Quantitative Detection of Toxic Elements in Food Samples by Inductively Coupled Plasma Mass Spectrometry (ICP-MS)
Abstract
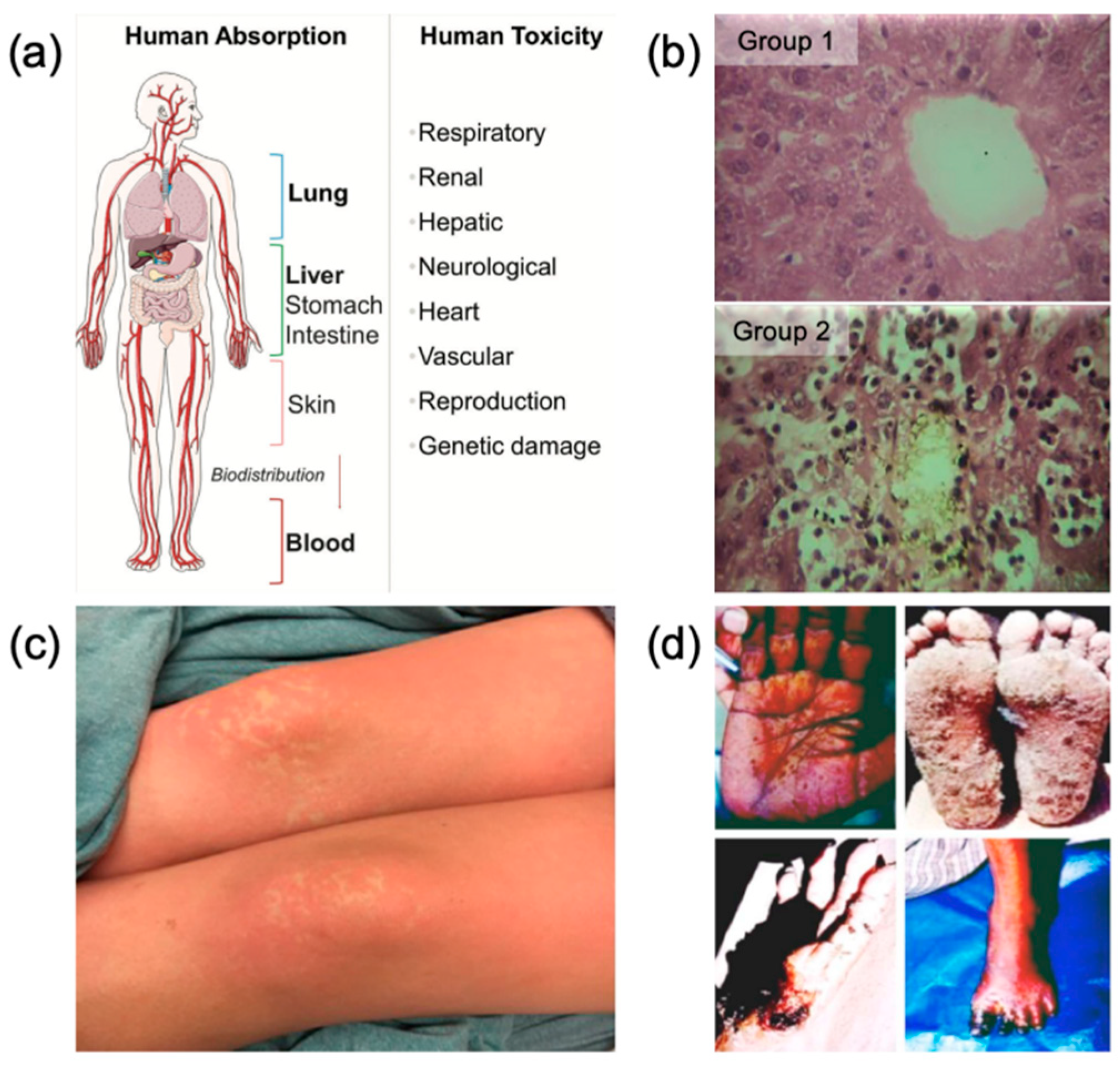
1. Introduction
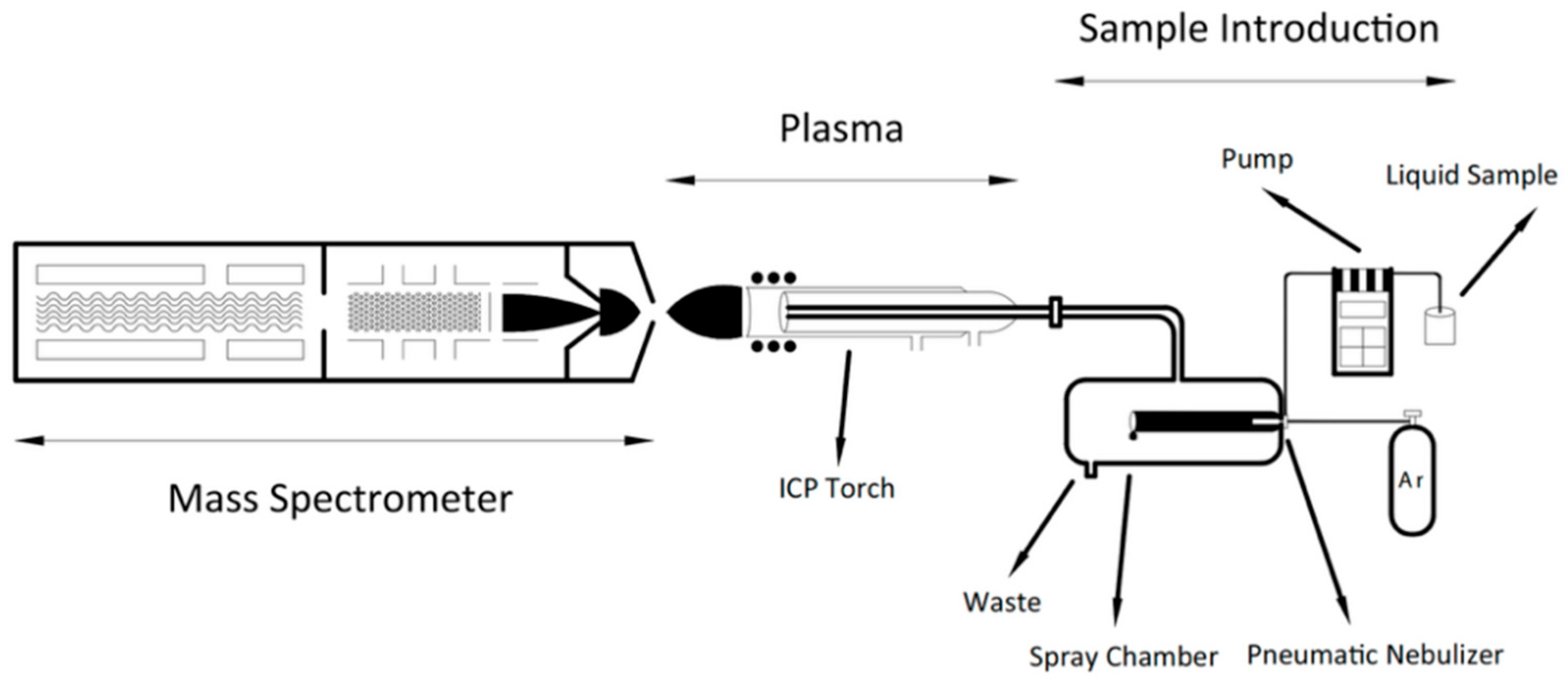
2. Principle of ICP-MS
3. Sample Preparation
3.1. Conventional Sample Preparation Process
3.2. Polyatomic Interferences Correction
3.3. Recent Improvements in Sample Preparation Process
3.3.1. Environmental Friendly
3.3.2. Optimization Strategies for Pretreatment Under the Complexity of Food Matrices
3.3.3. Interference Cancellation and Correction
4. The Detection of Special Elements
4.1. As
4.2. Pb
4.3. Cd
4.4. Hg
4.5. Cr
5. Detection of Toxic Elements in Different Food
5.1. Seafood
5.2. Cereals
5.3. Dairy
5.4. Potable Water
5.5. Vegetables
5.6. Honey
6. Applications of LA-ICP-MS
6.1. Element Quantification by LA-ICP-MS
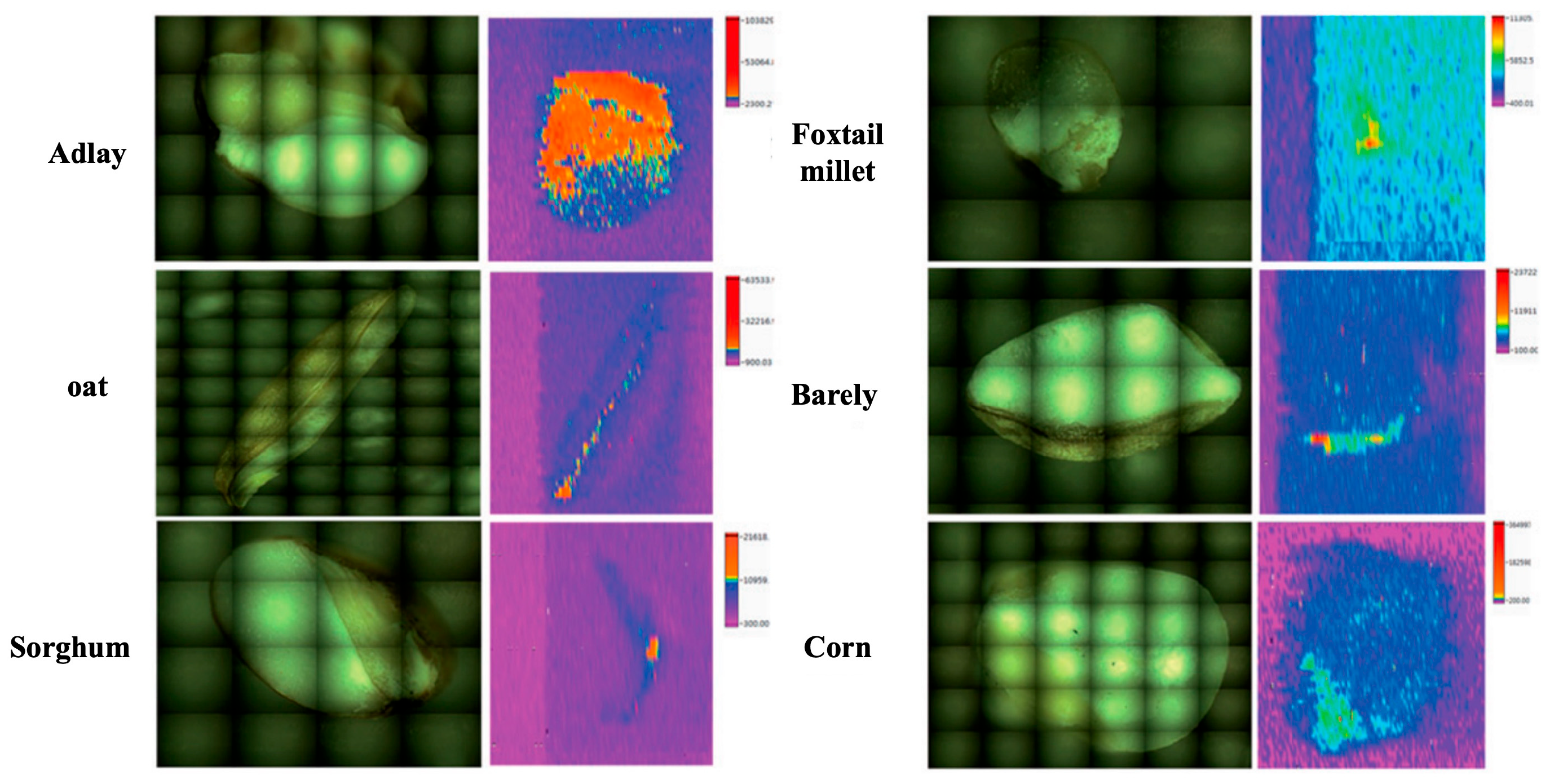
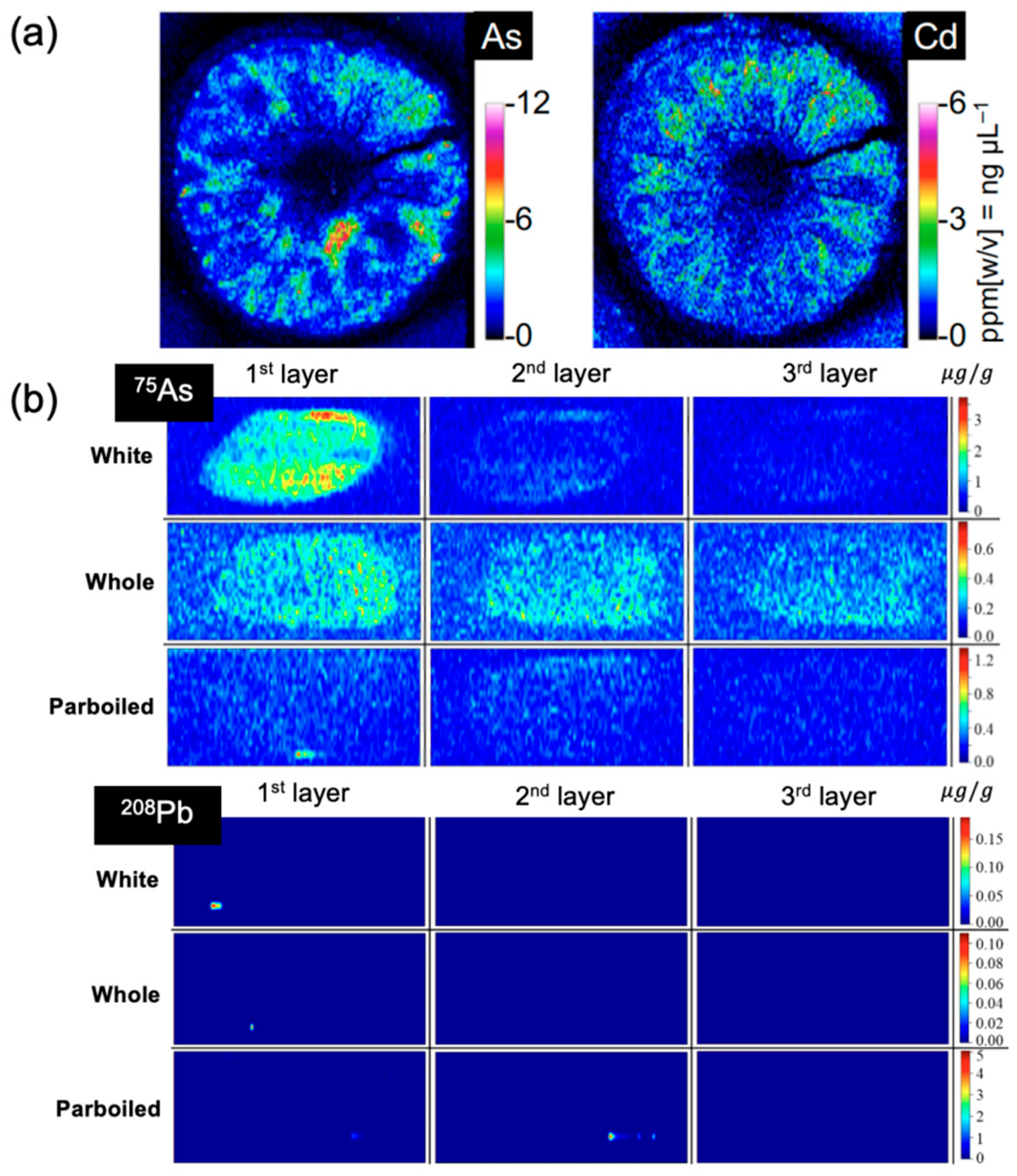
6.2. Element Imaging by LA-ICP-MS
7. Challenges and Opportunities of ICP-MS
7.1. Challenges
7.2. Opportunities
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AFS | Atomic Fluorescence Spectroscopy |
| AsB | Arsenobetaine |
| AsC | Arsenocholine |
| BEC | Background Equivalent Concentration |
| BS | British Standard |
| Cd-MTs | Cadmium-Metallothioneins |
| CE | Capillary Electrophoresis |
| CH3Hg+ | Methylmercury |
| CRC | Collision/Reaction Cell |
| CRM | Certified reference materials |
| CV-AAS | Cold Vapor–Atomic Absorption Spectrometry |
| CVG | Chemical Vapor Generation |
| DMA | Di-methylarsinic Acid |
| DPASV | Differential Pulse Anodic Stripping Voltammetry |
| DRC | Dynamic Reaction Cell |
| EC | Electrical Conductivity |
| EDTA | Ethylenediaminetetraacetic Acid |
| EFSA | European Food Safety Authority |
| EPA | Environmental Protection Agency |
| ETV | Electrothermal Vaporization |
| FAAS | Flame Atomic Absorption Spectroscopy |
| FDA | Food and Drug Administration |
| fs-LA-ICP-MS | Femtosecond–Laser Ablation–ICP-MS |
| GED | Gas Exchange Device |
| GFAAS | Graphite Furnace Atomic Absorption Spectroscopy |
| H2 | Hydrogen |
| HAE | Heat-Assisted Extraction |
| He | Helium |
| Hg2+ | Inorganic Mercury |
| HPLC | High-Performance Liquid Chromatography |
| HR-ICP-MS | High-Resolution–ICP-MS |
| ICP-MS | Inductively Coupled Plasma–Mass Spectrometry |
| ICP-OES | Inductively Coupled Plasma–Optical Emission Spectrometry |
| IDMS | Isotope Dilution Mass Spectrometry |
| iAs | Inorganic Arsenic |
| IFS | Interference Standard Method |
| IS | Internal Standard |
| JECFA | Joint FAO/WHO Expert Committee on Food Additives |
| KED | Kinetic Energy Discrimination |
| LA-ICP-MS | Laser Ablation–ICP-MS |
| LOD | Limit of Detection |
| LOQ | Limit of Quantification |
| LPME | Liquid Phase Microextraction |
| MAC | Maximum Allowable Concentration |
| MAE | Microwave-Assisted Extraction |
| MD | Membrane Desolvation |
| MeHg⁺ | Methylmercury |
| MICAP | Microwave-sustained, Inductively Coupled, Atmospheric Pressure-based nitrogen plasma |
| MMA | Mono-Methylarsonic Acid |
| MPI | Metal Pollution Index |
| MSC | Multi-Signal Calibration method |
| MSPE | Magnetic Solid Phase Extraction |
| MTs | Metallothioneins |
| MW-UV | Microwave–Ultraviolet Degradation |
| NADES | Natural Deep-Eutectic Solvents |
| PhHg⁺ | Phenylmercury |
| ppb | Parts Per Billion |
| ppt | Parts Per Trillion |
| PTMI | Provisional Tolerable Monthly Intake |
| RSD | Relative Standard Deviation |
| SAM | Standard Addition Method |
| SEC | Size Exclusion Chromatography |
| SPME | Solid-Phase Microextraction |
| TDS | Total Dissolved Solids |
| TF | Transfer Factor |
| THQ | Target Hazard Quotient |
| TQMS | Triple Quadrupole Mass Spectrometry |
| TWI | Tolerable Weekly Intake |
| UV-vis | Ultraviolet–Visible Spectroscopy |
| VSG | Volatile Species Generation |
| WHO | World Health Organization |
| YMWE | Yemen Ministry of Water and Environment |
References
- Pauli, B.J. The Flint water crisis. Wiley Interdiscip. Rev. Water 2020, 7, e1420. [Google Scholar] [CrossRef]
- Hüsken, A.; Arent, L.; Lohmayer, R. Cadmium Maximum Levels and Residue Situation in the German Wheat and Rye Harvest from 1975 to 2021. J. Agric. Food Chem. 2024, 72, 16496–16505. [Google Scholar] [CrossRef]
- Orzoł, A.; Gołębiowski, A.; Szultka-Młyńska, M.; Głowacka, K.; Pomastowski, P.; Buszewski, B. ICP-MS Analysis of Cadmium Bioaccumulation and Its Effect on Pea Plants (Pisum sativum L.). Pol. J. Environ. Stud. 2022, 31, 4779–4787. [Google Scholar] [CrossRef] [PubMed]
- Aoshima, K. Itai-itai disease: Renal tubular osteomalacia induced by environmental exposure to cadmium—Historical review and perspectives. Soil Sci. Plant Nutr. 2016, 62, 319–326. [Google Scholar] [CrossRef]
- Li, H.; Bei, Q.; Zhang, W.; Marimuthu, M.; Hassan, M.M.; Haruna, S.A.; Chen, Q. Ultrasensitive fluorescence sensor for Hg2+ in food based on three-dimensional upconversion nanoclusters and aptamer-modulated thymine-Hg2+-thymine strategy. Food Chem. 2023, 422, 136202. [Google Scholar] [CrossRef]
- Caravati, E.M.; Erdman, A.R.; Christianson, G.; Nelson, L.S.; Woolf, A.D.; Booze, L.L.; Cobaugh, D.J.; Chyka, P.A.; Scharman, E.J.; Manoguerra, A.S.; et al. Elemental mercury exposure: An evidence-based consensus guideline for out-of-hospital management. Clin. Toxicol. 2008, 46, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, T.W.; Magos, L.; Myers, G.J. The Toxicology of Mercury—Current Exposures and Clinical Manifestations. N. Engl. J. Med. 2003, 349, 1731–1737. [Google Scholar] [CrossRef]
- Eto, K. Minamata disease. Neuropathology 2000, 20, 14–19. [Google Scholar] [CrossRef]
- Atkins, P.; Hassan, M.; Dunn, C. Poisons, pragmatic governance and deliberative democracy: The arsenic crisis in Bangladesh. Geoforum 2007, 38, 155–170. [Google Scholar] [CrossRef]
- Maietta, I.; Otero-Martínez, C.; Fernández, S.; Sánchez, L.; González-Fernández, Á.; Polavarapu, L.; Simón-Vázquez, R. The Toxicity of Lead and Lead-Free Perovskite Precursors and Nanocrystals to Human Cells and Aquatic Organisms. Adv. Sci. 2025, 12, e2415574. [Google Scholar] [CrossRef]
- Athmouni, K.; Belhaj, D.; El Feki, A.; Ayadi, H. Optimization, antioxidant properties and GC–MS analysis of Periploca angustifolia polysaccharides and chelation therapy on cadmium-induced toxicity in human HepG2 cells line and rat liver. Int. J. Biol. Macromol. 2018, 108, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Atti, S.K.; Silver, E.M.; Chokshi, Y.; Casteel, S.; Kiernan, E.; Dela Cruz, R.; Kazzi, Z.; Geller, R.J. All that glitters is not gold: Mercury poisoning in a family mimicking an infectious illness. Curr. Probl. Pediatr. Adolesc. Health Care 2020, 50, 100758. [Google Scholar] [CrossRef] [PubMed]
- Sambu, S.; Wilson, R. Arsenic in food and water—A brief history. Toxicol. Ind. Health 2008, 24, 217–226. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, G.; Xie, S.; Zhao, X.; Zhang, Y. Comparison of high-performance liquid chromatography and ultraviolet-visible spectrophotometry to determine the best method to assess Levofloxacin released from mesoporous silica microspheres/nano-hydroxyapatite composite scaffolds. Exp. Ther. Med. 2019, 17, 2694–2702. [Google Scholar] [CrossRef]
- Hu, B.; Shengqing, L.; Guoqiang, X.; Man, H.; Jiang, Z. Recent Progress in Electrothermal Vaporization–Inductively Coupled Plasma Atomic Emission Spectrometry and Inductively Coupled Plasma Mass Spectrometry. Appl. Spectrosc. Rev. 2007, 42, 203–234. [Google Scholar] [CrossRef]
- Ammann, A.A. Inductively coupled plasma mass spectrometry (ICP MS): A versatile tool. J. Mass Spectrom. 2007, 42, 419–427. [Google Scholar] [CrossRef]
- Wu, D.; Pichler, T. Simultaneous speciation analysis of As, Sb and Se redox couples by SF-ICP-MS coupled to HPLC. Anal. Methods 2014, 6, 5112–5119. [Google Scholar] [CrossRef]
- Sylvester, P.J. A brief history of laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS). Elements 2016, 12, 307–310. [Google Scholar] [CrossRef]
- Gao, F.; Hao, X.; Zeng, G.; Guan, L.; Wu, H.; Zhang, L.; Wei, R.; Wang, H.; Li, H. Identification of the geographical origin of Ecolly (Vitis vinifera L.) grapes and wines from different Chinese regions by ICP-MS coupled with chemometrics. J. Food Compos. Anal. 2022, 105, 104248. [Google Scholar] [CrossRef]
- Kashani, A.; Mostaghimi, J. Aerosol characterization of concentric pneumatic nebulizer used in inductively coupled plasma-mass spectrometry(ICP-MS). At. Sprays 2010, 20, 415–433. [Google Scholar] [CrossRef]
- Brito, T.A.; Costa, F.S.; Oliveira, R.C.; Amaral, C.D.; Labuto, G.; Gonzalez, M.H. Green extraction using natural deep eutectic solvents for determination of As, Cd, and Pb in plant and food matrices by ICP-MS. Food Chem. 2025, 464, 141922. [Google Scholar] [CrossRef]
- Cerveira, C.; Hermann, P.; Pereira, J.; Pozebon, D.; Mesko, M.; Moraes, D. Evaluation of microwave-assisted ultraviolet digestion method for rice and wheat for subsequent spectrometric determination of As, Cd, Hg and Pb. J. Food Compos. Anal. 2020, 92, 103585. [Google Scholar] [CrossRef]
- Patel, A.; Chakraborty, S.; Misra, S.K.; Datta, B. Hydrolytic Enzyme-Facilitated Mass Spectrometric Investigation of Metals in Processed Food Matrices. ACS Food Sci. Technol. 2024, 4, 1152–1165. [Google Scholar] [CrossRef]
- Peters, R.; Herrera-Rivera, Z.; Undas, A.; van der Lee, M.; Marvin, H.; Bouwmeester, H.; Weigel, S. Single particle ICP-MS combined with a data evaluation tool as a routine technique for the analysis of nanoparticles in complex matrices. J. Anal. At. Spectrom. 2015, 3, 1274–1285. [Google Scholar] [CrossRef]
- Ghaffour, D.; Leufroy, A.; Jitaru, P. A novel method for multi-matrix arsenic speciation analysis by anion-exchange HPLC-ICP-MS in the framework of the third (French) total diet study: A novel method for multi-matrix arsenic speciation analysis by anion-exchange HPLC-ICP-MS in the framework of the third (French) total diet study. Anal. Bioanal. Chem. 2025, 417, 1519–1530. [Google Scholar] [CrossRef] [PubMed]
- Beltrami, D.; Calestani, D.; Maffini, M.; Suman, M.; Melegari, B.; Zappettini, A.; Zanotti, L.; Casellato, U.; Careri, M.; Mangia, A. Development of a combined SEM and ICP-MS approach for the qualitative and quantitative analyses of metal microparticles and sub-microparticles in food products. Anal. Bioanal. Chem. 2011, 401, 1401–1409. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, X.; Nan, K.; Chen, B.; He, M.; Hu, B. Sample pre-treatment techniques for use with ICP-MS hyphenated techniques for elemental speciation in biological samples. J. Anal. At. Spectrom. 2017, 32, 58–77. [Google Scholar] [CrossRef]
- Silva, J.S.; Heidrich, G.M.; Poletto, B.O.; Paniz, J.N.G.; Dressler, V.L.; Flores, E.M.M. Mercury determination in bioresorbable calcium phosphate using a new electrothermal vaporization system coupled to ICP-MS. J. Anal. At. Spectrom. 2023, 38, 1–16. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, R.; Yang, L. Application of chemical vapor generation in ICP-MS: A review. Chin. Sci. Bull. 2013, 58, 1980–1991. [Google Scholar] [CrossRef]
- Subramanian, R.; Muthuganesan, N.; Venu, S.; Gopika, S.K.; Thummar, U.G.; Chakkaravarthi, A.; Singh, P.S.; Singh, S. Integrated membrane process for pretreating and desolventizing hexane-soybean oil miscella—A pilot plant study. J. Membr. Sci. 2024, 704, 122808. [Google Scholar] [CrossRef]
- Kilic, S.; Soylak, M. Determination of trace element contaminants in herbal teas using ICP-MS by different sample preparation method. J. Food Sci. Technol. 2020, 57, 927–933. [Google Scholar] [CrossRef]
- Shen, K.; Ni, Z.; Xiaoming, Y.; Zhongxi, L.; Ying, Z.; Zhou, T. Dry Ashing Preparation of (Quasi)solid Samples for the Determination of Inorganic Elements by Atomic/Mass Spectrometry. Appl. Spectrosc. Rev. 2015, 50, 304–331. [Google Scholar] [CrossRef]
- Pereira, L.S.F.; Enders, M.S.P.; Iop, G.D.; Mello, P.A.; Flores, E.M.M. Determination of Cl, Br and I in soils by ICP-MS: Microwave-assisted wet partial digestion using H2O2 in an ultra-high pressure system. J. Anal. At. Spectrom. 2018, 33, 649–657. [Google Scholar] [CrossRef]
- Nurubeyli, T.K.; Ahmadova, K.N. The role of the spectral matrix effect in the element analysis of biological fluids in ICP-MS. Mod. Phys. Lett. B 2020, 35, 2150094. [Google Scholar] [CrossRef]
- Kilic, M.; Kilic, S.; Yenisoy-Karakaş, S. The method development for elimination of matrix interferences in seawater monitoring to determine elements by ICP-MS. Environ. Monit. Assess. 2023, 195, 180. [Google Scholar] [CrossRef]
- Sugiyama, N. Attenuation of doubly charged ion interferences on arsenic and selenium by ICP-MS under low kinetic energy collision cell conditions with hydrogen cell gas. J. Anal. At. Spectrom. 2021, 36, 294–302. [Google Scholar] [CrossRef]
- Lum, T.-S.; Sze-Yin Leung, K. Strategies to overcome spectral interference in ICP-MS detection. J. Anal. At. Spectrom. 2016, 31, 178–188. [Google Scholar] [CrossRef]
- Ni, W.; Mao, X.; Xiao, F.; Guo, X.; Wang, L.; Wu, J.; Wang, T. Determination of Ultra-Trace Rhodium in Geological Samples by High Radio Frequency Power Cold Plasma and Kinetic Energy Discrimination Collision Cell-Inductively Coupled Plasma-Mass Spectrometry (KED-ICP-MS). Anal. Lett. 2024, 57, 1816–1828. [Google Scholar] [CrossRef]
- Ohata, M.; Nishiguchi, K. Research Progress on Gas to Particle Conversion–Gas Exchange ICP-MS for Direct Analysis of Ultra-trace Metallic Compound Gas. Anal. Sci. 2018, 34, 657–666. [Google Scholar] [CrossRef]
- Yamada, N. Kinetic energy discrimination in collision/reaction cell ICP-MS: Theoretical review of principles and limitations. Spectrochim. Acta Part B At. Spectrosc. 2015, 110, 31–44. [Google Scholar] [CrossRef]
- Kuznetsova, O.V.; Mokhodoeva, O.B.; Maksimova, V.V.; Dzhenloda, R.K.; Jarosz, M.; Shkinev, V.M.; Timerbaev, A.R. High-resolution ICP-MS approach for characterization of magnetic nanoparticles for biomedical applications. J. Pharm. Biomed. Anal. 2020, 189, 113479. [Google Scholar] [CrossRef] [PubMed]
- Schoof, R.A.; Yost, L.J.; Eickhoff, J.; Crecelius, E.A.; Cragin, D.W.; Meacher, D.M.; Menzel, D.B. A Market Basket Survey of Inorganic Arsenic in Food. Food Chem. Toxicol. 1999, 37, 839–846. [Google Scholar] [CrossRef]
- Nawrocka, A.; Durkalec, M.; Michalski, M.; Posyniak, A. Simple and reliable determination of total arsenic and its species in seafood by ICP-MS and HPLC-ICP-MS. Food Chem. 2022, 379, 132045. [Google Scholar] [CrossRef]
- Wang, Z.; Gui, H.; Luo, Z.; Sarakiotia, I.L.; Yan, C.; Laing, G.D. Arsenic release: Insights into appropriate disposal of arsenic-loaded algae precipitated from arsenic contaminated water. J. Hazard. Mater. 2020, 384, 121249. [Google Scholar] [CrossRef] [PubMed]
- Norton, G.; Deacon, C.; Mestrot, A.; Feldmann, J.; Jenkins, P.; Baskaran, C.; Meharg, A.A. Arsenic Speciation and Localization in Horticultural Produce Grown in a Historically Impacted Mining Region. Environ. Sci. Technol. 2013, 47, 6164–6172. [Google Scholar] [CrossRef] [PubMed]
- Norton, G.J.; Pinson, S.R.; Alexander, J.; Mckay, S.; Hansen, H.; Duan, G.L.; Rafiqul Islam, M.; Islam, S.; Stroud, J.L.; Zhao, F.J.; et al. Variation in grain arsenic assessed in a diverse panel of rice (Oryza sativa) grown in multiple sites. New Phytol. 2012, 193, 650–664. [Google Scholar] [CrossRef]
- Arsenic. Available online: https://www.who.int/news-room/fact-sheets/detail/arsenic (accessed on 27 July 2025).
- Arsenic in Food. Available online: https://www.fda.gov/food/environmental-contaminants-food/arsenic-food (accessed on 27 July 2025).
- Arsenic. Available online: https://food.ec.europa.eu/food-safety/chemical-safety/contaminants/catalogue/arsenic_en (accessed on 27 July 2025).
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Scientific Opinion on Lead in Food. EFSA J. 2010, 8, 1570. [Google Scholar] [CrossRef]
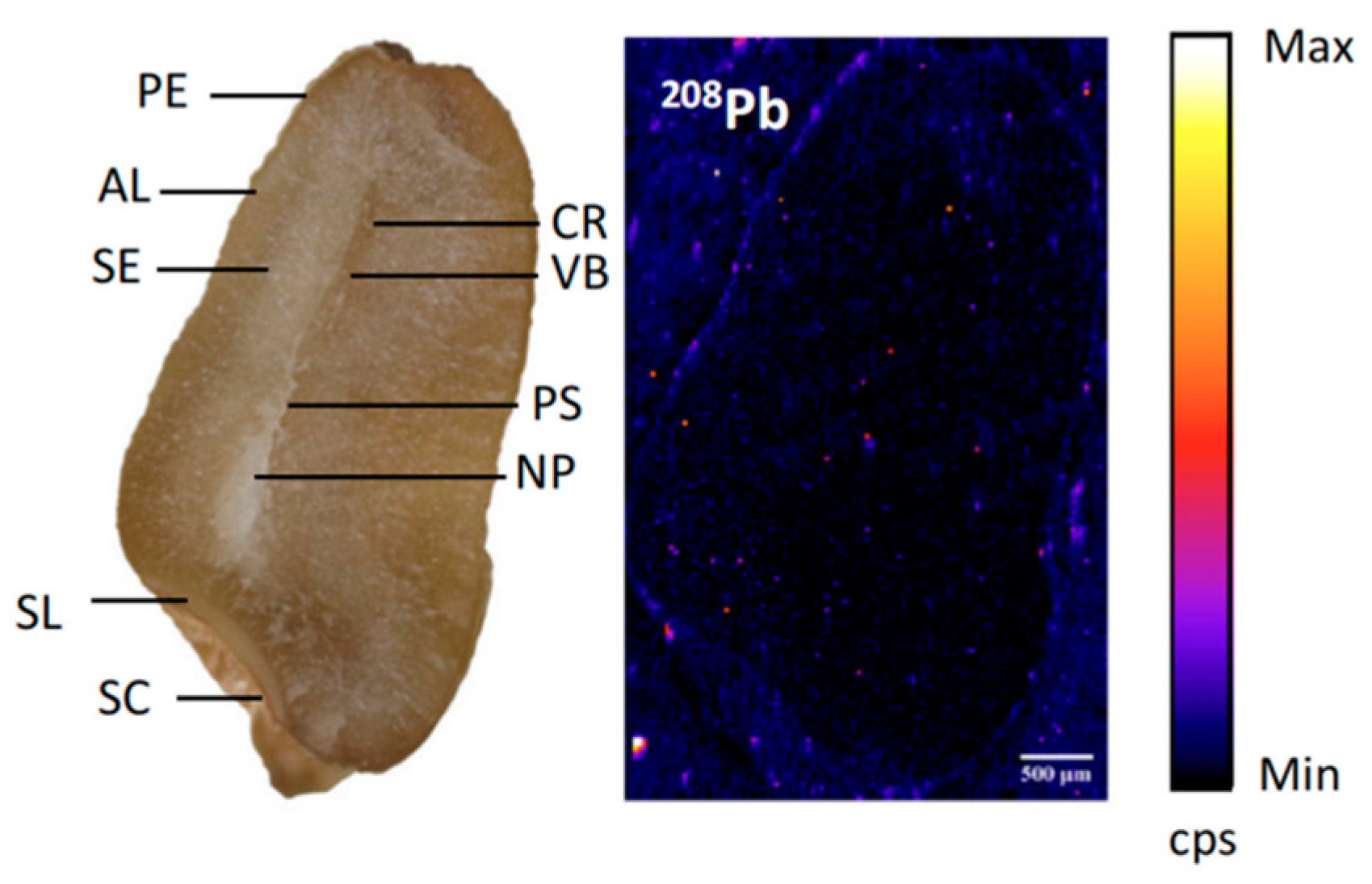
- Zhang, X.-Y.; Geng, L.-P.; Gao, P.-P.; Dong, J.-W.; Zhou, C.; Li, H.-B.; Chen, M.-M.; Xue, P.-Y.; Liu, W.-J. Bioimaging of Pb by LA-ICP-MS and Pb isotopic compositions reveal distributions and origins of Pb in wheat grain. Sci. Total Environ. 2022, 802, 149729. [Google Scholar] [CrossRef]
- Lead Poisoning and Health. Available online: https://www.who.int/news-room/fact-sheets/detail/lead-poisoning-and-health (accessed on 27 July 2025).
- Lead in Food and Foodwares. Available online: https://www.fda.gov/food/environmental-contaminants-food/lead-food-and-foodwares (accessed on 27 July 2025).
- EASECAST NEWS—Lead Regulation in the, EU. Available online: https://www.rheinmetall.com/Rheinmetall%20Group/Systeme%20und%20Produkte/KS%20Gleitlager/Datenbl%C3%A4tter/EASECAST%20NEWS%20en.pdf (accessed on 27 July 2025).
- Wang, R.; Sang, P.; Guo, Y.; Jin, P.; Cheng, Y.; Yu, H.; Xie, Y.; Yao, W.; Qian, H. Cadmium in food: Source, distribution and removal. Food Chem. 2023, 405, 134666. [Google Scholar] [CrossRef]
- Liu, M.; Fan, D.; Liao, Y.; Chen, B.; Yang, Z. Heavy metals in surficial sediments of the central Bohai Sea: Their distribution, speciation and sources. Acta Oceanol. Sin. 2016, 35, 98–110. [Google Scholar] [CrossRef]
- Álvarez-Llamas, G.; Fernández de la Campa, M.R.; Sanz-Medel, A. An alternative interface for CE–ICP–MS cadmium speciation in metallothioneins based on volatile species generation. Anal. Chim. Acta 2005, 546, 236–243. [Google Scholar] [CrossRef]
- Wei, S.; Guo, B.; Feng, L.; Jiang, T.; Li, M.; Wei, Y. Cadmium Distribution and Characteristics of Cadmium-binding Proteins in Rice (Oryza sativa L.) Kernel. Food Sci. Technol. Res. 2017, 23, 661–668. [Google Scholar] [CrossRef]
- Suzuki, K.T.; Sasakura, C.; Ohmichi, M. Binding of Endogenous and Exogenous Cadmium to Glutelin in Rice Grains as Studied by HPLC/ICP-MS with Use of a Stable Isotope. J. Trace Elem. Med. Biol. 1997, 11, 71–76. [Google Scholar] [CrossRef]
- World Health Organization. Exposure to Cadmium: A Major Public Health Concern. 2019. Available online: https://iris.who.int/bitstream/handle/10665/329480/WHO-CED-PHE-EPE-19.4.3-eng.pdf?sequence=1 (accessed on 27 July 2025).
- Cadmium in Food and Foodwares. Available online: https://www.fda.gov/food/environmental-contaminants-food/cadmium-food-and-foodwares (accessed on 27 July 2025).
- Commission Regulation (EU) 2021/1323. Available online: https://eur-lex.europa.eu/eli/reg/2021/1323/oj/eng (accessed on 27 July 2025).
- Nogara, P.A.; Farina, M.; Aschner, M.; Rocha, J.B.T. Mercury in Our Food. Chem. Res. Toxicol. 2019, 32, 1459–1461. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Chen, B.; He, M.; Huang, T.; Hu, B. Speciation of mercury in water and fish samples by HPLC-ICP-MS after magnetic solid phase extraction. Talanta 2017, 171, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Anil, A.S.; Alam, S.; Thakur, L.K. Optimized mercury speciation analysis using LC-ICP-MS and microwave assisted extraction for precise determination of methylmercury in fish, rice and soil. J. Food Compos. Anal. 2024, 129, 106092. [Google Scholar] [CrossRef]
- Tian, X.; Wang, Y.; Xu, T.; Guo, Y.; Bi, Y.; Liu, Y.; Liang, Y.; Cui, W.; Liu, Y.; Hu, L.; et al. Bioconcentration of Inorganic and Methyl Mercury by Algae Revealed Using Dual-Mass Single-Cell ICP-MS with Double Isotope Tracers. Environ. Sci. Technol. 2024, 58, 7860–7869. [Google Scholar] [CrossRef] [PubMed]
- Mercury and Health. Available online: https://www.who.int/news-room/fact-sheets/detail/mercury-and-health (accessed on 27 July 2025).
- Mercury in Food. Available online: https://www.fda.gov/food/environmental-contaminants-food/mercury-food (accessed on 27 July 2025).
- Mercury. Available online: https://environment.ec.europa.eu/topics/chemicals/mercury_en (accessed on 27 July 2025).
- Pluháček, T.; Pechancová, R.; Milde, D.; Bettencourt da Silva, R.J.N. Bottom-up uncertainty evaluation of complex measurements from correlated performance data: Determination of total Cr in yeast by ICP-MS after acid digestion. Food Chem. 2023, 404, 134466. [Google Scholar] [CrossRef]
- Lee, J.; Park, Y.-S.; Lee, H.-J.; Koo, Y.E. Microwave-assisted digestion method using diluted nitric acid and hydrogen peroxide for the determination of major and minor elements in milk samples by ICP-OES and ICP-MS. Food Chem. 2022, 373, 131483. [Google Scholar] [CrossRef]
- WHO. Background document for development of WHO guideline for drinking water quality. Version Public Rev. Issued WHO 2022, 9, 29. [Google Scholar]
- Kuplulu, O.; Iplikcioglu Cil, G.; Korkmaz, S.D.; Aykut, O.; Ozansoy, G. Determination of Metal Contamination in Seafood from the Black, Marmara, Aegean and Mediterranean Sea Metal Contamination in Seafood. J. Hell. Vet. Med. Soc. 2018, 69, 749–758. [Google Scholar] [CrossRef]
- Jovičić, K.; Nikolić, D.M.; Višnjić-Jeftić, Ž.; Đikanović, V.; Skorić, S.; Stefanović, S.M.; Lenhardt, M.; Hegediš, A.; Krpo-Ćetković, J.; Jarić, I. Mapping differential elemental accumulation in fish tissues: Assessment of metal and trace element concentrations in wels catfish (Silurus glanis) from the Danube River by ICP-MS. Environ. Sci. Pollut. Res. Int. 2015, 22, 3820–3827. [Google Scholar] [CrossRef]
- Choi, S.H.; Kim, J.Y.; Mi Choi, E.; Lee, M.Y.; Yang, J.Y.; Ho Lee, G.; Su Kim, K.; Yang, J.-S.; Russo, R.E.; Yoo, J.H.; et al. Heavy Metal Determination by Inductively Coupled Plasma—Mass Spectrometry (ICP-MS) and Direct Mercury Analysis (DMA) and Arsenic Mapping by Femtosecond (fs)—Laser Ablation (LA) ICP-MS in Cereals. Anal. Lett. 2019, 52, 496–510. [Google Scholar] [CrossRef]
- Thabit, T.M.A.M.; Shokr, S.A.; Elgeddawy, D.I.H.; El-Naggar, M.A.H. Determination of Heavy Metals in Wheat and Barley Grains Using ICP-MS/MS. J. AOAC Int. 2020, 103, 1277–1281. [Google Scholar] [CrossRef]
- Sel, S.; Koyuncu, İ. Microwave-Assisted Sample Preparation for Simultaneous Determination of Trace Elements in Vegan Foods with Inductively Coupled Plasma Mass Spectrometry. Food Anal. Methods 2025, 18, 428–441. [Google Scholar] [CrossRef]
- Jain, H.V.; Dhiman, S.; Ansari, N.G. Divulging the unexpected: Insight investigation of minor and toxic elements in diverse millet. Microchem. J. 2025, 213, 113636. [Google Scholar] [CrossRef]
- Fioroto, A.M.; Albuquerque, L.G.R.; Carvalho, A.A.C.; Oliveira, A.P.; Rodrigues, F.; Oliveira, P.V. Hydroponic growth test of maize sprouts to evaluate As, Cd, Cr and Pb translocation from mineral fertilizer and As and Cr speciation. Environ. Pollut. 2020, 262, 114216. [Google Scholar] [CrossRef] [PubMed]
- Silalahi, E.M.; Lioe, H.N.; Faridah, D.N. Heavy Metals Cd, Hg, and Pb in Fresh Milk from Dairy Farms in South Jakarta Analyzed by ICP-MS. Trop. Anim. Sci. J. 2023, 46, 502–508. [Google Scholar] [CrossRef]
- Pacquette, L.H.; Thompson, J.J.; Malaviole, I.; Zywicki, R.; Woltjes, F.; Ding, Y.; Mittal, A.; Ikeuchi, Y.; Sadipiralla, B.; Kimura, S.; et al. Minerals and Trace Elements in Milk, Milk Products, Infant Formula, and Adult/Pediatric Nutritional Formula, ICP-MS Method: Collaborative Study, AOAC Final Action 2015.06, ISO/DIS 21424, IDF 243. J. AOAC Int. 2018, 101, 536–561. [Google Scholar] [CrossRef] [PubMed]
- Başaran, B. An assessment of heavy metal level in infant formula on the market in Turkey and the hazard index. J. Food Compos. Anal. 2022, 105, 104258. [Google Scholar] [CrossRef]
- Balaram, V.; Copia, L.; Kumar, U.S.; Miller, J.; Chidambaram, S. Pollution of water resources and application of ICP-MS techniques for monitoring and management—A comprehensive review. Geosyst. Geoenviron. 2023, 2, 100210. [Google Scholar] [CrossRef]
- Alhagri, I.A.; Al-Hakimi, A.N.; Al-Hazmy, S.M.; Albadri, A.E. Determination of trace and heavy metals in bottled drinking water in Yemen by ICP-MS. Results Chem. 2024, 8, 101558. [Google Scholar] [CrossRef]
- Surucu, O. Trace determination of heavy metals and electrochemical removal of lead from drinking water. Chem. Pap. 2021, 75, 4227–4238. [Google Scholar] [CrossRef]
- İslamoğlu, A.H.; Kahvecioğlu, T.; Bönce, G.; Gedik, E.; Güneş, F. Determination of heavy metals in some fruits, vegetables and fish by ICP-MS. Eurasian J. Food Sci. Technol. 2021, 5, 67–76. [Google Scholar]
- İzol, E.; Kaya, E.; Karahan, D. Investigation of some metals in honey samples produced in Different Regions of Turkey’s Bingöl province by ICP-MS. Mellifera 2021, 21, 1–17. [Google Scholar]
- Scivicco, M.; Squillante, J.; Velotto, S.; Esposito, F.; Cirillo, T.; Severino, L. Dietary exposure to heavy metals through polyfloral honey from Campania region (Italy). J. Food Compos. Anal. 2022, 114, 104748. [Google Scholar] [CrossRef]
- Limbeck, A.; Galler, P.; Bonta, M.; Bauer, G.; Nischkauer, W.; Vanhaecke, F. Recent advances in quantitative LA-ICP-MS analysis: Challenges and solutions in the life sciences and environmental chemistry. Anal. Bioanal. Chem. 2015, 407, 6593–6617. [Google Scholar] [CrossRef]
- Wang, H.A.O.; Grolimund, D.; Giesen, C.; Borca, C.N.; Shaw-Stewart, J.R.H.; Bodenmiller, B.; Günther, D. Fast Chemical Imaging at High Spatial Resolution by Laser Ablation Inductively Coupled Plasma Mass Spectrometry. Anal. Chem. 2013, 85, 10107–10116. [Google Scholar] [CrossRef]
- Cruz-Alonso, M.; Lores-Padín, A.; Valencia, E.; González-Iglesias, H.; Fernández, B.; Pereiro, R. Quantitative mapping of specific proteins in biological tissues by laser ablation–ICP-MS using exogenous labels: Aspects to be considered. Anal. Bioanal. Chem. 2019, 411, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Fernández, B.; Rodríguez-González, P.; García Alonso, J.I.; Malherbe, J.; García-Fonseca, S.; Pereiro, R.; Sanz-Medel, A. On-line double isotope dilution laser ablation inductively coupled plasma mass spectrometry for the quantitative analysis of solid materials. Anal. Chim. Acta 2014, 851, 64–71. [Google Scholar] [CrossRef]
- Michaliszyn, L.; Ren, T.; Röthke, A.; Rienitz, O. A new method for the SI-traceable quantification of element contents in solid samples using LA-ICP-MS. J. Anal. At. Spectrom. 2020, 35, 126–135. [Google Scholar] [CrossRef]
- Pollak-Kowa, E.; Telk, A.; Wieczorek, M. Evaluating solid standards for LA-ICP-MS quantitative imaging of organisms with calcareous skeletons: Accuracy, homogeneity, and laser wavelength effects. Analyst 2025, 12, 2564–2573. [Google Scholar] [CrossRef]
- Neff, C.; Becker, P.; Hattendorf, B.; Günther, D. LA-ICP-MS using a nitrogen plasma source. J. Anal. At. Spectrom. 2021, 36, 1750–1757. [Google Scholar] [CrossRef] [PubMed]
- Rua-Ibarz, A.; Van Acker, T.; Bolea-Fernandez, E.; Boccongelli, M.; Vanhaecke, F. A comparison of calibration strategies for quantitative laser ablation ICP-mass spectrometry (LA-ICP-MS) analysis of fused catalyst samples. J. Anal. At. Spectrom. 2024, 39, 888–899. [Google Scholar] [CrossRef]
- Pisonero, J.; Fernández, B.; Günther, D. Critical revision of GD-MS, LA-ICP-MS and SIMS as inorganic mass spectrometric techniques for direct solid analysis. J. Anal. At. Spectrom. 2009, 24, 1145–1160. [Google Scholar] [CrossRef]
- Pozebon, D.; Scheffler, G.L.; Dressler, V.L. Recent applications of laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS) for biological sample analysis: A follow-up review. J. Anal. At. Spectrom. 2017, 32, 89–919. [Google Scholar] [CrossRef]
- Yamaji, N.; Ma, J.F. Bioimaging of multiple elements by high-resolution LA-ICP-MS reveals altered distribution of mineral elements in the nodes of rice mutants. Plant J. Cell Mol. Biol. 2019, 99, 1254–1263. [Google Scholar] [CrossRef]
- Pereira, C.K.; Neves, V.M.; Hidrich, G.M.; Faccin, H.; Pozebon, D.; Dressler, V.L. Imaging of Elements Distribution in Rice by Laser Ablation Inductively Coupled Plasma Mass Spectrometry. Braz. J. Anal. Chem 2023, 10, 79–91. [Google Scholar] [CrossRef]
- Thyssen, G.M.; Keil, C.; Wolff, M.; Sperling, M.; Kadow, D.; Haase, H.; Karst, U. Bioimaging of the elemental distribution in cocoa beans by means of LA-ICP-TQMS. J. Anal. At. Spectrom. 2018, 33, 187–194. [Google Scholar] [CrossRef]
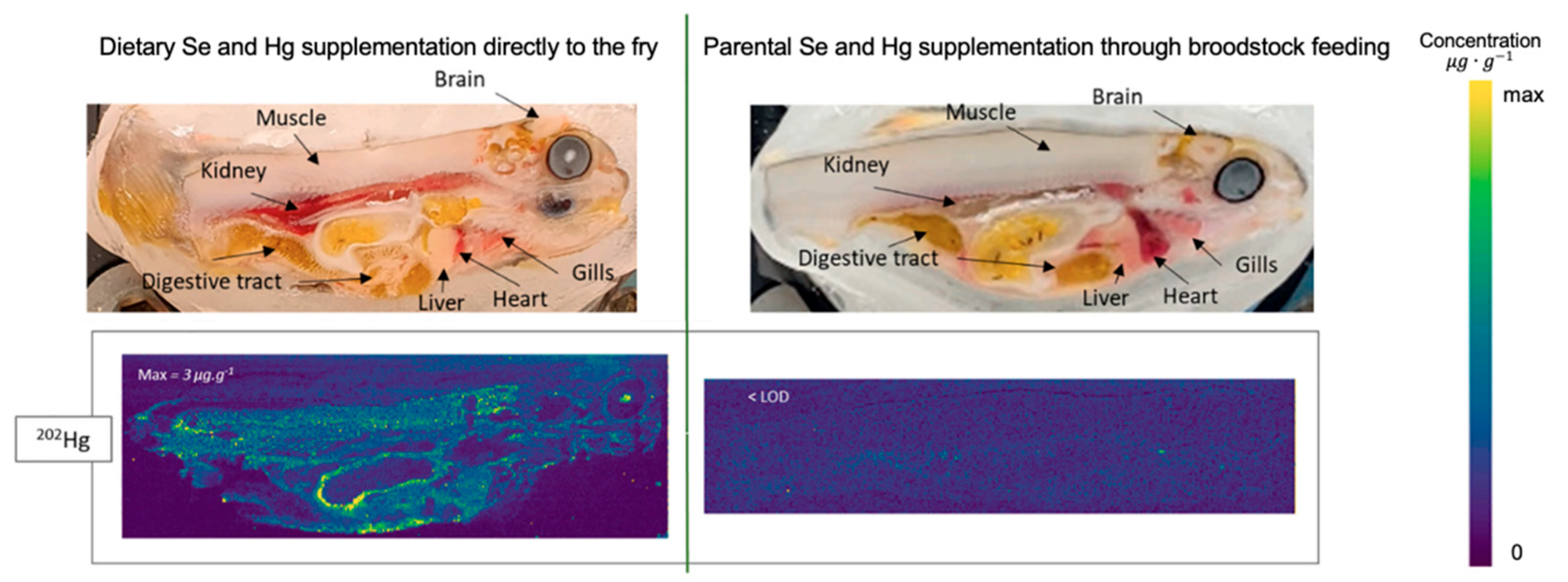
- Labeyrie, L.; Vallverdu, G.S.; Michau, D.; Fontagné-Dicharry, S.; Mounicou, S. Development of polymer films and biological matrices standards for selenium, mercury and endogenous elements quantitative LA-ICP MS imaging in entire rainbow trout fry. Microchem. J. 2023, 194, 109204. [Google Scholar] [CrossRef]
- Liu, J.; Cui, J.; Wei, X.; Li, W.; Liu, C.; Li, X.; Chen, M.; Fan, Y.; Wang, J. Investigation on selenium and mercury interactions and the distribution patterns in mice organs with LA-ICP-MS imaging. Anal. Chim. Acta 2021, 1182, 338941. [Google Scholar] [CrossRef] [PubMed]
- Najafabadipour, A.; Hassanzadeh, F.; Kordestani, M. Advanced deep learning models for predicting elemental concentrations in iron ore mine using XRF data: A cost-effective alternative to ICP-MS methods. Environ. Geochem. Health 2025, 47, 104. [Google Scholar] [CrossRef] [PubMed]
- Evans, E.H.; Pisonero, J.; Smith, C.M.; Taylor, R.N. Atomic spectrometry update: Review of advances in atomic spectrometry and related techniques. J. Anal. At. Spectrom. 2021, 36, 868–891. [Google Scholar] [CrossRef]
- Herwig, N.; Stephan, K.; Panne, U.; Pritzkow, W.; Vogl, J. Multi-element screening in milk and feed by SF-ICP-MS. Food Chem. 2011, 124, 1223–1230. [Google Scholar] [CrossRef]
- Ogra, Y. Development of miniaturized HPLC-ICP-MS for speciation of bio-trace elements. Biomed. Res. Trace Elem. 2008, 19, 34–42. [Google Scholar]
- Pungjunun, K.; Praphairaksit, N.; Chailapakul, O. A facile and automated microfluidic electrochemical platform for the in-field speciation analysis of inorganic arsenic. Talanta 2023, 265, 124906. [Google Scholar] [CrossRef] [PubMed]






| Categorization | Technical Name | Applicable Samples/Scenarios | Advantages | Limitations | References | |
|---|---|---|---|---|---|---|
| Conventional sample preparation process | Dry ashing | High-organic samples (biological tissues and plant materials) | Minimal reagent use; low contamination risk | Loss of volatile elements; analyte adsorption on crucible surfaces | - | |
| Acid digestion | Complex matrices (environmental solids and food) | Handles organic/inorganic components, short time; less volatile loss | Risk of contamination; matrix effects from residual salts/acids may require dilution | - | ||
| Improved methods | ||||||
| Eco-friendly optimization | NADES (natural deep-eutectic solvents)–ultrasonic/microwave-assisted extraction | Toxic elements (As, Cd, and Pb) in food/plant samples | Biodegradable; nitric acid-free; high recovery; low environmental burden | Requires water content optimization to reduce viscosity | [21] | |
| Microwave–ultraviolet degradation (MW-UV) | Cereals (rice and wheat) | Reduced reagent toxicity; low residual carbon/acidity; digestate directly analyzable | Requires controlled radiation conditions | [22] | ||
| Enzymatic digestion | Food matrices | Avoids strong acids, reduces waste/volatile loss; aligns with atom economy | Enzyme activity sensitive to pH/temperature | [23] | ||
| Complex matrix optimization | Enzyme-ultrasonication-dilution | High-fat (cooking oil); high-fiber (spinach) | Prevents nanoparticle dissolution/agglomeration; reduces salt/protein/fat interference | Dilution may reduce sensitivity; requires optimization | [24] | |
| Hot water extraction–As speciation preservation | Seafood, cereals, and vegetables | Preserves As speciation; As(III) recovery up to 93–97% | Requires precise temperature/time control | [25] | ||
| Enzyme-extraction-graded filtration | Wheat, flour, and high-fat samples (butter cookies) | Separates micron/submicron particles; ethanol pretreatment prevents membrane fouling | Multi-step process; complex handling | [26] | ||
| Interference mitigation | Non-instrumental methods | Dilution | Universal | Simple, rapid matrix effect reduction | Reduces target sensitivity; may introduce impurities | - |
| Microextraction (LPME/SPME/MSPE) | Biological samples | Efficient matrix removal; automated operation | Requires optimized adsorbents | [27] | ||
| Introduction techniques | Electrothermal vaporization (ETV) | Calcium phosphate-based bio-matrices | Avoids co-evaporation; reduces memory effects | Requires optimized carrier gas flow/heating program | [28] | |
| Chemical vapor generation (CVG) | Hg and As in complex matrices | Eliminates spectral/non-spectral interferences | Limited to volatile elements | [29] | ||
| Membrane desolvation (MD) | Vegetable oils | Reduces interferences under non-thermal conditions; enhances solvent recovery | Membrane stability critical | [30] | ||
| Instrumental improvements | Cold plasma–collision cell (KED) | High-salt/organic matrices | Direct interference elimination without mathematical correction | Requires high RF power | [31] | |
| Gas modification | Gaseous metal compounds | Eliminates polyatomic/non-reactive gas interferences | Requires gas exchange device (GED) | [32] | ||
| High-resolution ICP-MS (HR-ICP-MS) | Complex biological matrices (e.g., serum) | Interference-free quantification combined with ultrafiltration | High instrument cost | [33] | ||
| Emerging techniques | Tandem ICP-MS | Ultra-trace analysis (LOD: ng/L level) | High efficiency/accuracy; ideal for nanomaterial quantification | Requires optimization of reaction gases | [34] | |
| Digestion Reagent Combination | Typical Recovery Target Range | Potential Issues Affecting Recovery | Reference |
|---|---|---|---|
| HNO3 (pure or primary) | 85–115% | Low recovery of refractory elements; incomplete digestion of some species (e.g., Cr3+) | [35] |
| HNO3 + H2O2 | 85–115% | Impurities in H2O2 may cause contamination; vigorous reaction: risk of splattering/loss | |
| HNO3 + HCl (Aqua Regia) | 80–110% | Cl− interference: severe polyatomic interferences (e.g., ArCl+ interferes with 75As); insoluble chloride precipitation: Ag+, Pb2+ (PbCl2), and Hg+ (Hg2Cl2); enhanced matrix effects from residual Cl− | |
| HNO3 + HCl + HF (Inverse Aqua Regia) | 75–110% | F−/Cl− interference; high corrosivity (requires specialized vessels); insoluble fluoride precipitation; residual HF damages instrumentation and is hazardous |
| Morphology of As | Food Categories | Concentration Range (mg/kg) | Detection Limit (mg/kg) | Recovery Rate (%) | Key Findings and Method Performance | References |
|---|---|---|---|---|---|---|
| Total As | Seafood | >10 | 0.05 (ICP-MS) | 92–105 | Exceeds safety limits; validated via CRM DORM-4 | [42] |
| Algae | 5–120 * | 0.03 (ICP-MS) | 85–98 | * Eutrophic conditions increase levels by 3–5×; CRM NIES-19 verified | [44] | |
| iAs | Algae | 0.5–35 | 0.01 (HPLC-ICP-MS) | 88–102 | Converts inorganic to organic forms; spike recovery: 94 ± 6% | [44] |
| Cereals | 0.02–0.35 | 0.005 (HPLC-ICP-MS) | 90–104 | Dominant in rice (60–80% of total As); inter-lab RSD <15% | [43] | |
| As5+ | Seafood | <0.1 | 0.008 (HPLC-ICP-MS) | 84–97 | Minor oxidation state; co-elution resolved via anion-exchange | [43] |
| AsB | Seafood | 1–85 | 0.005 (HPLC-ICP-MS) | 95–108 | >90% total As; quantified using CRM BCR-627 | [43] |
| DMA | Seafood | <0.05 | 0.003 (HPLC-ICP-MS) | 89–101 | Trace levels; separation confirmed with ESI-MS | [43] |
| Cereals | 0.01–0.20 | 0.004 (HPLC-ICP-MS) | 91–106 | Major form in wheat (30–50% total As); precision RSD 8.2% | [46] | |
| MMA | Cereals | <0.01 | 0.002 (HPLC-ICP-MS) | 86–99 | Rarely detected; LOD verified via NIST SRM 1568b | [46] |
| Morphology of Pb | Food Categories | Sources/Sites | Concentration Range (mg/kg) | Detection Limit (mg/kg) | Recovery Rate (%) | Pollution Mechanisms and Risks | Reference |
|---|---|---|---|---|---|---|---|
| Total Pb | Plant-based food | Crops | 0.15–2.3 Limit exceedance: 33% | 0.002 (ICP-MS) | 88–102 | Soil adsorption predominates, with 33% of wheat Pb exceeding the limit in industrial areas (exceeding the Chinese limit) | [51] |
| Animal products | Meat/seafood | 0.02–0.45 | 0.001 (ICP-MS) | 91–106 | Enrichment through the food chain with high bio-availability | ||
| Particulate Pb | Cereals | Wheat bran | 1.8–12.5 (Industrial = 3.5× transport zones) | 0.005 (μ-XRF: micro-X-ray fluorescence) | 85–98 | Atmospheric deposition of PbSO4 particles directly adsorbed, accounting for 56% of the bran lead source | |
| Soluble Pb2+ | Cereals | Wheat flour | 0.05–0.82 | 0.003 (AAS) | 89–104 | Highly efficient transfer of foliar absorption (leaf→seed); high water solubility and bio-availability | |
| Soil-bound Pb | Cereal roots | Wheat root | 0.8–3.1 | 0.01 (XANES: X-ray absorption near-edge structure) | 82–96 | Root uptake less efficient than atmospheric deposition pathway | |
| Organic Pb | All foods | <0.001 (TEL: Tetraethyl lead) | 0.0001 (GC-MS) | 75–88 | Low natural presence and low pollution contribution | ||
| Morphology of Cd | Food Categories | Concentration Range | LOD (mg/kg) | Recovery (%) | References |
|---|---|---|---|---|---|
| Non-residual Cd (exchange + carbonate bound) | Marine sediment | 12–85 mg/kg 80–90% of total Cd | 0.05 (BCR-SEP: BCR–sequential extraction protocol) | 92–105 | [56] |
| Cd-MTs | Animal offal | 0.5–8.7 mg/kg Liver/kidney enrichment | 0.01 (HPLC-ICP-MS) | 85–98 | [57] |
| Globulin/Albumin-bound Cd | Rice seed | Binding capacity: globulin > albumin > glutamate > alcohol-soluble proteins | 0.003 (SEC-ICP-MS: size exclusion chromatography–ICP-MS) | 88–102 | [59] |
| Gluten-bound Cd | Rice endosperm | 0.15–2.1 mg/kg Primary residue form | 0.002 (SEC-ICP-MS) | 90–104 | [51] |
| Free Cd2+ | Plant-based food | 0.01–0.8 mg/kg | 0.001 (DPASV: differential pulse anodic stripping voltammetry) | 94–108 | [55] |
| Particulate Cd (CdS/CdO) | Contaminated area food | 0.3–6.5 mg/kg | 0.02 (μ-XRF) | 78–92 | [55] |
| Morphology of Hg | Food Categories | Sources/Mechanisms | Concentration Range | LOD | Recovery (%) | References |
|---|---|---|---|---|---|---|
| CH3Hg+ | Fish/aquaculture | Food chain bio-accumulation | 0.1–1.8 mg/kg >80% total Hg | 0.003 (GC-ICP-MS: gas chromatography-ICP-MS) | 92–107 | [63] |
| Hg2+ | Rice/sediment | Contaminated soil migration | 0.02–0.35 mg/kg <20% total Hg | 0.005 (CV-AAS: cold vapor–atomic absorption spectrometry) | 85–98 | [65] |
| PhHg+ | Food from industrially contaminated areas | Pesticide/fungicide residues | <0.01 mg/kg | 0.001 (HPLC-CV-AFS: HPLC–cold vapor–atomic fluorescence spectrometry) | 75–90 | [64] |
| Hg0 | Processed food | Equipment contamination | <0.001 mg/kg | 0.0003 (Au-amalgamation CV-AAS) | 65–82 | [66] |
| Heterogeneous accumulation of Hg | Algae | Single-cell uptake differences | Macro-algae: 0.05–0.8 mg/kg Micro-algae: 0.2–1.5 mg/kg | 0.002 (CV-AAS) | 88–103 | [66] |
| Elements | Rural Water | Well Water | Urban Water | BS 6920 Limit | Reference |
|---|---|---|---|---|---|
| Al | 1.725 ± 0.360 | 2.456 ± 0.140 | 2.247 ± 0.260 | 200.00 | [85] |
| Cr | 1.854 ± 0.010 | 0.092 ± 0.010 | 2.006 ± 0.018 | 50.00 | |
| Mn | <LSL | 16.711 ± 0.008 | 0.156 ± 0.080 | 50.00 | |
| Fe | <LSL | 1.608 ± 0.200 | 1.766 ± 0.320 | 200.00 | |
| Ni | <LSL | 0.570 ± 0.004 | 0.857 ± 0.071 | 20.00 | |
| Se | <LSL | 0.283 ± 0.120 | 0.393 ± 0.130 | 10.00 | |
| Cd | <LSL | 0.592 ± 0.007 | <LSL | 5.00 | |
| Sb | 0.967 ± 0.015 | 3.082 ± 0.014 | 1.306 ± 0.019 | 5.00 | |
| Ba | 28.790 ± 0.008 | 226.597 ± 0.001 | 122.120 ± 0.012 | 1000.00 | |
| Pb | 0.290 ± 0.160 | 18.732 ± 0.310 | 0.897 ± 0.061 | 25.00 |
| Food Categories | Specific Foods/Samples | Main Metal Elements of Concern | References |
|---|---|---|---|
| Seafood | Fish, shellfish, and shrimp from the four seas of Turkey (13 species) | Pb, Cd, Hg, As | [73] |
| Sturgeon | Pb, Cd, Cu, Fe, Se, Hg | [74] | |
| Cereals | Barley, oats, millet, corn, sorghum, Job’s tears | Pb, Cd, Hg, As | [75] |
| Oat milk | Pb, Cd, As | [76] | |
| Diverse millet | Pb, Cd, Hg, As, Al | [78] | |
| Corn seedling (hydroponic experiment) | Pb, Cd, As | [77] | |
| Dairy | Fresh milk (South Jakarta traditional farm) | Pb, Cd, Hg, As | [80] |
| Infant formula (multi-brand) | Pb, Cd, Hg, As | [81,82] | |
| Potable water | Bottled drinking water | Hg | [84] |
| Rural water | Pb, As | [85] | |
| Well water | Pb | ||
| Vegetables | Spinach | Pb, Cd | [86] |
| Carrot | - | ||
| Potato | - | ||
| Honey | Turkish honey | - | [87] |
| Italian honey | Cr, As, Ni | [88] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, M.; Li, X. Quantitative Detection of Toxic Elements in Food Samples by Inductively Coupled Plasma Mass Spectrometry (ICP-MS). Processes 2025, 13, 3361. https://doi.org/10.3390/pr13103361
Huang M, Li X. Quantitative Detection of Toxic Elements in Food Samples by Inductively Coupled Plasma Mass Spectrometry (ICP-MS). Processes. 2025; 13(10):3361. https://doi.org/10.3390/pr13103361
Chicago/Turabian StyleHuang, Mengtian, and Xin Li. 2025. "Quantitative Detection of Toxic Elements in Food Samples by Inductively Coupled Plasma Mass Spectrometry (ICP-MS)" Processes 13, no. 10: 3361. https://doi.org/10.3390/pr13103361
APA StyleHuang, M., & Li, X. (2025). Quantitative Detection of Toxic Elements in Food Samples by Inductively Coupled Plasma Mass Spectrometry (ICP-MS). Processes, 13(10), 3361. https://doi.org/10.3390/pr13103361







