Vapor Pressure Measurement of 2-Pentenenitrile and Vapor–Liquid Equilibrium for Its Mixtures with 2-Methyl-3-Butenenitrile and 2-Methyl-2-Butenenitrile
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
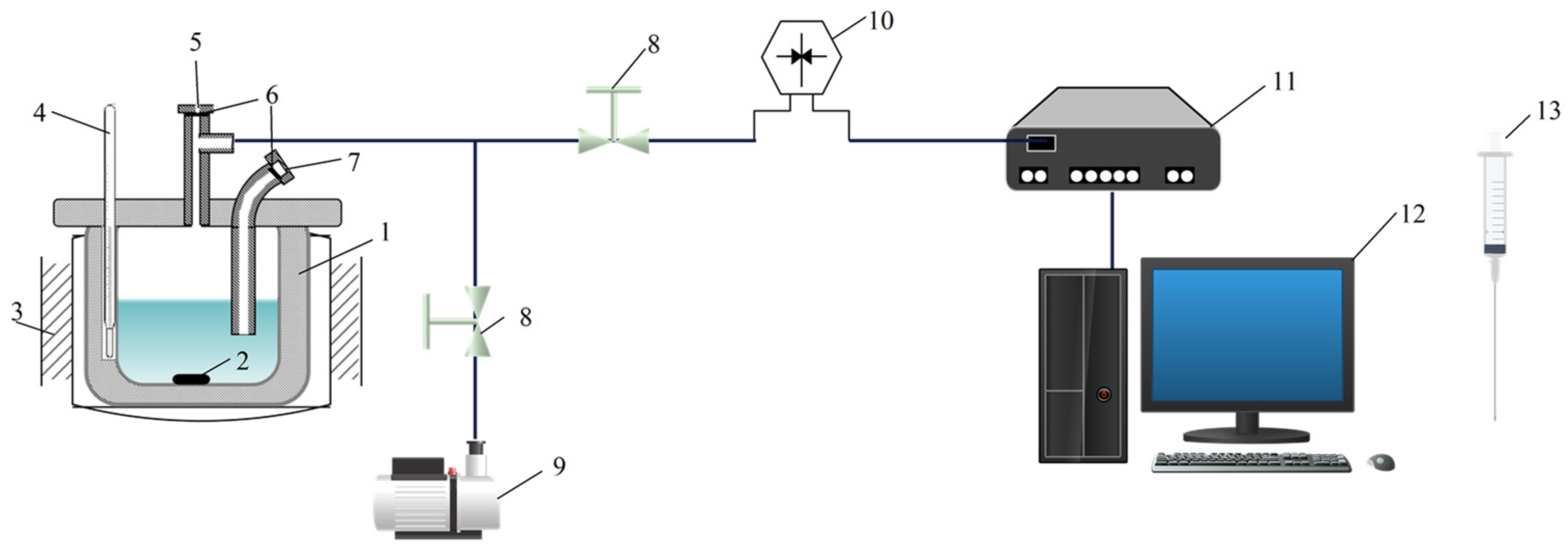
2.2. Apparatus and Procedure
2.3. Analysis
3. Results and Discussion
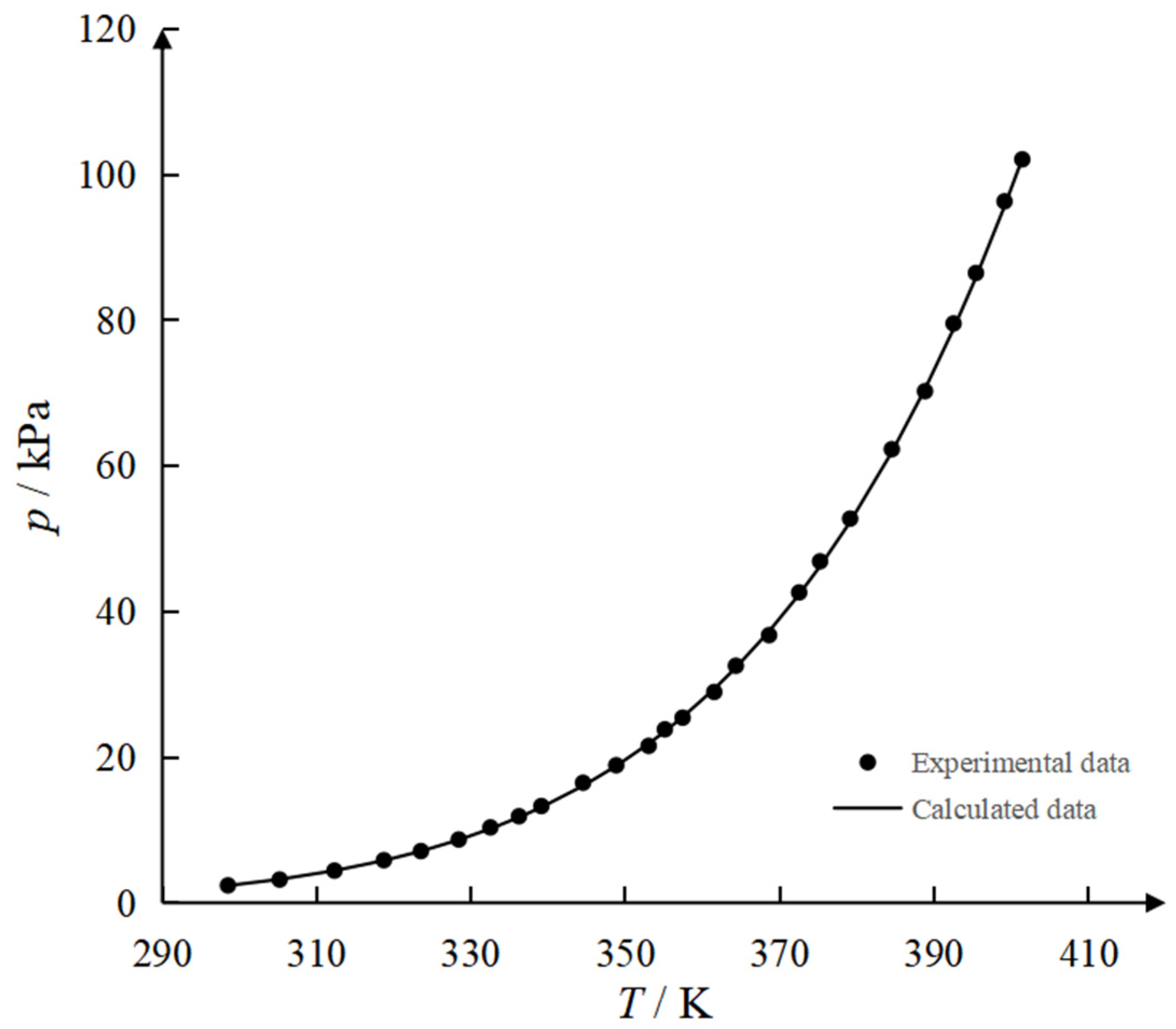
3.1. Pure Component Vapor Pressures
3.2. Isobaric Binary Systems
3.3. Thermodynamic Consistency Test
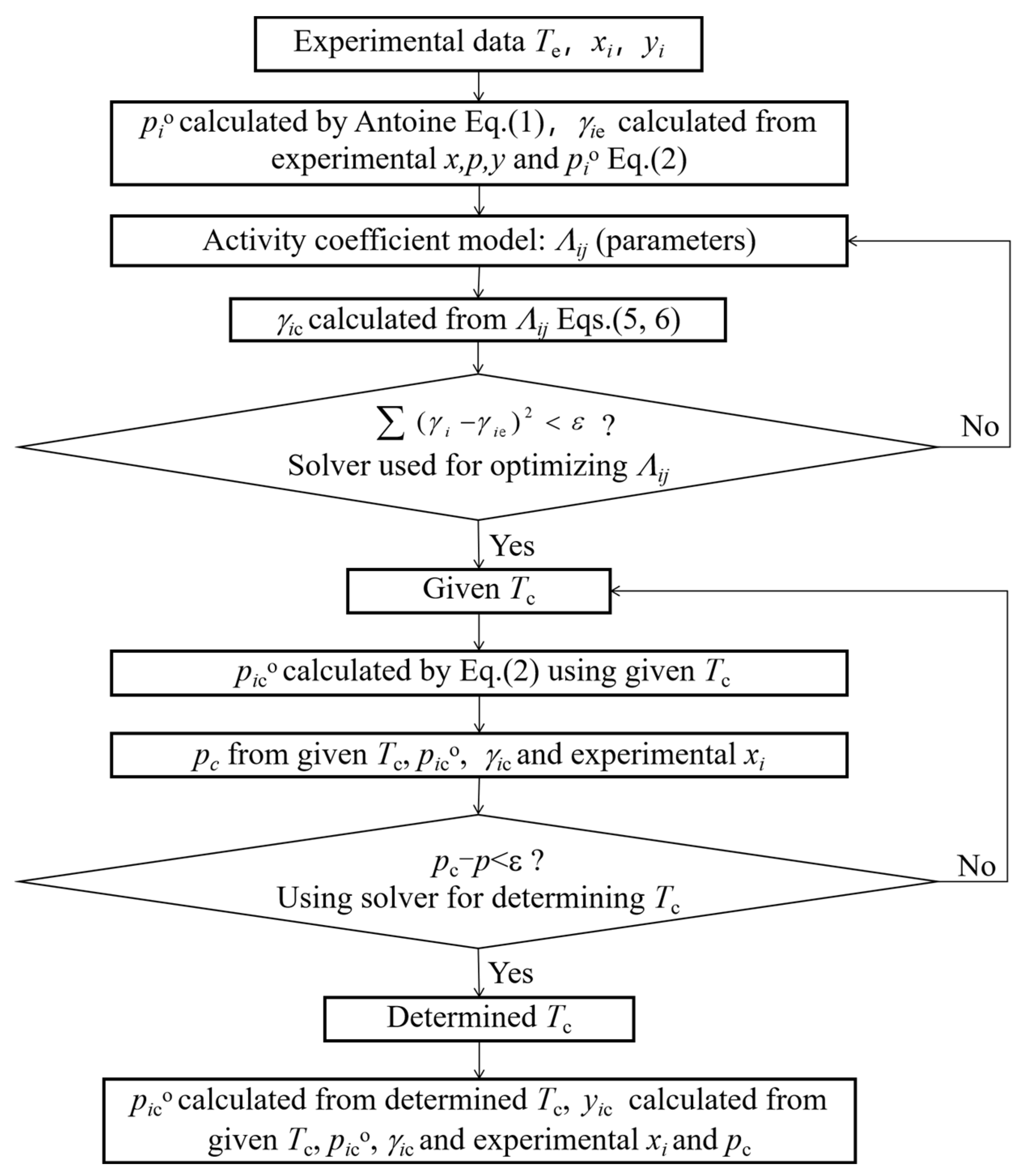
3.4. Correlation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| p | Pressure |
| A, B, C | Antoine coefficients |
| po | Saturated vapor pressure |
| T | Temperature |
| x, y | Mole fraction |
| g | Binary interaction parameter NRTL |
| n | Number of components |
| RMS(T) | Root-mean-square deviation of temperature |
| RMS(p) | Root-mean-square deviation of pressure |
| RMS(y) | Root-mean-square deviation of mole fraction in vapor phase |
| N | Number of data points |
| γ | Activity coefficient |
| α | Non-randomness parameter of NRTL model |
| Λ | Binary interaction parameter of Wilson model |
| i | Component i |
| j | Component j |
| m | Number of experimental data |
| ie | Experimental for ith component |
| ic | Calculated for ith component |
References
- Mathison, R.; Rani, E.; Patel, M.K.; Cerrato, A.L.; Bloomquist, C.K.; Modestino, M.A. Accelerated analysis of the electrochemical production route for biomass-derived adiponitrile. Chem. Catal. 2024, 4, 100998. [Google Scholar] [CrossRef]
- Su, J.S.; Huang, S.C.; Tsai, M.C.; Yen, C.H.; Lin, C.Y. Efficient and selective electrosynthesis of adiponitrile by electrohydrodimerization of acrylonitrile over a bismuth nanosheet modified electrode. Green Chem. 2024, 26, 8220–8229. [Google Scholar] [CrossRef]
- Guo, T.; Yan, F.Y.; Wang, Y.F.; Xu, X.F.; Jia, Q.Z.; Xu, B.H. Boosted nitrilation of dimethyl adipate with NH3 to adiponitrile over bimetallic oxide: Synergetic effect between Nb and W. Chem. Eng. Sci. 2023, 281, 119121. [Google Scholar] [CrossRef]
- Tessonnier, J.P. When bio- and electrocatalysis meet: A leap forward in the sustainable production of adiponitrile. Chem. Catal. 2024, 4, 101008. [Google Scholar] [CrossRef]
- Lv, Y.; Cui, H.; Liu, P.; Hao, F.; Xiong, W.; Luo, H. Functionalized multi-walled carbon nanotubes supported Ni-based catalysts for adiponitrile selective hydrogenation to 6-aminohexanenitrile and 1,6-hexanediamine: Switching selectivity with [Bmim]OH. J. Catal. 2019, 372, 330–351. [Google Scholar] [CrossRef]
- Bini, L.; Houben, E.J.E.; Pidko, E.A.; Müller, C.; Vogt, D. Nickel-catalyzed isomerization of 2-methyl-3-butenenitrile to 3-pentenenitrile: A kinetic study using in situ FTIR-ATR spectroscopy. Catal. Today 2010, 155, 271–278. [Google Scholar] [CrossRef]
- Liu, K.; Xin, H.; Han, M. Elucidation of key factors in nickel-diphosphines catalyzed isomerization of 2-methyl-3-butenenitrile. J. Catal. 2019, 377, 13–19. [Google Scholar] [CrossRef]
- Liu, K.; Wang, T.; Han, M. Rational design of efficient steric catalyst for isomerization of 2-methyl-3-butenenitrile. Mol. Catal. 2020, 498, 111259. [Google Scholar] [CrossRef]
- Thiyagarajan, S.; Diskin-Posner, Y.; Montag, M.; Milstein, D. Manganese-catalyzed base-free addition of saturated nitriles to unsaturated nitriles by template catalysis. Chem. Sci. 2024, 15, 2571–2577. [Google Scholar] [CrossRef] [PubMed]
- Horijan, B.; Kundu, A.; Nebapure, S.M.; Mandal, A.; Patanjali, N.; Mukhopadhyay, A.; Yadava, D.K.; Singh, A. Bioactive volatiles of Allium sativum and Brassica juncea for management of Tribolium castaneum and Corcyra cephalonica: Comprehensive in-silico and in-vitro analysis. J. Stored Prod. Res. 2025, 114, 102736. [Google Scholar] [CrossRef]
- Li, T.; Jones, W.D. DFT Calculations of the Isomerization of 2-Methyl-3-butenenitrile by [Ni(bisphosphine)] in Relation to the DuPont Adiponitrile Process. Organometallics 2011, 30, 547–555. [Google Scholar] [CrossRef]
- Hann, E.C.; Sigmund, A.E.; Fager, S.K.; Cooling, F.B.; Gavagan, J.E.; Bramucci, M.G.; Chauhan, S.; Payne, M.S.; DiCosimo, R. Regioselective biocatalytic hydrolysis of (E,Z)-2-methyl-2-butenenitrile for production of (E)-2-methyl-2-butenoic acid. Tetrahedron 2004, 60, 577–581. [Google Scholar] [CrossRef]
- Musser, M.T.; Streitwieser, A., Jr. Selective Removal of 2-Pentenenitrile and 2-Methyl-2-Butenenetrile from 3-Pentenenitrile. U.S. Patent 3865865, 11 February 1975. [Google Scholar]
- Cao, Y.; Dai, X.; Song, H.; Yao, S.; Lan, X. Vapour pressures and isobaric vapour-liquid equilibria for binary mixtures of (ZE-2-methyl-2-butenenitrile and 2-methyl-3-butenenitrile. J. Chem. Eng. Data 2016, 61, 1573–1577. [Google Scholar] [CrossRef]
- Zanghelini, G.; Athès, V.; Esteban-Decloux, M.; Glampaoli, P.; Vitu, S. Isobaric vapour-liquid equilibrium of α-terpineol highly diluted in hydroalcoholic mixtures at 101.3 kPa: Experimental measurements and thermodynamic modeling. J. Chem. Thermodyn. 2022, 171, 106806. [Google Scholar] [CrossRef]
- Khairutdinova, V.F.; Khabrieva, I.S.; Akhmetzyanova, T.R.; Yarullina, L.Y.; Gabitova, F.R.; Polishukb, I.; Abdulagatov, I.M. Experimental study and modeling of the isothermal VLE properties of ethylbenzene in supercritical solvents (CO2 and C3H8). J. Supercrit. Fluids 2023, 203, 106060. [Google Scholar] [CrossRef]
- Moghimia, M.; Roosta, A.; Hekayati, J.; Rezaei, N. Estimating VLE behavior from SLE data in aqueous mixtures of choline chloride-sorbitol deep eutectic solvents: Experimental investigation and thermodynamic modeling using the e-NRTL model. J. Mol. Liq. 2023, 371, 121126. [Google Scholar] [CrossRef]
- Arnautovic, Z.; Weith, T.; Heberle, F.; Brüggemann, D. Isobaric vapor-liquid equilibrium for ethanol/water and binary linear siloxane mixtures at 100 kPa. Fluid Phase Equilib. 2022, 556, 113371. [Google Scholar] [CrossRef]
- Henao, J.D.; Velásquez, J.A.; Cardona, L.F.; Forero, L.A. Modeling and experimental data of LLE, VLE, kinematic Viscosity, and density for the 2-Phenylethanol+n-Heptane mixture at low pressure. J. Chem. Thermodyn. 2025, 205, 107459. [Google Scholar] [CrossRef]
- Ganesan, J.; van der Ham, A.G.J.; Brilman, D.W.F. Experimental validation of kinetics and VLE of carbon di-oxide absorption in aqueous MEA at deep removal conditions. Chem. Eng. Sci. 2026, 320, 122487. [Google Scholar] [CrossRef]
- Khabriev, I.S.; Khairutdinov, V.F.; Akhmetzyanov, T.R.; Radifovich, G.I.; Polishuk, I.; Abdulagatov, I.M. Partial molar and microstructural properties of binary propane + o-toluidine system near the critical point of pure solvent based on the VLE measurements and modeling with CP-PC-SAFT and mg-SAFT equation of states. J. Chem. Thermodyn. 2025, 201, 107395. [Google Scholar] [CrossRef]
- Liao, Y.; Dai, Y.; Xu, C. Experimental investigation on vapor-liquid equilibrium for 1,1-difluoroethane(R152a) +trans-1,3,3,3-tetrafluoropropene (R1234ze (E)) binary systems. J. Chem. Thermodyn. 2022, 175, 106899. [Google Scholar] [CrossRef]
- Peng, S.; Li, S.; Yang, Z.; Duan, Y. Vapor-liquid equilibrium measurements for the binary mixtures of pentafluoroethane (R125) with 2,3,3,3-Tetrafluoroprop-1-ene (R1234yf) and 3,3,3-Trifluoropropene (R1243zf). Int. J. Refrig. 2022, 134, 115–125. [Google Scholar] [CrossRef]
- Nwokoye, C.; Nath, D.; Abdi, M.; Khalifi, M.; Hassanzadeh, H. Vapor-liquid equilibria (VLE) of the binary mixture of normal hexane and water at P = 2.5 MPa and T = (456.85–487.85 K). Fluid Phase Equilib. 2023, 572, 113837. [Google Scholar] [CrossRef]
- Turkman, S.; Nath, D.; Abdi, M.; Hassanzadeh, H. Vapor–liquid equilibria (VLE), density, and viscosity of the ternary mixtures of ethane, water, and bitumen at T = 190–210 °C and P = 2.5 MPa—Measurements and CPA-EoS modeling. Fluid Phase Equilib. 2025, 590, 114285. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, Z.; Lu, C.; Wang, X. Vapor-liquid equilibrium measurements of 3,3,3-trifluoropropene with pentaerythritol tetraheptanoate and pentaerythritol tetranonanoate. J. Chem. Thermodyn. 2022, 174, 106874. [Google Scholar] [CrossRef]
- Sharma, A.; Lee, B.S.; Shin, H.Y. Assessing suitability of glycerol-derived green solvent for the separation of n-hexane + ethanol azeotropic mixture, accompanied by VLE studies using machine learning. J. Mol. Liq. 2025, 423, 127003. [Google Scholar] [CrossRef]
- Guo, J.; Hu, B.; Li, Z.; Zheng, Y.; Zhou, C.; Li, Q. Vapor-liquid equilibrium experiment and extractive distillation process design for the azeotrope ethyl propionate n-propanol using ionic liquid. J. Mol. Liq. 2022, 350, 118492. [Google Scholar] [CrossRef]
- International Association for the Properties of Water and Steam. Revised Release on the IAPWS Industrial Formulation 1997 for the Thermodynamic Properties of Water and Steam; IAPWS: Lucerne, Switzerland, 2007. [Google Scholar]
- Wang, D.C.; Yao, S.; Cao, Y.; Yao, T.; Song, H. Vapor pressures and isobaric (vapor + liquid) equilibrium data for the binary system of (RS-4-vinyl-1-cyclohexene + ZE-3-pentenenitrile) at (50.0 and 100.0) kPa. J. Chem. Thermodyn. 2016, 92, 55–59. [Google Scholar] [CrossRef]
- Wang, C.; Li, H.; Zhu, L.; Han, S. Isothermal and isobaric vapor + liquid equilibria of N,N-dimethylformamide + n-propanol + n-butanol. Fluid Phase Equilib. 2001, 189, 119–127. [Google Scholar] [CrossRef]
- Wisniak, J.; Ortega, J.; Fernández, L. A fresh look at the thermodynamic consistency of vapour-liquid equilibria data. J. Chem. Thermodyn. 2017, 105, 385–395. [Google Scholar] [CrossRef]
- Fredenslund, A.; Gmehling, J.; Rasmussen, P. Vapour-Liquid Equilibria Using UNIFAC a Group-Contribution Method, 1st ed.; Elsevier: Amsterdam, The Netherlands, 1977. [Google Scholar]
- Van Ness, H.C. Thermodynamics in the treatment of capor/liquid equilibrium (VLE) data. Pure Appl. Chem. 1995, 67, 859–872. [Google Scholar] [CrossRef]
- Wilson, G.M. Vapor-liquid equilibria XI. A new expression for the excess free energy of mixing. J. Am. Chem. Soc. 1964, 86, 127–130. [Google Scholar] [CrossRef]
- Renon, H.; Prausnitz, J.M. Local compositions in thermodynamics for liquid mixtures. AIChE J. 1968, 14, 135–144. [Google Scholar] [CrossRef]

 , calculated data on mole fraction by NRTL model.
, calculated data on mole fraction by NRTL model.
 , calculated data on mole fraction by NRTL model.
, calculated data on mole fraction by NRTL model.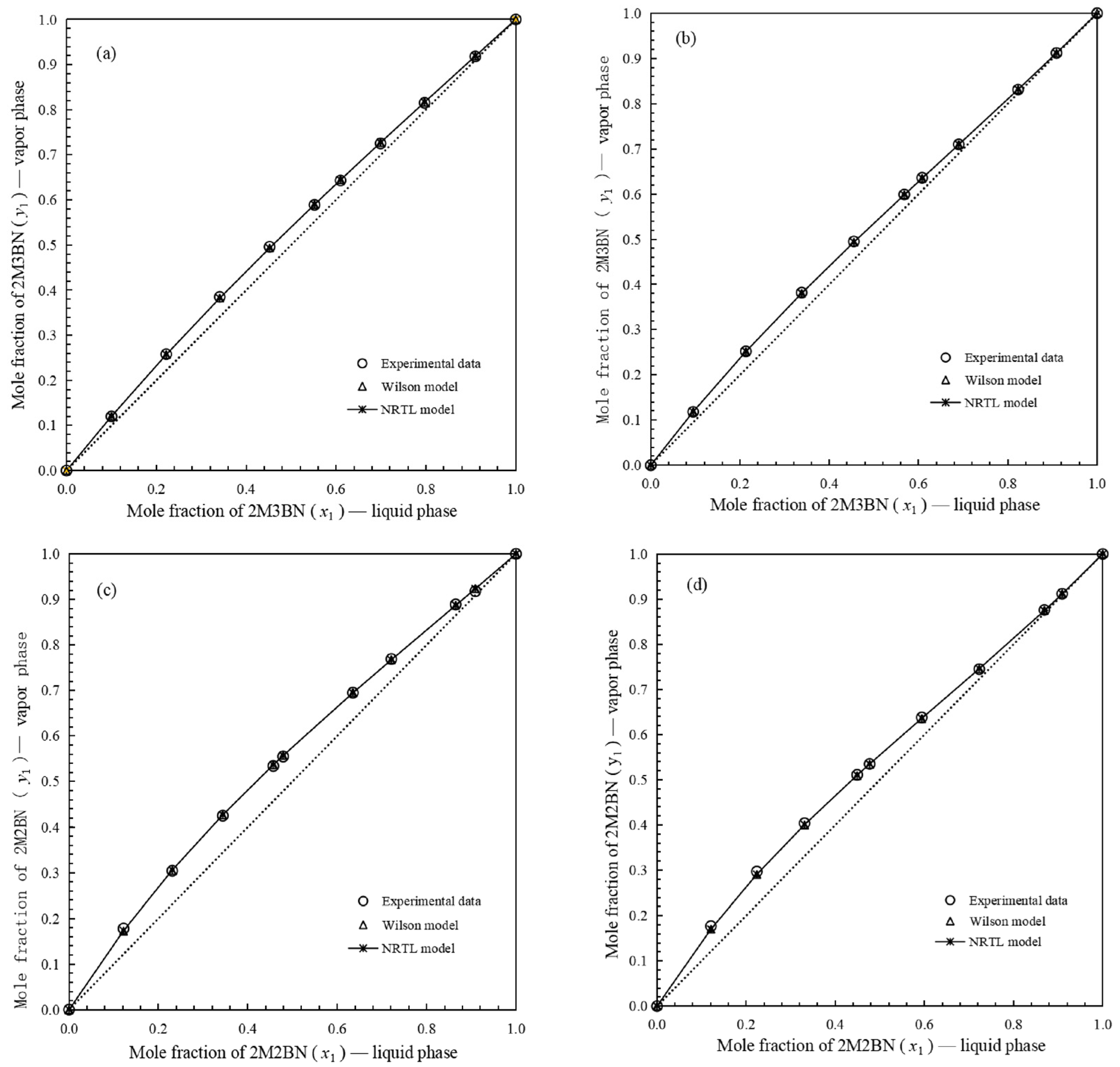

| Compound | CAS No. | Initial Mass Fraction Purity a | Purification Method | Final Mass Fraction Purity a |
|---|---|---|---|---|
| 2M3BN | 16529-56-9 | 0.9163 | Distillation | 0.9937 |
| 2M2BN | 4403-61-6 | 0.7686 | Distillation | 0.9873 |
| 2PN | 13284-42-9 | 0.9167 | Distillation | 0.9905 |
| T/K | pe/kPa | pc/kPa | Deviation/kPa | T/K | pe/kPa | pc/kPa | Deviation/kPa |
|---|---|---|---|---|---|---|---|
| 298.5 | 2.395 | 2.374 | 0.021 | 357.4 | 25.422 | 25.503 | 0.081 |
| 305.2 | 3.197 | 3.228 | −0.031 | 361.5 | 28.956 | 29.372 | 0.416 |
| 312.3 | 4.424 | 4.420 | 0.004 | 364.3 | 32.555 | 32.297 | −0.258 |
| 318.7 | 5.857 | 5.809 | 0.048 | 368.6 | 36.755 | 37.278 | 0.523 |
| 323.5 | 7.107 | 7.090 | 0.017 | 372.5 | 42.621 | 42.355 | −0.266 |
| 328.4 | 8.697 | 8.646 | 0.051 | 375.2 | 46.887 | 46.210 | −0.677 |
| 332.5 | 10.357 | 10.170 | 0.187 | 379.1 | 52.754 | 52.308 | −0.446 |
| 336.2 | 11.890 | 11.742 | 0.148 | 384.5 | 62.286 | 61.886 | −0.400 |
| 339.1 | 13.290 | 13.118 | 0.172 | 388.8 | 70.253 | 70.552 | 0.299 |
| 344.5 | 16.490 | 16.059 | 0.431 | 392.5 | 79.551 | 78.822 | −0.729 |
| 348.8 | 18.890 | 18.795 | 0.095 | 395.4 | 86.484 | 85.869 | −0.615 |
| 353.0 | 21.556 | 21.850 | −0.294 | 399.1 | 96.320 | 95.637 | −0.683 |
| 355.1 | 23.823 | 23.533 | 0.290 | 401.4 | 102.083 | 102.175 | 0.092 |
| Compound | A | B | C | Relative Deviation/% | ||
|---|---|---|---|---|---|---|
| Ave. | Max. | Min. | ||||
| 2PN | 18.158 | 6400.096 | 71.584 | 0.92 | 2.66 | 0.09 |
| 2M3BN a | 16.690 | 5136.422 | 29.330 | 0.91 | 2.51 | 0.02 |
| 2M2BN a | 13.329 | 2945.380 | −55.112 | 1.96 | 6.76 | 0.06 |
| p/kPa | T/K | x1 | y1 | g1 | g2 | △T b/K | △y1 b | △T c/K | △y1 c |
|---|---|---|---|---|---|---|---|---|---|
| 50.0 | 377.1 | 0.100 | 0.120 | 1.0432 | 0.9957 | 0.22 | −0.0002 | 0.22 | −0.0002 |
| 376.3 | 0.222 | 0.258 | 1.0358 | 0.9963 | 0.22 | 0.0010 | 0.22 | 0.0010 | |
| 375.6 | 0.341 | 0.385 | 1.0285 | 0.9969 | 0.23 | 0.0028 | 0.23 | 0.0028 | |
| 375.0 | 0.452 | 0.496 | 1.0186 | 1.0016 | 0.22 | 0.0027 | 0.22 | 0.0028 | |
| 374.5 | 0.552 | 0.589 | 1.0062 | 1.0152 | 0.21 | −0.0007 | 0.21 | −0.0007 | |
| 374.2 | 0.61 | 0.643 | 1.0034 | 1.0228 | 0.17 | −0.0014 | 0.17 | −0.0014 | |
| 373.8 | 0.699 | 0.725 | 0.9999 | 1.0341 | 0.15 | −0.0020 | 0.15 | −0.0020 | |
| 373.4 | 0.797 | 0.815 | 0.9984 | 1.0449 | 0.13 | −0.0015 | 0.13 | −0.0015 | |
| 373.0 | 0.91 | 0.918 | 0.9975 | 1.0583 | 0.13 | −0.0007 | 0.13 | −0.0008 | |
| 100.0 | 399.8 | 0.095 | 0.118 | 1.1067 | 0.9987 | 0.13 | −0.0004 | 0.13 | −0.0004 |
| 398.7 | 0.213 | 0.252 | 1.0870 | 1.0054 | 0.03 | 0.0005 | 0.03 | 0.0005 | |
| 397.7 | 0.338 | 0.382 | 1.0680 | 1.0165 | −0.11 | 0.0016 | −0.11 | 0.0016 | |
| 397.0 | 0.455 | 0.495 | 1.0486 | 1.0298 | −0.15 | 0.0016 | −0.15 | 0.0016 | |
| 396.5 | 0.568 | 0.599 | 1.0309 | 1.0467 | −0.14 | 0.0006 | −0.14 | 0.0007 | |
| 396.4 | 0.608 | 0.636 | 1.0255 | 1.0502 | −0.08 | 0.0011 | −0.08 | 0.0011 | |
| 396.2 | 0.690 | 0.710 | 1.0145 | 1.0642 | −0.01 | 0.0004 | −0.01 | 0.0005 | |
| 396.0 | 0.823 | 0.831 | 1.0012 | 1.0925 | 0.12 | −0.0003 | 0.12 | −0.0003 | |
| 395.9 | 0.909 | 0.912 | 0.9976 | 1.1098 | 0.16 | 0.0001 | 0.15 | 0.0001 |
| p/kPa | T/K | x1 | y1 | g1 | g2 | △T b/K | △y1 b | △T c/K | △y1 c |
|---|---|---|---|---|---|---|---|---|---|
| 50.0 | 375.9 | 0.122 | 0.178 | 1.1531 | 0.9906 | 0.21 | 0.0055 | 0.25 | 0.0046 |
| 374.6 | 0.231 | 0.304 | 1.0797 | 0.9984 | 0.39 | −0.0009 | 0.44 | −0.0016 | |
| 373.2 | 0.344 | 0.425 | 1.0556 | 1.0115 | 0.32 | −0.0016 | 0.38 | −0.0018 | |
| 372.0 | 0.457 | 0.534 | 1.0340 | 1.0297 | 0.29 | −0.0027 | 0.34 | −0.0025 | |
| 371.8 | 0.479 | 0.555 | 1.0313 | 1.0315 | 0.30 | −0.0022 | 0.35 | −0.0019 | |
| 370.6 | 0.635 | 0.695 | 1.0093 | 1.0495 | 0.44 | −0.0001 | 0.47 | 0.0005 | |
| 370.0 | 0.721 | 0.769 | 1.0012 | 1.0605 | 0.48 | 0.0013 | 0.50 | 0.0019 | |
| 369.0 | 0.865 | 0.889 | 0.9939 | 1.0884 | 0.43 | 0.0019 | 0.44 | 0.0022 | |
| 368.7 | 0.909 | 0.918 | 0.9855 | 1.2046 | 0.39 | −0.0056 | 0.40 | −0.0054 | |
| 100.0 | 398.6 | 0.121 | 0.177 | 1.2604 | 0.9933 | 0.18 | 0.0058 | 0.11 | 0.0068 |
| 397.4 | 0.224 | 0.297 | 1.1773 | 0.9951 | 0.41 | 0.0062 | 0.35 | 0.0062 | |
| 396.3 | 0.331 | 0.404 | 1.1143 | 1.0105 | 0.49 | 0.0045 | 0.45 | 0.0034 | |
| 395.3 | 0.449 | 0.511 | 1.0657 | 1.0366 | 0.52 | 0.0026 | 0.50 | 0.0009 | |
| 395.1 | 0.477 | 0.535 | 1.0556 | 1.0446 | 0.52 | 0.0018 | 0.50 | 0.0001 | |
| 394.3 | 0.594 | 0.638 | 1.0317 | 1.0725 | 0.46 | 0.0035 | 0.48 | 0.0021 | |
| 393.5 | 0.723 | 0.745 | 1.0103 | 1.1338 | 0.26 | −0.0002 | 0.29 | −0.0008 | |
| 392.9 | 0.869 | 0.876 | 1.0038 | 1.1867 | 0.07 | 0.0011 | 0.11 | 0.0015 | |
| 392.8 | 0.909 | 0.912 | 1.0017 | 1.2160 | 0.04 | 0.0001 | 0.06 | 0.0005 |
| Binary System | Pressure | Point Test of Fredenslund et al. | RMS δln(γ1/γ2) c | Consistency Index | |
|---|---|---|---|---|---|
| Dy1 < 0.01 a | Dp b | Direct Test of Van Ness | |||
| 2M3BN (1) + 2PN (2) | 50.0 kPa | 0.0009 | 0.0634 | 0.0084 | 1 |
| 100.0 kPa | 0.0011 | 0.2202 | 0.0039 | 1 | |
| 2M2BN (3) + 2PN (2) | 50.0 kPa | 0.0030 | 0.1116 | 0.0298 | 2 |
| 100.0 kPa | 0.0025 | 0.3999 | 0.0204 | 1 | |
| Binary System | Pressure | Equation Parameters | Deviations | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Wilson | NRTL | Wilson | NRTL | ||||||
| L12 | L21 | g12 − g11 /(J·mol−1) | g21 − g22 /(J·mol−1) | RMS(T)/K | RMS (y1) | RMS (T) T/K | RMS (y1) | ||
| 2M3BN (1) + 2PN (2) | 50.0 kPa | 0.9798 | 0.9608 | 0.0233 | 0.0369 | 0.20 | 0.0024 | 0.20 | 0.0024 |
| 100.0 kPa | 0.9404 | 0.9302 | 0.0605 | 0.0724 | 0.11 | 0.0013 | 0.11 | 0.0013 | |
| 2M2BN (3) + 2PN (2) | 50.0 kPa | 0.9590 | 0.8935 | 0.0645 | 0.0947 | 0.37 | 0.0043 | 0.40 | 0.0041 |
| 100.0 kPa | 0.7385 | 1.0305 | 0.0386 | 0.2169 | 0.38 | 0.0051 | 0.36 | 0.0048 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, Y.; Liu, F.; Jiang, Z.; Li, T.; Tian, H.; Song, H.; Yao, S. Vapor Pressure Measurement of 2-Pentenenitrile and Vapor–Liquid Equilibrium for Its Mixtures with 2-Methyl-3-Butenenitrile and 2-Methyl-2-Butenenitrile. Processes 2025, 13, 3588. https://doi.org/10.3390/pr13113588
Cao Y, Liu F, Jiang Z, Li T, Tian H, Song H, Yao S. Vapor Pressure Measurement of 2-Pentenenitrile and Vapor–Liquid Equilibrium for Its Mixtures with 2-Methyl-3-Butenenitrile and 2-Methyl-2-Butenenitrile. Processes. 2025; 13(11):3588. https://doi.org/10.3390/pr13113588
Chicago/Turabian StyleCao, Yu, Fanjing Liu, Zongting Jiang, Ting Li, Hui Tian, Hang Song, and Shun Yao. 2025. "Vapor Pressure Measurement of 2-Pentenenitrile and Vapor–Liquid Equilibrium for Its Mixtures with 2-Methyl-3-Butenenitrile and 2-Methyl-2-Butenenitrile" Processes 13, no. 11: 3588. https://doi.org/10.3390/pr13113588
APA StyleCao, Y., Liu, F., Jiang, Z., Li, T., Tian, H., Song, H., & Yao, S. (2025). Vapor Pressure Measurement of 2-Pentenenitrile and Vapor–Liquid Equilibrium for Its Mixtures with 2-Methyl-3-Butenenitrile and 2-Methyl-2-Butenenitrile. Processes, 13(11), 3588. https://doi.org/10.3390/pr13113588







