Efficient Recovery of Lithium and Cobalt from Spent LCO Using Mechanochemical Activation and Ammoniacal Leaching
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Mechanochemical Activation
2.3. Ammonia Leaching Procedure
2.4. Analytical Methods
2.5. Thermodynamic Modeling
3. Results and Discussion
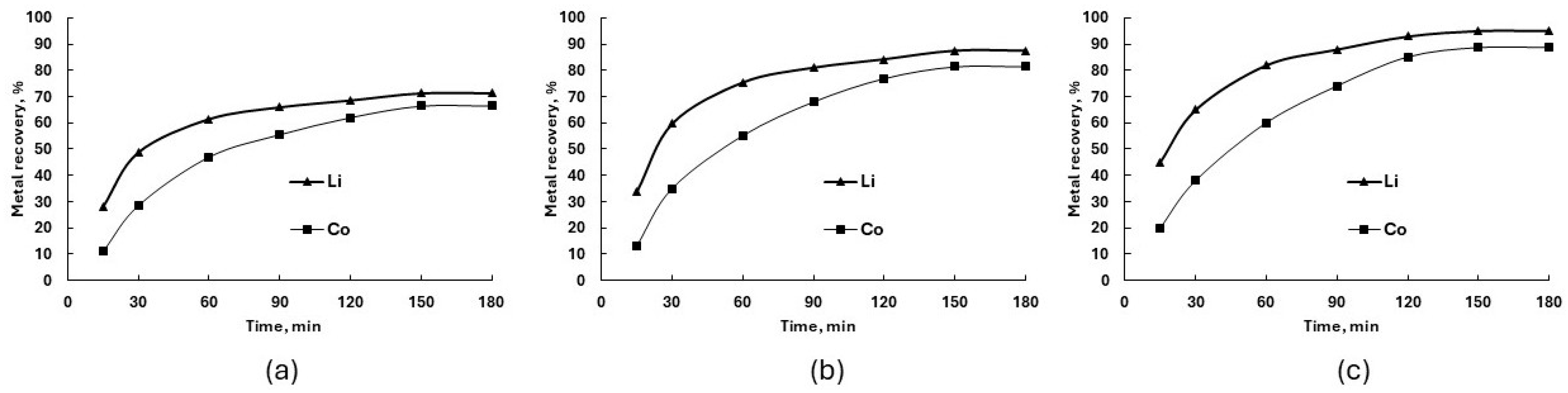
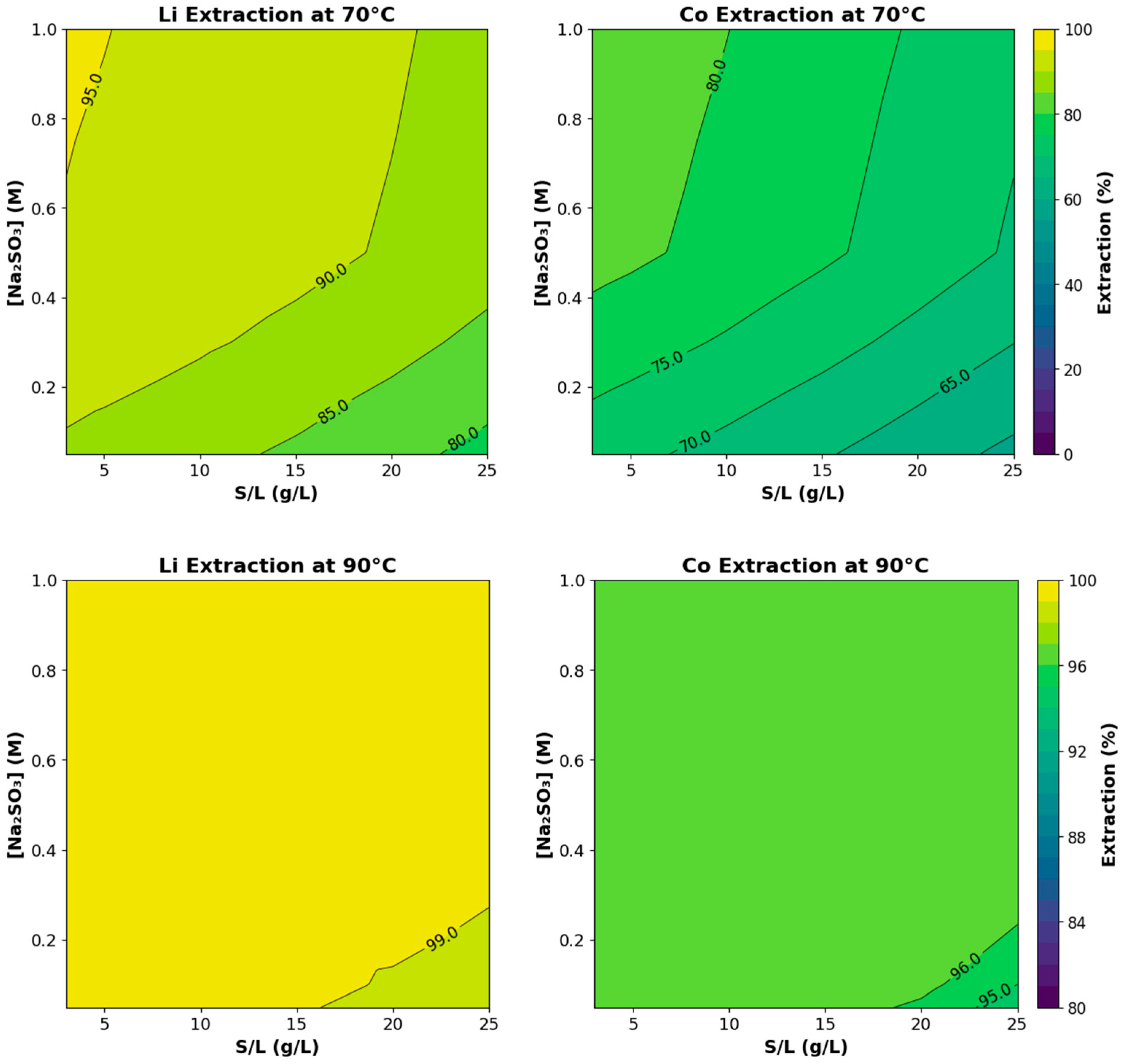
3.1. Ammonia Leaching Behavior
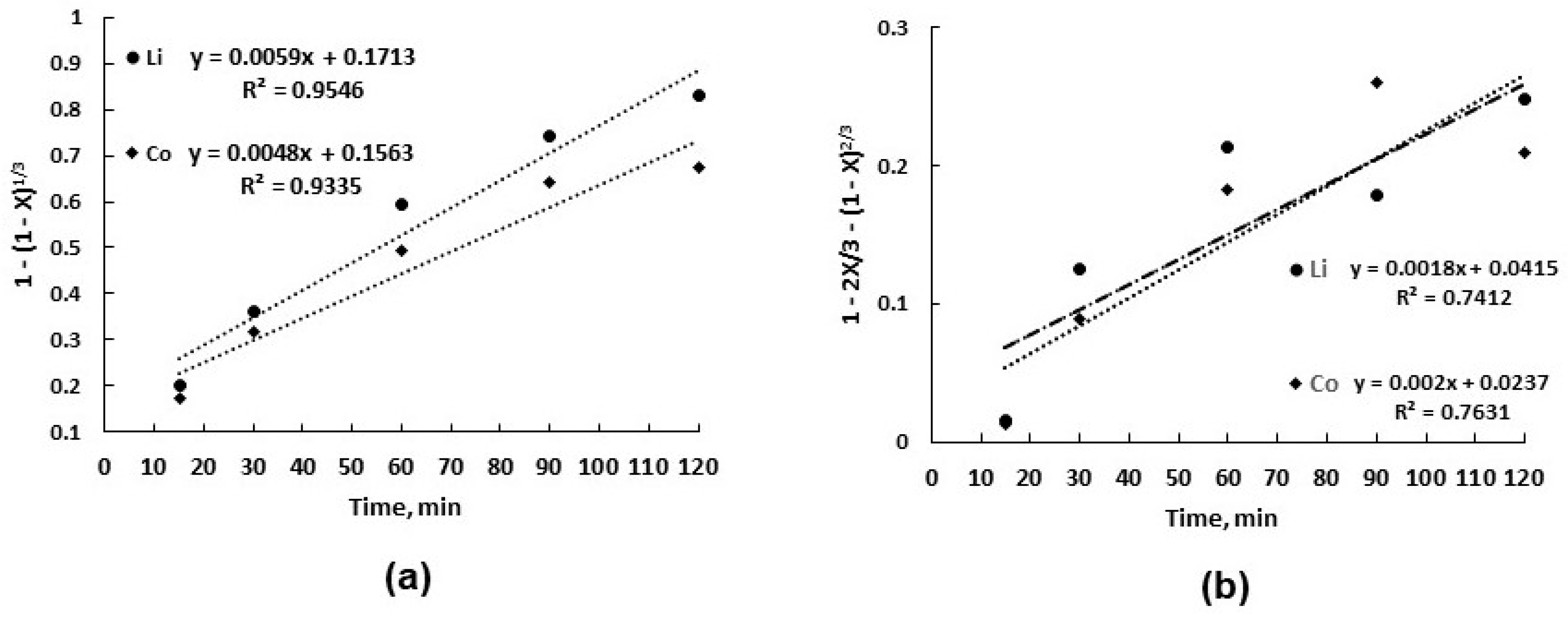
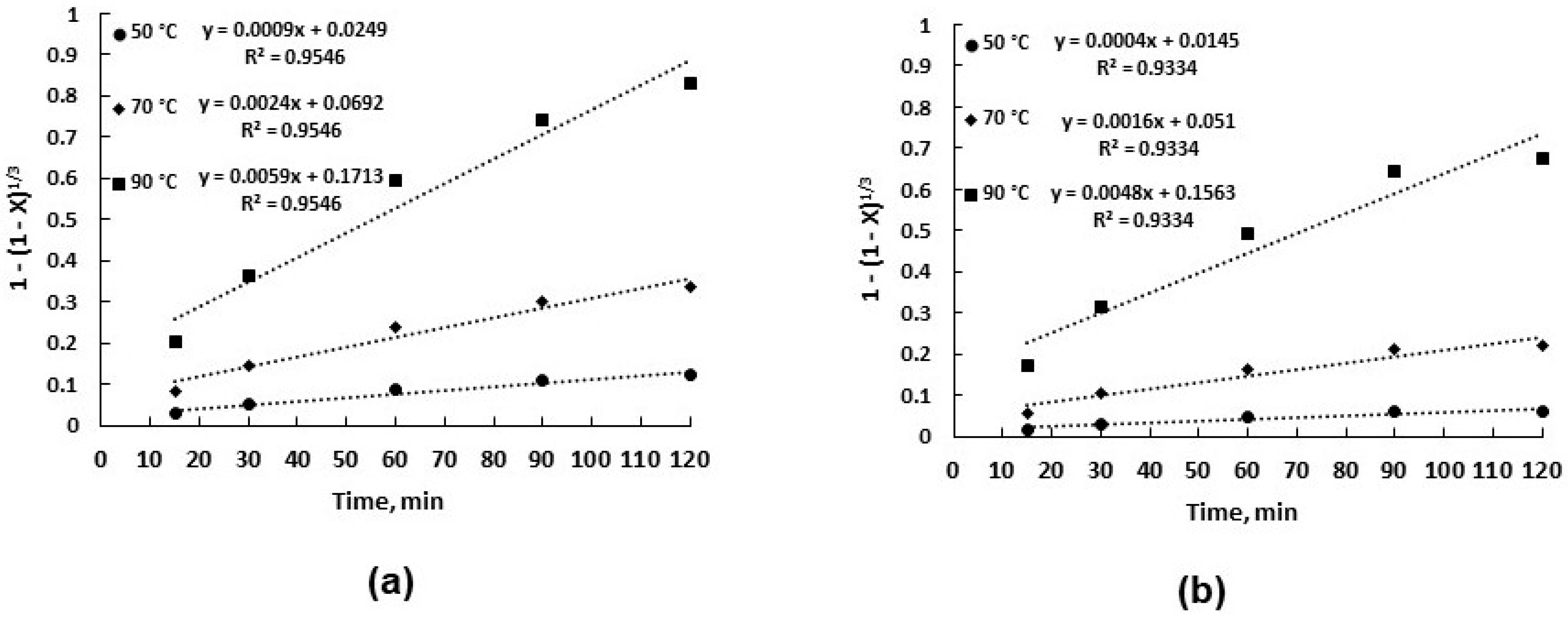
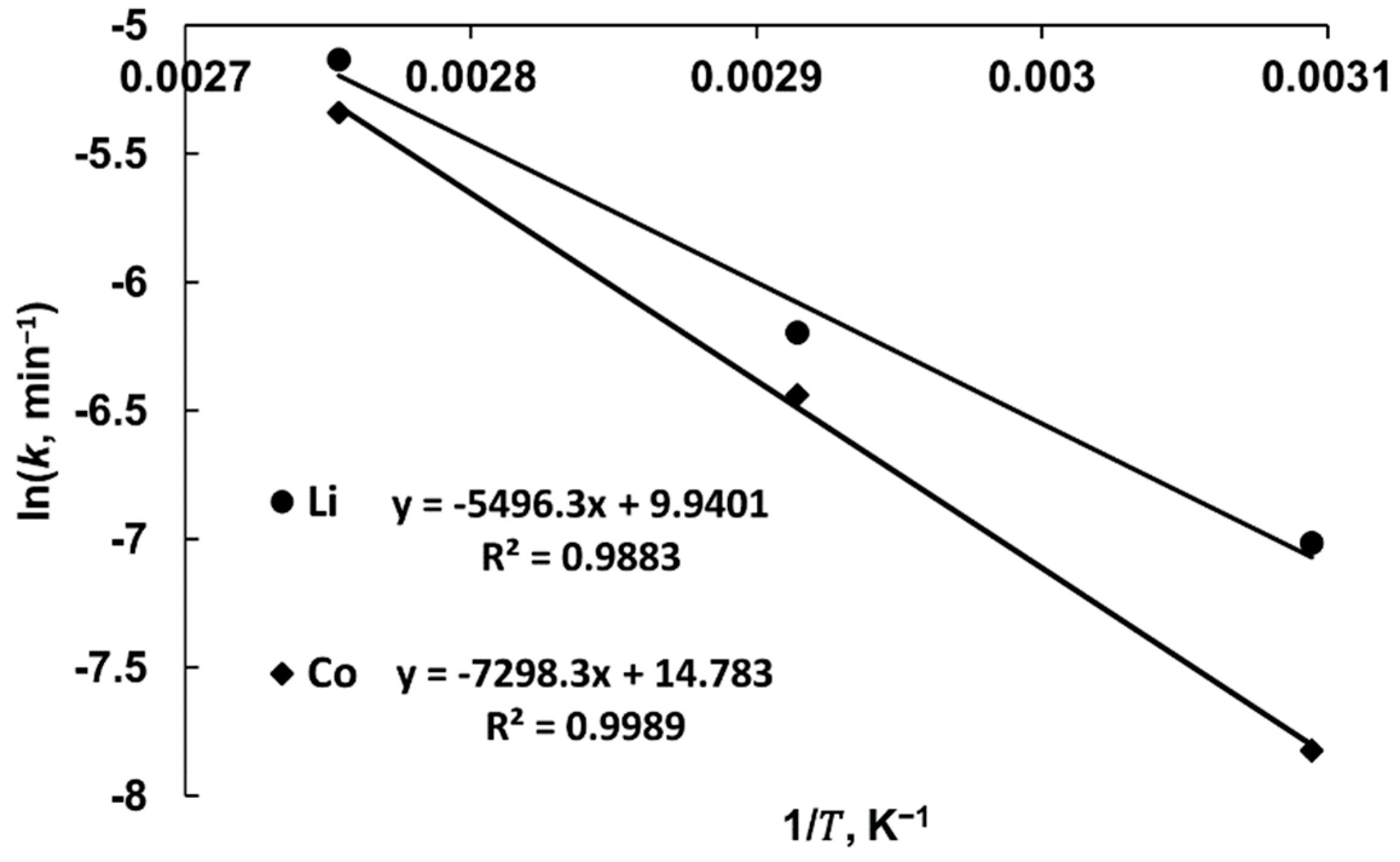
3.2. Leaching Kinetics
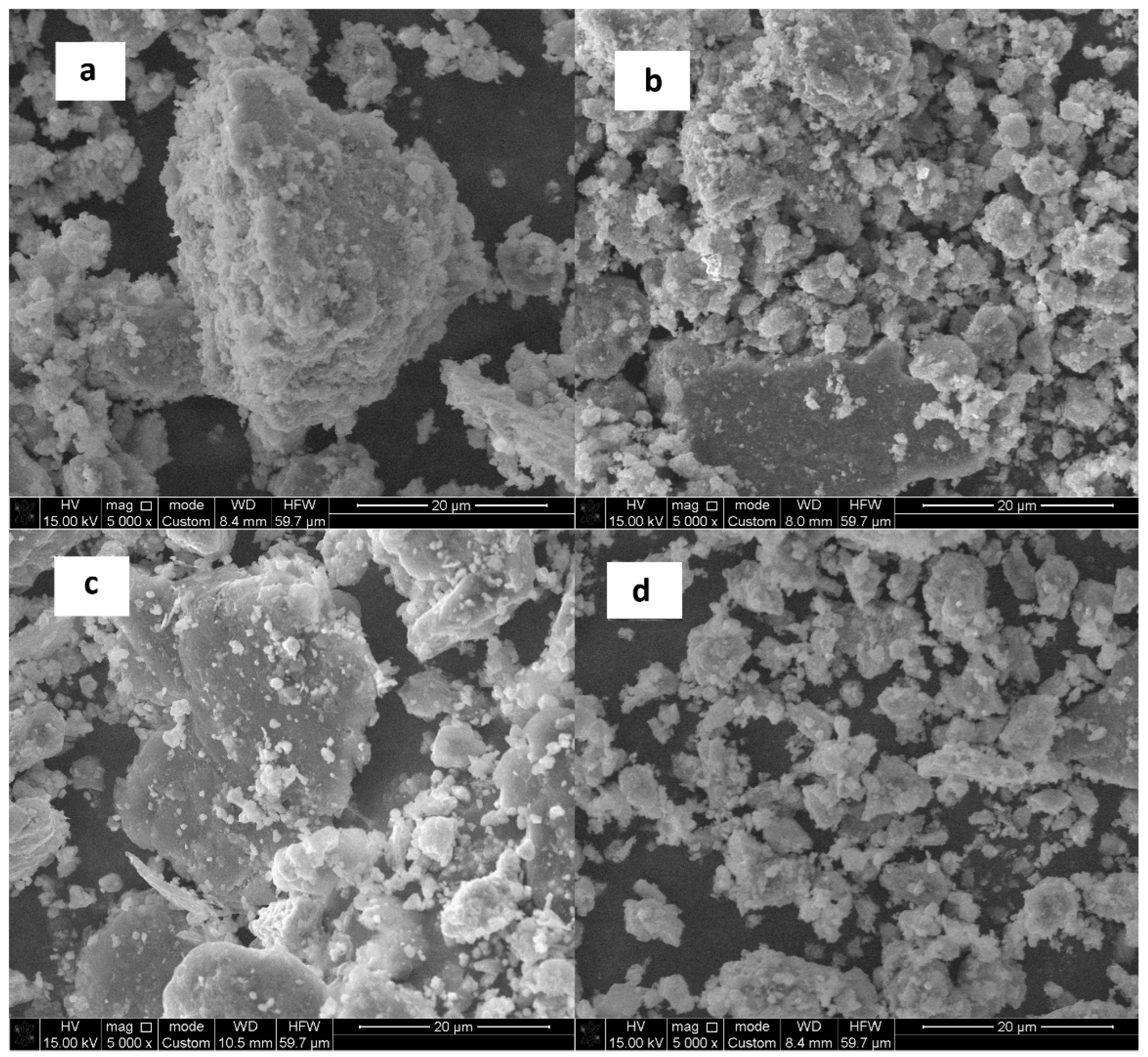
3.3. Material Characterization
3.4. Comparative Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zanoletti, A.; Carena, E.; Ferrara, C.; Bontempi, E. A review of lithium-ion battery recycling: Technologies, sustainability, and open issues. Batteries 2024, 10, 38. [Google Scholar] [CrossRef]
- Thompson, D.L.; Hartley, J.M.; Lambert, S.M.; Shiref, M.; Harper, G.D.; Kendrick, E.; Anderson, P.; Ryder, K.S.; Gaines, L.; Abbott, A.P. The importance of design in lithium ion battery recycling—A critical review. Green Chem. 2020, 22, 7585–7603. [Google Scholar] [CrossRef]
- Mao, J.; Ye, C.; Zhang, S.; Xie, F.; Zeng, R.; Davey, K.; Guo, Z.; Qiao, S. Toward practical lithium-ion battery recycling: Adding value, tackling circularity and recycling-oriented design. Energy Environ. Sci. 2022, 15, 2732–2752. [Google Scholar] [CrossRef]
- Flamme, B.; Garcia, G.R.; Weil, M.; Haddad, M.; Phansavath, P.; Ratovelomanana-Vidal, V.; Chagnes, A. Guidelines to design organic electrolytes for lithium-ion batteries: Environmental impact, physicochemical and electrochemical properties. Green Chem. 2017, 19, 1828–1849. [Google Scholar] [CrossRef]
- Mrozik, W.; Rajaeifar, M.A.; Heidrich, O.; Christensen, P. Environmental impacts, pollution sources and pathways of spent lithium-ion batteries. Energy Environ. Sci. 2021, 14, 6099–6121. [Google Scholar] [CrossRef]
- Vandepaer, L.; Cloutier, J.; Amor, B. Environmental impacts of Lithium Metal Polymer and Lithium-ion stationary batteries. Renew. Sustain. Energy Rev. 2017, 78, 46–60. [Google Scholar] [CrossRef]
- Li, M.; Mo, R.; Ding, A.; Zhang, K.; Guo, F.; Xiao, C. Electrochemical technology to drive spent lithium-ion batteries (LIBs) recycling: Recent progress, and prospects. Energy Mater. 2024, 4, 400070. [Google Scholar] [CrossRef]
- Chen, X.; Chen, Y.; Zhou, T.; Liu, D.; Hu, H.; Fan, S. Hydrometallurgical recovery of metal values from sulfuric acid leaching liquor of spent lithium-ion batteries. Waste Manag. 2015, 38, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Sun, R.; Xu, J.; Chen, Z.; Kang, M. Recovery of cobalt from spent lithium ion batteries using sulphuric acid leaching followed by solid–liquid separation and solvent extraction. RSC Adv. 2016, 6, 85303–85311. [Google Scholar] [CrossRef]
- Vieceli, N.; Casasola, R.; Lombardo, G.; Ebin, B.; Petranikova, M. Hydrometallurgical recycling of EV lithium-ion batteries: Effects of incineration on the leaching efficiency of metals using sulfuric acid. Waste Manag. 2021, 125, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Hamuyuni, J.; Wilson, B.P.; Lundström, M. Selective reductive leaching of cobalt and lithium from industrially crushed waste Li-ion batteries in sulfuric acid system. Waste Manag. 2018, 76, 582–590. [Google Scholar] [CrossRef]
- Xuan, W.; de Souza Braga, A.; Korbel, C.; Chagnes, A. New insights in the leaching kinetics of cathodic materials in acidic chloride media for lithium-ion battery recycling. Hydrometallurgy 2021, 204, 105705. [Google Scholar] [CrossRef]
- Guo, Y.; Li, F.; Zhu, H.; Li, G.; Huang, J.; He, W. Leaching lithium from the anode electrode materials of spent lithium-ion batteries by hydrochloric acid (HCl). Waste Manag. 2016, 51, 227–233. [Google Scholar] [CrossRef]
- Barik, S.P.; Prabaharan, G.; Kumar, L. Leaching and separation of Co and Mn from electrode materials of spent lithium-ion batteries using hydrochloric acid: Laboratory and pilot scale study. J. Clean. Prod. 2017, 147, 37–43. [Google Scholar] [CrossRef]
- Jung, Y.J.; Park, S.C.; Kim, Y.H.; Yoo, B.Y.; Lee, M.S.; Son, S.H. A study on optimization of nitric acid leaching and roasting process for selective lithium leaching of spent batreries cell powder. Resour. Recycl. 2021, 30, 43–52. [Google Scholar] [CrossRef]
- Lee, C.K.; Rhee, K.-I. Reductive leaching of cathodic active materials from lithium ion battery wastes. Hydrometallurgy 2003, 68, 5–10. [Google Scholar] [CrossRef]
- Vieceli, N.; Benjamasutin, P.; Promphan, R.; Hellström, P.; Paulsson, M.; Petranikova, M. Recycling of lithium-ion batteries: Effect of hydrogen peroxide and a dosing method on the leaching of LCO, NMC oxides, and industrial black mass. ACS Sustain. Chem. Eng. 2023, 11, 9662–9673. [Google Scholar] [CrossRef]
- Zheng, X.; Gao, W.; Zhang, X.; He, M.; Lin, X.; Cao, H.; Zhang, Y.; Sun, Z. Spent lithium-ion battery recycling—Reductive ammonia leaching of metals from cathode scrap by sodium sulphite. Waste Manag. 2017, 60, 680–688. [Google Scholar] [CrossRef]
- Chen, X.; Guo, C.; Ma, H.; Li, J.; Zhou, T.; Cao, L.; Kang, D. Organic reductants based leaching: A sustainable process for the recovery of valuable metals from spent lithium ion batteries. Waste Manag. 2018, 75, 459–468. [Google Scholar] [CrossRef]
- Punt, T.; Akdogan, G.; Bradshaw, S.; van Wyk, P. Development of a novel solvent extraction process using citric acid for lithium-ion battery recycling. Miner. Eng. 2021, 173, 107204. [Google Scholar] [CrossRef]
- Punt, T.; van Wyk, A.P.; Bradshaw, S.M.; Akdogan, G. Citric Acid Leaching Performance at High Solid-to-Liquid Ratios for Lithium-Ion Battery Recycling. Mining, Met. Explor. 2024, 41, 3463–3474. [Google Scholar] [CrossRef]
- Rouquette, L.M.; Petranikova, M.; Vieceli, N. Complete and selective recovery of lithium from EV lithium-ion batteries: Modeling and optimization using oxalic acid as a leaching agent. Sep. Purif. Technol. 2023, 320, 124143. [Google Scholar] [CrossRef]
- Cheng, Q.; Marchetti, B.; Chen, M.; Li, J.-T.; Wu, J.; Liu, X.; Zhou, X.D. Novel approach for in situ recovery of cobalt oxalate from spent lithium-ion batteries using tartaric acid and hydrogen peroxide. J. Mater. Cycles Waste Manag. 2023, 25, 1534–1548. [Google Scholar] [CrossRef]
- Ketegenov, T.; Kamunur, K.; Mussapyrova, L.; Batkal, A.; Nadirov, R. Enhanced recovery of lithium and cobalt from spent lithium-ion batteries using ultrasound-assisted deep eutectic solvent leaching. Metals 2024, 14, 1052. [Google Scholar] [CrossRef]
- Batkal, A.; Kamunur, K.; Mussapyrova, L.; Mukanov, Y.; Nadirov, R. Efficient Extraction of Lithium, Cobalt, and Nickel from Nickel-Manganese-Cobalt Oxide Cathodes with Cholin Chloride/Pyrogallol-Based Deep Eutectic Solvent. Recycling 2025, 10, 88. [Google Scholar] [CrossRef]
- Saim, A.K. Ammoniacal leaching for the extraction of valuable metals from secondary resources: A review. Miner. Process. Extr. Met. Rev. 2025, 46, 284–305. [Google Scholar] [CrossRef]
- Yu, J.; Ma, B.; Qiu, Z.; Wang, C.; Chen, Y. Separation and recovery of valuable metals from ammonia leaching solution of spent lithium-ion batteries. ACS Sustain. Chem. Eng. 2023, 11, 9738–9750. [Google Scholar] [CrossRef]
- Batkal, A.; Kamunur, K.; Mussapyrova, L.; Milikhat, B.; Nadirov, R. Optimized Ammonia Leaching and Energy-Efficient Stripping for Lithium and Cobalt Recovery from Spent LiCoO2 Cathodes. Metals 2025, 15, 690. [Google Scholar] [CrossRef]
- Liu, C.; Liu, P.; Xu, H.; Zeng, G.; Luo, X.; Wang, Z.; Yang, L.; Deng, C.; He, J. Oriented conversion of spent LiCoO2-lithium battery cathode materials to high-value products via thermochemical reduction with common ammonium oxalate. Resour. Conserv. Recycl. 2023, 190, 106782. [Google Scholar] [CrossRef]
- Xiao, J.; Niu, B.; Xu, Z. Ammonia reduction system for the diversity of cathode processing of Li-ion batteries. ACS Sustain. Chem. Eng. 2021, 9, 12091–12099. [Google Scholar] [CrossRef]
- Odebiyi, O.S.; Du, H.; Lasisi, K.H.; Liu, B.; Wang, S.; Nwakanma, C.C.; Nnyia, M.O. Effect of ball mill parameters’ variation on the particles of a mechanical activation-assisted leaching: A hydrometallurgical mechanics. Mater. Circ. Econ. 2021, 3, 23. [Google Scholar] [CrossRef]
- Golik, V.I.; Klyuev, R.V.; Martyushev, N.V.; Brigida, V.; Efremenkov, E.A.; Sorokova, S.N.; Mengxu, Q. Tailings utilization and zinc extraction based on mechanochemical activation. Materials 2023, 16, 726. [Google Scholar] [CrossRef]
- Baláž, P. Mechanical activation in hydrometallurgy. Int. J. Miner. Process. 2003, 72, 341–354. [Google Scholar] [CrossRef]
- Alex, T.C.; Kumar, R.; Roy, S.K.; Mehrotra, S.P. Mechanical activation of Al-oxyhydroxide minerals–a review. Miner. Process. Extr. Met. Rev. 2016, 37, 15–19. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, C.; Zhang, X.; Ma, E. A mechanochemical method for one-step leaching of metals from spent LIBs. Waste Manag. 2023, 161, 245–253. [Google Scholar] [CrossRef]
- Dolotko, O.; Hlova, I.Z.; Mudryk, Y.; Gupta, S.; Balema, V.P. Mechanochemical recovery of Co and Li from LCO cathode of lithium-ion battery. J. Alloy. Compd. 2020, 824, 153876. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, X.; Yan, S.; Ou, Y.; Zhou, T. Mechanochemistry-induced recycling of spent lithium-ion batteries for synergistic treatment of mixed cathode powders. Green Chem. 2022, 24, 5987–5997. [Google Scholar] [CrossRef]
- Algül, H.; Algül, F. Role of Mechanical Activation in Enhancing Li and Co Recovery from Spent Li-ion Batteries through Citric Acid Leaching. Sak. Univ. J. Sci. 2024, 28, 1000–1009. [Google Scholar] [CrossRef]
- Eom, Y.; Alorro, R.D.; Gamutan, J.; Nikoloski, A.N. Lithium Extraction from Spent Lithium-Ion Batteries (LIBs) Using Mechanochemical Process: A Comprehensive Review. Resour. Recycl. 2023, 32, 3–17. [Google Scholar] [CrossRef]
- Meng, X.; Hao, J.; Cao, H.; Lin, X.; Ning, P.; Zheng, X.; Chang, J.; Zhang, X.; Wang, B.; Sun, Z. Recycling of LiNi1/3Co1/3Mn1/3O2 cathode materials from spent lithium-ion batteries using mechanochemical activation and solid-state sintering. Waste Manag. 2019, 84, 54–63. [Google Scholar] [CrossRef]
- Wang, S.; Yan, S.; Chen, Z.; Ou, Y.; Mahara, B.; Chen, X.; Yang, Y.; Zhou, T. Boosting the sustainable recycling of spent lithium-ion batteries through mechanochemistry. Green Chem. 2025, 27, 9917–9926. [Google Scholar] [CrossRef]
- Wang, C.; Qiu, X.; Shen, G.; Chen, X.; Wang, J.; Xie, L.; Han, Q.; Zhu, L.; Li, J.; Cao, X. Driving the rapid regeneration of LiFePO4 from spent lithium-ion batteries through one-pot mechanochemical activation. Green Chem. 2024, 26, 1501–1510. [Google Scholar] [CrossRef]
- Mussapyrova, L.; Milikhat, B.; Baláž, M.; Batkal, A.; Kamunur, K.; Nadirov, R. Mechanochemical Activation as a Key Step for Enhanced Ammonia Leaching of Spent LiCoO2 Cathodes. Metals 2025, 15, 1021. [Google Scholar] [CrossRef]
- Jin, W.; Cha, H.; Choi, S.; Song, G. Electrode-level strategies for high-Ni cathodes in high-energy-density batteries beyond material design. Energy Mater. 2025, 5, 500130. [Google Scholar] [CrossRef]
- Zhang, M.; Dong, X.; Li, X.; Jiang, Y.; Li, Y.; Liang, Y. Review of separation methods for the determination of ammonium/ammonia in natural water. Trends Environ. Anal. Chem. 2020, 27, e00098. [Google Scholar] [CrossRef]
- Riechers, S.L.; Ilton, E.S.; Qafoku, O.; Du, Y.; Kerisit, S.N. Cobalt hydroxide–cobalt carbonate competitive growth on carbonate surfaces. Chem. Geol. 2022, 605, 120951. [Google Scholar] [CrossRef]
- Wang, H.; Li, Z.; Meng, Q.; Duan, J.; Xu, M.; Lin, Y.; Zhang, Y. Ammonia leaching of valuable metals from spent lithium ion batteries in NH3-(NH4)2SO4-Na2SO3 system. Hydrometallurgy 2022, 208, 105809. [Google Scholar] [CrossRef]
- Naseri, T.; Mousavi, S.M. Improvement of Li and Mn bioleaching from spent lithium-ion batteries, using step-wise addition of biogenic sulfuric acid by Acidithiobacillus thiooxidans. Heliyon 2024, 10, e37447. [Google Scholar] [CrossRef]
- Wu, C.; Li, B.; Yuan, C.; Ni, S.; Li, L. Recycling valuable metals from spent lithium-ion batteries by ammonium sulfite-reduction ammonia leaching. Waste Manag. 2019, 93, 153–161. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Lv, C.; Wei, G.; Meng, L.; Fu, J.; Yu, Z.; Qu, J. Sustainable ammonia leaching for efficient nickel and cobalt recovery from mixed hydroxide precipitate. J. Environ. Chem. Eng. 2025, 13, 115731. [Google Scholar] [CrossRef]
- Nadirov, R.; Karamyrzayev, G. Selective ozone-assisted acid leaching of copper from copper smelter slag by using isopropanol as a solvent. Minerals 2022, 12, 1047. [Google Scholar] [CrossRef]
- Nadirov, R.; Karamyrzayev, G. Enhancing Synthetic Zinc Ferrite Hydrochloric Acid Leaching by Using Isopropanol as a Solvent. Mining, Met. Explor. 2022, 39, 1743–1751. [Google Scholar] [CrossRef]
- Su, F.; Zhou, X.; Liu, X.; Yang, J.; Tang, J.; Yang, W.; Li, Z.; Wang, H.; Zhang, Y.; Ma, Y. Recovery of valuable metals from spent lithium-ion batteries by complexation-assisted ammonia leaching from reductive roasting residue. Chemosphere 2023, 312, 137230. [Google Scholar] [CrossRef]
- Qi, Y.; Meng, F.; Yi, X.; Shu, J.; Chen, M.; Sun, Z.; Sun, S.; Xiu, F.R. A novel and efficient ammonia leaching method for recycling waste lithium ion batteries. J. Clean. Prod. 2020, 251, 119665. [Google Scholar] [CrossRef]
- Perdana, I.; Rahman, M.I.; Aprilianto, D.R.; Petrus, H.T.B.M.; Kinanti, D.H. Kinetics and Thermodynamics Study of Ammonia Leaching on Spent LMR-NMC Battery Cathodes. Indones. J. Chem. 2024, 24, 876–892. [Google Scholar] [CrossRef]
- Dávila-Pulido, G.I.; Salinas-Rodríguez, A.; Carrillo-Pedroza, F.R.; González-Ibarra, A.A.; Méndez-Nonell, J.; Garza-García, M. Leaching kinetics of electronic waste for the recovery of copper: Rate-controlling step and rate process in a multisize particle system. Int. J. Chem. Kinet. 2021, 53, 379–389. [Google Scholar] [CrossRef]
- Fu, Y.; He, Y.; Qu, L.; Feng, Y.; Li, J.; Liu, J.; Zhang, G.; Xie, W. Enhancement in leaching process of lithium and cobalt from spent lithium-ion batteries using benzenesulfonic acid system. Waste Manag. 2019, 88, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhang, B.; Ou, X.; Zhang, J.; Meng, K.; Ji, G.; Li, P.; Xu, J. Ammonia leaching mechanism and kinetics of LiCoO2 material from spent lithium-ion batteries. Chin. Chem. Lett. 2021, 32, 2333–2337. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, C.; Zhang, X.; Ma, E. Recovery of Li and Co from spent Li-Ion batteries by mechanochemical integration with NH4Cl. ACS Sustain. Chem. Eng. 2022, 10, 5611–5620. [Google Scholar] [CrossRef]
- Liu, X.; Huang, K.; Xiong, H.; Dong, H. Ammoniacal leaching process for the selective recovery of value metals from waste lithium-ion batteries. Environ. Technol. 2023, 44, 211–225. [Google Scholar] [CrossRef]




| Li, wt % | Co, wt % | Al, wt % | Cu, wt % |
|---|---|---|---|
| 6.8 | 52.5 | 0.8 | 0.4 |
| MA Conditions | Leaching Conditions | Co Recovery (%) | Li Recovery (%) | Refs. |
|---|---|---|---|---|
| High-energy ball milling (wet, zirconia media), 5 h | NH3-Na2SO3-NH4Cl solution, pH ≈ 10, 80 °C, ~4 h (after MA; vs. 48 h without MA) | 98.22 | 89.86 | [58] |
| Ball milling with NH4Cl, 500 rpm, 120 min (in-situ chlorination) | 1 M H2SO4 + 0.03 M NH4Cl, 80 °C, 120 min, L/S ≈ 10 mL·g−1 | 99.22 | 100 | [59] |
| Planetary ball milling, 800 rpm, 60 min, BPR ≈ 50:1, co-milled with 5 wt% Al + 2.5 wt% C | (NH4)2CO3-NH3 solution: 3.0 M NH3·H2O + 1.0 M (NH4)2CO3, 60 °C, 6 h, L/S = 25 mL·g−1 | 83.7 | 94.6 | [43] |
| Planetary ball milling, 800 rpm, 60 min, BPR = 50:1 | 4.0 M NH3·H2O + 1.5 M (NH4)2SO4 + 0.5 M Na2SO3, S/L = 10 g/L, 90 °C, 120 min | 96.5 | 99.5 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milikhat, B.; Batkal, A.; Kamunur, K.; Mussapyrova, L.; Mukanov, Y.; Nadirov, R. Efficient Recovery of Lithium and Cobalt from Spent LCO Using Mechanochemical Activation and Ammoniacal Leaching. Processes 2025, 13, 3345. https://doi.org/10.3390/pr13103345
Milikhat B, Batkal A, Kamunur K, Mussapyrova L, Mukanov Y, Nadirov R. Efficient Recovery of Lithium and Cobalt from Spent LCO Using Mechanochemical Activation and Ammoniacal Leaching. Processes. 2025; 13(10):3345. https://doi.org/10.3390/pr13103345
Chicago/Turabian StyleMilikhat, Bagdatgul, Aisulu Batkal, Kaster Kamunur, Lyazzat Mussapyrova, Yerzhan Mukanov, and Rashid Nadirov. 2025. "Efficient Recovery of Lithium and Cobalt from Spent LCO Using Mechanochemical Activation and Ammoniacal Leaching" Processes 13, no. 10: 3345. https://doi.org/10.3390/pr13103345
APA StyleMilikhat, B., Batkal, A., Kamunur, K., Mussapyrova, L., Mukanov, Y., & Nadirov, R. (2025). Efficient Recovery of Lithium and Cobalt from Spent LCO Using Mechanochemical Activation and Ammoniacal Leaching. Processes, 13(10), 3345. https://doi.org/10.3390/pr13103345






