Research on Os-Modified C3N Nanosheets for Sensing and Adsorbing Dissolved Gases in 10 kV Distribution Transformer Oil for Fault Diagnosis
Abstract
1. Introduction
2. Computational Details
3. Results and Discussion
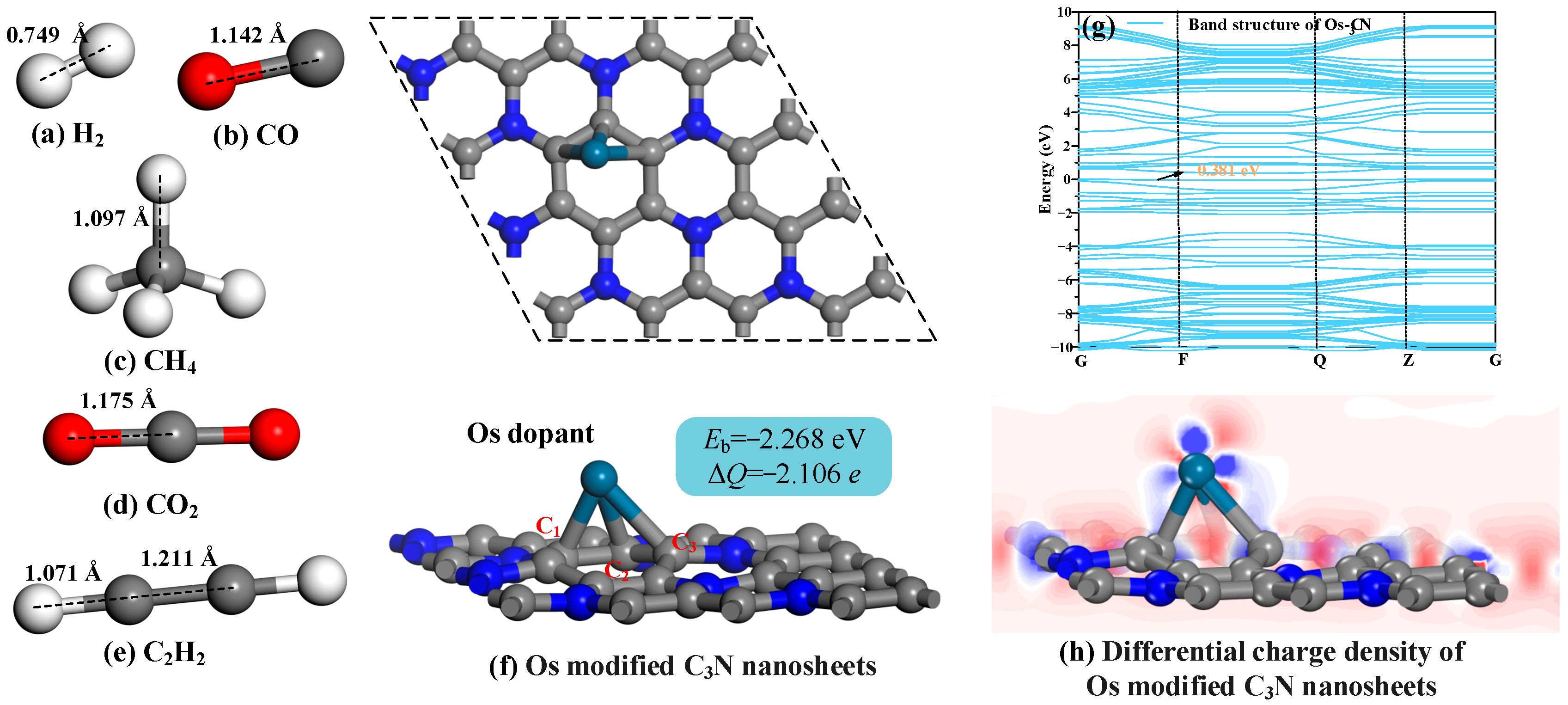
3.1. The Most Stable Geometries and Electronic Properties of Os Modified C3N Nanosheets
3.2. Adsorption Structures of Pure C3N and Os Modified C3N to Dissolved Gases in Oil
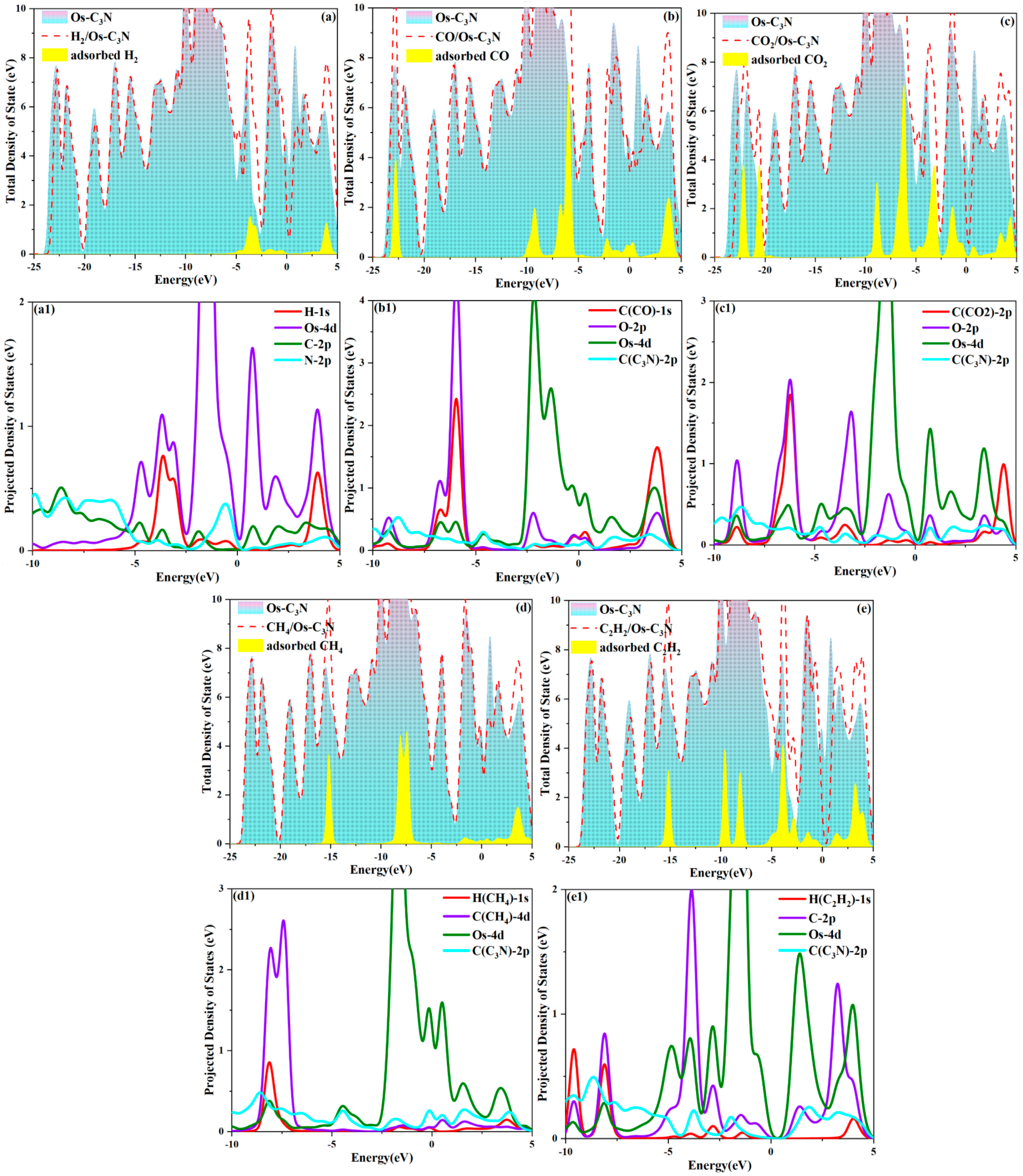
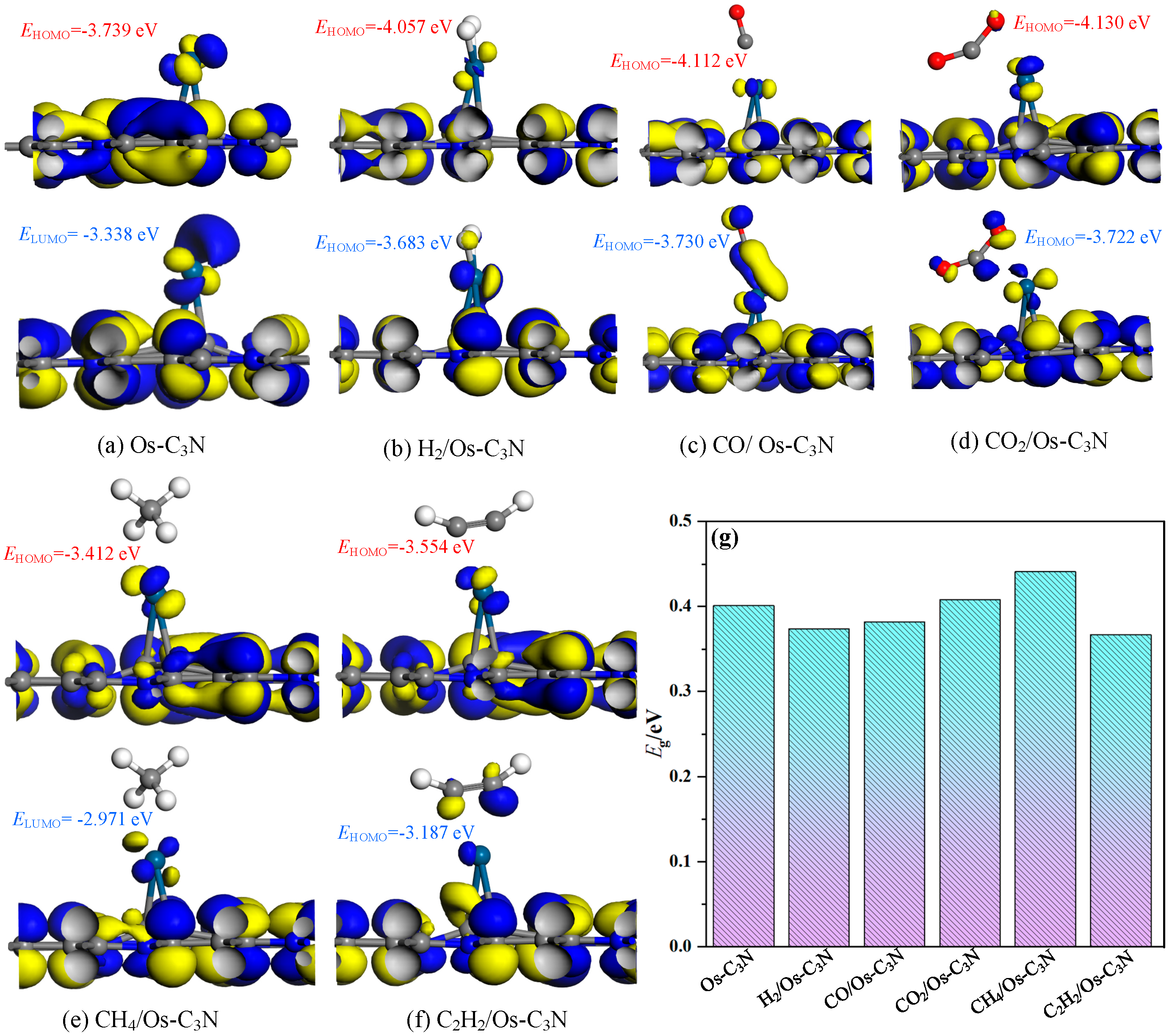
3.3. The Electronic Properties of Different Adsorption Systems
3.4. Exploration and Application of Sensing Properties
4. Conclusions
- (1)
- A 4 × 4 × 1 k-point mesh and an SCF convergence threshold of 1.0 × 10−6 Ha were employed in the simulations. After Os nanoparticle doping, no physical deformation occurred on the C3N surface, demonstrating that the integration of nanoparticles yields adsorption sites while maintaining the material’s structural integrity. Additionally, the band gap of C3N was significantly reduced from 1.274 eV to 0.381 eV, enhancing the system’s conductivity.
- (2)
- Pure C3N exhibits poor adsorption performance for dissolved gases in oil and lacks significant improvement in electronic properties. This suggests the potential for surface modifications to enhance its gas capture capability.
- (3)
- Os-modified C3N shows a marked improvement in gas capture capability for dissolved gases in 10 kV distribution TO, with the adsorption strength ranked as C2H2 > CO > H2 > CO2 > CH4. Notably, the adsorption of C2H2 is significantly enhanced, accompanied by molecular activation. Furthermore, gas adsorption induces a leftward shift in the overall DOS, while the system’s resistivity decreases post-adsorption, promoting the efficient acquisition and juxtaposition of electrical signals.
- (4)
- For the CO system, the band gap remains almost unchanged. In contrast, the band gap growth rates for H2, CO2, CH4, and C2H2 after surface reactions are 117%, 64%, 18%, and 99%, respectively, indicating varying degrees of enhanced system conductivity.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, Q.; Chen, S.; Huang, W.; Zhang, W.; Li, K. Research on Inversion Model of Winding Hot Spot Temperature of 10-kV Oil-Immersed Three-Dimensional Coiled Core Transformer. IEEE Access 2024, 12, 97691–97700. [Google Scholar] [CrossRef]
- Xie, Y.; Ruan, J.; Shi, Y.; Jin, S.; Tian, Y.; Zhu, L. Inversion Detection Method for Resistivity of Oil-Immersed Paper in Transformer. IEEE Trans. Power Deliv. 2019, 34, 1757–1765. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, S.; Lin, F.; Li, H. Extracting Frequency Spectroscopy of Oil-Immersed Paper Based on Havriliak-Negami Model Without Known Insulation Structure of Transformer. IEEE Trans. Instrum. Meas. 2022, 71, 6003312. [Google Scholar] [CrossRef]
- Lelekakis, N.; Martin, D.; Guo, W.; Wijaya, J. Comparison of Dissolved Gas-in-Oil Analysis Methods Using a Dissolved Gas-in-Oil Standard. IEEE Electr. Insul. Mag. 2011, 27, 29–35. [Google Scholar] [CrossRef]
- Gao, S.H.; Zeng, X.S.; Zhang, G.W. Effects of Metal Passivator Degradation on the Dissolved Gases Characteristics of Oil in Oil-immersed Transformers. IEEE Trans. Dielectr. Electr. Insul. 2021, 28, 1735–1742. [Google Scholar] [CrossRef]
- Peng, R.; Zeng, W.; Zhou, Q. Adsorption and gas sensing of dissolved gases in transformer oil onto Ru3-modified SnS2: A DFT study. Appl. Surf. Sci. 2023, 615, 156445. [Google Scholar] [CrossRef]
- Jiang, T.Y.; Zhang, W.T.; Zhang, T.; Yuan, H.X.; Bi, M.Q.; Zhou, X. Adsorption and gas-sensing performances of C2H2, C2H4, CO, H2 in transformer oil on Pt-doped MoTe2 monolayer: A DFT study. Phys. E-Low-Dimens. Syst. Nanostructures 2023, 146, 115568. [Google Scholar] [CrossRef]
- Sang, T.Y.; Li, T.; Wang, S.J.; Xu, H.J.; Hu, X.Q.; Yang, Y.H.; Zhang, Z.X.; Song, R.M.; Wang, Z.Y.; Tian, H.Y.; et al. nTiO2 cluster (n = 3) modified GaNNT: A potential candidate for online monitoring for oil-immersed transformers. Appl. Surf. Sci. 2023, 607, 154811. [Google Scholar] [CrossRef]
- Faiz, J.; Soleimani, M. Dissolved Gas Analysis Evaluation in Electric Power Transformers using Conventional Methods a Review. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 1239–1248. [Google Scholar] [CrossRef]
- Faiz, J.; Soleimani, M. Assessment of Computational Intelligence and Conventional Dissolved Gas Analysis Methods for Transformer Fault Diagnosis. IEEE Trans. Dielectr. Electr. Insul. 2018, 25, 1798–1806. [Google Scholar] [CrossRef]
- Zhao, P.P.; Tang, M.C.; Zhang, D.Z. Adsorption of dissolved gas molecules in the transformer oil on metal (Ag, Rh, Sb)-doped PdSe2 momlayer: A first-principles study. Appl. Surf. Sci. 2022, 600, 154054. [Google Scholar] [CrossRef]
- Tang, M.C.; Zhao, P.P.; Wang, L.; Zhu, L.; Wu, Z.F.; Zhang, D.Z. Density Functional Theory Study of Adsorption of Dissolved Gas in Transformer Oil on a Metal (Ag, Pd, and Pt)-Doped NbSe2 Monolayer. Acs Appl. Nano Mater. 2023, 6, 5517–5526. [Google Scholar] [CrossRef]
- Tong, C.; Zhu, Z.W.; Zhang, Y.N.; Zhou, M.Y.; Peng, S.; Shan, S.X.; Wang, P.; Liu, Y.T.; Tong, T.; Zeng, L.L. Online Monitoring Data Processing Method of Transformer Oil Chromatogram Based on Association Rules. IEEE Trans. Electr. Electron. Eng. 2022, 17, 354–360. [Google Scholar] [CrossRef]
- He, H.X.; Li, X.; Chen, P.; Chen, J.; Liu, M.; Wu, L. Efficiently localizing system anomalies for cloud infrastructures: A novel Dynamic Graph Transformer based Parallel Framework. J. Cloud Comput.-Adv. Syst. Appl. 2024, 13, 115. [Google Scholar] [CrossRef]
- Bakar, N.A.; Abu-Siada, A.; Islam, S. A Review of Dissolved Gas Analysis Measurement and Interpretation Techniques. IEEE Electr. Insul. Mag. 2014, 30, 39–49. [Google Scholar] [CrossRef]
- Wang, Z.Z.; Zhang, Y.J.; Huang, X.J.; Wang, H.; Zhao, J.K.; Li, C.X.; Zhu, M.; Chen, K. Miniature mid-infrared photoacoustic gas sensor for detecting dissolved carbon dioxide in seawater. Sens. Actuators B Chem. 2024, 405, 135370. [Google Scholar] [CrossRef]
- El-kenawy, E.S.M.; Albalawi, F.; Ward, S.A.; Ghoneim, S.S.M.; Eid, M.M.; Abdelhamid, A.A.; Bailek, N.; Ibrahim, A. Feature Selection and Classification of Transformer Faults Based on Novel Meta-Heuristic Algorithm. Mathematics 2022, 10, 3144. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, X.X.; Zhang, G.Z.; Tang, J. Pd-doped MoS2 monolayer: A promising candidate for DGA in transformer oil based on DFT method. Appl. Surf. Sci. 2019, 470, 1035–1042. [Google Scholar] [CrossRef]
- Darwish, M.M.F.; Hassan, M.H.A.; Abdel-Gawad, N.M.K.; Lehtonen, M.; Mansour, D.E.A. A new technique for fault diagnosis in transformer insulating oil based on infrared spectroscopy measurements. High Volt. 2024, 9, 319–335. [Google Scholar] [CrossRef]
- Krishna, K.G.; Parne, S.; Pothukanuri, N.; Kathirvelu, V.; Gandi, S.; Joshi, D. Nanostructured metal oxide semiconductor-based gas sensors: A comprehensive review. Sens. Actuators A-Phys. 2022, 341, 113578. [Google Scholar] [CrossRef]
- Ji, H.C.; Zeng, W.; Li, Y.Q. Gas sensing mechanisms of metal oxide semiconductors: A focus review. Nanoscale 2019, 11, 22664–22684. [Google Scholar] [CrossRef]
- Luo, Y.F.; Zhang, C.; Zheng, B.B.; Geng, X.; Debliquy, M. Hydrogen sensors based on noble metal doped metal-oxide semiconductor: A review. Int. J. Hydrogen Energy 2017, 42, 20386–20397. [Google Scholar] [CrossRef]
- Wang, J.F.; Jin, X.X.; Li, C.H.; Wang, W.J.; Wu, H.; Guo, S.Y. Graphene and graphene derivatives toughening polymers: Toward high toughness and strength. Chem. Eng. J. 2019, 370, 831–854. [Google Scholar] [CrossRef]
- Cui, G.; Bi, Z.X.; Zhang, R.Y.; Liu, J.G.; Yu, X.; Li, Z.L. A comprehensive review on graphene-based anti-corrosive coatings. Chem. Eng. J. 2019, 373, 104–121. [Google Scholar] [CrossRef]
- Schwierz, F.; Pezoldt, J.; Granzner, R. Two-dimensional materials and their prospects in transistor electronics. Nanoscale 2015, 7, 8261–8283. [Google Scholar] [CrossRef]
- Burch, K.S.; Mandrus, D.; Park, J.G. Magnetism in two-dimensional van der Waals materials. Nature 2018, 563, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, B. Ultra high stiffness and thermal conductivity of graphene like C3N. Carbon 2017, 118, 25–34. [Google Scholar] [CrossRef]
- Xu, J.T.; Mahmood, J.; Dou, Y.H.; Dou, S.X.; Li, F.; Dai, L.M.; Baek, J.B. 2D Frameworks of C2N and C3N as New Anode Materials for Lithium-Ion Batteries. Adv. Mater. 2017, 29, 1702007. [Google Scholar] [CrossRef]
- Shirazi, A.H.N.; Abadi, R.; Izadifar, M.; Alajlan, N.; Rabczuk, T. Mechanical responses of pristine and defective C3N nanosheets studied by molecular dynamics simulations. Comput. Mater. Sci. 2018, 147, 316–321. [Google Scholar] [CrossRef]
- Khatun, R.; Rocky, M.H.; Roy, D.; Al Roman, A.; Ahmed, M.T. Ab initio study of Ti-doped C3N nanosheet as COCl2, O3, and HCN gas sensor. Comput. Theor. Chem. 2024, 1239, 114769. [Google Scholar] [CrossRef]
- Yan, Z.Q.; Tao, C.; Bai, Y.; Liu, S.P. Adsorption of nitrogen based gas molecules on noble metal functionalized carbon nitride nanosheets: A theoretical investigation. Comput. Theor. Chem. 2021, 1194, 112950. [Google Scholar] [CrossRef]
- Xu, C.; Hu, Y.X.; Wang, W.; Ma, J.Y. Adsorption of toxic gases on metal doped C3N monolayer: A theoretical study. Comput. Theor. Chem. 2022, 1208, 113559. [Google Scholar] [CrossRef]
- Yu, H.; Zhou, Y.L.; Liang, Z. Adsorption of CO2 and CH4 molecules on the Pd-decorated C3N based sensors: A first-principles study. Phys. E-Low-Dimens. Syst. Nanostructures 2021, 129, 114622. [Google Scholar] [CrossRef]
- Cui, H.P.; Zheng, K.; Zhang, Y.Y.; Ye, H.Y.; Chen, X.P. Superior Selectivity and Sensitivity of C3N Sensor in Probing Toxic Gases NO2 and SO2. IEEE Electron Device Lett. 2018, 39, 284–287. [Google Scholar] [CrossRef]
- Peng, R.C.; Zhou, Q.; Zeng, W. First-Principles Insight into Pd-Doped C3N Monolayer as a Promising Scavenger for NO, NO2 and SO2. Nanomaterials 2021, 11, 1267. [Google Scholar] [CrossRef]
- Cui, H.; Yan, C.; Jia, P.F.; Cao, W. Adsorption and sensing behaviors of SF6 decomposed species on Ni-doped C3N monolayer: A first-principles study. Appl. Surf. Sci. 2020, 512, 145759. [Google Scholar] [CrossRef]
- Sang, T.Y.; Li, T.; Xu, H.J.; Wang, C.D.; Wang, Z.Y.; Sun, H.; Tian, H.Y.; Wu, K.J.; Yin, Z.X.; Li, M.; et al. Rhn (n = 1, 2) modified C3N: A potential candidate for H2S, SOF2, SO2F2 and SO2 detection and scavenging. Diam. Relat. Mater. 2022, 128, 109304. [Google Scholar] [CrossRef]
- Bing, Q.M.; Liu, J.Y. Transition metal single atom anchored C3N for highly efficient formic acid dehydrogenation: A DFT study. Appl. Surf. Sci. 2021, 562, 150186. [Google Scholar] [CrossRef]
- Medvedev, M.G.; Bushmarinov, I.S.; Sun, J.W.; Perdew, J.P.; Lyssenko, K.A. Density functional theory is straying from the path toward the exact functional. Science 2017, 355, 49. [Google Scholar] [CrossRef]
- Cui, H.; Liu, T.; Zhang, Y.; Zhang, X.X. Ru-InN Monolayer as a Gas Scavenger to Guard the Operation Status of SF6 Insulation Devices: A First-Principles Theory. IEEE Sens. J. 2019, 19, 5249–5255. [Google Scholar] [CrossRef]
- Wang, J.C.; Zhang, X.X.; Liu, L.; Wang, Z.T. Dissolved gas analysis in transformer oil using Ni-Doped GaN monolayer: A DFT study. Superlattices Microstruct. 2021, 159, 107055. [Google Scholar] [CrossRef]
- Yang, G.L.; Jiang, X.L.; Liu, L.; Han, X.B.; Li, T.; Liao, Y.; Bi, C.L.; Li, Y.T.; Ren, X.D. Adsorption Properties of Metal Oxides (ZnO, TiO2, Ag2O) Modified SnS2 Toward Dissolved Gas Analysis in Transformer Oil. IEEE Sens. J. 2023, 23, 20992–21000. [Google Scholar] [CrossRef]
- Patil, A.M.; Jadhav, A.A.; Chodankar, N.R.; Avatare, A.T.; Hong, J.; Dhas, S.D.; Patil, U.M.; Jun, S.C. Recent progress of MXene synthesis, properties, microelectrode fabrication techniques for microsupercapacitors and microbatteries energy storage devices and integration: A comprehensive review. Coord. Chem. Rev. 2024, 517, 216020. [Google Scholar] [CrossRef]


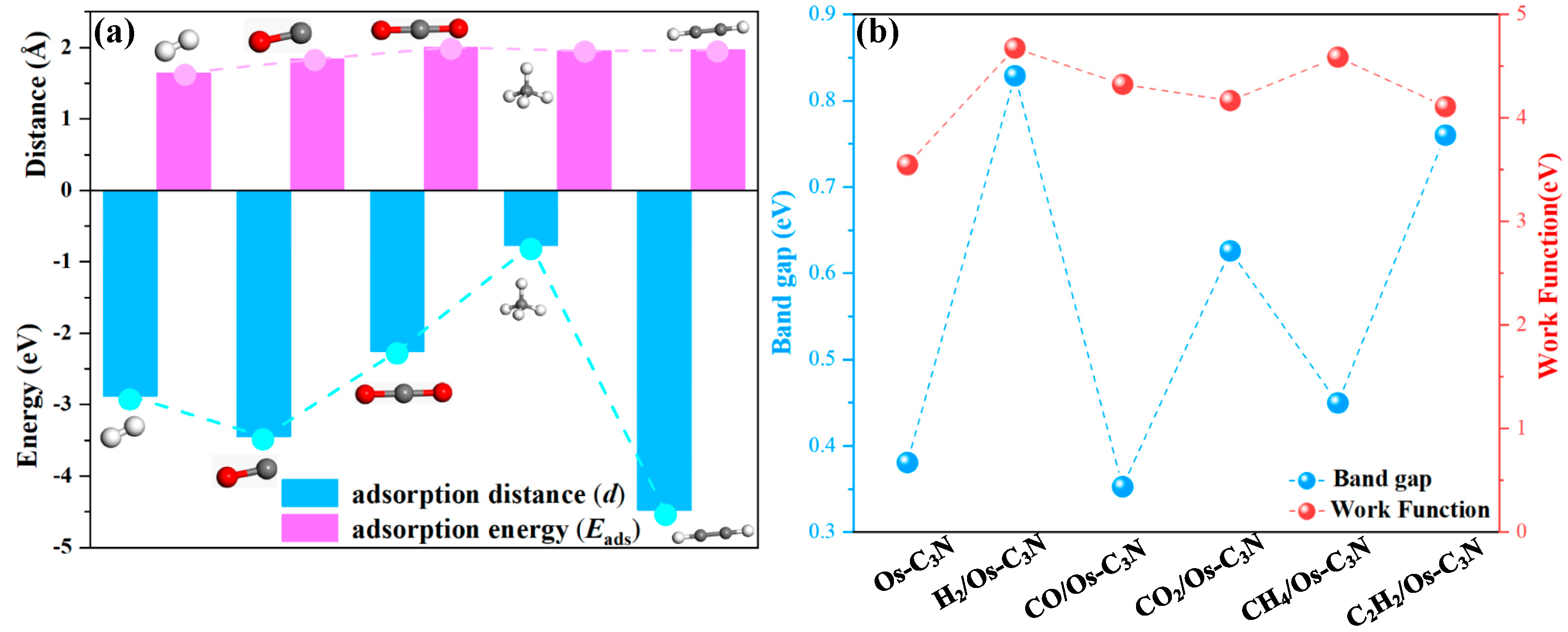
| Adsorption System | Structure | Eads (eV) | D (Å) | Qtrans (e) |
|---|---|---|---|---|
| H2/C3N | M1 | −0.052 | 2.858 | −0.001 |
| CO/C3N | M2 | −0.003 | 3.148 | −0.015 |
| CO2/C3N | M3 | −0.005 | 3.247 | −0.002 |
| CH4/C3N | M4 | −0.005 | 2.980 | −0.080 |
| C2H2/C3N | M5 | −0.113 | 3.278 | 0.004 |
| H2/Os-C3N | N1 | −2.878 | 1.644 | −0.098 |
| CO/Os-C3N | N2 | −3.447 | 1.841 | −0.067 |
| CO2/Os-C3N | N3 | −2.252 | 2.003 | −0.373 |
| CH4/Os-C3N | N4 | −0.773 | 1.952 | −0.078 |
| C2H2/Os-C3N | N5 | −4.479 | 1.966 | −0.151 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Y.; Wang, H.; Wang, F.; Zou, H. Research on Os-Modified C3N Nanosheets for Sensing and Adsorbing Dissolved Gases in 10 kV Distribution Transformer Oil for Fault Diagnosis. Processes 2025, 13, 3517. https://doi.org/10.3390/pr13113517
Zheng Y, Wang H, Wang F, Zou H. Research on Os-Modified C3N Nanosheets for Sensing and Adsorbing Dissolved Gases in 10 kV Distribution Transformer Oil for Fault Diagnosis. Processes. 2025; 13(11):3517. https://doi.org/10.3390/pr13113517
Chicago/Turabian StyleZheng, Yuanhao, Haixia Wang, Fei Wang, and Hongbo Zou. 2025. "Research on Os-Modified C3N Nanosheets for Sensing and Adsorbing Dissolved Gases in 10 kV Distribution Transformer Oil for Fault Diagnosis" Processes 13, no. 11: 3517. https://doi.org/10.3390/pr13113517
APA StyleZheng, Y., Wang, H., Wang, F., & Zou, H. (2025). Research on Os-Modified C3N Nanosheets for Sensing and Adsorbing Dissolved Gases in 10 kV Distribution Transformer Oil for Fault Diagnosis. Processes, 13(11), 3517. https://doi.org/10.3390/pr13113517





