The Efficient PAE Degradation by Glutamicibacter sp. FR1 and Its Molecular Mechanism
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Strains
2.2. Isolation and Identification of Bacteria
2.3. PAE Degradation Characteristics of Strain FR1
2.4. Complete-Genome Sequencing of Strain FR1
2.5. Cloning of Candidate Genes of PAE Hydrolases
2.6. Expression and Purification of Hydrolase DphGB1 and MphGB2
2.7. Functional Identification of Hydrolases DphGB1 and MphGB2
2.8. Molecular Docking
2.9. Analytic Method
3. Results
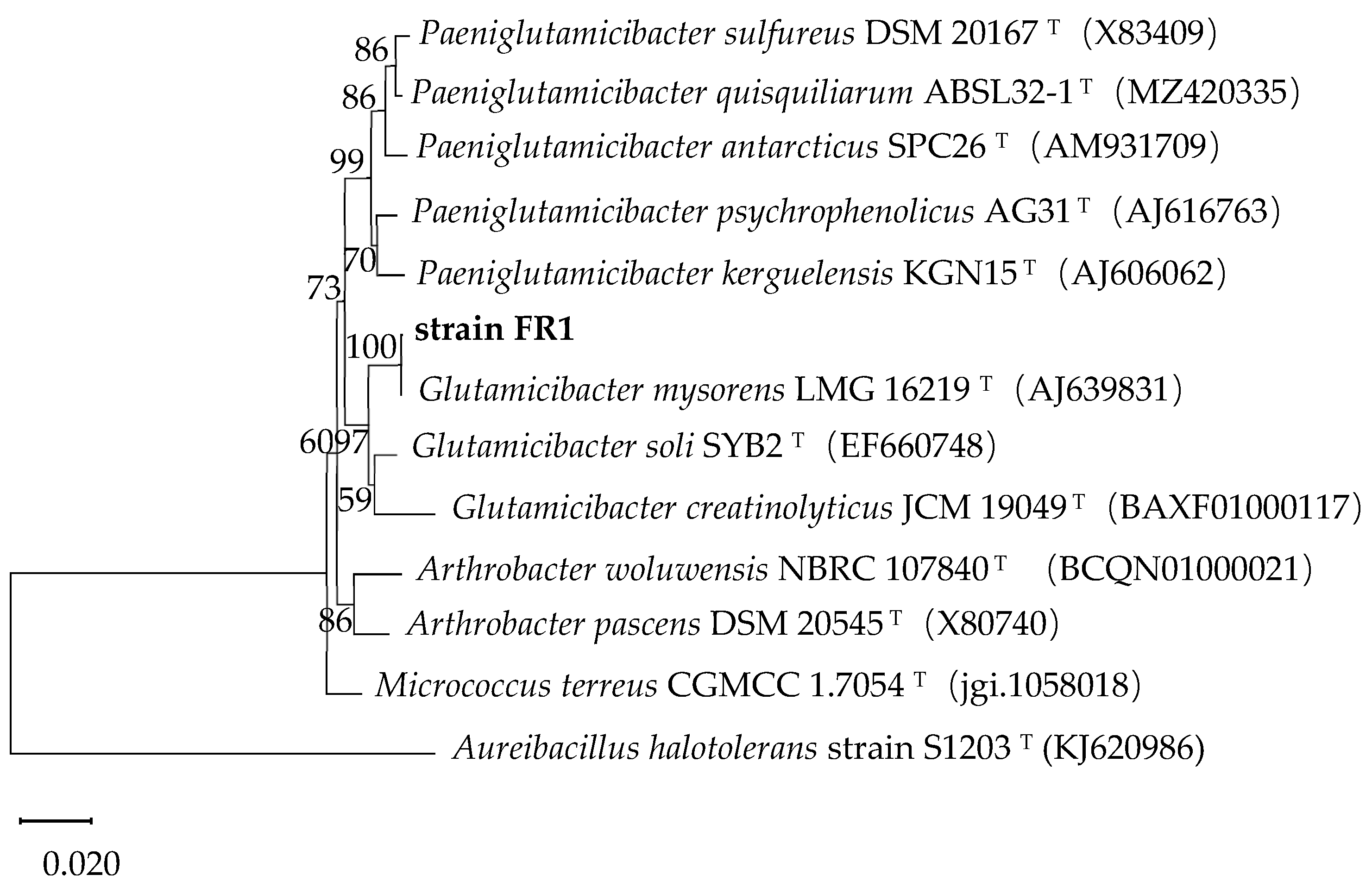
3.1. Isolation and Identification of the DBP-Degrading Strain
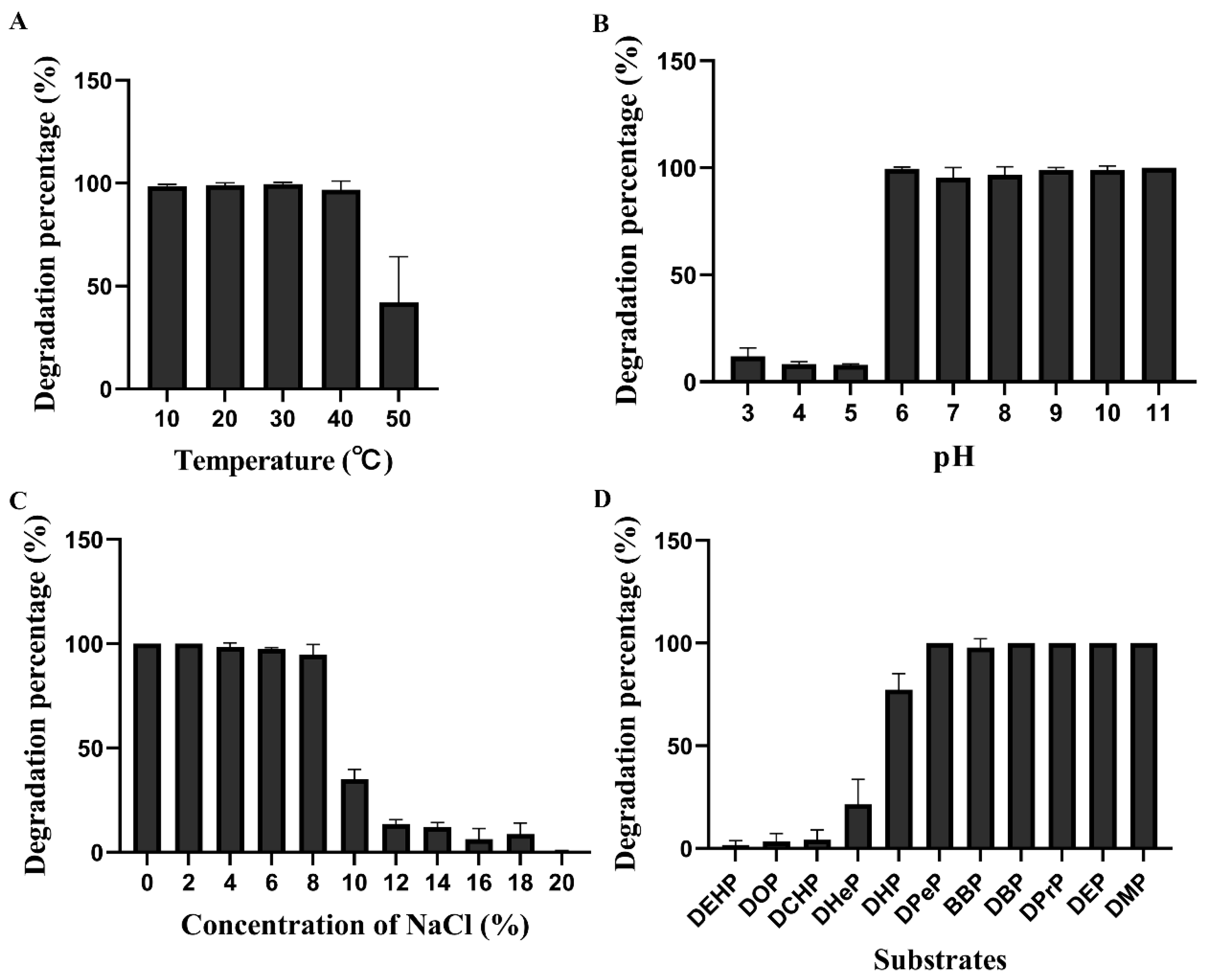
3.2. Degradation of PAEs by Glutamicibacter sp. FR1
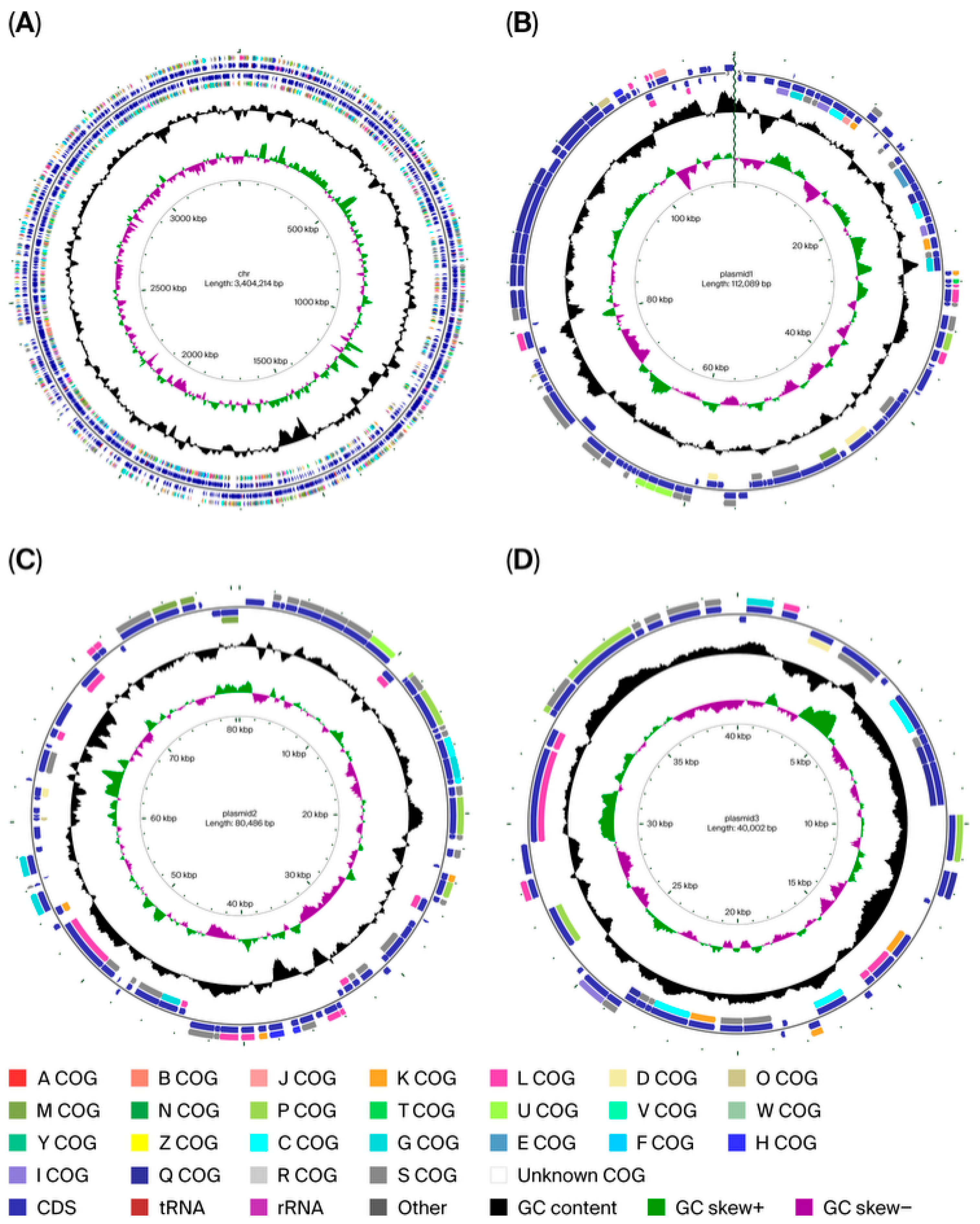
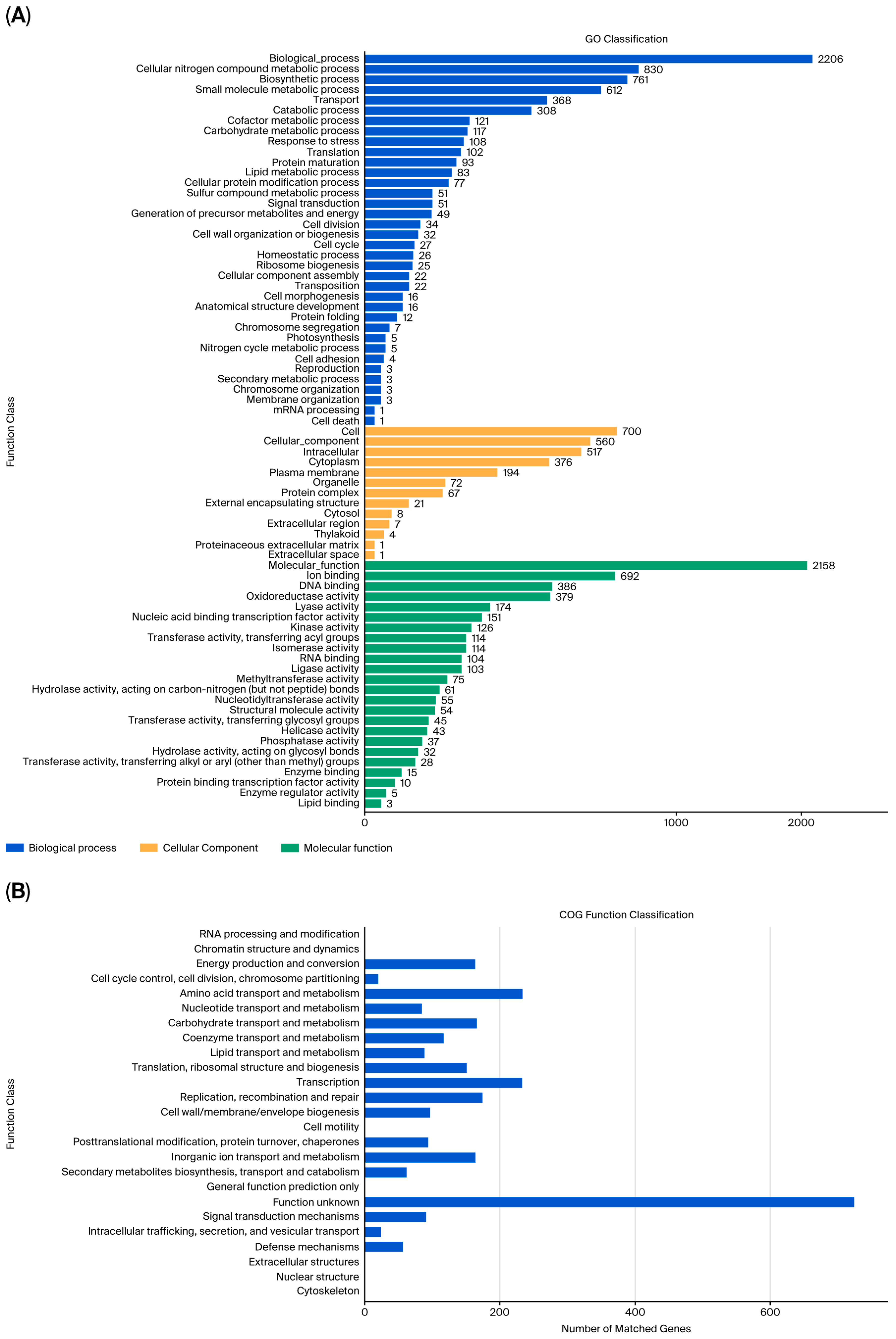
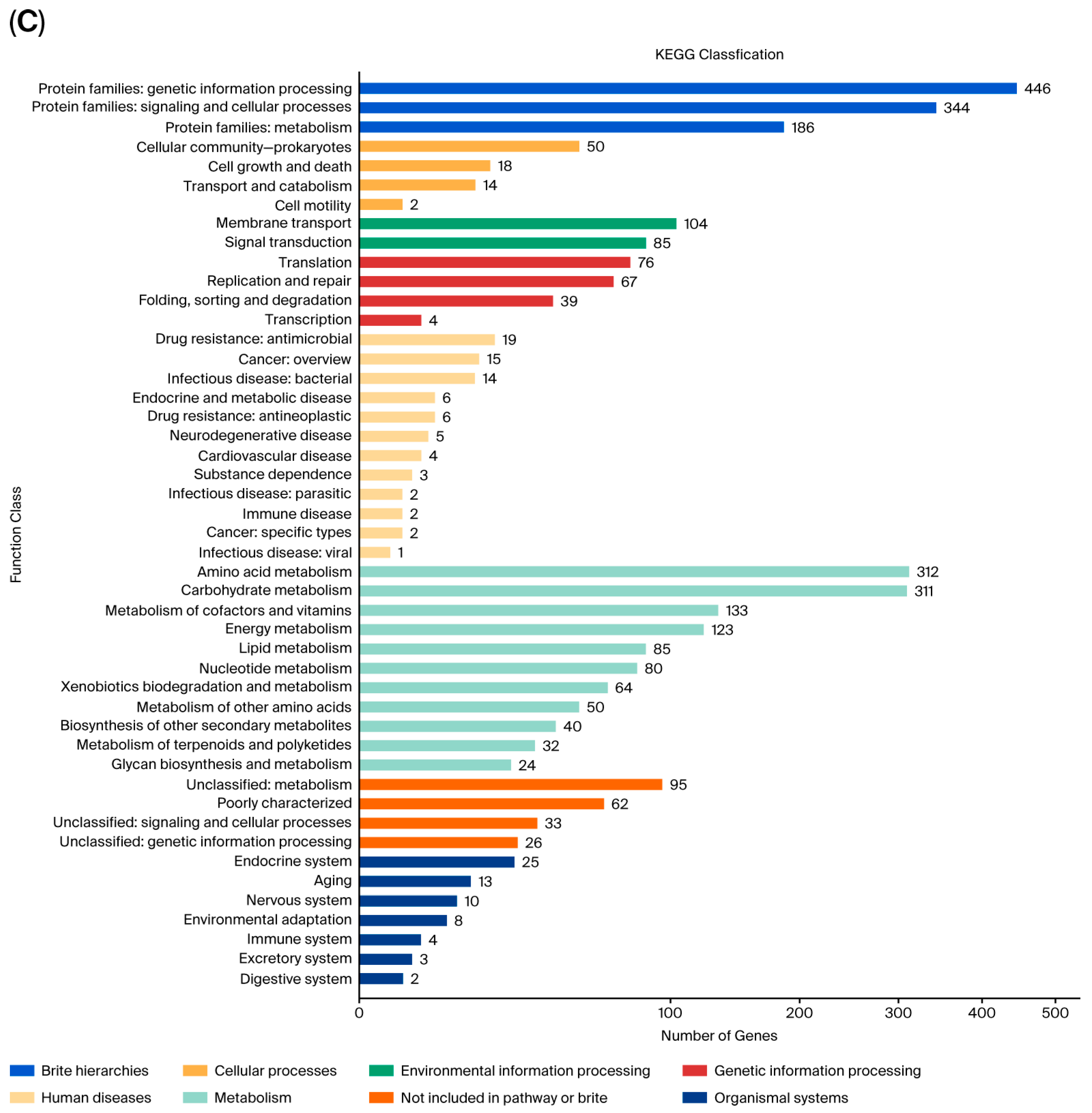
3.3. Complete-Genome Sequencing and Genomics Analysis of Strain FR1
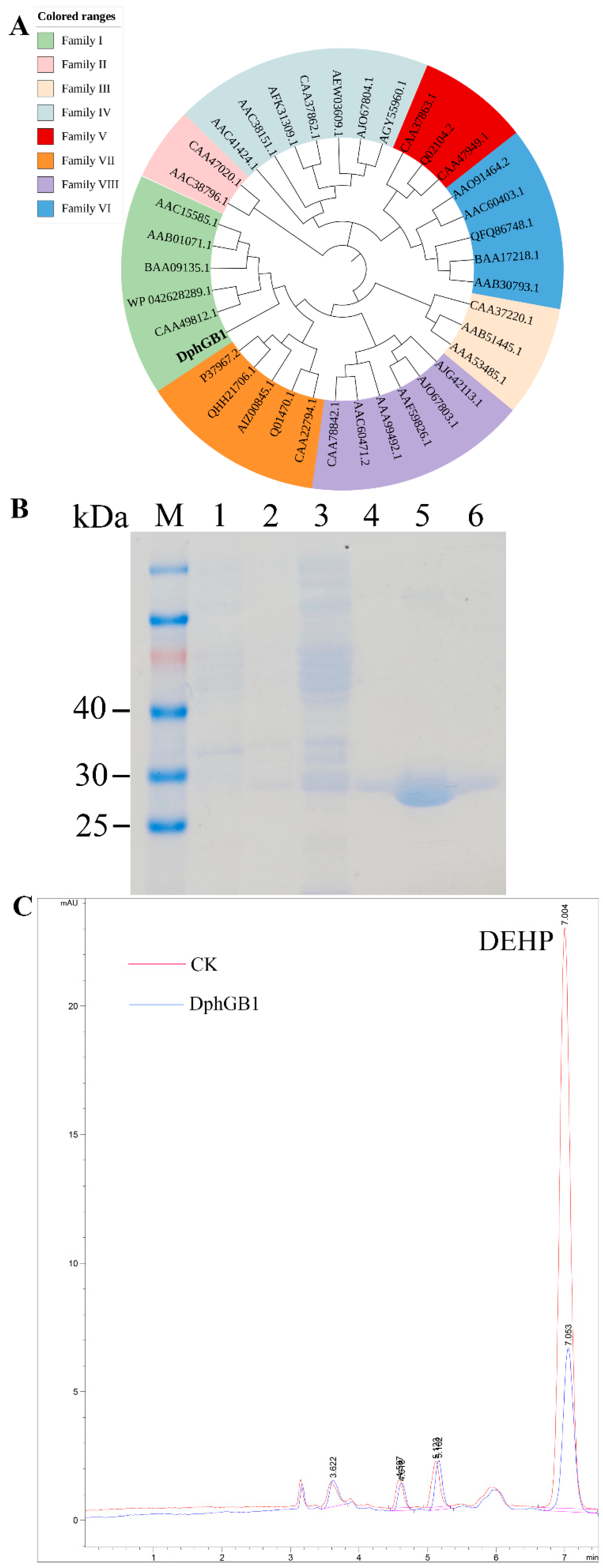
3.4. Key Genes Associated with PAE Degradation
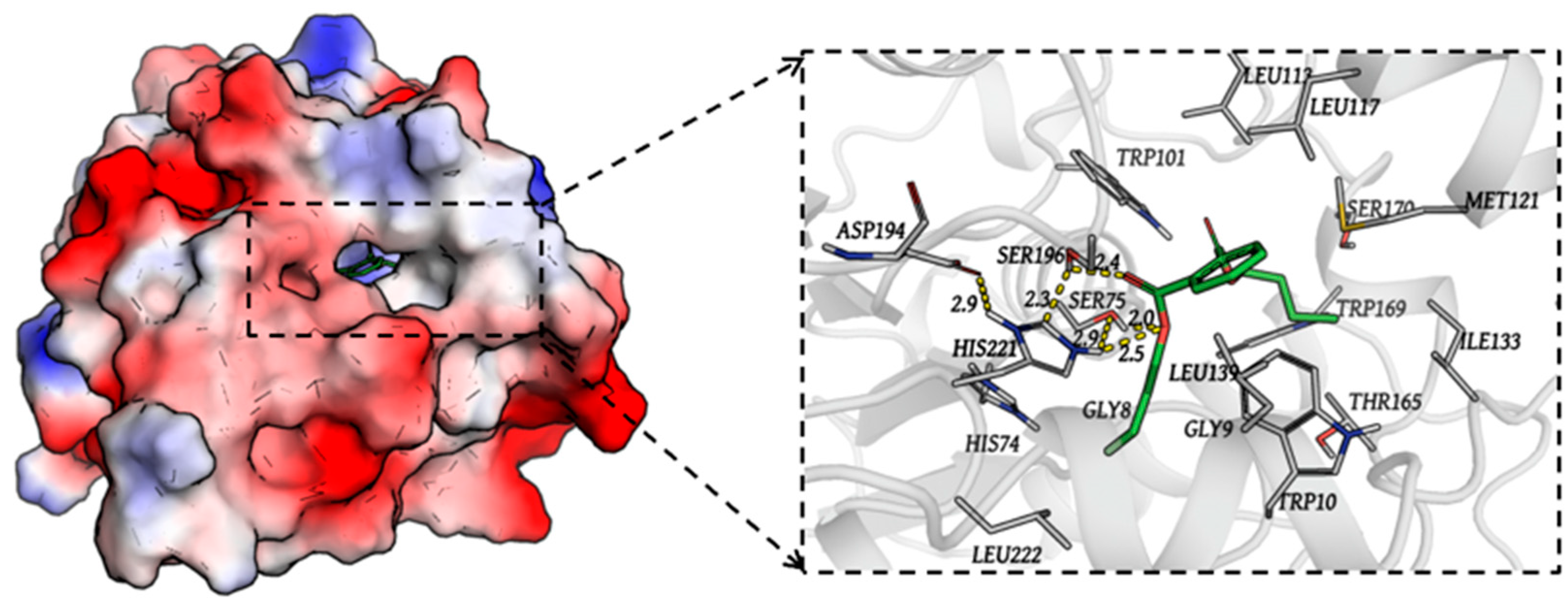
3.5. The Interaction Between DphGB1 and DBP
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Net, S.; Sempere, R.; Delmont, A.; Paluselli, A.; Ouddane, B. Occurrence, fate, behavior and ecotoxicological state of phthalates in different environmental matrices. Environ. Sci. Technol. 2015, 49, 4019–4035. [Google Scholar] [CrossRef]
- He, L.; Gielen, G.; Bolan, N.S.; Zhang, X.; Qin, H.; Huang, H.; Wang, H. Contamination and remediation of phthalic acid esters in agricultural soils in China: A review. Agron. Sustain. Dev. 2015, 35, 519–534. [Google Scholar] [CrossRef]
- Mo, C.; Cai, Q.; Li, Y.; Zeng, Q. Occurrence of priority organic pollutants in the fertilizers, China. J. Hazard. Mater. 2008, 152, 1208–1213. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lin, Q.; Wang, J.; Lu, X.; Wang, G. Effect of wetland reclamation and tillage conversion on accumulation and distribution of phthalate esters residues in soils. Ecol. Eng. 2013, 51, 10–15. [Google Scholar] [CrossRef]
- Gao, D.; Li, Z.; Wen, Z.; Ren, N. Occurrence and fate of phthalate esters in full-scale domestic wastewater treatment plants and their impact on receiving waters along the songhua river in china. Chemosphere 2014, 95, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Wang, Y.; Zhu, J.; Gu, M.; Chen, J.; Jiang, L.; Kong, D.; Zhang, Z.; Zhang, W. Effects of phthalic acid esters released from long-term agricultural film mulching on soil properties, bacterial communities, and functions in xinjiang cotton field. J. Environ. Manag. 2025, 392, 126582. [Google Scholar] [CrossRef]
- Zhou, B.; Zhao, L.; Sun, Y.; Li, X.; Weng, L.; Li, Y. Contamination and human health risks of phthalate esters in vegetable and crop soils from the huang-huai-hai region of china. Sci. Total Environ. 2021, 778, 146281. [Google Scholar] [CrossRef]
- Xia, B.; Zhu, Q.; Zhao, Y.; Ge, W.; Zhao, Y.; Song, Q.; Zhou, Y.; Shi, H.; Zhang, Y. Phthalate exposure and childhood overweight and obesity: Urinary metabolomic evidence. Environ. Int. 2018, 121, 159–168. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, J.; Wang, T.; Qin, C.; Hu, X.; Mosa, A.; Ling, W. Environmental health risks induced by interaction between phthalic acid esters (paes) and biological macromolecules: A review. Chemosphere 2023, 328, 138578. [Google Scholar] [CrossRef]
- Medic-Stojanoska, M.; Milošević, N.; Milic, N.; Abenavoli, L. The influence of phthalates and bisphenol a on the obesity development and glucose metabolism disorders. Endocrine 2017, 55, 666–681. [Google Scholar] [CrossRef]
- Staples, C.A.; Peterson, D.R.; Parkerton, T.F.; Adams, W.J. The environmental fate of phthalate esters: A literature review. Chemosphere 1997, 35, 667–749. [Google Scholar] [CrossRef]
- Peng, C.; Tang, J.; Yu, X.; Zhou, X.; Wang, M.; Zhang, Y.; Zhou, H.; Huang, S.; Wen, Q.; Chen, S.; et al. Biodegradation of various phthalic acid esters at high concentrations by gordonia alkanivorans gh-1 and its degradation mechanism. Environ. Technol. Innov. 2025, 38, 104066. [Google Scholar] [CrossRef]
- Mohan, H.; Muthukumar, S.P.; Acharya, S.; Jeong, H.J.; Lee, G.M.; Park, J.H.; Seralathan, K.K.; Oh, B.T. Harnessing landfill-derived bacillus subtilis (lls-04) for bio-electrodegradation of di-butyl phthalate: Comprehensive toxicity assessment across multiple biological models. J. Hazard. Mater. 2025, 481, 136480. [Google Scholar] [CrossRef]
- Fan, S.; Guo, J.; Han, S.; Du, H.; Wang, Z.; Fu, Y.; Han, H.; Hou, X.; Wang, W. A novel and efficient phthalate hydrolase from acinetobacter sp. Lunf3: Molecular cloning, characterization and catalytic mechanism. Molecules 2023, 28, 6738. [Google Scholar] [CrossRef]
- Ren, L.; Jia, Y.; Ruth, N.; Qiao, C.; Wang, J.; Zhao, B.; Yan, Y. Biodegradation of phthalic acid esters by a newly isolated mycobacterium sp. Yc-rl4 and the bioprocess with environmental samples. Environ. Sci. Pollut. Res. 2016, 23, 16609–16619. [Google Scholar] [CrossRef]
- Hou, Z.; Pan, H.; Gu, M.; Chen, X.; Ying, T.; Qiao, P.; Cao, J.; Wang, H.; Hu, T.; Zheng, L.; et al. Simultaneously degradation of various phthalate esters by rhodococcus sp. Ah-zy2: Strain, omics and enzymatic study. J. Hazard. Mater. 2024, 474, 134776. [Google Scholar] [CrossRef]
- Zhao, H.; Hu, R.; Chen, X.; Chen, X.; Lü, H.; Li, Y.; Li, H.; Mo, C.; Cai, Q.; Wong, M. Biodegradation pathway of di-(2-ethylhexyl) phthalate by a novel rhodococcus pyridinivorans xb and its bioaugmentation for remediation of dehp contaminated soil. Sci. Total Environ. 2018, 640–641, 1121–1131. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Liao, X.; Yu, F.; Wei, Z.; Yang, L. Cloning of a dibutyl phthalate hydrolase gene from acinetobacter sp. Strain m673 and functional analysis of its expression product in escherichia coli. Appl. Microbiol. Biotechnol. 2013, 97, 2483–2491. [Google Scholar] [CrossRef] [PubMed]
- Hara, H.; Stewart, G.R.; Mohn, W.W. Involvement of a novel abc transporter and monoalkyl phthalate ester hydrolase in phthalate ester catabolism by rhodococcus jostii rha1. Appl. Environ. Microbiol. 2010, 76, 1516–1523. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liang, R.; Dai, Q.; Jin, D.; Wang, Y.; Chao, W. Complete degradation of di-n-octyl phthalate by biochemical cooperation between gordonia sp. Strain jdc-2 and arthrobacter sp. Strain jdc-32 isolated from activated sludge. J. Hazard. Mater. 2010, 176, 262–268. [Google Scholar] [CrossRef]
- Lu, M.; Jiang, W.; Gao, Q.; Zhang, M.; Hong, Q. Degradation of dibutyl phthalate (dbp) by a bacterial consortium and characterization of two novel esterases capable of hydrolyzing paes sequentially. Ecotoxicol. Environ. Saf. 2020, 195, 110517. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, X.; Zhao, J.; Huang, H.; Wu, M.; Li, X.; Li, W.; Sun, X.; Sun, B. An efficient phthalate ester-degrading bacillus subtilis: Degradation kinetics, metabolic pathway, and catalytic mechanism of the key enzyme. Environ. Pollut. 2021, 273, 116461. [Google Scholar] [CrossRef]
- Qiu, J.; Yang, H.; Yan, Z.; Shi, Y.; Zou, D.; Ding, L.; Shao, Y.; Li, L.; Khan, U.; Sun, S.; et al. Characterization of xtjr8: A novel esterase with phthalate-hydrolyzing activity from a metagenomic library of lotus pond sludge. Int. J. Biol. Macromol. 2020, 164, 1510–1518. [Google Scholar] [CrossRef]
- Sarkar, J.; Dutta, A.; Pal Chowdhury, P.; Chakraborty, J.; Dutta, T.K. Characterization of a novel family viii esterase estm2 from soil metagenome capable of hydrolyzing estrogenic phthalates. Microb. Cell Factories 2020, 19, 77. [Google Scholar] [CrossRef]
- Fan, S.; Wang, J.; Li, K.; Yang, T.; Jia, Y.; Zhao, B.; Yan, Y. Complete genome sequence of gordonia sp. Yc-jh1, a bacterium efficiently degrading a wide range of phthalic acid esters. J. Biotechnol. 2018, 279, 55–60. [Google Scholar] [CrossRef]
- Xu, Y.; Zhao, J.; Huang, H.; Guo, X.; Li, X.; Zou, W.; Li, W.; Zhang, C.; Huang, M. Biodegradation of phthalate esters by pantoea dispersa bjq0007 isolated from baijiu. J. Food Compos. Anal. 2022, 105, 104201. [Google Scholar] [CrossRef]
- Qiu, J.; Zhang, Y.; Shi, Y.; Jiang, J.; Wu, S.; Li, L.; Shao, Y.; Xin, Z. Identification and characterization of a novel phthalate-degrading hydrolase from a soil metagenomic library. Ecotoxicol. Environ. Saf. 2020, 190, 110148. [Google Scholar] [CrossRef]
- Fan, S.; Li, C.; Guo, J.; Johansen, A.; Liu, Y.; Feng, Y.; Xue, J.; Li, Z. Biodegradation of phthalic acid esters (paes) by bacillus sp. Lunf1 and characterization of a novel hydrolase capable of catalyzing paes. Environ. Technol. Innov. 2023, 32, 103269. [Google Scholar] [CrossRef]
- Feng, N.X.; Li, D.W.; Zhang, F.; Bin, H.; Huang, Y.T.; Xiang, L.; Liu, B.L.; Cai, Q.Y.; Li, Y.W.; Xu, D.L.; et al. Biodegradation of phthalate acid esters and whole-genome analysis of a novel streptomyces sp. Fz201 isolated from natural habitats. J. Hazard. Mater. 2024, 469, 133972. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Feng, Z.; Xu, M.; Shi, Z.; Zhang, Y.; Zhang, P.; Hou, X. The efficient pae-degrading performance and complete genome sequencing of gordonia sp. Lunf6. Processes 2025, 13, 731. [Google Scholar] [CrossRef]
- Patel, R.K.; Jain, M. Ngs qc toolkit: A toolkit for quality control of next generation sequencing data. PLoS ONE 2012, 7, e30619. [Google Scholar] [CrossRef]
- Chin, C.; Peluso, P.; Sedlazeck, F.; Nattestad, M.; Concepcion, G.; Clum, A.; Dunn, C.; O’Malley, R.; Figueroa-Balderas, R.; Morales-Cruz, A.; et al. Phased diploid genome assembly with single-molecule real-time sequencing. Nat. Methods 2016, 13, 1050–1054. [Google Scholar] [CrossRef]
- Koren, S.; Walenz, B.P.; Berlin, K.; Miller, J.R.; Bergman, N.H.; Phillippy, A.M. Canu: Scalable and accurate long-read assembly via adaptive k-mer weighting and repeat separation. Genome Res. 2017, 27, 722–736. [Google Scholar] [CrossRef] [PubMed]
- Walker, B.J.; Abeel, T.; Shea, T.; Priest, M.; Abouelliel, A.; Sakthikumar, S.; Cuomo, C.A.; Zeng, Q.; Wortman, J.; Young, S.K.; et al. Pilon: An integrated tool for comprehensive microbial variant detection and genome assembly improvement. PLoS ONE 2014, 9, e112963. [Google Scholar] [CrossRef] [PubMed]
- Almagro, A.J.; Tsirigos, K.D.; Sonderby, C.K.; Petersen, T.N.; Winther, O.; Brunak, S.; von Heijne, G.; Nielsen, H. Signalp 5.0 improves signal peptide predictions using deep neural networks. Nat. Biotechnol. 2019, 37, 420–423. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. Autodock vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Hong, D.K.; Jang, S.; Lee, C. Gene cloning and characterization of a psychrophilic phthalate esterase with organic solvent tolerance from an arctic bacterium sphingomonas glacialis pamc 26605. J. Mol. Catal. B Enzym. 2016, 133, S337–S345. [Google Scholar] [CrossRef]
- Ren, C.; Wang, Y.; Wu, Y.; Zhao, H.P.; Li, L. Complete degradation of di-n-butyl phthalate by glutamicibacter sp. Strain 0426 with a novel pathway. Biodegradation 2024, 35, 87–99. [Google Scholar] [CrossRef]
- Wang, X.; Shen, S.; Wu, H.; Wang, H.; Wang, L.; Lu, Z. Acinetobacter tandoii zm06 assists glutamicibacter nicotianae zm05 in resisting cadmium pressure to preserve dipropyl phthalate biodegradation. Microorganisms 2021, 9, 1417. [Google Scholar] [CrossRef]
- Li, H.; Zhou, H.; Fan, L.; Meng, L.; Zhao, Y.; Zhao, L.; Wang, B. Glutamicibacter nicotianae at6: A new strain for the efficient biodegradation of tilmicosin. J. Environ. Sci. 2024, 142, 182–192. [Google Scholar] [CrossRef]
- Yastrebova, O.V.; Malysheva, A.A.; Plotnikova, E.G. Halotolerant terephthalic acid-degrading bacteria of the genus glutamicibacter. Appl. Biochem. Microbiol. 2022, 58, 590–597. [Google Scholar] [CrossRef]
- Lian, D.; Zhuang, S.; Shui, C.; Zheng, S.; Ma, Y.; Sun, Z.; Porras-Domínguez, J.R.; öner, E.T.; Liang, M.; Van den Ende, W. Characterization of inulolytic enzymes from the jerusalem artichoke–derived glutamicibacter mishrai njau-1. Appl. Microbiol. Biotechnol. 2022, 106, 5525–5538. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.; Yadav, M.P.; Li, X. Biodegradability and biodegradation pathway of di-(2-ethylhexyl) phthalate by burkholderia pyrrocinia b1213. Chemosphere 2019, 225, 443–450. [Google Scholar] [CrossRef]
- Chen, F.; Chen, Y.; Chen, C.; Feng, L.; Dong, Y.; Chen, J.; Lan, J.; Hou, H. High-efficiency degradation of phthalic acid esters (paes) by pseudarthrobacter defluvii e5: Performance, degradative pathway, and key genes. Sci. Total Environ. 2021, 794, 148719. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Yang, C.; Hou, Z.; Liu, T.; Mei, X.; Zheng, L.; Zhong, W. Phthalate esters metabolic strain gordonia sp. Gz-yc7, a potential soil degrader for high concentration di-(2-ethylhexyl) phthalate. Microorganisms 2022, 10, 641. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Gao, D.; Wu, W. Biodegradation and kinetic analysis of phthalates by an arthrobacter strain isolated from constructed wetland soil. Appl. Microbiol. Biotechnol. 2014, 98, 4683–4690. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Liu, Y.; Chen, Q.; Luo, H.; Zhu, Z.; Chen, W.; Chen, J.; Mo, Y. Biochemical pathways and enhanced degradation of di-n-octyl phthalate (dop) in sequencing batch reactor (sbr) by arthrobacter sp. Slg-4 and rhodococcus sp. Slg-6 isolated from activated sludge. Biodegradation 2018, 29, 171–185. [Google Scholar] [CrossRef]
- Deng, Q.; Zhang, P.; Li, X.; Shen, Z.; Mi, X.; Cai, Z.; Gu, L. Effect of seawater salinity on the fretting corrosion behavior of nickel-aluminum bronze (nab) alloy. Tribol. Int. 2024, 193, 109357. [Google Scholar] [CrossRef]
- Li, D.; Yan, J.; Wang, L.; Zhang, Y.; Liu, D.; Geng, H.; Xiong, L. Characterization of the phthalate acid catabolic gene cluster in phthalate acid esters transforming bacterium-gordonia sp. Strain hs-nh1. Int. Biodeterior. Biodegrad. 2016, 106, 34–40. [Google Scholar] [CrossRef]
- Hu, R.; Zhao, H.; Xu, X.; Wang, Z.; Yu, K.; Shu, L.; Yan, Q.; Wu, B.; Mo, C.; He, Z.; et al. Bacteria-driven phthalic acid ester biodegradation: Current status and emerging opportunities. Environ. Int. 2021, 154, 106560. [Google Scholar] [CrossRef]
- Liu, T.; Li, J.; Qiu, L.; Zhang, F.; Linhardt, R.; Zhong, W. Combined genomic and transcriptomic analysis of dibutyl phthalate metabolic pathway in arthrobacter sp. Zjutw. Biotechnol. Bioeng. 2020, 117, 3712–3726. [Google Scholar] [CrossRef] [PubMed]
- Feng, N.; Yu, J.; Mo, C.; Zhao, H.; Li, Y.; Wu, B.; Cai, Q.; Li, H.; Zhou, D.; Wong, M. Biodegradation of di-n-butyl phthalate (dbp) by a novel endophytic bacillus megaterium strain yjb3. Sci. Total Environ. 2018, 616–617, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Mu, B.; Sadowski, P.; Te’O, J.; Patel, B.; Pathiraja, N.; Dudley, K. Identification and characterisation of moderately thermostable diisobutyl phthalate degrading esterase from a great artesian basin bacillus velezensis np05. Biotechnol. Rep. 2024, 42, e840. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, P.; Fan, S.; Xu, M.; Liu, L.; Zhang, X.; Feng, Z.; Du, H.; Wang, Z.; Qin, Q.; Feng, W.; et al. The Efficient PAE Degradation by Glutamicibacter sp. FR1 and Its Molecular Mechanism. Processes 2025, 13, 3245. https://doi.org/10.3390/pr13103245
Peng P, Fan S, Xu M, Liu L, Zhang X, Feng Z, Du H, Wang Z, Qin Q, Feng W, et al. The Efficient PAE Degradation by Glutamicibacter sp. FR1 and Its Molecular Mechanism. Processes. 2025; 13(10):3245. https://doi.org/10.3390/pr13103245
Chicago/Turabian StylePeng, Peng, Shuanghu Fan, Meiting Xu, Liyuan Liu, Xiaolin Zhang, Zihan Feng, Haina Du, Zimeng Wang, Qiao Qin, Weiming Feng, and et al. 2025. "The Efficient PAE Degradation by Glutamicibacter sp. FR1 and Its Molecular Mechanism" Processes 13, no. 10: 3245. https://doi.org/10.3390/pr13103245
APA StylePeng, P., Fan, S., Xu, M., Liu, L., Zhang, X., Feng, Z., Du, H., Wang, Z., Qin, Q., Feng, W., Liu, H., & Guo, J. (2025). The Efficient PAE Degradation by Glutamicibacter sp. FR1 and Its Molecular Mechanism. Processes, 13(10), 3245. https://doi.org/10.3390/pr13103245





