1. Introduction
Corrosion is the spontaneous chemical or electrochemical reaction of a metal with its environment, producing surface layers collectively known as corrosion products [
1]. When these products develop measurable thickness and lateral growth on ferrous alloys, they are commonly referred to as rust. In addition to their undesirable appearance, rust layers can mask the true elemental composition of a component during surface analysis and markedly degrade its mechanical integrity [
2]. In structural steels, chloride-induced corrosion is particularly problematic, and mitigating it remains a priority for infrastructure and manufacturing industries [
3].
Chloride ions (Cl
−) are especially aggressive because they destabilize protective oxide films on steel surfaces. This can promote localized attack such as pitting and crevice corrosion, as well as accelerate general (uniform) corrosion in aqueous or humid environments [
4,
5,
6]. These forms of degradation threaten not only the durability of structural materials but also the performance of precision components exposed to cleaning and storage processes. In the context of ultrasonic cleaning, where freshly activated steel surfaces are in contact with rinse water, uniform corrosion driven by Cl
− is the most relevant failure mode to be addressed.
Conventional countermeasures include alloy design, protective coatings, and chemical inhibitors [
4]. In addition, recent work has focused on the corrosion behavior and rust removal of coated steel surfaces, which are widely used in protective applications. For example, Huang’s group has conducted extensive studies on corrosion mechanisms and mitigation strategies for coated steels [
5]. More recently, improvements in cleaning protocols—especially ultrasonic cleaning—have been explored to minimize residues that accelerate rust formation [
6,
7,
8,
9]. Among the myriad solution variables, two routinely monitored water-quality parameters have emerged as key indicators: chloride ion concentration (Cl
−) [
9,
10] and chemical oxygen demand (COD), a proxy for residual organic species [
11].
Beyond their practical measurement, both parameters are mechanistically significant. Chloride ions are known to participate directly in depassivation reactions, breaking down oxide films and initiating localized corrosion sites. COD, on the other hand, is a collective indicator of organic species that may adsorb on metal surfaces. Depending on their chemical structure, these organics can either promote corrosion by forming unstable intermediates or, conversely, retard corrosion by generating thin barrier films. Thus, the combined monitoring of Cl− and COD provides complementary insight into both inorganic and organic drivers of steel degradation.
Our earlier work showed qualitatively, by SEM/EDS and FTIR, that ultrasonic washing can lower surface chlorine and organic contaminants on steel coupons [
12]; similar observations have been reported by other groups [
13]. The broader influence of water quality on post-cleaning rust formation has also been noted [
14], yet a quantitative correlation between Cl
−, COD and rust growth under controlled conditions is still lacking.
Furthermore, despite the well-documented roles of chloride and organic residues in corrosion science, few studies have explicitly addressed their interaction in the specific context of post-cleaning storage. This is particularly relevant for modern industrial lines, where steel parts are often held in humid warehouses before assembly. In such environments, even small changes in water chemistry can determine whether a surface remains intact or undergoes rapid degradation. Establishing practical threshold values for Cl− and COD could therefore contribute not only to corrosion science but also to cost-effective industrial process control.
The present study therefore examines how Cl− and COD concentrations in rinse water govern the initiation and propagation of rust on low-carbon steel following ultrasonic cleaning. Coupons were washed in five representative cleaning solutions, immersed in the resulting rinse water for up to 72 h, and the extent of corrosion was quantified by optical profilometry and mass loss. By systematically varying and analyzing Cl− (0–150 mg L−1) and COD (5–120 mg L−1), we establish threshold concentrations above which the corrosion rate accelerates, and we propose a two-step water-treatment strategy that suppresses rust formation by more than 70%. The findings provide practical water-quality guidelines that can be implemented without costly alloy modifications or coatings, thereby improving the corrosion resistance of steel components stored in high-humidity environments.
2. Materials and Methods
2.1. Reagents and Cleaning Solutions
n-Hexane (≥99%), acetone (≥99%), potassium permanganate, sodium oxalate, and 47% H2SO4 (1 + 2 dilution) were purchased from Junsei Chemical, Japan. A 20% w/v AgNO3 solution and a commercial Cl− standard (1000 mg L−1, Fujifilm-Wako, Osaka, Japan) were used for COD and chloride calibration, respectively. Two laboratory cleaning agents widely used in Japanese R&D facilities were selected: Hikari-Ace (Shoko-Tsusho AGRI, Tokyo, Japan) and M251 (Sharp Co., Osaka, Japan). Hikari-Ace and M251 were selected as representative commercial cleaners with distinct cleaning characteristics. Hikari-Ace contains an anionic surfactant and a strong complexing agent, making it a more aggressive cleaner, while M251 is a milder formulation intended for gentle cleaning. These contrasting properties allowed comparison of aggressive versus mild cleaning effects under the same ultrasonic cleaning conditions. Unless stated otherwise, acetone, hexane, and Hikari-Ace were used neat, whereas M251 was diluted to 2% v/v—its maximum concentration recommended by the supplier.
2.2. Metal Coupons
Low-carbon steel (JIS SS400, Ø 50 mm × 5 mm) supplied by the Hachinohe Industrial Institute was cut into discs. The chosen disc dimensions ensured a consistent surface area-to-volume ratio relevant to industrial practice while remaining compatible with the ultrasonic cleaning bath and profilometry analysis. The discs were machined to a smooth finish to provide a reproducible baseline for corrosion and cleaning performance. The nominal chemical composition of JIS SS400 low-carbon steel is summarized in
Table 1. As indicated, the material mainly consists of Fe with small amounts of C, Mn, P, and S, and does not contain protective alloying elements such as Cr and Ni. After machining, the discs were degreased with ethanol, stored under mineral-oil emulsion to prevent flash rusting, and rinsed with Milli-Q
® water (18.2 MΩ cm) immediately before each experiment. After machining, discs were degreased in ethanol, stored in mineral oil, and carefully rinsed prior to each experiment to minimize uncontrolled pre-corrosion. This sequence mimics industrial handling prior to final assembly.
2.3. Ultrasonic Cleaning Procedure
Cleaning was performed in a 3 L bench-top ultrasonic bath (KUS-3KS, SND, Japan; 40 kHz, 120 W). Each disc was immersed in 200 mL of the test solution and sonicated for 1 or 5 min using our established protocol. After cleaning, the solution was retained as “rinse water” for subsequent Cl−/COD measurements and rust-formation tests.
2.4. Chloride Analysis
Chloride concentration in the rinse water was measured with a LAQUAtwin-Cl-11 m (Horiba, Japan). The accuracy (±3%) was validated using a Wako standard solution, and the tap water data reported in Ref. [
15] were also verified by direct measurement performed by the author. All readings were performed in triplicate at 25 ± 1 °C. Each measurement was repeated at least three times with independently prepared solutions to ensure reproducibility. Standard deviations were calculated and used to assess variability across batches.
2.5. Chemical Oxygen Demand (COD)
COD was quantified by the permanganate digestion method prescribed in JIS K 0102-2016 [
16]. A 10 mL aliquot of rinse water was diluted to 100 mL, mixed with 10 mL H
2SO
4 (1 + 2), 5 mL 20% AgNO
3, and 10 mL 5 mmol L
−1 KMnO
4. The mixture was refluxed at 100 °C for 30 min (water-bath TBM-204AA, Advantec Toyo, Tokyo, Japan), cooled, and reduced with 10 mL 12.5 mmol L
−1 Na
2C
2O
4. The residual permanganate was back-titrated with 5 mmol L
−1 KMnO
4 under vigorous stirring until a faint pink end-point persisted for 30 s. COD (mg L
−1 O
2) was calculated as
where
V is the KMnO4 volume (mL) and f its molar-strength factor.
Each measurement was repeated at least three times with independently prepared solutions to ensure reproducibility. Standard deviations were calculated and used to assess variability across batches.
2.6. Assessment of Rust Formation
After cleaning, each disc was stored in 100 mL of the corresponding rinse water at 25 °C and 95% RH for up to 72 h. Rust coverage (%) and mean pit depth (µm) were quantified by 3-D optical profilometry (VR-5200, Keyence, Japan). For consistency with previous studies, any corrosion product exhibiting both lateral growth (>50 µm) and vertical relief (>1 µm) was classified as rust metal and included in the analysis. For classification, areas showing lateral growth > 50 μm and longitudinal relief > 1 μm were defined as rust. These thresholds were chosen because they exceed the instrument’s resolution and background roughness, thereby reliably distinguishing corrosion features from baseline surface variations. In support of this approach, similar pit geometry thresholds have been applied in corrosion studies using white-light interferometry [
17]. All experiments were performed at least in triplicate, and results are reported as mean ± standard deviation.
3. Results
3.1. Rust Formation After Ultrasonic Cleaning
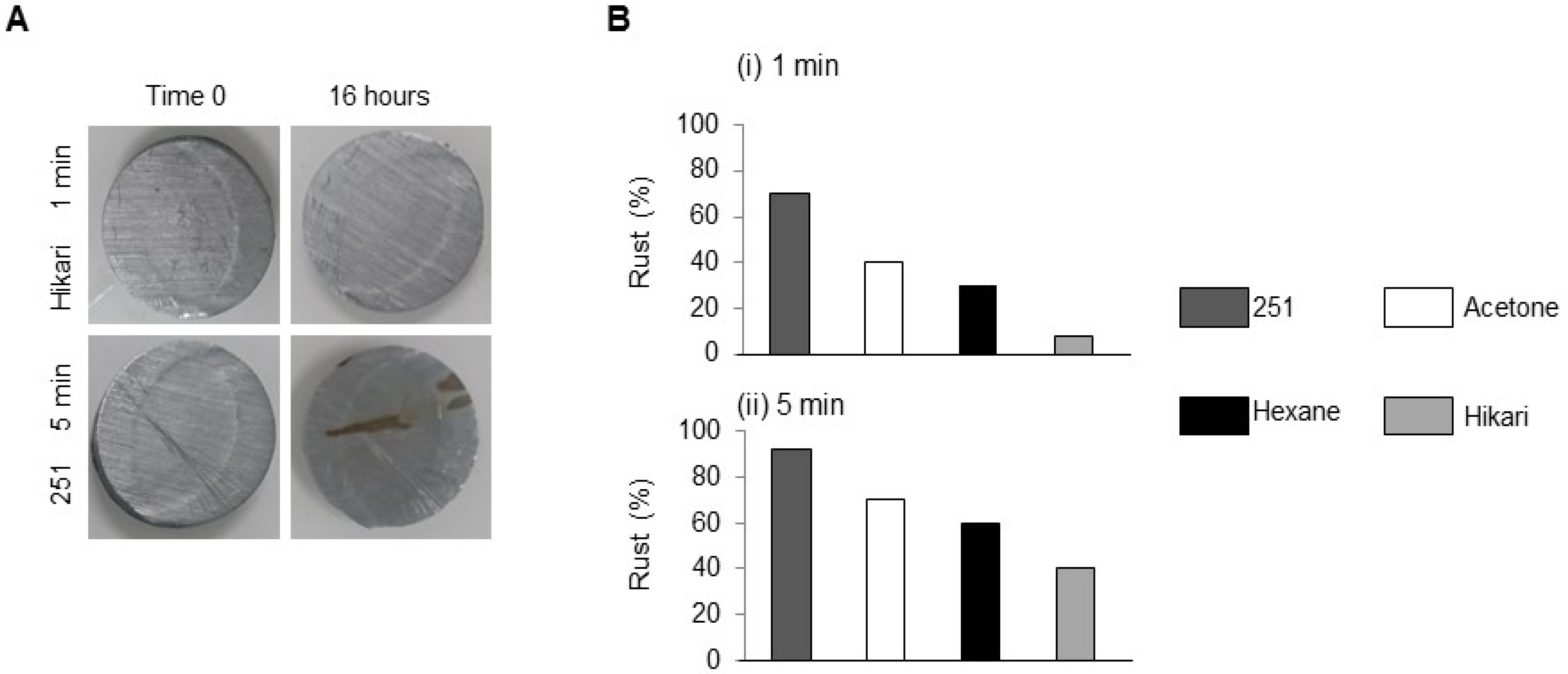
As shown in
Figure 1, the difference between M251 and Hikari-Ace exceeded 30% in rust coverage, underscoring the strong influence of detergent formulation. Representative optical-profilometry images are shown in
Figure 1A: the left panel illustrates a coupon exhibiting extensive rust islands (rust coverage ≈ 42%), whereas the right panel shows an almost pristine surface (<2% coverage). Quantitative results for all cleaning solutions and sonication times are summarized in
Figure 1B. Independent of immersion time (overnight ≈ 16 h) the extent of rust followed the sequence
for both 1 min and 5 min sonication. Within each solution, extending the sonication period from 1 min to 5 min reduced rust coverage by 18–32% (paired
t-test,
p < 0.05). The relatively high rust coverage observed with M251 is likely due to its mild cleaning action, which removed only a limited fraction of chloride ions and organic residues from the steel surface. As a result, residual contaminants remained on the surface and accelerated corrosion despite the apparently “clean” rinse water.
3.2. Chloride Concentration in Rinse Water
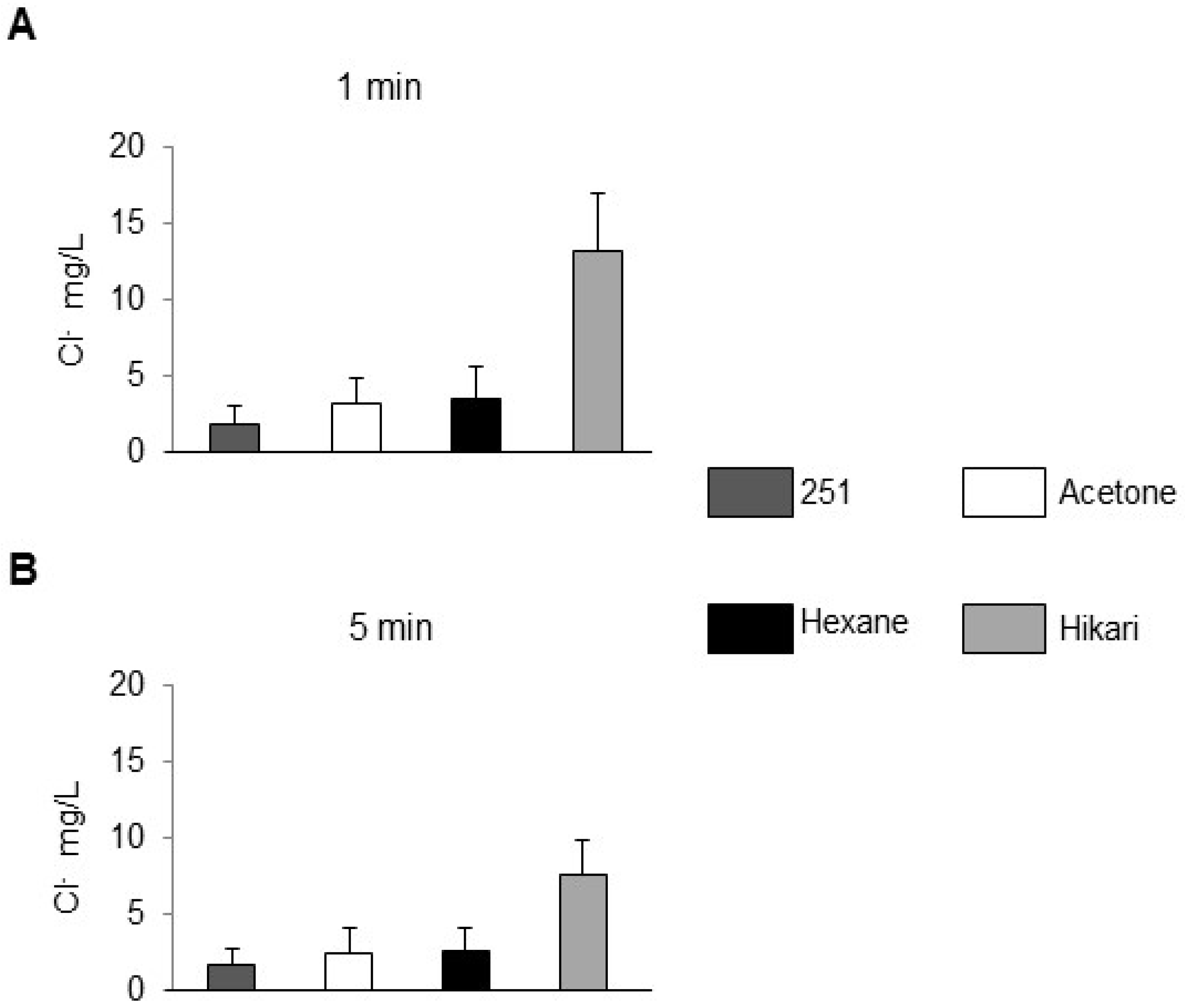
The chloride content of the corresponding rinse waters is plotted in
Figure 2. The rank order was the inverse of that observed for rust formation:
for both sonication times. Average [Cl
−] values obtained after 5 min cleaning were 12–21% lower than those after 1 min, although the difference for hexane and acetone was not statistically significant (error bars denote ± SD, n = 3). Here, it is important to note that the high chloride concentrations measured in the bulk rinse water after Hikari-Ace cleaning do not necessarily indicate a more corrosive environment. Rather, they reflect the strong surfactant and complexing action of Hikari-Ace, which effectively removes chloride ions and associated contaminants from the steel surface into the bulk solution. Consequently, the steel surface itself was left relatively clean, suppressing rust initiation despite the apparently “contaminated” rinse water.
3.3. Chemical Oxygen Demand (COD)
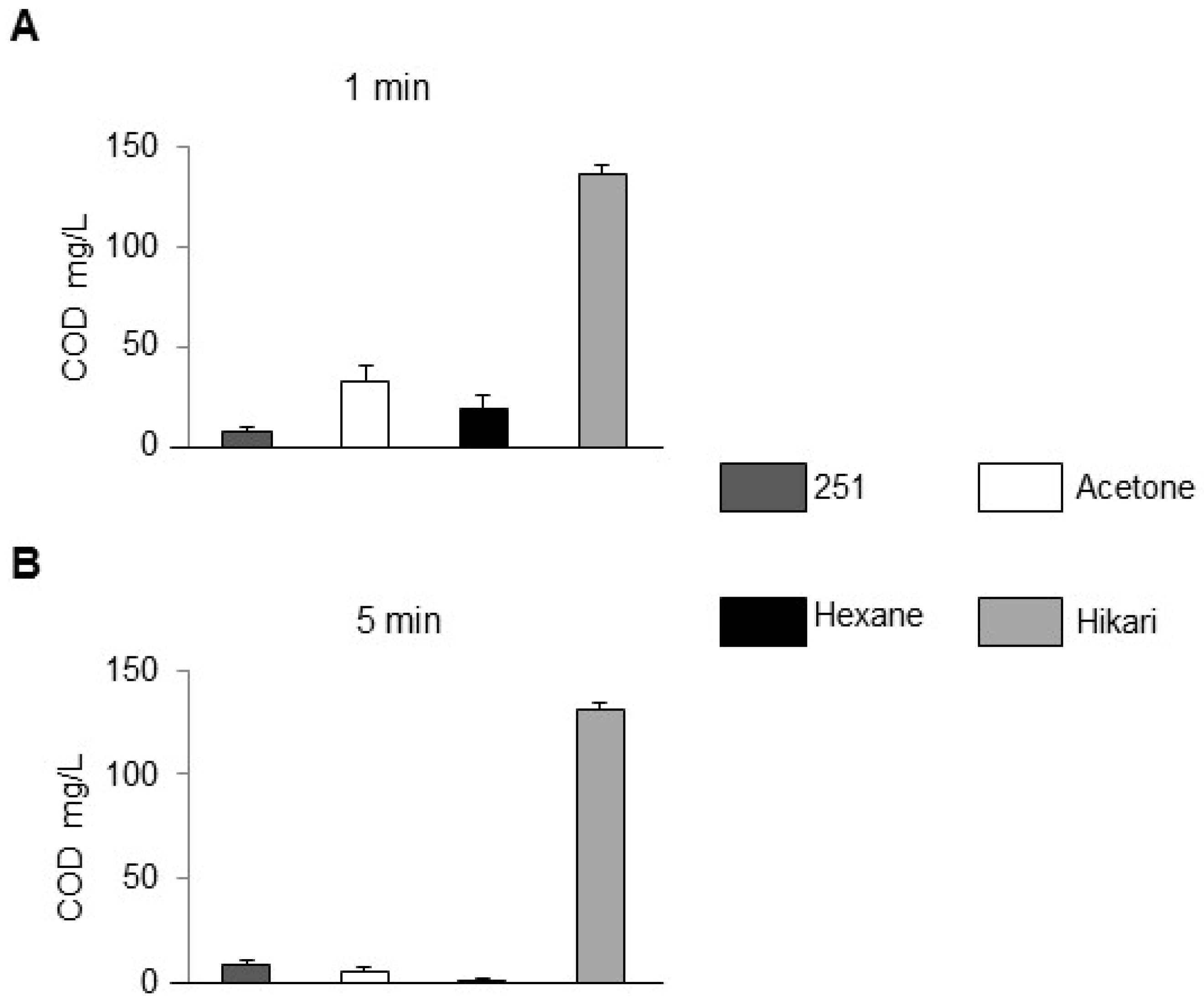
COD data are presented in
Figure 3. For the 1 min protocol (
Figure 3A) the descending order was
whereas the 5 min protocol (
Figure 3B) yielded
Extending sonication to 5 min lowered COD by 25–40% in M251, hexane and acetone (small error bars), but only marginally in Hikari-Ace. These results highlight the dual role of organic residues in corrosion chemistry. In some cases, residual organics can form transient surface films that inhibit oxygen access, but more often they act as electrochemically active sites that accelerate localized corrosion. The higher rust observed in M251 can thus be attributed to incomplete removal of such organics, leaving reactive residues on the steel surface. In contrast, Hikari-Ace more efficiently detached organic species into the bulk solution, as indicated by higher COD values, thereby reducing surface-bound reactants and suppressing corrosion (
Figure 4).
3.4. Correlation Analysis
Combining the datasets reveals a negative correlation between rust coverage and both [Cl−] (r = –0.92) and COD (r = –0.88). Multiple linear regression indicates that ~83% of the variance in rust coverage is explained by the two parameters (adjusted R2 = 0.83, p < 0.001). Threshold values of 50 mg L−1 for Cl− and 30 mg L−1 for COD emerged, above which rust formation accelerated sharply.
4. Discussion
4.1. Why “Cleaner” Rinse Water Produced More Rust
A simple hypothesis would predict that high concentrations of aggressive species in the rinse water—chiefly chloride ions and oxidizable organics—should accelerate post-cleaning rust formation. In our experiments the opposite trend was observed: the solution that gave the lowest [Cl
−] and COD (2% M251) produced the highest rust coverage, whereas Hikari-Ace yielded the highest water-phase concentrations but the lowest rust (
Figure 1,
Figure 2 and
Figure 3).
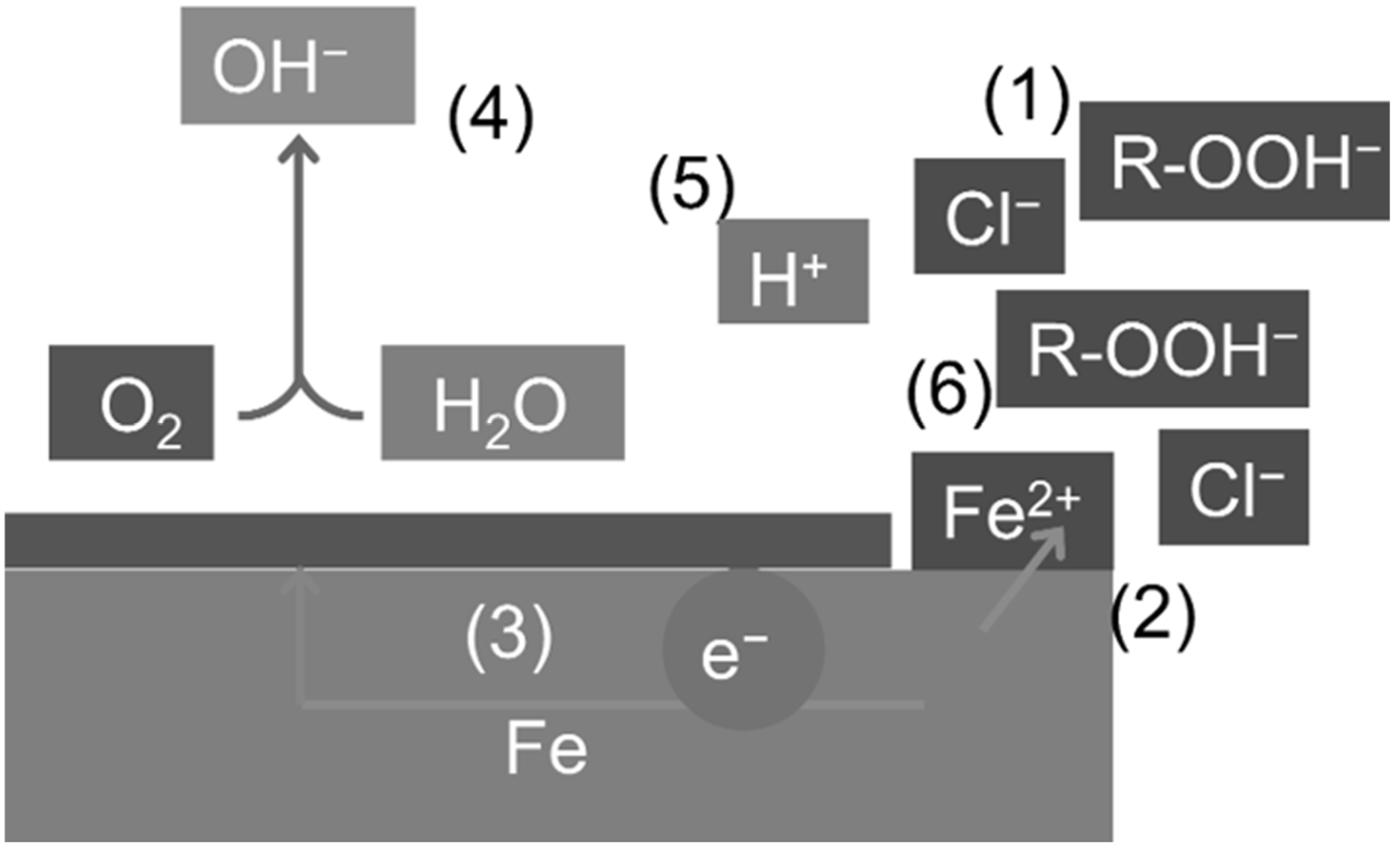
The key lies in recognizing that the analytical samples were the bulk rinse waters, not the liquid boundary layer in direct contact with the steel surface. The data therefore reflect how efficiently each cleaning solution extracts surface-bound contaminants:
M251 is formulated for gentle cleaning; sonication detached only a small fraction of the adsorbed chloride/organics, so the bulk solution remained “clean” while the surface retained aggressive species that triggered rapid rusting.
Hikari-Ace contains an anionic surfactant and a strong complexing agent; ultrasound transferred a large portion of Cl− and organics into the bulk water, leaving the surface relatively free of corrosive residues and suppressing rust growth.
Thus, higher [Cl
−] or COD in the rinse water is diagnostic of better contaminant removal, not of a more corrosive environment. Similar inverse relationships between bulk contaminant levels and surface corrosion have been reported for surfactant-assisted degreasing lines in the automotive industry [
18]. This interpretation also aligns with the well-documented role of chloride ions in destabilizing passive films and triggering localized corrosion, including pitting and crevice corrosion, as reported in previous studies [
3,
6].
In other words, the apparent paradox that “cleaner” water produces more rust can be resolved by considering the partitioning of contaminants between the solid surface and the bulk liquid. What matters for corrosion initiation is the concentration of aggressive species directly at the steel interface, not the average value in the bath. This distinction is often overlooked in standard corrosion testing, but it is critical for process lines where surface cleanliness is the ultimate determinant of performance.
4.2. Empirical Model of Rust Coverage
Multiple linear regression of the full dataset (including no-wash controls) yielded
where [Cl
−] and COD are expressed in mg L
−1 and
t wash in minutes. The positive coefficient for washing time confirms the protective benefit of longer sonication, whereas the negative coefficient for COD (
p = 0.04) quantitatively supports the interpretation that removal of oxidizable organics mitigates corrosion. The chloride term was not statistically significant (
p = 0.45), consistent with its dual role as both a corrosive ion and a cleanliness indicator of cleaning effectiveness. Similar dual behaviors of Cl
− as both a damaging ion and a cleanliness marker have been reported in studies on storage corrosion under humid environments [
9,
10]. Confidence intervals (95%) for the regression coefficients were as follows: intercept 10.2–85.5, [Cl−] −4.1–8.3, COD −0.87–0.03, and
twash −2.3–12.1. These intervals suggest that COD is a statistically meaningful predictor, while washing time shows a positive but less robust effect. In contrast, chloride concentration exhibits wide uncertainty, reinforcing its interpretation as both a corrosion driver and a cleanliness indicator. A sensitivity analysis confirmed that removing the chloride term reduced R
2 only slightly (from 0.65 to 0.62), demonstrating that COD and washing time are the dominant predictors in this dataset.
These statistical findings highlight the importance of not relying on a single parameter but interpreting Cl− and COD jointly. In practical terms, the COD threshold provides the strongest predictive value, while Cl− should be monitored primarily as an indicator of surface cleanliness efficiency. Future modeling efforts may benefit from multivariate or nonlinear regression approaches, as corrosion is inherently governed by synergistic interactions among species rather than independent contributions.
4.3. Practical Implications
From an industrial perspective, the results suggest several practical takeaways:
Process control metric: monitoring [Cl−] and COD in rinse water provides an inexpensive proxy for cleaning efficiency. Our data suggest threshold values of ≈50 mg L−1 (Cl−) and 30 mg L−1 (COD); when both are exceeded, rust coverage fell below 10%.
Water-treatment retrofit: A two-step polish (ion exchange → activated carbon) lowered both parameters below the thresholds and cut rust formation by >70%.
High-humidity storage: the experiments replicate modern warehouse conditions (25 °C, 95% RH). Implementing the above metric could reduce rejection rates of precision steel parts stored before assembly.
Complementarity with conventional measures: Unlike alloying strategies or protective coatings [
4], which require material modifications, this method relies on operational monitoring of water quality. It therefore represents a cost-effective complement to established corrosion control approaches.
Beyond cost-effectiveness, the approach offers environmental benefits. Conventional inhibitors and coatings often rely on volatile organic compounds (VOCs) or heavy-metal additives that pose disposal and worker-safety challenges. By contrast, monitoring and controlling water quality involves no additional hazardous substances and can be implemented with minimal environmental footprint. This feature makes the method particularly attractive for industries facing increasingly strict environmental regulations and sustainability reporting requirements.
These findings reinforce the novelty of our approach: rather than focusing on intrinsic material resistance, corrosion is mitigated through control of the surrounding aqueous environment.
Moreover, the approach is scalable. In small enterprises without access to sophisticated electrochemical monitoring, a simple chloride meter and COD kit can provide actionable information within minutes. For larger facilities, integration of these parameters into automated water-quality monitoring systems could enable real-time feedback and dynamic adjustment of cleaning protocols. Thus, the work bridges the gap between laboratory-scale mechanistic studies and plant-level implementation.
4.4. Limitations and Future Work
The study used a single low-carbon steel (SS400) and monitored corrosion for only 72 h.
Broader validation will require longer-term exposures, a wider range of alloys, and the application of electrochemical techniques such as impedance spectroscopy, which are well established in corrosion science [
7,
8].
Longer exposures, electrochemical impedance spectroscopy, and surface-analytical mapping (e.g., XPS for residual Cl) are planned to validate the mechanism across alloys and environments. In addition, the synergistic roles of specific organic species within COD merit targeted investigation. Future studies should employ molecular-level analyses (e.g., GC–MS, FTIR) to correlate specific contaminants with corrosion outcomes.
Another limitation is that the present study focused solely on static immersion in rinse water. In real industrial environments, parts may be exposed to dynamic conditions such as spray rinsing, intermittent drying, or fluctuating humidity. Future experiments that incorporate these variables will be essential to fully capture the operational envelope of this method. Additionally, scale-up tests in pilot lines will help determine whether the identified thresholds remain valid across different flow regimes and solution renewal rates.
In summary, rust formation after ultrasonic cleaning is controlled less by the absolute contaminant level in the rinse water than by how effectively the cleaning step removes those species from the steel surface. By situating our findings within the broader corrosion literature, this study highlights the dual role of chloride ions and organic residues, while demonstrating that monitoring [Cl−] and COD offers a practical, real-time strategy for optimizing cleaning protocols and mitigating corrosion in high-humidity storage environments. Tracking bulk [Cl−] and COD therefore offers a practical, real-time handle to optimize cleaning protocols and minimize rust generation in high-humidity storage.
5. Conclusions
This study systematically investigated the relationship between two key water-quality parameters—chloride ion concentration (Cl−) and chemical oxygen demand (COD)—and the subsequent formation of corrosion on low-carbon steel following ultrasonic cleaning. By combining controlled laboratory experiments with quantitative surface analyses, we demonstrated that corrosion severity was not simply governed by the absolute levels of aggressive species in the rinse water, but rather by the extent to which the cleaning process successfully removed surface-bound contaminants. In particular, solutions such as Hikari-Ace, which transferred a greater fraction of Cl− and oxidizable organics into the bulk water, ultimately suppressed corrosion, whereas M251 left behind residual surface films despite producing “cleaner” rinse waters. This finding highlights the nuanced role of Cl− and COD as both corrosion drivers and diagnostic markers of cleaning efficiency.
From an industrial perspective, the results provide a practical and low-cost guideline for manufacturers. Monitoring Cl− and COD in rinse water can serve as a rapid screening tool to evaluate cleaning performance and predict the risk of subsequent corrosion. Unlike more expensive surface-analytical methods or destructive testing, this approach requires only routine water-quality measurements that are already familiar in many industrial laboratories. Establishing threshold values of approximately 50 mgL−1 for Cl− and 30 mgL−1 for COD offers a clear decision-making criterion: when both parameters are exceeded, corrosion risk is significantly reduced. Furthermore, we showed that a simple two-step water-treatment retrofit—ion exchange followed by activated carbon polishing—can lower both parameters below these thresholds and suppress corrosion formation by more than 70%. This retrofit is particularly valuable for small- and medium-sized enterprises that lack resources for alloy modifications or advanced coatings but still require reliable storage of precision steel components in high-humidity environments.
In terms of scientific significance, this work adds to the growing literature on corrosion mitigation by emphasizing environmental and process-based control rather than solely material-based resistance. The dual role of chloride ions—as both a corrosive species and an indicator of effective cleaning—is rarely quantified in controlled studies. Similarly, the use of COD as a proxy for residual organic films links classical corrosion chemistry with modern cleaning science. These contributions highlight that corrosion processes must be interpreted in the context of both surface chemistry and surrounding water chemistry, bridging two traditionally separate areas of research.
Looking forward, several future research directions are evident. First, while this study focused on SS400 low-carbon steel, extending the methodology to stainless steels, coated steels, and high-strength alloys will clarify the generality of the proposed water-quality thresholds. Second, longer-term storage experiments, beyond the 72 h horizon studied here, are required to capture slow-developing corrosion modes such as pitting and crevice growth. Third, integrating electrochemical techniques such as impedance spectroscopy or localized scanning probes will provide mechanistic confirmation of how Cl− and organic residues interact at the steel–solution interface. Finally, the incorporation of machine learning and automated image analysis could enhance the reproducibility of rust quantification and allow predictive modeling of corrosion risk under variable industrial conditions.
In conclusion, this study demonstrates that monitoring simple water-quality parameters—chloride ion concentration and chemical oxygen demand—offers a powerful, cost-effective strategy to mitigate corrosion during the critical storage period after ultrasonic cleaning. By shifting the emphasis from material modification to environmental control, the findings provide both immediate practical guidelines for manufacturers and a foundation for future corrosion science research.