Influence of Guar Gum and Xanthan Gum on the Rheological Behavior, Texture, and Microstructure of Probiotic Low-Fat Yogurt
Abstract
1. Introduction
2. Materials and Methods
2.1. Overall Experimental Design
2.2. Low-Fat Yoghurt Manufacturing
2.3. Proximate Composition
2.4. Textural Analysis
2.5. Viscosity Determination
2.6. Syneresis
2.7. Color Characteristics
2.8. Microbiological Analysis
2.9. Sensory Evaluation
2.10. Rheological Characteristics
2.11. Microstructure
2.12. Statistical Analysis
3. Results and Discussion
3.1. Proximate Composition
3.2. Hardness
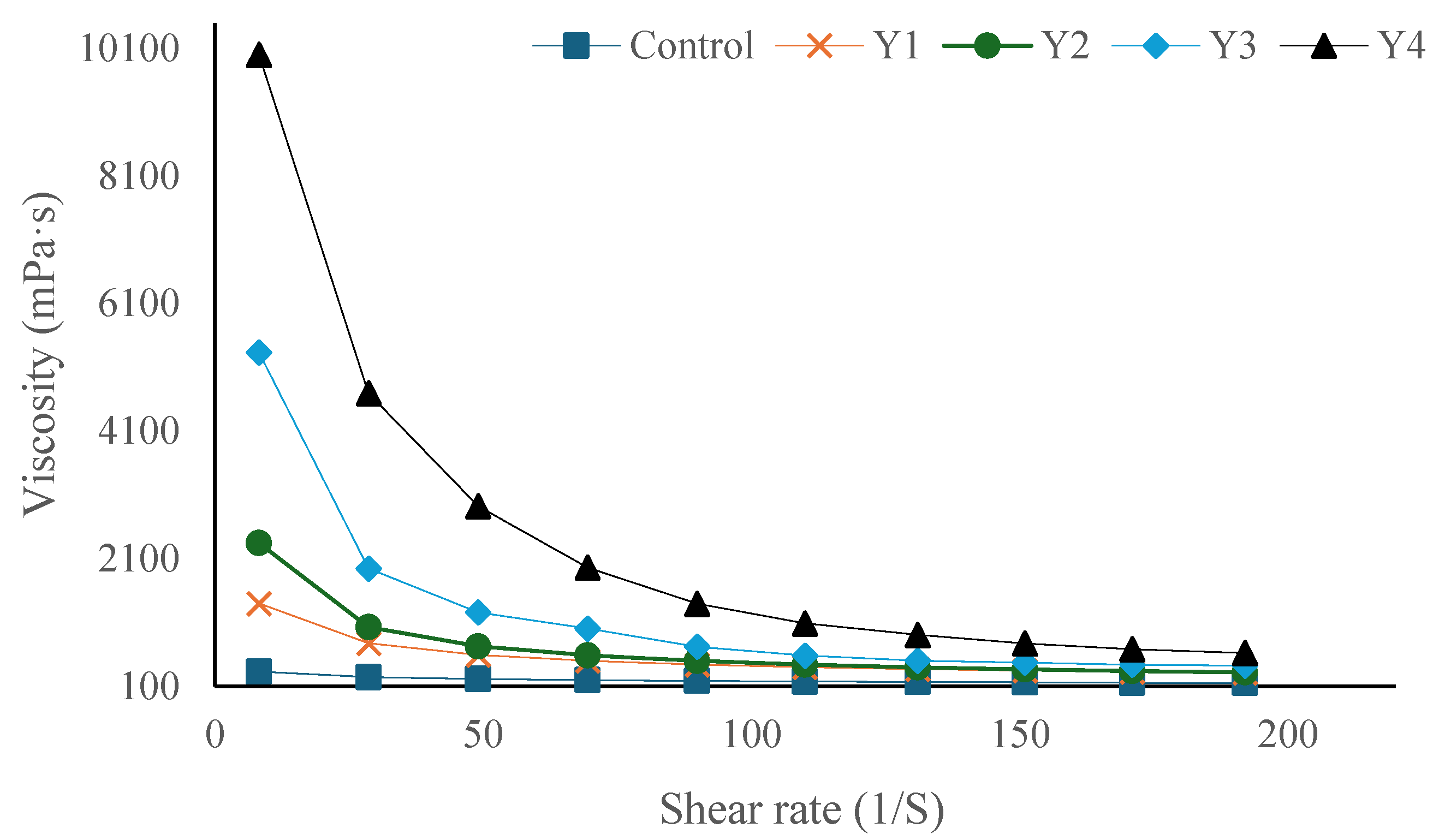
3.3. Viscosity
3.4. Syneresis
3.5. Color Characteristics
3.6. Microbiological Characteristics
3.7. Sensory Analysis
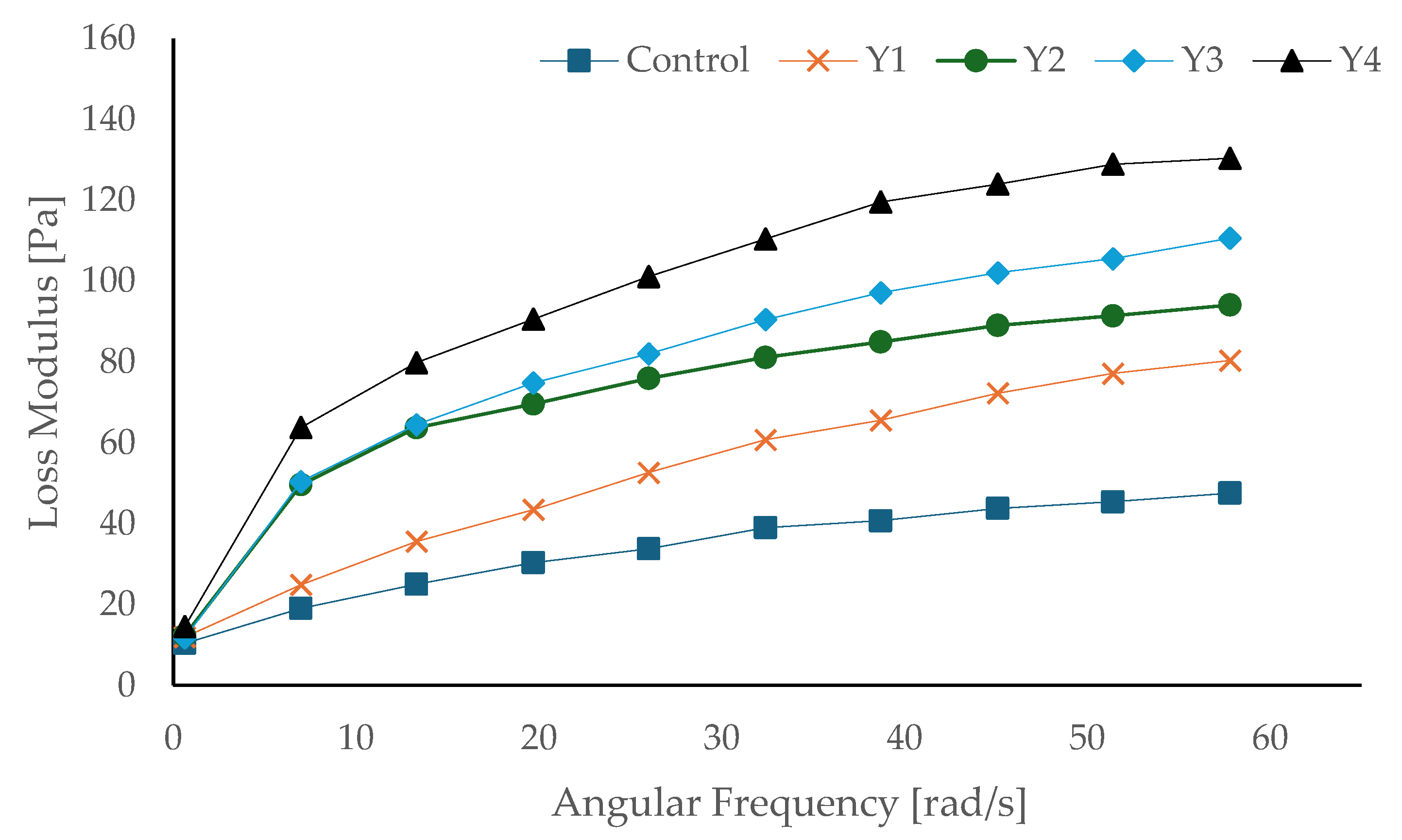
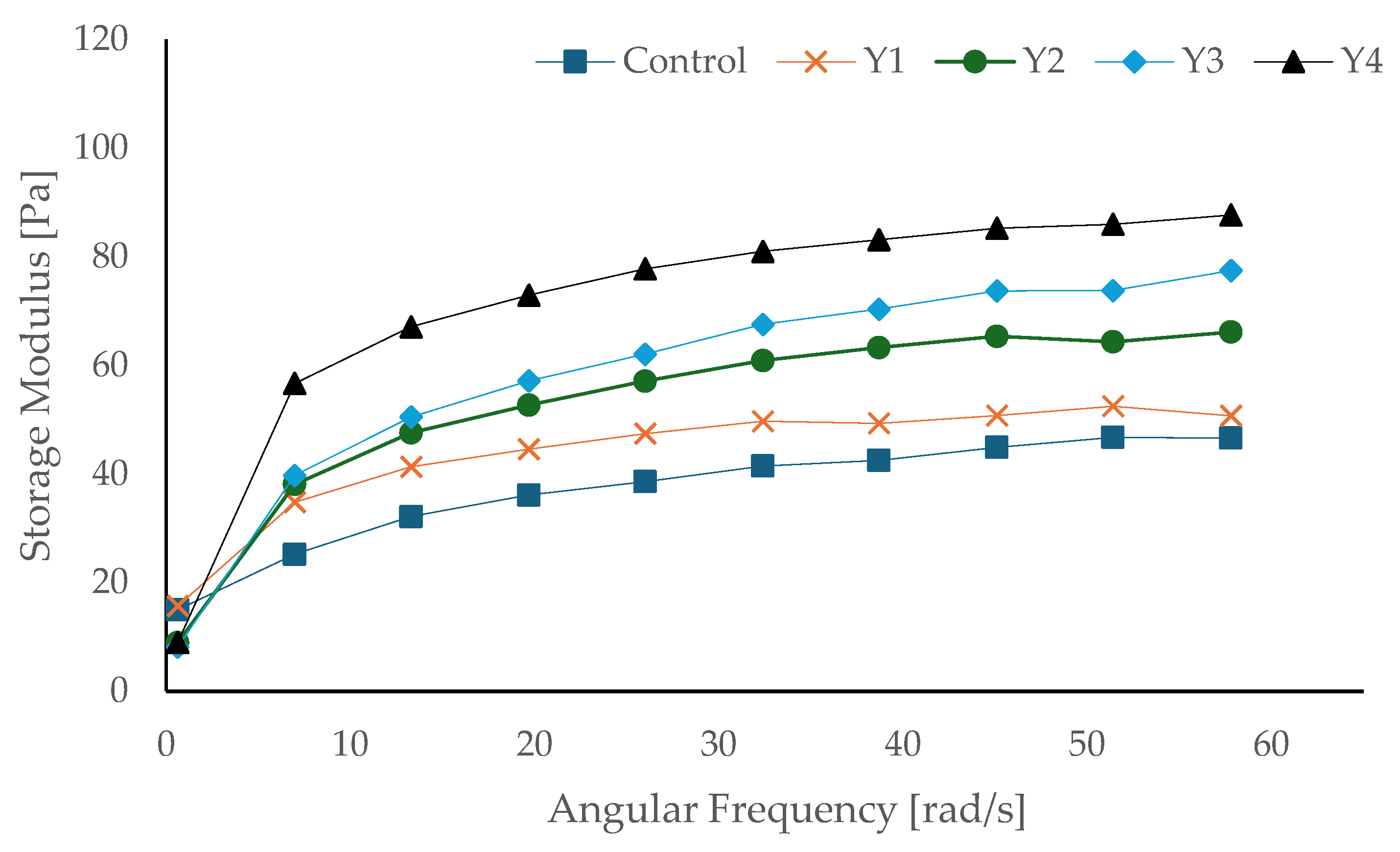
3.8. Rheological Properties
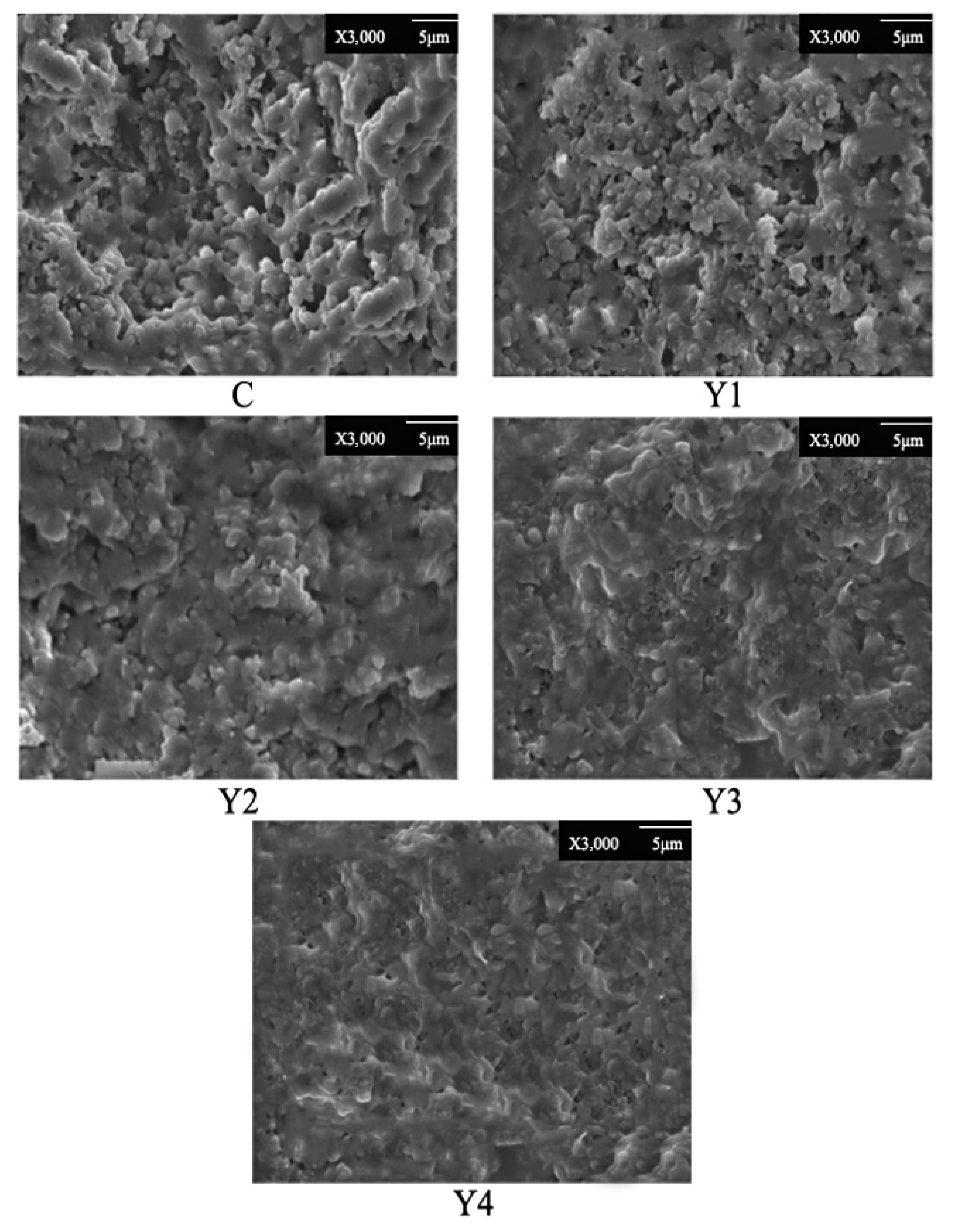
3.9. Microstructure
4. Conclusions
5. Study Limitations
6. Future Research
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| XG | Xanthan Gum |
| GG | Guar Gum |
| EMCC | Egyptian Microbial Culture Collection |
| LFY | Low-fat yogurt |
| TP | Total Protein |
| TS | Total Solids |
| CPS | Centipoise |
| TBC | Total Bacterial Count |
| CFU | Colony Forming Unit |
| ANOVA | Analysis of variance |
| GLM | General Linear Model |
| LSD | Least Significant Difference |
References
- Granato, D.; Branco, G.F.; Cruz, A.G.; de Faria, J.A.F.; Shah, N.P. The global dairy industry has experienced significant growth and innovation, with yogurt emerging as a key player in the functional food market. Compr. Rev. Food Sci. Food Saf. 2010, 9, 455–470. [Google Scholar] [CrossRef] [PubMed]
- Susarla, G. Health Influence on the Development of Low Fat Dairy Products: A Thesis Presented in Partial Fulfilment of the Requirements for the Degree of Master of Philosophy in Food Technology at Massey University. Master’s Thesis, Massey University, Turitea, New Zealand, 1994. [Google Scholar]
- Achayuthakan, P.; Suphantharika, M. Pasting and rheological properties of waxy corn starch as affected by guar gum and xanthan gum. Carbohydr. Polym. 2008, 71, 9–17. [Google Scholar] [CrossRef]
- Goh, K.K.T.; Teo, A.; Sarkar, A.; Singh, H. Milk protein-polysaccharide interactions. In Milk Proteins; Elsevier: Amsterdam, The Netherlands, 2020; pp. 499–535. [Google Scholar] [CrossRef]
- Abbas, H.M.; El-Gawad, M.A.M.A.; Kassem, J.M.; Salama, M. Application of fat replacers in dairy products: A review. Foods Raw Mater. 2023, 12, 319–333. [Google Scholar] [CrossRef]
- Chandan, R.C.; Gandhi, A.; Shah, N.P. Yogurt: Historical background, health benefits, and global trade. In Yogurt in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2017; pp. 3–29. [Google Scholar] [CrossRef]
- Bagheri, R.; Seyedein, S.M. The effect of adding rice bran fibre on wheat dough performance and bread quality. World Appl. Sci. J. 2011, 14, 121–125. Available online: http://www.idosi.org/wasj/wasj14(Food&Environment)11/18.pdf (accessed on 24 June 2024).
- Pannerchelvan, S.; Rios-Solis, L.; Wasoh, H.; Sobri, M.Z.M.; Wong, F.W.F.; Mohamed, M.S.; Mohamad, R.; Halim, M. Functional yogurt: A comprehensive review of its nutritional composition and health benefits. Food Funct. 2024, 15, 10927–10955. [Google Scholar] [CrossRef] [PubMed]
- Arab, M.; Yousefi, M.; Khanniri, E.; Azari, M.; Ghasemzadeh-Mohammadi, V.; Mollakhalili-Meybodi, N. A comprehensive review on yogurt syneresis: Effect of processing conditions and added additives. J. Food Sci. Technol. 2022, 60, 1656–1665. [Google Scholar] [CrossRef]
- Yousefi, M.; Jafari, S.M. Recent advances in application of different hydrocolloids in dairy products to improve their techno-functional properties. Trends Food Sci. Technol. 2019, 88, 468–483. [Google Scholar] [CrossRef]
- Tahmouzi, S.; Meftahizadeh, H.; Eyshi, S.; Mahmoudzadeh, A.; Alizadeh, B.; Mollakhalili-Meybodi, N.; Hatami, M. Application of guar (Cyamopsis tetragonoloba L.) gum in food technologies: A review of properties and mechanisms of action. Food Sci. Nutr. 2023, 11, 4869–4897. [Google Scholar] [CrossRef]
- Leal, M.R.S.; Albuquerque, P.B.S.; Rodrigues, N.E.R.; dos Santos Silva, P.M.; de Oliveira, W.F.; dos Santos Correia, M.T.; Kennedy, J.F.; Coelho, L.C.B.B. A review on the use of polysaccharides as thickeners in yogurts. Carbohydr. Polym. Technol. Appl. 2024, 8, 100547. [Google Scholar] [CrossRef]
- Krishna Leela, J.; Sharma, G. Studies on xanthan production from Xanthomonas campestris. Bioprocess Eng. 2000, 23, 687–689. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Z.; Zheng, Y.; Hong, Q.; Wang, Q.; Xu, X. Synergistic effects of microcrystalline cellulose and xanthan gum on the stability of milk fat-based UHT whipping cream. LWT 2023, 184, 114966. [Google Scholar] [CrossRef]
- Alsaleem, K.A.; Hamouda, M.E.A. Enhancing Low-Fat Probiotic Yogurt: The Role of Xanthan Gum in Functionality and Microbiological Quality. Processes 2024, 12, 990. [Google Scholar] [CrossRef]
- AOAC Official Method 2015.01 Heavy Metals in Food. (n.d.). Available online: https://www.academia.edu/30141101/AOAC_Official_Method_2015_01_Heavy_Metals_in_Food (accessed on 24 June 2024).
- AOAC Official Method 990.20. (n.d.). Solids (Total) in Milk by Direct Forced Air Oven Drying. Available online: https://www.scirp.org/reference/ReferencesPapers?ReferenceID=1687699 (accessed on 24 June 2024).
- Ling, E.R. Textbook of Dairy Chemistry; Chapman & Hall: London, UK, 1963. [Google Scholar]
- Gharibzahedi, S.M.T.; Emam-Djomeh, Z.; Razavi, S.H.; Jafari, S.M. Mechanical behavior of lentil seeds in relation to their physicochemical and microstructural characteristics. Int. J. Food Prop. 2014, 17, 545–558. [Google Scholar] [CrossRef]
- Keogh, M.K.; O’Kennedy, B.T. Rheology of stirred yogurt as affected by added milk fat, protein and hydrocolloids. J. Food Sci. 1998, 63, 108–112. [Google Scholar] [CrossRef]
- Guler, Z.; Park, Y.W. Evaluation of chemical and color index characteristics of goat milk, its yoghurt and salted yoghurt. Trop. Subtrop. Agroecosyst. 2009, 11, 37–39. Available online: https://www.redalyc.org/pdf/939/93913000009.pdf (accessed on 24 June 2024).
- Wehr, H.M.; Frank, J.F. Standard Methods for the Examination of Dairy Products; Ignatius Press: San Francisco, CA, USA, 2004. [Google Scholar] [CrossRef]
- De Man, J.C.; Rogosa, M.; Sharpe, M.E. A Medium for the Cultivation of Lactobacilli. J. Appl. Bacteriol. 1960, 23, 130–135. [Google Scholar] [CrossRef]
- Brewer, J.H. Clear liquid mediums for the aerobic cultivation of anaerobes. J. Am. Med. Assoc. 1940, 115, 598–600. [Google Scholar] [CrossRef]
- Hamdy, A.M.; Ahmed, M.E.; Mehta, D.; Elfaruk, M.S.; Hammam, A.R.A.; El-Derwy, Y.M.A. Enhancement of low-fat Feta cheese characteristics using probiotic bacteria. Food Sci. Nutr. 2020, 9, 62–70. [Google Scholar] [CrossRef]
- Wu, T.; Deng, C.; Luo, S.; Liu, C.; Hu, X. Effect of rice bran on properties of yogurt: Comparison between addition of bran before fermentation and after fermentation. Food Hydrocoll. 2023, 135, 108122. [Google Scholar] [CrossRef]
- Matumoto-Pintro, P.T.; Rabiey, L.; Robitaille, G.; Britten, M. Use of modified whey protein in yoghurt formulations. Int. Dairy J. 2011, 21, 21–26. [Google Scholar] [CrossRef]
- Gomez, K.A.; Gomez, A.A. Statistical Procedures for Agricultural Research; John Wiley & Sons: Hoboken, NJ, USA, 1984. [Google Scholar]
- Coggins, P.C.; Rowe, D.E.; Wilson, J.C.; Kumari, S. Storage and temperature effects on appearance and textural characteristics of conventional milk yogurt. J. Sens. Stud. 2010, 25, 549–576. [Google Scholar] [CrossRef]
- Costa, M.P.; Rosario, A.I.L.S.; Silva, V.L.M.; Vieira, C.P.; Conte-Junior, C.A. Rheological, physical and sensory evaluation of low-fat cupuassu goat milk yogurts supplemented with fat replacer. Food Sci. Anim. Resour. 2022, 42, 210. [Google Scholar] [CrossRef]
- Cunha, P.L.R.; de Paula, R.C.M.; Feitosa, J.P.A. Purification of guar gum for biological applications. Int. J. Biol. Macromol. 2007, 41, 324–331. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, R.; Zhang, F.; Wang, B.; Liu, Y. Storage stability of texture, organoleptic, and biological properties of goat milk yogurt fermented with probiotic bacteria. Front. Nutr. 2023, 9, 1093654. [Google Scholar] [CrossRef]
- Terpiłowski, K.; Lange, I.; Kowalczyk, K.; Tomczyńska-Mleko, M.; Sapiga, V.; Wesołowska-Trojanowska, M.; Mleko, S.; Pérez-Huertas, S. Impact of Storage Conditions of Yogurt Dry Ingredients on the Physicochemical Properties of the Final Product. Appl. Sci. 2023, 13, 13201. [Google Scholar] [CrossRef]
- Sattar, M.Z.; Zaeem Shan, Z.U.; Saleem, M.; Sohail, M.A.; Murtaza, S.; Shahbaz, M. Effect of Gums on the Quality Parameters of Low Fat Yoghurt. Pak. J. Multidiscip. Innov. 2022, 1, 45–57. [Google Scholar] [CrossRef]
- Pei, R.; DiMarco, D.M.; Putt, K.K.; Martin, D.A.; Chitchumroonchokchai, C.; Bruno, R.S.; Bolling, B.W. Premeal low-fat yogurt consumption reduces postprandial inflammation and markers of endotoxin exposure in healthy premenopausal women in a randomized controlled trial. J. Nutr. 2018, 148, 910–916. [Google Scholar] [CrossRef]
- Javed, M.; Ahmed, W.; Shahbaz, H.M.; Rashid, S.; Javed, H. Techno-Functional Assay and Quality Assessment of Yogurt Supplemented with Basil Seed Gum Powder. Braz. Arch. Biol. Technol. 2022, 65, e22200814. [Google Scholar] [CrossRef]
- Ismaiel AEl Basheer, E.O.; Elhassan, I.H.; Alnor, M.A.; Abdullatif, G.; Ali, M.; Ahmed, S.Y.; Babekir, W.S. Effect of Arabic Gum (AG) on Physical Characteristics of yoghurt. IJISET-Int. J. Innov. Sci. Eng. Technol. 2022, 9, 8. Available online: www.ijiset.com (accessed on 24 June 2024).
- Publishing, B.; Lucey, J.A. Cultured dairy products: An overview of their gelation and texture properties. Int. J. Dairy Technol. 2004, 57, 77–84. [Google Scholar] [CrossRef]
- Tamime, A.Y.; Robinson, R.K. Tamime and Robinson’s Yoghurt: Science and Technology; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar] [CrossRef]
- Sahan, N.; Yasar, K.; Hayaloglu, A.A. Physical, chemical and flavour quality of non-fat yogurt as affected by a β-glucan hydrocolloidal composite during storage. Food Hydrocoll. 2008, 22, 1291–1297. [Google Scholar] [CrossRef]
- Aziznia, S.; Khosrowshahi, A.; Madadlou, A.; Rahimi, J. Whey protein concentrate and gum tragacanth as fat replacers in nonfat yogurt: Chemical, physical, and microstructural properties. J. Dairy Sci. 2008, 91, 2545–2552. [Google Scholar] [CrossRef]
- Everett, D.W.; McLeod, R.E. Interactions of polysaccharide stabilisers with casein aggregates in stirred skim-milk yoghurt. Int. Dairy J. 2005, 15, 1175–1183. [Google Scholar] [CrossRef]
- Mistry, V.V.; Hassan, H.N. Manufacture of nonfat yogurt from a high milk protein powder. J. Dairy Sci. 1992, 75, 947–957. [Google Scholar] [CrossRef]
- Ahmed, M.E.; Rathnakumar, K.; Awasti, N.; Elfaruk, M.S.; Hammam, A.R.A. Influence of probiotic adjunct cultures on the characteristics of low-fat Feta cheese. Food Sci. Nutr. 2021, 9, 1512–1520. [Google Scholar] [CrossRef]
- Alsaleem, K.A.; Hamouda, M.E.A.; Alayouni, R.R.; Elfaruk, M.S.; Hammam, A.R.A. Effect of Skim Milk Powder and Whey Protein Concentrate Addition on the Manufacture of Probiotic Mozzarella Cheese. Fermentation 2023, 9, 948. [Google Scholar] [CrossRef]
- Ahmed, M.E.; Hamdy, A.M.; El-Derway, Y.M.A.; El-Gazzar, F.E.; El-Naga, I.G.A. Impact of Probiotic Bacteria on the Chemical Characteristics of Low-fat Soft White Cheese. Assiut J. Agric. Sci. 2020, 51, 91. [Google Scholar] [CrossRef]
- Alsaleem, K.A.; Hamouda, M.E.A. Optimizing Probiotic Low-Fat Yogurt: The Benefits of Incorporating Defatted Rice Bran for Enhanced Quality and Functionality. Food Sci. Nutr. 2024, 12, 10242–10254. [Google Scholar] [CrossRef]
- Kim, S.Y.; Hyeonbin, O.; Lee, P.; Kim, Y.-S. The quality characteristics, antioxidant activity, and sensory evaluation of reduced-fat yogurt and nonfat yogurt supplemented with basil seed gum as a fat substitute. J. Dairy Sci. 2020, 103, 1324–1336. [Google Scholar] [CrossRef]
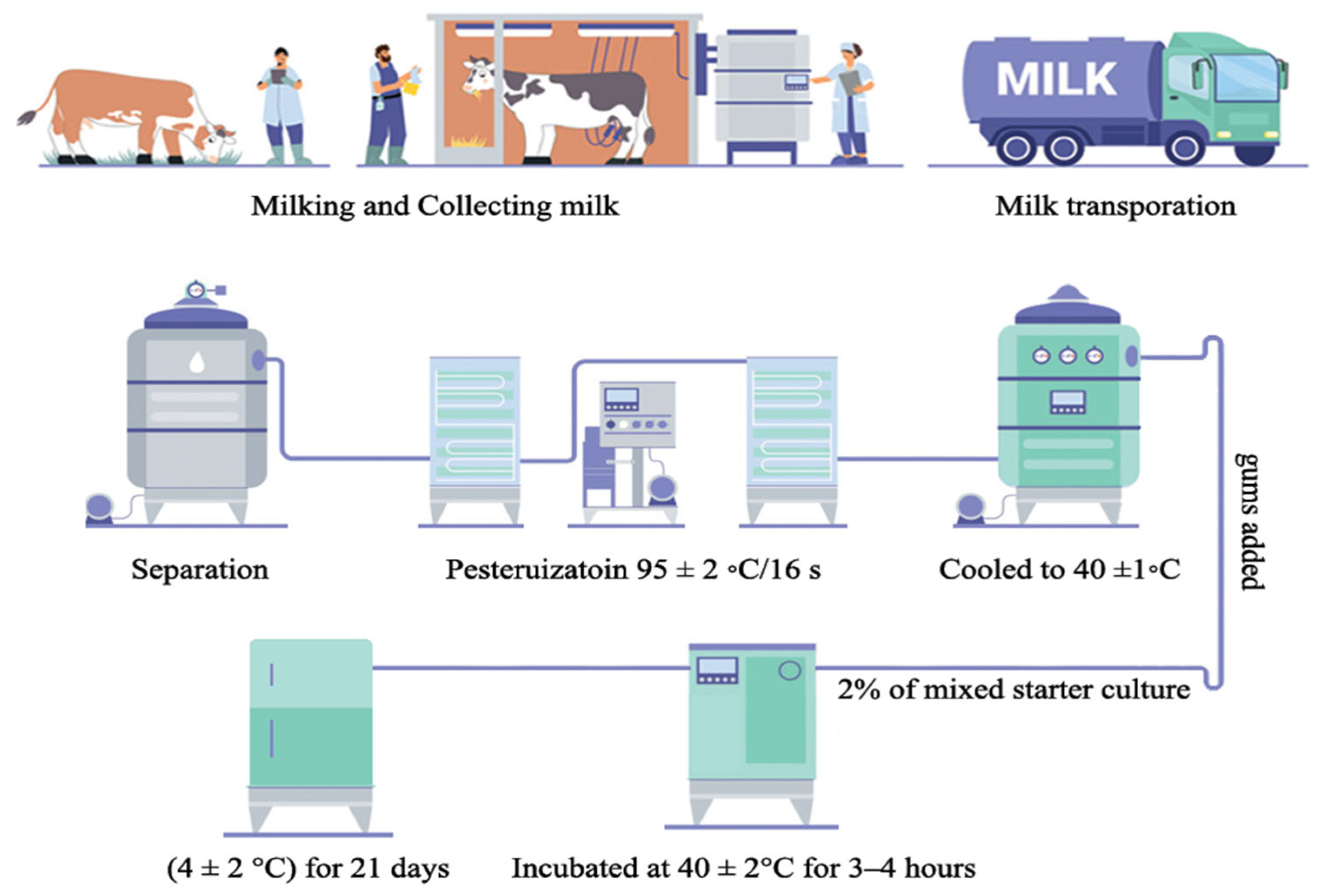
| Treatments | GG % | XG % |
|---|---|---|
| Control | - | - |
| Y1 | 0.5 | - |
| Y2 | 1 | - |
| Y3 | - | 0.5 |
| Y4 | - | 1 |
| Treatments 1 | Storage 2 | pH | TS% | Ash% | Protein% |
|---|---|---|---|---|---|
| Control | 0 | 4.60 ± 0.01 | 11.30 ± 0.05 | 0.79 ± 0.00 | 4.54 ± 0.20 |
| 7 | 4.44 ± 0.03 | 11.29 ± 0.04 | 0.76 ± 0.00 | 4.23 ± 0.08 | |
| 14 | 4.40 ± 0.01 | 11.32 ± 0.06 | 0.82 ± 0.00 | 4.47 ± 0.14 | |
| 21 | 4.03 ± 0.01 | 11.28 ± 0.05 | 0.78 ± 0.00 | 4.54 ± 0.11 | |
| Y1 | 0 | 4.60 ± 0.01 | 11.35 ± 0.04 | 0.87 ± 0.00 | 4.95 ± 0.01 |
| 7 | 4.42 ± 0.01 | 11.33 ± 0.06 | 0.84 ± 0.00 | 4.93 ± 0.02 | |
| 14 | 4.30 ± 0.01 | 11.31 ± 0.05 | 0.83 ± 0.00 | 4.88 ± 0.01 | |
| 21 | 3.90 ± 0.01 | 11.34 ± 0.04 | 0.88 ± 0.00 | 4.94 ± 0.01 | |
| Y2 | 0 | 4.60 ± 0.02 | 11.38 ± 0.05 | 0.93 ± 0.00 | 5.20 ± 0.01 |
| 7 | 4.39 ± 0.01 | 11.37 ± 0.04 | 0.91 ± 0.00 | 5.14 ± 0.01 | |
| 14 | 4.23 ± 0.01 | 11.36 ± 0.05 | 0.94 ± 0.00 | 5.13 ± 0.01 | |
| 21 | 3.54 ± 0.02 | 11.34 ± 0.05 | 0.92 ± 0.00 | 5.28 ± 0.01 | |
| Y3 | 0 | 4.60 ± 0.01 | 11.40 ± 0.05 | 0.96 ± 0.01 | 4.56 ± 0.06 |
| 7 | 4.36 ± 0.01 | 11.38 ± 0.04 | 0.98 ± 0.02 | 4.62 ± 0.10 | |
| 14 | 4.13 ± 0.01 | 11.36 ± 0.06 | 0.93 ± 0.00 | 4.58 ± 0.01 | |
| 21 | 3.37 ± 0.01 | 11.35 ± 0.05 | 0.96 ± 0.00 | 4.61 ± 0.01 | |
| Y4 | 0 | 4.60 ± 0.02 | 11.30 ± 0.05 | 1.08 ± 0.02 | 4.81 ± 0.10 |
| 7 | 4.20 ± 0.01 | 11.29 ± 0.04 | 1.10 ± 0.10 | 4.79 ± 0.00 | |
| 14 | 3.60 ± 0.01 | 11.32 ± 0.06 | 1.06 ± 0.01 | 4.84 ± 0.00 | |
| 21 | 3.03 ± 0.01 | 11.28 ± 0.05 | 1.10 ± 0.02 | 4.80 ± 0.00 | |
| Treatments | (<0.05) * | NS | (<0.05) * | (<0.05) * | |
| Storage | (<0.05) * | NS | NS | NS | |
| (Treatments × Storage) | (<0.05) * | NS | NS | NS |
| Treatments 1 | Storage 2 | Hardness (g) | Viscosity (cp) | Syneresis% | L* | b* | a* |
|---|---|---|---|---|---|---|---|
| Control | 0 | 0.66 ± 0.03 | 1659.80 ± 3.05 | 38.50 ± 0.45 | 62.78 ± 0.30 | 22.35 ± 0.32 | 12.40 ± 0.26 |
| 7 | 0.61 ± 0.07 | 1690.21 ± 2.07 | 40.50 ± 0.10 | 63.10 ± 0.07 | 22.45 ± 0.24 | 12.60 ± 0.19 | |
| 14 | 0.58 ± 0.01 | 1730.23 ± 4.71 | 44.50 ± 0.10 | 61.85 ± 0.16 | 21.75 ± 0.30 | 12.10 ± 0.15 | |
| 21 | 0.56 ± 0.02 | 1810.25 ± 4.12 | 51.50 ± 0.10 | 62.40 ± 0.20 | 22.50 ± 0.27 | 12.55 ± 0.35 | |
| Y1 | 0 | 0.71 ± 0.05 | 2026.32 ± 7.88 | 35.50 ± 0.10 | 68.90 ± 0.01 | 22.50 ± 0.08 | 12.80 ± 0.44 |
| 7 | 0.70 ± 0.04 | 2094.05 ± 6.17 | 36.80 ± 0.10 | 60.15 ± 0.28 | 21.70 ± 0.31 | 12.85 ± 0.14 | |
| 14 | 0.65 ± 0.08 | 2130.55 ± 4.12 | 39.50 ± 0.10 | 59.70 ± 0.12 | 23.05 ± 0.04 | 13.15 ± 0.11 | |
| 21 | 0.62 ± 0.01 | 2153.45 ± 5.37 | 46.50 ± 0.10 | 61.20 ± 0.60 | 21.90 ± 0.40 | 11.75 ± 0.25 | |
| Y2 | 0 | 0.79 ± 0.01 | 2174.21 ± 4.47 | 33.50 ± 0.10 | 64.45 ± 0.12 | 22.60 ± 0.05 | 12.45 ± 0.03 |
| 7 | 0.75 ± 0.04 | 2194.25 ± 10.47 | 35.00 ± 0.90 | 61.55 ± 0.55 | 22.05 ± 0.95 | 11.15 ± 1.00 | |
| 14 | 0.71 ± 0.07 | 2203.98 ± 3.16 | 37.50 ± 0.10 | 64.10 ± 0.25 | 23.00 ± 0.40 | 13.35 ± 0.25 | |
| 21 | 0.68 ± 0.03 | 2240.55 ± 10.87 | 43.50 ± 0.10 | 63.00 ± 0.15 | 22.15 ± 0.30 | 12.25 ± 0.10 | |
| Y3 | 0 | 0.85 ± 0.02 | 2359.21 ± 12.84 | 30.50 ± 0.10 | 59.50 ± 0.60 | 22.15 ± 0.20 | 11.80 ± 0.45 |
| 7 | 0.83 ± 0.12 | 2403.25 ± 13.67 | 32.50 ± 0.45 | 60.50 ± 0.45 | 23.00 ± 0.50 | 13.50 ± 0.35 | |
| 14 | 0.79 ± 0.05 | 2426.97 ± 5.32 | 35.00 ± 0.10 | 63.40 ± 0.20 | 22.20 ± 0.70 | 12.15 ± 0.40 | |
| 21 | 0.75 ± 0.01 | 2457.65 ± 2.97 | 40.50 ± 0.10 | 62.20 ± 0.25 | 21.50 ± 0.30 | 12.60 ± 0.20 | |
| Y4 | 0 | 0.91 ± 0.05 | 2537.28 ± 8.54 | 24.50 ± 0.90 | 63.00 ± 0.45 | 22.30 ± 0.90 | 13.20 ± 0.50 |
| 7 | 0.91 ± 0.09 | 2602.09 ± 10.87 | 26.50 ± 0.10 | 61.75 ± 0.10 | 21.40 ± 0.20 | 12.10 ± 0.12 | |
| 14 | 0.87 ± 0.01 | 2624.85 ± 7.83 | 30.00 ± 0.10 | 59.70 ± 0.30 | 22.05 ± 0.25 | 12.15 ± 0.60 | |
| 21 | 0.81 ± 0.02 | 2680.05 ± 6.08 | 32.00 ± 0.10 | 62.75 ± 0.50 | 22.20 ± 0.50 | 12.70 ± 0.45 | |
| Treatments | (<0.05) * | (<0.05) * | (<0.05) * | NS | NS | NS | |
| Storage | (<0.05) * | (<0.05) * | (<0.05) * | NS | NS | NS | |
| (Treatments × Storage) | (<0.05) * | (<0.05) * | (<0.05) * | NS | NS | NS |
| Treatments 1 | Storage 2 | Total Bacterial Counts (log CFU/g) | Lactobacillus dlebreuckii subsp. bulgaricus (log CFU/g) | Streptococcus thermophilus Counts (log CFU) | Bifidobacterium bifidum Counts (log CFU) |
|---|---|---|---|---|---|
| Control | 0 | 7.77 ± 0.05 | 5.62 ± 0.05 | 6.95 ± 0.03 | 3.94 ± 0.08 |
| 7 | 8.17 ± 0.12 | 6.82 ± 0.06 | 7.23 ± 0.04 | 4.07 ± 0.05 | |
| 14 | 8.27 ± 0.02 | 5.51 ± 0.02 | 6.64 ± 0.03 | 4.23 ± 0.06 | |
| 21 | 7.38 ± 0.14 | 5.47 ± 0.02 | 6.45 ± 0.06 | 3.77 ± 0.05 | |
| Y1 | 0 | 8.23 ± 0.07 | 8.32 ± 0.03 | 8.28 ± 0.01 | 5.95 ± 0.03 |
| 7 | 8.31 ± 0.12 | 8.52 ± 0.04 | 8.71 ± 0.03 | 6.26 ± 0.02 | |
| 14 | 8.43 ± 0.02 | 7.17 ± 0.02 | 8.23 ± 0.06 | 6.37 ± 0.05 | |
| 21 | 8.07 ± 0.03 | 8.21 ± 0.02 | 7.94 ± 0.03 | 5.71 ± 0.04 | |
| Y2 | 0 | 8.37 ± 0.07 | 8.57 ± 0.05 | 8.37 ± 0.05 | 6.47 ± 0.02 |
| 7 | 8.50 ± 0.03 | 8.71 ± 0.03 | 8.82 ± 0.07 | 6.75 ± 0.02 | |
| 14 | 8.72 ± 0.10 | 7.51 ± 0.01 | 8.05 ± 0.04 | 6.82 ± 0.04 | |
| 21 | 7.86 ± 0.06 | 8.48 ± 0.05 | 7.88 ± 0.08 | 7.17 ± 0.05 | |
| Y3 | 0 | 8.49 ± 0.07 | 8.80 ± 0.06 | 9.03 ± 0.02 | 8.07 ± 0.04 |
| 7 | 8.63 ± 0.11 | 8.93 ± 0.03 | 9.05 ± 0.06 | 8.08 ± 0.05 | |
| 14 | 8.82 ± 0.10 | 8.86 ± 0.02 | 8.93 ± 0.04 | 8.21 ± 0.03 | |
| 21 | 8.26 ± 0.12 | 8.61 ± 0.05 | 7.67 ± 0.05 | 7.66 ± 0.05 | |
| Y4 | 0 | 6.94 ± 0.09 | 8.88 ± 0.02 | 8.32 ± 0.05 | 7.18 ± 0.03 |
| 7 | 6.91 ± 0.04 | 9.17 ± 0.07 | 8.68 ± 0.08 | 7.30 ± 0.02 | |
| 14 | 6.91 ± 0.08 | 8.95 ± 0.02 | 8.22 ± 0.02 | 7.56 ± 0.01 | |
| 21 | 5.74 ± 0.15 | 8.73 ± 0.01 | 8.16 ± 0.06 | 7.16 ± 0.08 | |
| Treatments | (<0.05) * | (<0.05) * | (<0.05) * | (<0.05) * | |
| Storage | (<0.05) * | (<0.05) * | (<0.05) * | (<0.05) * | |
| (Treatments × Storage) | (<0.05) * | (<0.05) * | (<0.05) * | (<0.05) * |
| Treatments 1 | Storage 2 | Color and Appearance (15) | Body and Texture (35) | Flavor (50) | Total (100) |
|---|---|---|---|---|---|
| Control | 0 | 6.765 | 20.975 | 36.385 | 64.125 |
| 14 | 5.885 | 18.625 | 35.785 | 60.295 | |
| 21 | 5.885 | 16.805 | 34.445 | 57.135 | |
| Y1 | 0 | 7.485 | 24.825 | 30.385 | 62.695 |
| 14 | 6.885 | 21.825 | 32.085 | 60.795 | |
| 21 | 6.885 | 14.825 | 28.785 | 50.495 | |
| Y2 | 0 | 9.385 | 27.825 | 25.135 | 62.345 |
| 14 | 8.685 | 25.825 | 27.635 | 62.145 | |
| 21 | 7.885 | 20.825 | 21.085 | 49.795 | |
| Y3 | 0 | 10.885 | 30.625 | 41.845 | 83.355 |
| 14 | 10.885 | 28.725 | 44.505 | 84.115 | |
| 21 | 9.885 | 22.925 | 37.085 | 69.895 | |
| Y4 | 0 | 14.385 | 32.225 | 42.885 | 89.495 |
| 14 | 13.885 | 30.825 | 45.885 | 90.595 | |
| 21 | 13.685 | 26.825 | 40.235 | 80.745 | |
| Treatments | (<0.05) * | (<0.05) * | (<0.05) * | (<0.05) * | |
| Storage | (<0.05) * | (<0.05) * | (<0.05) * | (<0.05) * | |
| (Treatments × Storage) | (<0.05) * | (<0.05) * | (<0.05) * | (<0.05) * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elderwy, Y.; Kalita, R.; Hamouda, M.E.A.; Chaudhary, P.; Elfaruk, M.S.; Don, P.U.; Abdelsater, O.A.A. Influence of Guar Gum and Xanthan Gum on the Rheological Behavior, Texture, and Microstructure of Probiotic Low-Fat Yogurt. Processes 2025, 13, 3301. https://doi.org/10.3390/pr13103301
Elderwy Y, Kalita R, Hamouda MEA, Chaudhary P, Elfaruk MS, Don PU, Abdelsater OAA. Influence of Guar Gum and Xanthan Gum on the Rheological Behavior, Texture, and Microstructure of Probiotic Low-Fat Yogurt. Processes. 2025; 13(10):3301. https://doi.org/10.3390/pr13103301
Chicago/Turabian StyleElderwy, Yaser, Ratul Kalita, Mahmoud E. A. Hamouda, Pratibha Chaudhary, Mohamed S. Elfaruk, Pramith U. Don, and Omar A. A. Abdelsater. 2025. "Influence of Guar Gum and Xanthan Gum on the Rheological Behavior, Texture, and Microstructure of Probiotic Low-Fat Yogurt" Processes 13, no. 10: 3301. https://doi.org/10.3390/pr13103301
APA StyleElderwy, Y., Kalita, R., Hamouda, M. E. A., Chaudhary, P., Elfaruk, M. S., Don, P. U., & Abdelsater, O. A. A. (2025). Influence of Guar Gum and Xanthan Gum on the Rheological Behavior, Texture, and Microstructure of Probiotic Low-Fat Yogurt. Processes, 13(10), 3301. https://doi.org/10.3390/pr13103301






