A Comparative Techno-Economic Analysis of Waste Cooking Oils and Chlorella Microalgae for Sustainable Biodiesel Production
Abstract
1. Introduction
2. Materials and Methodology
2.1. Raw Materials
2.2. Production Process of Biodiesel from Microalgae
2.3. Production of Biodiesel from Waste Cooking Oils
- Type of alcohol: In the case of transesterification of domestic WCO, which commonly consists of sunflower oil, ethanol was selected over the use of methanol. This is because it is less toxic and causes less environmental and health hazards in case of spill or evaporation. But in the case of restaurant WCO, typically methanol was utilized based on its increased reactivity in transesterification and its lower cost.
- Catalyst type: The catalyst to oil molar ratio forms a very important parameter, which determines the quantity and quality of biodiesel obtained during transesterification. The transesterification reaction may be catalyzed with alkalis, acids, or enzyme catalysts. Studies have shown that the use of alkali-catalyzed reactions, usually with catalysts such as KOH and NaOH, yields a higher amount and purity of biodiesel. These reactions occur over a smaller time span as compared to acid-based or enzymatic processes [42,47]. The present study used NaOH as the base catalyst for the esterification process.
- Alcohol to oil ratio: Regarding restaurant WCO, the biodiesel yield was experimentally measured by varying the methanol/oil molar ratio using 2 wt% of NaOH catalyst at a constant temperature of 60 °C for one hour. Equation (1) represents the transesterification reaction. During this reaction, the triglyceride supplied by WCO undergoes a reaction with alcohol (methanol or ethanol) in the presence of a catalyst (NaOH) to form biodiesel (fatty acid methyl esters) and glycerol. In this reaction, the triglycerides in restaurant WCO (palm oil) and household WCO (sunflower oil) were transformed into biodiesel, where it was more efficient in the household WCO, since it is less viscous and can combine better with ethanol.

- Transesterification temperature and duration: Regarding previous research works, the esterification process using ethanol is carried out at a temperature range of 55–65 °C compared to a range of 54–60 °C in the case of using methanol [48]. Thus, for domestic WCO, transesterification reaction with ethanol (boiling point of 78.37 °C) in the presence of NaOH catalyst was performed at a temperature range of 55–65 °C. However, this reaction was carried out at a range of 54–60 °C in the case of restaurant WCO, which used methanol (boiling point of 64.7 °C) for the esterification process. The duration of the transesterification reaction for both domestic and restaurant WCO was one hour.
- Considering the optimum alcohol to oil molar ratio of 6:1, 22.69 g of methanol or 32.09 g of ethanol was needed to react with 100 g of the pretreated WCO. A quantity of 100 g of the pretreated WCO was put in a 250 mL Erlenmeyer flask, while the required amount of alcohol was kept in another flask of 100 mL.
- The appropriate amount of catalyst (NaOH or KOH) was then added carefully to the alcohol (e.g., methanol or ethanol) to avoid skin contact, since alkali catalysts are potentially irritating or burn the skin. The catalyst was then added to the alcohol and thoroughly dissolved using a magnetic stirrer at a speed of around 500 rpm, and a temperature of 50 °C, to produce a homogeneous alkoxide solution. The flask holding the solution was wrapped with aluminum foil after full dissolution to avoid light and moisture exposure. In the meantime, the flask with the filtered WCO was warmed to 60 °C with mild mixing by a heated magnetic stirrer with the aim of establishing a homogenous temperature and supporting the subsequent transesterification process.
- The catalyst–alcohol solution (e.g., sodium methoxide in methanol) was slowly added to the hot WCO placed in a reaction flask by adding the prepared solution through a 90 mm funnel. Aluminum foil was then added to cover the flask and prevent exposure of the mixture to light and the moisture, after which the contents were mixed with a magnetic stirrer adjusted to around 6.5 rpm to enable mixing during the transesterification reaction.
- Once the transesterification reaction had achieved completion, the resulting reaction mixture was settled in a 250 mL separatory funnel for approximately 12 h. In this time two different layers were formed due to the lack of solubility between biodiesel and glycerol, which creates a clear separation of glycerol and biodiesel. Glycerol appeared as the lower layer due to its higher density. The lower layer was drained out of the funnel, leaving biodiesel (fatty acid methyl esters) as the upper layer in the separatory funnel to be further processed. The biodiesel was finally decanted into a round-bottom flask and heated in a heating mantle with reduced pressure. A condenser was used to recover any remaining excess methanol at about 60–70 °C, just above the methanol boiling point of 64.7 °C. This procedure extracts any remaining methanol in the biodiesel to a concentration that can be reused in the same process of biodiesel manufacturing. The obtained biodiesel was then further purified, e.g., water washed, to free it of impurities.
2.4. Analysis and Statistical Experimental Design
2.5. Analytical Methods for Biodiesel Characterization
| Property | Unit | Measured Value | Limits | Test Method | |
|---|---|---|---|---|---|
| Min. | Max. | ||||
| Minimum ester content | wt% | 98.2 | 96.5 | - | EN 14103:2020 [54] |
| Density at 15 °C | g/mL | 0.88 | 0.86 | 0.90 | ISO 12185:2024 |
| Viscosity at 40 °C | mPa·s | 3.87 | 2.9 | 6 | ISO 3104:2023 |
| Flash point | °C | 152 | 120 | - | ISO 3679:2022 [55] |
| Carbon residue | wt% | 0.1 | - | 0.3 | ISO 10370:2014 [56] |
| Water content | mg/kg | 482 | - | 500 | ISO 12937:2000 [57] |
| Acid value | mg KOH/g | 0.42 | - | 0.5 | EN 14104:2021 [58] |
| Iodine value | g I2/100 g | 111.5 | - | 120 | EN 14111:2022 [59] |
| Linolenic acid methyl ester | wt% | 5.7 | - | 12 | EN 14103:2020 |
| Methanol content | wt% | 0.08 | - | 0.2 | EN 14110:2019 [60] |
3. Results and Discussion
3.1. Biodiesel Production from Chlorella sp. Microalgae
3.2. Biodiesel from Waste Cooking Oils
3.2.1. Biodiesel from Household WCO and Ethanol
3.2.2. Biodiesel from Restaurant WCO and Methanol
3.3. RSM and ANOVA Analysis
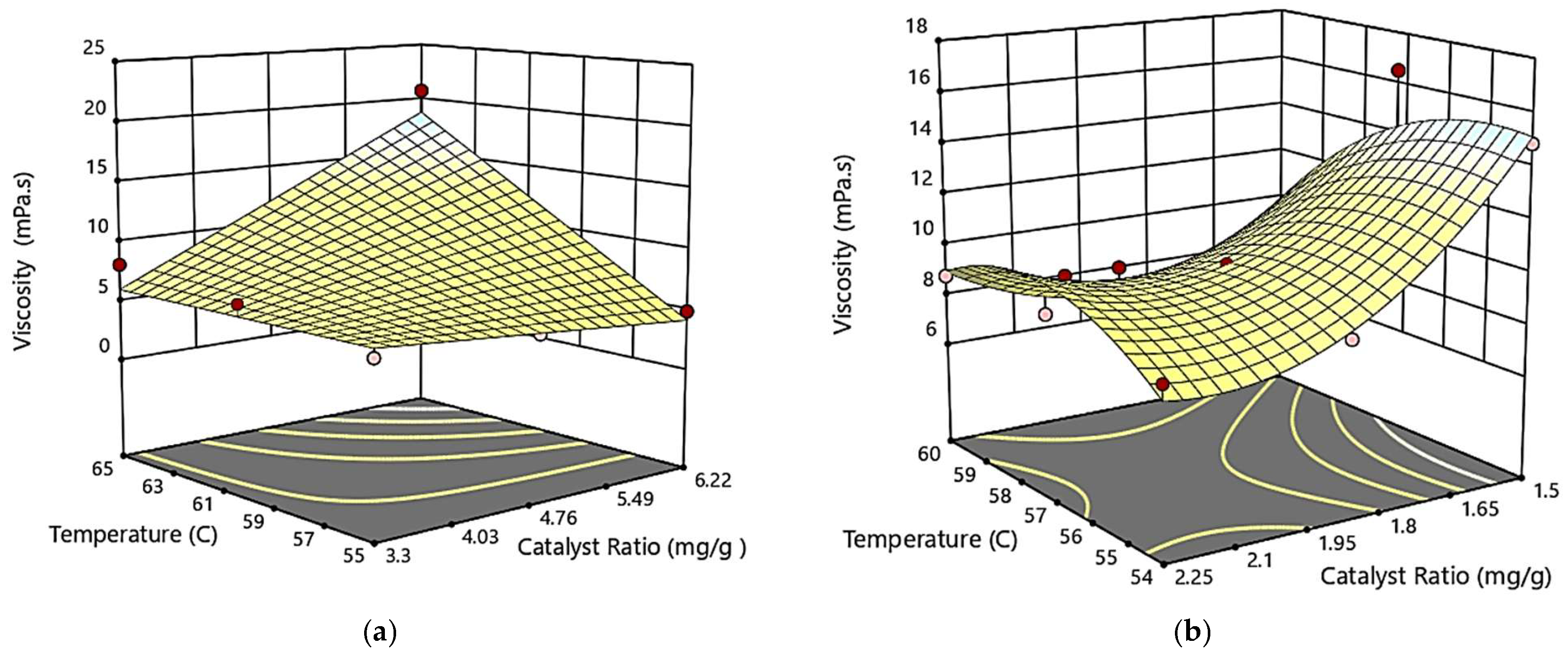
3.3.1. Combined Effect of Temperature and Catalyst Ratio on Biodiesel Viscosity
−0.195477*T2 + 17.01833*CR2
3.3.2. The Combined Effect of Catalyst Ratio and Temperature on Biodiesel Yield
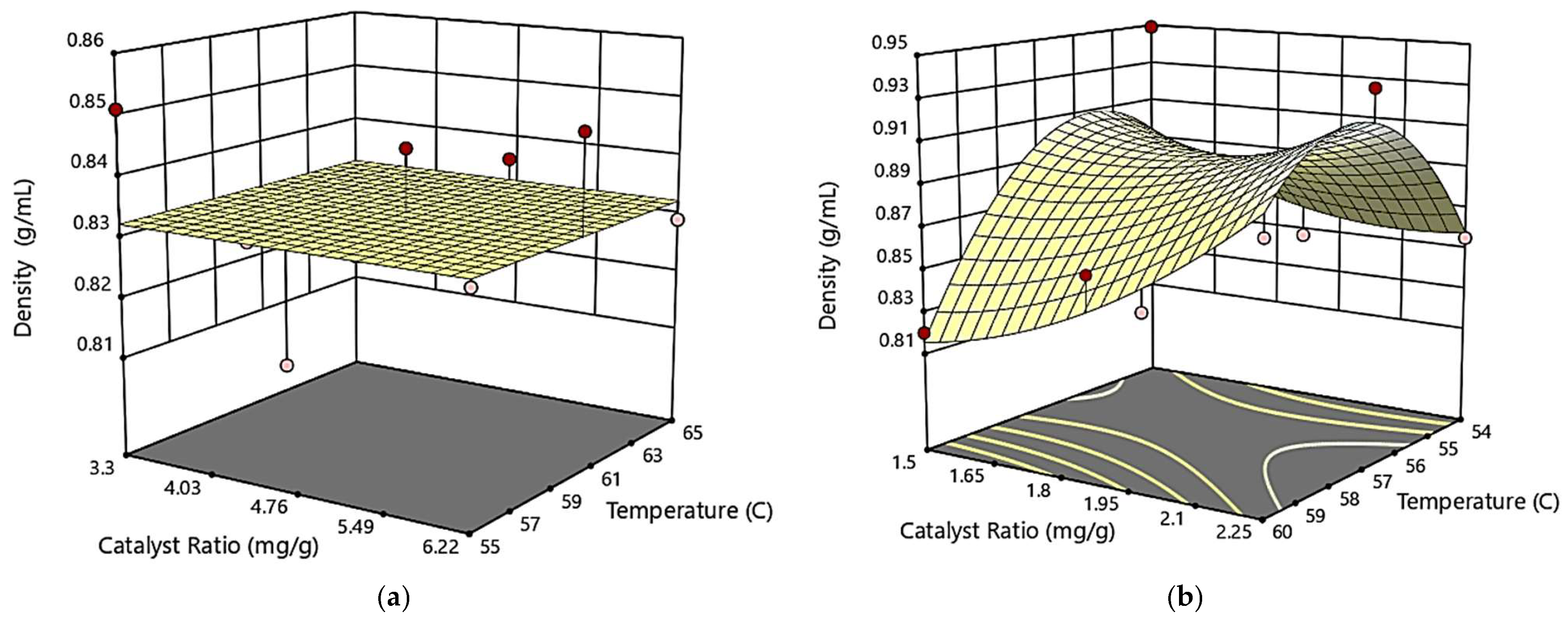
3.3.3. The Combined Effect of Catalyst Ratio and Temperature on Biodiesel Density
−0.005065*T2 + 0.1016*CR2
3.4. Comprehensive Analysis of WCOs and Chlorella sp. Microalgae as Feedstocks for Biodiesel Production
3.5. Economic Comparison of Biodiesel Production from WCO and Microalgae
| Parameter | Household WCO | Restaurant WCO | Microalgae (Chlorella) |
|---|---|---|---|
| Capital Costs | |||
| Filtration Systems | USD 60,000 [107] | USD 80,000 [107] | - |
| Heating Units | USD 30,000 [107] | USD 40,000 [107] | - |
| Transesterification Reactors | USD 100,000 [107] | USD 100,000 [107] | USD 100,000 [108] |
| Photobioreactors/Glass Pools | - | - | USD 480,000 [108] |
| Harvesting Equipment | - | - | USD 140,000 [108] |
| Total Capital Costs | USD 190,000 | USD 220,000 | USD 720,000 |
| Operational Costs (per L) | |||
| Feedstock | USD 0.05 [109] | USD 0.07 [109] | USD 0.10–0.20 [108] |
| Pretreatment | USD 0.05–0.10 [109] | USD 0.10–0.15 [109] | - |
| Transesterification | USD 0.10–0.20 [109] | USD 0.10–0.20 [109] | USD 0.10–0.20 [109] |
| Cultivation (Nutrients, CO2, Light) | - | - | USD 0.70–0.90 [108] |
| Harvesting and Drying | - | - | USD 0.30–0.50 [110] |
| Energy (Pretreatment and Processing) | USD 0.05–0.10 [106] | USD 0.05–0.10 [106] | USD 0.20–0.30 [106] |
| Total Operational Costs (per L) | USD 0.25–0.45 | USD 0.32–0.52 | USD 1.40–2.10 |
| Byproduct Revenue (per L) | |||
| Glycerol | USD 0.05–0.07 [111] | USD 0.05–0.07 [111] | USD 0.05–0.07 [111] |
| Algal Biomass | - | - | USD 0.10–0.20 [112] |
| Total Byproduct Revenue (per L) | USD 0.05–0.07 [111] | USD 0.05–0.07 [111] | USD 0.15–0.27 [111,112] |
| Net Production Costs (per L) | USD 0.20–0.38 [109,111] | USD 0.27–0.45 [109,111] | USD 1.25–1.83 [108,110,112] |
| Biodiesel Revenue (per L) | USD 0.70–1.00 [105] | USD 0.70–1.00 [105] | USD 0.70–1.00 [105] |
| Annual Cash Flow | USD 320,000–800,000 | USD 250,000–730,000 | (−USD 1,130,000)–(−USD 250,000) |
| ROI (%) | 168.42–421.05% | 113.64–331.82% | (−156.94)–(−34.72) |
| Payback Period (Years) | 0.24–0.59 | 0.3–0.88 | Negative cash flow |
4. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- International Energy Agency. Governments Have Unleashed a Wave of Clean Energy Policies to Benefit from the New Energy Economy. IEA News. 26 September 2024. Available online: https://www.iea.org/news/governments-have-unleashed-a-wave-of-clean-energy-policies-to-benefit-from-the-new-energy-economy (accessed on 23 June 2022).
- El-Araby, R. Biofuel production: Exploring renewable energy solutions for a greener future. Biotechnol. Biofuels 2024, 17, 129. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Energy. Biofuel Basics. Energy.gov. Available online: https://www.energy.gov/eere/bioenergy/biofuel-basics (accessed on 23 June 2022).
- Saravanan, A.; Deivayanai, V.C.; Kumar, P.S.; Rangasamy, G.; Varjani, S. CO2 Bio-Mitigation Using Genetically Modified Algae and Biofuel Production towards a Carbon Net-Zero Society. Bioresour. Technol. 2022, 363, 127982. [Google Scholar] [CrossRef]
- Siddiki, S.Y.A.; Mofijur, M.; Kumar, P.S.; Ahmed, S.F.; Inayat, A.; Kusumo, F.; Badruddin, I.A.; Yunus Khan, T.M.; Nghiem, L.D.; Ong, H.C.; et al. Microalgae Biomass as a Sustainable Source for Biofuel, Biochemical and Biobased Value-Added Products: An Integrated Biorefinery Concept. Fuel 2022, 307, 121782. [Google Scholar] [CrossRef]
- Paladino, O.; Neviani, M. Sustainable Biodiesel Production by Transesterification of Waste Cooking Oil and Recycling of Wastewater Rich in Glycerol as a Feed to Microalgae. Sustainability 2022, 14, 273. [Google Scholar] [CrossRef]
- Goh, B.H.H.; Ong, H.C.; Cheah, M.Y.; Chen, W.-H.; Yu, K.L.; Mahlia, T.M.I. Sustainability of Direct Biodiesel Synthesis from Microalgae Biomass: A Critical Review. Renew. Sustain. Energy Rev. 2019, 107, 59–74. [Google Scholar] [CrossRef]
- Maroa, S.; Inambao, F. Waste to Energy Feedstock Sources for the Production of Biodiesel as Fuel Energy in Diesel Engine—A Review. Adv. Sci. Technol. Eng. Syst. 2021, 6, 409–446. [Google Scholar]
- Ulukardesler, A.H. Biodiesel Production from Waste Cooking Oil Using Different Types of Catalysts. Processes 2023, 11, 2035. [Google Scholar] [CrossRef]
- Cerón Ferrusca, M.; Romero, R.; Martínez, S.L.; Ramírez-Serrano, A.; Natividad, R. Biodiesel Production from Waste Cooking Oil: A Perspective on Catalytic Processes. Processes 2023, 11, 1952. [Google Scholar] [CrossRef]
- Manikandan, G.; Kanna, P.R.; Taler, D.; Sobota, T. Review of Waste Cooking Oil (WCO) as a Feedstock for Biofuel—Indian Perspective. Energies 2023, 16, 1739. [Google Scholar] [CrossRef]
- Nazir, M.H.; Ayoub, M.; Shamsuddin, R.B.; Malik, Z. Production of Biodiesel from Waste Cooking Oil Using Sugarcane Bagasse Derived Acid Activated Catalyst and Microwave as Heating Source. Solid. State Technol. 2020, 63, 3839–3846. [Google Scholar]
- Bharti, M.K.; Chalia, S.; Thakur, P.; Sridhara, S.N.; Thakur, A.; Sharma, P.B. Nanoferrites Heterogeneous Catalysts for Biodiesel Production from Soybean and Canola Oil: A Review. Environ. Chem. Lett. 2021, 19, 3727–3746. [Google Scholar] [CrossRef]
- Yaakob, M.A.; Mohamed, R.M.S.R.; Al-Gheethi, A.; Aswathnarayana Gokare, R.; Ambati, R.R. Influence of Nitrogen and Phosphorus on Microalgal Growth, Biomass, Lipid, and Fatty Acid Production: An Overview. Cells 2021, 10, 393. [Google Scholar] [CrossRef]
- Faruque, M.O.; Razzak, S.A.; Hossain, M.M. Application of Heterogeneous Catalysts for Biodiesel Production from Microalgal Oil—A Review. Catalysts 2020, 10, 1025. [Google Scholar] [CrossRef]
- Praveena, V.; Martin, L.J.; Matijošius, J.; Aloui, F.; Pugazhendhi, A.; Varuvel, E.G. A Systematic Review on Biofuel Production and Utilization from Algae and Waste Feedstocks—A Circular Economy Approach. Renew. Sustain. Energy Rev. 2024, 192, 114178. [Google Scholar] [CrossRef]
- Gaurav, K.; Neeti, K.; Singh, R. Microalgae-Based Biodiesel Production and Its Challenges and Future Opportunities: A Review. Green. Technol. Sustain. 2024, 2, 100060. [Google Scholar] [CrossRef]
- Pandey, S.; Narayanan, I.; Selvaraj, R.; Varadavenkatesan, T.; Vinayagam, R. Biodiesel Production from Microalgae: A Comprehensive Review on Influential Factors, Transesterification Processes, and Challenges. Fuel 2024, 367, 131547. [Google Scholar] [CrossRef]
- Bošnjaković, M.; Sinaga, N. The Perspective of Large-Scale Production of Algae Biodiesel. Appl. Sci. 2020, 10, 8181. [Google Scholar] [CrossRef]
- Lee, J.-C.; Lee, B.; Heo, J.; Kim, H.-W.; Lim, H. Techno-Economic Assessment of Conventional and Direct-Transesterification Processes for Microalgal Biomass to Biodiesel Conversion. Bioresour. Technol. 2019, 294, 122173. [Google Scholar] [CrossRef]
- Mittal, A.; Negi, G.S.; Singh, P.; Kaur, S.; Dayawati; Kumar, A.V. Green Catalysts for Sustainable Biodiesel Production from Waste Cooking Oil. E3S Web Conf. 2024, 511, 01019. [Google Scholar] [CrossRef]
- Salam, K.A.; Velasquez-Orta, S.B.; Harvey, A.P. A Sustainable Integrated In Situ Transesterification of Microalgae for Biodiesel Production and Associated Co-Products—A Review. Renew. Sustain. Energy Rev. 2016, 65, 1179–1198. [Google Scholar] [CrossRef]
- Chong, J.W.R.; Khoo, K.S.; Chew, K.W.; Ting, H.-Y.; Show, P.L. Trends in Digital Image Processing of Isolated Microalgae by Incorporating Classification Algorithm. Biotechnol. Adv. 2023, 63, 108095. [Google Scholar] [CrossRef]
- Khan, M.I.; Chin, J.H.; Rashmi; Kim, J.D. The Promising Future of Microalgae: Current Status, Challenges, and Optimization of a Sustainable and Renewable Industry for Biofuels, Feed, and Other Products. Microb. Cell Fact. 2018, 17, 36. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Zhu, L.; Li, S.; Hu, T.; Chu, R.; Mo, F.; Hu, D.; Liu, C.; Li, B. A Comprehensive Review on Cultivation and Harvesting of Microalgae for Biodiesel Production: Environmental Pollution Control and Future Directions. Bioresour. Technol. 2020, 301, 122804. [Google Scholar] [CrossRef]
- Salami, R.; Kordi, M.; Bolouri, P.; Delangiz, N.; Lajayer, B.A. Algae-Based Biorefinery as a Sustainable Renewable Resource. Circ. Econ. Sustain. 2021, 1, 1349–1365. [Google Scholar] [CrossRef]
- Sudhanthiran, M.C.; Perumalsamy, M. Bioremediation of Dairy Industry Wastewater and Assessment of Nutrient Removal Potential of Chlorella vulgaris. Biomass Conv. Bioref. 2024, 14, 10335–10346. [Google Scholar]
- Taparia, T.; Manjari, M.V.S.S.; Mehrotra, R.; Shukla, P.; Mehrotra, S. Developments and Challenges in Biodiesel Production from Microalgae: A Review. Biotechnol. Appl. Biochem. 2016, 63, 715–726. [Google Scholar] [CrossRef]
- Razzak, S.A.; Bahar, K.; Islam, K.M.O.; Haniffa, A.K.; Faruque, M.O.; Hossain, S.M.Z.; Hossain, M.M. Microalgae Cultivation in Photobioreactors: Sustainable Solutions for a Greener Future. Green Chem. Eng. 2024, 5, 418–439. [Google Scholar] [CrossRef]
- Gülyurt, M.Ö.; Özçimen, D.; İnan, B. Biodiesel Production from Chlorella protothecoides Oil by Microwave-Assisted Transesterification. Int. J. Mol. Sci. 2016, 17, 579. [Google Scholar] [CrossRef] [PubMed]
- Mandotra, S.K.; Kumar, R.; Suseela, M.R.; Ramteke, P.W. Fresh Water Green Microalga Scenedesmus abundans: A Potential Feedstock for High Quality Biodiesel Production. Bioresour. Technol. 2014, 156, 42–47. [Google Scholar] [CrossRef]
- Osman, M.E.H.; Abo-Shady, A.M.; Gheda, S.F.; Desoki, S.M.; Elshobary, M.E. Unlocking the Potential of Microalgae Cultivated on Wastewater Combined with Salinity Stress to Improve Biodiesel Production. Environ. Sci. Pollut. Res. 2023, 30, 114610–114624. [Google Scholar] [CrossRef] [PubMed]
- Karpagam, R.; Rani, K.; Ashokkumar, B.; Moorthy, I.G.; Dhakshinamoorthy, A.; Varalakshmi, P. Green Energy from Coelastrella sp. M-60: Bio-Nanoparticles Mediated Whole Biomass Transesterification for Biodiesel Production. Fuel 2020, 279, 118490. [Google Scholar] [CrossRef]
- Risjani, Y. Editorial: Marine Microalgae: From Biodiversity to Biotechnology. Front. Mar. Sci. 2025, 12, 1579115. [Google Scholar] [CrossRef]
- Ranjan, S.; Gupta, P.K.; Gupta, S.K. Comprehensive Evaluation of High-Rate Algal Ponds: Wastewater Treatment and Biomass Production. In Application of Microalgae in Wastewater Treatment; Gupta, S., Bux, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2019; Volume 2, pp. 531–552. [Google Scholar] [CrossRef]
- Voloshin, R.; Rodionova, M.V.; Zharmukhamedov, S.K.; Veziroglu, T.N.; Allakhverdiev, S.I. Review: Biofuel Production from Plant and Algal Biomass. Altern. Energy Ecol. (ISJAEE) 2019, 7–9, 12–31. [Google Scholar] [CrossRef]
- Tien Thanh, N.; Mostapha, M.; Lam, M.K.; Ishak, S.; Dasan, Y.K.; Lim, J.W.; Tan, I.S.; Lau, S.Y.; Chin, B.L.F.; Hadibarata, T. Fundamental Understanding of In-Situ Transesterification of Microalgae Biomass to Biodiesel: A Critical Review. Energy Convers. Manag. 2022, 270, 116212. [Google Scholar] [CrossRef]
- Bibi, F.; Ali, M.I.; Ahmad, M.; Bokhari, A.; Khoo, K.S.; Zafar, M.; Asif, S.; Mubashir, M.; Han, N.; Show, P.L. Production of Lipids Biosynthesis from Tetradesmus nygaardii Microalgae as a Feedstock for Biodiesel Production. Fuel 2022, 326, 124985. [Google Scholar] [CrossRef]
- Hegel, P.; Martín, L.; Popovich, C.; Damiani, C.; Pancaldi, S.; Pereda, S.; Leonardi, P. Biodiesel Production from Neochloris oleoabundans by Supercritical Technology. Chem. Eng. Process. Process Intensif. 2017, 121, 232–239. [Google Scholar] [CrossRef]
- Huang, Y.; Ding, W.; Zhou, X.; Jin, W.; Han, W.; Chi, K.; Chen, Y.; Zhao, Z.; He, Z.; Jiang, G. Sub-Pilot Scale Cultivation of Tetradesmus dimorphus in Wastewater for Biomass Production and Nutrients Removal: Effects of Photoperiod, CO2 Concentration and Aeration Intensity. J. Water Process Eng. 2022, 49, 103003. [Google Scholar] [CrossRef]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for Biodiesel Production and Other Applications: A Review. Renew. Sustain. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef]
- Encinar, J.M.; González, J.F.; Rodríguez-Reinares, A. Ethanolysis of used frying oil: Biodiesel preparation and characterization. Fuel Process. Technol. 2007, 88, 513–522. [Google Scholar] [CrossRef]
- Blair, M.F.; Kokabian, B.; Gude, V.G. Light and Growth Medium Effect on Chlorella vulgaris Biomass Production. J. Environ. Chem. Eng. 2014, 2, 665–674. [Google Scholar] [CrossRef]
- Neag, E.; Stupar, Z.; Maicaneanu, S.A.; Roman, C. Advances in Biodiesel Production from Microalgae. Energies 2023, 16, 1129. [Google Scholar] [CrossRef]
- Brennan, L.; Owende, P. Biofuels from Microalgae—A Review of Technologies for Production, Processing, and Extractions of Biofuels and Co-Products. Renew. Sustain. Energy Rev. 2010, 14, 557–577. [Google Scholar] [CrossRef]
- Cazarolli, J.C.; Silva, T.L.; Lobato, M.R.; de Brito, J.R.; Rampelotto, P.H.; de Souza Rocha, J.V.; de Azambuja, A.O.; Mann, M.B.; Ferrão, M.F.; Peralba, M.C.R.; et al. Impact of water content on microbial growth in Brazilian biodiesel during simulated storage. Fuel 2021, 297, 120761. [Google Scholar] [CrossRef]
- Efavi, J.K.; Kanbogtah, D.; Apalangya, V.; Nyankson, E.; Tiburu, E.K.; Dodoo-Arhin, D.; Onwona-Agyeman, B.; Yaya, A. The Effect of NaOH Catalyst Concentration and Extraction Time on the Yield and Properties of Citrullus vulgaris Seed Oil as a Potential Biodiesel Feedstock. S. Afr. J. Chem. Eng. 2018, 25, 97–102. [Google Scholar] [CrossRef]
- Elgharbawy, A.A.; Sadik, W.; Sadek, O.; Kasaby, M. Transesterification Reaction Conditions and Low-Quality Feedstock Treatment Processes for Biodiesel Production—A Review. J. Pet. Min. Eng. 2021, 23, 89–94. [Google Scholar] [CrossRef]
- Meher, L.C.; Vidya Sagar, D.V.; Naik, S.N. Technical aspects of biodiesel production by transesterification—A review. Renew. Sustain. Energy Rev. 2006, 10, 248–268. [Google Scholar] [CrossRef]
- Myers, R.H.; Montgomery, D.C.; Anderson-Cook, C.M. Response Surface Methodology: Process and Product Optimization Using Designed Experiments, 4th ed.; Wiley: Hoboken, NJ, USA, 2016. [Google Scholar]
- Box, G.E.P.; Wilson, K.B. On the Experimental Attainment of Optimum Conditions. In Breakthroughs in Statistics; Kotz, S., Johnson, N.L., Eds.; Springer Series in Statistics; Springer: New York, NY, USA, 1992. [Google Scholar] [CrossRef]
- ISO 12185:2024; Crude Petroleum and Petroleum Products—Determination of Density—Oscillating U-Tube Method. ISO: Geneva, Switzerland, 2024.
- ISO 3104:2023; Petroleum Products—Transparent and Opaque Liquids—Determination of Kinematic Viscosity and Calculation of Dynamic Viscosity. ISO: Geneva, Switzerland, 2023.
- EN 14103:2020; Fat and Oil Derivatives—Fatty Acid Methyl Esters (FAME)—Determination of Ester and Linolenic Acid Methyl Ester Contents. CEN: Brussels, Belgium, 2020.
- ISO 3679:2022; Determination of Flash Point—Rapid Equilibrium Closed Cup Method. ISO: Geneva, Switzerland, 2022.
- ISO 10370:2014; Petroleum Products—Determination of Carbon Residue—Micro Method. ISO: Geneva, Switzerland, 2014.
- ISO 12937:2000; Petroleum Products—Determination of Water—Coulometric Karl Fischer Titration Method. ISO: Geneva, Switzerland, 2000.
- EN 14104:2021; Fat and Oil Derivatives—Fatty Acid Methyl Esters (FAME)—Determination of Acid Value. CEN: Brussels, Belgium, 2021.
- EN 14111:2022; Fat and Oil Derivatives—Fatty Acid Methyl Esters (FAME)—Determination of Iodine Value. CEN: Brussels, Belgium, 2022.
- EN 14110:2019; Fat and Oil Derivatives—Fatty Acid Methyl Esters (FAME)—Determination of Methanol Content. CEN: Brussels, Belgium, 2019.
- Milano, J.; Ong, H.C.; Masjuki, H.H.; Chong, W.T.; Lam, M.K.; Loh, P.K.; Vellayan, V. Microalgae biofuels as an alternative to fossil fuel for power generation. Renew. Sustain. Energy Rev. 2016, 58, 180–197. [Google Scholar] [CrossRef]
- EN 14214:2012+A2:2019; Liquid Petroleum Products—Fatty Acid Methyl Esters (FAME) for Use in Diesel Engines and Heating Applications—Requirements and Test Methods. CEN: Brussels, Belgium, 2019.
- ASTM D6751-23a; Standard Specification for Biodiesel Fuel Blend Stock (B100) for Middle Distillate Fuels. ASTM International: West Conshohocken, PA, USA, 2023.
- Saber, H.; Galal, H.R.; Abo-Eldahab, M.; Alwaleed, E. Enhancing the biodiesel production in the green alga Chlorella vulgaris by heavy metal stress and prediction of fuel properties from fatty acid profiles. Environ. Sci. Pollut. Res. 2024, 31, 35952–35968. [Google Scholar] [CrossRef]
- Rasoul-Amini, S.; Montazeri-Najafabady, N.; Mobasher, M.A.; Hoseini-Alhashemi, S.; Ghasemi, Y. Chlorella sp.: A new strain with highly saturated fatty acids for biodiesel production in bubble-column photobioreactor. Appl. Energy 2011, 88, 3354–3356. [Google Scholar] [CrossRef]
- Scion Instruments. Determination of Total FAME and Linolenic Acid Methyl Esters in Biodiesel According to EN 14103. Scion Instruments Application Note 2023, AN076v2, 1–3. Available online: https://scioninstruments.com/wp-content/uploads/2024/02/AN076v2-Determination-of-Total-FAME-and-Linolenic-Acid-Methyl-Esters-in-Biodiesel-According-to-EN14103.pdf (accessed on 5 July 2025).
- Thawornprasert, J.; Duangsuwan, W.; Somnuk, K. Investigating the Effect of a Diesel–Refined Crude Palm Oil Methyl Ester–Hydrous Ethanol Blend on the Performance and Emissions of an Unmodified Direct Injection Diesel Engine. ACS Omega 2023, 8, 9275–9290. [Google Scholar] [CrossRef]
- Suzihaque, M.U.H.; Alwi, H.; Ibrahim, U.K.; Abdullah, S.; Haron, N. Biodiesel production from waste cooking oil: A brief review. Mater. Today Proc. 2022, 63, S490–S495. [Google Scholar] [CrossRef]
- Degfie, T.A.; Mamo, T.T.; Mekonnen, Y.S. Optimized Biodiesel Production from Waste Cooking Oil (WCO) using Calcium Oxide (CaO) Nano-catalyst. Sci. Rep. 2019, 9, 18982. [Google Scholar]
- Park, S.H.; Khan, N.; Lee, S.; Zimmermann, K.; Derosa, M.; Hamilton, L.; Hudson, W.; Hyder, S.A.; Serratos, M.; Sheffield, E.C.; et al. Biodiesel Production from Locally Sourced Restaurant Waste Cooking Oil and Grease: Synthesis, Characterization, and Performance Evaluation. ACS Omega 2019, 4, 7775–7784. [Google Scholar] [CrossRef]
- Monika; Banga, S.; Pathak, V.V. Biodiesel production from waste cooking oil: A comprehensive review on the application of heterogenous catalysts. Energy Nexus 2023, 10, 100209. [Google Scholar] [CrossRef]
- Ahmed, S.L.; Bai, M.T.; Sridevi, V.; Moyila, S. Effect of Mole Ratio and Catalyst Concentration on Yield and Properties of Biodiesel. I-Manag. J. Future Eng. Technol. 2015, 10, 22–27. [Google Scholar] [CrossRef]
- Ramírez-Verduzco, L.F. Density and viscosity of biodiesel as a function of temperature: Empirical models. Renew. Sustain. Energy Rev. 2013, 19, 652–665. [Google Scholar] [CrossRef]
- Scientific Net. Viscosity of Three Biodiesel Fuels: Experimental Data vs. Prediction Models. Adv. Mater. Res. 2011, 236–238, 3032. [Google Scholar] [CrossRef]
- Pinzi, S.; Gandia, L.M.; Arzamendi, G.; Ruiz, J.J.; Dorado, M.P. Influence of Vegetable Oils Fatty Acid Composition on Reaction Temperature and Glycerides Conversion to Biodiesel during Transesterification. Bioresour. Technol. 2011, 102, 1044–1050. [Google Scholar] [CrossRef]
- Worapun, I.; Pianthong, K.; Thaiyasuit, P. Optimization of biodiesel production from crude palm oil using ultrasonic irradiation assistance and response surface methodology. J. Chem. Technol. Biotechnol. 2012, 87, 189–197. [Google Scholar] [CrossRef]
- Leung, D.Y.C.; Wu, X.; Leung, M.K.H. A review on biodiesel production using catalyzed transesterification. Appl. Energy 2010, 87, 1083–1095. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, S.; Yang, R.; Yan, Y. Biodiesel production from vegetable oil using heterogenous acid and alkali catalyst. Fuel 2010, 89, 2939–2944. [Google Scholar] [CrossRef]
- Demirbas, A. Biodiesel: A Realistic Fuel Alternative for Diesel Engines, 1st ed.; Springer: London, UK, 2008; ISBN 9781846289941. [Google Scholar]
- Yaakob, Z.; Mohammad, M.; Alherbawi, M.; Alam, Z.; Sopian, K. Overview of the production of biodiesel from waste cooking oil. Renew. Sustain. Energy Rev. 2013, 18, 184–193. [Google Scholar] [CrossRef]
- Atadashi, I.M.; Aroua, M.K.; Aziz, A.A. High quality biodiesel and its diesel engine application: A review. Renew. Sustain. Energy Rev. 2010, 14, 1999–2008. [Google Scholar] [CrossRef]
- Tran, N.N.; Tišma, M.; Budžaki, S.; McMurchie, E.J.; Gonzalez, O.M.M.; Hessel, V.; Ngothai, Y. Scale-up and economic analysis of biodiesel production from recycled grease trap waste. Appl. Energy 2018, 229, 142–150. [Google Scholar] [CrossRef]
- Tat, M.E.; Van Gerpen, J.H. Kinematic viscosity of biodiesel and its blends with diesel fuel. J. Amer. Oil Chem. Soc. 1999, 76, 1511–1513. [Google Scholar] [CrossRef]
- Issariyakul, T.; Dalai, A.K. Biodiesel from vegetable oils. Renew. Sustain. Energy Rev. 2014, 31, 446–471. [Google Scholar] [CrossRef]
- Vicente, G.; Martinez, M.; Aracil, J. Optimisation of integrated biodiesel production. Part I. A study of the biodiesel purity and yield. Bioresour. Technol. 2007, 98, 1724–1733. [Google Scholar] [CrossRef]
- Ahmad, G.; Imran, S.; Farooq, M.; Shah, A.N.; Anwar, Z.; Rehman, A.U.; Imran, M. Biodiesel Production from Waste Cooking Oil Using Extracted Catalyst from Plantain Banana Stem via RSM and ANN Optimization for Sustainable Development. Sustainability 2023, 15, 13599. [Google Scholar] [CrossRef]
- Nabizadeh, R.; García, I.L.; Sadjadi, S.; Yaghmaeian, K.; Mahvi, A.H.; Yunesian, M.; Baghani, A.N. Biodiesel production from supernatant waste cooking oil by a simple one-step technique: Calorific value optimization using response surface methodology. J. Mater. Cycles Waste Manag. 2023, 25, 3367–3383. [Google Scholar] [CrossRef]
- Danane, F.; Bessah, R.; Alloune, R.; Tebouche, L.; Madjene, F.; Kheirani, A.Y.; Bouabibsa, R. Experimental optimization of Waste Cooking Oil ethanolysis for biodiesel production using Response Surface Methodology (RSM). Sci. Technol. Energy Transit. 2022, 77, 14. [Google Scholar] [CrossRef]
- Bajwa, W.; Ikram, A.; Malik, M.A.I.; Razzaq, L.; Khan, A.R.; Latif, A.; Hussain, F.; Qazi, A. Optimization of biodiesel yield from waste cooking oil and sesame oil using RSM and ANN techniques. Heliyon 2024, 10, e34804. [Google Scholar] [CrossRef]
- Hamze, H.; Akiaa, M.; Yazdani, F. Optimization of biodiesel production from waste cooking oil by response surface methodology. Process Saf. Environ. Prot. 2015, 94, 1–10. [Google Scholar] [CrossRef]
- Yahya, S.; Wahab, S.K.M.; Harun, F.W. Optimization of biodiesel production from waste cooking oil using Fe-Montmorillonite K10 by response surface methodology. Renew. Energy 2020, 157, 164–172. [Google Scholar] [CrossRef]
- Milano, J.; Shamsuddin, A.H.; Silitonga, A.S.; Sebayang, A.H.; Siregar, M.A.; Masjuki, H.H.; Pulungan, M.A.; Chia, S.R.; Zamri, M.F.M.A. Tribological study on the biodiesel produced from waste cooking oil, waste cooking oil blend with Calophyllum inophyllum and its diesel blends on lubricant oil. Energy Rep. 2022, 8, 1578–1590. [Google Scholar] [CrossRef]
- Kulkarni, M.G.; Dalai, A.K. Waste Cooking Oils an Economical Source for Biodiesel: A Review. Ind. Eng. Chem. Res. 2006, 45, 2901–2913. [Google Scholar] [CrossRef]
- Xue, J.; Grift, T.E.; Hansen, A.C. Effect of Biodiesel on Engine Performances and Emissions. Renew. Sustain. Energy Rev. 2011, 15, 1098–1116. [Google Scholar] [CrossRef]
- Chhetri, A.B.; Watts, K.C.; Islam, M.R. Waste Cooking Oil as an Alternate Feedstock for Biodiesel Production. Energies 2008, 1, 3–18. [Google Scholar] [CrossRef]
- Kemp, W.H. Biodiesel Basics and Beyond: A Comprehensive Guide to Production and Use for the Home and Farm; Aztext Press: Tamworth, ON, Canada, 2006. [Google Scholar]
- Wijffels, R.H.; Barbosa, M.J. An Outlook on Microalgal Biofuels. Science 2010, 329, 796–799. [Google Scholar] [CrossRef] [PubMed]
- Lardon, L.; Helias, A.; Sialve, B.; Steyer, J.P.; Bernard, O. Life-Cycle Assessment of Biodiesel Production from Microalgae. Environ. Sci. Technol. 2009, 43, 6475–6481. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, A.L.; Kazamia, E.; Dennis, J.S.; Howe, C.J.; Scott, S.A.; Smith, A.G. Life-Cycle Assessment of Potential Algal Biodiesel Production in the United Kingdom: A Comparison of Raceways and Air-Lift Tubular Bioreactors. Energy Fuels 2010, 24, 4062–4077. [Google Scholar] [CrossRef]
- Demirbas, A. Biodiesel Production from Vegetable Oils via Catalytic and Non-Catalytic Supercritical Methanol Transesterification Methods. Prog. Energy Combust. Sci. 2005, 31, 466–487. [Google Scholar] [CrossRef]
- Msangi, S.; Rosegrant, M.W. Agriculture and the Environment: Linkages, Trade-Offs and Opportunities. Geo. Int’l Envtl. L. Rev. 2007, 19, 699–728. [Google Scholar]
- Yang, H.-H.; Chien, S.-M.; Lo, M.-Y.; Lan, J.C.-W.; Lu, W.-C.; Ku, Y.-Y. Effects of Biodiesel on Emissions of Regulated Air Pollutants and Polycyclic Aromatic Hydrocarbons under Engine Durability Testing. Atmos. Environ. 2007, 41, 7232–7240. [Google Scholar] [CrossRef]
- Sivasamy, A.; Cheah, K.Y.; Fornasiero, P.; Kemausour, F.; Zinoviev, S.; Miertus, S. Catalytic Applications in the Production of Biodiesel from Vegetable Oils. ChemSusChem 2009, 2, 278–300. [Google Scholar] [CrossRef]
- Rocha-Meneses, L.; Hari, A.; Inayat, A.; Yousef, L.A.; Alarab, S.; Abdallah, M.; Abdallah, S.; Ghenai, C.; Shanmugam, S.; Kikas, T. Recent advances on biodiesel production from waste cooking oil (WCO): A review of reactors, catalysts, and optimization techniques impacting the production. Fuel 2023, 348, 128514. [Google Scholar] [CrossRef]
- Avinash, A.; Murugesan, A. Economic analysis of biodiesel production from waste cooking oil. Energy Sources B Econ. Plan. Policy 2017, 12, 890–894. [Google Scholar] [CrossRef]
- Aboelazayem, O.; Gadalla, M.; Saha, B. Design and Simulation of an Integrated Process for Biodiesel Production from Waste Cooking Oil Using Supercritical Methanolysis. Energy 2018, 161, 299–307. [Google Scholar] [CrossRef]
- Haas, M.J.; McAloon, A.J.; Yee, W.C.; Foglia, T.A. A Process Model to Estimate Biodiesel Production Costs. Bioresour. Technol. 2006, 97, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Norsker, N.-H.; Barbosa, M.J.; Vermuë, M.H.; Wijffels, R.H. Microalgal Production—A Close Look at the Economics. Biotechnol. Adv. 2011, 29, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dubé, M.A.; McLean, D.D.; Kates, M. Biodiesel Production from Waste Cooking Oil: 2. Economic Assessment and Sensitivity Analysis. Bioresour. Technol. 2003, 90, 229–240. [Google Scholar] [CrossRef]
- Sharma, A.K.; Jaryal, S.; Sharma, S.; Dhyani, A.; Tewari, B.S.; Mahato, N. Biofuels from Microalgae: A Review on Microalgae Cultivation, Biodiesel Production Techniques and Storage Stability. Processes 2025, 13, 488. [Google Scholar] [CrossRef]
- César, A.d.S.; Werderits, D.E.; de Oliveira Saraiva, G.L.; Guabiroba, R.C.d.S. The potential of waste cooking oil as supply for the Brazilian biodiesel chain. Renew. Sust. Energy Rev. 2017, 72, 246–253. [Google Scholar] [CrossRef]
- Thomassen, G.; Egiguren Vila, U.; Van Dael, M.; Lemmens, B.; Van Passel, S. A techno-economic assessment of an algal-based biorefinery. Clean Technol. Environ. Policy 2016, 18, 1849–1862. [Google Scholar] [CrossRef]
- Aboelazayem, O.; Gadalla, M.; Saha, B. Valorisation of High Acid Value Waste Cooking Oil into Biodiesel Using Supercritical Methanolysis: Experimental Assessment and Techno-Economic Analysis. Energy 2018, 162, 408–420. [Google Scholar] [CrossRef]
- Kumar, D.; Singh, B. Algal Biorefinery: An Integrated Approach for Sustainable Biodiesel Production. Biomass Bioenergy 2019, 131, 105398. [Google Scholar] [CrossRef]
- Yang, J.; Fujiwara, T.; Geng, Q. Life cycle assessment of biodiesel fuel production from waste cooking oil in Okayama City. J. Mater. Cycles Waste Manag. 2017, 19, 1457–1467. [Google Scholar] [CrossRef]
- Mobin, S.M.A.; Alam, F.; Chowdhury, H. Environmental impact of algae-based biofuel production: A review. AIP Conf. Proc. 2022, 2681, 020084. [Google Scholar] [CrossRef]

| Nutrients for One Liter of Chlorella | Trace Element Solution Composition | ||
|---|---|---|---|
| Chemical | g L−1 | Chemical | g L−1 |
| KNO3 | 1.011 | H3BO3 | 0.0618 |
| NaH2PO4 | 0.0399 | MnSO4·H2O | 0.151 |
| Na2HPO4 | 0.0709 | ZnSO4·7H2O | 0.2875 |
| MgSO4·7H2O | 0.0246 | CuSO4·5H2O | 0.0024 |
| CaCl2·2H2O | 0.0017 | (NH4)6Mo7O24·4H2O | 0.0135 |
| Fe-complex | 1 mL | ||
| Trace element solution | 1 mL | ||
| Total volume | 1000 mL | ||
| pH | 6.8 | ||
| Run | House WCO | Restaurant WCO | ||
|---|---|---|---|---|
| mg Catalyst/g (WCO + Alcohol) | Temperature (°C) | mg Catalyst/g (WCO + Alcohol) | Temperature (°C) | |
| 1 | 3.3 | 60 | 1.5 | 54 |
| 2 | 6.22 | 60 | 2.25 | 57 |
| 3 | 6.22 | 55 | 1.88 | 60 |
| 4 | 6.22 | 55 | 2.25 | 60 |
| 5 | 6.22 | 65 | 1.5 | 54 |
| 6 | 4.76 | 55 | 2 | 60 |
| 7 | 3.3 | 55 | 1.88 | 57 |
| 8 | 4.76 | 65 | 2.25 | 54 |
| 9 | 6.22 | 65 | 1.5 | 60 |
| 10 | 4.76 | 60 | 1.88 | 54 |
| 11 | 3.3 | 65 | 2.25 | 54 |
| 12 | 3.3 | 55 | 1.5 | 57 |
| Run | Catalyst Ratio (mg/g) | Temperature (°C) | Viscosity (mPa·s) | (%) Yield | Density (g/mL) |
|---|---|---|---|---|---|
| 1 | 3.3 | 60 | 7.06 ± 0.1 | 96.31 ± 0.3 | 0.823 ± 0.0015 |
| 2 | 6.22 | 60 | 6.57 ± 0.1 | 92.81 ± 0.3 | 0.849 ± 0.0015 |
| 3 | 6.22 | 55 | 4.82 ± 0.1 | 88.54 ± 0.3 | 0.831 ± 0.0015 |
| 4 | 6.22 | 55 | 4.82 ± 0.1 | 88.54 ± 0.3 | 0.831 ± 0.0015 |
| 5 | 6.22 | 65 | 20.75 ± 0.1 | 91.23 ± 0.3 | 0.829 ± 0.0015 |
| 6 | 4.76 | 55 | 5.06 ± 0.1 | 91.52 ± 0.3 | 0.814 ± 0.0015 |
| 7 | 3.3 | 55 | 5.48 ± 0.1 | 99.08 ± 0.3 | 0.851 ± 0.0015 |
| 8 | 4.76 | 65 | 7.14 ± 0.1 | 91.32 ± 0.3 | 0.836 ± 0.0015 |
| 9 | 6.22 | 65 | 20.75 ± 0.1 | 91.23 ± 0.3 | 0.829 ± 0.0015 |
| 10 | 4.76 | 60 | 7.82 ± 0.1 | 94.08 ± 0.3 | 0.843 ± 0.0015 |
| 11 | 3.3 | 65 | 8.05 ± 0.1 | 97.65 ± 0.3 | 0.814 ± 0.0015 |
| 12 | 3.3 | 55 | 5.48 ± 0.1 | 99.08 ± 0.3 | 0.851 ± 0.0015 |
| Run | Temperature (°C) | Catalyst Ratio (mg/g) | Viscosity (mPa·s) | Yield (%) | Density (g/mL) |
|---|---|---|---|---|---|
| 1 | 54 | 1.5 | 14.85 ± 0.14 | 96.615 ± 0.5 | 0.949 ± 0.0021 |
| 2 | 57 | 2.25 | 9.0 ± 0.14 | 94.6 ± 0.5 | 0.938 ± 0.0021 |
| 3 | 60 | 1.88 | 8.0 ± 0.14 | 90.05 ± 0.5 | 0.8586 ± 0.0021 |
| 4 | 60 | 2.25 | 8.75 ± 0.14 | 70.82 ± 0.5 | 0.885 ± 0.0021 |
| 5 | 54 | 1.5 | 14.85 ± 0.14 | 96.615 ± 0.5 | 0.849 ± 0.0021 |
| 6 | 60 | 2.0 | 8.0 ± 0.14 | 78.94 ± 0.5 | 0.8455 ± 0.0021 |
| 7 | 57 | 1.88 | 9.8 ± 0.14 | 94.755 ± 0.5 | 0.8605 ± 0.0021 |
| 8 | 54 | 2.25 | 8.4 ± 0.14 | 88.135 ± 0.5 | 0.8552 ± 0.0021 |
| 9 | 60 | 1.5 | 8.25 ± 0.14 | 94.0 ± 0.5 | 0.82 ± 0.0021 |
| 10 | 54 | 1.88 | 8.7 ± 0.14 | 95.668 ± 0.5 | 0.847 ± 0.0021 |
| 11 | 54 | 2.25 | 8.4 ± 0.14 | 88.135 ± 0.5 | 0.8552 ± 0.0021 |
| 12 | 57 | 1.5 | 16.5 ± 0.14 | 95.515 ± 0.5 | 0.8877 ± 0.0021 |
| Aspect | Household WCO | Restaurant WCO | Chlorella sp. Microalgae |
|---|---|---|---|
| Characteristics | Cleaner, less contaminated | More polluted, higher impurities | High oil content (30–50%), cultivated |
| Alcohol Used | Ethanol | Methanol | Methanol |
| Temperature Range | 55–65 °C | 54–60 °C | ~60 °C |
| Catalyst Concentration | 3.3–6.22 mg/g (NaOH) | 1.5–2.25 mg/g (NaOH) | NaOH (unspecified) |
| Biodiesel Yield | Up to 99.08% | Up to 96.61% | 28.6% (semi-open) |
| Biodiesel Viscosity | 4.82–5.49 mPa·s | 8.25 mPa·s | Within EN 14214 |
| Biodiesel Density | 0.851 g/ml | 0.82–0.949 g/ml | Within EN 14214 |
| Processing Challenges | Slight pretreatment | Extensive purification needed | Complex cultivation and extraction |
| Economic Viability | High (low preprocessing cost) | Moderate (higher processing cost) | Low (high cultivation cost) |
| Environmental Impact | Reduces waste, low emissions | Reduces waste, low emissions | Carbon neutral, high energy use |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhran, A.A. A Comparative Techno-Economic Analysis of Waste Cooking Oils and Chlorella Microalgae for Sustainable Biodiesel Production. Processes 2025, 13, 3526. https://doi.org/10.3390/pr13113526
Bhran AA. A Comparative Techno-Economic Analysis of Waste Cooking Oils and Chlorella Microalgae for Sustainable Biodiesel Production. Processes. 2025; 13(11):3526. https://doi.org/10.3390/pr13113526
Chicago/Turabian StyleBhran, Ahmed A. 2025. "A Comparative Techno-Economic Analysis of Waste Cooking Oils and Chlorella Microalgae for Sustainable Biodiesel Production" Processes 13, no. 11: 3526. https://doi.org/10.3390/pr13113526
APA StyleBhran, A. A. (2025). A Comparative Techno-Economic Analysis of Waste Cooking Oils and Chlorella Microalgae for Sustainable Biodiesel Production. Processes, 13(11), 3526. https://doi.org/10.3390/pr13113526






