Assessing Different Passive Treatment Pathways of Acid Mine Drainage in an Ecologically Engineered Wetland After a Veldfire
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection and Analysis
2.2. Physical–Chemical Sampling
2.3. Drifting Macroalgae Mat Sampling
2.4. Metal Biosorption Analysis of Drifting Macroalgae Mats
2.5. Wetland Substrate Cover, Rainfall and Erosion
2.6. Statistical Methods
3. Results and Discussion
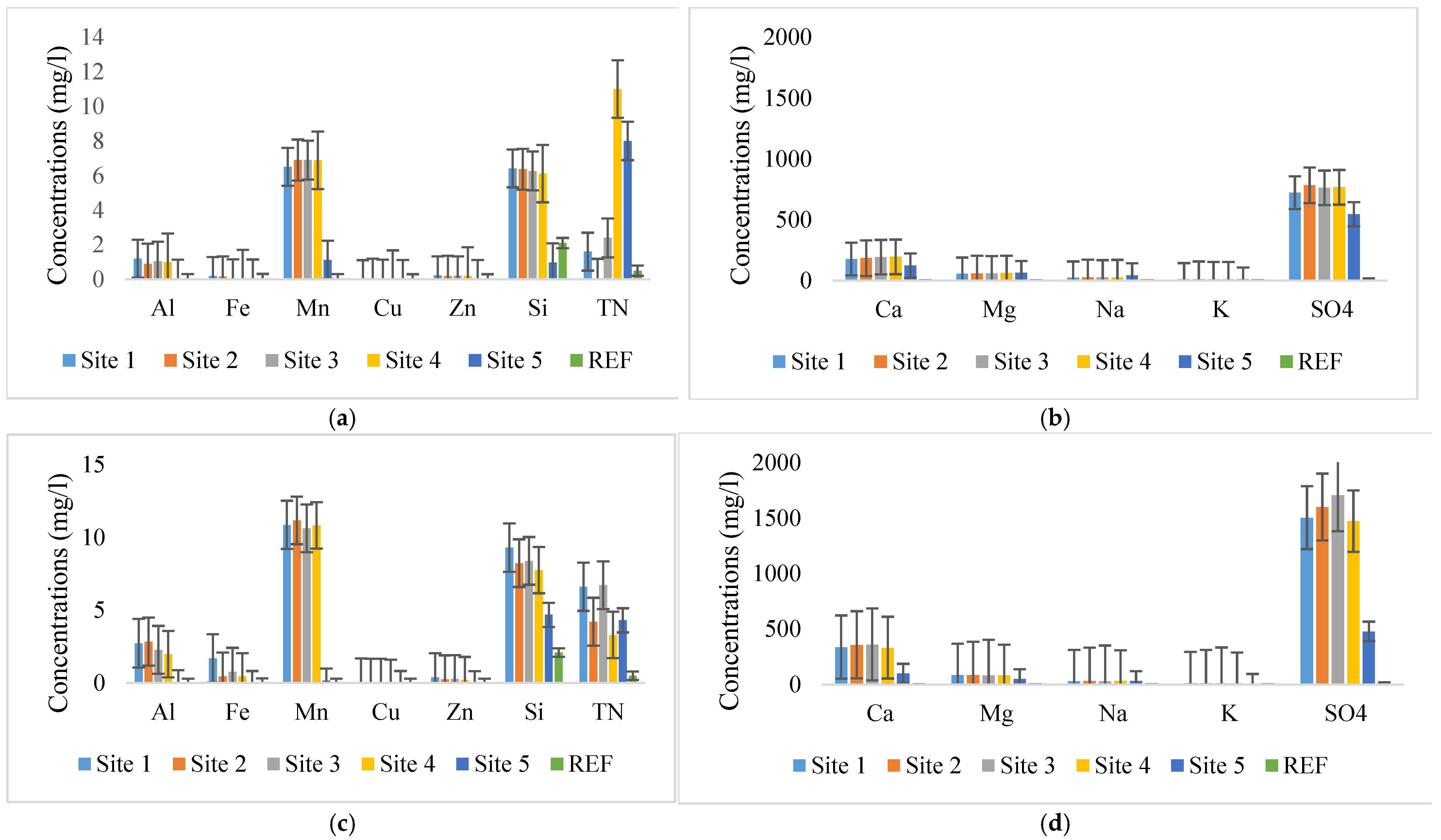
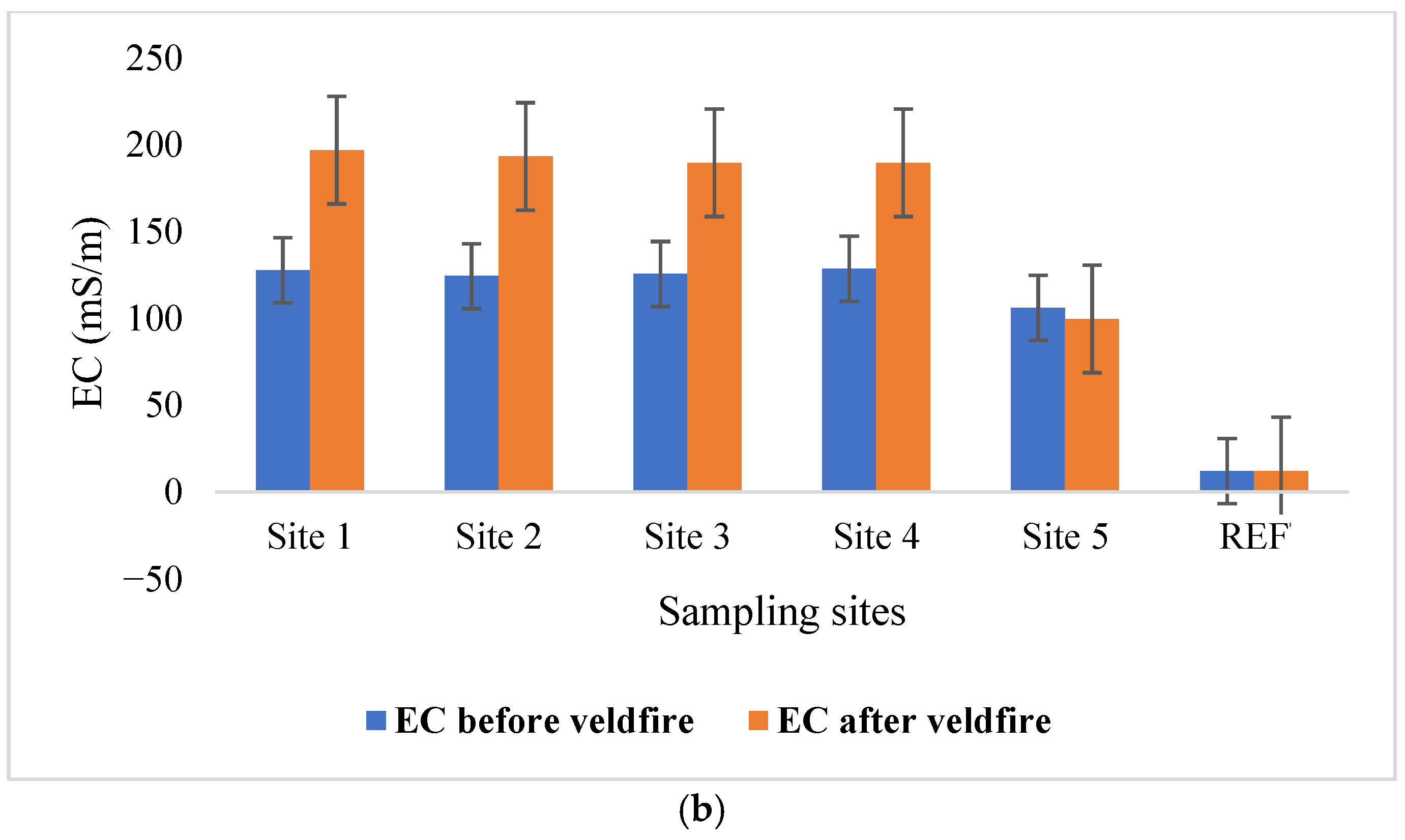
3.1. Selected Parameter Concentrations Before and After Veldfire
3.2. Rainfall and Overland Flows
3.3. Drifting Algae Mats and Metal Bioaccumulation (Phycoremediation)
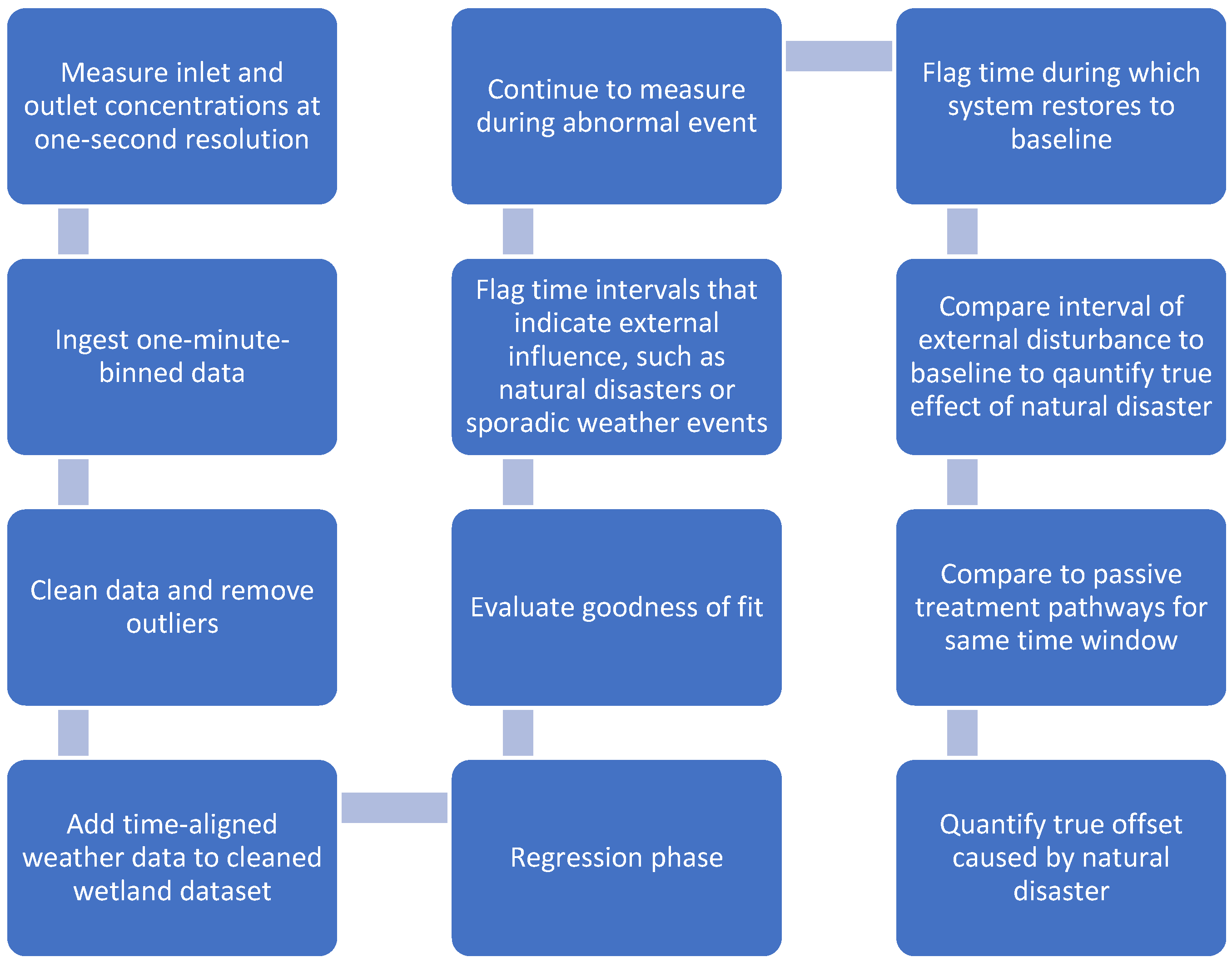
3.4. Towards a Framework of Twinning Wetlands to Quantify Passive Treatment Pathways After Future Catastrophes
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Bands | Resolution | Wavelength | Description |
|---|---|---|---|
| Band 1 | 20 m | 443 nm | Ultra-Blue (Coastal and Aerosol) |
| Band 2 | 10 m | 490 nm | Blue |
| Band 3 | 10 m | 560 nm | Green |
| Band 4 | 10 m | 665 nm | Red |
| Band 8 | 10 m | 842 nm | Visible Near-Infrared |
References
- de Klerk, A.; Oberholster, P.; van Wyk, J.; Truter, J.; Schaefer, L.; Botha, A.-M. The effect of rehabilitation measures on ecological infrastructure in response to acid mine drainage from coal mining. Ecol. Eng. 2016, 95, 463–474. [Google Scholar] [CrossRef]
- Akcil, A.; Koldas, S. Acid Mine Drainage (AMD): Causes, treatment and case studies. J. Clean. Prod. 2006, 14, 1139–1145. [Google Scholar] [CrossRef]
- Xu, X.; Wu, Y.; Rao, Y.; Fu, T.; Wu, X. Influence of litter decomposition on iron and manganese in the sediments of wetlands for acid mine drainage treatments. Acta Geochim. 2019, 38, 68–77. [Google Scholar] [CrossRef]
- Gorjão, L.R.; Maritz, J.J. The stochastic nature of power-grid frequency in South Africa. Phys. Complex. 2023, 4, 15007. [Google Scholar] [CrossRef]
- Ochieng, G.M.; Seanego, E.S.; Nkwonta, O.I. Impacts of mining on water resources in South Africa: A review. Sci. Res. Essays 2010, 5, 3351–3357. [Google Scholar]
- Singh, S.; Chakraborty, S. Performance of organic substrate amended constructed land treating acid mine drainage (AMD) of North-Eastern India. J. Hazard. Mater. 2020, 397, 122719. [Google Scholar] [CrossRef] [PubMed]
- Bwapwa, J.; Jaiyeola, A.; Chetty, R. Bioremediation of acid mine drainage using algae strains: A review. S. Afr. J. Chem. Eng. 2017, 24, 62–70. [Google Scholar] [CrossRef]
- Oberholster, P.J.; McMillan, P.; Durgapersad, K.; Botha, A.M.; de Klerk, A.R. The development of a wetland classification and risk assessment index (WCRAI) for non-wetland specialists for the management of natural freshwater wetland ecosystems. Water Air Soil Pollut. 2014, 225, 1833. [Google Scholar] [CrossRef]
- Adeeyo, A.O.; Ndlovu, S.S.; Ngwagwe, L.M.; Mudau, M.; Alabi, M.A.; Edokpayi, J.N. Wetland Resources in South Africa: Threats and Metadata Study. Resources 2022, 11, 54. [Google Scholar] [CrossRef]
- Blodau, C. A review of acidity generation and consumption in acidic coal mine lakes and their watersheds. Sci. Total Environ. 2006, 369, 307–332. [Google Scholar] [CrossRef]
- Kuyucak, N. Role of microorganisms in mining: Generation of acid rock drainage and its mitigation and treatment. Ejmp Ep (Eur. J. Miner. Process. Environ. Prot.) 2002, 2, 179–196. [Google Scholar]
- Kefeni, K.K.; Msagati, T.A.M.; Mamba, B.B. Acid mine drainage: Prevention, treatment options, and resource recovery: A review. J. Clean. Prod. 2017, 151, 475–493. [Google Scholar] [CrossRef]
- Staebe, K. Determination of the Bacterial Diversity of a Natural Freshwater Land Impacted by Acid Mine Drainage. Ph.D. Thesis, Stellenbosch University, Stellenbosch, South Africa, 2015. [Google Scholar]
- Oberholster, P.J.; De Klerk, A.R.; Chamier, J.; Cho, M.; Crafford, J.; De Klerk, L.P.; Dini, J.A.; Harris, K.; Holness, S.D.; Le Roux, W.; et al. Assessment of the Ecological Integrity of the Zaalklapspruit Land in Mpumalanga (South Africa) Before and After Rehabilitation: The Grootspruit Case Study: Report to the Water Research Commission, Pretoria, South Africa. 2016. Available online: https://wrcwebsite.azurewebsites.net/wp-content/uploads/mdocs/2230%20Volume%202%20-%20Jo.pdf (accessed on 10 September 2025).
- Forsyth, G.; Kruger, F.; Le Maitre, D. National Veldfire Risk Assessment: Analysis of Exposure of Social, Economic and Environmental Assets to Veldfire Hazards in South Africa; National Resources and the Environment CSIR, Fred Kruger Consulting cc: Stellenbosch, South Africa, 2010. [Google Scholar]
- Kotze, D. The effects of fire on wetland structure and functioning. Afr. J. Aquat. Sci. 2013, 38, 237–247. [Google Scholar] [CrossRef]
- Staebe, K.; Botes, M.; Madlala, T.; Oberholster, P.J.; Cloete, T.E. Microbial community diversity as a potential bioindicator of AMD and steel plant effluent in a channeled valley bottom wetland. Water Air Soil Pollut. 2018, 229, 397. [Google Scholar] [CrossRef]
- Jansen van Vuuren, M.; Schoeman, Y.; Botha, A.-M.; Oberholster, P.J. Revealing the Protective Dynamics of an Ecologically Engineered Wetland against Acid Mine Drainage: A Case Study in South Africa. Appl. Sci. 2024, 14, 7441. [Google Scholar] [CrossRef]
- Shelton, L.; Capel, P. Guidelines for Collecting and Processing Samples of Stream Bed Sediment for Analysis of Trace Elements and Organic Contaminants for the National Water-Quality Assessment Program; U.S. Geological Survey: Reston, VA, USA, 1994. [CrossRef]
- Apello, C.A.J.; Potsma, D. Geochemistry, Groundwater and Pollution, 2nd ed.; Buaikema Publishers: Leiden, The Netherlands, 2005. [Google Scholar]
- Girts, M.A.; Kleinmann, R.L.P.; Erickson, P.E. Performance data on Typha and Sphagnum wetlands constructed to treat coal mine drainage. In Proceedings of the 8th Annual Surface Mine Drainage Task Force Symposium, Morgantown, WV, USA, 7–8 April 1987. [Google Scholar]
- Wieder, R.K. Ion input:output budgets for five wetlands constructed for acid coal mine drainage treatment. Water Air Soil Pollut. 1993, 71, 231–270. [Google Scholar] [CrossRef]
- Ostenfeld, C.H.; Nygaard, G. On the phytoplankton of the Gatun lake, Panama Canal. Dan. Bot. Ark. 1925, 4, 1–16. [Google Scholar]
- Truter, E. An Aid to the Identification of the Dominant and Commonly Occurring Genera of Algae Observed in Some South African Impoundments; Department of Water Affairs: Pretoria, South Africa, 1987; pp. 1–97.
- Komarek, J.; Anagnostidis, K. Cyanoprokaryota: Chroococcales. In Susswasserflora von Mitteleuropa 19/1; Ettl, H., Gartner, G., Heynig, H., Mollenhauer, D., Eds.; Gustav Fischer: Stutgart, Germany, 1999. [Google Scholar]
- Wehr, J.D.; Sheath, R.G. Freshwater Algae of North America Ecology and Classification; Academic Press: San Diego, CA, USA, 2003. [Google Scholar]
- American Public Health Association (APHA). Standard Methods for Examination of Water and Wastewater, 20th ed.; American Public Health Association: Washington, DC, USA, 2006. [Google Scholar]
- Berger, W.H.; Parker, F.L. Diversity of planktonic Foraminifera in deep sea sediments. Science 1970, 168, 1345–1347. [Google Scholar] [CrossRef]
- Vanhaecke, F.; Vanhoe, H.; Dams, R.; Vandecasteele, C. The use of internal standards in ICP-MS. Talanta 1992, 39, 737–742. [Google Scholar] [CrossRef]
- Stevenson, R.J.; Bahls, L.L. Periphyton protocols. In Rapid Bioassessment Protocols for Use in Streams and Wadeable Rivers: Periphyton, Benthic Macroinvertebrates and Fish, 2nd ed.; EPA 841-B-99-002; Barbour, M.T., Gerritren, J., Snyder, B.D., Stribling, J.B., Eds.; U.S. Environmental Protection Agency: Washington, DC, USA, 1999; Volume 6, pp. 1–22. [Google Scholar]
- Dube, T.; Wepener, V.; van Vuren, J.; Smit, N.; Brendonck, L. The case for environmental flow determination for the Phongolo River, South Africa. Afr. J. Aquat. Sci. 2015, 40, 269–276. [Google Scholar] [CrossRef]
- Brown, T.J.; Hall, B.L.; Westerling, A.L. The Impact of Twenty-First Century Climate Change on Wildland Fire Danger in the Western United States: An Applications Perspective. Clim. Change 2004, 62, 365–388. [Google Scholar] [CrossRef]
- Qian, Y.; Miao, S.L.; Gu, B.; Li, Y.C. Effects of Burn Temperature on Ash Nutrient Forms and Availability from Cattail (Typha domingensis) and Sawgrass (Cladium jamaicense) in the Florida Everglades. J. Environ. Qual. 2009, 38, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Gluns, D.R.; Toews, D.A. Effect of a major wildfire on water quality in southeastern British Columbia. In Proceedings of the Symposium on Headwaters Hydrology, Bethesda, MD, USA, 27–30 June 1989; American Water Resources Association: Middleburg, VA, USA; pp. 487–499. [Google Scholar]
- Ranalli, A.J. A Summary of the Scientific Literature on the Effects of Fire on the Concentration of Nutrients in Surface Waters; US Geological Survey: Reston, VA, USA, 2004; 23p. [Google Scholar]
- Spencer, C.N.; Hauer, F.R. Phosphorus and nitrogen dynamics in streams during a wildfire. J. N. Am. Benthol. Soc. 1991, 10, 24–30. [Google Scholar] [CrossRef]
- Du Plessis, J.A.; Van Zyl, H. The effect of veld fires on the hydrological response of streamflow. Water SA 2021, 47, 185–193. [Google Scholar] [CrossRef]
- Kutiel, P.; Inbar, M. Fire impacts on soil nutrients and soil nutrients and soil erosion in a Mediterranean pine forest plantation. Catena 1993, 20, 129–139. [Google Scholar] [CrossRef]
- Mackay, S.M.; Robinson, G. Effects of wildfire and logging on stream water chemistry and cation exports of small forested catchments in south-eastern New South Wales, Australia. Hydrol. Process. 1987, 1, 359–384. [Google Scholar] [CrossRef]
- Oberholster, P.J.; Madlala, T.; Oberholster, P.F. Post Restoration Monitoring of the Zaalklapspruit Wetland with Special Reference to Groundwater-Surface Water Interaction; Coaltech Technical Report: E2019-7; Coaltech: Johannesburg, South Africa, 2019; pp. 1–21. [Google Scholar]
- Oberholster, P.J.; Botha, A.-M.; Hill, L.; Strydom, W.F. River catchment responses to anthropogenic acidification in relationship with sewage effluent: An ecotoxicology screening application. Chemosphere 2017, 189, 407–417. [Google Scholar] [CrossRef]
- Wang, Y.; Wai, K.M.; Gao, J.; Liu, X.H.; Wang, T.; Wang, W.X. The impacts of anthropogenic emissions on the precipitation chemistry at an elevated site in North-eastern China. Atmos. Environ. 2008, 42, 2959–2970. [Google Scholar] [CrossRef]
- Josipovic, M.; Annegarn, H.J.; Kneen, M.A.; Pienaar, J.J.; Piketh, S.J. Atmospheric dry and wet deposition of sulphur and nitrogen species and assessment of critical loads of acidic deposition exceedance in South Africa. S. Afr. J. Sci. 2011, 107, 10. [Google Scholar] [CrossRef]
- Behra, R.; Landwehrjohann, R.; Vogel, K.; Wagner, B.; Sigg, L. Copper and zinc content of periphyton from two rivers as a function of dissolved metal concentration. Aquat. Sci. 2002, 64, 300–306. [Google Scholar] [CrossRef]
- Hill, W. Effects of light. In Algal Ecology: Freshwater Benthic Ecosystems; Stevenson, R., Bothwell, M., Lowe, R., Eds.; Academic Press: Cambridge, UK, 1996; pp. 121–148. [Google Scholar]
- Meylan, S.; Behra, R.; Sigg, L. Influence of metal speciation in natural freshwater on bioaccumulation of copper and zinc in periphyton: A microcosm study. Environ. Sci. Technol. 2004, 38, 3104–3111. [Google Scholar] [CrossRef]
- Brouwer, J.F.C.; Deckere, E.M.G.T.; Stal, L.J. Distribution of extracellular carbohydrates in three intertidal mudflats in Western Europe. Estuar. Coast. Shelf Sci. 2003, 56, 313–324. [Google Scholar] [CrossRef]
- Pal, A.; Paul, A.K. Microbial extracellular polymeric substances: Central elements in heavy metal bioremediation. Indian J. Microbiol. 2008, 48, 49–64. [Google Scholar] [CrossRef]
- Valente, T.M.; Gomes, C.L. The role of two acidophilic algae as ecological indicators of acid mine drainage sites. J. Iber. Geol. 2007, 33, 283–294. [Google Scholar]
- Stevens, A.E.; McCathy, B.C.; Vis, M.L. Metal content of Klebsormidium-dominated (Chlorophyta) algal mats from acid mine drainage waters in southeastern. J. Torrey Bot. Soc. 2001, 128, 226–233. [Google Scholar] [CrossRef]
- Boshoff, G.; Duncan, J.; Rose, P.D. The use of micro-algal biomass as a carbon source for biological sulphate reducing systems. Water Res. 2004, 38, 2659–2666. [Google Scholar] [CrossRef] [PubMed]
- Molwantwa, J.B.; Molipane, N.P.; Rose, P.D. Biological sulfate reduction utilizing algal extracellular products as a carbon source. In Proceedings of the WISA 2000 Biennial Conference, Sun City, South Africa, 20 May 2000; Volume 28. [Google Scholar]
- Van Hille, R.P.; Boshoff, G.A.; Rose, P.D.; Duncan, J.R. A continuous process for the biological treatment of heavy metal contaminated acid mine water. Resour. Conserv. Recycl. 1999, 27, 157–167. [Google Scholar] [CrossRef]
- Nordi, C.; Vieira, A.; Nascimento, O. The metal binding capacity of Anabaena spiroides extracellular polysaccharide: An EPR study. Process Biochem. 2005, 40, 2215–2224. [Google Scholar] [CrossRef]
- Greenway, M. Nutrient content of wetland plants in constructed wetlands receiving municipal effluent in tropical Australia. Water Sci. Technol. 1997, 35, 135–142. [Google Scholar] [CrossRef]
- Christakis, N.; Cross, M.; Patel, M.K.; Tüzün, U. A Hybrid Approach for the Modelling of Complex Systems: Methodologies and Applications. J. Algorithms Comput. Technol. 2013, 7, 113–143. [Google Scholar] [CrossRef]
- Rojas Cala, E.F.; Béjar, R.; Mateu, C.; Borri, E.; Romagnoli, A.; Cabeza, L.F. Modeling of TES Tanks by Means of CFD Simulation Using Neural Networks. Energies 2025, 18, 511. [Google Scholar] [CrossRef]
- Rousseau, D.P.; Vanrolleghem, P.A.; De Pauw, N. Model-based design of horizontal subsurface flow constructed treatment wetlands: A review. Water Res. 2004, 38, 1484–1493. [Google Scholar] [CrossRef]
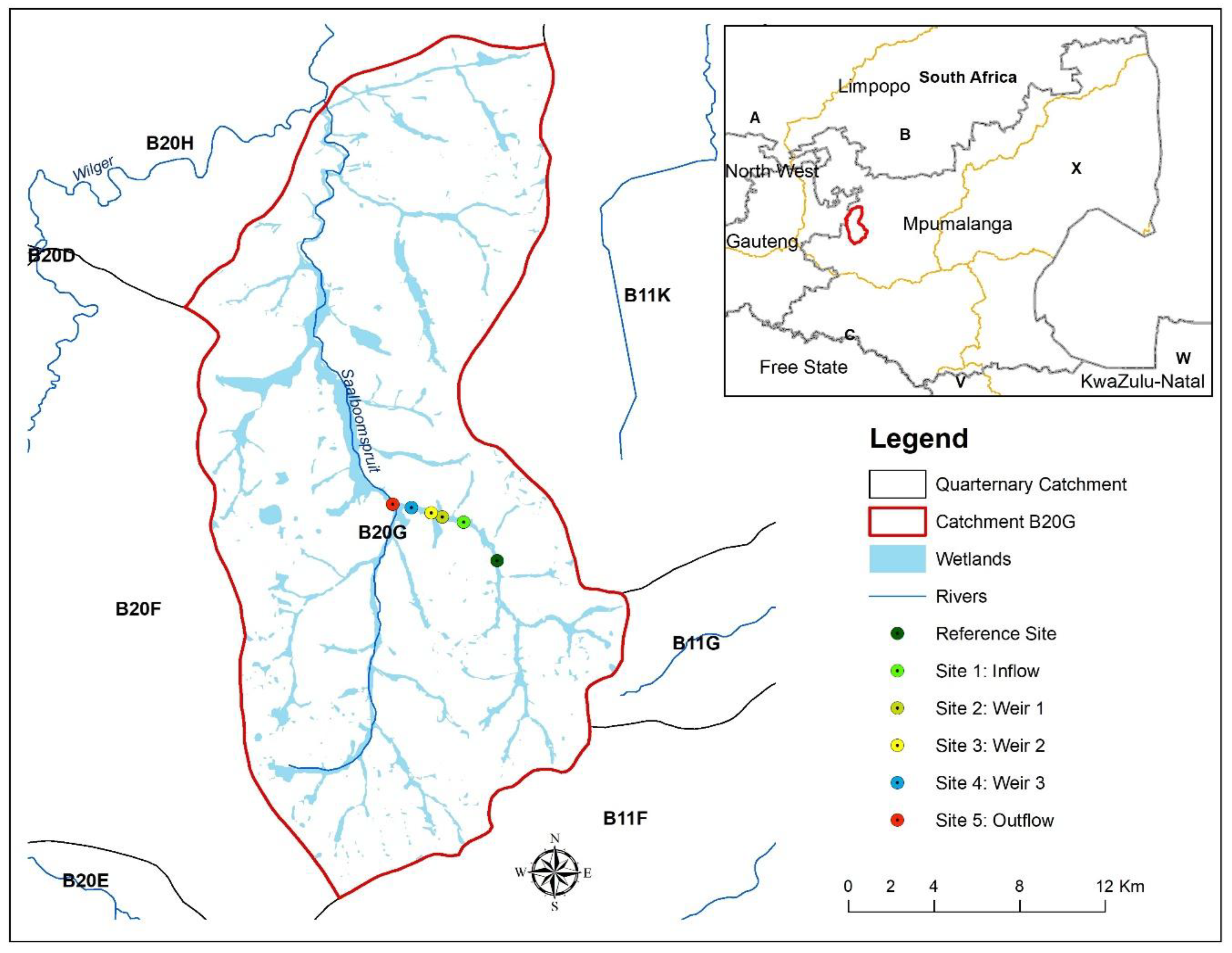




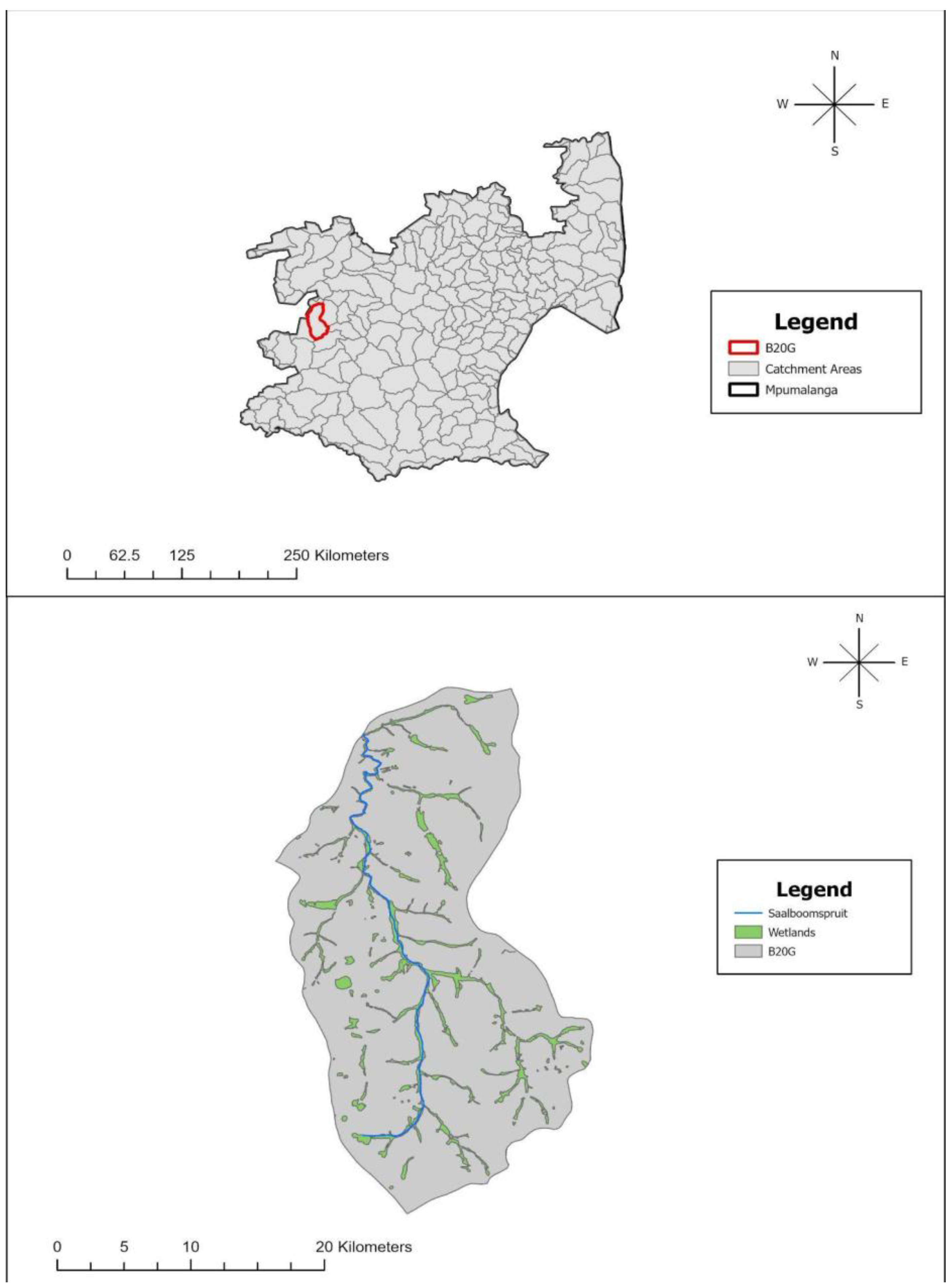
| Sites | Bottom Substrate Characteristic | Average Water Column Depth Per Site (cm) | Source of Land-Use Impacts | Ash Slurry | Macroalgae Mat Formation After Veldfire (>5 m2) at Each Sampling Site | Average Thickness (mm) of Bottom Hydroxide Precipitate Observed |
|---|---|---|---|---|---|---|
| Reference site | Sand, silt | 51 cm | Agriculture activities and sand mining upstream | None | 0 | 0 mm |
| Site 1 (inflow) | Sand | 17 cm | AMD effluent from coal mining | Low quantities | 6 m2 | 0 mm |
| Site 2 | Silt and clay | 15 cm | Agriculture and AMD effluent from coal mining | Low quantities | 4 m2 | 1 mm |
| Site 3 | Clay | 11 cm | Agriculture and AMD effluent from coal mining | Medium quantities | 9 m2 | 1 mm |
| Site 4 | Clay, Sand | 13 cm | Agriculture and AMD effluent from coal mining | Medium quantities | 8 m2 | 1 mm |
| Site 5 (outflow) | Clay | 10 cm | Agriculture and AMD effluent from coal mining | Large quantities | 15 m2 | 0 mm |
| Parameters | Units | RQOs | TWQR | Mean and (SD) Before Veldfire Across Sites 1–5 | Mean and (SD) After Veldfire Across Sites 1–5 | Before Veldfire (REF Site) | After Veldfire (REF Site) |
|---|---|---|---|---|---|---|---|
| pH | 9 | 4.71 (0.82) | 4.31 (1.38) | 8.9 | 8.9 | ||
| EC | mS/m | 70–250 | 122.42 (8.32) | 173.77 (37.18) | 12 | 12 | |
| SO4 | mg/L | 200 | N/A | 715.90 (87.81) | 1351.19 (443.84) | 16 | 16 |
| TN | mg/L | N/A | 4.60 (4.18) | 5.02 (1.38) | 0.5 | 0.5 | |
| Al | mg/L | 0.105 | 0.005 | 0.83 (0.41) | 1.98 (1.00) | 0.01 | 0.01 |
| Mn | mg/L | 0.99 | 0.014 | 5.66 (2.27) | 8.73 (4.28) | 0.01 | 0.01 |
| Fe | mg/L | 0.0001 | 0.09 (0.06) | 0.68 (0.56) | 0.02 | 0.02 | |
| Si | mg/L | N/A | N/A | 5.22 (2.38) | 7.66 (1.76) | 2.1 | 2.1 |
| Na | mg/L | N/A | 28.90 (7.08) | 31.59 (1.62) | |||
| Ca | mg/L | N/A | 174.3 (26.09) | 281.16 (105.33) | |||
| Cl | mg/L | 3.1 free Cl | 0.0002 | 18.90 (2.66) | 23.73 (3.08) | ||
| Zn | mg/L | 25.2 | 0.002 | 0.16 (0.08) | 0.28 (0.07) | ||
| Cu | mg/L | 6 | 0.0012 | 0.01(0.00) | 0.02 (0.00) | ||
| Measurable | Accuracy | Resolution | Time Scales Captured and Represented in Complex System (or Black Box) |
|---|---|---|---|
| Optimally based on complex mix | Every 1 s, long-term online monitoring | Hourly, daily and seasonal effects | |
| Optimally based on complex mix | Every 1 s, long-term online monitoring | Hourly, daily and seasonal effects | |
| ±0.01 m/s | Every 1 s, long-term online monitoring | Hourly, daily and seasonal effects Macro- + micro-governing dynamics | |
| Localized weather (temperature, humidity at different heights and windspeeds) | for digital temperature and for humidity and windspeed | Every 1 s, long-term online monitoring | Humidity rallies, stratification, abnormal transient weather effects |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oberholster, P.; Schoeman, Y.; Botha, A.-M.; Oberholster, P.; Maritz, J. Assessing Different Passive Treatment Pathways of Acid Mine Drainage in an Ecologically Engineered Wetland After a Veldfire. Processes 2025, 13, 3494. https://doi.org/10.3390/pr13113494
Oberholster P, Schoeman Y, Botha A-M, Oberholster P, Maritz J. Assessing Different Passive Treatment Pathways of Acid Mine Drainage in an Ecologically Engineered Wetland After a Veldfire. Processes. 2025; 13(11):3494. https://doi.org/10.3390/pr13113494
Chicago/Turabian StyleOberholster, Paul, Yolandi Schoeman, Anna-Maria Botha, Petri Oberholster, and Jacques Maritz. 2025. "Assessing Different Passive Treatment Pathways of Acid Mine Drainage in an Ecologically Engineered Wetland After a Veldfire" Processes 13, no. 11: 3494. https://doi.org/10.3390/pr13113494
APA StyleOberholster, P., Schoeman, Y., Botha, A.-M., Oberholster, P., & Maritz, J. (2025). Assessing Different Passive Treatment Pathways of Acid Mine Drainage in an Ecologically Engineered Wetland After a Veldfire. Processes, 13(11), 3494. https://doi.org/10.3390/pr13113494








