Abstract
The sustainable conversion of biogenic waste into high-value materials presents a promising approach for addressing environmental and industrial challenges. This work reports an advancement into antioxidant-enriched phosphate minerals derived from green conversion of biogenic calcium carbonates of crustaceans. We demonstrate the effectiveness of Raman technology in controlling conversion using phosphoric acid treatment. The effects of reaction parameters—including acid stoichiometry, granular size distribution, and thermal treatment at 700 °C and 1200 °C—were systematically evaluated. Raman spectroscopy results validated by X-ray diffraction (XRD) and SEM-EDX analyses revealed mixed-phase minerals monetite, brushite, whitlockite or hydroxylapatite, respectively. Notably, reducing particle size enhanced conversion efficiency by increasing the reactive surface area, while the use of excess phosphoric acid facilitated conversion to monocalcium phosphate and promoted the degradation of the organic matrix. Thermal treatment further altered the product composition: heating at 700 °C produced a whitlockite-rich phase, whereas treatment at 1200 °C shifted the balance toward hydroxylapatite. The synthesized calcium phosphate compounds, including hydroxylapatite, monocalcium phosphate, whitlockite, and brushite, hold significant practical utility in biomedical applications (such as bone grafts and dental implants), agriculture, and industrial processing. Moreover, we have proven that by controlling the reaction parameters the final product composition can be tailored according to the specific needs. A greener approach yields brushite, monetite, or monocalcium phosphate, while a more energy-demanding process, including heating to 1200 °C, yields a high-purity hydroxylapatite. This research offers a sustainable analytical route for producing high-purity calcium phosphate materials from wasted biomaterials, contributing to both the bioeconomy as well as scientific innovation.
1. Introduction
In recent times, sustainable biomaterials technologies have garnered significant scientific attention. The prospect of converting biogenic waste into high-value products has fuelled many recent innovations [1,2,3] within the blue bioeconomy concept. Among these products, calcium phosphates play pivotal roles in various industrial and biomedical applications. For instance, calcium phosphates are employed in water treatment processes [4,5,6]; in the food industry, calcium phosphates serve multiple roles in food processing [7,8]; while in agriculture, these minerals are processed into fertilizers that supply essential phosphorus for plant growth [9,10,11]. In biomedicine, calcium phosphate-based biomaterials are extensively used in orthopaedics and dentistry for bone grafts and implants due to their biocompatibility and structural similarity to natural bone, which facilitates osteoconduction and supports new bone formation [12,13,14,15,16,17]. Although hydroxyapatite (HA), beta-tricalcium phosphate (β-TCP), and brushite are the most popular bone substitution materials, recent studies have demonstrated the viability of other calcium phosphates such as monetite or whitlockite [18,19,20,21,22,23,24,25]. Despite a growing body of research on calcium phosphates, only a small fraction of published papers focus on their application as bone replacement materials, and an even smaller portion investigates the use of biogenic calcium carbonate sources as precursors for calcium phosphate [26,27,28,29] (see meta-analysis in Figure 1). In the present work, we propose the use of an abundant yet scarcely utilized biomaterial—wasted post-consumption crab shells—as a precursor for conversion into calcium phosphate.
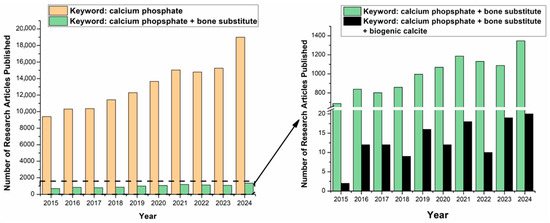
Figure 1.
Number of research articles published in the last 10 years according to Science Direct database (Accessed 3 March 2025), when using the search keywords: ‘calcium phosphate’, ‘calcium phosphate + bone substitute’ (left) and calcium phosphate + bone subsitute’, ‘calcium phosphate + bone substitute + biogenic calcite’ (right). The low number of reports involving biogenic calcite to produce phosphate minerals is highlighted with an arrow indicated in the y zoom range.
As anticipated in our previous paper [3] and subsequently reported results [30,31,32,33,34,35], the biogenic carbonate derived from crustacean exploitation is a promising material with wider applicability, due to its amazing nanoarchitecture as a 3D plywood-like Bouligand pattern, which naturally supplies nanochannels and nanopores in the mineral-organic composite. Moreover, their organic counterpart naturally embeds astaxanthin, a valuable oxygenated carotenoid with powerful antioxidant properties, which confers attractive advantages compared to the use of geogenic calcium carbonate. These materials are highly abundant and yet little translated to industrial exploitation, although their structure-morphology is the key to developing new drug nanocarriers [30,31], new biostimulants [32], efficient pollutant adsorbents [33], pharmaceutical formulations for slow release of active ingredient [30], and many more.
In addressing this research gap, our study focuses on the conversion of biogenic calcium carbonate derived from wasted crab carapace into high-value calcium phosphate compounds. Our aim was twofold: first, to develop and optimize a sustainable chemical and thermal processing route that transforms biogenic calcite into various calcium phosphate phases; and second, to demonstrate that Raman Spectroscopy serves as an effective, real-time, in situ analytical tool for monitoring the conversion process.
Raman spectroscopy is an invaluable tool for characterizing calcium phosphate phases due to its sensitivity to subtle differences in molecular vibrations associated with phosphate groups, allowing their identification through characteristic Raman bands. For example, brushite (CaHPO4•2H2O), a hydrated form of calcium hydrogen phosphate, features a prominent band in the region of 985–990 cm−1, which is attributed to the symmetric stretching (ν1) mode of the HPO4 group, as well as a weaker band at 875 cm−1 [36]. Monetite (CaHPO4), the anhydrous form of calcium hydrogen phosphate, displays a strong band in the 950–980 cm−1 region corresponding to the symmetric stretching vibration of the HPO4 unit; in some cases, this band exhibits splitting—indicative of subtle structural distortions or heterogeneity—and an additional weaker band at approximately 900 cm−1 further distinguishes monetite from brushite [37]. Hydroxylapatite (Ca5(PO4)3OH) is characterized by a sharp and intense band near 960 cm−1, corresponding to the symmetric stretching (ν1) mode of the PO43− group; additional bands arising from phosphate bending modes (ν2 and ν4) appear around 430–450 cm−1 and 580–610 cm−1, while the ν3 antisymmetric stretching mode is observed between 1000 and 1100 cm−1 [38]. Whitlockite (Ca9(PO4)6(PO3OH)) exhibits a main phosphate band slightly shifted to around 968 cm−1, often accompanied by subtle shoulders or additional peaks that reflect the unique local environment of the phosphate groups and may indicate the presence of minor ionic substitutions, such as magnesium [39].
By systematically varying reaction parameters such as acid stoichiometry, particle size, and thermal treatment, we explore the optimal conditions under which the conversion of the biogenic precursor occurs. In parallel, in situ Raman measurements allow us to track the evolution of specific vibrational bands associated with both the starting material and the emerging phosphate phases. This dual focus enhances the practical utility of the final calcium phosphate products, which have wide-ranging applications in fields such as biomedicine, agriculture, water treatment, and food processing.
2. Materials and Methods
2.1. Materials
Specimens of fresh Atlantic blue crabs, C. sapidus (carapace width 10−15 cm), were caught by traps in Parila Lagoon in the Neretva estuary (Croatia), as shown in previous papers [3,34,35]. Post-cooking and consumption resulted in biogenic waste, which allowed us to selectively collect the intact red carapace, which was subject to cleaning, removing the soft tissue remains, carefully washed in distilled water, and further subject to grinding and powdering.
Ortho-phosphoric acid 25% (PanReac AppliChem, ITW Reagents) and 85% was acquired from Nordic chemicals (Cluj-Napoca, Romania) and was used as such or further diluted in distilled water. Phosphoric acid dilution did not affect the final reaction products.
Standard calcium carbonate powder (99% purity, Chemreactiv SRL Bucureşti) was acquired from Nordic chemicals (Cluj-Napoca, Romania) and was used without further purification. Ultrapure water (18.2 8.2 MΩ cm) was used for solutions.
2.2. Methods of Biomaterial Exposure to Phosphoric Acid
To produce shell powders with various particle sizes and granulation, from large grains of 1.5 cm to microparticles (50–250 μm range), each sample was first crushed by hand and then ball-milled using a Retsch Mixer Mill MM 400 ball mill, Retsch, Haan, Germany. The 50 mL hardened steel jars were used, with 6 balls of 10 mm steel diameter per jar. Different milling times and frequencies were used: 30 Hz × 15 min, 20 Hz × 12 min, and 20 Hz × 6 min, to compare the effect of the particle size on the phosphate conversion reaction.
In the following, we named the methods referring to various grain size or microparticle size material from 1 to 5, as summarized in Table 1.

Table 1.
Summarized table presenting the phosphate phase transitions across different acid ratios, temperatures, and particle sizes.
For the initial experiment (Method 1), 1 g (0.01 mol) of standard CaCO3 was treated with 2 mL of ortho-phosphoric acid 25% (0.006 mol acid) without further dilution, according to the following stoichiometry:
10CaCO3 + 6H3PO4 → Ca10(PO4)6(OH)2 + 8H2O + 10CO2
The hydroxylapatite (HAP) stoichiometry was used as a reference in the experiments, as HAP holds the most significant value among the calcium phosphate minerals in bone tissue engineering.
Next experiment (Method 2) considered 2 g of waste post-consumption crab shell biomaterial treated with 4 mL of ortho-phosphoric acid 25% mixed with 1 mL of distilled water (2 g material in 5 mL liquid being completely covered). The dilution was performed to achieve enough reagent volume for proper stirring and mixing. The crab shells were incrementally added to the phosphoric acid solution over a period of one hour to minimize the effect of bubbling on the reaction.
To investigate the reaction products when excess phosphoric acid is used in the reaction (compared to Equation (1)), 1 g of biomaterial was treated with 5 mL (Method 3) and 8 mL (Method 4) of ortho-phosphoric acid 25%, respectively.
Experiments were performed with (Methods 2, 3, 4) and without (1, 5) stirring and heating to 90 °C, according to the synthesis of phosphate minerals using calcium carbonate [40,41,42].
Finally, Method 5, considering 1 g of waste post-consumption crab shell biomaterial and 0.405 mL of ortho-phosphoric acid 85% was hand-milled using a mortar and pestle system. Distilled water was added in small portions of 0.5 mL to enhance the reaction rate and the CO2 evolution. The resulting paste was dried in an open atmosphere.
Separate samples were calcined at 700 °C and 1200 °C, respectively.
2.3. Instruments and Data Processing
Confocal Raman spectra were acquired using a Renishaw InVia Confocal Raman System and a Cobolt DPSS laser emitting at 532 nm, as well as a high-power NIR diode laser emitting at 785 nm. During Raman microscopy, the 5× (NA 0.12, WD 13.2 mm), 20× (NA 0.35, WD 2 mm), and 100× (NA 0.9, WD 3.4 mm) collecting objectives were used with theoretical spatial resolutions of 2.7, 0.927, and 0.36 μm, respectively. An edge filter has been employed to record spectra in the 50−1840 cm−1 spectral range with 0.5 cm−1 resolution. The signal has been detected using an ultra-fast Centrus CCD detector, and data acquisition and processing have been achieved with WIRE 5.6 and OriginPro 2021 software.
Reaction monitoring was achieved using a fast, portable, i-Raman Plus B&W TEK spectrometer, equipped with a 785 nm laser line. The i-Raman Plus system was coupled with a fiber optic probe and can be used with a cuvette holder, a video microscope BAC 151X, an XYZ positioning stage probe holder, and our proprietary BWIQ® multivariate analysis software and BWID identification software. Multiple measurements were performed at regular intervals starting from 30 min after the beginning of the reaction. Both the trigger feature (ε-Grade Raman Trigger probe) of the spectrometer as well as various microscope objectives (20×, 50×, 100×) for micro-Raman were used for point measurements, yielding the higher quality Raman spectra in the 100–4000 cm−1 with 4 cm−1 spectral resolution.
The composition of the reaction products was probed by X-ray diffraction (XRD), using a Bruker D8 Advance diffractometer in Bragg−Brentano geometry with a Cu tube with λKα = 0.15418 nm, Ni filter, and a LynxEye detector. Corundum (NIST SRM1976a) was used as an internal standard. The data were collected on a 10−64° 2θ interval at a 0.02° 2θ step, measuring each step for 1 s. The identification of mineral phases was performed with Diffrac.Eva 2.1 software (Bruker AXS) using the PDF2 (Release 2023) database. Rietveld refinement using the Profex software [43] was performed for the quantification of the phases obtained.
An Equinox 55 Bruker FT-IR spectrometer with an integrated FRA 106S Raman module was employed to acquire FT-Raman spectra of bulk material. Excitation was achieved using a Nd:YAG laser operating at 1064 nm, with an output power of 350 mW. A Ge detector operating at liquid nitrogen temperature was used for detection. 350 scans were co-added for data acquisition with a spectral resolution of 2 cm−1.
SEM-EDX analyses of the shell powder and reaction products were conducted using a Hitachi SU8230 cold-field emission electron microscope. The samples were deposited on a carbon stub holder sputter-coated with a 7–10 nm thick Au film prior to imaging.
Calcination of the hand-milled product was performed in a Carbolite laboratory chamber furnace, using ceramic laboratory crucibles.
3. Results
Table 1 provides a comprehensive overview of the experiments conducted to convert biogenic calcium carbonate derived from waste cooked crab shells into calcium phosphate minerals. The starting CaCO3-based biomaterial was subjected to controlled reactions with phosphoric acid (H3PO4) in an effort to optimize the conversion efficiency and understand the influence of different reaction parameters on the identity of the reaction product mineral.
To assess the impact of particle size on the reaction kinetics and final product composition, the crab shell material was prepared in varying granulations, ranging from large macroscopic fragments (>2 cm) to finely ground powder (<250 μm). In addition to particle size variations, different reagent quantities were explored, from maintaining strict molar stoichiometry between calcium carbonate and phosphoric acid (Equation (1)) to using an excess of H3PO4.
These variations aimed to enhance reaction completeness and yield a range of calcium phosphate phases.
Experiments were performed under different physical conditions as described in Methods 1–5, to evaluate their impact on reaction dynamics. The initial reactions were carried out at room temperature with no stirring, yielding low conversion rates. The subsequent reactions were then carried out under continuous stirring and were conducted at elevated temperatures (~90 °C) according to previous studies [40,41,42].
A combination of advanced analytical techniques was employed to monitor and characterize the reaction process. Raman Spectroscopy and X-Ray Diffraction (XRD) were the primary tools used for phase identification, and various techniques provided specific advantages for the aimed characterization, as summarized:
- -
- BWTEK Portable Raman Spectrometer: Chosen for its rapid response time and flexibility, this instrument was used for real-time monitoring of the reaction, providing immediate feedback on changes in chemical composition as the reaction progressed.
- -
- FT-Raman Spectrometer: Applied to bulk sample analysis, this spectrometer enabled a more comprehensive evaluation of phosphate conversion throughout the entire volume of the sample, ensuring a representative assessment of reaction completion.
- -
- Renishaw Micro-Raman Spectrometer: This high-resolution system, equipped with an ultrafast Centrus CCD detector, was used to investigate fine spectral details, enabling precise identification of different calcium phosphate phases and distinguishing subtle structural differences in the reaction products at the microscale.
- -
- X-Ray Diffraction (XRD): Employed as a complementary technique, XRD provided crystallographic validation of the Raman data, confirming the phase composition of the calcium phosphate products and ensuring accurate interpretation of spectral findings.
Table 1 shows the summarized data recorded using Raman spectroscopy tools and XRD results from the five methods of conversion of biogenic carbonates to phosphate minerals.
As summarized in Table 1, a variety of calcium phosphate compounds were obtained throughout the experimental series, depending on the employed specific reaction conditions.
The following sections will provide a detailed discussion of each experiment, including the observed reaction trends, the influence of material properties, and the resulting phosphate phase composition of the synthesized calcium phosphate material.
Initially, we investigated the behavior of standard calcium carbonate (CaCO3) powder when treated with phosphoric acid (H3PO4) under controlled molar stoichiometry—Method 1 (Figure 2). Despite carefully maintaining the molar ratios, the reaction did not yield a pure calcium phosphate product. Instead, the resulting material was a heterogeneous mixture primarily composed of unreacted calcium carbonate in the form of calcite, along with monetite (CaHPO4) and minor traces of amorphous calcium phosphate.
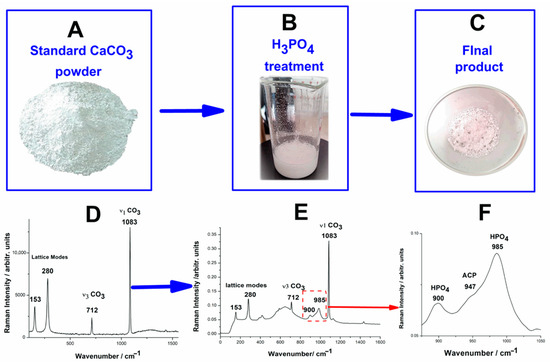
Figure 2.
Pictures of standard calcium carbonate powder (A), CaCO3 + H3PO4 reaction bowl (B), reaction product (C), along with Raman spectra of the standard calcium carbonate powder (D) and the reaction product of standard calcium carbonate powder and phosphoric acid, full spectral range (E), region of interest (F), 850–1050 cm−1.
Spectroscopic analysis revealed that the carbonate functional groups remained dominant within the reaction product. Specifically, the main CO32− stretching mode at 1083 cm−1 appeared as a highly intense peak in the spectra. Additionally, other characteristic vibrational bands of calcite were clearly visible, including the pronounced band at 712 cm−1 and the lattice modes observed in the low-wavenumber region at 153 cm−1 and 280 cm−1. These spectral features confirmed the persistence of calcium carbonate in the reaction product, indicating incomplete conversion into calcium phosphate.
A key observation during the reaction process was the high degree of effervescence, caused by the rapid release of carbon dioxide (CO2) gas. This vigorous bubbling likely disrupted the reaction pathway, preventing the full transformation of calcium carbonate into calcium phosphate. As a result, calcite remained the dominant phase within the final product.
In the spectral region between 800 and 1100 cm−1, where characteristic vibrational modes of calcium phosphate compounds are expected, additional features were identified. As shown in Figure 2F, two distinct bands at 985 cm−1 and 900 cm−1 were assigned to monetite (CaHPO4), indicating partial formation of a calcium phosphate phase. Furthermore, a subtle shoulder at 947 cm−1 suggested the presence of amorphous calcium phosphate, though only in minor quantities. These results confirm that the reaction led to a mixed-phase product rather than a complete transformation into a pure calcium phosphate compound.
Following the initial experiments in which standard calcium carbonate powder was treated with phosphoric acid, we proceeded to investigate the behaviour of biogenic calcium carbonate derived from waste cooked crab shells. This transition allowed us to evaluate the impact of the natural organic matrix present in the shells on the conversion process and the resulting calcium phosphate phases. A schematic figure of the experiments performed on wasted crustacean shells is presented in Figure 3.
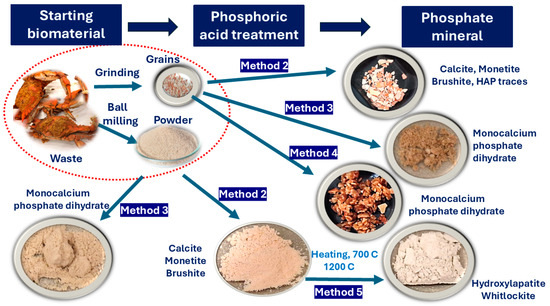
Figure 3.
Schematic presentation of the experiments performed on the wasted crustacean shells, along with the reaction products.
To prepare the biogenic calcium carbonate for reaction, the crab shells were first ground into a powder using a planetary ball mill, which ensured uniform particle size reduction. The resulting powder facilitated a more efficient reaction with phosphoric acid due to the increased surface area.
Figure 4D presents the Raman spectra of the untreated biogenic calcium carbonate powder, highlighting its composite nature. As evident from the spectra, the powder is primarily composed of a calcium carbonate matrix, with an organic component interspersed within it. Among the organic constituents, chitin and carotenoids are the most prominent. Chitin exhibits its strongest Raman band at 954 cm−1, along with a series of less intense bands spanning the 1200–1500 cm−1 region [34]. Carotenoids, which contribute to the shell’s natural pigmentation, display two strong Raman bands at 1153 cm−1 and 1515 cm−1, with an additional weaker band around 1004 cm−1.
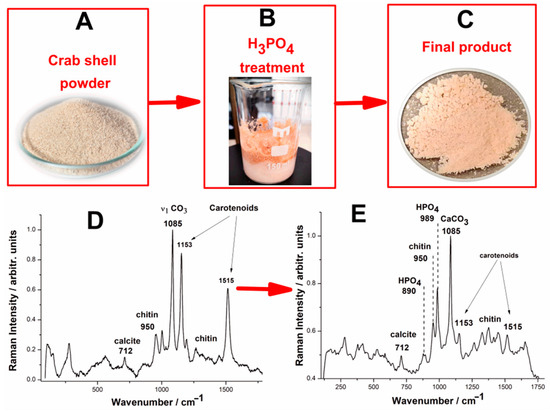
Figure 4.
Pictures of the biogenic calcium carbonate conversion to calcium phosphate minerals, wasted crustacean shell powder (A), CaCO3 + H3PO4 reaction bowl (B), reaction product (C), along with the FT-Raman spectra of the biogenic calcite powder (D) and the reaction product resulting from biogenic calcite powder from crustacean waste under phosphoric acid treatment (E).
Upon treatment with phosphoric acid—Method 5, under no stirring and heating, the Raman spectrum of the reaction product (Figure 4E) reveals significant spectral changes, indicating partial transformation of the calcium carbonate into calcium phosphate. However, the reaction does not proceed to full conversion, as evidenced by the persistent presence of calcite alongside the newly formed phosphate phases. The characteristic bands of chitin and carotenoids remain visible in the reaction product, although the intensities of the carotenoid bands are notably reduced, suggesting partial degradation or leaching of the organic components during the acid treatment. The strong calcite band at 1085 cm−1 is still present, though with reduced intensity compared to the original spectrum, indicating that a significant fraction of the calcium carbonate remains unreacted. The formation of calcium phosphate is evidenced by the band at 985 cm−1, which corresponds to the symmetric stretching mode of the HPO42− phosphate ion in brushite mineral (CaHPO4•2H2O). However, its lower intensity relative to the calcite band suggests that calcite remains the dominant phase in the reaction product. The presence of the weak band at 890 cm−1 could also be attributed to residual phosphoric acid, further indicating the acid excess, which is explainable considering that the surface passivation of the phosphate minerals by calcium phosphate could take place, similarly with the previous reports [44] on reverse anionic flotation of phosphate ores using phosphoric acid.
Comparing these results with the previous experiment—where pure, synthetic calcium carbonate was used—it appears that the biogenic calcium carbonate exhibits a higher conversion rate. This conclusion is drawn based on the relative intensities of the phosphate and carbonate bands in the Raman spectra. The improved conversion efficiency could be attributed to the porous structure of the biogenic material, which allows for a higher effective reaction surface.
When using standard calcium carbonate as the starting material—Method 1, the reaction product consists primarily of a mixture of calcite and monetite (CaHPO4), with monetite the dominant phase (63.4%), the calcite peaks suggesting, however, an incomplete conversion of the starting material into calcium phosphate. While Raman spectroscopy hinted at the possible presence of amorphous calcium phosphate in the reaction product, this phase could not be detected in the XRD analysis. This is likely due to the inherent limitations of XRD in detecting amorphous or poorly crystalline phases, which do not produce sharp diffraction peaks.
Figure 5B presents the XRD pattern of the reaction product obtained when using biogenic calcium carbonate sourced from waste cooked crab shells—Method 5. The analysis reveals that the final material is a mixture of calcite and brushite (CaHPO4•2H2O), a hydrated calcium phosphate phase. Interestingly, while the Raman spectra suggested that calcite was still the predominant compound, the quantitative composition resulting from the Rietveld refinement indicates a major participation of brushite, as 70.5% of the original material has been transformed.
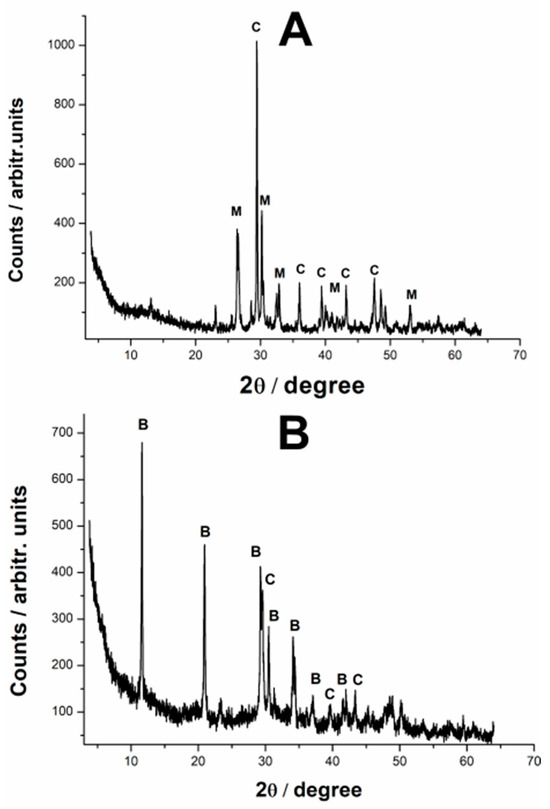
Figure 5.
XRD patterns of the reaction products resulting from the reaction of standard calcium carbonate and phosphoric acid (A) and the reaction product resulting from biogenic calcite powder from crustacean waste under phosphoric acid treatment (B). The peaks are marked as follows: C—calcite, M—monetite, B—brushite.
This discrepancy between the Raman and XRD results may be attributed to differences in the techniques’ sensitivity to various phases. Raman spectroscopy is highly sensitive to molecular vibrations and may detect smaller amounts of a compound more effectively, whereas XRD provides a more quantitative analysis of crystalline materials.
Although the precise calcite-to-phosphate ratio in the final reaction product remains uncertain, the data strongly suggests that biogenic calcium carbonate exhibited a higher conversion rate compared to the synthetic calcium carbonate. This improved reactivity may be due to several factors:
- -
- Inherent Porosity and Surface Area: Biogenic calcium carbonate, particularly from crab shells, often possesses a more porous and heterogeneous microstructure compared to synthetic CaCO3, which may facilitate greater acid penetration and reaction efficiency.
- -
- Organic Matrix Influence: The presence of organic compounds such as chitin and carotenoids in the biogenic material could alter the reaction dynamics by either stabilizing phosphate formation or modifying the surface interactions between reactants.
3.1. Carbonate to Phosphate Conversion Dependence on the Particle Size
Several stocks of starting materials were prepared, consisting of grained biogenic carbonate from crab exoskeleton waste (post-consumption = thermal treated [34]) to investigate the most favourable path to chemically convert into phosphate mineral resulting from biogenic magnesian calcite derived from crustacean exoskeleton. Our objective was to identify the most favourable reaction conditions for transforming biogenic magnesian calcite, naturally present in crustacean exoskeletons, into phosphate minerals.
The reactions were carried out according to Method 2, under continuous heating at 90 °C and stirring to ensure optimum conversion as described in the literature [40,41,42]. One key factor that appeared to play a critical role in the conversion process was the granular size of the starting material. To systematically assess this effect, we prepared samples with a range of particle sizes, from large macroscopic fragments (>1.5 cm) to finely ground powder (<250 μm). These powders were reacted under stoichiometric conditions (Equation (1)), ensuring the proper molar ratios of calcium carbonate and phosphoric acid to allow for an effective conversion.
Figure 6A presents a stacked image of the averaged Raman spectra obtained for each particle size category, recorded using the BWTEK portable Raman spectrometer. 5 Raman spectra were acquired for each particle size category, which were subsequently background subtracted, normalized and averaged. This figure provides a clear visual representation of how the spectral features evolve as the granulation of the starting material decreases. One of the most striking trends observed in the Raman spectra is the gradual reduction and eventual disappearance of the main carbonate band as the particle size decreases. This suggests that finer powder undergoes a more complete reaction with phosphoric acid, leading to a higher degree of carbonate-to-phosphate conversion. In addition to the diminishing carbonate signal, variations in phosphate-related bands are also evident across different particle sizes. The band at 989 cm−1, attributed to HPO42− ions, becomes more intense as particle size decreases, indicating increased formation of phosphate phases in finely ground samples. The characteristic phosphate band at 960 cm−1, corresponding to the symmetric stretching of the PO43− group, appears in some spectra, suggesting localized crystallization of hydroxyapatite (Ca10(PO4)6(OH)2). However, distinguishing this signal is challenging due to its proximity to the chitin band at 950 cm−1, making direct comparison with the 989 cm−1 or 1083 cm−1 (carbonate) bands less straightforward.
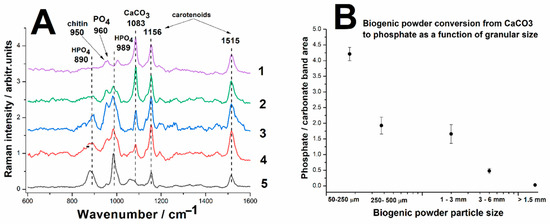
Figure 6.
(A) Micro-Raman spectra of the reaction products resulted from biogenic calcite from crustacean waste under phosphoric acid treatment under different granulation, as follows. 1—grain sizes > 1.5 mm, 2—grain sizes ~ 3–6 mm, 3—grain sizes ~ 1–3 mm, 4—powder particles 250–500 µm, 5—powder particles 50–250 µm. (B) Phosphate-to-carbonate conversion efficiency expressed as the Raman band ratio of the main stretching modes of respective minerals 989 cm−1/1083 cm−1 as a function of the biogenic calcite powder particle size distribution.
Throughout all reaction products, organic compounds—primarily chitin and carotenoids—are still detectable in the Raman spectra, though with reduced band intensities. This suggests that while some portion of the organic matrix persists post-reaction, its concentration diminishes. These results suggest that finer granulations of biogenic calcium carbonate are more favorable for efficient conversion into calcium phosphate phases.
To quantitatively assess the effect of granular size on the conversion efficiency of calcium carbonate to calcium phosphate, we analyzed the Raman intensity ratios of two key spectral bands:
- -
- The HPO42− phosphate band at 989 cm−1, indicative of phosphate formation.
- -
- The CO32− carbonate band at 1083 cm−1, representative of unreacted calcium carbonate.
By computing the intensity ratio (I989/I1083) and plotting it as a function of particle size, we aimed to establish a clear correlation between granular size and conversion efficiency. The results are summarized in Figure 6B.
The particle size distribution of the starting biogenic calcium carbonate material was determined using a series of precision strainers with varying mesh sizes. The mesh gaps ranged from 500 μm down to 50 μm.
For enhanced readability and better visualization of trends, the X-axis of the graph in Figure 6B is presented on a logarithmic scale. This scaling approach effectively highlights the sharp variations in reaction behavior that occur over multiple orders of magnitude in particle size. The Y-axis represents the reaction conversion rate, expressed as the Raman intensity ratio (I989/I1083), providing a quantitative measure of the extent to which the carbonate has been transformed into phosphate.
Figure 6B reveals a distinct logarithmic dependence between the conversion efficiency and particle size. Larger granules exhibit a low I989/I1083 ratio, indicating that the conversion of calcium carbonate to phosphate is limited. This suggests that the reaction occurs primarily on the outer surface of the granules, with restricted diffusion of phosphoric acid into the interior. Intermediate-sized particles (1–6 mm) show a moderate increase in conversion efficiency, suggesting that as the granules become smaller, the reaction progresses more thoroughly, possibly due to an increased surface area-to-volume ratio. Finely ground powder (<250 μm) demonstrates a significantly higher I989/I1083 ratio, indicating a much greater degree of carbonate-to-phosphate conversion. The sharp rise in conversion at this size range suggests that reaction kinetics are strongly dependent on particle size, with finer particles facilitating near-complete transformation due to enhanced acid penetration and a larger available reactive surface area.
These results confirm that particle size plays a crucial role in determining the efficiency of calcium carbonate conversion into calcium phosphate. The logarithmic correlation observed in Figure 6B suggests that reducing particle size yields exponentially greater conversion rates, reinforcing the idea that surface area accessibility is a primary limiting factor in the reaction process.
Furthermore, high-resolution scanning electron microscopy (SEM) images were obtained from the reaction products resulting from biogenic calcite from crustacean waste with powder particles < 250 um under phosphoric acid treatment to analyze the morphological characteristics and structural transformations during the conversion process.
Figure 7A depicts the SEM images of the reaction product, showcasing a distinct morphological transformation. The highly organized porous network of the starting material has been replaced by well-defined, dominant, monoclinic brushite crystals, which are evident throughout the samples. The presence of this characteristic dominant brushite mineral coexisting with monetite is supported by the XRD data and the layered elemental distribution (Figure 7B), reinforcing the successful conversion of biogenic calcium carbonate into calcium phosphate. The EDX data of three sets of samples clearly showed two main peaks of P and C whose ratio r = Wt(Ca)/Wt(P) was 1.24, 1.26 and 1.32 (average 1.27); 1.2, 1.19 and 1.18 (average 1.19); and 1.3, 1.3 and 1.01 (average 1.2), respectively, close enough to the theoretical Ca/P weight ratio of brushite or monetite which is 1.29. In addition, EDX mapping highlights the co-existent organic phase associated with the C distribution over the mapped area.
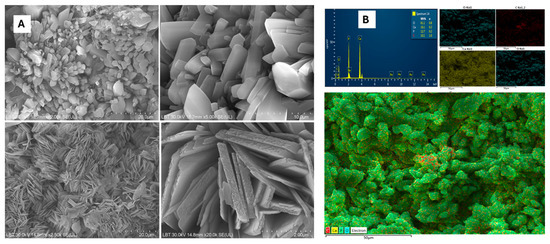
Figure 7.
High-resolution SEM images collected from the brushite—monetite minerals (A) resulted from biogenic calcite from crustacean waste with powder particles < 250 µm under phosphoric acid treatment. The layered EDX mapping of an SEM image area showing the elemental distribution of P, Ca, O, and C, and the corresponding EDX spectrum (B). Scale bars: 20, 10, 2 µm in SEM (A) and 50 µm layered EDX map (B) and individual components.
3.2. Reaction Products with Increased Phosphoric Acid Amount
To ensure the complete conversion of biogenic calcite derived from wasted crab shells into calcium phosphate minerals, we conducted experiments in which the starting material was treated with phosphoric acid in excess, when compared to HAP stoichiometry (Equation (1)), following Methods 3 and 4. The goal was to determine whether an increased acid concentration could drive the reaction to full completion, eliminating any residual calcium carbonate and maximizing phosphate yield.
Additionally, we investigated whether granular size influenced the reaction efficiency and final product composition. This was particularly important given previous findings that particle size plays a role in reaction kinetics when acid is used in stoichiometric quantities.
Experiments were conducted using two types of biogenic calcite starting materials, larger grains (>1.5 cm) and finely ground powder (50–250 μm). The resulting products were characterized using Raman spectroscopy (Figure 8A) and X-ray diffraction (XRD) (Figure 8B), to determine the phase composition and evaluate the effectiveness of the conversion.
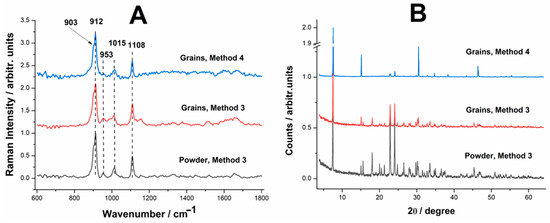
Figure 8.
(A) Raman spectra of the reaction product resulting from biogenic calcite from crustacean waste as grains (fragments) or fine powder following Method 3 and Method 4. (B) XRD patterns of the reaction product (monocalcium phosphate) resulting from biogenic calcite from crustacean waste as grains (fragments) or fine powder following Method 3 and Method 4.
The Raman spectra of the reaction products (Figure 8A) confirm that, under both acid excess conditions, the primary product formed was monocalcium phosphate (Ca(H2PO4)2). However, differences in the spectral features indicate a progressive degradation of organic material as the acid concentration increased. In the first case (Method 3), the chitin band at 950 cm−1 is still clearly visible, indicating that a portion of the organic matrix remains intact. This suggests that, while the reaction successfully converted the calcium carbonate into phosphate, the acid treatment did not fully break down the chitinous material embedded within the crab shell structure. In the second case (Method 4), the chitin band is barely detectable, with only a weak residual peak at 950 cm−1. This indicates that the higher acid concentration almost completely degraded the organic components, leaving behind a purer phosphate product.
The X-ray diffraction (XRD) patterns of the reaction products (Figure 8B) further confirm that the reaction was fully completed in both cases. In both experiments, the XRD data identified monocalcium phosphate (Ca(H2PO4)2) as the sole crystalline phase present in the final product. No detectable traces of calcite (CaCO3) were found, confirming that the excess phosphoric acid successfully dissolved all residual carbonate material. The XRD patterns did not show significant variations between the two acid concentrations, reinforcing that increasing the acid quantity does not substantially alter the phosphate phase composition—rather, it mainly affects the degradation of the organic matrix.
Interestingly, granular size did not appear to have a significant influence on the final reaction product under these conditions of acid excess. This suggests that, unlike in previous experiments, the presence of excess acid allowed for sufficient penetration into the organic matrix, ensuring complete reaction even in larger granules. In other words, while finer powders may still enhance reaction kinetics in stoichiometric conditions, when a large excess of acid is used, it effectively diffuses through larger grains, ensuring full conversion regardless of initial particle size.
By increasing the phosphoric acid concentration, we achieved complete conversion of biogenic calcite into monocalcium phosphate, demonstrating that using excess phosphoric acid is an effective strategy for achieving high-purity phosphate conversion.
3.3. Phosphate Minerals Obtained at High Temperature (700 °C, 1200 °C)
To achieve full conversion of biogenic calcium carbonate from wasted crab shells into stable calcium phosphate without relying on excess phosphoric acid, we subjected the reaction product (obtained from fine biogenic powder treated with phosphoric acid) to a controlled thermal treatment process. The samples were first heated to 700 °C, followed by further heating to 1200 °C, to facilitate phase transitions and enhance crystallinity.
The structural and compositional changes resulting from this thermal transformation were analysed using Micro-Raman spectroscopy and X-ray diffraction (XRD). The following figures provide a detailed spectroscopic and crystallographic comparison of the materials obtained at different temperatures:
- -
- Figure 9—Displays a series of Micro-Raman spectra collected from the surface of the sample heated to 700 °C (Method 5), alongside reference spectra of hydroxylapatite and whitlockite (Ca9(PO4)6PO3OH) for comparison. This analysis helps identify the presence of these key calcium phosphate phases and assess their spectral signatures.
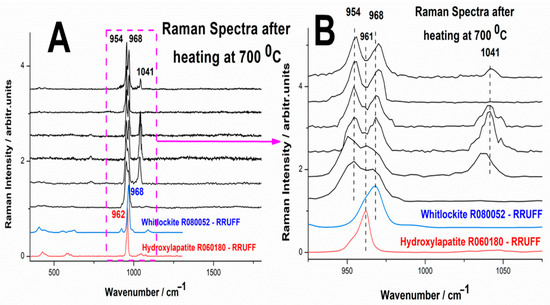 Figure 9. Series of single particle micro-Raman spectra of reaction product after heating at 700 °C, (A) and the detailed spectral range 900–1100 cm−1 (B). The bottom spectra correspond to the hydroxylapatite (R060180) and whitlockite (R070675).
Figure 9. Series of single particle micro-Raman spectra of reaction product after heating at 700 °C, (A) and the detailed spectral range 900–1100 cm−1 (B). The bottom spectra correspond to the hydroxylapatite (R060180) and whitlockite (R070675). - -
- Figure 10—Presents Micro-Raman spectra collected from the surface of the sample heated to 1200 °C (Method 5), using different collecting objectives to analyze potential structural and compositional variations across different regions of the sample. Reference spectra of hydroxylapatite and whitlockite are included for direct comparison.
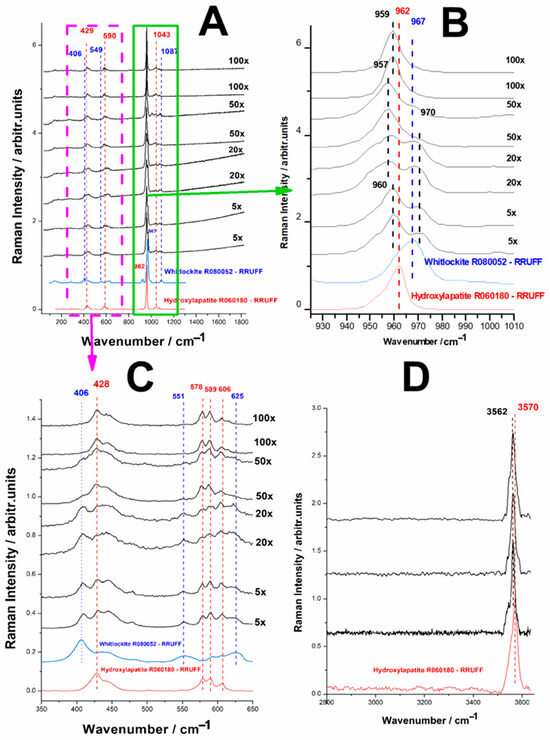 Figure 10. Series of micro-Raman spectra of the phosphate mineral particles obtained from biogenic calcite from crustacean waste under phosphoric acid treatment, thermally treated to 1200 °C: (A) 0–1800 cm−1 range, (B) 925–1010 cm−1 range, (C) 350–650 cm−1 range, (D) high wavenumber range 2800–3600 cm−1. The two bottom spectra correspond to the reference of hydroxylapatite and whitlockite from the RRUFF database, as indicated.
Figure 10. Series of micro-Raman spectra of the phosphate mineral particles obtained from biogenic calcite from crustacean waste under phosphoric acid treatment, thermally treated to 1200 °C: (A) 0–1800 cm−1 range, (B) 925–1010 cm−1 range, (C) 350–650 cm−1 range, (D) high wavenumber range 2800–3600 cm−1. The two bottom spectra correspond to the reference of hydroxylapatite and whitlockite from the RRUFF database, as indicated. - -
- Figure 11—Shows X-ray diffraction (XRD) patterns for both 700 °C and 1200 °C samples (Method 5), providing complementary crystallographic data to confirm the structural changes detected via Raman spectroscopy. Additionally, reference XRD diffractograms of hydroxylapatite and whitlockite are included, facilitating phase identification and comparison of crystallinity levels between the different samples.
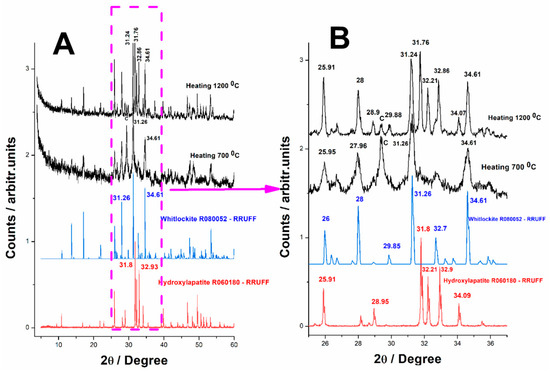 Figure 11. XRD patterns of the phosphate minerals obtained from biogenic calcite from crustacean waste under phosphoric acid treatment after heating at 700 °C and 1200 °C, (A) 0–60° range, (B) 25–37° range. The bottom diffractograms correspond to the reference diffractograms of hydroxylapatite (R060180) and whitlockite (R070675) from the RRUFF database.
Figure 11. XRD patterns of the phosphate minerals obtained from biogenic calcite from crustacean waste under phosphoric acid treatment after heating at 700 °C and 1200 °C, (A) 0–60° range, (B) 25–37° range. The bottom diffractograms correspond to the reference diffractograms of hydroxylapatite (R060180) and whitlockite (R070675) from the RRUFF database.
Figure 9 provides clear evidence of chemical and structural inhomogeneity in the phosphate mineral obtained from the reaction process. Raman spectroscopic analysis reveals variability in spectral features across different sampling points, indicating a non-uniform distribution of phosphate phases and potentially varying degrees of crystallinity.
According to the RRUFF mineral database, the primary Raman marker band for whitlockite is located at 968 cm−1, which is indeed present in the spectra of the reaction product. However, in addition to this characteristic whitlockite band, two additional spectral features are observed at 954 cm−1 and 1041 cm−1. These bands exhibit significant variations in intensity depending on the sampling location, further reinforcing the notion of inhomogeneity in the mineral phase distribution.
The band at 954 cm−1 closely corresponds to the main Raman signature of chitin, a key organic component of the original biogenic material. However, an important observation is that no other characteristic chitin bands (typically appearing in the 1200–1500 cm−1 region) are detectable in the spectra. This suggests that the 954 cm−1 band cannot be directly attributed to residual chitin.
Instead, as previously reported by Zhai et al. [39], the internal PO4 symmetric stretching mode (ν1) can undergo splitting, resulting in the emergence of two separate peaks at 954 cm−1 and 968 cm−1. This phenomenon could be indicative of structural distortions or subtle compositional variations within the phosphate mineral, possibly due to factors such as partial hydration, magnesium incorporation, or lattice disorder.
The third prominent band at 1041 cm−1 corresponds to the ν3 asymmetric stretching mode of the PO4 group [45]. Interestingly, this band exhibits high variability in intensity across different sampling points, suggesting that certain areas of the material contain a greater degree of structural order, while others may be more disordered or amorphous. Moreover, the polycrystalline orientation may favor more or less orientation for randomly quenched intensity due to the symmetry of the vibrational mode (asymmetric stretching) with polarizability more or less perpendicular to the incident electric field.
One of the most intriguing findings is that despite heating the sample to 700 °C, there is no detectable Raman signature of hydroxylapatite (HA) in the final product. Based on prior literature, thermal treatment of calcium phosphate phases at this temperature often promotes the formation of hydroxylapatite, typically characterized by a strong Raman peak around 960 cm−1 (PO4 symmetric stretch). However, in our case, the expected 960 cm−1 band of hydroxylapatite is completely absent, suggesting that either hydroxylapatite did not form under these conditions or it exists in too small a quantity to be detected by Raman spectroscopy. The dominant 968 cm−1 peak of whitlockite remains prominent, on a strong Raman background, regardless of acquisition parameter changes, implying that the phosphate phase is resistant to transformation under the applied heat treatment conditions. The presence of spectral splitting in the PO4 bands (954 and 968 cm−1) suggests that even after heating, structural distortions or chemical substitutions (such as magnesium incorporation) may be stabilizing the whitlockite-like phase and preventing the formation of hydroxylapatite.
Figure 10 presents the Raman spectroscopic data collected from the surface of the conversion product after it underwent thermal treatment at 1200 °C, using different collecting objectives to analyze variations in sample composition at different spatial scales. The spectral analysis reveals significant inhomogeneity in the material, with the observed band positions indicating the presence of both hydroxylapatite (HAP) and whitlockite as major components of the reaction product.
When comparing the experimentally obtained Raman spectra to RRUFF reference spectra for hydroxylapatite and whitlockite, a clear phase distribution pattern emerges. Each distinct Raman band detected in the sample can be confidently attributed to either HAP or whitlockite across all three key spectral regions of interest, confirming that the final product consists of a heterogeneous mixture of these two calcium phosphate phases.
By varying the collection objective magnification, we observed differences in the spectral composition, suggesting a degree of spatial separation between HAP and whitlockite domains. With a High-magnification objective (100×), when focusing on smaller regions of the sample, the spectra indicate that HAP crystals could be selectively isolated, suggesting localized crystallization of hydroxylapatite in specific regions. This is evident from the spectral data collected at higher magnification, where the characteristic Raman bands of HAP appear more pronounced, with minimal contribution from whitlockite. When using lower magnification objectives, the sampling volume is significantly larger, leading to the detection of both HAP and whitlockite bands in the spectra. This suggests that the material consists of a heterogeneous distribution of both crystalline phases, rather than a uniform, single-phase product.
A particularly noteworthy feature in Figure 10B is the shift in the position of the main PO4 symmetric stretching band. The ν1(PO43−) mode, which is typically centred around 960 cm−1 in pure hydroxylapatite, exhibits slight variations in peak position (ranging from 957 cm−1 to 960 cm−1) across different sampling points. This could be indicative of variations in local crystal chemistry, potentially due to minor compositional differences in Ca/P ratio or the presence of structural substitutions, as well as different degrees of crystallization, where slight changes in peak position and bandwidth can reflect variations in crystal size, ordering, and structural strain within the sample.
The relative Raman band intensities of hydroxylapatite and whitlockite provide insights into the phase distribution within the sample. As observed in Figure 10B, the intensity of the HAP-specific bands is consistently higher than that of whitlockite, suggesting that HAP is the dominant phase in the mixture. While whitlockite is present, its lower relative intensity indicates it is a secondary phase rather than the primary crystalline product.
Figure 11 presents the X-ray diffraction (XRD) patterns for the samples subjected to thermal treatment at 700 °C and 1200 °C, providing valuable insights into the phase composition and crystallinity of the reaction products. The XRD data is largely consistent with the Raman spectroscopy results, confirming the presence of the key calcium phosphate phases. However, certain discrepancies between the two techniques highlight the complexities of phase distribution and material heterogeneity.
According to Raman spectroscopy, the sample heated at 700 °C appears to be composed entirely of whitlockite with no detectable traces of calcium carbonate (calcite) or other calcium phosphate phases. This suggests that the reaction successfully converted the majority of the biogenic calcite into whitlockite through the thermal treatment process.
However, the XRD diffractogram of the same sample presents a different picture. While whitlockite remains the dominant phase (74.9%), a fraction of residual calcite is still present in the material (13.3%), as well as traces of HAP (11.7%). The peaks corresponding to calcite (marked as “C” in Figure 11) indicate that not all of the original biogenic carbonate has reacted, suggesting an incomplete transformation during the heating process.
This discrepancy between Raman spectroscopy and XRD results can likely be attributed to a number of factors. In terms of sample heterogeneity, Raman spectroscopy is a localized technique with a small sampling volume, meaning that specific regions analyzed under the microscope may have higher concentrations of whitlockite, while calcite-rich domains may not have been sampled. In contrast, XRD provides bulk compositional information, averaging signals from the entire sample, which allows it to detect phases that may not have been observed locally with Raman spectroscopy; local crystallization effects, the transformation of calcite into phosphate phases during heating may be spatially uneven, with some regions undergoing full conversion while others retain unreacted carbonate; when comparing differences in sensitivity, Raman spectroscopy is highly sensitive to certain vibrational modes, particularly those of phosphate species, whereas XRD is more effective at identifying crystalline phases, making it more reliable for detecting minor residual calcite.
Following additional heating to 1200 °C, the material undergoes further structural changes, as reflected in the Raman spectra and validated by the XRD results, which indicate a composition of 57.5% whitlockite and 41.4% hydroxylapatite, with only a minor trace of calcite (1.1%) very close to the detection limit. The XRD pattern indicates that most of the remaining calcite has been consumed, with only a minor percentage still detectable. This suggests that heating at this higher temperature enhanced the conversion process, driving the transformation of residual calcium carbonate into calcium phosphate phases. Raman spectroscopy confirms that the predominant phases in the sample are hydroxylapatite (HAP) and whitlockite, aligning well with the XRD findings. Interestingly, despite the significant reduction in calcite content, XRD still detects trace amounts of calcite, reinforcing the idea that complete conversion was not achieved.
Although the reaction and thermal treatment at 1200 °C resulted in a high degree of conversion, it did not reach 100% efficiency, as evidenced by the minor residual calcite in the XRD data. Further increasing the temperature beyond 1200 °C could potentially eliminate the remaining calcite, fully converting it into calcium phosphate, improve crystallinity, potentially leading to a purer HAP phase with reduced whitlockite content, as well as enhance material homogeneity, ensuring that conversion occurs uniformly across the entire sample.
4. Conclusions and Outlook
This study demonstrates the feasibility of converting biogenic calcium carbonate, sourced from wasted crab shells, into high-value calcium phosphate minerals through controlled chemical and thermal processing. Our findings underscore that reaction parameters—such as calcite to phosphoric acid ratio, granular size, and thermal treatment—are critical in dictating phase composition and conversion efficiency. Under stoichiometric conditions, the reaction produced mixed-phase products containing residual calcite alongside monetite, brushite, or whitlockite. Finer granulations enhanced phosphate formation due to increased surface area, while the use of excess phosphoric acid facilitated near-complete conversion to monocalcium phosphate, with higher acid concentrations promoting more thorough organic matrix degradation.
Thermal treatment further refined the product composition. Heating at 700 °C resulted predominantly in a whitlockite-rich phase, while treatment at 1200 °C shifted the balance toward a dominant hydroxylapatite phase, as confirmed by both Raman spectroscopy and X-ray diffraction. Although minor residual calcite was detected even after high-temperature processing, these findings suggest that further optimization of thermal parameters could yield products of higher purity. The drawback of thermal treatment is the complete disappearance of the organic matrix, particularly astaxanthin and chitin.
As outlined 10 years ago [46], although CaP-based biomaterials are extensively used today, their full potential is far from being reached.
Importantly, all the calcium phosphate compounds produced in this study—hydroxylapatite, monocalcium phosphate, monetite, whitlockite, and brushite—have significant practical utility. Hydroxylapatite is widely used in biomedical applications, including bone grafts, dental implants, and tissue engineering, due to its excellent biocompatibility and osteoconductivity. Monocalcium phosphate finds application as a leavening agent in food processing and as a component in fertilizer formulations, contributing essential phosphorus nutrients to crops. Whitlockite and brushite are also of considerable interest in medical and dental fields; whitlockite’s unique properties are being explored for bone regeneration and implant coatings, while brushite is valued for its resorbability and role in controlled drug delivery systems. Moreover, we have proven that by varying the reaction parameters, the final product can be adapted to fit specific needs. Greener, less energy-consuming approaches lead to the formation of brushite and monetite mixtures, while preserving the organic constituents of the initial powder. Both brushite and monetite are being used as bone replacement materials; hence, the presence of carotenoids and their antioxidant properties could potentially provide wasted crab shell-based bone replacements with superior properties compared to the currently existing products. While raising the phosphoric acid amount in the reaction destroys the organic components of the shell powders, it still provides a relatively straightforward and energy-efficient method of producing monocalcium phosphate, widely used as a fertilizer and leavening agent. On the other hand, if hydroxylapatite is the desired product, we have proven that heating to 1200 °C yields relatively high-purity hydroxylapatite; further optimization of the thermal parameters would likely increase the purity of the final product.
Throughout the experiments, Raman Spectroscopy has proven to be a valuable tool in both monitoring and controlling the reaction process as well as in the investigation in situ of various reaction products as a function of conditions, ratio of ingredients and biogenic powder quality, highlighting the central point of implementing Raman technology for such roadmap, which would overcome many traditional steps to validate final products, thus, with promising perspectives for process upscaling.
From an economic perspective, as nicely highlighted in a recent review on food waste conversion in value-added products [47], biogenic waste derived from crustaceans was globally estimated to be about six to eight million tons [47,48]. Such waste, mostly landfilled, incinerated, or discarded in the ocean or wastewaters, poses a negative impact on the environment; thus, knowledge-based valorization within innovative processes is imperative for enhancing circularity and sustainability. For example, a dedicated study [49] conducted an economic analysis, suggesting that the valorization process of meat-bone meal into high-quality monocalcium phosphate. It is highly profitable, with an annual production capacity of 21,700 tons at a cost of $924 per ton, while the market price reported was $1400 per ton [49]. At this point, yet difficult to estimate the cost for upscaling the conversion of biogenic calcite from crustaceans into phosphate minerals without running additional environmental-relevant dedicated study, this study focused to avoid as much as possible energy consumption and pollution, while achieving sustainability through innovative implementation of Raman technology to control the process, thus saving significant costs of the traditional calcination and analytical costs for traditionally validating the final product.
Overall, this research not only advances our understanding of the conversion of biogenic waste into functional calcium phosphate materials but also paves the way for their sustainable production and practical application across diverse fields, from biomedicine and regenerative medicine to other fields with high phosphate mineral demand, such as various industries, agriculture, and others. Finally, implementing Raman technology for such process control would provide innovative pathways while saving costs and energy load, typical for traditional waste conversion processes.
Author Contributions
G.L.: Formal Analysis, Data Curation, Writing—Original Draft Preparation; T.T.: Formal analysis, Data Curation; L.B.-T.: Formal Analysis, Data Curation; M.M.V.: Formal Analysis, Data Curation, Writing—Review; I.B.: Formal analysis, Data Curation; S.C.P.: Conceptualization, Methodology, Formal Analysis, Supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data will be made available on request from the corresponding author.
Acknowledgments
Geza Lazar acknowledges the financial support of Babes-Bolyai University in the form of a PhD scholarship.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mishra, B.; Mohanta, Y.K.; Reddy, C.N.; Reddy, S.D.M.; Mandal, S.K.; Yadavalli, R.; Sarma, H. Valorization of agro-industrial biowaste to biomaterials: An innovative circular bioeconomy approach. Circ. Econ. 2023, 2, 100050. [Google Scholar] [CrossRef]
- Pal, P.; Singh, A.K.; Srivastava, R.K.; Rathore, S.S.; Sahoo, U.K.; Subudhi, S.; Sarangi, P.K.; Prus, P. Circular Bioeconomy in Action: Transforming Food Wastes into Renewable Food Resources. Foods 2024, 13, 3007. [Google Scholar] [CrossRef] [PubMed]
- Nekvapil, F.; Aluas, M.; Barbu-Tudoran, L.; Suciu, M.; Bortnic, R.A.; Glamuzina, B.; Cîntă Pinzaru, S. From Blue Bioeconomy toward Circular Economy through High-Sensitivity Analytical Research on Waste Blue Crab Shells. ACS Sustain. Chem. Eng. 2019, 7, 16820–16827. [Google Scholar] [CrossRef]
- Lyczko, N.; Nzihou, A.; Sharrok, P. Calcium Phosphate Sorbent for Environmental Application. Procedia Eng. 2014, 83, 423–431. [Google Scholar] [CrossRef]
- da Silva, E.A.B.; Costa, C.A.E.; Vilar, V.J.P.; Botelho, C.M.S.; Larosi, M.B.; Saracho, J.M.P.; Boaventura, R.A.R. Water remediation using calcium phosphate derived from marine residues. Water Air. Soil Pollut. 2012, 223, 989–1003. [Google Scholar] [CrossRef]
- Amjad, Z. (Ed.) Calcium Phosphates in Biological and Industrial Systems; Springer Science & Business Media: Berlin, Germany, 2013. [Google Scholar]
- Enax, J.; Meyer, F.; Schulze zur Wiesche, E.; Epple, M. On the Application of Calcium Phosphate Micro- and Nanoparticles as Food Additive. Nanomaterials 2022, 12, 4075. [Google Scholar] [CrossRef]
- Laohavisuti, N.; Boonchom, B.; Boonmee, W.; Chaiseeda, K.; Seesanong, S. Simple recycling of biowaste eggshells to various calcium phosphates for specific industries. Sci. Rep. 2021, 11, 15143. [Google Scholar] [CrossRef]
- Fellet, G.; Pilotto, L.; Marchiol, L.; Braidot, E. Tools for Nano-Enabled Agriculture: Fertilizers Based on Calcium Phosphate, Silicon, and Chitosan Nanostructures. Agronomy 2021, 11, 1239. [Google Scholar] [CrossRef]
- Carmona, F.J.; Guagliardi, A.; Masciocchi, N. Nanosized Calcium Phosphates as Novel Macronutrient Nano-Fertilizers. Nanomaterials 2022, 12, 2709. [Google Scholar] [CrossRef]
- Weeks, J.J., Jr.; Hettiarachchi, G.M. A Review of the Latest in Phosphorus Fertilizer Technology: Possibilities and Pragmatism. J. Environ. Qual. 2019, 48, 1300–1313. [Google Scholar] [CrossRef]
- Gong, Y.; Zhang, Y.; Cao, Z.; Ye, F.; Lin, Z.; Li, Y. Development of CaCO3 microsphere-based composite hydrogel for dual delivery of growth factor and Ca to enhance bone regeneration. Biomater. Sci. 2019, 7, 3614–3626. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Zhang, L.; Zhou, Z.; Luo, X.; Wang, T.; Zhao, X.; Lu, B.; Chen, F.; Zheng, L. Calcium Phosphate-Based Biomaterials for Bone Repair. J. Funct. Biomater. 2022, 13, 187. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, H.; Ma, Y.; Jiang, Y. Calcium Phosphate-Based Nanomaterials: Preparation, Multifunction, and Application for Bone Tissue Engineering. Molecules 2023, 28, 4790. [Google Scholar] [CrossRef]
- Albulescu, R.; Popa, A.-C.; Enciu, A.-M.; Albulescu, L.; Dudau, M.; Popescu, I.D.; Mihai, S.; Codrici, E.; Pop, S.; Lupu, A.-R.; et al. Comprehensive In Vitro Testing of Calcium Phosphate-Based Bioceramics with Orthopedic and Dentistry Applications. Materials 2019, 12, 3704. [Google Scholar] [CrossRef]
- Das, A.; Ghosh, S.; Ringu, T.; Pramanik, N. A Focus on Biomaterials Based on Calcium Phosphate Nanoparticles: An Indispensable Tool for Emerging Biomedical Applications. BioNanoScience 2023, 13, 795–818. [Google Scholar] [CrossRef]
- Ślósarczyk, A.; Czechowska, J.; Cichoń, E.; Zima, A. New Hybrid Bioactive Composites for Bone Substitution. Processes 2020, 8, 335. [Google Scholar] [CrossRef]
- Vijayan, A.; Vishnu, J.; Revathi, A.; Shankar, B.; Sambhudevan, S. A review on hydroxyapatite fabrication: From powders to additive manufactured scaffolds. Biomater. Sci. 2025, 13, 913–945. [Google Scholar] [CrossRef]
- Skibiński, S.; Czechowska, J.; Guzik, M.; Vivcharenko, V.; Przekora, A.; Szymczak, P.; Zima, A. Scaffolds based on β tricalcium phosphate and polyhydroxyalkanoates as biodegradable and bioactive bone substitutes with enhanced physicochemical properties. Sustain. Mater. Technol. 2023, 38, e00722. [Google Scholar] [CrossRef]
- Kattimani, V.S.; Kondaka, S.; Lingamaneni, K.P. Hydroxyapatite—Past, Present, and Future in Bone Regeneration. Bone Tissue Regen. Insights 2016, 7. Available online: https://journals.sagepub.com/doi/10.4137/BTRI.S36138 (accessed on 23 October 2025). [CrossRef]
- Tanaka, T.; Komaki, H.; Chazono, M.; Kitasato, S.; Kakuta, A.; Akiyama, S.; Marumo, K. Basic research and clinical application of beta-tricalcium phosphate (β-TCP). Morphologie 2017, 101, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Haiping, L.; Yinghong, Z.; Yaping, M.; Lan, X.; Wenjun, J.; Yi, Z.; Xin, W. Current Application of Beta-Tricalcium Phosphate in Bone Repair and Its Mechanism to Regulate Osteogenesis. Front. Mater. 2021, 8, 698915. [Google Scholar] [CrossRef]
- Imaniyyah, A.G.; Sunarso, E.H. Monetite as a potential ideal bone substitute: A short review on fabrication and properties. Mater. Today Proc. 2022, 66, 2762–2766. [Google Scholar] [CrossRef]
- Bauer, L.; Antunović, M.; Rogina, A.; Ivanković, M.; Ivanković, H. Bone-mimetic porous hydroxyapatite/whitlockite scaffolds: Preparation, characterization and interactions with human mesenchymal stem cells. J. Mater. Sci. 2021, 56, 3947–3969. [Google Scholar] [CrossRef]
- Ćurković, L.; Žmak, I.; Kurajica, S.; Tonković, M.E.; Šokčević, Z.; Renjo, M.M. From eggshells biowaste to hydroxyapatite biomaterial. Materialwissenschaft und Werkstofftechnik 2017, 48, 797. [Google Scholar] [CrossRef]
- Dinatha, I.K.H.; Diputra, A.H.; Partini, J.; Wihadmadyatami, H.; Yusuf, Y. 3D pure bioceramic scaffold from biogenic sand lobster (Panulirus homarus) shell waste for enhancing in vitro cell osteogenic differentiation. Ceram. Int. 2024, 51, 11188–11200. [Google Scholar] [CrossRef]
- Irfa, M.A.; Muryanto, S.; Prihanto, A.; Pusparizkita, Y.M.; Ismail, R.; Jamari, J.; Bayuseno, A.P.; Show, P.L. Microwave-assisted hydrothermal synthesis of carbonated apatite with calcium and phosphate resources derived from green mussel shell and bovine bone wastes. Environ. Adv. 2024, 17, 100582. [Google Scholar] [CrossRef]
- Lee, S.J.; Oh, S.H. Fabrication of calcium phosphate bioceramics by using eggshell and phosphoric acid. Mater. Lett. 2003, 57, 4570–4574. [Google Scholar] [CrossRef]
- Raya, I.; Mayasari, E.; Yahya, A.; Syahrul, M.; Latunra, A.I. Shynthesis and Characterizations of Calcium Hydroxyapatite Derived from Crabs Shells (Portunus pelagicus) and Its Potency in Safeguard against to Dental Demineralizations. Int. J. Biomater. 2015, 2015, 469176. [Google Scholar] [CrossRef] [PubMed]
- Lazar, G.; Nekvapil, F.; Glamuzina, B.; Tamaș, T.; Barbu-Tudoran, L.; Suciu, M.; Cinta Pinzaru, S. pH-Dependent Behavior of Novel 5-FU Delivery System in Environmental Conditions Comparable to the Gastro-Intestinal Tract. Pharmaceutics 2023, 15, 1011. [Google Scholar] [CrossRef]
- Lazar, G.; Nekvapil, F.; Hirian, R.; Glamuzina, B.; Tamas, T.; Barbu-Tudoran, L.; Cinta Pinzaru, S. Novel Drug Carrier: 5-Fluorouracil Formulation in Nanoporous Biogenic Mg-calcite from Blue Crab Shells—Proof of Concept. ACS Omega 2021, 6, 27781–27790. [Google Scholar] [CrossRef] [PubMed]
- Nekvapil, F.; Ganea, V.; Ciorîță, A.; Hirian, R.; Ogresta, L.; Glamuzina, B.; Roba, C.; Cintă Pinzaru, S. Wasted Biomaterials from Crustaceans as a Compliant Natural Product Regarding Microbiological, Antibacterial Properties and Heavy Metal Content for Reuse in Blue Bioeconomy: A Preliminary Study. Materials 2021, 14, 4558. [Google Scholar] [CrossRef]
- Nekvapil, F.; Stegarescu, A.; Lung, I.; Hirian, R.; Cosma, D.; Levei, E.; Soran, M.-L. A Novel Nanoporous Adsorbent for Pesticides Obtained from Biogenic Calcium Carbonate Derived from Waste Crab Shells. Nanomaterials 2023, 13, 3042. [Google Scholar] [CrossRef]
- Nekvapil, F.; Pinzaru, S.C.; Barbu–Tudoran, L.; Suciu, M.; Glamuzina, B.; Tamaș, T.; Chiș, V. Color-specific porosity in double pigmented natural 3d-nanoarchitectures of blue crab shell. Sci. Rep. 2020, 10, 3019. [Google Scholar] [CrossRef]
- Ogresta, L.; Nekvapil, F.; Tǎmaş, T.; Barbu-Tudoran, L.; Suciu, M.; Hirian, R.; Aluaş, M.; Lazar, G.; Levei, E.; Glamuzina, B.; et al. Rapid and Application-Tailored Assessment Tool for Biogenic Powders from Crustacean Shell Waste: Fourier Transform-Infrared Spectroscopy Complemented with X-ray Diffraction, Scanning Electron Microscopy, and Nuclear Magnetic Resonance Spectroscopy. ACS Omega 2021, 6, 27773–27780. [Google Scholar] [CrossRef]
- Frost, R.L.; Xi, Y.; Pogson, R.E.; Millar, G.J.; Tan, K.; Palmer, S.J. Raman spectroscopy of synthetic CaHPO4·2H2O– and in comparison with the cave mineral brushite. J. Raman Spectrosc. 2012, 43, 571–576. [Google Scholar] [CrossRef]
- Casciani, F.; Condrate, R.A. The Raman spectrum of monetite, CaHPO4. J. Solid State Chem. 1980, 34, 385–388. [Google Scholar] [CrossRef]
- Timchenko, P.E.; Timchenko, E.V.; Pisareva, E.V.; Vlasov, M.Y.; A Red’kin, N.; O Frolov, O. Spectral analysis of allogeneic hydroxyapatite powders. J. Phys. Conf. Ser. 2017, 784, 012060. [Google Scholar] [CrossRef]
- Zhai, S.; Wu, X.; Xue, W. Pressure-dependent Raman spectra of β-Ca3(PO4)2 whitlockite. Phys. Chem. Miner. 2015, 42, 303–308. [Google Scholar] [CrossRef]
- Engin, A.; Girgin, I. Synthesis of hydroxyapatite by using calcium carbonate and phosphoric acid in various water-ethanol solvent systems. Open Chem. 2009, 7, 745–751. [Google Scholar] [CrossRef]
- Minh, D.P.; Rio, S.; Sharrock, P.; Sebei, H.; Lyczko, N.; Tran, N.D.; Raii, M.; Nzihou, A. Hydroxyapatite starting from calcium carbonate and orthophosphoric acid: Synthesis, characterization, and applications. J. Mater. Sci. 2014, 49, 4261–4269. [Google Scholar] [CrossRef]
- Minh, D.P.; Martínez, M.G.; Nzihou, A.; Sharrock, P. Thermal behavior of apatitic calcium phosphates synthesized from calcium carbonate and orthophosphoric acid or potassium dihydrogen orthophosphate. J. Therm. Anal. Calorim. 2013, 112, 1145–1155. [Google Scholar] [CrossRef]
- Doebelin, N.; Kleeberg, R. Profex: A graphical user interface for the Rietveld refinement program BGMN. J. Appl. Crystallogr. 2015, 48, 1573–1580. [Google Scholar] [CrossRef] [PubMed]
- Filippov, L.O.; Filippova, I.V.; Kaba, O.B.; Fornasiero, D. In-Situ study of the kinetics of phosphoric acid interaction with calcite and fluorapatite by Raman spectroscopy and flotation. Miner. Eng. 2021, 162, 106729. [Google Scholar] [CrossRef]
- de Aza, P.N.; Santos, C.; Pazo, A.; de Aza, S.; Cuscó, R.; Artús, L. Vibrational Properties of Calcium Phosphate Compounds. 1. Raman Spectrum of β-Tricalcium Phosphate. Chem. Mater. 1997, 9, 912–915. [Google Scholar] [CrossRef]
- Habraken, W.; Habibovic, P.; Epple, M.; Bohner, M. Calcium phosphates in biomedical applications: Materials for the future? Mater. Today 2016, 19, 69–87. [Google Scholar] [CrossRef]
- Topić Popović, N.; Lorencin, V.; Strunjak-Perović, I.; Čož-Rakovac, R. Shell Waste Management and Utilization: Mitigating Organic Pollution and Enhancing Sustainability. Appl. Sci. 2023, 13, 623. [Google Scholar] [CrossRef]
- Liu, Z.; de Souza, T.S.P.; Holland, B.; Dunshea, F.; Barrow, C.; Suleria, H.A.R. Valorization of Food Waste to Produce Value-Added Products Based on Its Bioactive Compounds. Processes 2023, 11, 840. [Google Scholar] [CrossRef]
- Kowalski, Z.; Wilkosz-Język, A.; Makara, A. Production of Food-Grade Monocalcium Phosphate from Meat-Bone Meal. Materials 2025, 18, 4653. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).