Abstract
The development of photocatalyst material is vital for the practical application of environmental purification, solar energy conversion, and chemical production. In this work, a spherical Bi(C6N7O3) photocatalyst is successfully prepared by replacing K+ of rod-shaped K3[C6N7O3] with Bi3+ ions through the ion exchange method, which demonstrates an improved homogeneous photodegradation efficiency over antibiotics in water compared to pristine K3[C6N7O3]. Under visible light irradiation, Bi(C6N7O3) degraded 81% of tetracycline hydrochloride within 60 min, and the rate constant was 1.8 and 79.1 times greater than K3[C6N7O3] and bulk g-C3N4, respectively. Scavenger experiments revealed that superoxide radicals and holes are the primary active species in the photocatalytic process. This study presents a promising route for designing high-performance visible-light photocatalysts by a simple ion-exchange approach for environmental applications.
1. Introduction
Environmental pollution caused by antibiotic misuse directly threatens human life and health [1], which is one of the key problems around the world and needs to be addressed urgently [2,3,4,5,6]. The traditional methods dealing with antibiotic pollution are mainly physical adsorption and microbiological treatment [7]. However, these methods have been labeled as insufficient due to the unconquerable drawbacks such as limited choice of adsorbents, potential for secondary contamination, high cost, long treatment time, and ineffective removal. Developing a green approach is vital for the efficient control of antibiotic pollution. Photocatalytic oxidation directly employs solar energy as a light source to drive catalysis [8], which has become a green and ideal approach to monitor the antibiotic pollutants [9,10,11,12,13].
Graphitic phase carbon nitride (g-C3N4) is a prospective polymer photocatalyst with the advantages of strong visible-light response, ease of preparation, and chemical stability, which has been intensively studied and broadly applied in visible-light photocatalysis, such as hydrogen production via water splitting and degradation of the emerging pollutant [14]. However, the practical application is severely restricted due to the drawbacks, such as a lack of active sites and a faint visible-light response. Great efforts have been made to overcome these defects, and strategies such as constructing hollow or porous structures have been developed to enlarge the specific area recently [15]. Nevertheless, most of the currently reported g-C3N4 photocatalysts are heterogeneous phases. They are still suffering from the main insufficiencies such as limited specific surface area and active sites, which hinder the practical applications of g-C3N4-type photocatalyst [14,16].
On the contrary, homogeneous photocatalysis deals with a photocatalytic reaction where the catalyst and reactants exist in the same phase, facilitating the complete access of reactants toward the active sites, which may be an alternative approach to optimize the photocatalytic performance. The construction of homogeneous photocatalysts has thrived in recent years. In 2018, Huang et al. successfully synthesized a homogeneous CN-based photocatalyst, which demonstrated an improved photocatalytic activity in comparison with that of the heterogeneous counterpart [17]. However, the prepared photocatalyst is solely soluble in certain corrosive solvents, including CH3SO2OH and H2SO4, which limits its wide application in water. Given the urgent demand for water purification and pollutant decomposition, the fabrication of water-soluble photocatalysts remains challenging. Recently, a series of CN derivatives such as basic cyanurate of K3[C6N7(NCN)3] and C6N7O3H3 with distinct optical properties have been developed via alkali treatment, which are water-soluble compounds [18,19]. In 2021, Yang et al. successfully prepared rod-shaped K3[C6N7O3] compounds from top to bottom by reacting melon polymer with a hot strong base and applied it as a homogeneous photocatalyst in removing antibiotic residues in water for the first time [20]. The obtained photocatalyst showed 10-fold higher photocatalytic activity than that of bulk g-C3N4. However, a further improvement of activity is necessary for the practical application of homogeneous photocatalysis.
In this study, Bi(C6N7O3) was successfully synthesized by replacing the K+ ion in K3[C6N7O3] with the Bi3+ ion from an ion-exchange process under solvothermal conditions, which showed improved photocatalytic activity and excellent stability toward the visible-light degradation of antibiotic residue in aqueous solutions. The substitution of K+ with Bi3+ may introduce a high-valence metal center, effectively modulating the electronic structure and facilitating charge carrier separation, which promotes the photocatalytic behaviors. The as-prepared Bi(C6N7O3) shows 79.1 and 1.8-fold greater activity than those of g-C3N4 and K3[C6N7O3], respectively. Several techniques have been utilized to explore the factors contributing to the enhanced properties and stability. The active species are identified, and a reaction mechanism is proposed in the end. This work offers an easy strategy of ion exchange to manipulate the photocatalytic properties, which may spur the development and application of homogeneous photocatalysis.
2. Experimental
2.1. Chemicals and Materials
Melamine (C3N3(NH2)3, 99%) was bought from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Potassium hydroxide (KOH, 85%) was obtained from Aladdin Industrial Corporation. (Shanghai, China). N,N-dimethylformamide (HCON(CH3)2, 99.5%) and bismuth nitrate pentahydrate (Bi(NO3)3·5H2O, 99%) were obtained from Hangzhou Mick Chemical Reagent Co., Ltd. (Hangzhou, China). Methanol (CH3OH, 99.7%) was obtained from Hangzhou Gaojing Fine Chemical Industry Co., Ltd. (Hangzhou, China). All the reagents were directly used after purchase.
2.2. Preparation
Preparation of rod-shaped K3[C6N7O3]. The preparation of K3[C6N7O3] is followed by the previous report [20]. Typically, 14.0 g of KOH and 5.0 g of melon powder were added to 50.0 mL of aqueous solution, mixed thoroughly, and transferred to a 150 mL round-bottom flask. Subsequently, the flask was positioned in an oil bath and refluxed at 130 °C for 2 h under magnetic stirring until the yellow-colored solution turned transparent. The hot solution was filtered, and the filtrate was stored in a refrigerator for 12 h. Finally, the precipitated solid was separated via vacuum filtration and washed repeatedly with absolute ethanol, and then dried under vacuum at 70 °C for 6 h to yield the product of potassium cyanurate K3[C6N7O3].
Preparation of Bi(C6N7O3) nanospheres. 1.455 g Bi(NO3)3·5H2O and 1.005 gas-prepared K3[C6N7O3] were dispersed in 50 mL of mixed solution (DMF:CH3OH = 3:1) and stirred for 30 min to form a homogeneous solution. Subsequently, the solution was sealed in an autoclave equipped with polytetrafluoroethylene and heated at 120 °C for 16 h at a rate of 1 °C per minute. After that, the synthesized Bi(C6N7O3) was washed with absolute ethyl alcohol multiple times and dried at 70 °C for 12 h under vacuum.
2.3. Characterization
X-ray diffraction (XRD) pattern was detected on the a Panalytical X’Pert’3 Powder instrument (Malvern Panalytical, Almelo, The Netherlands) applying Cu Kα (λ = 1.5406 Å) as the source. Fourier transform infrared spectroscopy (FTIR) was performed on a Bruker Tensor 27 spectrometer (Bruker Corporation, Billerica, MA, USA). Scanning electron microscopy (SEM) pictures and energy dispersive X-ray (EDX) patterns were carried out on a ZEISS Sigma 300 microscope (Carl Zeiss AG, Oberkochen, Germany). X-ray photoelectron spectroscopy (XPS) was recorded on a Thermo Scientific K-Alpha instrument (Thermo Fisher Scientific, Waltham, MA, USA). UV-visible diffuse spectrum (UV-vis DRS) was performed on a Shimadzu UV-3600 UV-visible spectrophotometer (Shimadzu Corporation, Kyoto, Japan). Full-wavelength UV-visible absorption spectra were recorded on the UV-2600 UV-visible spectrophotometer (Shanghai Sunny Hengping Scientific Instrument Co., Ltd., Shanghai, China).
2.4. Photocatalytic Degradation Measurements
TC-HCl was employed as the typical antibiotic residue to evaluate the photocatalytic activities of the prepared samples. For simulated visible light, a 300 W Xenon lamp equipped with a 420-nm cut-off filter was employed, with a spectral irradiance of 272.0 mW/cm2. Moreover, a circulating cooling system was applied to maintain the reaction temperature at around 20 °C. Briefly, 20.0 mg of the prepared photocatalyst was dispersed in 100 mL of TC-HCl solution (10, 20, and 50 mg/L) using a 150 mL beaker as the reactor. The solution was stirred in the dark for 30 min to reach adsorption–desorption equilibrium. Then, the solution was positioned under the simulated light source, with the bottom of the reactor positioned at a vertical distance of 13 cm from the lamp, and the lamp was turned on for illumination with constant magnetic stirring (550 rpm). The initial pH of the solution before the photocatalytic reaction was measured to be 6.76. 5.0 mL of solution was collected every 10 min, and the supernatant after centrifugation was taken for measurement on a UV-2600 spectrophotometer. All experiments were repeated at least three times to ensure reproducibility.
3. Results and Discussion
The crystal structure of Bi(C6N7O3) was analyzed using XRD, as shown in Figure 1a. The diffraction pattern was compared to that of K3[C6N7O3], considering the absence of a reference XRD pattern for Bi(C6N7O3). The prominent peak at 28.12° corresponded to the interlayer stacking of aromatic units, while the diffraction peak at approximately 14.02° was characteristic of the melon structure, which was ascribed to the cohesive and ordered arrangement of cyanuric acid units [21]. A notable rightward shift in the diffraction peak positions for Bi(C6N7O3) was observed when compared to the JCPDS pattern of K3[C6N7O3]. This shift is attributed to the substitution of Bi3+ ions, which have a smaller ionic radius than K+ ions. The introduction of smaller dopant atoms into the lattice leads to a reduction in the unit cell parameters, thereby causing the rightward shift of the XRD peaks.
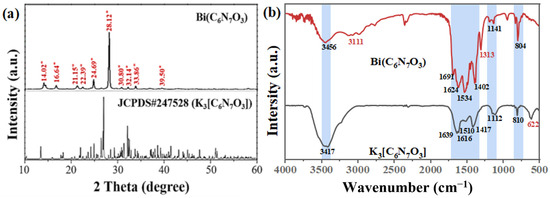
Figure 1.
(a) XRD patterns of Bi(C6N7O3) and K3[C6N7O3], (b) Recorded FTIR spectra of K3[C6N7O3] and Bi(C6N7O3). The black and red lines correspond to the K3[C6N7O3] and Bi(C6N7O3) samples, respectively.
As displayed in Figure 1b, the FTIR spectra of K3[C6N7O3] and Bi(C6N7O3) were consistent with previous studies [18]. The peak at 804 cm−1 corresponded to the bending vibration associated with the S-triazine unit. In the range of 1000–1600 cm−1, the observed band was attributed to the stretching vibration of the C-N heterocyclic ring [22]. The peaks at 1624, 1534, and 1402 cm−1 were associated with the stretching vibration of heptazine-derived repeating units, respectively [23]. Additionally, the broad band between 3111 and 3456 cm−1 was attributed to the stretching vibration of N-H bonds and the presence of absorbed water [24]. These characteristic features collectively confirm the identity of the prepared sample as Bi(C6N7O3).
The chemical composition and valence states of Bi(C6N7O3) were investigated using XPS measurement. As shown in Figure 2a, the presence of all constituent elements, including carbon (C), nitrogen (N), oxygen (O), and bismuth (Bi), corroborated the above discussion. No peak belonging to potassium (K) was observed, indicating the complete substitution of K by Bi. The fine C 1s spectrum and its deconvoluted peaks, presented in Figure 2b, revealed two principal peaks at 284.8 eV (C-C) and 288.6 eV (N-C=N), corresponding to different carbon environments within Bi(C6N7O3) [25]. In addition, the peak at 286.5 eV is likely ascribed to the presence of C-O-Bi groups. Figure 2c displayed the N 1s spectrum with a peak at 398.4 eV, characteristic of the sp2 hybridized N atom in C=N-C bonds [26]. The peaks at 400.0 eV and 401.1 eV are tentatively assigned to N atoms in N-(C)3 and N-H configurations, respectively [27]. The faint peak at 404.8 eV was related to π-excitation [28]. The O 1s (Figure 2d) exhibited a peak at 531.2 eV, which corresponded to C-O-Bi bonds in Bi(C6N7O3) [29]. Figure 2e revealed two distinct peaks at 159.4 eV (Bi 4f7/2) and 164.7 eV (Bi 4f5/2), which were indicative of the bismuth species present within the compound. These XPS results confirm that the prepared Bi(C6N7O3) has an aromatic skeleton composed of heptazine units, suggesting the successful synthesis of the target compound.
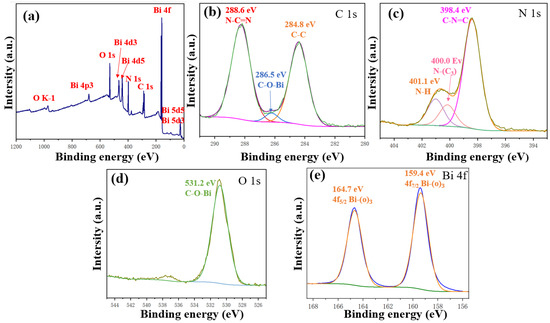
Figure 2.
XPS spectra of Bi(C6N7O3): (a) survey spectrum, (b) C 1s spectrum, (c) N 1s spectrum, (d) O 1s spectrum, and (e) Bi 4f spectrum.
Figure 3a shows the SEM image of the Bi(C6N7O3) sample. The prepared Bi(C6N7O3) had an irregular spherical shape with diameters close to 5 μm. As seen in Figure 3b, the chemical composition of Bi was via EDS and peaks at ~0.3, ~0.4, ~0.5, and ~2.4 keV were detected in the EDS spectra, assigned to the elements C, N, O, and Bi, respectively, indicating that C, N, O, and Bi atoms coexisted in Bi(C6N7O3) [30]. According to the EDS analysis, the atomic contents of C and N were 31.53% and 36.28%, respectively. The ratio of C to N atoms was 0.87, which is consistent with the structural formula. The EDS elemental mappings of Bi(C6N7O3), as demonstrated in Figure 3c–f confirmed the uniform distribution of all the elements within Bi(C6N7O3). These results further confirm the successful preparation of Bi(C6N7O3).
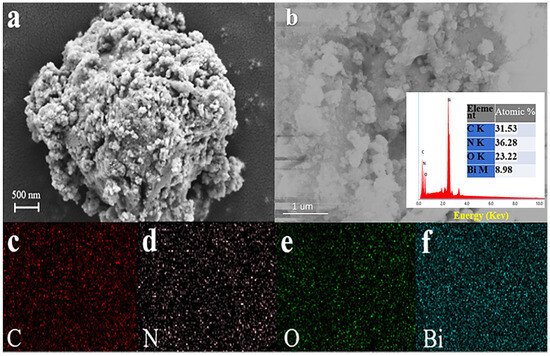
Figure 3.
(a) SEM image of Bi(C6N7O3), (b–f) EDS elemental mappings of Bi(C6N7O3).
Figure 4 presents the homogeneous degradation profiles of tetracycline hydrochloride (TC-HCl) in aqueous solution under visible-light irradiation using different dosages of photocatalysts. As depicted in Figure 4a, g-C3N4 exhibited minimal degradation of TC-HCl under visible light, with only a 2.4% reduction observed. In contrast, significant degradation was achieved with K3[C6N7O3] and Bi(C6N7O3) as photocatalysts, resulting in efficiencies of 63% and 81%, respectively, within 60 min. Further investigation into the photocatalytic degradation efficiency of Bi(C6N7O3) on TC-HCl solutions with different concentrations was shown in Figure 4b. Photocatalytic degradation efficiencies of TC-HCl solutions with initial concentrations of 20, 30, and 50 mg/L were 81%, 75%, and 55%, respectively. These results confirm that the synthesized Bi(C6N7O3) is an effective photocatalyst for homogeneous processes, demonstrating its potential for practical applications in water treatment.
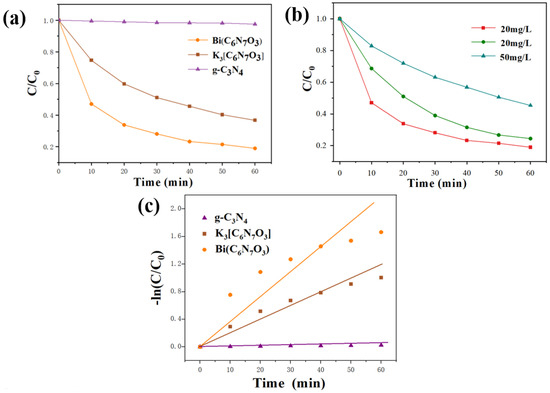
Figure 4.
(a) Photocatalytic degradation efficiency of TC-HCl under the visible light irradiation using g-C3N4, K3[C6N7O3], and Bi(C6N7O3); (b) Degradation efficiency of TC-HCl solutions with different concentrations (20, 30, and 50 mg/L) using Bi(C6N7O3); (c) Pseudo-first-order kinetic fitting of TC-HCl degradation over the three photocatalysts.
The photocatalytic activity of the obtained samples was quantified using a pseudo-first-order model, Equation (1).
where C0 represented the initial TC-HCl concentration, and Ct represented the TC-HCl concentration following t min of irradiation. The optimum slope, corresponding to the apparent rate constant kapp, was obtained by the method of least squares [28]. In Figure 4c, the calculated kapp values for g-C3N4, K3[C6N7O3], and Bi(C6N7O3) photocatalysts were 0.00042 min−1, 0.01873 min−1, and 0.03323 min−1, respectively. Obviously, the kapp value of Bi(C6N7O3) is 79.1 and 1.8-fold higher than that of g-C3N4 and K3[C6N7O3], demonstrating the superior photocatalytic activity of Bi(C6N7O3).
The photocatalytic performance of Bi(C6N7O3) was compared with that of the recently reported photocatalysts. As summarized in Table 1, the Bi(C6N7O3) catalyst exhibits a notably higher kapp than most of the compared materials, underscoring its superior activity and reinforcing the advancement presented in this study.

Table 1.
Comparison of the kapp for various photocatalysts reported in recent studies.
The reusability and sustainability of photocatalysts are crucial for their practical applications. The Bi(C6N7O3) photocatalyst in ethanol was collected and washed after the initial photocatalytic run and then subjected to an additional three cycles of the same procedure, as illustrated in Figure 5. A decline in photocatalytic activity with each cycle was observed, which could be attributed to the gradual leaching of Bi3+ from the (C6N7O3)3− framework as the cycles progress. Additionally, Bi(C6N7O3) has good solubility, readily soluble in water, leading to noticeable mass loss during the recovery process in ethanol, considering that ethanol contains a small amount of water.
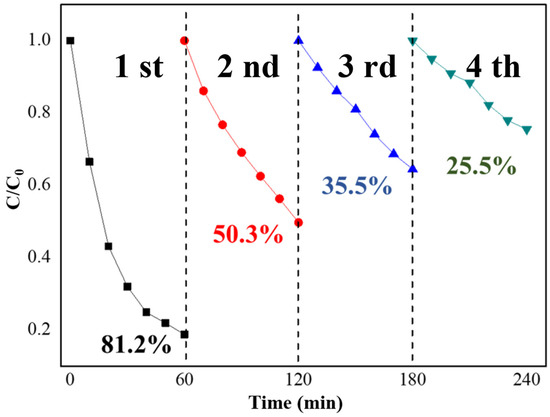
Figure 5.
Cyclic stability tests of Bi(C6N7O3) for TC-HCl photodegradation.
The underlying mechanism of the enhanced photocatalytic property of Bi(C6N7O3) was investigated. The UV-Vis diffuse reflection absorption spectra for the synthesized photocatalysts were measured, as depicted in Figure 6a. Both K3[C6N7O3] and Bi(C6N7O3) demonstrated strong absorption across the UV-visible spectrum, with distinct absorption edges observed at 438 and 486 nm. These edges suggest that the samples can capture a fraction of the visible light. The bandgap of the K3[C6N7O3] and Bi(C6N7O3) was calculated using the Tauc plot, as shown in Figure 6b, yielding bandgap values of 2.8 eV for K3[C6N7O3] and 2.2 eV for Bi(C6N7O3). Compared to K3[C6N7O3], the band gap of Bi(C6N7O3) was reduced, allowing it to absorb more light energy and generate more electron-hole pairs, increasing its photosensitivity and photovoltaic effect.
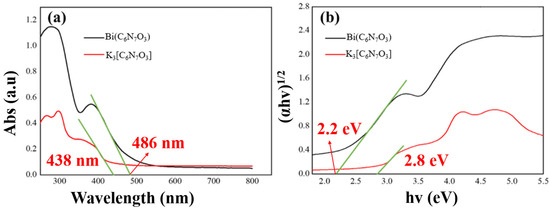
Figure 6.
(a) UV-vis absorption spectrum of K3[C6N7O3] and Bi(C6N7O3); (b) Tauc plots derived from UV-Vis spectra.
Photocurrent measurement upon light illumination is a robust indicator of the efficiency of photogenerated carrier separation and transport, which are key factors in photocatalytic performance. Figure 7a showed the photocurrent responses of g-C3N4, K3[C6N7O3], and Bi(C6N7O3) under visible light irradiation in ethanol, considering the water solubility of the latter two compounds. Upon turning on the light source, both K3[C6N7O3] and Bi(C6N7O3) exhibited significant photocurrent, with Bi(C6N7O3) exhibiting the highest response. This suggests that Bi(C6N7O3) might have a more efficient separation and migration of photoexcited carriers compared to g-C3N4 and K3[C6N7O3]. Furthermore, EIS was utilized to evaluate the electron transfer characteristics of the prepared samples, as depicted in Figure 7b. Bi(C6N7O3) had the smallest arc diameter among these samples, indicating the lowest electron transfer resistance. These results show that Bi(C6N7O3) has a narrow band gap, a high charge separation capability, and a low electron transfer resistance, which collectively improve its photocatalytic performance.
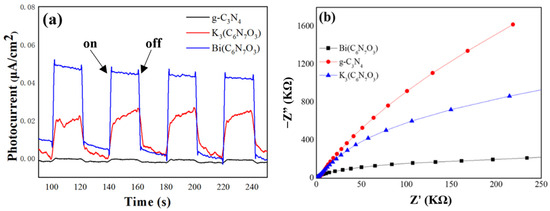
Figure 7.
(a) Transient photocurrent response under visible light irradiation and (b) Electrochemical impedance spectroscopy (EIS) Nyquist plots of g-C3N4, K3[C6N7O3], and Bi(C6N7O3).
As shown in Figure 8, radical trapping experiments were conducted to identify the active substances involved in the photocatalytic reactions. Ethylenediamine tetraacetic acid (EDTA-2Na), p-benzoquinone (BQ, 0.5 mM), and tert-butanol (TBA, 2 mM) were employed as the scavengers of photogenerated holes (h+), superoxide radicals (·O2−), and hydroxyl radicals (·OH), respectively. The low concentration of BQ used (0.5 mM) is well-established in the literature to specifically quench ∙O2− without significant side reactions under standard photocatalytic conditions [36,37]. Similarly, TBA is a highly specific scavenger for ∙OH, whose steric hindrance makes reactions with ∙O2− or h+ unlikely [37]. The addition of BQ and EDTA-2Na resulted in a significant decrease in photocatalytic activity, indicating that both ·O2− and h+ were primary reactive species in the photocatalytic process. In contrast, the introduction of TBA did not affect the degradation efficiency, suggesting that ·OH had an insignificant effect in the photocatalytic process [38].
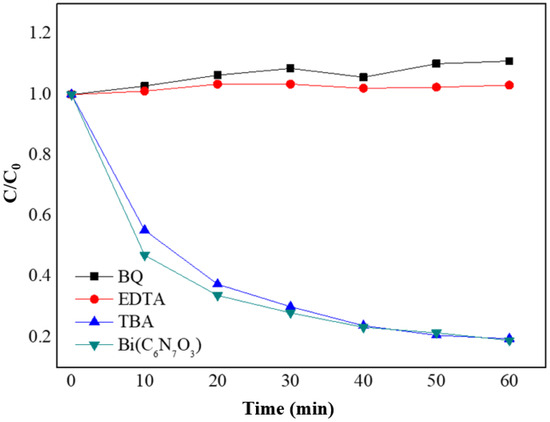
Figure 8.
Effects of different radical scavengers (TBA for ·OH, EDTA-2Na for h+, BQ for ·O2−) on the photocatalytic degradation of TC-HCl over Bi(C6N7O3).
The photocatalytic performance of the Bi(C6N7O3) nanospheres was compared with a recently reported Fe-TiO2/biochar composite, similarly identifying ∙O2− as the predominant reactive species [39]. The kapp of our Bi(C6N7O3) catalyst for TC-HCl degradation was calculated to be 1.99 h−1 (from 0.03323 min−1), which is higher than the kapp of 0.202 h−1 on Fe-TiO2/biochar composite.
The Mott–Schottky plot for Bi(C6N7O3) displayed a positive slope, as illustrated in Figure 9a, which indicates that the sample is an n-type semiconductor. With respect to the normal hydrogen electrode, the conduction band (CB) energy level of an n-type semiconductor is measured to be −0.62 V. Typically, the conduction band energy level of an n-type semiconductor is approximately 0.1 eV less negative than the conduction band energy level of the flat-band potential [40]. Consequently, the conduction band level of Bi(C6N7O3) is estimated to be −0.72 V with respect to the normal hydrogen electrode (NHE), and the position of the valence band(VB) is obtained according to Equation (2) [41]:
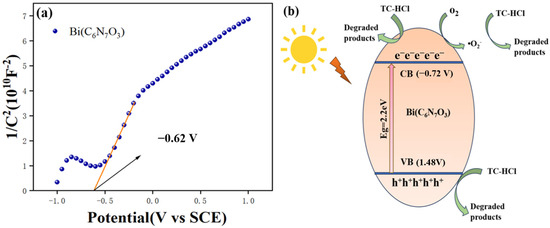
Figure 9.
(a) Mott–Schottky curve of Bi(C6N7O3) and (b) schematic illustration of the proposed mechanism for photocatalytic degradation of TC-HCl over Bi(C6N7O3) nanospheres under visible light irradiation.
Based on the above results, a plausible mechanism for the photocatalytic degradation is proposed. As illustrated in Figure 9b, upon illumination with visible light, the electrons (e−) in Bi(C6N7O3) are excited to a conduction band, leaving behind holes (h+) in the valence band. This process results in the formation of electron-hole pairs, where the holes possess sufficient positive potential to produce ·OH radicals. However, the radical scavenger test confirms that ·OH radicals have an insignificant role in the photocatalytic process. Rather, it is the holes at the surface of Bi(C6N7O3) that are primarily engaged in the photocatalytic activity. In conclusion, the active species, including superoxide radicals, photogenerated electron-positive hole pairs, are crucially involved in the photodegradation of TC-HCl.
4. Conclusions
Bi (C6N7O3) nanospheres are successfully prepared by replacing K+ of as-synthesized K3[C6N7O3] with Bi3+ ions, which are applied as a prominent homogeneous photocatalyst for dealing with the emerging pollutant in water for the first time. The as-prepared compound can combine with three surroundings (C6N7O3)3− ions because Bi3+ has three positive charges. Therefore, metal ions and organic ligands can be connected by self-assembly to form a category of crystalline materials with a periodic network structure. Experiments with photocatalysis have shown that Bi (C6N7O3) demonstrates a greatly improved photocatalytic activity, which is 79.1- and 1.8-fold greater than that of g-C3N4 and K3[C6N7O3], respectively. The enhancement may be attributed to the increased optimized visible-light response and charge separation. This work offers a strategy to build high-performance photocatalysts by simple ion substitution and broadens the application of homogeneous photocatalysis in environmental control and other fields.
Author Contributions
Conceptualization, Z.C. and X.W. (Xiaojing Wang); methodology, R.J., Y.C. and J.C.; validation, Y.C., J.W., X.W. (Xiaozhuo Wang) and C.J.; formal analysis, H.X. and J.W.; investigation, J.W., X.W. (Xiaozhuo Wang), J.C., L.L. and C.J.; resources, R.J., H.X., L.L., C.J. and Z.C.; data curation, M.T. and M.H.; writing—original draft preparation, R.J., M.T., M.H. and Z.C.; writing—review and editing, R.J. and Z.C.; supervision, L.L. and Z.C.; project administration, Z.C. and X.W. (Xiaojing Wang); funding acquisition, Z.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work is financially supported by the National Key Research and Development Program of China (2023YFF0612601), the Key Research and Development Program of Zhejiang Province (2023C02038), the Key Research and Development Program of Ningbo (2022Z178), and the Zhejiang Provincial Natural Science Foundation of China (LQ23B010003).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Thakur, S.; Mutreja, V.; Tomar, A.; Nainwal, S.; Kaur, K.; Kaur, R.; Li, Q.; Ataya, F.S. Phytochemical-Assisted Fabrication of ZrO2/ZnO Nanocomposites: A Sustainable Approach for Efficient Photodegradation of Tetracycline Hydrochloride. J. Mol. Struct. 2025, 1330, 141350. [Google Scholar] [CrossRef]
- Zhou, D.; Chen, Z.; Yang, Q.; Dong, X.; Zhang, J.; Qin, L. In-Situ Construction of All-Solid-State Z-Scheme g-C3N4/TiO2 Nanotube Arrays Photocatalyst with Enhanced Visible-Light-Induced Properties. Sol. Energy Mater. Sol. Cells 2016, 157, 399–405. [Google Scholar] [CrossRef]
- Senthil, R.A.; Theerthagiri, J.; Selvi, A.; Madhavan, J. Synthesis and Characterization of Low-Cost g-C3N4/TiO2 Composite with Enhanced Photocatalytic Performance under Visible-Light Irradiation. Opt. Mater. 2017, 64, 533–539. [Google Scholar] [CrossRef]
- Mohini, R.; Lakshminarasimhan, N. Coupled Semiconductor Nanocomposite g-C3N4/TiO2 with Enhanced Visible Light Photocatalytic Activity. Mater. Res. Bull. 2016, 76, 370–375. [Google Scholar] [CrossRef]
- Sun, M.; Shen, S.; Wu, Z.; Tang, Z.; Shen, J.; Yang, J. Rice Spike-like g-C3N4/TiO2 Heterojunctions with Tight-Binding Interface by Using Sodium Titanate Ultralong Nanotube as Precursor and Template. Ceram. Int. 2018, 44, 8125–8132. [Google Scholar] [CrossRef]
- Liu, R.; Bie, Y.; Qiao, Y.; Liu, T.; Song, Y. Design of g-C3N4/TiO2 Nanotubes Heterojunction for Enhanced Organic Pollutants Degradation in Waste Water. Mater. Lett. 2019, 251, 126–130. [Google Scholar] [CrossRef]
- Liu, C.; Tan, L.; Zhang, L.; Tian, W.; Ma, L. A Review of the Distribution of Antibiotics in Water in Different Regions of China and Current Antibiotic Degradation Pathways. Front. Environ. Sci. 2021, 9, 692298. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, Y.; Huang, H. Layered Photocatalytic Nanomaterials for Environmental Applications. Chin. Chem. Lett. 2023, 34, 107523. [Google Scholar] [CrossRef]
- Qian, X.; Chen, Z.; Yang, X.; Zhao, W.; Liu, C.; Sun, T.; Zhou, D.; Yang, Q.; Wei, G.; Fan, M. Perovskite Cesium Lead Bromide Quantum Dots: A New Efficient Photocatalyst for Degrading Antibiotic Residues in Organic System. J. Clean. Prod. 2020, 249, 119335. [Google Scholar] [CrossRef]
- Ma, Z.; He, Y.; Li, X.; Zhou, C.; Deng, L. Ultrasonic-Assisted Efficient Degradation of Tetracycline over ZnO/BiOBr Heterojunctions: Synergistic Effect and Role of Oxidative Species. Mater. Res. Bull. 2022, 146, 111591. [Google Scholar] [CrossRef]
- Guo, S.; Luo, H.; Li, Y.; Chen, J.; Mou, B.; Shi, X.; Sun, G. Structure-Controlled Three-Dimensional BiOI/MoS2 Microspheres for Boosting Visible-Light Photocatalytic Degradation of Tetracycline. J. Alloys Compd. 2021, 852, 157026. [Google Scholar] [CrossRef]
- Kandi, D.; Behera, A.; Sahoo, S.; Parida, K. CdS QDs Modified BiOI/Bi2MoO6 Nanocomposite for Degradation of Quinolone and Tetracycline Types of Antibiotics towards Environmental Remediation. Sep. Purif. Technol. 2020, 253, 117523. [Google Scholar] [CrossRef]
- Jiang, W.; Li, Z.; Liu, C.; Wang, D.; Yan, G.; Liu, B.; Che, G. Enhanced Visible-Light-Induced Photocatalytic Degradation of Tetracycline Using BiOI/MIL-125(Ti) Composite Photocatalyst. J. Alloys Compd. 2021, 854, 157166. [Google Scholar] [CrossRef]
- Li, X.; Masters, A.; Maschmeyer, T. Polymeric Carbon Nitride for Solar Hydrogen Production. Chem. Commun. 2017, 53, 7438–7446. [Google Scholar] [CrossRef]
- Wang, D.; Huang, Y.; Yu, X.; Huang, X.; Zhong, Y.; Huang, X.; Liu, Z.; Feng, Q. Template-Free Synthesis of High Specific Surface Area Gauze-like Porous Graphitic Carbon Nitride for Efficient Photocatalytic Degradation of Tetracycline Hydrochloride. J. Mater. Sci. 2020, 56, 4641–4653. [Google Scholar] [CrossRef]
- Fang, L.J.; Li, Y.H.; Liu, P.F.; Wang, D.P.; Zeng, H.D.; Wang, X.L.; Yang, H.G. Facile Fabrication of Large-Aspect-Ratio g-C3N4 Nanosheets for Enhanced Photocatalytic Hydrogen Evolution. ACS Sustain. Chem. Eng. 2017, 5, 2039–2043. [Google Scholar] [CrossRef]
- Huang, C.; Wen, J.; Shen, Y.; He, F.; Mi, L.; Gan, Z.; Ma, J.; Liu, S.; Ma, H.; Zhang, Y. Dissolution and Homogeneous Photocatalysis of Polymeric Carbon Nitride. Chem. Sci. 2018, 9, 7912–7915. [Google Scholar] [CrossRef] [PubMed]
- Horvath-Bordon, E.; Kroke, E.; Svoboda, I.; Fuess, H.; Riedel, R.; Neeraj, S.; Cheetham, A.K. Alkalicyamelurates, M3[C6N7O3].xH2O, M = Li, Na, K, Rb, Cs: UV-Luminescent and Thermally Very Stable Ionic Tri-s-Triazine Derivatives. Dalton Trans. 2004, 22, 3900–3908. [Google Scholar] [CrossRef]
- Wang, Z.; Pei, G.X.; Qi, H.; Zhang, S.; Liu, C.; Li, Z.; Han, K. Solar Hydrogen Generation over Carbon Nitride Photocatalyst Promoted by Water-Soluble Carbon Dots. ChemPhotoChem 2022, 6, e202200176. [Google Scholar] [CrossRef]
- Wang, W.; Chen, Z.; Yang, X.; Audebert, P.; Sahoo, S.; Chen, J.; Liu, Y.; Pamir Alpay, S.; Xie, L.; Wei, G. Potassium Cyamelurate K3[C6N7O3] Rod: A New Visible-Light Photocatalyst for Homogeneous/Heterogeneous Degradation of Antibiotics. Appl. Catal. A Gen. 2022, 641, 118669. [Google Scholar] [CrossRef]
- Tian, Y.; Li, Q.; Zhang, M.; Nie, Y.; Tian, X.; Yang, C.; Li, Y. pH-Dependent Oxidation Mechanisms over FeCu Doped g-C3N4 for Ofloxacin Degradation via the Efficient Peroxymonosulfate Activation. J. Clean. Prod. 2021, 315, 128207. [Google Scholar] [CrossRef]
- Bao, J.; Jiang, X.; Huang, L.; Quan, W.; Zhang, C.; Wang, Y.; Wang, H.; Zeng, Y.; Zhang, W.; Ma, Y.; et al. Molybdenum disulfide loading on a Z-scheme graphitic carbon nitride and lanthanum nickelate heterojunction for enhanced photocatalysis: Interfacial charge transfer and mechanistic insights. J. Colloid Interface Sci. 2022, 611, 684–694. [Google Scholar] [CrossRef]
- Preeyanghaa, M.; Erakulan, E.S.; Thapa, R.; Ashokkumar, M.; Neppolian, B. Scrutinizing the Role of Tunable Carbon Vacancies in g-C3N4 Nanosheets for Efficient Sonophotocatalytic Degradation of Tetracycline in Diverse Water Matrices: Experimental Study and Theoretical Calculation. Chem. Eng. J. 2023, 452, 139437. [Google Scholar] [CrossRef]
- Wang, D.; Li, S.; Feng, Q. Supramolecular Self-Assembled Carbon Nitride for the Degradation of Tetracycline Hydrochloride. J. Mater. Sci. Mater. Electron. 2018, 29, 9380–9386. [Google Scholar] [CrossRef]
- Yu, H.; Shi, R.; Zhao, Y.; Bian, T.; Zhao, Y.; Zhou, C.; Waterhouse, G.I.N.; Wu, L.-Z.; Tung, C.-H.; Zhang, T. Alkali-Assisted Synthesis of Nitrogen Deficient Graphitic Carbon Nitride with Tunable Band Structures for Efficient Visible-Light-Driven Hydrogen Evolution. Adv. Mater. 2017, 29, 1605148. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; You, J.; Zhang, Q.; Liu, D.; Hu, S.; Gui, J. Preparation of Fe-Doped Graphitic Carbon Nitride with Enhanced Visible-Light Photocatalytic Activity. Editor. Off. Acta Phys.-Chim. Sin. 2014, 30, 1706–1712. [Google Scholar] [CrossRef]
- Zhang, S.; Zou, Y.; Chen, Z.; Li, B.; Gu, P.; Wen, T. Visible-Light-Driven Activation of Persulfate by RGO/g-C3N4 Composites for Degradation of BPA in Wastewater. J. Inorg. Mater. 2020, 35, 329–336. [Google Scholar] [CrossRef]
- Zhou, Y.; Jiang, D.; Wang, Z.; Yi, L.; Sun, J.; Liu, D.; Yu, X.; Chen, Y. Bandgap Engineering of Carbon Nitride by Formic Acid Assisted Thermal Treatment for Photocatalytic Degradation of Tetracycline Hydrochloride. Chem. Eng. J. 2024, 485, 149830. [Google Scholar] [CrossRef]
- Jourshabani, M.; Shariatinia, Z.; Badiei, A. Controllable Synthesis of Mesoporous Sulfur-Doped Carbon Nitride Materials for Enhanced Visible Light Photocatalytic Degradation. Langmuir 2017, 33, 7062–7078. [Google Scholar] [CrossRef]
- Zhang, A.; Guo, Y.; Xie, H.; Zhang, Y.; Fu, Y.; Ye, C.; Du, Y.; Zhu, M. Green and Controllable Synthesis of Kelp-like Carbon Nitride Nanosheets via an Ultrasound-Mediated Self-Assembly Strategy. J. Colloid Interface Sci. 2022, 628, 397–408. [Google Scholar] [CrossRef]
- Fan, X.; Zhang, Y.; Liang, H.; Zhang, M.; Sun, X.; Bai, J. Augmented Singlet Oxygen (1O2) Production via ZnO2/Bi2O2CO3 Heterojunction for Photodegradation of Tetracycline Hydrochloride through in-Situ Self-Fenton-like Mechanisms. Rare Met. 2025. [Google Scholar] [CrossRef]
- Ramteke, P.; Bhoyar, T.; Umare, S.S. Sulfamic Acid Coupled Polymeric Carbon Nitride: An Efficient Photocatalyst for Degradation of Tetracycline Hydrochloride and Supercapacitor Application. J. Nanoparticle Res. 2025, 27, 159. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Lee, T.; Nguyen, T.D. Synergistic Charge Separation via S-Scheme and Schottky Junctions in Ni-Decorated Melem Hydrate/g-C3N5 for Enhanced Photocatalytic Tetracycline Hydrochloride Degradation. J. Mater. Sci. 2025, 60, 19675–19694. [Google Scholar] [CrossRef]
- Li, F.; Cheng, Y.; Li, P.; Yu, G. Study on the Performance of BiOCl Photocatalyst for Degradation of Tetracycline Hydrochloride. Separations 2025, 12, 242. [Google Scholar] [CrossRef]
- Yang, X.; Li, Y.; Wang, J.; Li, M.; Zhang, H.; Yang, J.; Wei, L. Synthesis and Application of Transition Metal Ions Co-Doped CdS Photocatalyst for Tetracycline Hydrochloride Degradation. Res. Chem. Intermed. 2025, 51, 5315–5388. [Google Scholar] [CrossRef]
- Cao, H.; Shi, X.; Wang, Y. Construction of a Z-Scheme CoFe2O4/Bi4O7 Heterojunction for Enhanced Photocatalytic Degradation of Tetracycline Hydrochloride. Mater. Lett. 2025, 398, 138974. [Google Scholar] [CrossRef]
- Feng, F.; Zhang, X.; Xu, X.; Huang, Q.; Yin, H.; Li, R.; Wu, G.; Xing, W. Construction of 2D/3D Biochar Modified g-C3N4 for Efficient Removal of Tetracycline Hydrochloride via Photocatalytic PMS Activation. Opt. Mater. 2025, 167, 117246. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, X.; Yang, X.; Liu, C.; Qian, X.; Sun, T.; Chang, W.; Zhang, J.; Chen, Z. Synthesis of Novel 1T/2H-MoS2 from MoO3 Nanowires with Enhanced Photocatalytic Performance. Nanomaterials 2020, 10, 1124. [Google Scholar] [CrossRef]
- Rosa, D.; Remmani, R.; Bavasso, I.; Bracciale, M.P.; Di Palma, L. Biochar Supported Fe–TiO2 Composite for Wastewater Treatment: Solid-State Synthesis and Mechanistic Insights. Chem. Eng. Sci. 2025, 317, 122076. [Google Scholar] [CrossRef]
- Wang, D.; Yin, F.-X.; Cheng, B.; Xia, Y.; Yu, J.-G.; Ho, W.-K. Enhanced Photocatalytic Activity and Mechanism of CeO2 Hollow Spheres for Tetracycline Degradation. Rare Met. 2021, 40, 2369–2380. [Google Scholar] [CrossRef]
- Liang, H.; Wang, A.; Cheng, R.; Chen, F.; Kannan, P.; Molochas, C.; Tsiakaras, P. Bi, K Co-Doped Graphitic Phase Carbon Nitride for Efficient Photocatalytic H2O2 Production. Chem. Eng. J. 2024, 489, 151145. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).