Antibacterial Ability and Feature of Polyvinyl Alcohol/Chitosan/Montmorillonite/Copper Nanoparticle Composite Gel Beads
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
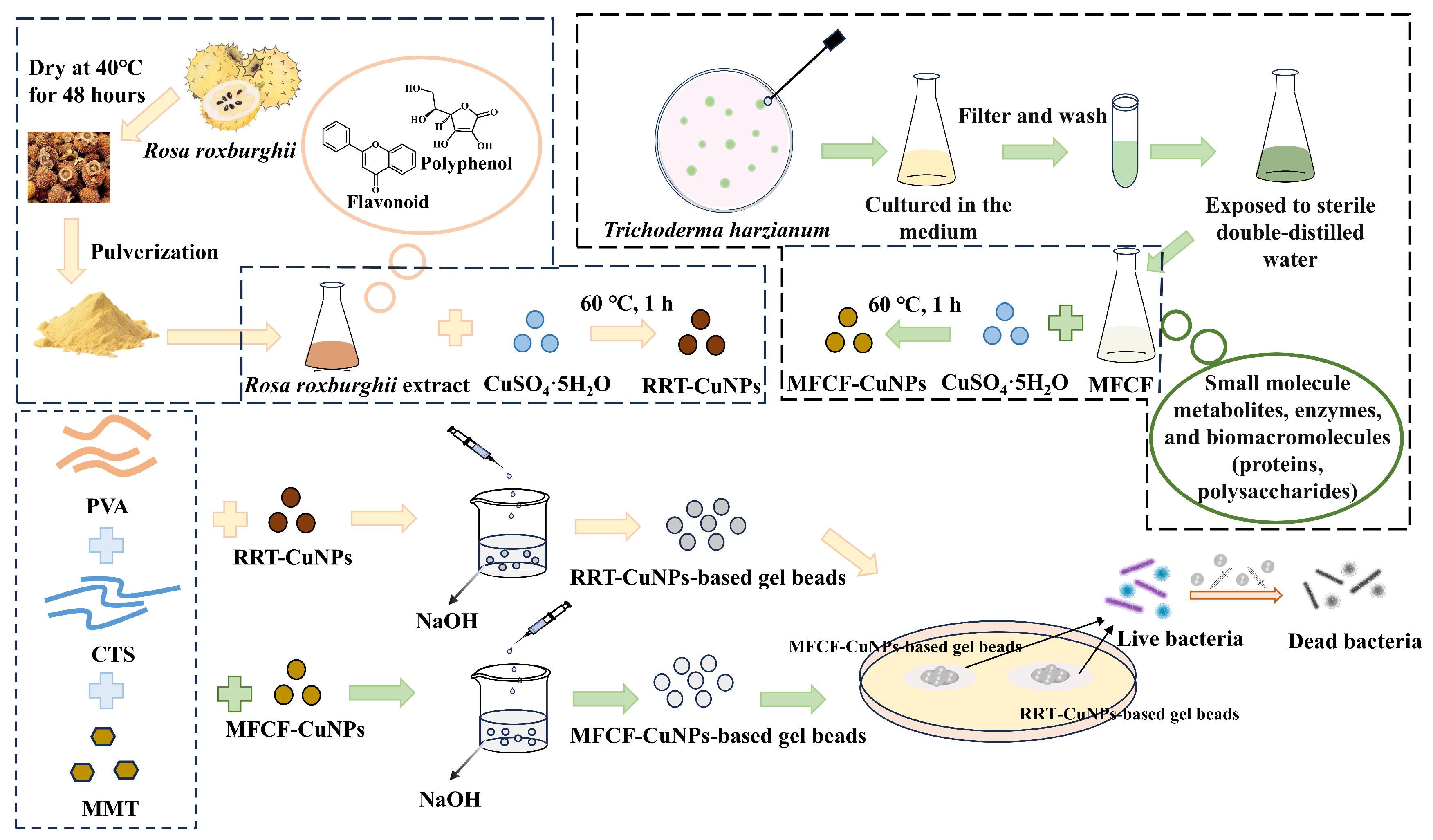
2.2. Preparation of Extracts, CuNPs, and Gel Beads
2.3. Antibacterial Testing
2.3.1. Agar Diffusion Method
2.3.2. Colony Count Method in Aqueous Solution
2.4. Characterization of Gel Beads
3. Results and Discussion
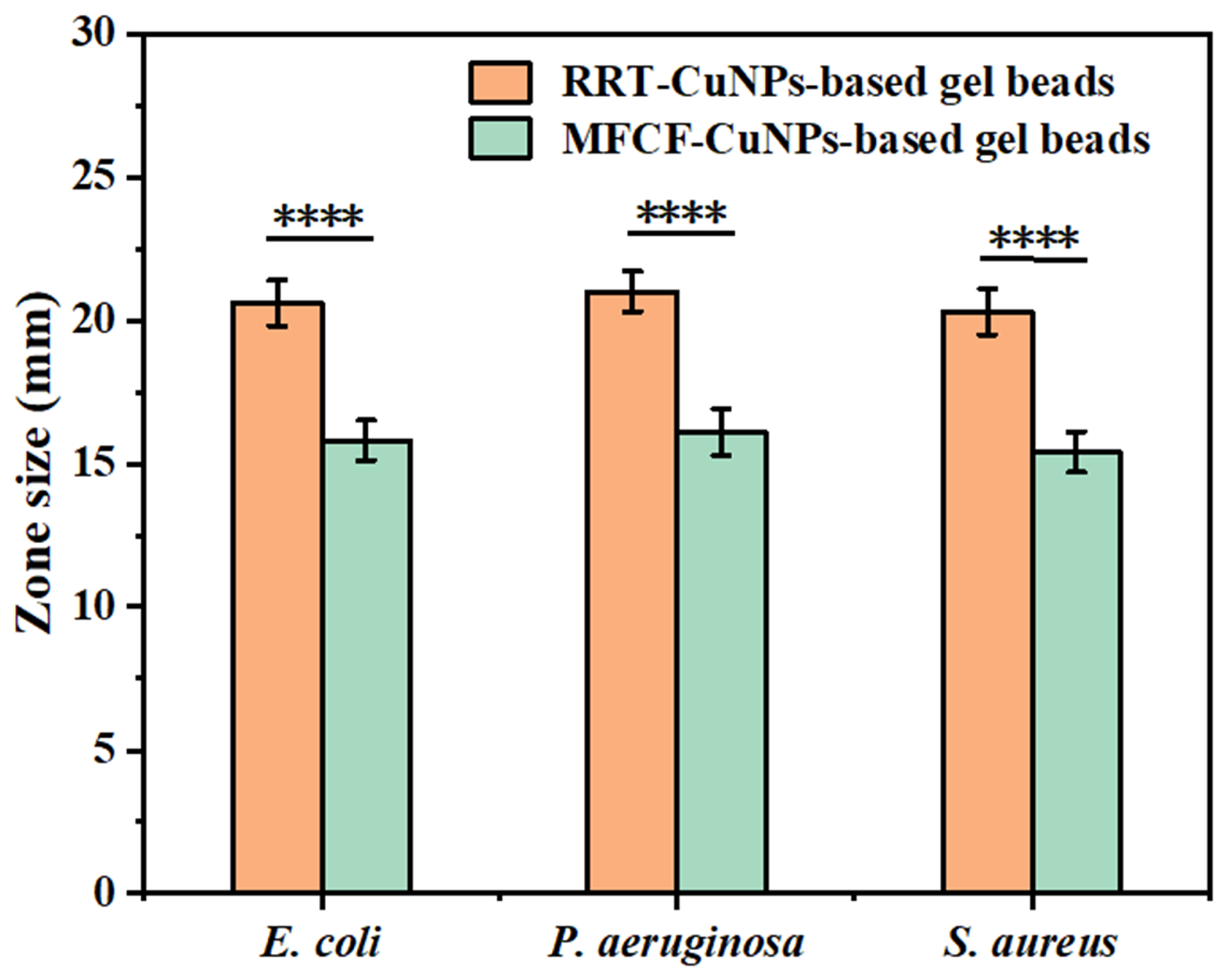
3.1. Comparing Antibacterial Activity of CuNPs by Using RRT or MFCF as a Natural Reducing Agent
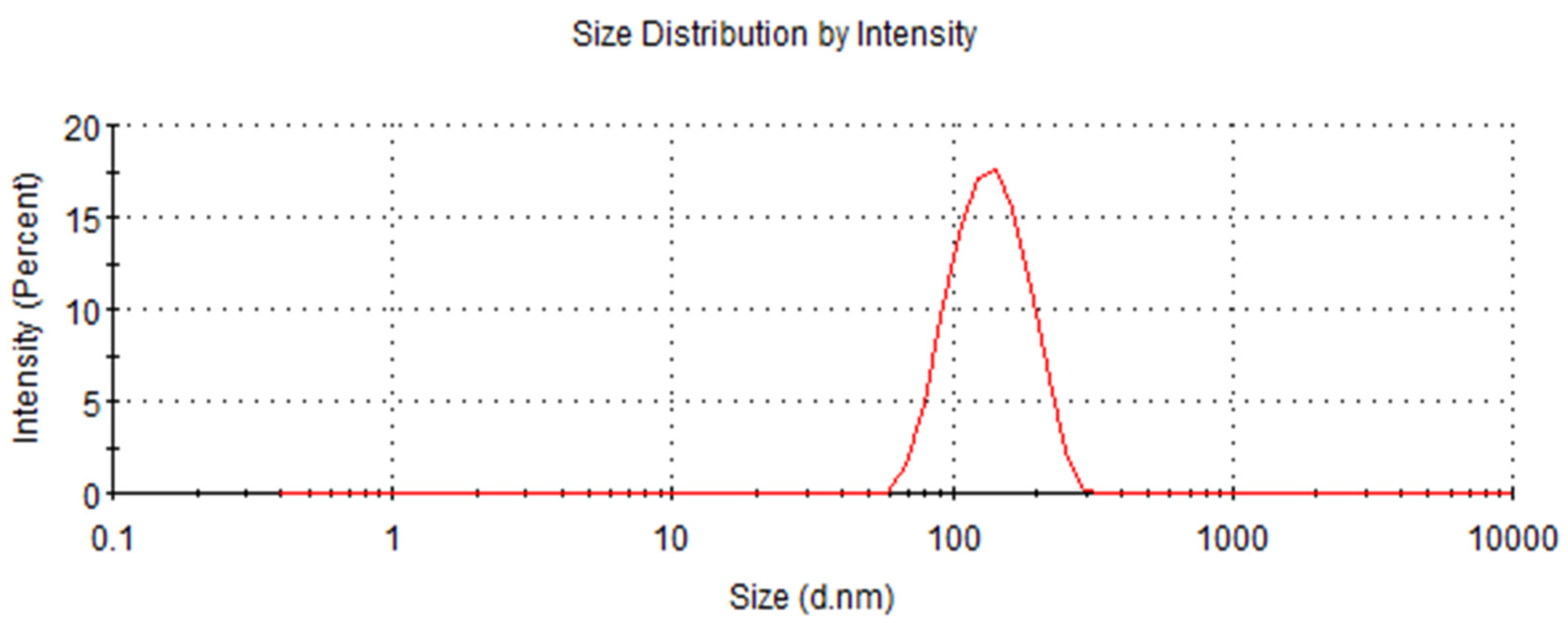
3.2. Particle Size Distribution of RRT-CuNPs
3.3. FTIR of CTS/PVA/MMT and CTS/PVA/MMT/RRT-CuNPs
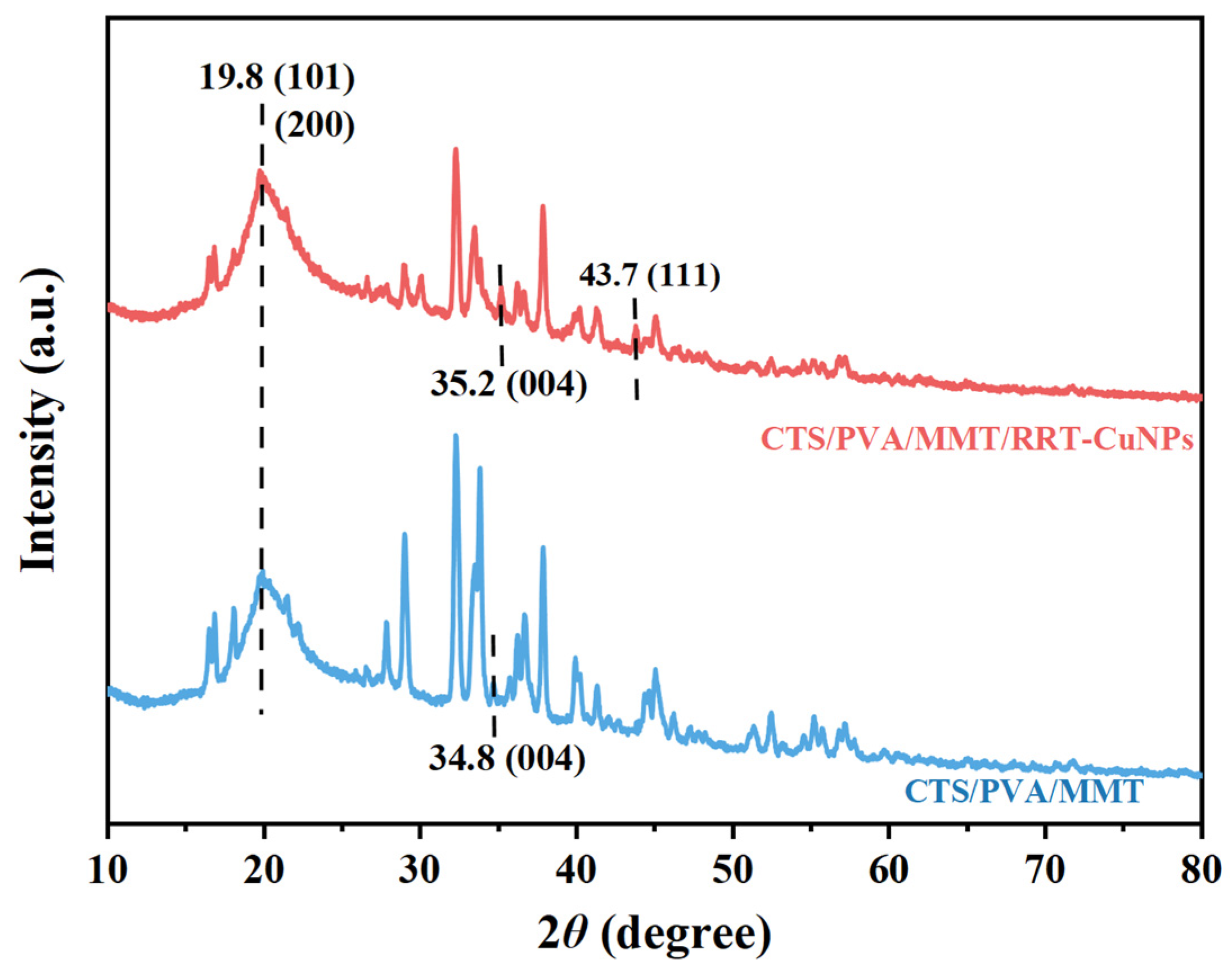
3.4. XRD of CTS/PVA/MMT and CTS/PVA/MMT/RRT-CuNPs
3.5. SEM of CTS/PVA/MMT and CTS/PVA/MMT/CuNPs
3.6. Effect of CTS, MMT, and CuNPs Loading on the Antibacterial Efficacy of Gel Beads
3.7. The Effect of the Dose and Action Duration of Gel Beads on the Bacteriostatic Efficacy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goswami, R.K.; Mehariya, S.; Verma, P.; Lavecchia, R.; Zuorro, A. Microalgae-based biorefineries for sustainable resource recovery from wastewater. J. Water Process Eng. 2021, 40, 101747. [Google Scholar] [CrossRef]
- Salim Dantas, M.; Rodrigues Barroso, G.; Corrêa Oliveira, S. Performance of sewage treatment plants and impact of effluent discharge on receiving water quality within an urbanized area. Environ. Monit. Assess. 2021, 193, 289. [Google Scholar] [CrossRef] [PubMed]
- Al Aukidy, M.; Verlicchi, P. Contributions of combined sewer overflows and treated effluents to the bacterial load released into a coastal area. Sci. Total. Environ. 2017, 607–608, 483–496. [Google Scholar] [CrossRef]
- Pistocchi, A.; Alygizakis, N.A.; Brack, W.; Boxall, A.; Cousins, I.T.; Drewes, J.E.; Finckh, S.; Gallé, T.; Launay, M.A.; McLachlan, M.S.; et al. European scale assessment of the potential of ozonation and activated carbon treatment to reduce micropollutant emissions with wastewater. Sci. Total. Environ. 2022, 848, 157124. [Google Scholar] [CrossRef]
- Wang, R.; Liu, Y.; Guan, J.; Zuo, C.; Dai, C.; Liu, X.; Liang, Z.; Zhao, G. Environmental justice of texas recreational water quality—The disproportionate E. coli levels and trends. J. Environ. Manag. 2024, 370, 122969. [Google Scholar] [CrossRef]
- Deepnarain, N.; Nasr, M.; Amoah, I.D.; Enitan-Folami, A.M.; Reddy, P.; Stenström, T.A.; Kumari, S.; Bux, F. Impact of sludge bulking on receiving environment using quantitative microbial risk assessment (QMRA)-based management for full-scale wastewater treatment plants. J. Environ. Manag. 2020, 267, 110660. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Liu, W.; Cao, J.; Wu, Z.; Yang, C. Foam fractionation for effective removal of Pseudomonas aeruginosa from water body: Strengthening foam drainage by artificially inducing foam evolution. J. Environ. Manag. 2021, 291, 112628. [Google Scholar] [CrossRef] [PubMed]
- Pattnaik, A.; Ghosh, P.; Poonia, A.K. An overview on advancements in hydrogels for effective wastewater treatment. J. Mol. Liq. 2025, 424, 127120. [Google Scholar] [CrossRef]
- Xie, L.; Zhang, Z.; He, Y. Antibacterial effect of polyvinyl alcohol/biochar–nano silver/sodium alginate gel beads. Processes 2023, 11, 2330. [Google Scholar] [CrossRef]
- Singh, S.; Khurana, P.; Kaur, G.; Prasad, S.; Thatai, S. Antibacterial activity of biogenic and chemically synthesized silver nanoparticles and its application in wastewater treatment. Inorg. Chem. Commun. 2024, 170, 113205. [Google Scholar] [CrossRef]
- Atkinson, S.; Thomas, S.F.; Goddard, P.; Bransgrove, R.M.; Mason, P.T.; Oak, A.; Bansode, A.; Patankar, R.; Gleason, Z.D.; Sim, M.K.; et al. Swirl Flow Bioreactor coupled with Cu-alginate beads: A system for the eradication of Coliform and Escherichia coli from biological effluents. Sci. Rep. 2015, 5, 9461. [Google Scholar] [CrossRef]
- Mukherjee, A.; Das, S.; Ahn, Y.-H. Highly recyclable and stable CdS-alginate hydrogel beads towards prevention of oxidative release of nanoparticles in complete photocatalytic disinfection of multidrug-resistant Escherichia coli. Environ. Sci. Water Res. Technol. 2023, 9, 2954–2964. [Google Scholar] [CrossRef]
- Li, J.L.; Jiang, X.; Liu, X.; He, C.; Di, Y.; Lu, S.; Huang, H.; Lin, B.; Wang, D.; Fan, B. Antibacterial anthraquinone dimers from marine derived fungus Aspergillus sp. Fitoterapia 2019, 133, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.-Y.; Song, Y.-Z. Synthesis of uniform zinc peroxide nanoparticles for antibacterial application. Indian J. Pharm. Sci. 2024, 86, 219–225. [Google Scholar]
- Chen, H.; Li, Y.; Chen, D.; Fang, Y.; Gong, X.; Wang, K.; Ma, C. Photothermally enhanced antibacterial wound healing using albumin-loaded tanshinone IIA and IR780 nanoparticles. Front. Bioeng. Biotechnol. 2024, 12, 1487660. [Google Scholar] [CrossRef]
- Zhao, Q.-M.; Sun, Y.-Y.; Wu, C.-S.; Yang, J.; Bao, G.-F.; Cui, Z.-M. Enhanced osteogenic activity and antibacterial ability of manganese–titanium dioxide microporous coating on titanium surfaces. Nanotoxicology 2019, 14, 289–309. [Google Scholar] [CrossRef] [PubMed]
- Sedighi, O.; Johnsen, T.; Badireddy, A.R.; Wargo, M.J.; Doiron, A.L. Synergistic effects of iron oxide nanoparticles and hydrogen peroxide in inhibiting Pseudomonas aeruginosa growth to combat bacterial contamination in water recovery systems. Environ. Sci. Nano 2025, 12, 2449–2461. [Google Scholar] [CrossRef]
- Zhang, W.; Hu, E.; Wang, Y.; Miao, S.; Liu, Y.; Hu, Y.; Liu, J.; Xu, B.; Chen, D.; Shen, Y. Emerging antibacterial strategies with application of targeting drug delivery system and combined treatment. Int. J. Nanomed. 2021, 16, 6141–6156. [Google Scholar] [CrossRef]
- Ortega-Nieto, C.; Losada-Garcia, N.; Pessela, B.C.; Domingo-Calap, P.; Palomo, J.M. Design and synthesis of copper nanobiomaterials with antimicrobial properties. ACS Bio Med Chem Au 2023, 3, 349–358. [Google Scholar] [CrossRef]
- Vodyashkin, A.; Stoinova, A.; Kezimana, P. Promising biomedical systems based on copper nanoparticles: Synthesis, characterization, and applications. Colloids Surf. B Biointerfaces 2024, 237, 113861. [Google Scholar] [CrossRef]
- Siddiqi, K.S.; Husen, A. Current status of plant metabolite-based fabrication of copper/copper oxide nanoparticles and their applications: A review. Biomater. Res. 2020, 24, 11. [Google Scholar] [CrossRef]
- Liu, W.; Li, N.; Hou, J.; Cao, R.; Jia, L.; Guo, Y.; Xu, J. Structure and antitumor activity of a polysaccharide from Rosa roxburghii. Int. J. Biol. Macromol. 2024, 273, 132807. [Google Scholar] [CrossRef]
- Wang, L.; Wei, T.; Zheng, L.; Jiang, F.; Ma, W.; Lu, M.; Wu, X.; An, H. Recent advances on main active ingredients, pharmacological activities of Rosa roxbughii and its development and utilization. Foods 2023, 12, 1051. [Google Scholar] [CrossRef]
- Tang, Z.; Chen, Y.; Cai, C.; Ma, S.; Tan, Z. CO2-responsive ionic liquid for the sustainable extraction and separation of Rosa roxburghii polysaccharides. Int. J. Biol. Macromol. 2025, 308, 142708. [Google Scholar] [CrossRef]
- Mishra, D.N.; Prasad, L.; Suyal, U. Synthesis of zinc oxide nanoparticles using Trichoderma harzianum and its bio-efficacy on Alternaria brassicae. Front. Microbiol. 2025, 16, 1506695. [Google Scholar] [CrossRef]
- Chand Mali, S.; Dhaka, A.; Sharma, S.; Trivedi, R. Review on biogenic synthesis of copper nanoparticles and its potential applications. Inorg. Chem. Commun. 2023, 149, 110448. [Google Scholar] [CrossRef]
- Han, M.; Zhang, Y.; Zhang, Y.; Ye, Q.; Sillanpää, M.; Zhu, X.; Yang, W. A mini-review on polyvinyl alcohol/lignin (nano)composites: Preparation, applications and perspectives. Sustain. Chem. Pharm. 2024, 42, 101861. [Google Scholar] [CrossRef]
- Wang, M.; Bai, J.; Shao, K.; Tang, W.; Zhao, X.; Lin, D.; Huang, S.; Chen, C.; Ding, Z.; Ye, J. Poly(vinyl alcohol) hydrogels: The Old and New Functional Materials. Int. J. Polym. Sci. 2021, 2021, 2225426. [Google Scholar] [CrossRef]
- Khadivi, H.; Sirousazar, M.; Abbasi-Chianeh, V.; Jalilnejad, E. Egg white/polyvinyl alcohol/clay bionanocomposite hydrogel adsorbents for dye removal. J. Polym. Environ. 2022, 30, 3186–3202. [Google Scholar] [CrossRef]
- Zhang, P.; Zou, K.; Yuan, L.; Liu, J.; Liu, B.; Qing, T.-P.; Feng, B. A biomass resource strategy for alginate-polyvinyl alcohol double network hydrogels and their adsorption to heavy metals. Sep. Purif. Technol. 2022, 301, 122050. [Google Scholar] [CrossRef]
- Enoch, K.; C․S, R.; Somasundaram, A.A. Thixotropic chitosan hydrogels for biomedical applications: Unravelling the effect of chitosan concentration on the mechanical behaviour. Surf. Interfaces 2024, 50, 104475. [Google Scholar] [CrossRef]
- Wang, J.; Yuan, Y.; Liu, Y.; Li, X.; Wu, S. Application of chitosan in fruit preservation: A review. Food Chem. X 2024, 23, 101589. [Google Scholar] [CrossRef]
- Wu, K.; Yan, Z.; Wu, Z.; Li, J.; Zhong, W.; Ding, L.; Zhong, T.; Jiang, T. Recent advances in the preparation, antibacterial mechanisms, and applications of chitosan. J. Funct. Biomater. 2024, 15, 318. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Qian, J. Recent advances in montmorillonite-based composites for biomedical applications: A review. J. Drug Deliv. Sci. Technol. 2025, 109, 107012. [Google Scholar] [CrossRef]
- Sarkar, A.; Mushahary, N.; Basumatary, F.; Das, B.; Basumatary, S.F.; Venkatesan, K.; Selvaraj, M.; Rokhum, S.L.; Basumatary, S. Efficiency of montmorillonite-based materials as adsorbents in dye removal for wastewater treatment. J. Environ. Chem. Eng. 2024, 12, 112519. [Google Scholar] [CrossRef]
- Tang, Z.; Guo, H.; Xu, J.; Li, Z.; Sun, G. Cationic poly(diallyldimethylammonium chloride) based hydrogel for effective anionic dyes adsorption from aqueous solution. React. Funct. Polym. 2022, 174, 105239. [Google Scholar] [CrossRef]
- Phu, N.A.M.M.; Wi, E.; Jeong, G.; Kim, H.; Singha, N.R.; Chang, M. Highly efficient dye adsorption by hierarchical porous SA/PVA/ZIF-8 composite microgels prepared via microfluidics. Carbohydr. Polym. 2024, 350, 123016. [Google Scholar] [CrossRef] [PubMed]
- Njuguna, D.G.; Schönherr, H. Smart and regeneratable Xanthan gum hydrogel adsorbents for selective removal of cationic dyes. J. Environ. Chem. Eng. 2022, 10, 107620. [Google Scholar] [CrossRef]
- Duru Kamacı, U.; Kamacı, M. Hydrogel beads based on sodium alginate and quince seed nanoparticles for the adsorption of methylene blue. Inorg. Chem. Commun. 2023, 160, 111919. [Google Scholar] [CrossRef]
- Zhou, X.; Qin, Y.; Wang, Y.; Wang, Y.; Qin, Z. Phytochemical profile and antioxidant characteristics of bound and free phenolics from Rosa roxburghii tratt. Food Biosci. 2024, 57, 103576. [Google Scholar] [CrossRef]
- Ahluwalia, V.; Kumar, J.; Sisodia, R.; Shakil, N.A.; Walia, S. Green synthesis of silver nanoparticles by Trichoderma harzianum and their bio-efficacy evaluation against Staphylococcus aureus and Klebsiella pneumonia. Ind. Crops Prod. 2014, 55, 202–206. [Google Scholar] [CrossRef]
- Chiu, C.-T.; Lai, C.-H.; Huang, Y.-H.; Yang, C.-H.; Lin, J.-N. Comparative analysis of gradient diffusion and disk diffusion with agar dilution for susceptibility testing of Elizabethkingia anopheles. Antibiotics 2021, 10, 450. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Hao, X.; Kang, Y.; He, X.; Zhao, H. Preparation and antibacterial properties of polyelectrolyte complexed nanoparticles aggregated from PHMG and sodium caffeate. ACS Appl. Bio Mater. 2024, 7, 6467–6476. [Google Scholar] [CrossRef] [PubMed]
- Ranaware, A.S.; Kushwaha, S.B.; Kunchge, N.; Prakash, G.; Lele, S.S. Effect of different size silver nanoparticles synthesized at varying pH of plant extract for germination improvement of tetraploid watermelon (Citrullus lanatus). J. Clust. Sci. 2025, 36, 101. [Google Scholar] [CrossRef]
- Sacourbaravi, R.; Ansari-Asl, Z.; Hoveizi, E.; Darabpour, E. Poly(vinyl alcohol)/chitosan hydrogel containing gallic acid-modified Fe, Cu, and Zn metal–organic frameworks (MOFs): Preparation, characterization, and biological applications. ACS Appl. Mater. Interfaces 2024, 16, 61609–61620. [Google Scholar] [CrossRef]
- Roufegari-Nejhad, E.; Sirousazar, M.; Abbasi-Chiyaneh, V.; Kheiri, F. Removal of methylene blue from aqueous solutions using poly(vinyl alcohol)/montmorillonite nanocomposite hydrogels: Taguchi optimization. J. Polym. Environ. 2019, 27, 2239–2249. [Google Scholar] [CrossRef]
- Zhou, C.; Qi, S.; Zhu, P.; Zhao, Y.; Xu, Y.; Dong, X.; Wang, D. The methylene infrared vibration and dielectric behavior monitored by amide group arrangement for long chain polyamides. Polymer 2020, 190, 122231. [Google Scholar] [CrossRef]
- Sanjay, V.; Rajashekara, K.M.; Pattar, V.; Murugendrappa, M.V. Effect on electrical and dielectric properties of Te nanoparticle-doped PVA composite. J. Mater. Sci. Mater. Electron. 2022, 33, 17382–17394. [Google Scholar] [CrossRef]
- Liu, J.; Song, Z.; Li, B.; Ren, J.; Chen, F.; Xiao, M. Experimental study on the microstructure of coal with different particle sizes. Energies 2022, 15, 4043. [Google Scholar] [CrossRef]
- Wu, Y.; Guan, Y.; Gao, H.; Zhou, L.; Peng, F. Novel high-strength montmorillonite/polyvinyl alcohol composite film enhanced by chitin nanowhiskers. J. Appl. Polym. Sci. 2020, 138, 50344. [Google Scholar] [CrossRef]
- Moja, T.N.; Phiri, Z.; Nure, J.F.; Nkambule, T.T.; de Kock, L. Insights into preparation of polycaprolactone–chitosan–montmorillonite composite for Pb(II) and Cd(II) removal. ChemistrySelect 2025, 10, e202500798. [Google Scholar] [CrossRef]
- Radoor, S.; Karayil, J.; Jayakumar, A.; Siengchin, S.; Parameswaranpillai, J. A low cost and eco-friendly membrane from polyvinyl alcohol, chitosan and honey: Synthesis, characterization and antibacterial property. J. Polym. Res. 2021, 28, 82. [Google Scholar] [CrossRef]
- Suneetha, G.; Ayodhya, D.; Srikanth, K.; Manjari, P.S. Fabrication of CuNPs using schiff base ligand and their catalytic reduction of pharmaceutical drugs, fluorescence selective detection of Cd2+, antimicrobial, and antioxidant activities. J. Fluoresc. 2023, 34, 1307–1318. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Singh, B.N.; Srivastava, P. Effect of copper nanoparticles on physico-chemical properties of chitosan and gelatin-based scaffold developed for skin tissue engineering application. 3 Biotech 2019, 9, 102. [Google Scholar] [CrossRef]
- Varma, R.; Vasudevan, S. Extraction, characterization, and antimicrobial activity of chitosan from horse mussel modiolus modiolus. ACS Omega 2020, 5, 20224–20230. [Google Scholar] [CrossRef] [PubMed]
- Laanoja, J.; Sihtmäe, M.; Vihodceva, S.; Iesalnieks, M.; Otsus, M.; Kurvet, I.; Kahru, A.; Kasemets, K. Synthesis and synergistic antibacterial efficiency of chitosan-copper oxide nanocomposites. Heliyon 2024, 10, e35588. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Machado, D.I.; López-Cervantes, J.; Vega-Cázarez, C.A.; Hernández-Ruiz, K.L.; Campas-Baypoli, O.N.; Soto-Cota, A.; Madera-Santana, T.J. Functional and antibacterial characterization of electrospun nanofiber membranes made of chitosan and polyvinyl alcohol. Results Chem. 2024, 7, 101314. [Google Scholar] [CrossRef]
- Liang, X.; Wang, W.; Tang, M.; Kang, Y.; Cui, M.; Zhao, H. Preparation, characterization and antibacterial activities of bio-based schiff base copper complex intercalated montmorillonite. Appl. Clay Sci. 2025, 271, 107809. [Google Scholar] [CrossRef]
- Huang, Y.; Wu, L.; Chang, L.; Peng, W.; Wang, W.; Cao, Y. Sodium alginate-crosslinked montmorillonite nanosheets hydrogel for efficient gallium recovery. Int. J. Biol. Macromol. 2025, 295, 139474. [Google Scholar] [CrossRef]
- Noppradit, B.; Uthaipan, N.; Klinnawee, L.; Kongtragoul, P.; Phengdaam, A. Antifungal copper nanocomposite-rubber compound for tree wound dressings. Ind. Crops Prod. 2024, 222, 119798. [Google Scholar] [CrossRef]
- Kazeminava, F.; Arsalani, N.; Ahmadi, R.; Kafil, H.S.; Geckeler, K.E. A facile approach to incorporate silver nanoparticles into solvent-free synthesized PEG-based hydrogels for antibacterial and catalytical applications. Polym. Test. 2021, 101, 106909. [Google Scholar] [CrossRef]
- Song, Z.; Huang, L.; Cui, S. Preparation of porous Ga-doped TiO2 composite aerogel and its bactericidal activity against Escherichia coli and Staphylococcus aureus. Adv. Eng. Mater. 2024, 26, 2302042. [Google Scholar] [CrossRef]
- Ma, S.; Chen, K.; Ding, Q.; Zhang, S.; Lu, Y.; Yu, T.; Ding, C.; Liu, W.; Liu, S. Quaternized oxidized sodium alginate injectable hydrogel with high antimicrobial and hemostatic efficacy promotes diabetic wound healing. Int. J. Pharm. 2024, 661, 124421. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Du, C.; Gao, B.; Xiang, G.; Huang, Y.; He, B. Dual enzymatic preparation of flexible biocomplex with full amino-acid composition. Polymer 2024, 294, 126713. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, M.; Zhang, T.; He, W.; He, Y. Antibacterial Ability and Feature of Polyvinyl Alcohol/Chitosan/Montmorillonite/Copper Nanoparticle Composite Gel Beads. Processes 2025, 13, 3518. https://doi.org/10.3390/pr13113518
Huang M, Zhang T, He W, He Y. Antibacterial Ability and Feature of Polyvinyl Alcohol/Chitosan/Montmorillonite/Copper Nanoparticle Composite Gel Beads. Processes. 2025; 13(11):3518. https://doi.org/10.3390/pr13113518
Chicago/Turabian StyleHuang, Meizi, Tingting Zhang, Wei He, and Yucai He. 2025. "Antibacterial Ability and Feature of Polyvinyl Alcohol/Chitosan/Montmorillonite/Copper Nanoparticle Composite Gel Beads" Processes 13, no. 11: 3518. https://doi.org/10.3390/pr13113518
APA StyleHuang, M., Zhang, T., He, W., & He, Y. (2025). Antibacterial Ability and Feature of Polyvinyl Alcohol/Chitosan/Montmorillonite/Copper Nanoparticle Composite Gel Beads. Processes, 13(11), 3518. https://doi.org/10.3390/pr13113518






