Influence of Drying Methods and Parameters on the Quality of Jasminum sambac (L.) Flower Extracts Obtained via Supercritical Fluid Extraction
Abstract
1. Introduction
2. Materials and Methods
2.1. Extraction Parameters
2.2. J. sambac Samples and Preparation
2.3. Drying Methods
2.4. J. sambac Extraction
2.5. GC–MS and FTIR Analysis of Extract and Sensory Evaluation
2.6. Literature Review of Compounds from GC–MS and FTIR Analysis for Odor Characterization
3. Results and Discussion
3.1. Effects of the Drying Method
3.2. SFE Extracts
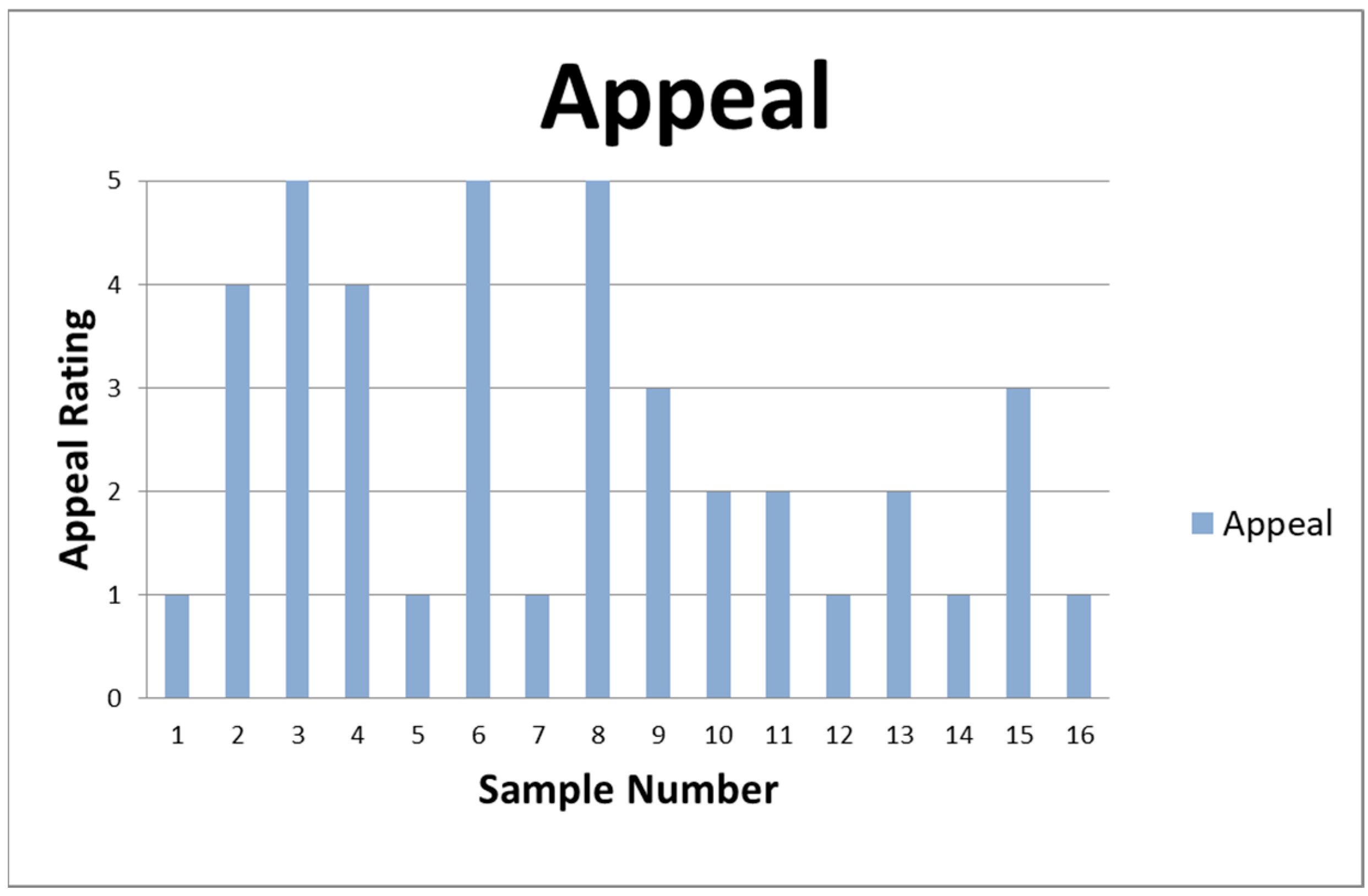
3.3. Sensory Evaluation
3.4. Compound Identification Through FTIR and GC–MS Analysis
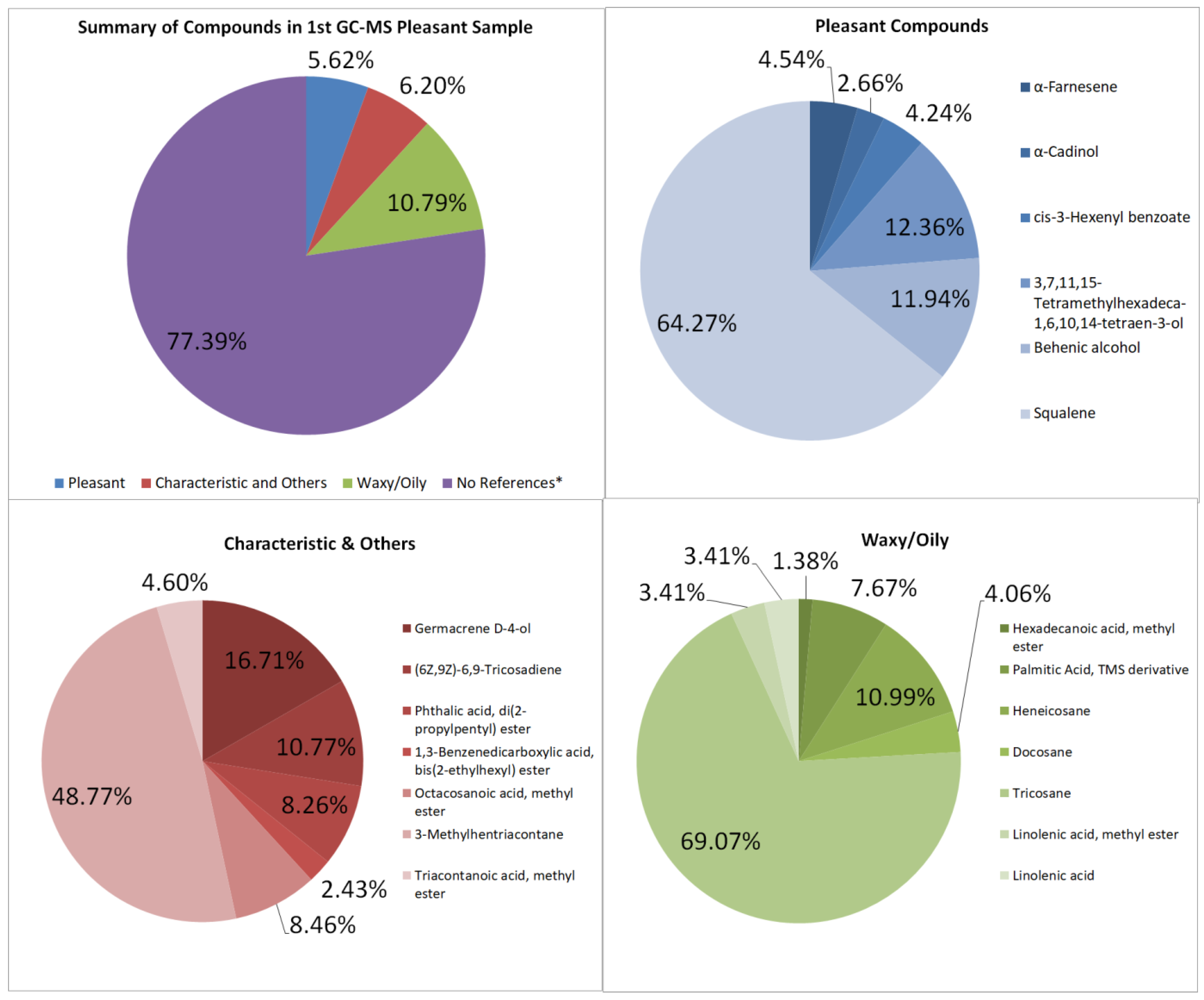
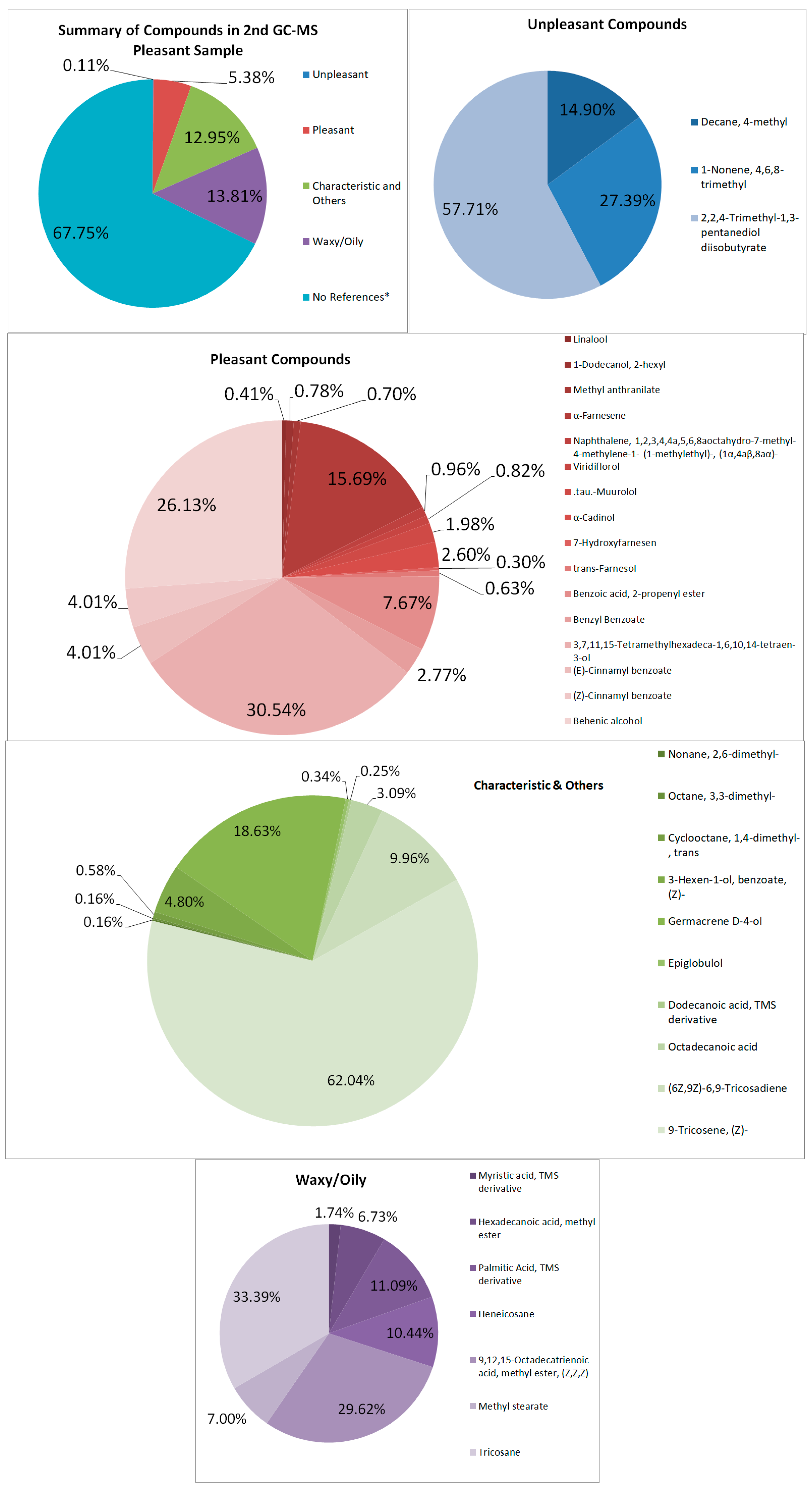
3.4.1. Pleasant-Smelling Samples
FTIR Analysis
GC–MS
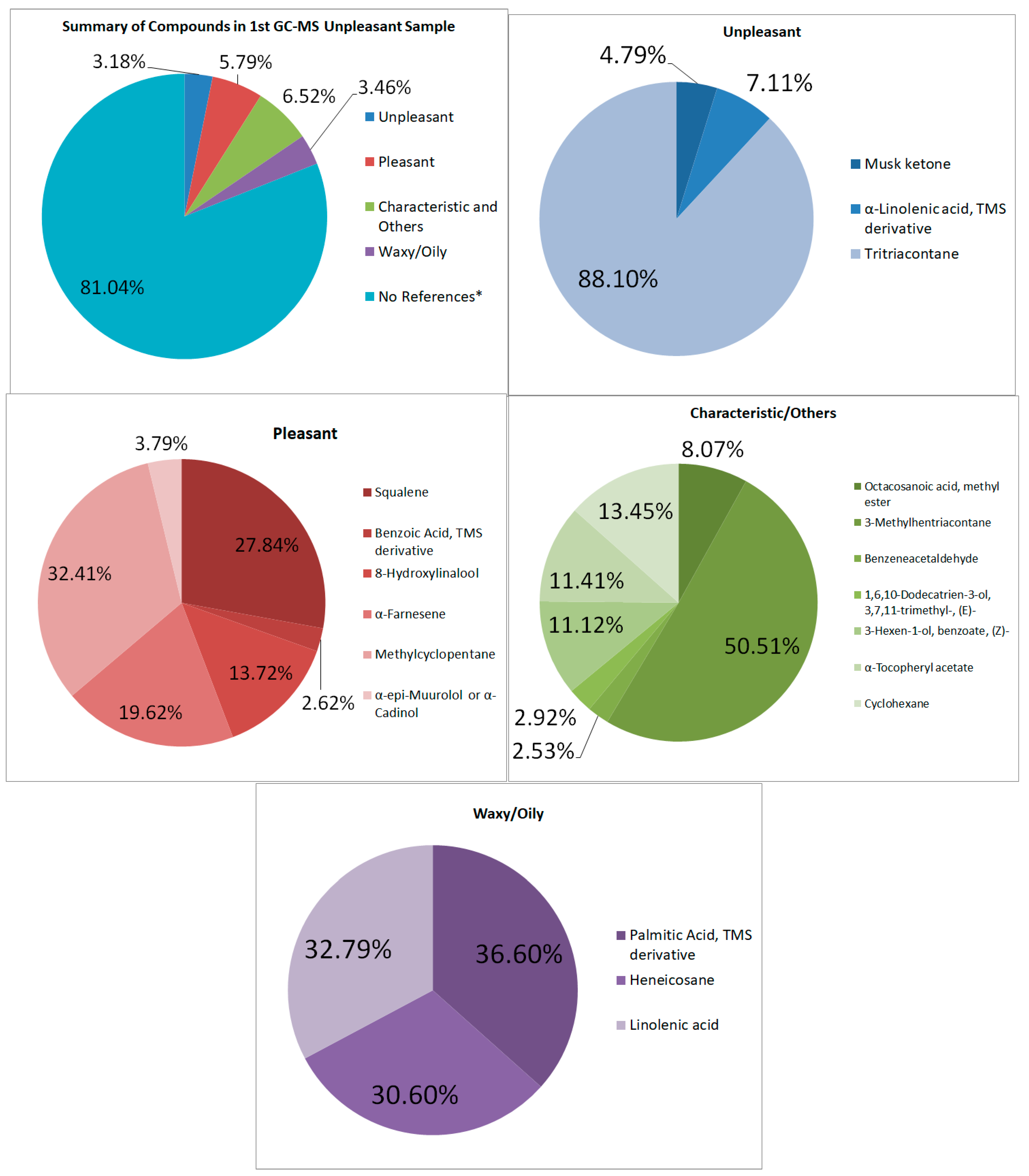
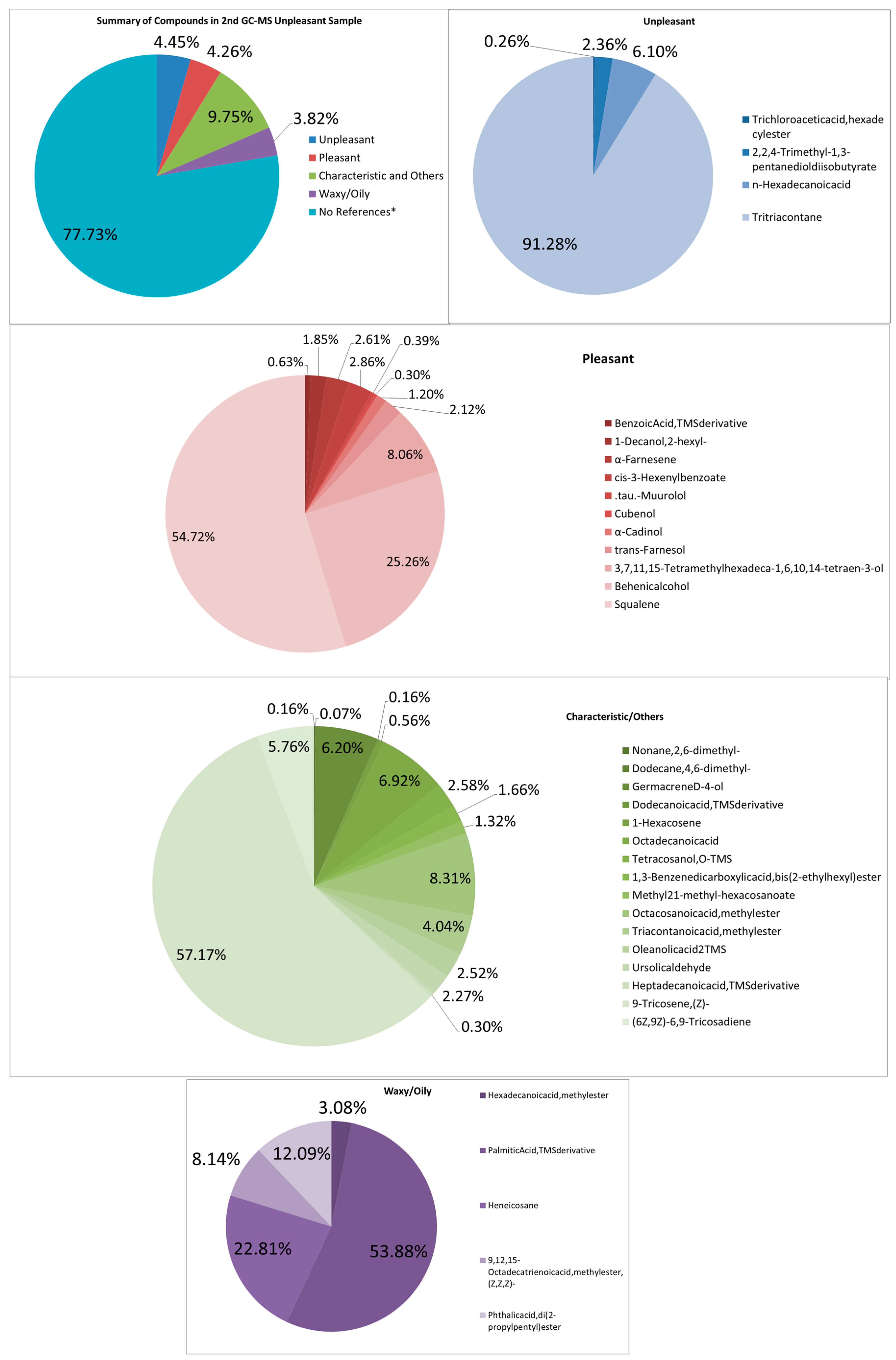
3.4.2. Unpleasant-Smelling Samples
FTIR
GC–MS
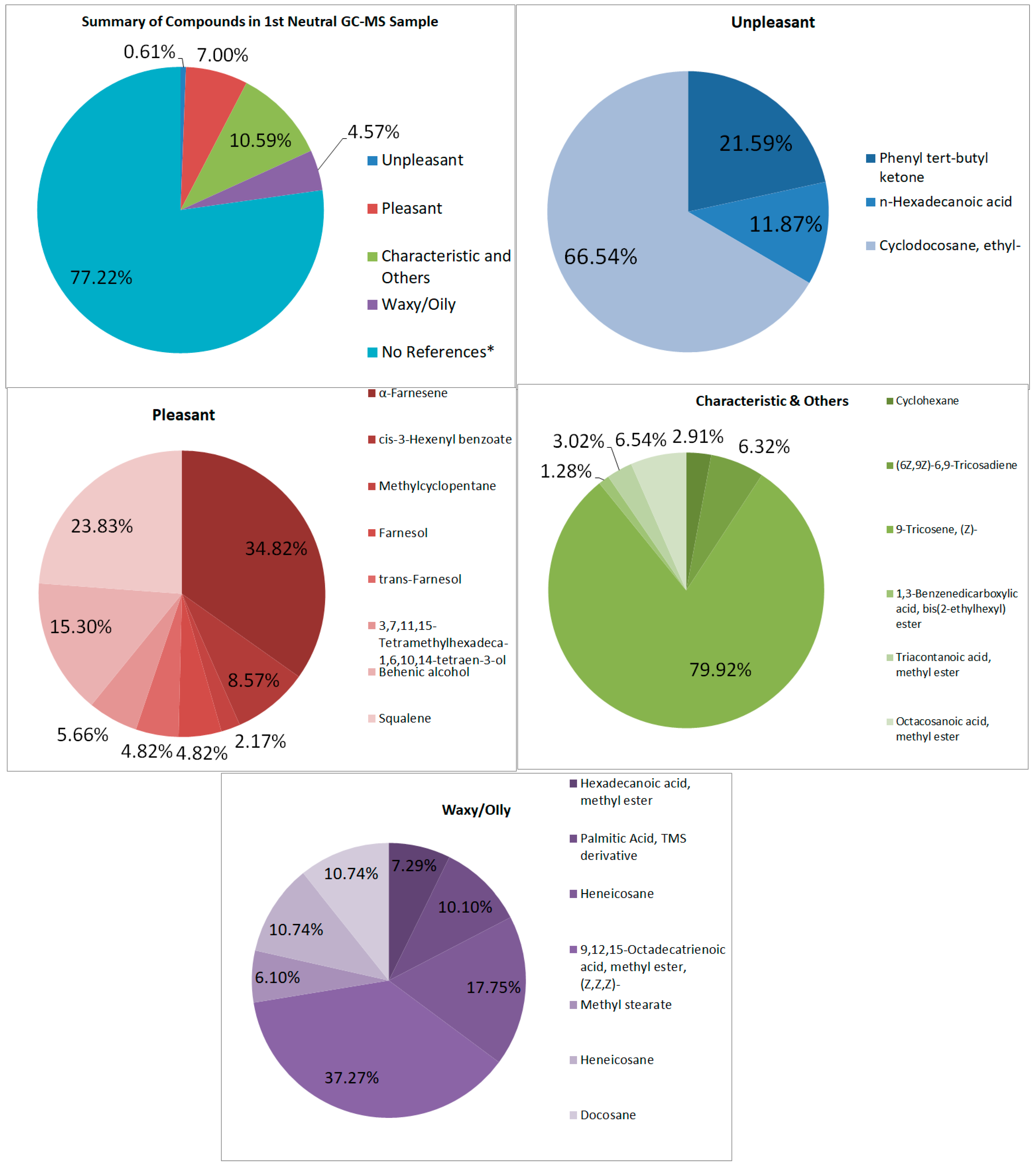
3.4.3. Neutral Samples
FTIR
GC–MS
3.5. Statistical Significance of Yield to Parameters
4. Conclusions and Recommendations
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A. List of Figures

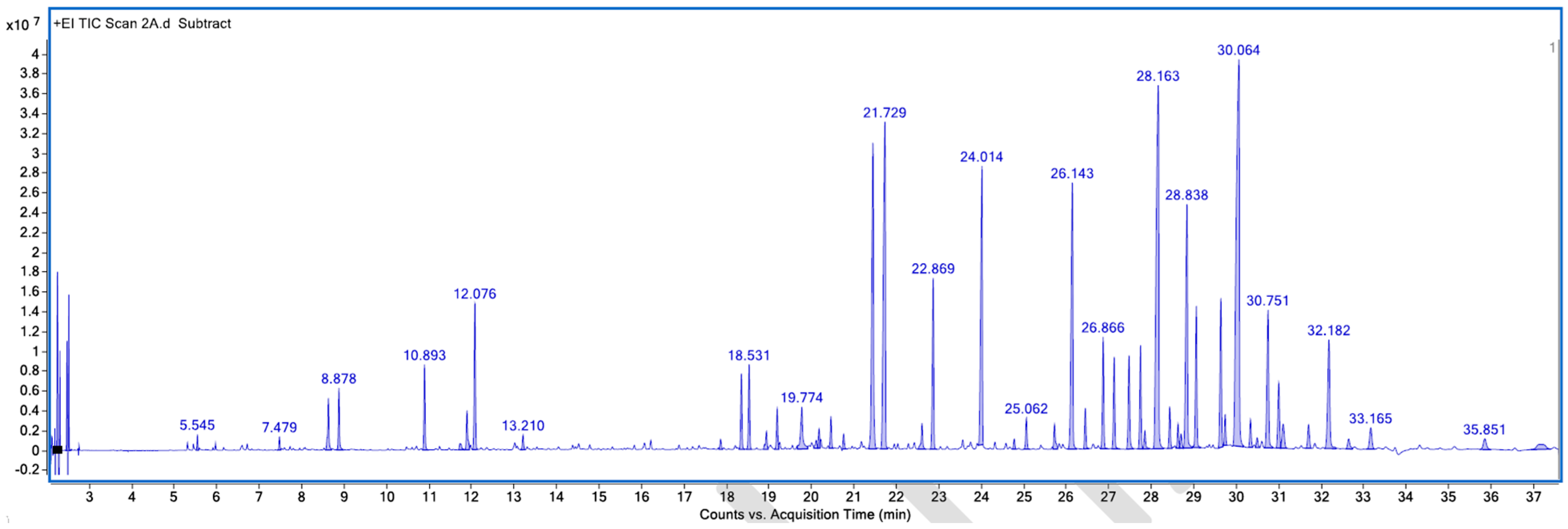
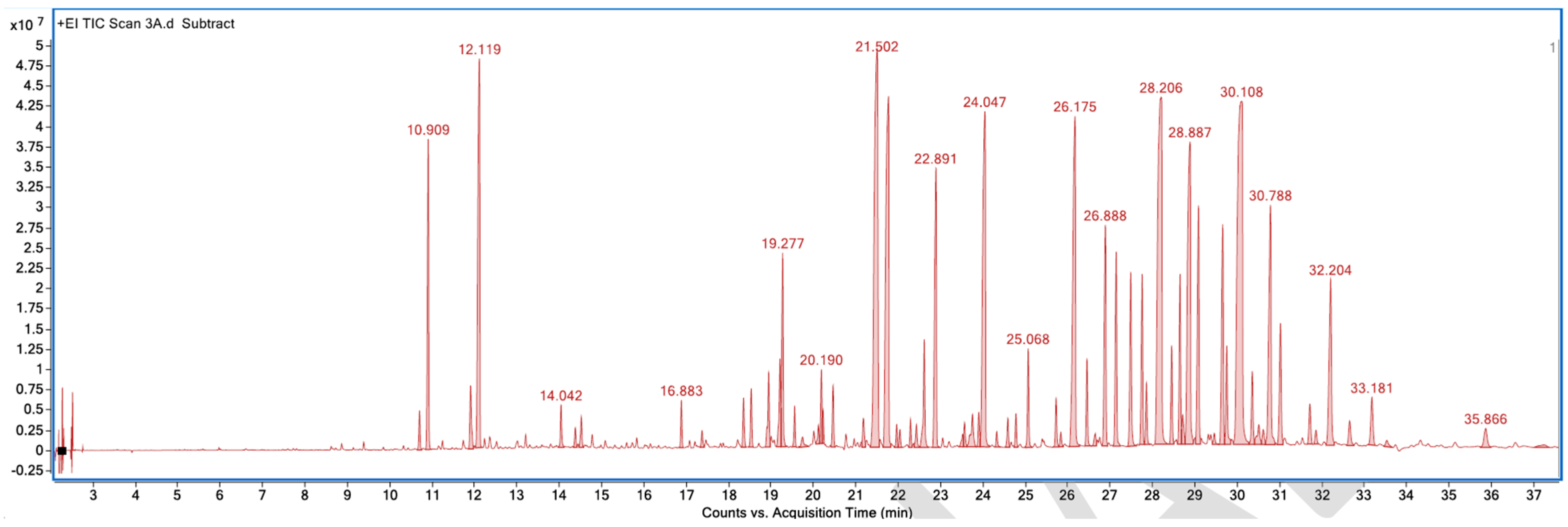
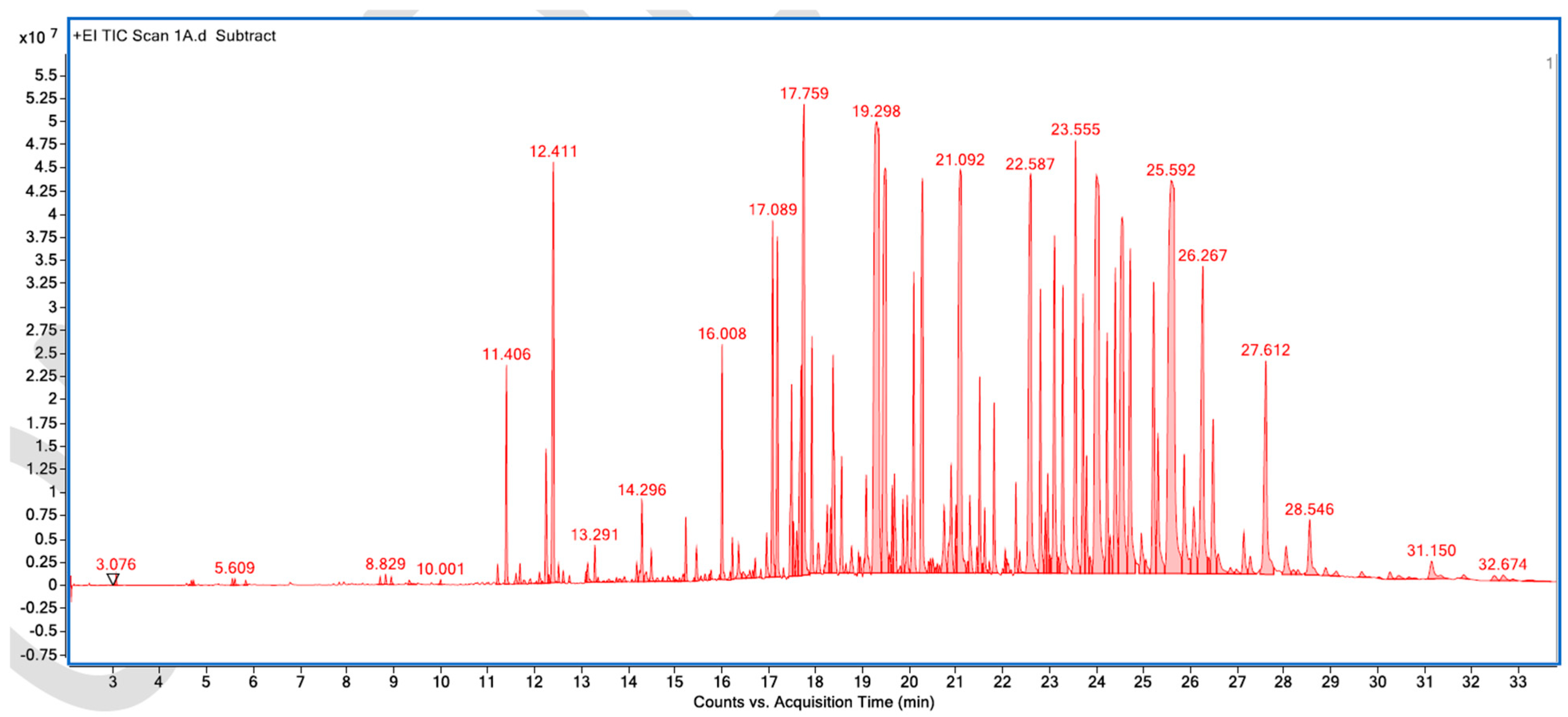
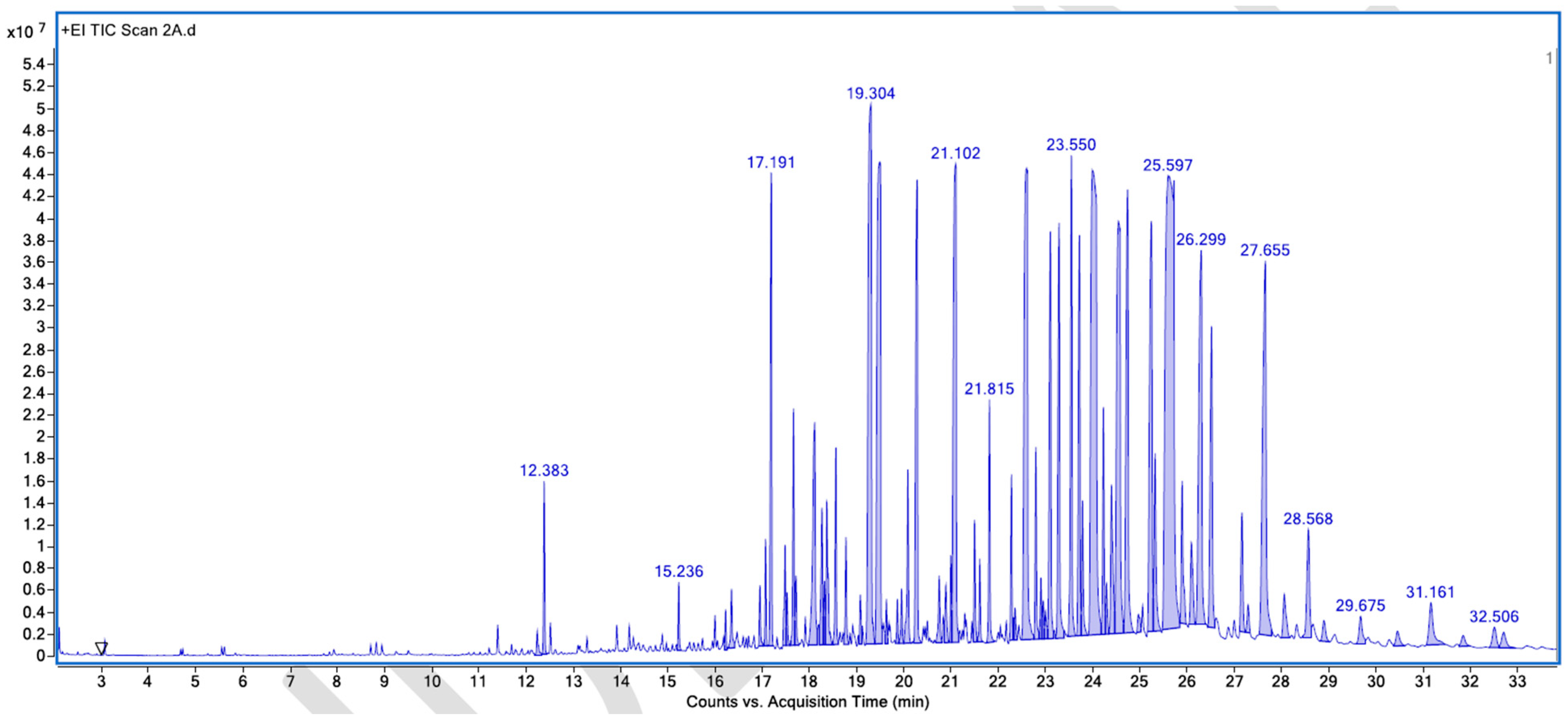
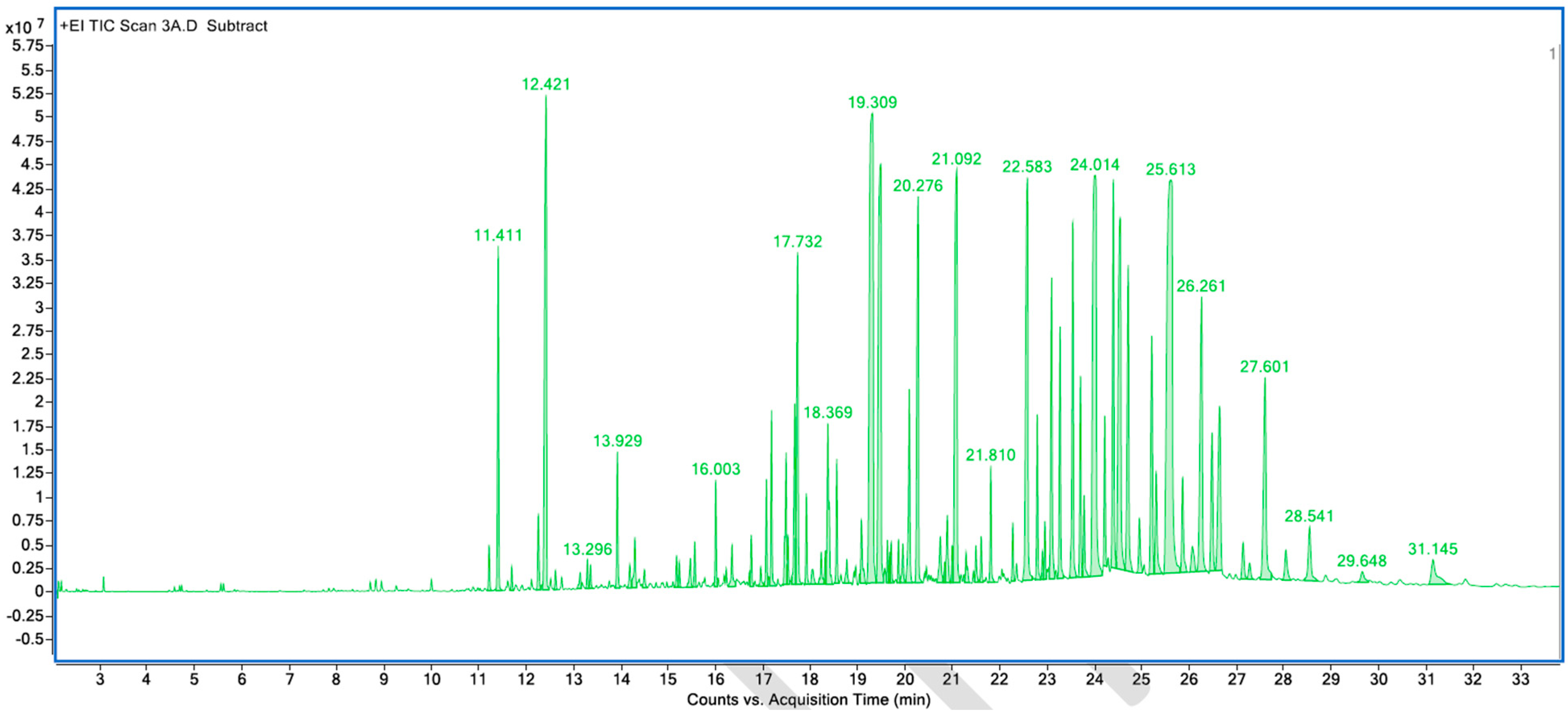
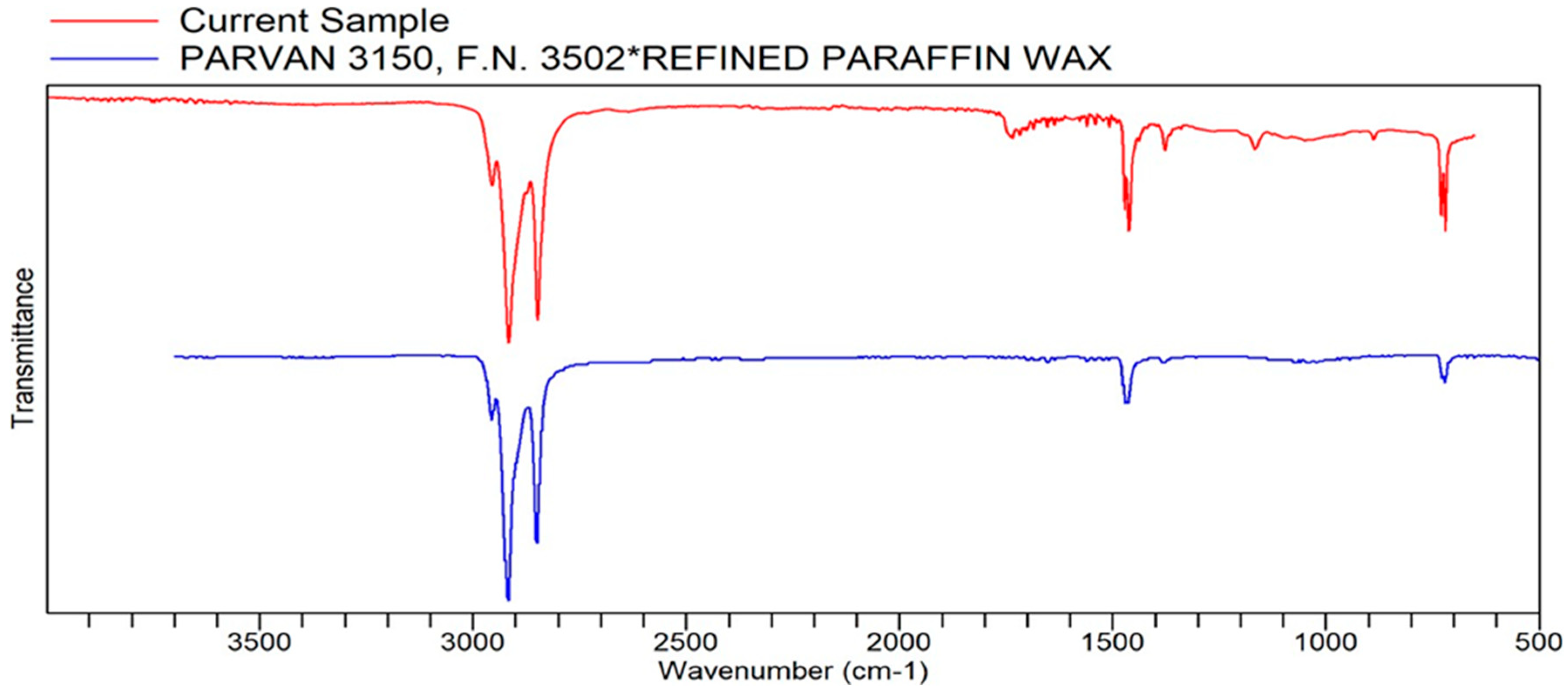
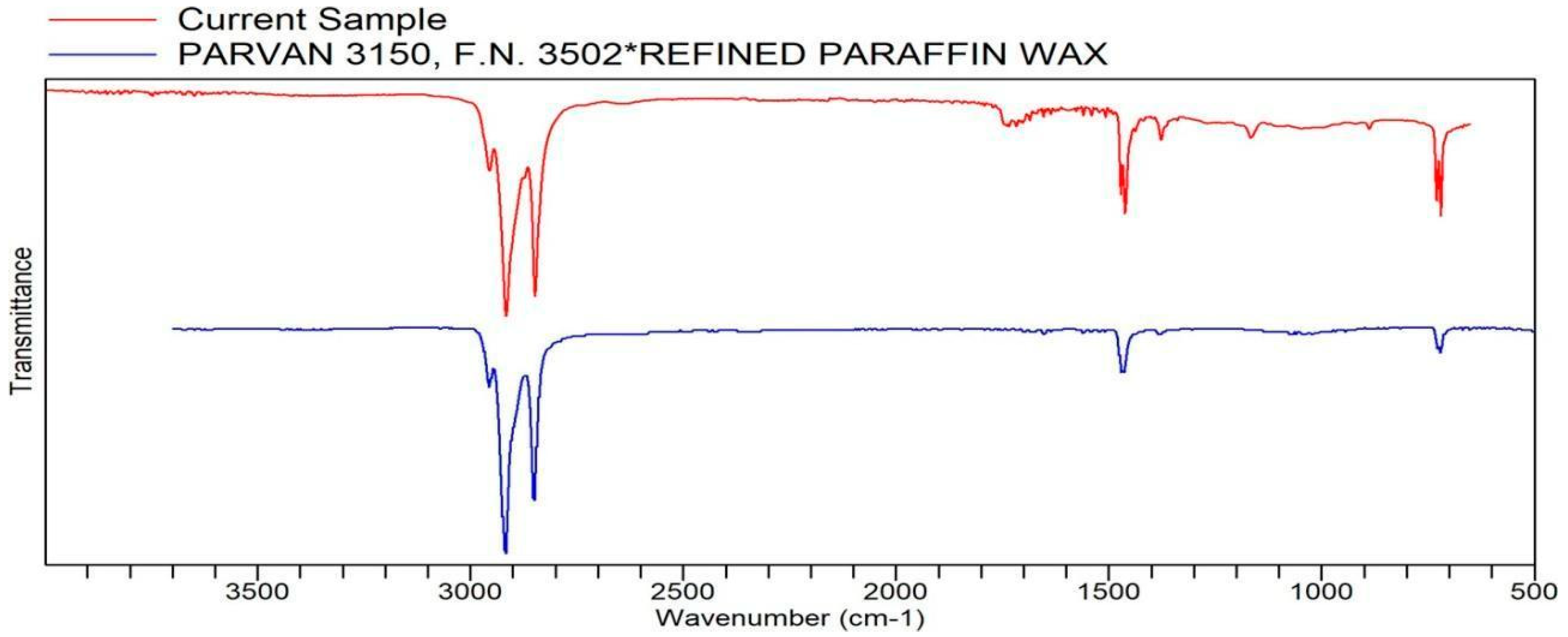
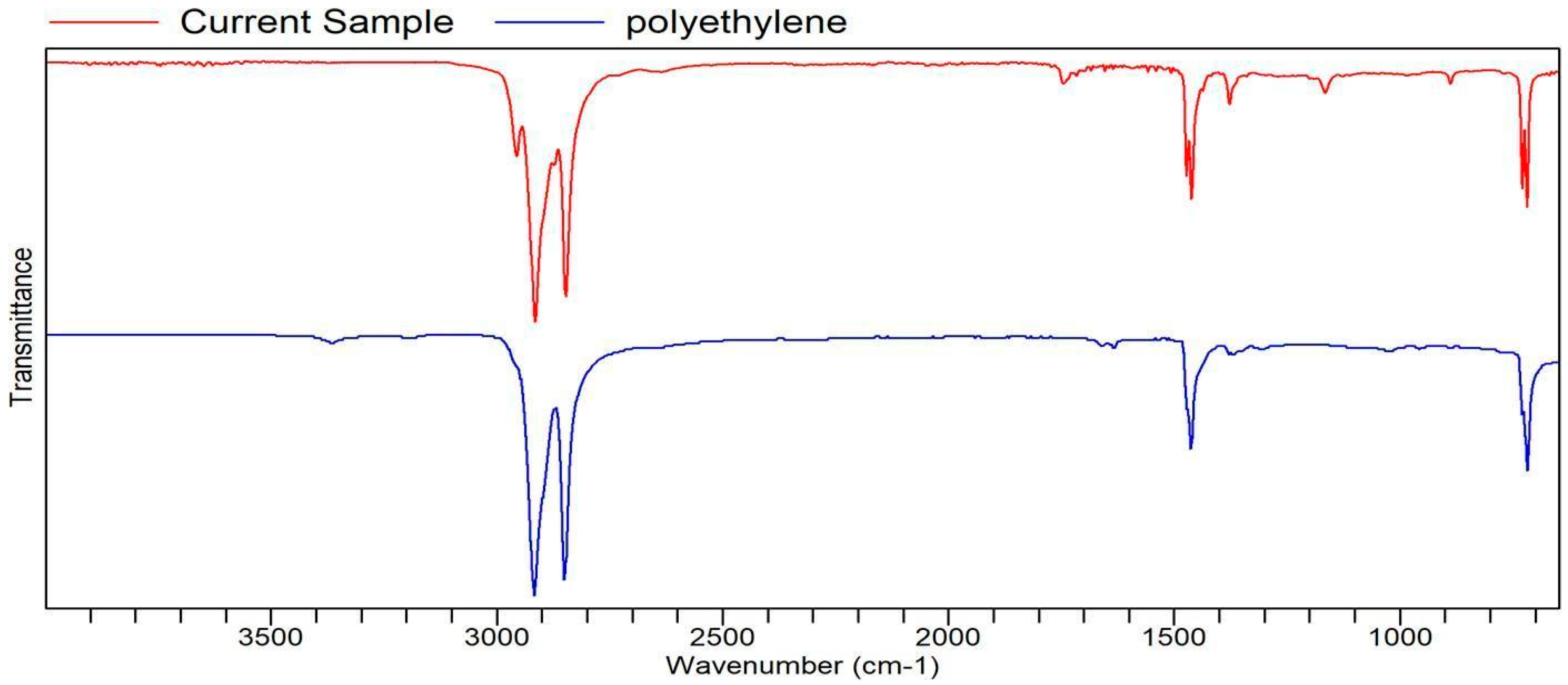
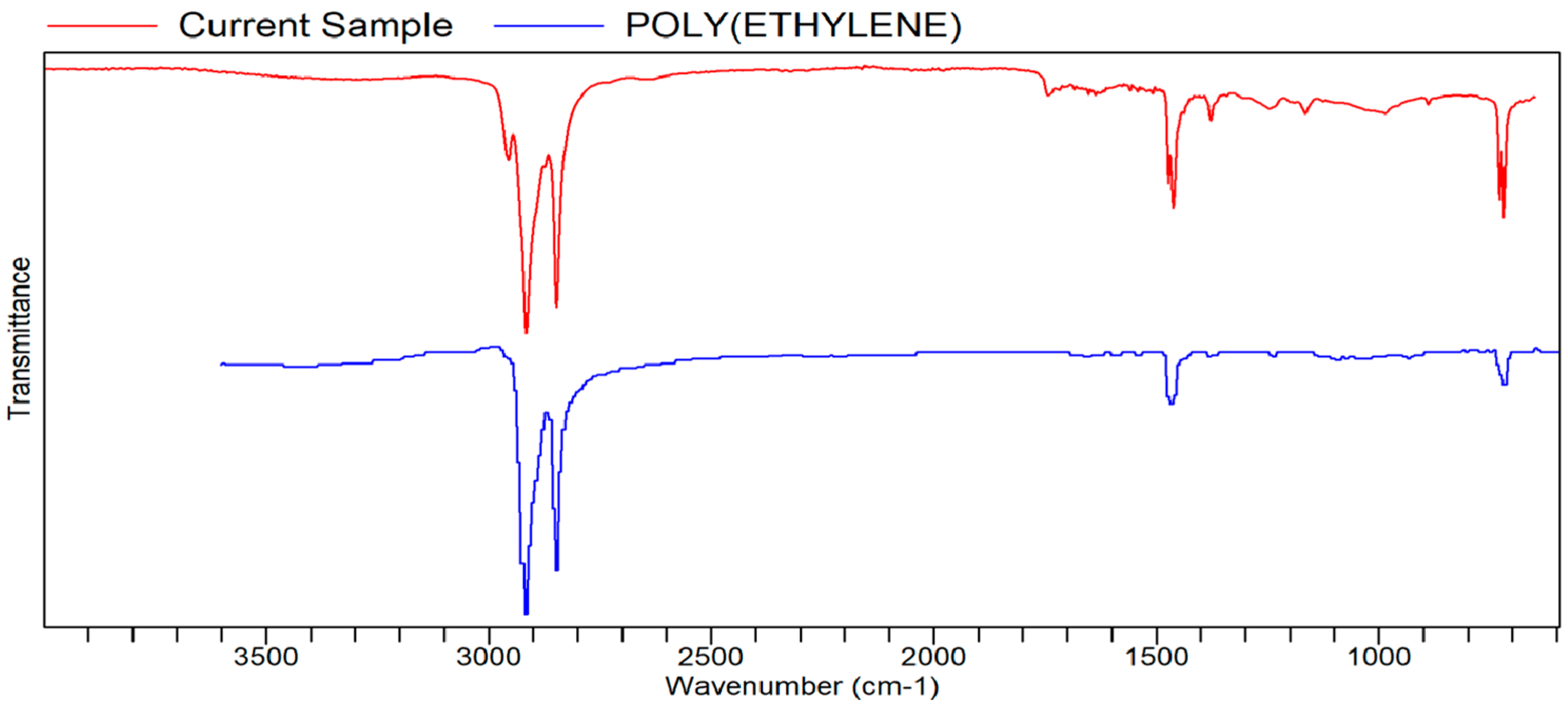
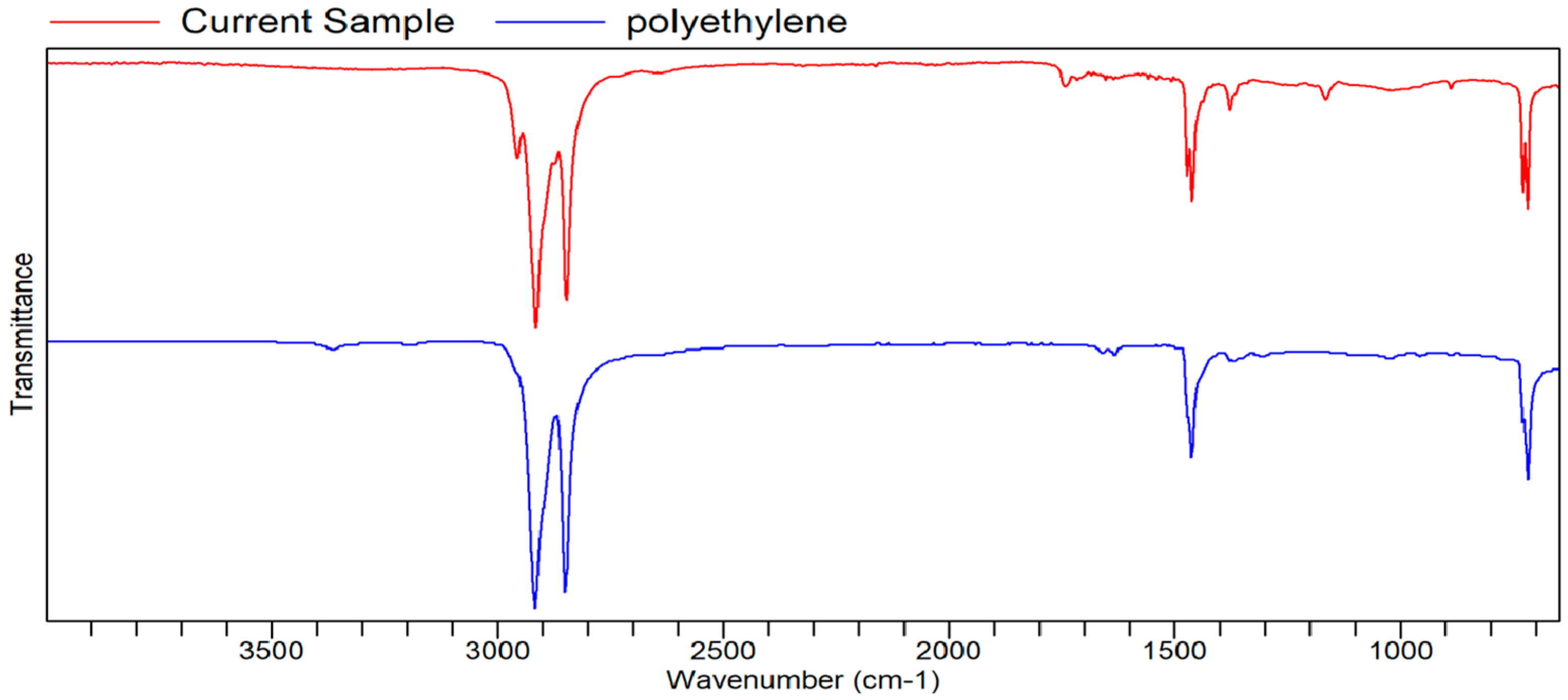
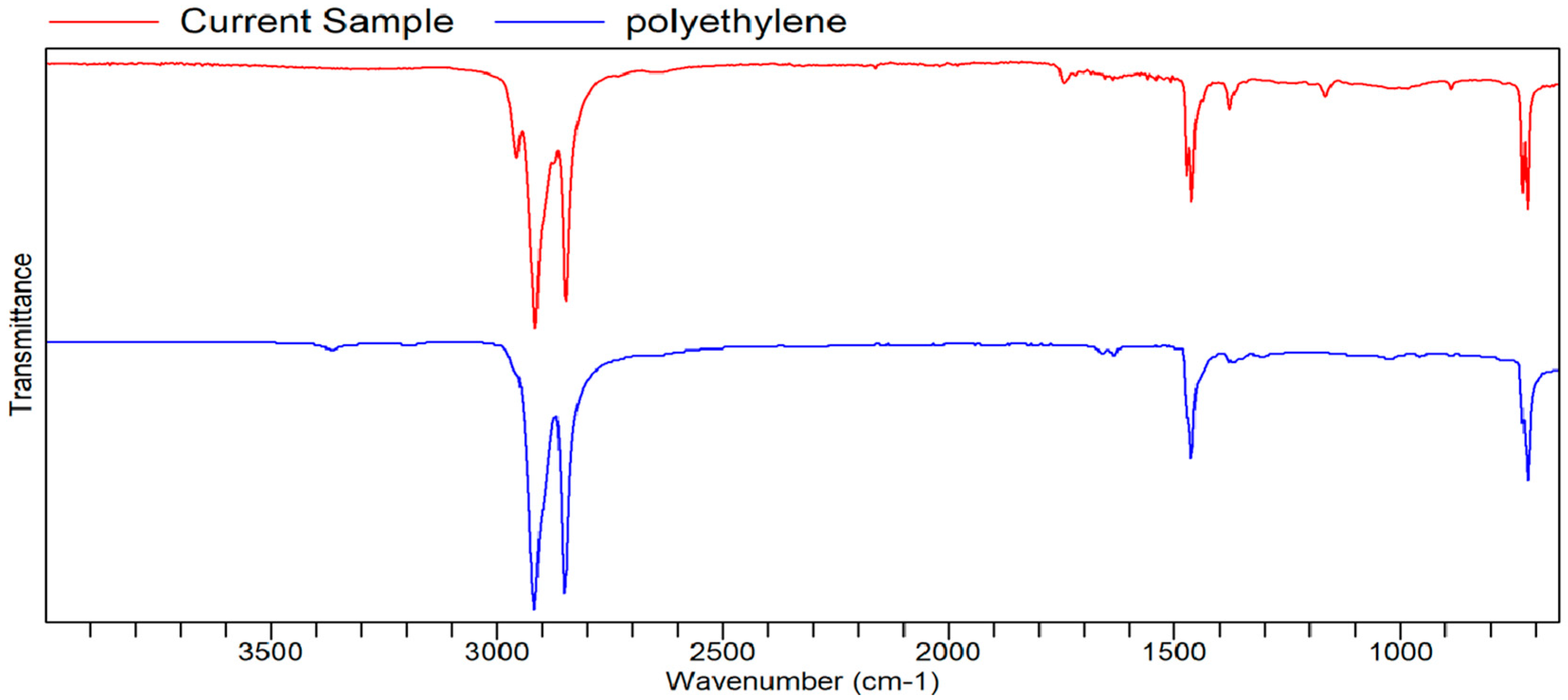
Appendix B. List of Tables
| Notes | Area | Percent (%) | |
|---|---|---|---|
| Unpleasant-Smelling Compounds | |||
| N/A | 0 | 0 | |
| Total | 0 | 0 | |
| Pleasant-Smelling Compounds | |||
| α-Farnesene | sweet, woody, and fruity smell | 5,180,291.48 | 0.2553 |
| α-Cadinol | floral, fresh, and fruity odor of tea flowers | 3,027,782.37 | 0.1492 |
| cis-3-Hexenyl benzoate | green, herbaceous, floral, and woody odor | 4,828,245.4 | 0.2380 |
| 3,7,11,15-Tetramethylhexadeca-1,6,10,14-tetraen-3-ol | floral | 14,087,864.88 | 0.6944 |
| Behenic alcohol | mild, pleasant, aromatic | 10,400,228.42 | 0.5127 |
| 3,209,425.88 | 0.1582 | ||
| Squalene | “faint agreeable odor” | 73,269,459.38 | 3.6116 |
| Total | 114,003,297.8 | 5.6195 | |
| Characteristic and Others | |||
| Germacrene D-4-ol | characteristic odor | 21,033,012.76 | 1.0368 |
| (6Z,9Z)-6,9-Tricosadiene | pheromone component | 5,128,708.79 | 0.2528 |
| 3,224,764.08 | 0.1590 | ||
| 5,199,288.16 | 0.2563 | ||
| Phthalic acid, di(2-propylpentyl) ester | “characteristic odor” | 10,398,735.97 | 0.5126 |
| 1,3-Benzenedicarboxylic acid, bis(2-ethylhexyl) ester | “slight odor” | 3,058,139.39 | 0.1507 |
| Octacosanoic acid, methyl ester | characteristic | 10,648,040.61 | 0.5249 |
| 3-Methylhentriacontane | pheromone component | 61,385,160.33 | 3.0258 |
| Triacontanoic acid, methyl ester | “characteristic odor” | 5,788,126.16 | 0.2853 |
| Total | 125,863,976.3 | 6.2041 | |
| Waxy or Oily Compounds | |||
| Hexadecanoic acid, methyl ester | weak “waxy” odor | 3,011,745.59 | 0.1485 |
| Palmitic Acid, TMS derivative, | slight waxiness | 16,795,154.37 | 0.8279 |
| Heneicosane | waxy | 15,165,101.58 | 0.7475 |
| waxy | 8,895,049.09 | 0.4385 | |
| Docosane | minimal waxiness, nearly odorless | 8,895,049.09 | 0.4385 |
| Tricosane | waxy | 151,194,085 | 7.4527 |
| Linolenic acid, methyl ester | oily, fatty, sometimes woodsy | 7,466,982.14 | 0.3681 |
| Linolenic acid | oily, fatty, sometimes woodsy | 7,466,982.14 | 0.3681 |
| Total | 218,890,149 | 10.7896 |
| Notes | Area | Percent (%) | |
|---|---|---|---|
| Unpleasant-Smelling Compounds | |||
| Decane, 4-methyl | pungent, acrid | 776,589.03 | 0.0170 |
| 1-Nonene, 4,6,8-trimethyl | unpleasant-smelling | 1,427,263.6 | 0.0312 |
| 2,2,4-Trimethyl-1,3-pentanediol diisobutyrate | musty odor | 3,007,628.3 | 0.0658 |
| Total | 5,211,480.93 | 0.1140 | |
| Pleasant-Smelling Compounds | |||
| Linalool | floral, citric, fresh, and sweet | 1,001,785.3 | 0.0219 |
| 1-Dodecanol, 2-hexyl | with a mild sweet odor | 1,914,146.1 | 0.0419 |
| Methyl anthranilate | grape-like smell | 1,730,390.1 | 0.0379 |
| α-Farnesene | sweet, woody, and fruity smell. | 38,575,207 | 0.8438 |
| Naphthalene, 1,2,3,4,4a,5,6,8aoctahydro-7-methyl-4-methylene-1-(1-methylethyl)-,(1α,4aβ,8aα)- | piquant | 2,371,798.2 | 0.0519 |
| Viridiflorol | sweet, green, herbal, fruity, tropical, minty odor. | 2,024,211 | 0.0443 |
| .tau.-Muurolol | herbal type odor; slight spicy | 4,878,448 | 0.1067 |
| α-Cadinol | floral, fresh, and fruity odor of tea flowers | 6,393,944.6 | 0.1399 |
| 7-Hydroxyfarnesen | citrus, flowery | 741,259.32 | 0.0162 |
| trans-Farnesol | floral scent | 1,549,129.3 | 0.0339 |
| Benzoic acid, 2-propenyl ester | fruity | 18,850,949 | 0.4123 |
| Benzyl Benzoate | weak, sweet-balsamic odor | 6,799,447.1 | 0.1487 |
| 3,7,11,15-Tetramethylhexadeca-1,6,10,14-tetraen-3-ol | floral | 75,092,203 | 1.6426 |
| (E)-Cinnamyl benzoate | sweet smell | 9,855,007.8 | 0.2156 |
| (Z)-Cinnamyl benzoate | sweet smell | 9,855,007.8 | 0.2156 |
| Behenic alcohol | mild, pleasant, aromatic | 64,254,257 | 1.4055 |
| Total | 245,887,190.6 | 5.3785 | |
| Characteristic and Others | |||
| Nonane, 2,6-dimethyl- | camphoreous | 931,876.01 | 0.0204 |
| Octane, 3,3-dimethyl- | VOC | 931,876.01 | 0.0204 |
| Cyclooctane, 1,4-dimethyl-, trans | VOC | 1,004,860 | 0.0220 |
| 1,222,376.3 | 0.0267 | ||
| Octadecyl octyl ether | octadecyls are odor maskers | 1,205,706.7 | 0.0264 |
| 3-Hexen-1-ol, benzoate, (Z)- | intense grassy-green odor | 28,400,595 | 0.6212 |
| Germacrene D-4-ol | characteristic odor | 110,319,891 | 2.4131 |
| Epiglobulol | characteristic | 2,024,211 | 0.0443 |
| Dodecanoic acid, TMS derivative | faint odor of bay oil | 1,479,934.6 | 0.0324 |
| Octadecanoic acid | “mild odor” | 18,301,603 | 0.4003 |
| (6Z,9Z)-6,9-Tricosadiene | pheromone component | 27,983,459 | 0.6121 |
| 14,381,094 | 0.3146 | ||
| 16,577,987 | 0.3626 | ||
| 9-Tricosene, (Z)- | pheromone component | 367,301,953 | 8.0343 |
| Total | 592,067,422.6 | 12.9508 | |
| Waxy Compounds | |||
| Myristic acid, TMS derivative | odorless, but some blends are oily | 10,978,338 | 0.2401 |
| Hexadecanoic acid, methyl ester | weak “waxy” odor | 42,472,332 | 0.9290 |
| Palmitic Acid, TMS derivative | slight waxiness | 70,031,808 | 1.5319 |
| Heneicosane | waxy | 65,893,071 | 1.4413 |
| 24,646,580 | 0.5391 | ||
| 9,12,15-Octadecatrienoic acid, methyl ester, (Z,Z,Z)- | oily | 162,366,628 | 3.5516 |
| Methyl stearate | waxy | 44,168,233 | 0.9661 |
| Tricosane | waxy | 210,780,830 | 4.6106 |
| Total | 631,337,820 | 13.8098 |
| Notes | Area | Percent (%) | |
|---|---|---|---|
| Unpleasant-Smelling Compounds | |||
| Musk ketone | highly tenacious musky aroma with a discreet animal note | 2,144,687.96 | 0.1525 |
| α-Linolenic acid, TMS derivative | some references say it is fishy/oily; some say it is odorless | 3,179,637.28 | 0.2260 |
| Tritriacontane | range in odor from odorless to a fuel-like odor | 39,412,448.51 | 2.8017 |
| Total | 44,736,773.75 | 3.1802 | |
| Pleasant-Smelling Compounds | |||
| Squalene | “faint agreeable odor” | 22,692,839.01 | 1.6132 |
| Benzoic Acid, TMS derivative | faint, pleasant odor | 2,137,985.27 | 0.1520 |
| 8-Hydroxylinalool | citrus-like, sweet, soapy, and lemon-like | 11,186,775.44 | 0.7952 |
| α-Farnesene | sweet, woody, and fruity smell | 15,990,851.96 | 1.1368 |
| Methylcyclopentane | sweet | 22,305,310.15 | 1.5856 |
| 4,107,283.42 | 0.2920 | ||
| α-epi-Muurolol | herbal | 3,086,232.01 | 0.2194 |
| α-Cadinol | floral, fresh, and fruity odor of tea flowers | ||
| Total | 81,507,277.26 | 5.7942 | |
| Characteristics and Others | |||
| Octacosanoic acid, methyl ester | characteristic | 6,529,256.25 | 0.4641 |
| 3-Methylhentriacontane | pheromone component | 40,871,050.72 | 2.9054 |
| Benzeneacetaldehyde | grassy | 2,046,250.26 | 0.1455 |
| 1,6,10-Dodecatrien-3-ol, 3,7,11-trimethyl-, (E)- | fresh bark odor | 2,365,617.83 | 0.1682 |
| 3-Hexen-1-ol, benzoate, (Z)- | intense grassy-green odor | 9,000,686.39 | 0.6398 |
| α-Tocopheryl acetate | characteristic | 9,231,315.14 | 0.6562 |
| Cyclohexane | sweet, pungent odor | 10,880,395.17 | 0.7735 |
| 10,797,874.96 | 0.7676 | ||
| Total | 91,722,446.72 | 6.5203 | |
| Waxy Compounds | |||
| Palmitic Acid, TMS derivative | slight waxiness | 17,838,641.56 | 1.2681 |
| Heneicosane | waxy | 8,440,458.89 | 0.6000 |
| 6,474,638.67 | 0.4603 | ||
| Linolenic acid | 15,983,006.75 | 1.1362 | |
| Total | 48,736,745.87 | 3.4646 |
| Notes | Area | Percent (%) | |
|---|---|---|---|
| Unpleasant-Smelling Compounds | |||
| Trichloroaceticacid, hexadecylester | pungent, sharp | 511,976.24 | 0.0116 |
| 2,2,4-Trimethyl-1,3-pentanedioldiisobutyrate | musty odor | 4,627,215.6 | 0.1050 |
| n-Hexadecanoicacid | ‘rancid’ and ‘pungent’ odors | 11,956,959 | 0.2713 |
| Tritriacontane | range in odor from odorless to a fuel-like odor | 178,875,511 | 4.0587 |
| Total | 195,971,661.8 | 4.4466 | |
| Pleasant-Smelling Compounds | |||
| BenzoicAcid,TMSderivative | faint, pleasant odor | 1,186,255.2 | 0.0269 |
| 1-Decanol,2-hexyl- | mild, sweet odor | 1,256,663.2 | 0.0285 |
| α-Farnesene | sweet, woody, and fruity smell | 4,894,050.6 | 0.1110 |
| 1-Decanol,2-hexyl- | mild, sweet odor | 951,428.3 | 0.0216 |
| cis-3-Hexenylbenzoate | green, herbaceous, floral, and woody odor | 5,362,932.1 | 0.1217 |
| .tau.-Muurolol | herbal type odor; slight spicy | 734,662.17 | 0.0167 |
| Cubenol | spicy and herbal | 567,901.47 | 0.0129 |
| α-Cadinol | floral, fresh, and fruity odor of tea flowers | 2,255,064 | 0.0512 |
| trans-Farnesol | floral scent | 3,979,945.1 | 0.0903 |
| 3,7,11,15-Tetramethylhexadeca-1,6,10,14-tetraen-3-ol | floral | 15,117,986 | 0.3430 |
| 1-Decanol,2-hexyl- | mild, sweet odor | 1,256,663.2 | 0.0285 |
| Behenicalcohol | mild, pleasant, aromatic | 34,546,007 | 0.7838 |
| 12,859,135 | 0.2918 | ||
| Squalene | “faint agreeable odor” | 102,663,839 | 2.3294 |
| Total | 187,632,532.3 | 4.2574 | |
| Characteristic and Others | |||
| Nonane,2,6-dimethyl- | camphoreous | 676,510.19 | 0.0153 |
| Dodecane,4,6-dimethyl- | strong sweet-corn-like aroma | 291,572.1 | 0.0066 |
| GermacreneD-4-ol | characteristic odor | 26,646,212 | 0.6046 |
| Dodecanoicacid,TMSderivative | faint odor of bay oil | 684,929.83 | 0.0155 |
| 1-Hexacosene | mild | 2,414,809.6 | 0.0548 |
| Octadecanoicacid | “mild odor” | 29,731,365 | 0.6746 |
| Tetracosanol,O-TMS | characteristic | 11,102,856 | 0.2519 |
| 1,3-Benzenedicarboxylicacid,bis(2-ethylhexyl)ester | “slight odor” | 7,125,228.9 | 0.1617 |
| Methyl21-methyl-hexacosanoate | fruity-type odor | 5,661,947.4 | 0.1285 |
| Octacosanoicacid,methylester | characteristic | 35,703,227 | 0.8101 |
| Triacontanoicacid,methylester | “characteristic odor” | 17,341,539 | 0.3935 |
| Oleanolicacid2TMS | characteristic | 10,807,484 | 0.2452 |
| Ursolicaldehyde | characteristic | 9,729,875 | 0.2208 |
| Heptadecanoicacid,TMSderivative | pheromone component | 1,297,625.3 | 0.0294 |
| 9-Tricosene,(Z)- | pheromone component | 4,600,337.6 | 0.1044 |
| 240,988,707 | 5.4680 | ||
| (6Z,9Z)-6,9-Tricosadiene | pheromone component | 8,844,206.6 | 0.2007 |
| 6,574,194.6 | 0.1492 | ||
| 9,318,822.5 | 0.2114 | ||
| Total | 429,541,449.6 | 9.7462 | |
| Waxy Odors | |||
| Hexadecanoicacid,methylester | weak “waxy” odor | 5,185,499.6 | 0.1177 |
| PalmiticAcid,TMSderivative | slight waxiness | 1,617,083.9 | 0.0367 |
| 89,000,228 | 2.0194 | ||
| Heneicosane | waxy | 38,354,308 | 0.8703 |
| 9,12,15-Octadecatrienoicacid,methylester,(Z,Z,Z)- | oily | 13,691,718 | 0.3107 |
| Phthalicacid,di(2-propylpentyl)ester | “characteristic odor” | 20,331,662 | 0.4613 |
| Total | 168,180,499.5 | 3.8160 |
| Notes | Area | Percent (%) | |
|---|---|---|---|
| Unpleasant-Smelling Compounds | |||
| Phenyl tert-butyl ketone | unpleasant | 9,004,534.49 | 0.2623 |
| n-Hexadecanoic acid | ‘rancid’ and ‘pungent’ odors | 4,951,027.32 | 0.1442 |
| Cyclodocosane, ethyl- | cyclodocosane is musty | 6,897,525.67 | 0.2010 |
| Total | 20,853,087.48 | 0.6075 | |
| Pleasant-Smelling Compounds | |||
| α-Farnesene | sweet, woody, and fruity smell | 83,701,909.86 | 2.4385 |
| cis-3-Hexenyl benzoate | green, herbaceous, floral, and woody odor | 20,593,293.36 | 0.6000 |
| Methylcyclopentane | sweet | 5,205,381.56 | 0.1517 |
| Farnesol | mild, light-woody, linden, floral | 11,598,405.13 | 0.3379 |
| trans-Farnesol | floral scent | 11,598,405.13 | 0.3379 |
| 3,7,11,15-Tetramethylhexadeca-1,6,10,14-tetraen-3-ol | floral | 13,608,158.22 | 0.3965 |
| Behenic alcohol | mild, pleasant, aromatic | 19,208,391.39 | 0.5596 |
| 7,212,273.25 | 0.2101 | ||
| 10,366,741.04 | 0.3020 | ||
| Squalene | “faint agreeable odor” | 57,289,446.95 | 1.6691 |
| Total | 240,382,405.9 | 7.0032 | |
| Characteristic and Others | |||
| Cyclohexane | sweet, pungent odor | 5,205,381.56 | 0.1517 |
| 5,389,937.01 | 0.1570 | ||
| (6Z,9Z)-6,9-Tricosadiene | pheromone component | 9,921,740.66 | 0.2891 |
| 9-Tricosene, (Z)- | pheromone component | 290,560,215.4 | 8.4651 |
| (6Z,9Z)-6,9-Tricosadiene | pheromone component | 5,689,718.8 | 0.1658 |
| (6Z,9Z)-6,9-Tricosadiene | pheromone component | 7,379,949.17 | 0.2150 |
| 1,3-Benzenedicarboxylic acid, bis(2-ethylhexyl) ester | “slight odor” | 4,646,143.26 | 0.1354 |
| Triacontanoic acid, methyl ester | “characteristic odor | 10,986,908.05 | 0.3201 |
| Octacosanoic acid, methyl ester | characteristic | 23,773,091.44 | 0.6926 |
| Total | 363,553,085.4 | 10.5916 | |
| Waxy Odors | |||
| Hexadecanoic acid, methyl ester | weak “waxy” odor | 11,445,635.5 | 0.3335 |
| Palmitic Acid, TMS derivative | slight waxiness | 15,861,788.16 | 0.4621 |
| Heneicosane | waxy | 27,873,257.53 | 0.8121 |
| 9,12,15-Octadecatrienoic acid, methyl ester, (Z,Z,Z)- | oily | 58,522,173 | 1.7050 |
| Methyl stearate | waxy | 9,570,314.44 | 0.2788 |
| Heneicosane | waxy | 16,865,047.04 | 0.4913 |
| Docosane | minimal waxiness, nearly odorless | 16,865,047.04 | 0.4913 |
| Total | 157,003,262.7 | 4.5741 |
| Notes | Area | Percent (%) | |
|---|---|---|---|
| Unpleasant-Smelling Compounds | |||
| Decane | gasoline-like odor | 604,738.63 | 0.0173 |
| n-Hexadecanoicacid | ‘rancid’ and ‘pungent’ odors | 10,695,786 | 0.3067 |
| Muskketone | highly tenacious musky aroma with a discreet animal note | 9,514,186.8 | 0.2728 |
| Phenyltert-butylketone | unpleasant | 12,772,890 | 0.3663 |
| Total | 33,587,601 | 0.9631 | |
| Pleasant-Smelling Compounds | |||
| α-Farnesene | sweet, woody, and fruity smell | 65,957,731 | 1.8913 |
| α-Cadinol | floral, fresh, and fruity odor of tea flowers | 4,801,024.4 | 0.1377 |
| 7-Hydroxyfarnesen | citrus, flowery | 4,021,592.9 | 0.1153 |
| Tonalid | sweet, amber, ambrette, fruity, musk | 8,403,166.6 | 0.2410 |
| 3,7,11,15-Tetramethylhexadeca-1,6,10,14-tetraen-3-ol | floral | 18,615,401 | 0.5338 |
| Behenicalcohol | mild, pleasant, aromatic | 46,458,265 | 1.3322 |
| Behenicalcohol | mild, pleasant, aromatic | 6,314,700.2 | 0.1811 |
| Squalene | “faint agreeable odor” | 80,224,892 | 2.3005 |
| Total | 234,796,773 | 6.7328 | |
| Characteristic and Others | |||
| Nonane,2,6-dimethyl- | camphoreous | 741,324.04 | 0.0213 |
| Humulene | earthy, woody, with spicy, herbal notes | 974,635.97 | 0.0279 |
| 3-Hexen-1-ol,benzoate,(Z)- | intense grassy-green odor | 15,485,274 | 0.4440 |
| (2E,4S,7E)-4-Isopropyl-1,7-dimethylcyclodeca-2,7-dienol | VOC | 154,419,161 | 4.4280 |
| BenzylBenzoate | weak, sweet-balsamic odor | 4,237,489 | 0.1215 |
| Octadecanoicacid | “mild odor” | 7,989,615.3 | 0.2291 |
| (6Z,9Z)-6,9-Tricosadiene | pheromone component | 16,355,475 | 0.4690 |
| 9-Tricosene,(Z)- | pheromone component | 282,558,255 | 8.1024 |
| Docosanoicacid,methylester | characteristic | 7,721,575.1 | 0.2214 |
| Tetracosanol,O-TMS | characteristic | 5,073,007 | 0.1455 |
| Triacontanoicacid,methylester | “characteristic odor” | 12,060,521 | 0.3458 |
| α-Tocopherylacetate | characteristic | 65,652,636 | 1.8826 |
| Octacosanoicacid,methylester | characteristic | 26,609,462 | 0.7630 |
| Total | 599,878,430 | 17.2015 | |
| Waxy Odors | |||
| PalmiticAcid,TMSderivative | slight waxiness | 30,867,835 | 0.8851 |
| Heneicosane | waxy | 40,643,834 | 1.1655 |
| Methylstearate | waxy | 16,035,763 | 0.4598 |
| Docosane | minimal waxiness, nearly odorless | 21,544,047 | 0.6178 |
| Total | 109,091,479 | 3.1282 |
| Quality | Library | Name |
|---|---|---|
| 0.92425 | Poly_D (68) | POLY(ETHYLENE) |
| 0.92256 | Agilent Polymer Handheld ATR Library (110) | Polyethylene, high density Approx Mw 125,000 Pellets CAS#25213-02-_2012-06-19T16-44-41 (Alfa Chemistry, New York, NY, USA) |
| 0.92256 | Agilent Polymer Handheld ATR Library (111) | Polyethylene, high density Approx Mw 125,000 Pellets CAS25213-02-9 (Alfa Chemistry, New York, NY, USA) |
| 0.92122 | Agilent Polymer Handheld ATR Library (106) | Polyethylene, chlorinated, Chlorine content 25 wt%, cast film CAS 64754-90-1 (Alfa Chemistry, New York, NY, USA) |
| 0.92041 | Agilent Polymer Handheld ATR Library (182) | Paraffin Wax and Polyvinyl Acetate Mixture (7% PVA Wax Coating) |
| Quality | Library | Name |
|---|---|---|
| 0.92251 | Intro_D (148) | PARVAN 3150, F.N. 3502*REFINED PARAFFIN WAX |
| 0.92145 | BioRad_Demo (47) | PARVAN 3150, F.N. 3502*REFINED PARAFFIN WAX |
| 0.92063 | Agilent Polymer Handheld ATR Library (106) | Polyethylene, chlorinated, Chlorine content 25 wt%, cast film CAS 64754-90-1 |
| 0.91934 | Poly_D (68) | POLY(ETHYLENE) |
| 0.91314 | Agilent Polymer Handheld ATR Library (182) | Paraffin Wax and Polyvinyl Acetate Mixture (7% PVA Wax Coating) |
| 0.90772 | Agilent Polymer Handheld ATR Library (110) | Polyethylene, high density Approx Mw 125,000 Pellets CAS#25213-02-_2012-06-19T16-44-41 |
| Quality | Library | Name |
|---|---|---|
| 0.91873 | Intro_D (148) | PARVAN 3150, F.N. 3502*REFINED PARAFFIN WAX |
| 0.91794 | BioRad_Demo (47) | PARVAN 3150, F.N. 3502*REFINED PARAFFIN WAX |
| 0.91770 | Agilent Polymer Handheld ATR Library (106) | Polyethylene, chlorinated, Chlorine content 25 wt%, cast film CAS 64754-90-1 |
| 0.91460 | Poly_D (68) | POLY(ETHYLENE) |
| 0.91025 | Agilent Polymer Handheld ATR Library (182) | Paraffin Wax and Polyvinyl Acetate Mixture (7% PVA Wax Coating) |
| 0.90567 | Agilent Polymer Handheld ATR Library (110) | Polyethylene, high density Approx Mw 125,000 Pellets CAS#25213-02-_2012-06-19T16-44-41 |
| Quality | Library | Name |
|---|---|---|
| 0.94096 | ATR Demo Library (41) | polyethylene |
| 0.93648 | Agilent Polymer Handheld ATR Library (23) | ETHYLENE PROPYLENE DIENE TERPOLYMER Ethylene 70% diene 4% Pellets CAS 25038-36-2 (Alfa Chemistry, New York, NY, USA) |
| 0.92137 | Poly_D (68) | POLY(ETHYLENE) |
| 0.91619 | Intro_D (148) | PARVAN 3150, F.N. 3502*REFINED PARAFFIN WAX |
| 0.91533 | BioRad_Demo (47) | PARVAN 3150, F.N. 3502*REFINED PARAFFIN WAX |
| 0.90404 | Agilent Polymer Handheld ATR Library (110) | Polyethylene, high density Approx Mw 125,000 Pellets CAS#25213-02-_2012-06-19T16-44-41 |
| Quality | Library | Name |
|---|---|---|
| 0.94139 | ATR Demo Library (41) | polyethylene |
| 0.94033 | Agilent Polymer Handheld ATR Library (23) | ETHYLENE PROPYLENE DIENE TERPOLYMER Ethylene 70% diene 4% Pellets CAS 25038-36-2 |
| 0.92766 | Agilent Polymer Handheld ATR Library (110) | Polyethylene, high density Approx Mw 125,000 Pellets CAS#25213-02-_2012-06-19T16-44-41 |
| 0.92766 | Agilent Polymer Handheld ATR Library (111) | Polyethylene, high density Approx Mw 125,000 Pellets CAS 25213-02-9 (Alfa Chemistry, New York, NY, USA) |
| 0.92558 | Intro_D (148) | PARVAN 3150, F.N. 3502*REFINED PARAFFIN WAX |
| Quality | Library | Name |
|---|---|---|
| 0.94393 | ATR Demo Library (41) | polyethylene |
| 0.94110 | Agilent Polymer Handheld ATR Library (23) | ETHYLENE PROPYLENE DIENE TERPOLYMER Ethylene 70% diene 4% Pellets CAS 25038-36-2 |
| 0.92479 | Poly_D (68) | POLY(ETHYLENE) |
| 0.92473 | Agilent Polymer Handheld ATR Library (110) | Polyethylene, high density Approx Mw 125,000 Pellets CAS#25213-02-_2012-06-19T16-44-41 |
| 0.92473 | Agilent Polymer Handheld ATR Library (111) | Polyethylene, high density Approx Mw 125,000 Pellets CAS 25213-02-9 |
References
- Darbre, P.D. Plant-based ingredients in personal care products. In Personal Care Products and Human Health; Elsevier: Amsterdam, The Netherlands, 2023; pp. 97–112. [Google Scholar] [CrossRef]
- Abdullahi, A.; Tijjani, A.; Abubakar, A.I.; Khairulmazmi, A.; Ismail, M.R. Plant biomolecule antimicrobials: An alternative control measures for food security and safety. In Herbal Biomolecules in Healthcare Applications; Elsevier: Amsterdam, The Netherlands, 2022; pp. 381–406. [Google Scholar] [CrossRef]
- Jasmine Oil—Jasmine Oil Manufacturer, Supplier, Wholesaler & Retailer, Kanpur, India. Available online: https://www.indiaaromaoils.com/jasmine-oil.html#:~:text=Jasmine%20oil%20has%20a%20medium (accessed on 20 February 2024).
- Pavela, R. History, presence and perspective of using plant extracts as commercial botanical insecticides and farm products for protection against insects—A review. Plant Prot. Sci. 2016, 52, 229–241. [Google Scholar] [CrossRef]
- Cimino, C.; Maurel, O.M.; Musumeci, T.; Bonaccorso, A.; Drago, F.; Souto, E.M.B.; Pignatello, R.; Carbone, C. Essential Oils: Pharmaceutical Applications and Encapsulation Strategies into Lipid-Based Delivery Systems. Pharmaceutics 2021, 13, 327. [Google Scholar] [CrossRef] [PubMed]
- Burger, P.; Plainfossé, H.; Brochet, X.; Chemat, F.; Fernandez, X. Extraction of Natural Fragrance Ingredients: History Overview and Future Trends. Chem. Biodivers. 2019, 16, e1900424. [Google Scholar] [CrossRef] [PubMed]
- Icamina, P. PH Lures Investors to Big Essential Oil Business. Available online: https://malaya.com.ph/news_business/ph-lures-investors-to-big-essential-oil-business/ (accessed on 20 February 2024).
- Sampaguita Livelihoods of Peri-Urban Metro Manila PDF Metro Manila Agriculture. Scribd. Available online: https://www.scribd.com/document/149692864/Sampaguita-Livelihoods-of-Peri-urban-Metro-Manila (accessed on 20 February 2024).
- National Museum of the Philippines. Philippine National Flower-Sampaguita—National Museum. 2021. Available online: https://www.nationalmuseum.gov.ph/2021/11/10/philippine-national-flower-Sampaguita/ (accessed on 20 February 2024).
- Uwineza, P.A.; Waśkiewicz, A. Recent Advances in Supercritical Fluid Extraction of Natural Bioactive Compounds from Natural Plant Materials. Molecules 2020, 25, 3847. [Google Scholar] [CrossRef] [PubMed]
- Flora, I.O. Lubao Mayor Eyes Boosting Local Sampaguita Production; SunStar Publishing Inc.: Cebu City, Philippines, 2023; Available online: https://www.sunstar.com.ph/pampanga/local-news/lubao-mayor-eyes-boosting-local-Sampaguita-production (accessed on 20 February 2024).
- Bacud, S.T.; Cardenas, V.R.; Velasco, L.R.I. The Sampaguita Livelihood System in Sta. Cruz, Laguna, Philippines: A Case of a Transformative Resilience Development. Procedia Econ. Financ. 2014, 18, 439–446. [Google Scholar] [CrossRef]
- Rassem, H.; Nour, A.; Yunus, R.; Zaki, Y.H.; Abdlrhman, H.S. Yield Optimization and Supercritical CO2 Extraction of Essential Oil from Jasmine Flower. Indones. J. Chem. 2019, 19, 479. [Google Scholar] [CrossRef]
- Braun, N.A. Jasminum grandiflorum: Influence of Flower Processing on Concrete and Absolute Composition. J. Essent. Oil Res. 2020, 15, 1934578X20960998. [Google Scholar]
- Arifan, F.; Adhy, S.; Broto, W.; Nuswantari, S.R. A Variation of Jasmine Essential Oil for a New Jasmine Aromatherapy Formulation. Ann. Trop. Med. Public Health 2021. [Google Scholar] [CrossRef]
- Capuzzo, A.; Maffei, M.; Occhipinti, A. Supercritical Fluid Extraction of Plant Flavors and Fragrances. Molecules 2013, 18, 7194–7238. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xiong, Y.; An, H.; Li, J.; Li, Q.; Huang, J.; Liu, Z. Analysis of Volatile Components of Jasmine and Jasmine Tea during Scenting Process. Molecules 2022, 27, 479. [Google Scholar] [CrossRef] [PubMed]
- Extract Winterization Background & Basics. Aptia Engineering. 2022. Available online: https://aptiaengineering.com/2022/03/07/extract-winterization-background-and-basics/ (accessed on 20 February 2024).
- Nascimento, L.D.D.; Silva, S.G.; Cascaes, M.M.; Costa, K.S.D.; Figueiredo, P.L.B.; Costa, C.M.L.; de Aguiar Andrade, E.H.; de Faria, L.J.G. Drying Effects on Chemical Composition and Antioxidant Activity of Lippia thymoides Essential Oil, a Natural Source of Thymol. Molecules 2021, 26, 2621. [Google Scholar] [CrossRef] [PubMed]
- Menezes, J.; Athmaselvi, K.A. Report on Edible Films and Coatings. In Food Packaging and Preservation; Elsevier: Amsterdam, The Netherlands, 2018; pp. 177–212. [Google Scholar] [CrossRef]
- Paraffin Wax|Candle Making, Cosmetic Uses, Industrial Applications|Britannica. Available online: https://www.britannica.com/science/paraffin-wax (accessed on 17 September 2024).
- Rassem, H.H.; Nour, A.H.; Yunus, R.M. GC-MS analysis of bioactive constituents of jasmine flower. J. Chem. Eng. Ind. Biotechnol. 2018, 4, 52–59. [Google Scholar] [CrossRef]
- Alpha-Cadinol (CHEBI:132905). Available online: https://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:132905 (accessed on 20 February 2024).
- Akram, A.; Younis, A.; Akhtar, G.; Ameer, K.; Farooq, A.; Hanif, M.A.; Saeed, M.; Lim, K.B. Comparative efficacy of various essential oil extraction techniques on oil yield and quality of Jasminum sambac L. Sci. Int. 2017, 5, 84–95. [Google Scholar] [CrossRef]
- Cis-3 Hexenyl Benzoate. Available online: https://www.perfumersworld.com/view.php?pro_id=3GN00227 (accessed on 20 February 2024).
- Geranyl Linalool, 68931-30-6. Available online: http://www.thegoodscentscompany.com/data/rw1428051.html (accessed on 20 February 2024).
- Development from Jasminum sambac Flower Extracts of Products with Floral Fragrance and Multiple Physiological Activities—PMC. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8378956/ (accessed on 17 September 2024).
- 6-Dimethyl-3-oxatricyclo(4.2.1.0*2,4*)nonane. Available online: http://www.thegoodscentscompany.com/data/rw1061581.html (accessed on 20 February 2024).
- PubChem. 2-Hexyl-1-decanol. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/95337 (accessed on 20 February 2024).
- Scentspiracy. Methyl Anthranilate. Available online: https://www.scentspiracy.com/fragrance-ingredients/p/methyl-anthranilate#:~:text=What%20is%20Methyl%20Anthranilate%3F,Its%20molecular%20formula%20is%20C%E2%82%88H%E2%82%89NO%E2%82%82 (accessed on 20 February 2024).
- Zhang, D.; Sun, G.; Zhang, X. Analysis of a Gas Chromatography/Mass Spectrometry Thermal Desorption System with Simultaneous Sniffing for Determination of Off-Odor Compounds and Volatile Organic Compounds in Polypropylene Compo-sites. Sci. Adv. Mater. 2019, 11, 1594–1603. [Google Scholar] [CrossRef]
- PubChem. 4-Methyldecane. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/17835 (accessed on 20 February 2024).
- Panel, C.E.; Cosmetic Ingredient Review Expert Panel. Final Report on the Safety Assessment of Squalane and Squalene. J. Am. Coll. Toxicol. 1990, 1, 37–56. [Google Scholar] [CrossRef]
- α-Tocopheryl Acetate, 58-95-7. Available online: http://www.thegoodscentscompany.com/data/rw1372861.html (accessed on 20 February 2024).
- Musk Ketone (81-14-1)—Synthetic Ingredient for Perfumery. Scentspiracy. Available online: https://www.scentspiracy.com/fragrance-ingredients/p/musk-ketone (accessed on 20 February 2024).
- Grosch, W. Evaluation of the key odorants of foods by dilution experiments, aroma models and omission. Chem. Senses 2001, 26, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Boelens, M.H.; van Gemert, L.J. Perceptual masking of odorants in binary mixtures. Chem. Senses 1993, 18, 355–367. [Google Scholar] [CrossRef]
- Hartati; Salleh, L.M.; Aziz, A.; Yunus, M.A.C. The Effect of Supercritical Fluid Extraction Parameters on the Swietenia Mahagoni Seed Oil Extraction and its Cytotoxic Properties. J. Teknol. Sci. Eng. 2014, 69, 51–53. [Google Scholar] [CrossRef]
- van Harreveld, A.P. Odor concentration decay and stability in gas sampling bags. J. Air Waste Manag. Assoc. 2003, 53, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Salvador, A.; Chisvert, A. Analysis of Cosmetic Products. 2007. Available online: http://inci-dic.com/wp-content/uploads/2018/05/Analysis-of-Cosmetic-Products.pdf (accessed on 20 February 2024).


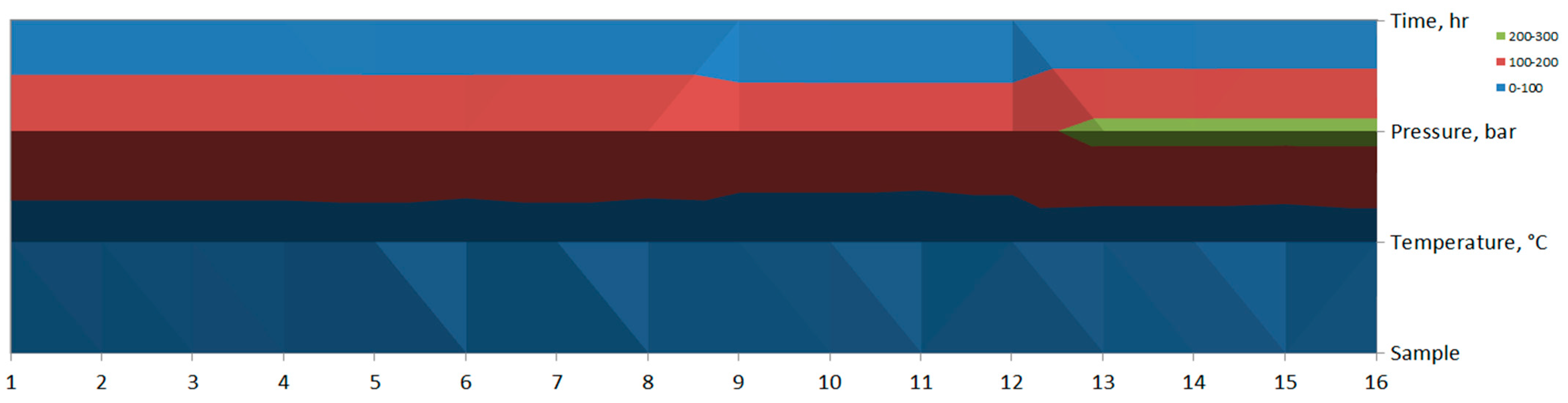
| Sample | Temperature (°C) | Pressure (Bar) | Time (h) |
|---|---|---|---|
| 1 | 40 | 200 | 2 |
| 2 | 40 | 200 | 2 |
| 3 | 40 | 200 | 2 |
| 4 | 40 | 200 | 2 |
| 5 | 45 | 200 | 1.5 |
| 6 | 35 | 200 | 1.5 |
| 7 | 45 | 200 | 2.5 |
| 8 | 35 | 200 | 2.5 |
| 9 | 40 | 175 | 2.5 |
| 10 | 40 | 175 | 1.5 |
| 11 | 35 | 175 | 2 |
| 12 | 45 | 175 | 2 |
| 13 | 40 | 225 | 2.5 |
| 14 | 40 | 225 | 1.5 |
| 15 | 35 | 225 | 2 |
| 16 | 45 | 225 | 2 |
| Drying Method | Description |
|---|---|
| No Drying | The J. sambac flower petals are plucked from their sepals and are extracted on the same day. No drying occurs. |
| Overnight Air Dry (A) | The J. sambac flower petals are air-dried AFTER being plucked. The extraction happens the next day. |
| Overnight Air Dry (B) | The J. sambac flowers are air-dried BEFORE being plucked. The plucking and extracting of samples happen the next day. |
| 2-day Air Drying (A) | The J. sambac flower petals are air-dried for two days after being plucked. The extraction of samples happens the next day. |
| Blow Dry (A) | The J. sambac flower petals are blow-dried AFTER being plucked. The dryness of the petals is not so dry that they become crisp; rather, it removes most of the moisture and still feels soft. The extraction of samples happens the next day. |
| Blow Dry (B) | The J. sambac flowers are blow-dried BEFORE the petals are plucked. The dryness of the petals is not so dry that they become crisp; rather, it removes most of the moisture and still feels soft. The extraction of samples happens the next day. |
| Drying Method | Sample | Petal Mass (g) | Extracted Oil Mass (g) | % Yield | Appearance | Odor |
|---|---|---|---|---|---|---|
| Overnight Air Dry (A) | 1 | 350.5 | 0.1784 | 0.0509 | Solid, yellowish, waxy | Smells slightly “off”; smells of an odd ‘browning’ Sampaguita |
| Overnight Air Dry (B) | 2 | 188 | 0.1231 | 0.0655 | Solid, yellowish, waxy | Strongly scented and aromatic |
| No Drying | 3 | 384.5 | 0.0621 | 0.0162 | Solid, yellowish, waxy | Pleasant scent and floral and grassy smells |
| Blow Dry (A) | 4 | 367.7 | 0.0598 | 0.0163 | Solid, yellowish, waxy | Smells a little bit of Sampaguita, nothing unpleasant detected |
| Blow Dry (A) | 5 | 408.3 | 0.1436 | 0.0352 | Solid, yellowish, waxy | Unpleasant, indescribable unpleasant scent |
| Blow Dry (A) | 6 | 401.3 | 0.0623 | 0.0155 | Solid, yellowish, waxy | Floral and smells pleasant |
| Blow Dry (B) | 7 | 358.1 | 0.1967 | 0.0549 | Solid, yellowish, waxy | Somehow smells like boiled petals in an unpleasant way |
| No Drying | 8 | 398.1 | 0.0562 | 0.0141 | Solid, yellowish, waxy | Not as strong as the other pleasant-smelling ones; more candy-like sweetness |
| Overnight Air Dry (A) | 9 | 350.8 | 0.1454 | 0.0414 | Solid, white with little yellow, waxy | Very little to no scent; some can detect that it smells pleasant, some cannot |
| Blow Dry (B) | 10 | 328 | 0.0329 | 0.0100 | Solid, white with little yellow, waxy | Neutral scent, little to no detectable scent |
| Blow Dry (B) | 11 | 297.5 | 0.0477 | 0.0160 | Solid, yellowish, waxy | Floral and smells pleasant, faint sweetness |
| Overnight Air Dry (A) | 12 | 400.6 | 0.148 | 0.0369 | Solid, slightly brown look, but mostly yellowish cream, waxy | Smells almost burnt |
| No Drying | 13 | 286.1 | 0.0561 | 0.0196 | Solid, yellowish, waxy | Very little to no scent is detectable |
| Overnight Air Dry (B) | 14 | 311 | 0.106 | 0.0341 | Solid, yellowish, has some red/brown tints, waxy | Smells almost burnt, grassy notes |
| 3-day Air Drying (A) | 15 | 260.5 | 0.0105 | 0.0040 | Solid, yellowish, has some red/brown tints, waxy | Smells almost burnt, floral notes |
| Overnight Air Dry (B) | 16 | 429 | 0.1329 | 0.0310 | Solid, yellowish, has some red/brown tints, waxy | Burnt quality, unpleasant but mellowed-out scent |
| Multiple R | 0.59076 |
| R Square | 0.34899 |
| Adjusted R Square | 0.18624 |
| Standard Error | 0.01604 |
| Observations | 16.00000 |
| df | SS | MS | F | Significance F | ||||
|---|---|---|---|---|---|---|---|---|
| Regression | 3.00000 | 0.00165 | 0.00055 | 2.14434 | 0.14785 | |||
| Residual | 12.00000 | 0.00309 | 0.00026 | |||||
| Total | 15.00000 | 0.00474 | ||||||
| Coefficient | Standard Error | t Stat | p-value | Lower 95% | Upper 95% | Lower 95% | Upper 95% | |
| Intercept | −0.08155 | 0.06815 | −1.19663 | 0.25456 | −0.23004 | 0.06694 | −0.23004 | 0.06694 |
| Temperature, °C | 0.00271 | 0.00113 | 2.39006 | 0.03413 | 0.00024 | 0.00518 | 0.00024 | 0.00518 |
| Pressure, bar | −0.00008 | 0.00023 | −0.34396 | 0.73683 | −0.00057 | 0.00042 | −0.00057 | 0.00042 |
| Time, h | 0.00880 | 0.01134 | 0.77611 | 0.45271 | −0.01590 | 0.03350 | −0.01590 | 0.03350 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinez, A.J.S.; Añonuevo, A.M.; Cruz, L.; Manayaga, D.; Tayo, L. Influence of Drying Methods and Parameters on the Quality of Jasminum sambac (L.) Flower Extracts Obtained via Supercritical Fluid Extraction. Processes 2025, 13, 3369. https://doi.org/10.3390/pr13103369
Martinez AJS, Añonuevo AM, Cruz L, Manayaga D, Tayo L. Influence of Drying Methods and Parameters on the Quality of Jasminum sambac (L.) Flower Extracts Obtained via Supercritical Fluid Extraction. Processes. 2025; 13(10):3369. https://doi.org/10.3390/pr13103369
Chicago/Turabian StyleMartinez, Aaron Juztine Santos, Andrea Mae Añonuevo, Lourdes Cruz, Danilo Manayaga, and Lemmuel Tayo. 2025. "Influence of Drying Methods and Parameters on the Quality of Jasminum sambac (L.) Flower Extracts Obtained via Supercritical Fluid Extraction" Processes 13, no. 10: 3369. https://doi.org/10.3390/pr13103369
APA StyleMartinez, A. J. S., Añonuevo, A. M., Cruz, L., Manayaga, D., & Tayo, L. (2025). Influence of Drying Methods and Parameters on the Quality of Jasminum sambac (L.) Flower Extracts Obtained via Supercritical Fluid Extraction. Processes, 13(10), 3369. https://doi.org/10.3390/pr13103369





