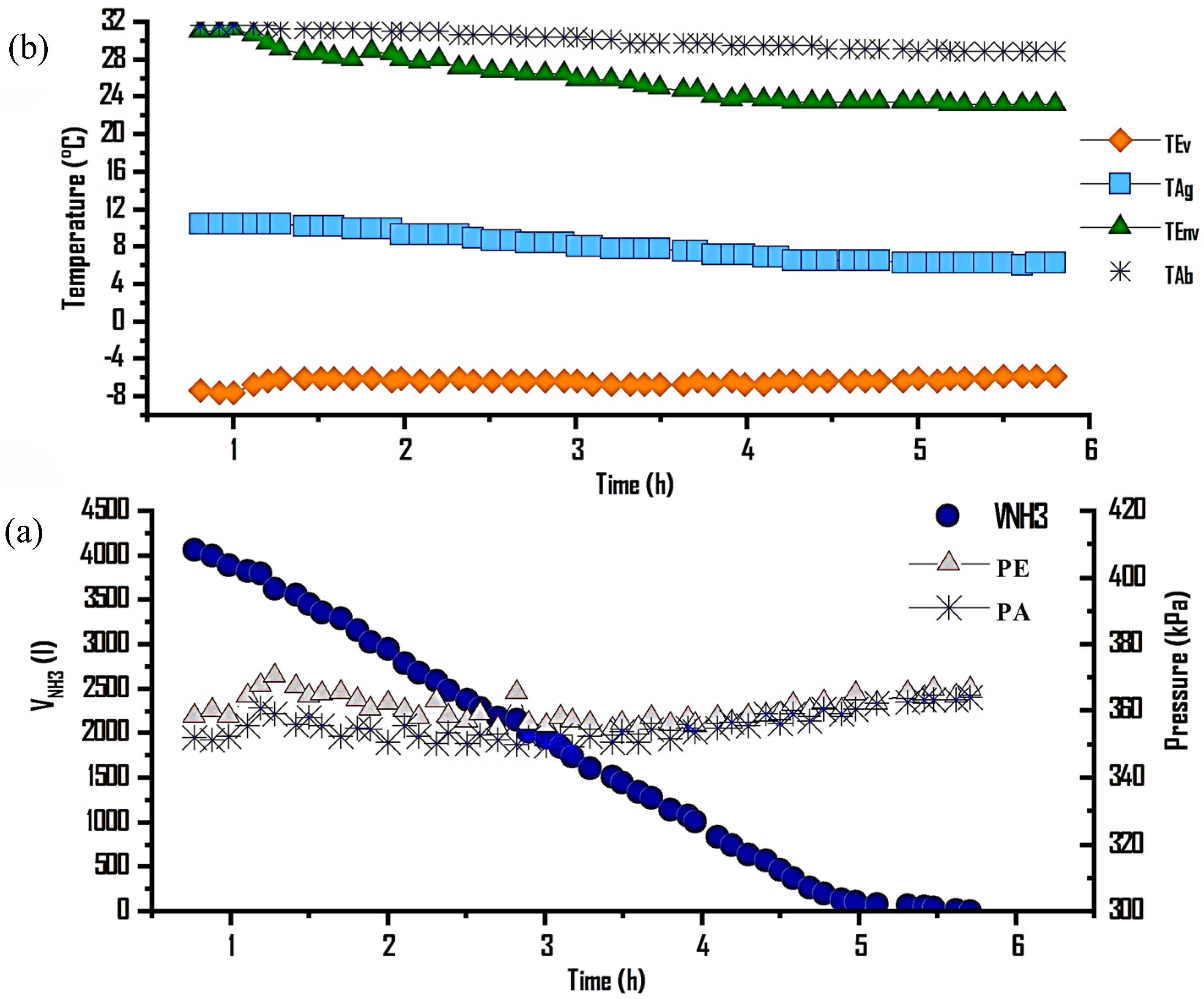
Figure 5a shows ammonia production at different generation/condensation temperatures. The first analysis was carried out at a condensation temperature (T
Co) of 30 °C, a generation temperature (T
Ge) of 55 °C, and an average irradiance of 850 W/m
2, achieving a production of 1000 mL of NH
3, equivalent to 15% of the generated refrigerant, in a 7 h operation period. In the second analysis, at T
Co = 25 °C and T
Ge = 57 °C, with an average irradiance of 750 W/m
2, 2800 mL of NH
3 were produced, representing 42% of the desorbed gas in 10 h, with a T
Hot between 30 and 66 °C. Finally, for the last analysis of this thermochemical cycle, at T
Co = 20 °C, T
Ge = 66 °C, and solar irradiance of 850 W/m
2, a production of 4200 mL of NH
3(l) was obtained, equivalent to 63% of desorbed gas in 6 h, with a T
Hot of 55 to 66 °C.
Figure 5b presents the characteristic generation/condensation process of the thermochemical cycle, showing the evolution of the heating temperatures in the solar collector field, desorption, and condensation as a function of the solar irradiance reached. This process was carried out during the day, from 8:00 a.m. to 5:00 p.m., with a heating and cooling water mass flow rate of 62 L/min in the reactor and 25 L/min in the condenser.
3.1.2. Evaporation–Absorption
This process analyzed the evaporation–absorption at evaporation temperatures ranging from −7 to −3 °C, showing the evolution of evaporation, absorption, cooling water, and ambient temperatures during this stage. Additionally, the volume of ammonia absorbed at constant pressure is presented to restart a new thermodynamic cycle. The evaporation/absorption process took place between 17:30 and 21:00 h. In
Figure 5a, the amount of NH
3 absorbed is shown, with average evaporation and absorption pressures of 358 and 355 kPa, respectively. A total of 4200 mL of ammonia was absorbed, with a water flow rate of 4 L/min in the evaporator and 25 L/min in the reactor, corresponding to an ammonia flow rate of 0.016 L/min, at ambient temperatures ranging from 25 to 32 °C.
Additionally, in
Figure 6b, the evolution of the evaporation temperature (T
Ev) is presented, which varied between −7 and −3 °C, maintaining a constant value of −7 °C. The absorption temperature (T
Ab) ranged from 25 to 32 °C, achieving a temperature difference (∆T) in chilled water production of 12 °C.
3.2. Determination of the Equilibrium Equations for the Compounds NH3(l)–NH3(v) and BaCl2(s)–NH3(v)
To determine the characteristic equilibrium equations for the compounds NH
3(l)–NH
3(v) and BaCl
2(s)–NH
3(v), a type A statistical analysis was performed on the experimental data obtained by evaluating the thermochemical solar refrigeration system and the representative equations of this working pair (1–17). A linear regression analysis, derived from the Clausius–Clapeyron equation, was used to establish the relationship between the equilibrium pressure (expressed as Ln P°) and the inverse temperature (−1000/T), describing each compound’s thermodynamic behavior. For this analysis, defining the uncertainties associated with each system was necessary. Experimental uncertainties, both for the independent and dependent variables, were estimated using a type A statistical analysis for the compounds NH
3(l)–NH
3(v) and BaCl
2(s)–NH
3(v). For each data point, error bars were included in the x (−1000/T) and y (Ln P°) directions, reflecting the experimental uncertainty, which was evaluated according to the linear regression model applied to each compound.
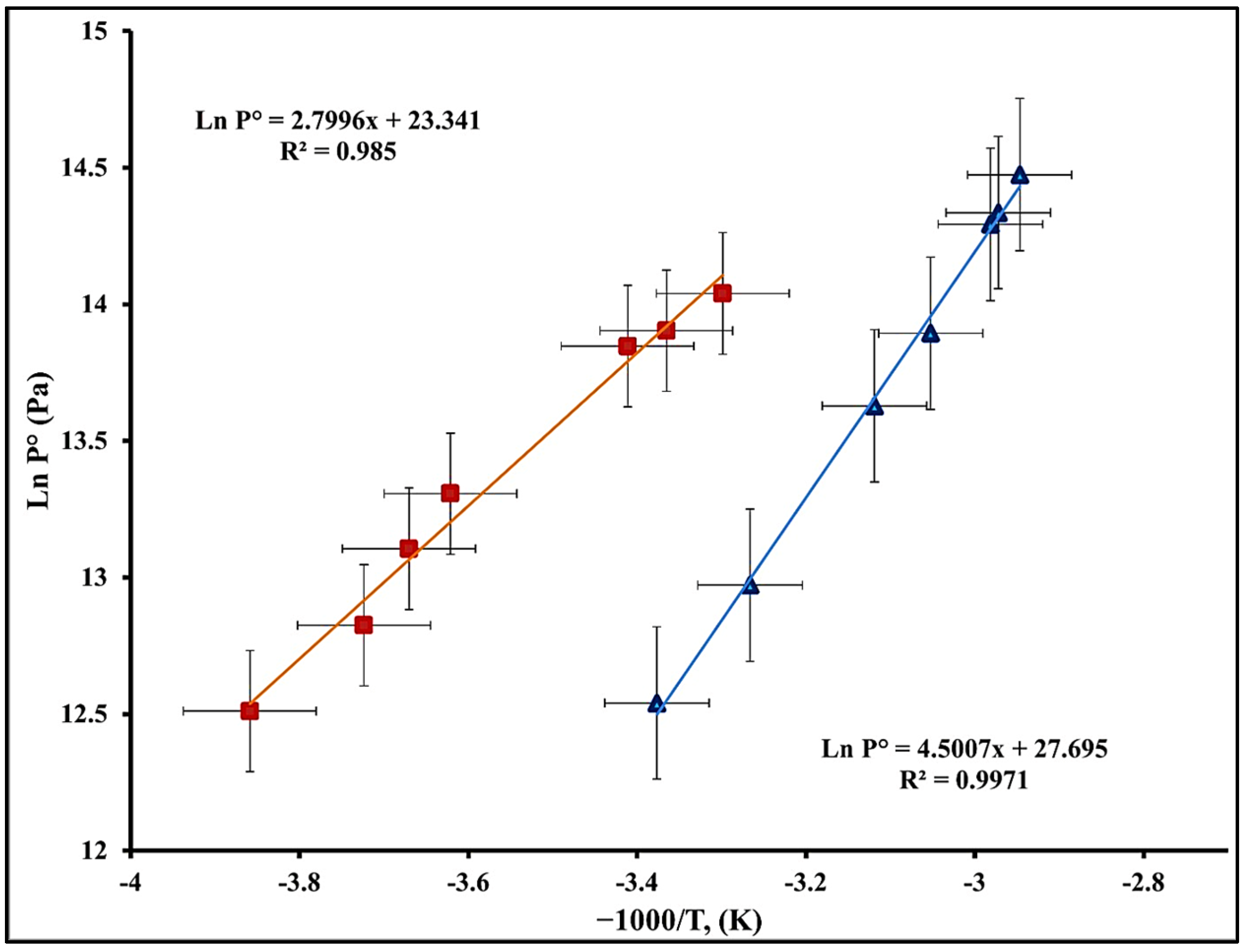
Figure 7, obtained from the experimental data, shows the thermodynamic behavior of the compounds.
Figure 7 shows the uncertainties associated with the variables (−1000/T) and (Ln P°), the coefficient of determination (R
2), and the respective equilibrium equations.
The statistical linear regression analysis results show thermodynamically consistent behavior for both compounds: liquid ammonia–vapor and barium chloride–ammonia. The equations shown accurately describe the dependence of the equilibrium pressure on temperature, demonstrating the linear trends observed in
Figure 6 for Ln P° versus (−1000/T).
For the NH3(l)–NH3(v) system, a slope of −2799.6 K was obtained, representing the enthalpy change associated with the vaporization process, with a coefficient of determination (R2 = 0.986). This indicates a significant relationship between the experimental data and the linear model obtained. However, this system exhibited relatively larger uncertainties on the −1000/T axes of ±0.079 and on the Ln P° axes of ±0.222, likely attributable to instrument sensitivity, thermal fluctuations during measurements, or the kinetics of liquid–vapor equilibrium.
However, the BaCl2(s)–NH3(v) compound exhibited a steeper slope (−4500.7 K) and a higher correlation coefficient (R2 = 0.997), indicating linear and predictable behavior. This steeper gradient corresponds to a higher ammonia desorption enthalpy relative to the solid compound, related to the interactions of the sorbent materials or solid chemical complexes.
While the uncertainty on the −1000/T axis was lower than that of the NH3(l)–NH3(v) compound at ±0.062, the uncertainty on the Ln P° axis was higher at ±0.279, perhaps related to hysteresis phenomena, complex stability, or a delay in reaching equilibrium.
These findings conclude that both compounds exhibit predictable responses to temperature variations but differ in terms of stability, sensitivity, and equilibrium complexity. These factors must be considered when applying these materials in practical applications, including thermal energy storage systems, absorption refrigeration, and solar refrigeration, where equilibrium pressure behavior is crucial for process efficiency.
The results demonstrate that both compounds behave according to the thermodynamically expected values. The barium chloride–ammonia system, characterized by a steeper slope in its equilibrium equation, exhibits a more energy-intensive desorption process than the vaporization of pure ammonia, an important distinction for absorption and thermal storage applications. The inclusion of uncertainties through type A analysis provides a quantitative measure of the reliability of the experimental data, reinforcing the validity of both equilibrium models presented through the following equations:
where P (Pa) and T (K).
3.2.1. Comparative Analysis of Temperature Versus Pressure Equilibrium Equations for the Compounds Barium Chloride–Ammonia
To perform a statistical and comparative analysis between the temperature–pressure characteristic equations of different authors, using the barium chloride–ammonia mixture as the working pair, it was necessary to reproduce the equations presented by the different authors and compare them with the equations proposed in this study, presented in
Table 2. Based on Equation (3), the following results are obtained.
Statistical Analysis of Barium Chloride–Ammonia Compounds by Different Authors
All models exhibit a coefficient of determination > 0.99, indicating that the Arrhenius-type relationship (logarithm of pressure vs. inverse of temperature) is reliable in describing the thermal dependence of pressure for both compounds. Also, the comparison of sorption/vaporization enthalpies (ΔH) is available. For the pure NH3 compound (liquid–vapor), all studies’ estimated vaporization enthalpy is around 23.1 kJ/mol. This value is consistent with the literature for ammonia.
On the other hand, the BaCl2–NH3 system’s sorption enthalpy is higher, around 37.42–45.96 kJ/mol. This is reliable because the interaction between barium chloride and ammonia is a chemical/physical sorption that requires more energy than just desorbing ammonia.
The constant of the independent term has a slight variation, ranging from ~23.0 to 23.4 for pure NH3, and 27.7 to 30.8 for BaCl2–NH3. This indicates stability in the pre-exponential term of the model. These models allow the equilibrium pressure to be predicted for temperatures within the experimental range (approximately −20 °C to 55 °C). This is essential for designing and optimizing refrigeration and absorption systems using BaCl2–NH3.
It is important to mention that all authors agree on a ΔH of NH3 evaporation around 23.2–23.4 kJ/mol. This validates the thermodynamic reliability of the compound NH3(l)–NH3(v).
And for BaCl2(s)–NH3(v), there is considerable variability in ΔH (37.4 to 46.0 kJ/mol), indicating possible differences in the solid phase, differences in measurement techniques, and different states and ammonia–metal bonds.
Finally, it should be noted that the higher enthalpy of BaCl
2–NH
3 means more energy is required to desorb ammonia (free it from the salt), an important factor in the cycle’s energy efficiency. As shown in this study, it reports lower ΔH values (37.42 kJ/mol), which means an improvement in the efficiency of the NH
3 thermal desorption process, which could be interpreted as better energy performance in practical applications (see
Table 2).
In addition,
Table 3 shows the power coefficients, the internal overall coefficient, and the overall efficiencies of the STRS operating at different temperatures, pressures, and flow rates. The experimental internal overall performance ranged between 0.244 and 0.307, while the overall efficiencies ranged from 0.146 to 0.206, using solar collector efficiencies of 0.6 to 0.67 to achieve evaporation temperatures suitable for ice production.
The experimental results validate the integration of STRS as a viable alternative for refrigeration applications in rural and fishing areas, where the availability of cold storage infrastructure is limited.
3.3. Novelty of the Research and Its Scientific, Technological, and Social Relevance
This study presents a substantial novelty in the design, characterization, and experimental validation of a solar thermochemical refrigeration system (STRS) based on the BaCl2–NH3 working pair using heat captured by vacuum solar collectors with heat pipes. Unlike previous work employing doped materials or additives (expanded graphite, vermiculite, Al2O3), this research demonstrated the technical feasibility of using industrial-grade barium chloride without additives, simplifying the synthesis process, reducing manufacturing costs, and avoiding unwanted chemical interactions. Furthermore, an experimental pressure–temperature equilibrium equation for the industrial-grade BaCl2–NH3 system was obtained, with type A statistical validation and a coefficient of determination R2 = 0.997, representing an original contribution to the thermodynamic modeling of thermochemical (solid–gas) systems applied to solar refrigeration. This equation allows for predicting the behavior of the reactive couple under real operating conditions and optimizing future thermochemical designs.
From a scientific perspective, this work expands our understanding of the thermal dynamics and overall efficiency of intermittent solar-powered thermochemical systems, providing experimental data that strengthens our comprehension of the BaCl
2–NH
3 desorption–absorption process under real irradiances (750–900 W/m
2). Technologically, the system achieves overall performance (COPₜ) values between 0.244 and 0.307 and overall efficiencies between 0.146 and 0.206, demonstrating that solar thermochemical refrigeration can compete with traditional absorption and adsorption technologies, but with greater simplicity, lower cost, and no direct electricity consumption. In social and environmental terms, the study is highly relevant for rural and coastal regions, where ice production and food preservation depend on intermittent energy sources. By eliminating the use of refrigerants with global warming potential (GWP ≈ 0) and operating with accessible materials, the proposal contributes to the transition towards sustainable refrigeration systems, in line with the Sustainable Development Goals (SDGs 7 and 13) [
46].
3.4. Comparative Assessment of BaCl2–NH3 Thermochemical Refrigeration Systems
Table 4 summarizes previous experimental and theoretical studies using the BaCl
2–NH
3 working pair for thermochemical or solar-powered refrigeration applications. The comparative analysis highlights the steady progress achieved over the last two decades in terms of performance, materials, and design simplicity.
Previous studies Dueñas et al., 2001 [
13] y Rivera et al., 2007 [
12] demonstrated the viability of BaCl
2–NH
3 systems at relatively low generation temperatures (50–60 °C), but provided limited efficiency data. Subsequent studies Le Pierrès et al., 2007 [
24]; 2008 [
23] y Stitou et al., 2012 [
22] required higher solar inlet temperatures (~70 °C) to achieve freezing or air-conditioned conditions, often using multi-stage configurations or absorbers composed of additives such as expanded graphite or vermiculite. These materials improved heat transfer but increased system complexity and cost.
In contrast, the present study achieves operation at temperatures of 55–66 °C using industrial-grade BaCl2 without additives, supported by evacuated tube collectors with heat pipes. This design allows for cooling at temperatures of −7 to −3 °C and an overall performance and efficiency of 0.24–0.31 and η = 14.6–20.6%, respectively, comparable to or better than values reported in the literature (COP = 0.05–0.40; η = 5–23%). The results demonstrate that high thermal efficiency can be achieved through geometric and thermal optimization, rather than chemical modification of the reactive medium.
Overall, the additive-free configuration represents a balance between performance, simplicity, and sustainability, offering a low-cost solution for ice production and refrigeration in rural areas with minimal environmental impact (GWP ≈ 0, ODP = 0). However, as with all intermittent systems, future research should focus on quasi-continuous operation and dynamic modelling to further improve scalability and energy utilization.
3.5. Discussion
The results obtained in the STRS confirm that the BaCl2–NH3 working pair can provide stable refrigeration cycles under real solar conditions without the need for conductivity-enhancing additives. The system’s performance indicators—ammonia desorption yield, coefficient of performance (COP), and overall efficiency (ηg)—demonstrate a consistent thermodynamic response to variations in solar radiation and ambient conditions.
3.5.1. Thermochemical Behavior and Energy Conversion Efficiency
The desorption–condensation data (
Figure 5) reveal that increasing the generation temperature from 55 °C to 66 °C while maintaining a condensation temperature close to 20 °C significantly increases ammonia production. The maximum desorption yield of 4.2 L (≈61%) at 66 °C confirms that low-enthalpy solar heat can efficiently drive the endothermic dissociation of the BaCl
2–NH
3 complex. This behavior is consistent with the equilibrium predicted from the Clausius–Clapeyron relation and demonstrates good thermal coupling between the evacuated tube collectors and the reactor.
The ammonia production curve shows a nearly linear response to increasing generation temperature, implying that the system operates close to its equilibrium limit and that heat transfer within the reaction bed is sufficiently effective. Despite using industrial-grade salt, no significant degradation in yield or limitations in mass transfer were observed—an important result considering the typically low thermal conductivity (≈0.15 W/m·K) of halide salts.
3.5.2. Cooling Performance and Absorption Dynamics
During the evaporation–absorption stage, the STRS achieved evaporation temperatures of −7 to −3 °C and absorption temperatures of 24 to 31 °C, generating a temperature difference of 12 °C in the chilled water circuit. This is sufficient for ice formation and cold storage. The absorption rate closely followed the desorption performance, confirming the high reversibility of the thermochemical reaction.
The internal COP ranged from 0.244 to 0.307, while the overall thermal efficiency varied from 14.6% to 20.6%, comparable to or higher than that of previous BaCl2–NH3 systems driven by flat-plate collectors (COP ≈ 0.05–0.40; η ≈ 5–23%). These figures highlight the role of vacuum tube collectors with heat pipes in maintaining stable generation temperatures and reducing heat losses. The high thermal efficiency achieved without composite additives demonstrates that geometric optimization and effective heat recovery can replace costly material modifications.
3.5.3. Thermodynamic Consistency and Equilibrium Analysis
The pressure–temperature equilibrium equations for NH3(l)–NH3(v) and BaCl2(s)–NH3(v) exhibit strong linearity (R2 = 0.986 and 0.997, respectively), confirming the reproducibility and consistency of the experimental data. The enthalpy of vaporization (ΔH ≈ 23.3 kJ mol−1) of pure ammonia is consistent with the existing literature, while the sorption enthalpy for BaCl2–NH3 (ΔH ≈ 37.4 kJ mol−1) indicates a moderate energy requirement for desorption, lower than that of most compound systems (40–46 kJ mol−1). This suggests that the industrial-grade salt exhibits favorable kinetics and a lower activation energy, possibly due to microstructural imperfections that enhance diffusion.
The lower desorption enthalpy directly contributes to higher system efficiency by reducing the thermal load during regeneration. This finding is crucial for the design of low-temperature solar reactors operating below 70 °C, eliminating the need for concentrating collectors.
3.5.4. Comparative and Practical Implications
The benchmarking (
Table 4) demonstrates that this system achieves performance equivalent to that of multi-stage or additive-based configurations, but with simpler construction and lower cost. Previous studies required higher solar inlet temperatures (≈70 °C) to reach freezing conditions, while the current prototype operates effectively between 55 and 66 °C. The simplicity of the additive-free BaCl
2–NH
3 mixture also minimizes environmental impact and improves system recyclability, in line with the principles of sustainable technology.
Furthermore, the successful operation under Mexican climatic conditions confirms the adaptability of STRS technology for rural and coastal regions where conventional refrigeration is limited by access to electricity.
According to the analysis, the industrial-grade BaCl2–NH3 pair balances performance, simplicity, and sustainability.
The high correlation coefficients, moderate desorption enthalpy, and repeatable operation throughout the cycles demonstrate the thermochemical process’s robustness and the STRS design’s practical feasibility.
These results provide a solid foundation for future optimization studies, including quasi-continuous systems.
4. Conclusions
Experimental research on a single-stage solar thermochemical cooling system (STRS), utilizing the BaCl2–NH3 working pair, demonstrated technical feasibility, achieving stable operation at low generation temperatures without using additives to enhance thermal conductivity. The system successfully integrated evacuated tube solar collectors with heat pipes, enabling operation at generation temperatures of 55–66 °C and evaporation temperatures between −7 and −3 °C, suitable for ice production and food preservation in rural areas with limited electricity access.
The STRS achieved a coefficient of performance (COP) ranging from 0.244 to 0.307 and an overall thermal efficiency of 0.146 to 0.206 under solar irradiance levels of 750–900 W/m2. An ammonia desorption rate of 61% was attained at 66 °C, confirming high thermochemical reversibility and efficient solar energy conversion. A key thermodynamic contribution of this work is the development of an empirical pressure–temperature equilibrium equation for industrial-grade BaCl2–NH3 (R2 = 0.997), which enhances predictive modeling and system optimization. Unlike previous studies that relied on doped or composite absorbents, the proposed design achieves comparable or superior performance while offering advantages in manufacturability, cost, and environmental compatibility—featuring a global warming potential (GWP) of approximately zero and zero ozone depletion potential (ODP). This study demonstrates that additive-free BaCl2–NH3 systems represent a cost-effective and sustainable solution for refrigeration and ice production in rural areas. Furthermore, the approach supports Sustainable Development Goals (SDGs) 7 and 13 by advancing clean and affordable energy technologies.
Future research efforts should focus on several complementary lines of work. The priority is the design of quasi-continuous or hybrid cycles to overcome the intermittency of solar energy and ensure stable operation. This development must be accompanied by thermogeometric optimization to improve heat and mass transfer, thereby increasing the efficiency of the thermochemical reaction. Likewise, it is crucial to implement artificial intelligence models, using neural networks and machine learning, to enable advanced system prediction and optimization. Finally, it will be essential to conduct a detailed techno-economic analysis and a life cycle assessment to quantify this sustainable technology’s long-term economic and environmental benefits.