Extraction pH Controls Assessed Biotoxicity of Chlorination Disinfection Byproducts from Amphoteric Precursors
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Experimental Procedures
2.2.1. Evaluation of Chlorination Kinetics
2.2.2. Quantitation of Aliphatic DBPs
2.2.3. Assessment of Biotoxicity
2.3. Analytical Methods
2.3.1. Analysis of Residual Precursors
2.3.2. Analysis of Aliphatic DBPs
2.3.3. Analysis of Biotoxicity
3. Results and Discussions
3.1. Chlorination Kinetics of Three Amphoteric Precursors
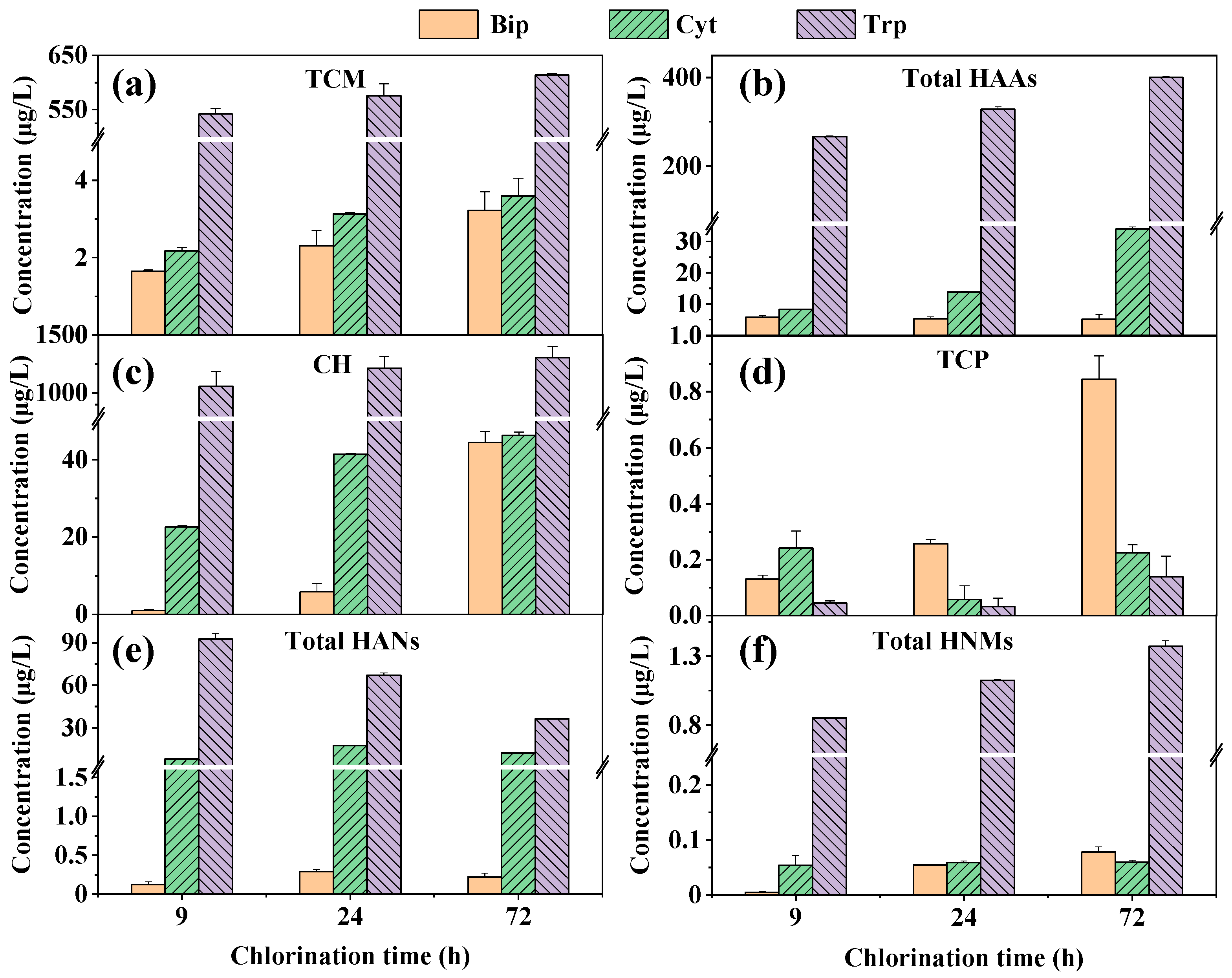
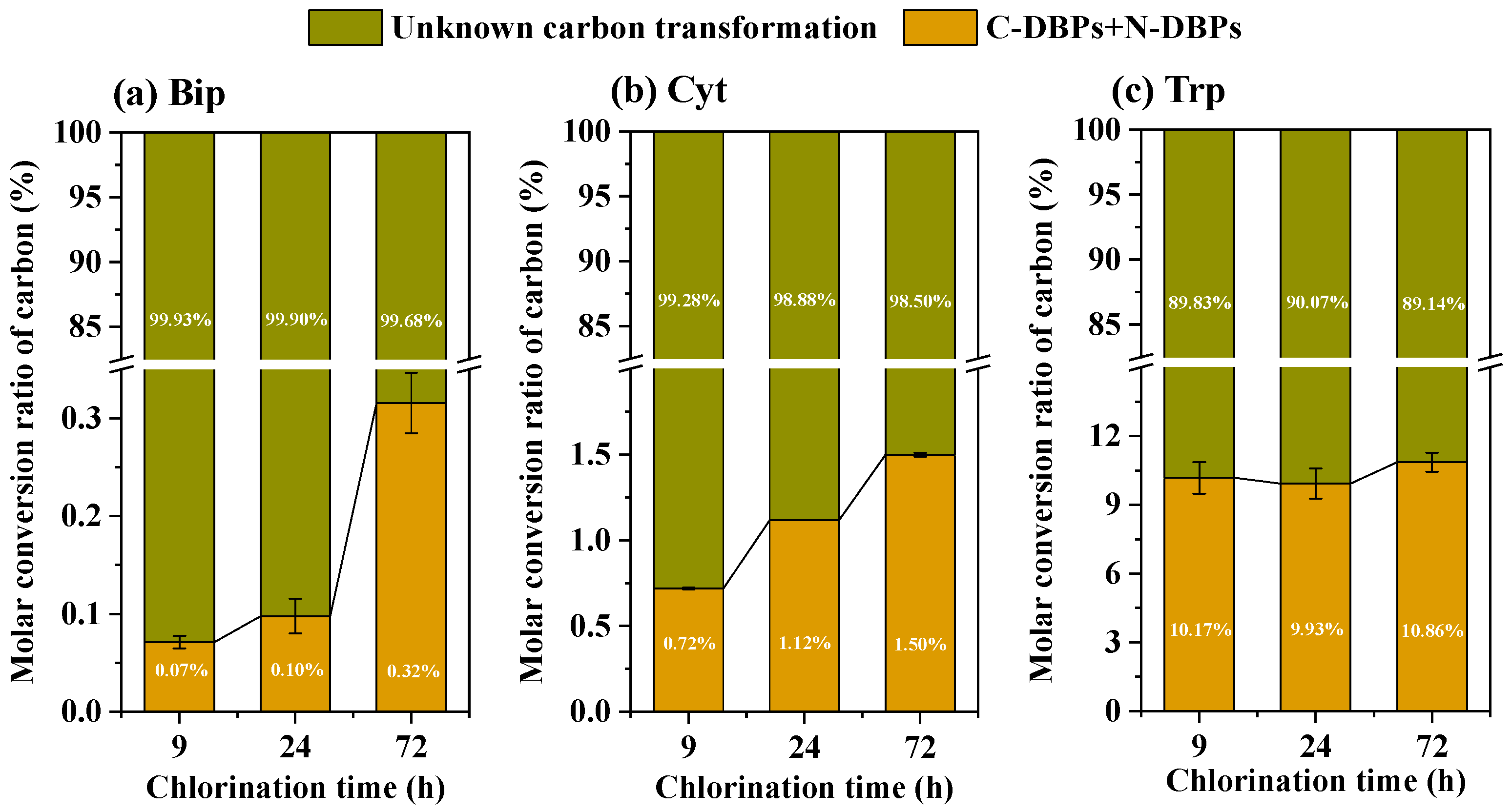
3.2. DBP Formation During Chlorination of Three Amphoteric Precursors
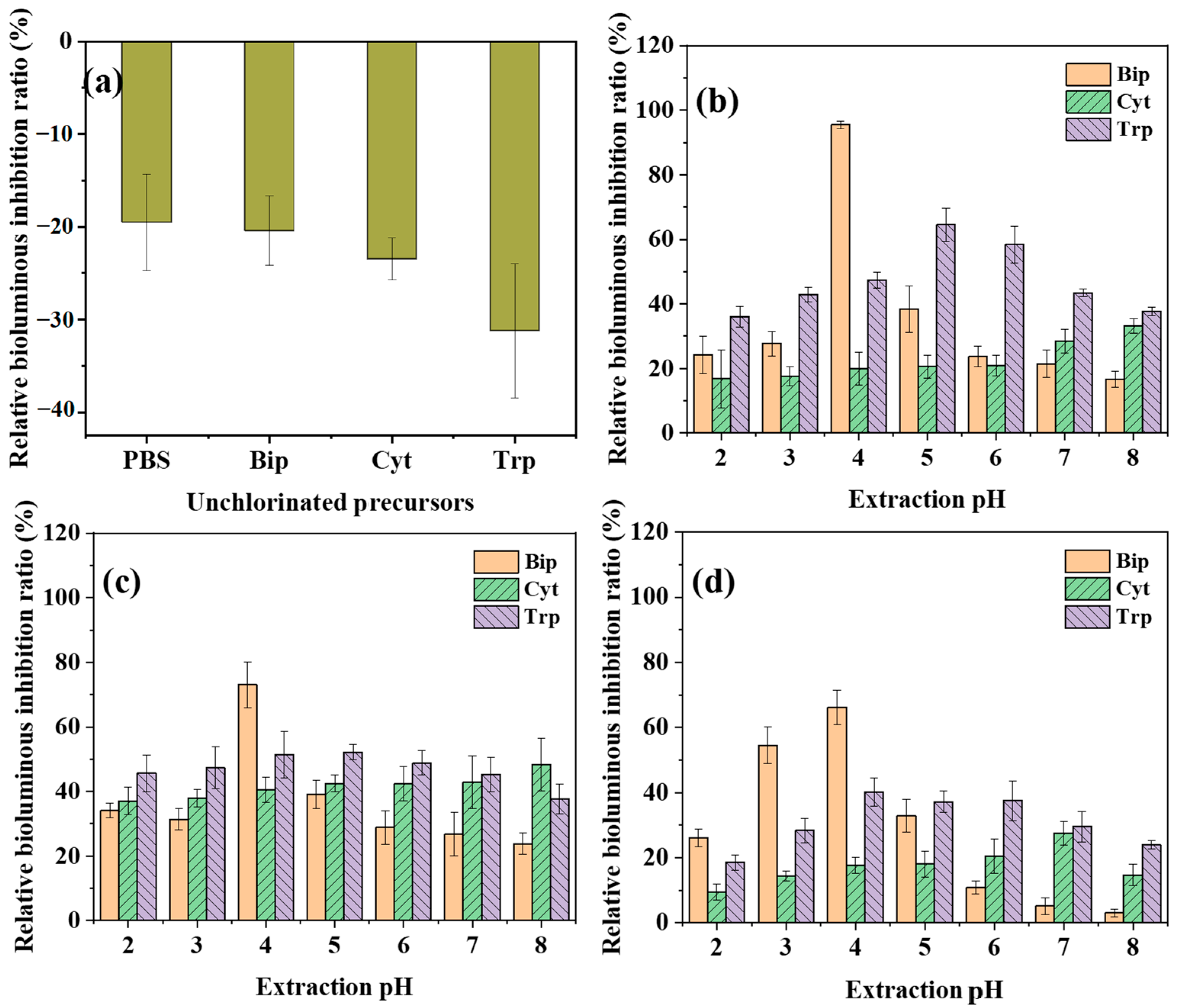
3.3. Biotoxicity of Extracts Related to Extraction pH During Chlorination of Three Amphoteric Precursors
4. Conclusions
- (1)
- The amphoteric precursors Bip, Cyt, and Trp were highly reactive with chlorine, readily forming a range of aliphatic DBPs, with formation yields following the order of Trp > Cyt > Bip.
- (2)
- A critical disconnect existed between the quantity of measured aliphatic DBPs and the biotoxicity of extracted non-volatile DBPs. Bip, which produced the lowest mass of monitored aliphatic DBPs, generated a mixture of non-volatile TPs that exhibited the highest biotoxicity.
- (3)
- Extraction pH was a variable that cannot be overlooked in the biotoxicity assessment of DBPs derived from amphoteric precursors. The measured toxicity of chlorinated extracts was profoundly pH-dependent, and the optimal pH for recovering the most potent toxic species varied significantly among the precursors.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, X.-F.; Mitch, W.A. Drinking Water Disinfection Byproducts (DBPs) and Human Health Effects: Multidisciplinary Challenges and Opportunities. Environ. Sci. Technol. 2018, 52, 1681–1689. [Google Scholar] [CrossRef]
- Hua, G.; Kim, J.; Reckhow, D.A. Disinfection Byproduct Formation from Lignin Precursors. Water Res. 2014, 63, 285–295. [Google Scholar] [CrossRef]
- Chuang, Y.H.; Lin, A.Y.; Wang, X.H.; Tung, H.H. The Contribution of Dissolved Organic Nitrogen and Chloramines to Nitrogenous Disinfection Byproduct Formation from Natural Organic Matter. Water Res. 2013, 47, 1308–1316. [Google Scholar] [CrossRef]
- Evlampidou, I.; Font-Ribera, L.; Rojas-Rueda, D.; Gracia-Lavedan, E.; Costet, N.; Pearce, N.; Vineis, P.; Jaakkola, J.J.K.; Delloye, F.; Makris, K.C.; et al. Trihalomethanes in Drinking Water and Bladder Cancer Burden in the European Union. Environ. Health Persp. 2020, 128, 017001. [Google Scholar] [CrossRef]
- Diana, M.; Felipe-Sotelo, M.; Bond, T. Disinfection Byproducts Potentially Responsible for the Association between Chlorinated Drinking Water and Bladder Cancer: A review. Water Res. 2019, 162, 492–504. [Google Scholar] [CrossRef]
- Aslani, H.; Hosseini, M.-S.; Mohammadi, S.; Naghavi-Behzad, M. Drinking Water Disinfection By-products and Their Carcinogenicity, A Review of Unseen Crisis. Int. J. Cancer Manag. 2019, 12, e88930. [Google Scholar] [CrossRef]
- Wu, Y.; Wei, W.; Luo, J.; Pan, Y.; Yang, M.; Hua, M.; Chu, W.; Shuang, C.; Li, A. Comparative Toxicity Analyses from Different Endpoints: Are New Cyclic Disinfection Byproducts (DBPs) More Toxic than Common Aliphatic DBPs? Environ. Sci. Technol. 2022, 56, 194–207. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Zhang, X.; Jiang, J.; Li, W. How Much of the Total Organic Halogen and Developmental Toxicity of Chlorinated Drinking Water Might Be Attributed to Aromatic Halogenated DBPs? Environ. Sci. Technol. 2021, 55, 5906–5916. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Guo, D.; Yao, W.; Wang, X.; Yang, H.; Xie, Y.F.; Komarneni, S.; Yu, G.; Wang, Y. Effects of Conventional Ozonation and Electro-peroxone Pretreatment of Surface Water on Disinfection By-product Formation during Subsequent Chlorination. Water Res. 2018, 130, 322–332. [Google Scholar] [CrossRef]
- Cheng, S.; Wu, Y.-P.; Young, T.R.; Dodd, M.C.; Wu, J.; Zhang, H.; Huo, Z.-L.; Qian, Y.-T.; Li, Y.; Li, W.-T.; et al. Rapid Determination of Trace Haloacetic Acids in Water and Wastewater using Non-suppressed Ion Chromatography with Electrospray Ionization-tandem Mass Spectrometry. Sci. Total Environ. 2021, 754, 142297. [Google Scholar] [CrossRef]
- Kalita, I.; Kamilaris, A.; Havinga, P.; Reva, I. Assessing the Health Impact of Disinfection Byproducts in Drinking Water. ACS ES&T Water 2024, 4, 1564–1578. [Google Scholar] [CrossRef] [PubMed]
- Kali, S.; Khan, M.; Ghaffar, M.S.; Rasheed, S.; Waseem, A.; Iqbal, M.M.; Bilal Khan Niazi, M.; Zafar, M.I. Occurrence, Influencing Factors, Toxicity, Regulations, and Abatement Approaches for Disinfection By-products in Chlorinated Drinking Water: A Comprehensive Review. Environ. Pollut. 2021, 281, 116950. [Google Scholar] [CrossRef]
- Li, W.; Han, J.; Zhang, X.; Chen, G.; Yang, Y. Contributions of Pharmaceuticals to DBP Formation and Developmental Toxicity in Chlorination of NOM-containing Source Water. Environ. Sci. Technol. 2023, 57, 18775–18787. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Zhang, X. Evaluating the Comparative Toxicity of DBP Mixtures from Different Disinfection Scenarios: A New Approach by Combining Freeze-Drying or Rotoevaporation with a Marine Polychaete Bioassay. Environ. Sci. Technol. 2018, 52, 10552–10561. [Google Scholar] [CrossRef] [PubMed]
- Method 552.3 Determination of Haloacetic Acids and Dalapon in Drinking Water by Liquid-Liquid Microextraction, Derivatization, and Gas Chromatography with Electron Capture Detection, United States Environmental Protection Agency. 2003. Available online: https://onesearch.library.wwu.edu/discovery/fulldisplay?docid=alma9993042367301453&context=L&vid=01ALLIANCE_WWU:WWU&lang=en&search_scope=DN_and_CI&adaptor=Local%20Search%20Engine&tab=Summit_Plus_Articles&query=sub,exact,Drinking%20water%20--%20Testing%20--%20Methodology&offset=0 (accessed on 15 September 2025).
- Tang, H.; Zhong, H.; Pan, Y.; Zhou, Q.; Huo, Z.; Chu, W.; Xu, B. A New Group of Heterocyclic Nitrogenous Disinfection Byproducts (DBPs) in Drinking Water: Role of Extraction pH in Unknown DBP Exploration. Environ. Sci. Technol. 2021, 55, 6764–6772. [Google Scholar] [CrossRef]
- Bond, T.; Mokhtar Kamal, N.H.; Bonnisseau, T.; Templeton, M.R. Disinfection By-product Formation from the Chlorination and Chloramination of Amines. J. Hazard. Mater. 2014, 278, 288–296. [Google Scholar] [CrossRef]
- Zhang, H.; Gao, P.; Liu, Y.; Du, Z.; Feng, L.; Zhang, L. Effects of Different Types of Nitrogen Sources in Water on the Formation Potentials of Nitrogenous Disinfection By-products in Chloramine Disinfection Process Based on Isotope Labeling. Sci. Total Environ. 2022, 842, 156692. [Google Scholar] [CrossRef]
- Sun, G.; Kaw, H.Y.; Zhou, M.; Guo, P.; Zhu, L.; Wang, W. Chlorinated Nucleotides and Analogs as Potential Disinfection Byproducts in Drinking Water. J. Hazard. Mater. 2023, 452, 131242. [Google Scholar] [CrossRef]
- Zuo, Y.; Cheng, S.; Han, Y.; Pu, L.; Du, E.; Peng, M.; Li, A.; Li, W. Chlorination of Biopterin in Water: Deciphering the Kinetics, Disinfection Byproducts, and Toxicity. Environ. Sci. Technol. 2024, 58, 20137–20146. [Google Scholar] [CrossRef]
- Siquerolo, L.V.; Ramos, R.M.B.; Monteiro, P.I.; Silveira, G.F.; Bassetti, F.d.J.; Coral, L.A.d.A. Impact of the UV/H2O2 Process on Assimilable Organic Carbon and Trihalomethane Formation in Cyanobacteria-Contaminated Waters. Processes 2025, 13, 23. [Google Scholar] [CrossRef]
- Tan, J.; Allard, S.; Gruchlik, Y.; McDonald, S.; Joll, C.A.; Heitz, A. Impact of bromide on halogen incorporation into organic moieties in chlorinated drinking water treatment and distribution systems. Sci. Total Environ. 2016, 541, 1572–1580. [Google Scholar] [CrossRef]
- Golaszewski, G.; Seux, R. The Kinetics of the Action of Chloroisocyanurates on Three Bacteria: Pseudomonas aeruginosa, Streptococcus faecalis and Staphylococcus aureus. Water Res. 1994, 28, 207–217. [Google Scholar] [CrossRef]
- Method 551.1 Determination of Chlorination Disinfection Byproducts, Chlorinated Solvents, and Halogenated Pesticides/Herbicides in Drinking Water by Liquid-Liquid Extraction and Gas Chromatography with Electron-Capture Detection, United States Environmental Protection Agency. 1995. Available online: https://www.epa.gov/sites/default/files/2015-06/documents/epa-551.1.pdf (accessed on 19 October 2025).
- Li, Y.; Zhang, X.; Shang, C. Effect of Reductive Property of Activated Carbon on Total Organic Halogen Analysis. Environ. Sci. Technol. 2010, 44, 2105–2111. [Google Scholar] [CrossRef]
- Hua, G.; Reckhow, D.A. Effect of Alkaline pH on the Stability of Halogenated DBPs. J. Am. Water Works Ass. 2012, 104, E107–E120. [Google Scholar] [CrossRef]
- Medici, A.; Luongo, G.; Di Fabio, G.; Zarrelli, A. Environmental Fate of Organic Sunscreens during Water Disinfection Processes: The Formation of Degradation By-Products and Their Toxicological Profiles. Molecules 2022, 27, 4467. [Google Scholar] [CrossRef]
- Lee, J.-S.; Hong, S.; Lee, J.; Choi, T.S.; Rhie, K.; Khim, J.S. Evaluation of Residual Toxicity of Hypochlorite-treated Water Using Bioluminescent Microbes and Microalgae: Implications for Ballast Water Management. Ecotox. Environ. Saf. 2019, 167, 130–137. [Google Scholar] [CrossRef]
- Wang, T.; Deng, L.; Tan, C.; Hu, J.; Singh, R.P. Comparative Analysis of Chlorinated Disinfection Byproducts Formation From 4-nitrophenol and 2-amino-4-nitrophenol During UV/ post-chlorination. Sci. Total Environ. 2024, 927, 172200. [Google Scholar] [CrossRef]
- Zhang, B.; Xian, Q.; Gong, T.; Li, Y.; Li, A.; Feng, J. DBPs Formation and Genotoxicity During Chlorination of Pyrimidines and Purines Bases. Chem. Eng. J. 2017, 307, 884–890. [Google Scholar] [CrossRef]
- Hua, L.-C.; Kim, E.; McCurry, D.L.; Huang, C.; Mitch, W.A. Novel Chlorination Byproducts of Tryptophan: Initial High-Yield Transformation Products versus Small Molecule Disinfection Byproducts. Environ. Sci. Technol. Lett. 2020, 7, 149–155. [Google Scholar] [CrossRef]
- Ding, S.; Wu, M.; Xiao, R.; Fang, C.; Wang, Q.; Xu, B.; Chu, W. Evaluation of N-acetylcysteine and Glutathione as Quenching Agents for the Analysis of Halogenated Disinfection By-products. J. Environ. Sci. 2022, 117, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Shen, Q.; Guo, W.; Peng, J.; Liang, Y. Precursors and nitrogen origins of trichloronitromethane and dichloroacetonitrile during chlorination/chloramination. Chemosphere 2012, 88, 25–32. [Google Scholar] [CrossRef]
- Shah, A.D.; Mitch, W.A. Halonitroalkanes, Halonitriles, Haloamides, and N-Nitrosamines: A Critical Review of Nitrogenous Disinfection Byproduct Formation Pathways. Environ. Sci. Technol. 2012, 46, 119–131. [Google Scholar] [CrossRef]
- Mazur, D.M.; Lebedev, A.T. Transformation of Organic Compounds during Water Chlorination/Bromination: Formation Pathways for Disinfection By-Products (A Review). J. Anal. Chem. 2022, 77, 1705–1728. [Google Scholar] [CrossRef]
- Huang, K.; MacKay, A.A. Microcystin-LR Degradation Kinetics during Chlorination: Role of Water Quality Conditions. Water Res. 2020, 185, 116305. [Google Scholar] [CrossRef]

| Precursors | Formula | Molecular Weight | Structures | pKa1 | pKa2 |
|---|---|---|---|---|---|
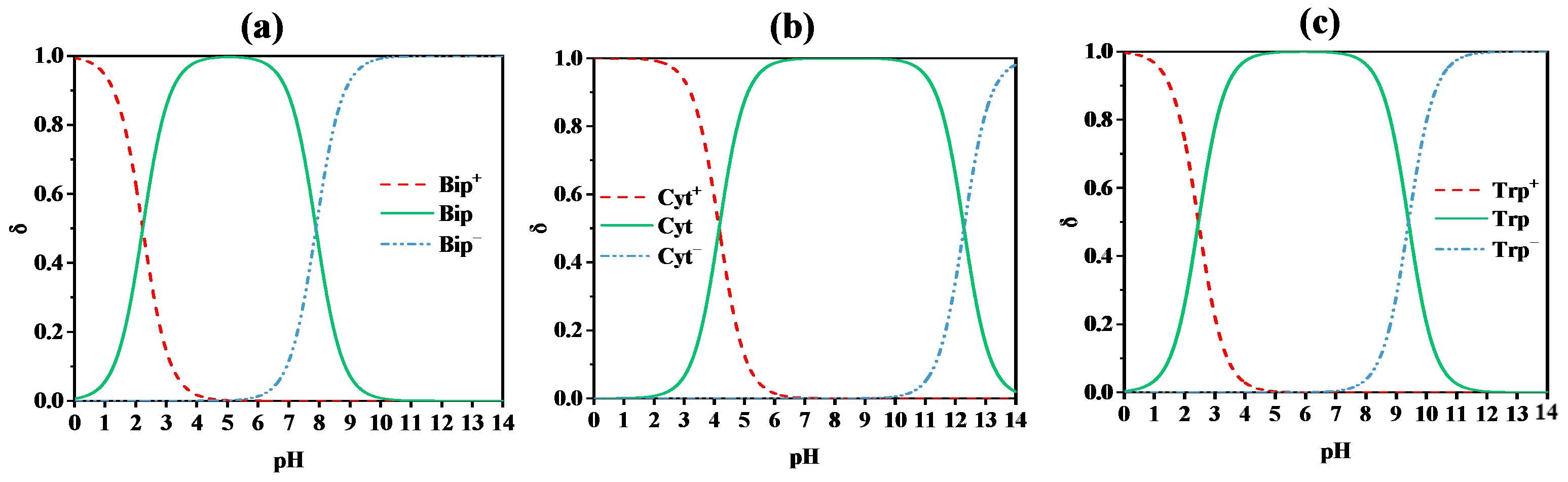
| Bip | C9H11N5O3 | 237.2 | 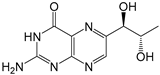 | 2.23 a | 7.89 a |
| Cyt | C4H5N3O | 111.1 | 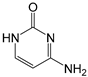 | 4.16 b | 12.28 b |
| Trp | C11H12N2O2 | 204.2 | 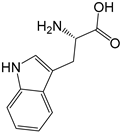 | 2.46 a | 9.41 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuo, Y.; Xu, S.; Wang, Z.; Zuo, J.; Fei, H.; Liu, H.; Bi, C.; Rui, G.; Cheng, S. Extraction pH Controls Assessed Biotoxicity of Chlorination Disinfection Byproducts from Amphoteric Precursors. Processes 2025, 13, 3355. https://doi.org/10.3390/pr13103355
Zuo Y, Xu S, Wang Z, Zuo J, Fei H, Liu H, Bi C, Rui G, Cheng S. Extraction pH Controls Assessed Biotoxicity of Chlorination Disinfection Byproducts from Amphoteric Precursors. Processes. 2025; 13(10):3355. https://doi.org/10.3390/pr13103355
Chicago/Turabian StyleZuo, Yanting, Senqi Xu, Zheng Wang, Jinhu Zuo, Hui Fei, Haolin Liu, Chenglu Bi, Guofen Rui, and Shi Cheng. 2025. "Extraction pH Controls Assessed Biotoxicity of Chlorination Disinfection Byproducts from Amphoteric Precursors" Processes 13, no. 10: 3355. https://doi.org/10.3390/pr13103355
APA StyleZuo, Y., Xu, S., Wang, Z., Zuo, J., Fei, H., Liu, H., Bi, C., Rui, G., & Cheng, S. (2025). Extraction pH Controls Assessed Biotoxicity of Chlorination Disinfection Byproducts from Amphoteric Precursors. Processes, 13(10), 3355. https://doi.org/10.3390/pr13103355






