Optimisation of Microwave-Assisted Extraction of Phenolic Compounds from Pithecellobium dulce Fruit Peels: Comparative Process Modelling Using RSM and ANN with Bioactivity Evaluation
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Sample Preparation
2.3. Chemicals and Reagents
2.4. Microwave Assisted Extraction
2.5. Experimental Design
2.6. Response Surface Methodology
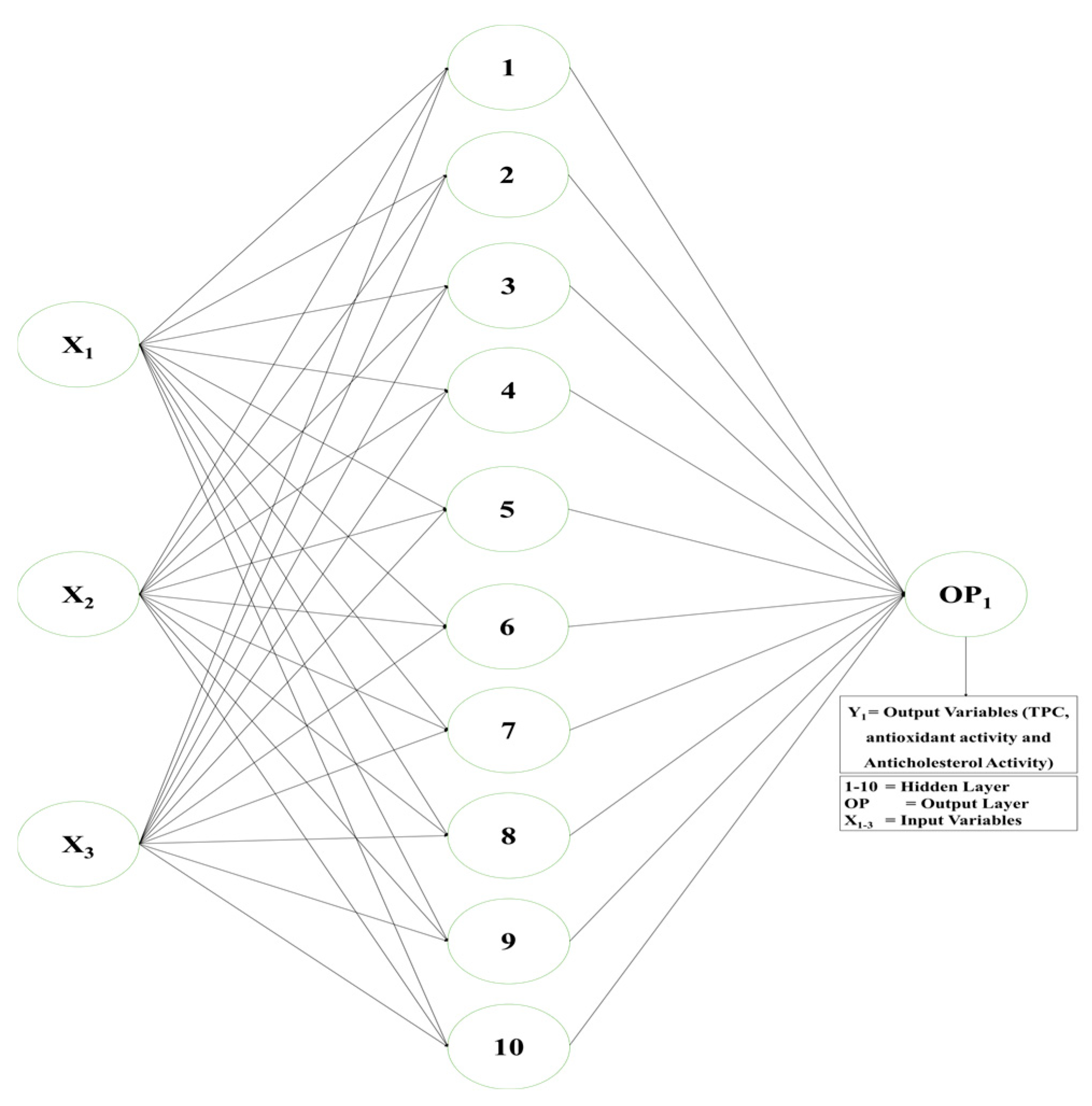
2.7. Artificial Neural Network (ANN) Modelling
2.8. Determination of Total Phenolic Contents
2.9. Determination of Antioxidant Activity
2.10. Determination of Anti-Cholesterol Activity
2.11. Statistical Analysis
3. Results and Discussion
3.1. Influence of the Various Process Parameters on MAE of Pithecellobium dulce
3.2. Model Fitting
3.3. Total Polyphenol Yield
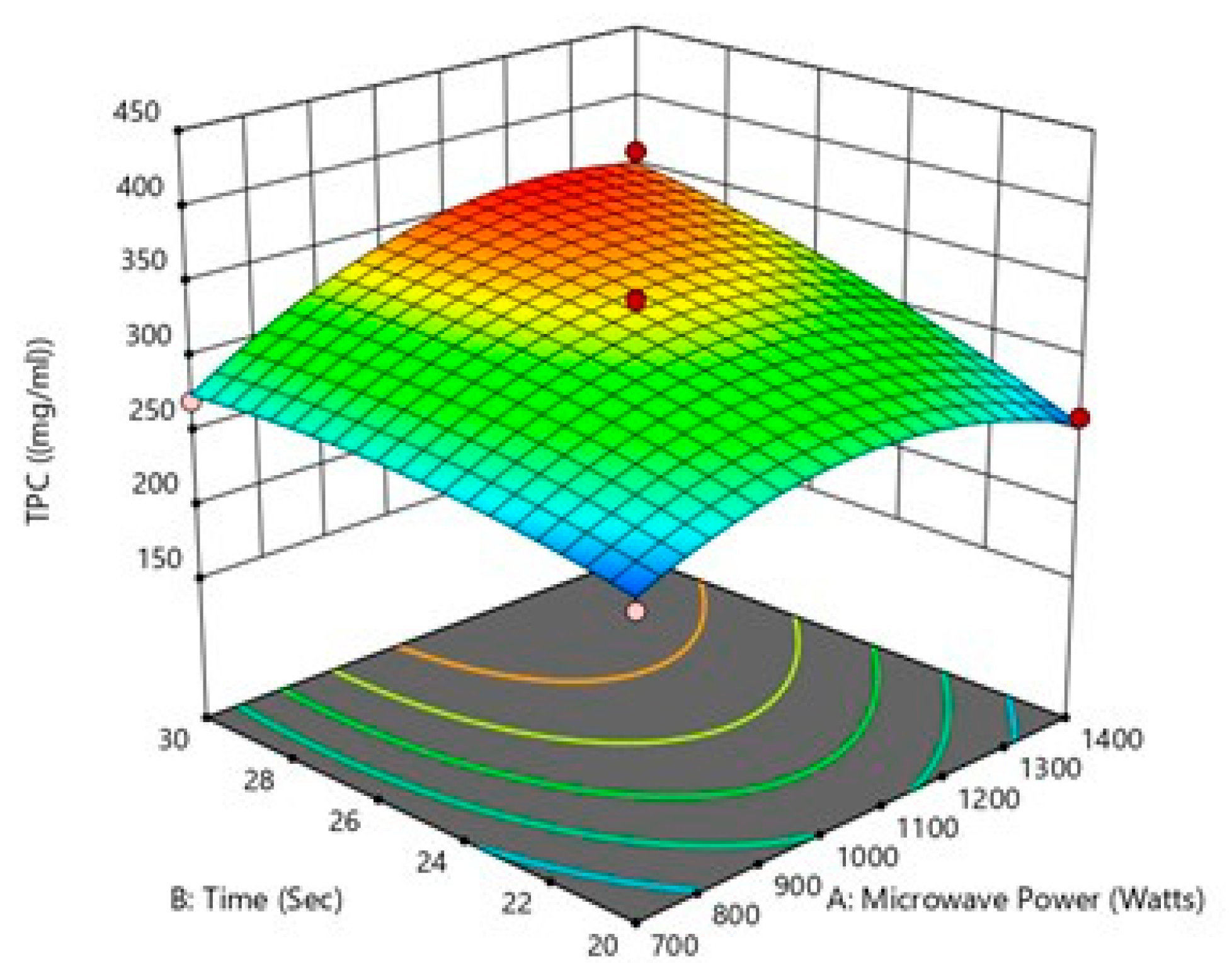
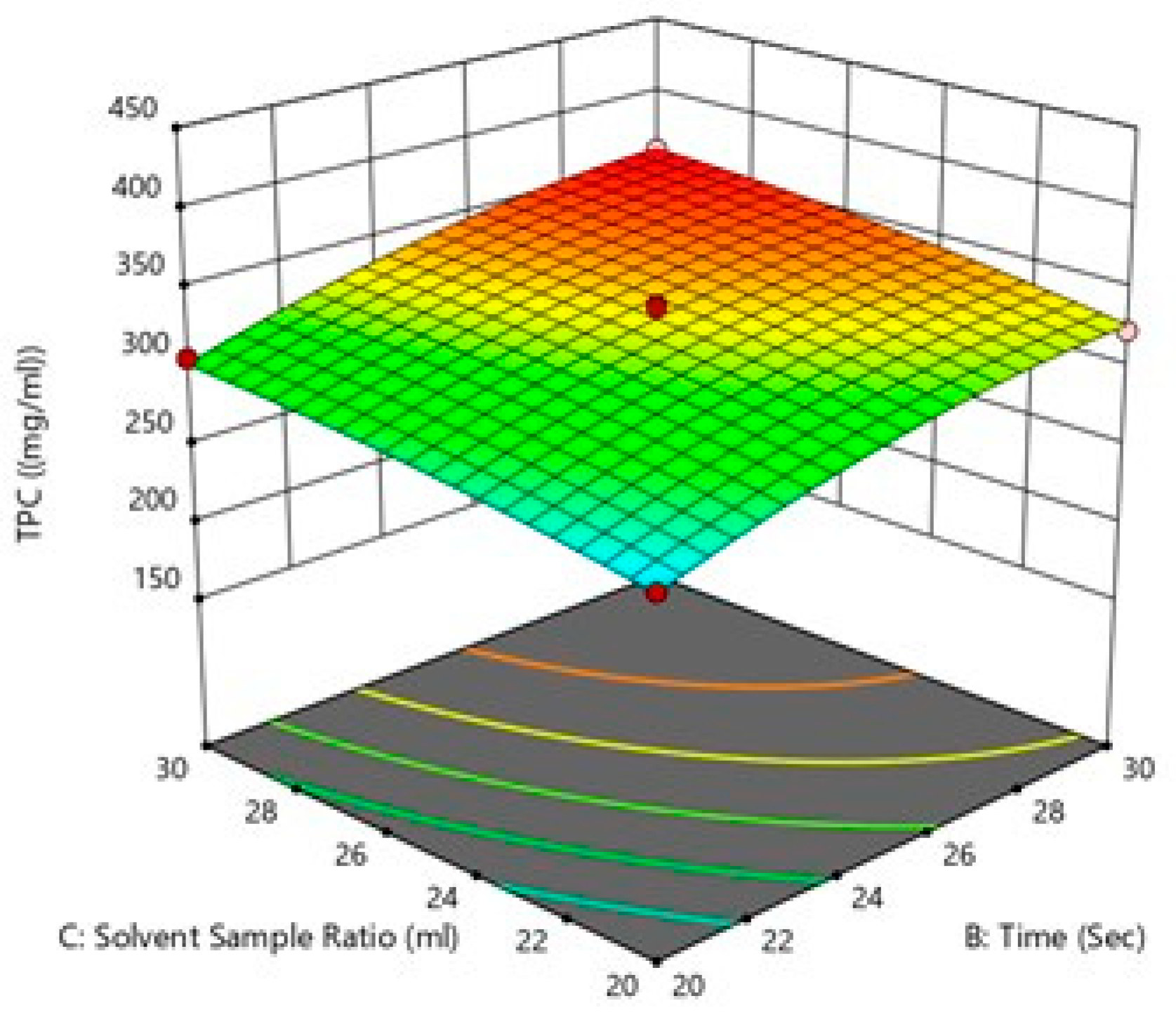
3.3.1. Response Surface Methodology
Influence of the Process Variables on Total Polyphenol Yield
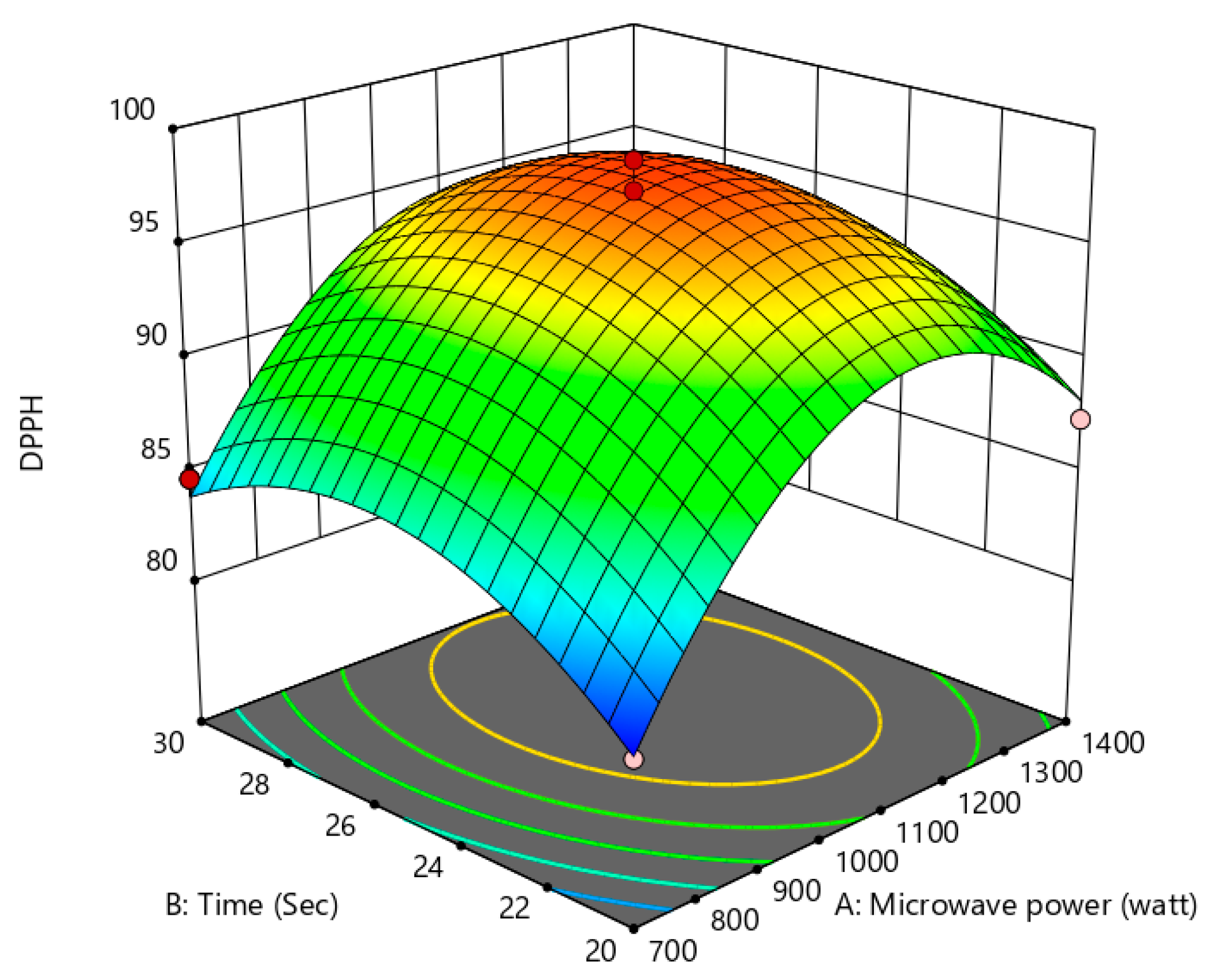
3.4. Antioxidant Activity
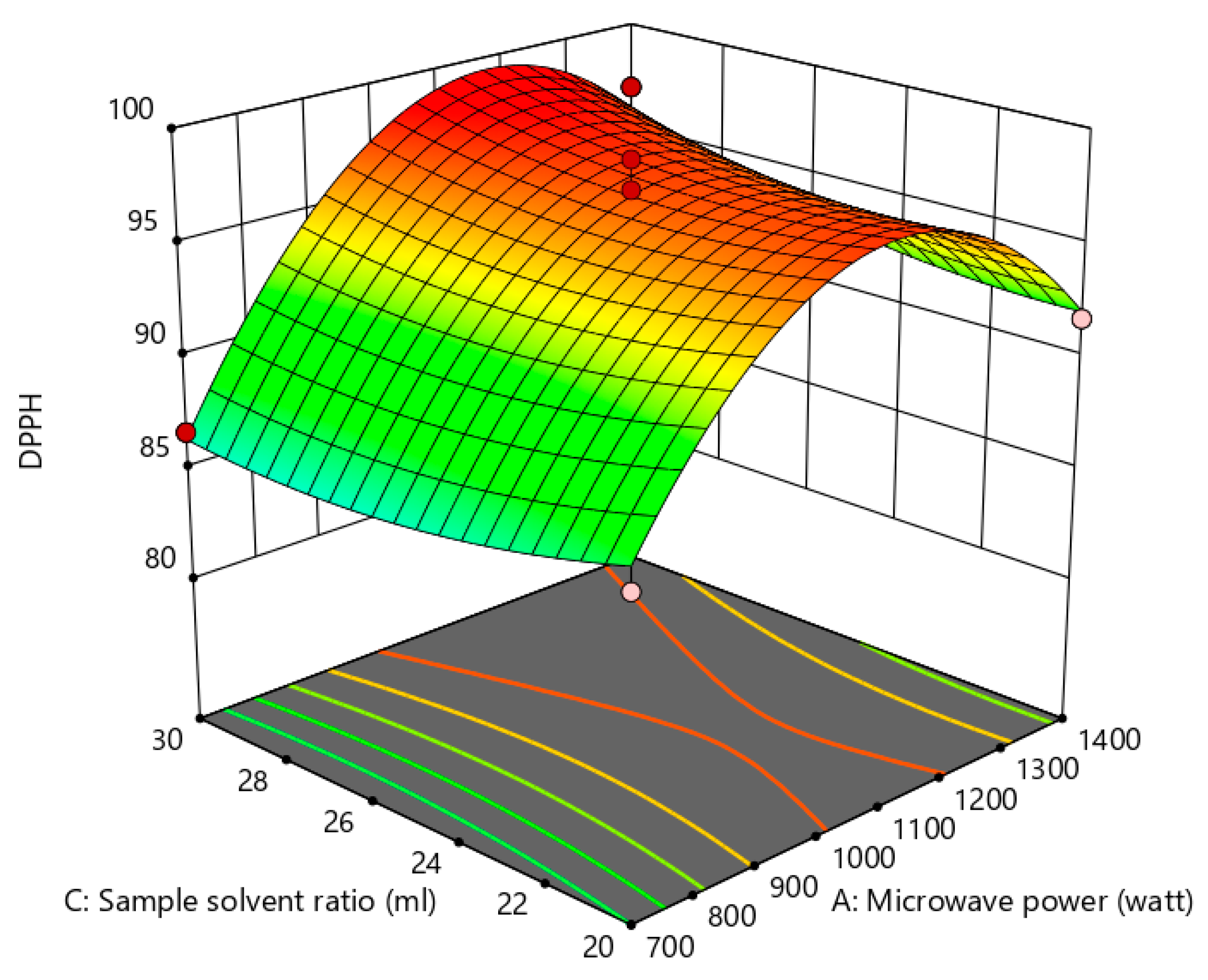
Influence of the Process Variables on Antioxidant Activity
3.5. Anti-Cholesterol Activity
Influence of the Process Variables on Anti-Cholesterol Activity
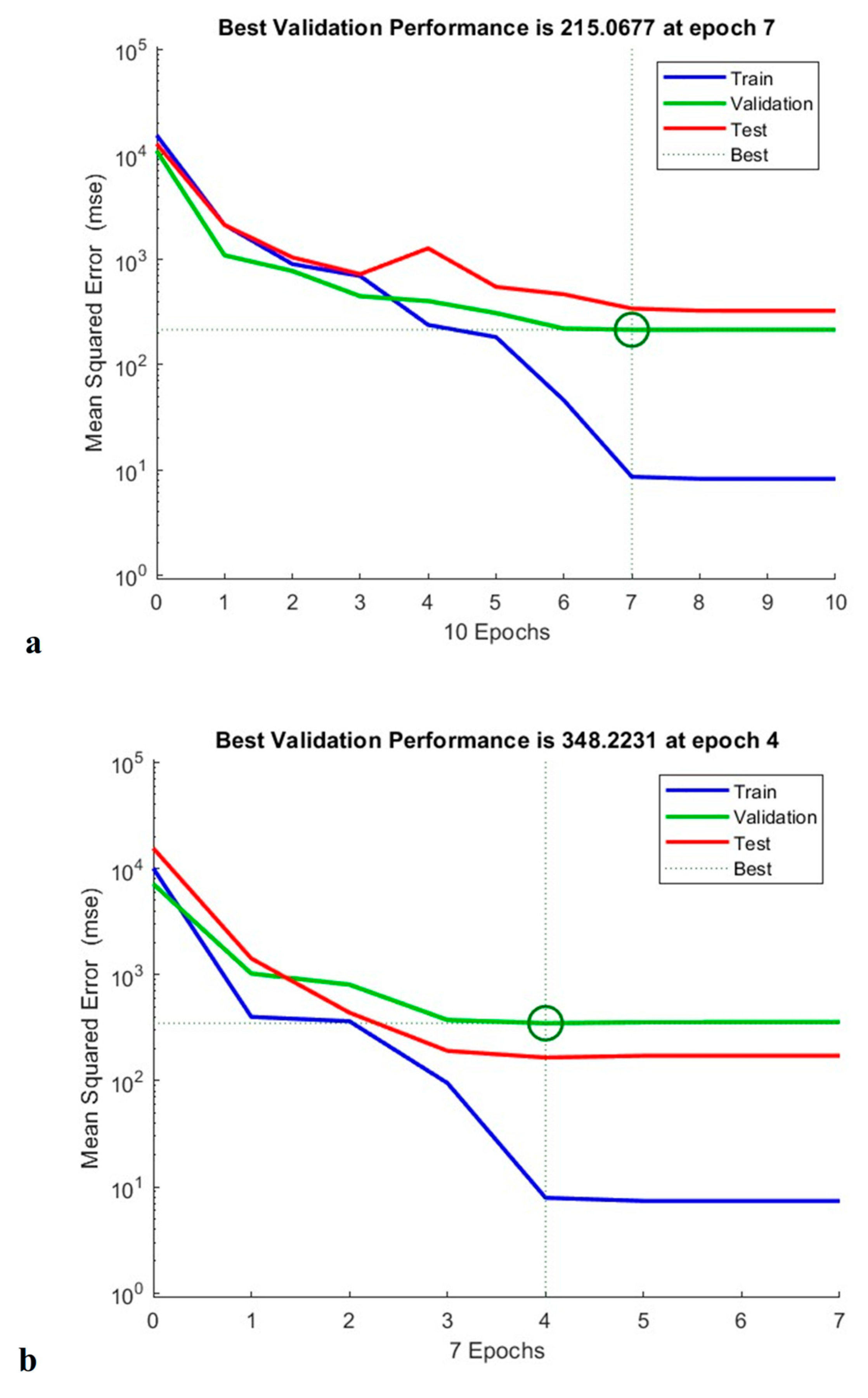
3.6. ANN Modelling
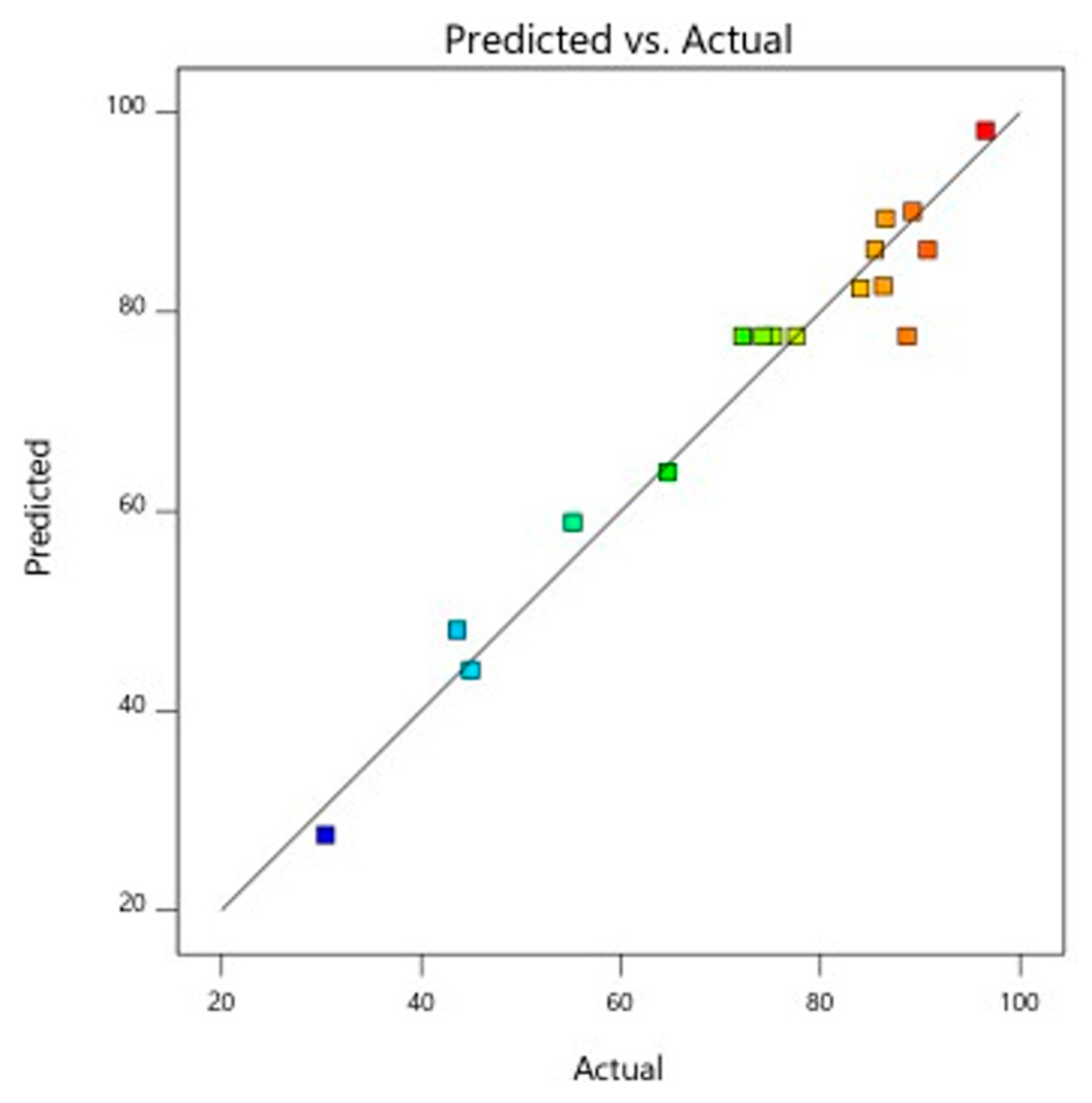
3.7. Comparison Between RSM and ANN Models
3.8. Optimisation of the Extraction Process
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Megala, J.; Geetha, A. Free radical-scavenging and H+, K+-ATPase inhibition activities of Pithecellobium dulce. Food Chem. 2010, 121, 1120–1128. [Google Scholar] [CrossRef]
- Tiwari, U.L.; Adhikari, B.S.; Rawat, G.S.; Chandola, S. Population Status of Commercially Important Medicinal Plants in Dehradun Forest Division, Uttarakhand (India). Not. Sci. Biol. 2013, 5, 175–182. [Google Scholar] [CrossRef]
- Murugesan, S.; Lakshmanan, D.K.; Arumugam, V.; Alexander, R.A. Nutritional and therapeutic benefits of medicinal plant Pithecellobium dulce (Fabaceae): A review. J. Appl. Pharm. Sci. 2019, 9, 130–139. [Google Scholar] [CrossRef]
- Kulkarni, K.V.; Jamakhandi, V.R. Medicinal uses of Pithecellobium dulce and its health benefits. J. Pharmacogn. Phytochem. 2018, 7, 700–704. [Google Scholar]
- Selvakumar, M.; Palanichamy, P.; Arumugam, V.; Venkatesan, M.; Aathmanathan, S.; Krishnamoorthy, H.; Pugazhendhi, A. In silico potential of nutraceutical plant of Pithecellobium dulce against GRP78 target protein for breast cancer. Appl. Nanosci. 2023, 13, 1737–1749. [Google Scholar] [CrossRef]
- Pal, P.B.; Pal, S.; Manna, P.; Sil, P.C. Traditional extract of Pithecellobium dulce fruits protects mice against CCl4 induced renal oxidative impairments and necrotic cell death. Pathophysiology 2012, 19, 101–114. [Google Scholar] [CrossRef]
- Adeniyi, A.; Asase, A.; Ekpe, P.K.; Asitoakor, B.K.; Adu-Gyamfi, A.; Avekor, P.Y. Ethnobotanical study of medicinal plants from Ghana; confirmation of ethnobotanical uses, and review of biological and toxicological studies on medicinal plants used in Apra Hills Sacred Grove. J. Herb. Med. 2018, 14, 76–87. [Google Scholar] [CrossRef]
- Alonso-Castro, A.J.; Domínguez, F.; Maldonado-Miranda, J.J.; Castillo-Pérez, L.J.; Carranza-Álvarez, C.; Solano, E.; Isiordia-Espinoza, M.A.; del Carmen Juárez-Vázquez, M.; Zapata-Morales, J.R.; Argueta-Fuertes, M.A.; et al. Use of medicinal plants by health professionals in Mexico. J. Ethnopharmacol. 2017, 198, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Orio, L.; Alexandru, L.; Cravotto, G.; Mantegna, S.; Barge, A. UAE, MAE, SFE-CO2 and classical methods for the extraction of Mitragynaspeciosa leaves. Ultrason. Sonochem. 2012, 19, 591–595. [Google Scholar] [CrossRef]
- Vinatoru, M.; Mason, T.J.; Calinescu, I. Ultrasonically assisted extraction (UAE) and microwave assisted extraction (MAE) of functional compounds from plant materials. TrAC Trends Anal. Chem. 2017, 97, 159–178. [Google Scholar] [CrossRef]
- Azwanida, N.N. A review on the extraction methods use in medicinal plants, principle, strength and limitation. Med. Aromat. Plants 2015, 4, 2167–2412. [Google Scholar]
- Sun-Waterhouse, D.; Wadhwa, S.S.; Waterhouse, G.I.N. Spray-drying microencapsulation of polyphenol bioactives: A comparative study using different natural fibre polymers as encapsulants. Food Bioprocess Technol. 2013, 6, 2376–2388. [Google Scholar] [CrossRef]
- Chan, C.-H.; Yusoff, R.; Ngoh, G.-C.; Kung, F.W.-L. Microwave-assisted extractions of active ingredients from plants. J. Chromatogr. A 2011, 1218, 6213–6225. [Google Scholar] [CrossRef]
- Murugesan, R.; Orsat, V. Spray drying for the production of nutraceutical ingredients—A review. Food Bioprocess Technol. 2012, 5, 3–14. [Google Scholar] [CrossRef]
- Kumar, M.; Nehra, K.; Duhan, J.S. Phytochemical analysis and antimicrobial efficacy of leaf extracts of Pithecellobium dulce. Asian J. Pharm. Clin. Res. 2013, 6, 70–76. [Google Scholar]
- da Rocha, C.B.; Noreña, C.P.Z. Microwave-assisted extraction and ultrasound-assisted extraction of bioactive compounds from grape pomace. Int. J. Food Eng. 2020, 16, 20190191. [Google Scholar] [CrossRef]
- Panchal, B.; Deshmukh, S.; Sharma, M. Optimization of oil extraction and characterization from Tamarindus indica Linn seed oil. Int. J. Oil Gas. Coal Eng. 2014, 2, 1–6. [Google Scholar] [CrossRef]
- Yolmeh, M.; Jafari, S.M. Applications of response surface methodology in the food industry processes. Food Bioprocess Technol. 2017, 10, 413–433. [Google Scholar] [CrossRef]
- Hunter, W.G.; Hunter, J.S. Statistics for Experimenters; Interscience: New York, NY, USA, 1978; Volume 453. [Google Scholar]
- Jaddu, S.; Abdullah, S.; Dwivedi, M.; Pradhan, R.C. Multipin cold plasma electric discharge on hydration properties of kodo millet flour: Modelling and optimization using response surface methodology and artificial neural network–Genetic algorithm. Food Chem. Mol. Sci. 2022, 5, 100132. [Google Scholar] [CrossRef]
- Patra, A.; Abdullah, S.; Pradhan, R.C. Application of artificial neural network-genetic algorithm and response surface methodology for optimization of ultrasound-assisted extraction of phenolic compounds from cashew apple bagasse. J. Food Process Eng. 2021, 44, e13828. [Google Scholar] [CrossRef]
- Fonteles, T.V.; Leite, A.K.; Silva, A.R.; Carneiro, A.P.; de Castro Miguel, E.; Cavada, B.S.; Fernandes, F.A.; Rodrigues, S. Ultrasound processing to enhance drying of cashew apple bagasse puree: Influence on antioxidant properties and in vitro bioaccessibility of bioactive compounds. Ultrason. Sonochem. 2016, 31, 237–249. [Google Scholar] [CrossRef]
- Hoskin, R.T.; Xiong, J.; Esposito, D.A.; Lila, M.A. Blueberry polyphenol-protein food ingredients: The impact of spray drying on the in vitro antioxidant activity, anti-inflammatory markers, glucose metabolism and fibroblast migration. Food Chem. 2019, 280, 187–194. [Google Scholar] [CrossRef]
- Swantini, D.; Nurenda, D.; Sugita, P. Fractionation and characterization of active compounds from Bangle (ZingibercassumunarRoxb.) as an activator of the enzyme cholesterol oxidase. In Simposium nasional Kimia BahanAlam XV.; Himpunan Kimia BahanAlam: Bogor, India, 2005. [Google Scholar]
- Vernès, L.; Vian, M.; Chemat, F. Ultrasound and microwave as green tools for solid-liquid extraction. In Liquid-Phase Extraction; Elsevier: Amsterdam, The Netherlands, 2020; pp. 355–374. [Google Scholar]
- Patra, A.; Abdullah, S.; Pradhan, R.C. Microwave-assisted extraction of bioactive compounds from cashew apple (Anacardium occidenatale L.) bagasse: Modeling and optimization of the process using response surface methodology. J. Food Meas. Charact. 2021, 15, 4781–4793. [Google Scholar] [CrossRef]
- Fang, Z.; Bhandari, B. Effect of spray drying and storage on the stability of bayberry polyphenols. Food Chem. 2011, 129, 1139–1147. [Google Scholar] [CrossRef] [PubMed]
- Preethi, S.; Saral, A.M. GC-MS analysis of microwave assisted ethanolic extract of Pithecellobium dulce. Malaya J. Biosci. 2014, 1, 242–247. [Google Scholar]
- Mestry, A.P.; Mujumdar, A.S.; Thorat, B.N. Optimization of spray drying of an innovative functional food: Fermented mixed juice of carrot and watermelon. Dry Technol. 2011, 29, 1121–1131. [Google Scholar] [CrossRef]
- Gupta, S.; Cox, S.; Abu-Ghannam, N. Effect of different drying temperatures on the moisture and phytochemical constituents of edible Irish brown seaweed. LWT-Food Sci. Technol. 2011, 44, 1266–1272. [Google Scholar] [CrossRef]
- Mishra, P.; Mishra, S.; Mahanta, C.L. Effect of maltodextrin concentration and inlet temperature during spray drying on physicochemical and antioxidant properties of amla (Emblica officinalis) juice powder. Food Bioprod. Process. 2014, 92, 252–258. [Google Scholar] [CrossRef]
- Alara, O.R.; Mudalip, S.K.A.; Olalere, O.A. Optimization of mangiferinextrated from Phaleriamacrocarpa fruits using response surface methodology. J. Appl. Res. Med. Aromat. Plants 2017, 5, 82–87. [Google Scholar]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Ciric, A.; Krajnc, B.; Heath, D.; Ogrinc, N. Response surface methodology and artificial neural network approach for the optimization of ultrasound-assisted extraction of polyphenols from garlic. Food Chem. Toxicol. 2020, 135, 110976. [Google Scholar] [CrossRef] [PubMed]
- Ameer, K.; Bae, S.-W.; Jo, Y.; Lee, H.-G.; Ameer, A.; Kwon, J.-H. Optimization of microwave-assisted extraction of total extract, stevioside and rebaudioside-A from Stevia rebaudiana (Bertoni) leaves, using response surface methodology (RSM) and artificial neural network (ANN) modelling. Food Chem. 2017, 229, 198–207. [Google Scholar] [CrossRef]
- Yang, Q.-Q.; Gan, R.-Y.; Zhang, D.; Ge, Y.-Y.; Cheng, L.-Z.; Corke, H. Optimization of kidney bean antioxidants using RSM & ANN and characterization of antioxidant profile by UPLC-QTOF-MS. Lebensm. Wiss. Technol. 2019, 114, 108321. [Google Scholar]
- Pilkington, J.L.; Preston, C.; Gomes, R.L. Comparison of response surface methodology (RSM) and artificial neural networks (ANN) towards efficient extraction of artemisinin from Artemisia annua. Ind. Crops Prod. 2014, 58, 15–24. [Google Scholar] [CrossRef]
- Al-Nemari, R.; Bacha, A.B.; Al-Senaidy, A.; Almutairi, M.H.; Arafah, M.; Al-Saran, H.; Abutaha, N.; Semlali, A. Cytotoxic effects of Annona squamosa leaves against breast cancer cells via apoptotic signaling proteins. J. King Saud. Univ. Sci. 2022, 34, 102013. [Google Scholar] [CrossRef]









| Category | Bioactive Compounds/Metabolites | Reported Biological Activities | References |
|---|---|---|---|
| Phenolic compounds | Total phenolic content (ethanolic peel extract)~19 mg GAE/g | Antioxidant, free radical scavenging, protection against oxidative stress-mediated diseases | [3] |
| Flavonoids | Flavonoid content~26 mg QE/g (quercetin equivalent, from peel ethanol extract) | Antioxidant, anti-inflammatory, enzyme inhibition (e.g., α-amylase), hypoglycaemic effects | [3] |
| Sterols | Stigmasterol, β-sitosterol | Potential cholesterol-lowering activity, general bioactivity of sterols in membranes, etc. | [3] |
| Flavonoid (isolated) | Quercetin (isolated from peel) | Antioxidant, various flavonoids known to contribute to anti-inflammatory, antioxidant, anticancer effects | [3] |
| Cyclitol | Pinitol | Osmoprotective, potentially antidiabetic or insulin-modulating (as known for pinitol in other plants) | [3] |
| Qualitative metabolites | Alkaloids, glycosides, anthraquinones, phenols, flavonoids, carbohydrates, proteins | These metabolite classes contribute to antioxidant, antimicrobial, enzyme inhibitory, anti-inflammatory activities | [3] |
| Nutrient/proximate data | Protein, carbohydrate content in peel extract | Nutritional/food value; could contribute to caloric/nutrient supply, possibly assist in functional food context | [3] |
| Independent Variables | Unit | Levels | |
|---|---|---|---|
| Microwave power, X1 | Watt | 700 | 1400 |
| Treatment time, X2 | s | 20 | 30 |
| Sample solvent ratio, X3 | w/v (g/mL) | 1:20 | 1:30 |
| S.No | Microwave Power | Time | Sample Solvent Ratio | TPC (mg GAE/g) | Anti-Cholesterol Activity (%) | Antioxidant Activity (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Watt | s | w/v (g/mL) | Experimental | RSM Predicted | ANN Predicted | Experimental | RSM Predicted | ANN Predicted | Experimental | RSM Predicted | ANN Predicted | |
| 1 | 1050 | 25 | 25 | 256.46 | 255.87 | 250.94 | 75.3 | 77.58 | 74.84 | 87.35 | 88.17 | 89.58 |
| 2 | 1050 | 20 | 30 | 249.53 | 253.8 | 250.94 | 86.31 | 82.59 | 86.39 | 96.76 | 97.09 | 96.89 |
| 3 | 700 | 30 | 25 | 336.51 | 341.99 | 276.61 | 30.41 | 27.55 | 47.37 | 94.75 | 94.53 | 95.12 |
| 4 | 1050 | 30 | 30 | 303.21 | 308.08 | 302.94 | 84.01 | 82.36 | 84.24 | 98.67 | 97.09 | 98.61 |
| 5 | 700 | 20 | 25 | 169.94 | 165.08 | 223.94 | 64.72 | 63.93 | 72.79 | 84.66 | 83.84 | 95.28 |
| 6 | 1400 | 30 | 25 | 183.6 | 193.35 | 183.38 | 85.45 | 86.24 | 85.43 | 94.97 | 96.1 | 95.04 |
| 7 | 1050 | 25 | 25 | 238.68 | 238.07 | 250.94 | 77.56 | 77.58 | 74.84 | 93.87 | 92.74 | 89.58 |
| 8 | 1400 | 20 | 25 | 234.01 | 229.74 | 234.02 | 86.51 | 89.37 | 86.5 | 97.36 | 97.09 | 97.37 |
| 9 | 700 | 25 | 20 | 328.31 | 317.96 | 328.31 | 44.94 | 44.08 | 44.97 | 80.51 | 80.61 | 80.5 |
| 10 | 1050 | 30 | 20 | 240.66 | 238.07 | 240.67 | 55.19 | 58.9 | 55.19 | 97 | 95.96 | 96.99 |
| 11 | 1400 | 25 | 20 | 205.18 | 215.53 | 205.67 | 90.74 | 86.23 | 90.72 | 89.76 | 89.67 | 89.75 |
| 12 | 700 | 25 | 30 | 230.56 | 238.07 | 230.21 | 43.6 | 48.1 | 43.61 | 86.68 | 86.37 | 86.72 |
| 13 | 1050 | 25 | 25 | 245.95 | 238.07 | 250.94 | 74.18 | 77.58 | 74.84 | 91.75 | 92.07 | 89.58 |
| 14 | 1050 | 20 | 20 | 180.41 | 174.92 | 192.66 | 96.53 | 98.18 | 96.57 | 92.94 | 93.16 | 96.62 |
| 15 | 1050 | 25 | 25 | 267.74 | 257.98 | 250.94 | 88.68 | 77.58 | 74.84 | 96.77 | 97.09 | 89.58 |
| 16 | 1400 | 25 | 30 | 234.49 | 238.07 | 233.73 | 89.21 | 90.08 | 89.4 | 95.89 | 97.09 | 96.04 |
| 17 | 1050 | 25 | 25 | 214.72 | 215.3 | 250.94 | 72.18 | 77.58 | 74.84 | 87.24 | 88.28 | 89.58 |
| Coefficients | TPC (mg GAE/g) | Anti-Cholesterol Activity (%) | Antioxidant Activity (%) |
|---|---|---|---|
| b0 | 331.65 | +77.58 | +97.09 |
| b1 | 19.12 *** | +21.03 *** | +3.35 *** |
| b2 | 28.93 *** | −9.88 ** | +1.18 * |
| b3 | 16.48 *** | +1.97 | +0.4965 |
| b12 | 18.57 ** | +8.31 * | −0.4336 |
| b13 | 41.06 ** | −0.0442 | +1.45 |
| b23 | 0.058 | +9.76 * | +0.2875 |
| b12 | −35.64 *** | −12.10 ** | −7.49 *** |
| b22 | −11.38 * | +1.29 | −4.03 *** |
| b32 | −5.01 *** | +1.64 | +1.07 |
| Lack of Fit | Not significant | Not significant | Not significant |
| R2 | 0.9767 | 0.9556 | 0.9760 |
| Adj. R2 | 0.9468 | 0.8984 | 0.9452 |
| Pred. R2 | 0.7546 | 0.7034 | 0.7543 |
| Coefficients | TPC (g/mg) | Anti-Cholesterol Activity (%) | Antioxidant Activity (%) | |||
|---|---|---|---|---|---|---|
| RSM | ANN | RSM | ANN | RSM | ANN | |
| AAD | 5.4582 | 2.2152 | 2.9982 | 7.5294 | 0.6435 | 0.4129 |
| MSE | 40.4479 | 20784 | 15.4041 | 8.4430 | 0.6232 | 0.5077 |
| MPE | 2.3236 | 0.9251 | 4.4904 | 2.5699 | 0.6926 | 0.4522 |
| RSME | 26.2224 | 18.797 | 16.1824 | 11.9805 | 3.2551 | 2.9378 |
| R2 | 0.9767 | 0.9813 | 0.9566 | 0.9655 | 0.9760 | 0.9711 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loganathan, V.; Vijayan, L.; Rengaraju, B. Optimisation of Microwave-Assisted Extraction of Phenolic Compounds from Pithecellobium dulce Fruit Peels: Comparative Process Modelling Using RSM and ANN with Bioactivity Evaluation. Processes 2025, 13, 3554. https://doi.org/10.3390/pr13113554
Loganathan V, Vijayan L, Rengaraju B. Optimisation of Microwave-Assisted Extraction of Phenolic Compounds from Pithecellobium dulce Fruit Peels: Comparative Process Modelling Using RSM and ANN with Bioactivity Evaluation. Processes. 2025; 13(11):3554. https://doi.org/10.3390/pr13113554
Chicago/Turabian StyleLoganathan, Veerapandi, Lekhashri Vijayan, and Balakrishnaraja Rengaraju. 2025. "Optimisation of Microwave-Assisted Extraction of Phenolic Compounds from Pithecellobium dulce Fruit Peels: Comparative Process Modelling Using RSM and ANN with Bioactivity Evaluation" Processes 13, no. 11: 3554. https://doi.org/10.3390/pr13113554
APA StyleLoganathan, V., Vijayan, L., & Rengaraju, B. (2025). Optimisation of Microwave-Assisted Extraction of Phenolic Compounds from Pithecellobium dulce Fruit Peels: Comparative Process Modelling Using RSM and ANN with Bioactivity Evaluation. Processes, 13(11), 3554. https://doi.org/10.3390/pr13113554






