Potential of Strawberry Leaves with Biostimulants: Repository of Metabolites and Bioethanol Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Strawberry Cultivation
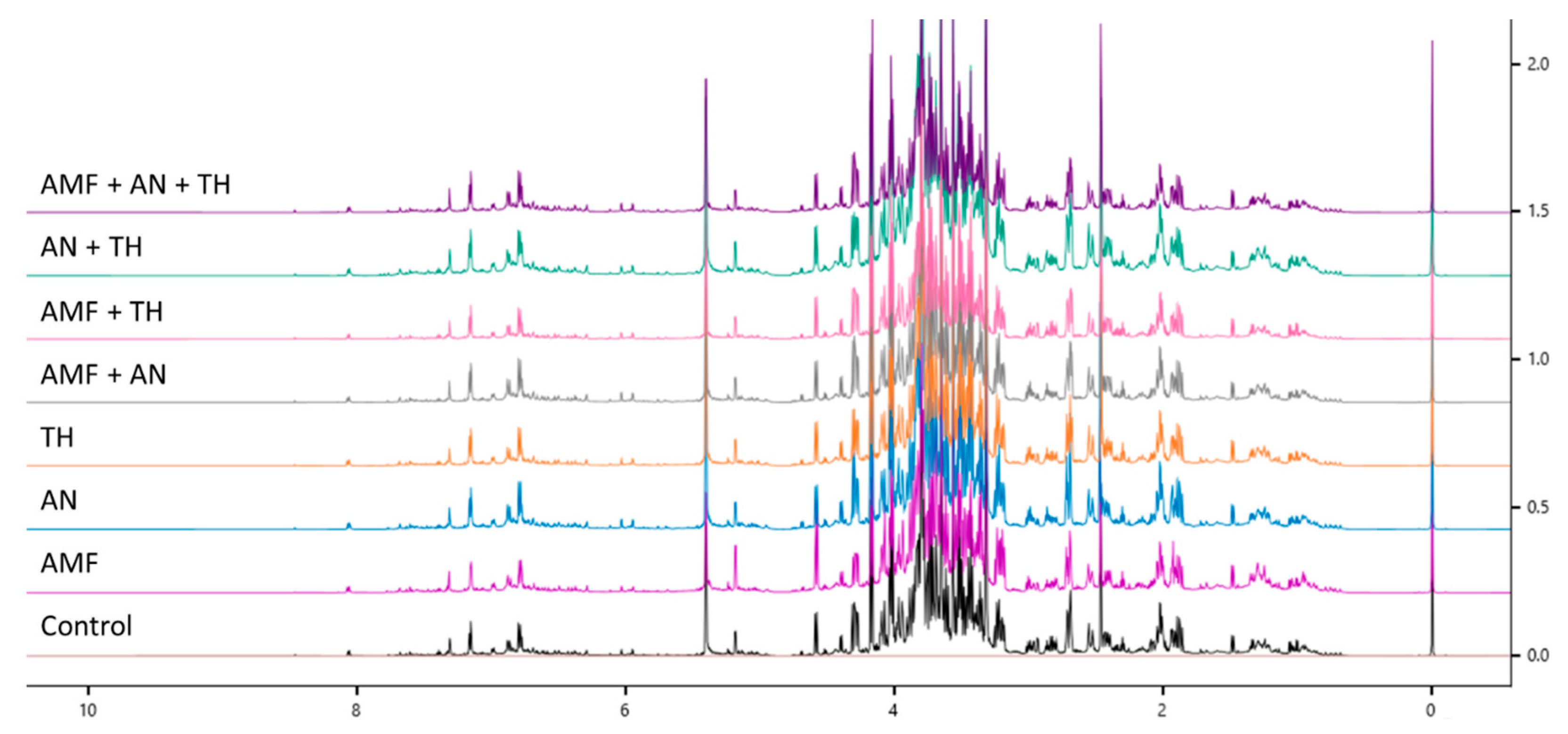
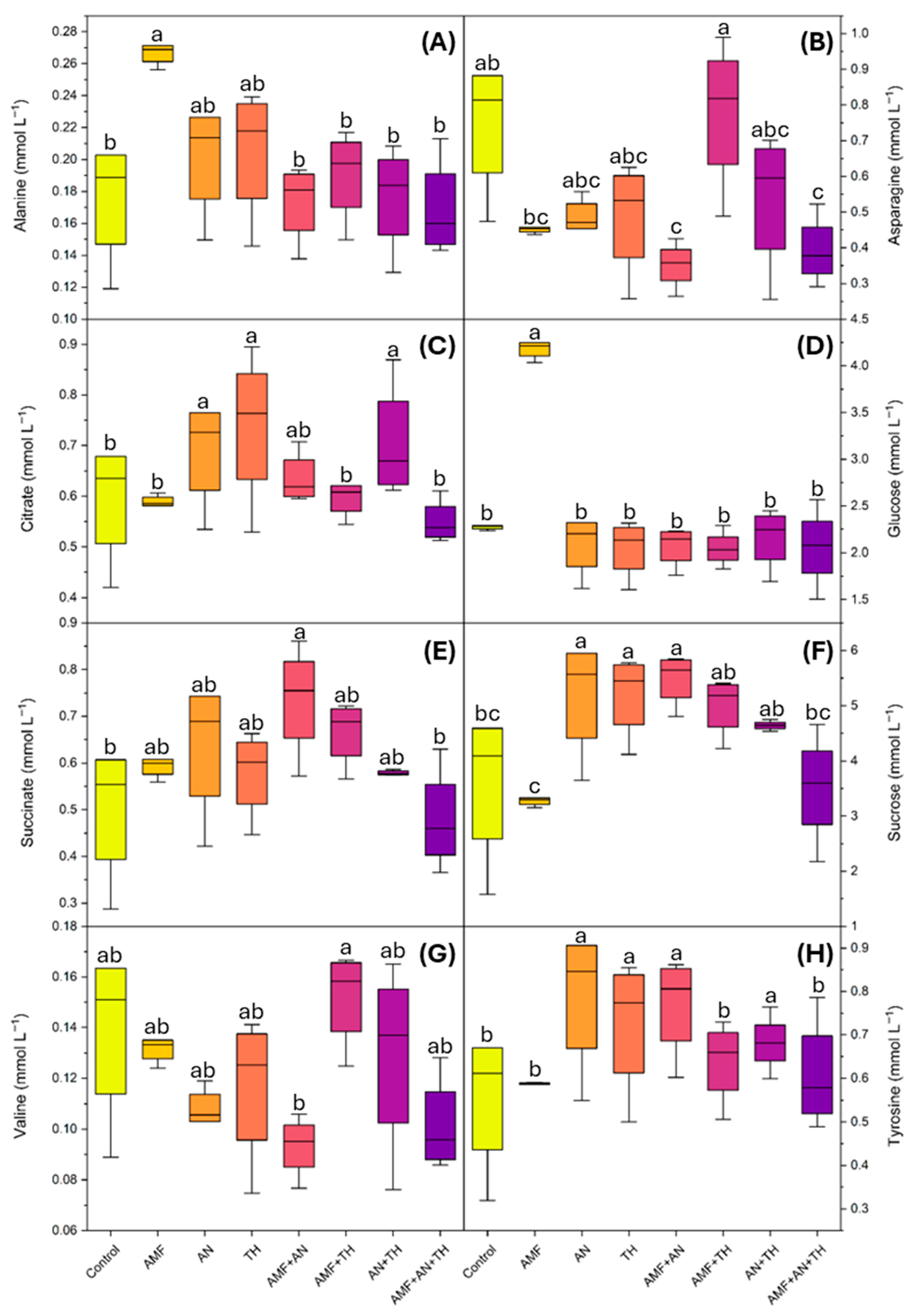
2.2. Metabolic Composition of Leaves by Nuclear Magnetic Resonance of Hydrogen
2.3. Bioethanol Production from Strawberry Leaves
2.3.1. Composition of Strawberry Leaves
2.3.2. Evaluation of Enzymes for the Saccharification of Strawberry Leaves
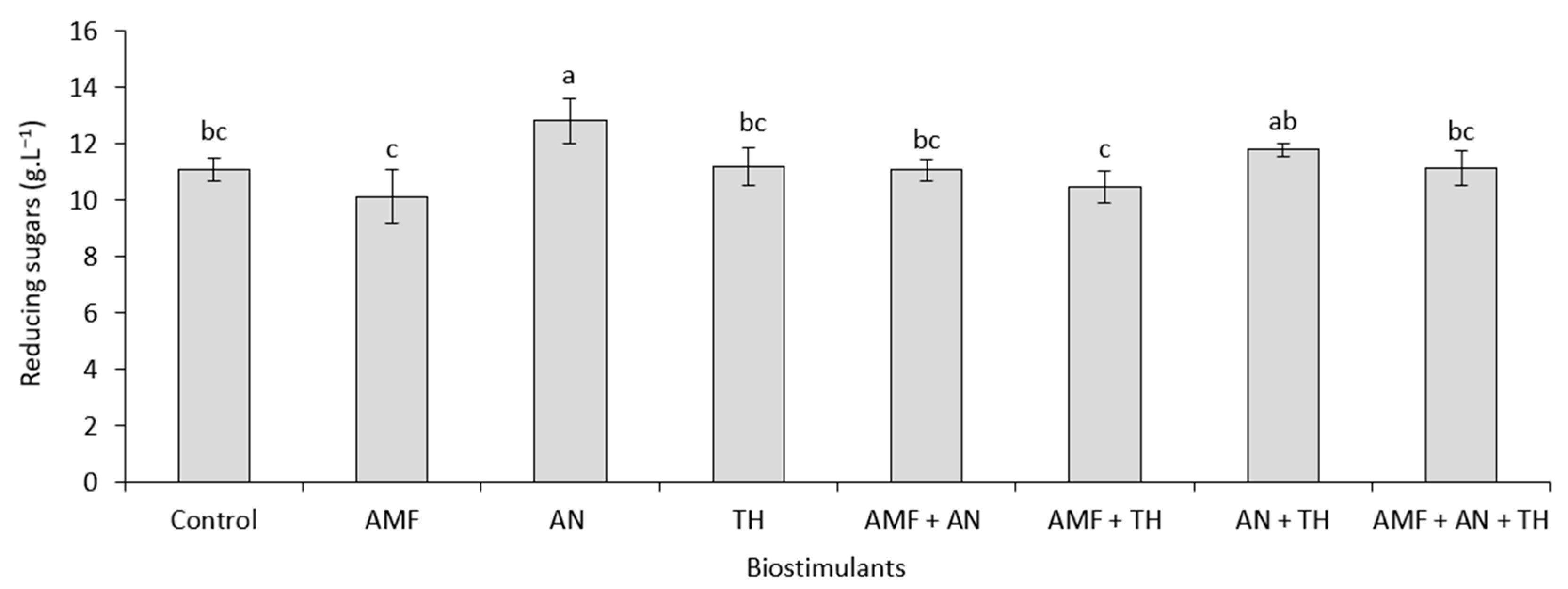
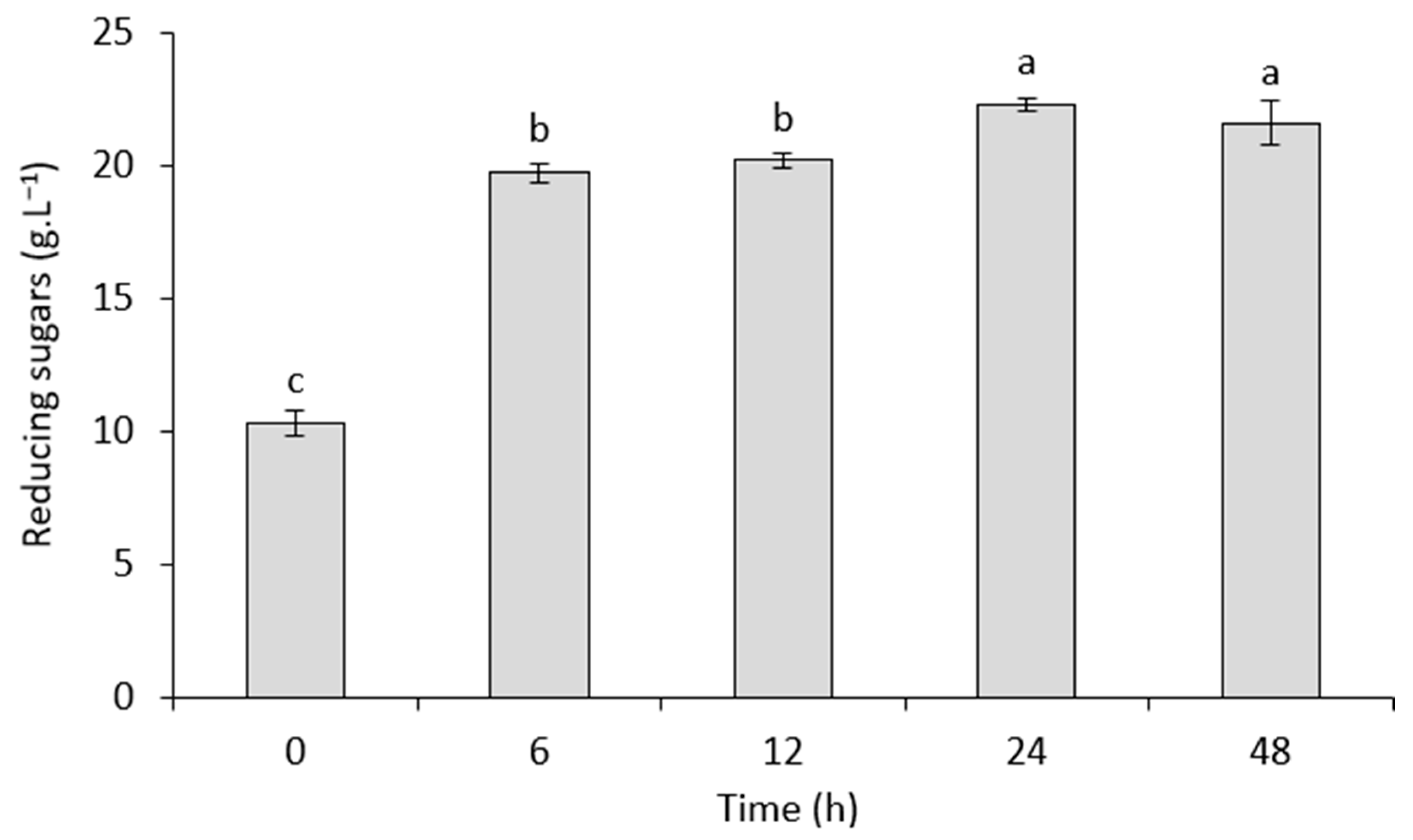
2.3.3. Enzymatic Hydrolysis
2.3.4. Fermentation and Bioethanol Production
3. Results
3.1. Metabolic Composition of Leaves by Nuclear Magnetic Resonance of Hydrogen
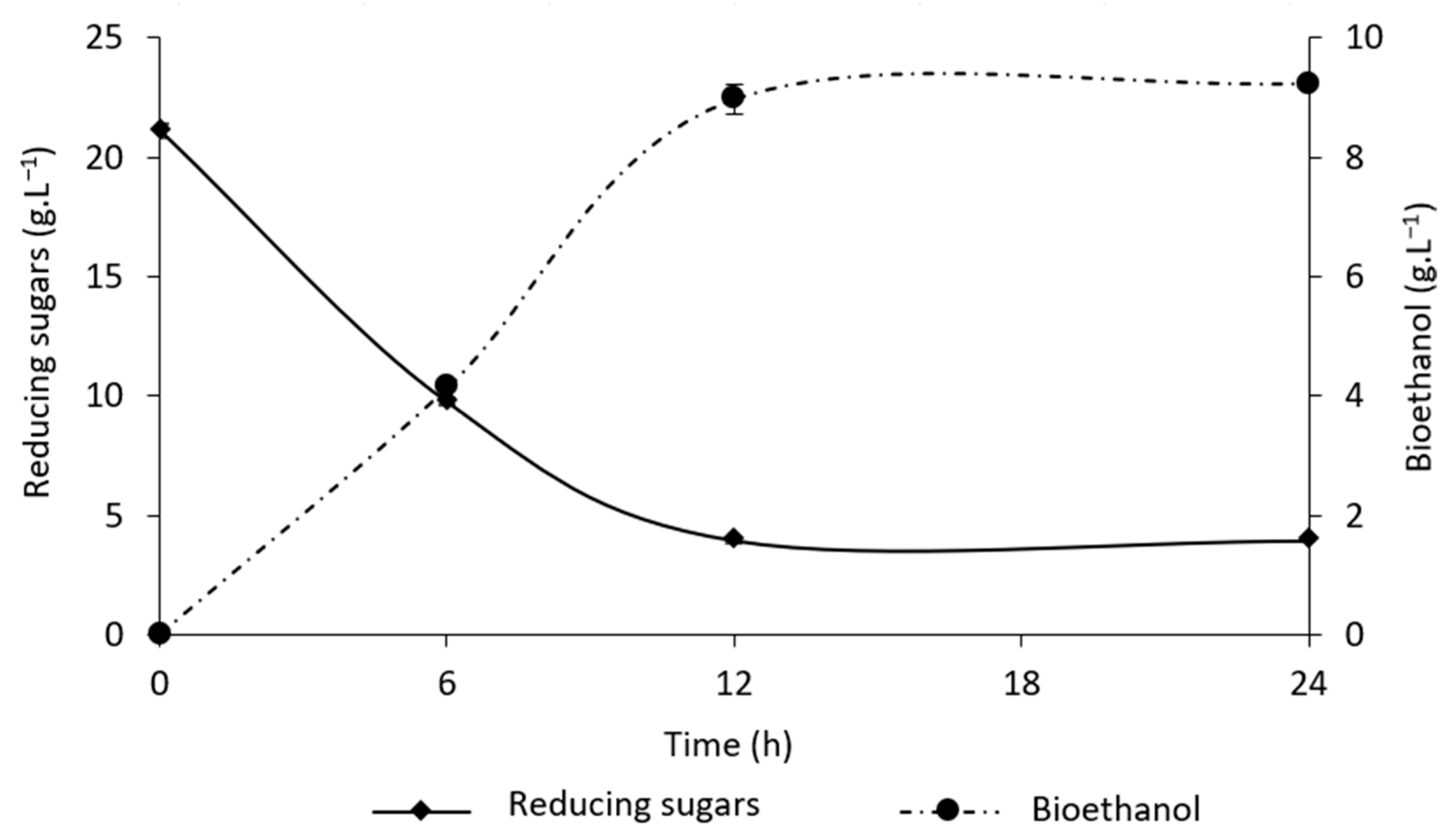
3.2. Bioethanol Production from Strawberry Leaves
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chiomento, J.L.T.; De Nardi, F.S.; Kujawa, S.C.; Deggerone, Y.S.; Fante, R.; Kaspary, I.J.; Dornelles, A.G.; Huzar-Novakowiski, J.; Trentin, T.S. Multivariate contrasts of seven strawberry cultivars in soilless cultivation and greenhouse in southern Brazil. Adv. Chem. Res. 2023, 2, 62–76. [Google Scholar] [CrossRef]
- Dornelles, A.G.; Trentin, T.S.; Petry, C.; Chiomento, J.L.T. Agronomic potential of nine strawberry cultivars for two consecutive cycles in a greenhouse. Sci. Plena 2024, 20, 040206. [Google Scholar] [CrossRef]
- Chiomento, J.L.T.; De Nardi, F.S.; Fante, R.; Dal Pizzol, E.; Trentin, T.S.; Borba, A.C.; Basílio, L.S.P.; Garcia, V.A.S.; Lima, G.P.P. Building the microbiota in strawberry soilless cultivation systems with on-farm AMF inoculants: Roles in yield, phytochemical profile, and root morphology. S. Afr. J. Bot. 2025, 178, 226–234. [Google Scholar] [CrossRef]
- Chiomento, J.L.T.; Fracaro, J.; Görgen, M.; Fante, R.; Dal Pizzol, E.; Welter, M.; Klein, A.P.; Trentin, T.S.; Suzana-Milan, C.S.; Palencia, P. Arbuscular mycorrhizal fungi, Ascophyllum nodosum, Trichoderma harzianum, and their combinations influence the phyllochron, phenology, and fruit quality of strawberry plants. Agronomy 2024, 14, 860. [Google Scholar] [CrossRef]
- Li, J.; Brecht, J.K.; Kim, J.; Bailey, L.S.; Kamat, M.N.; Basso, K.B.; Colee, J.C.; Zhao, X. Seaweed extract and microbial biostimulants show synergistic effects on improving organic strawberry production. HortScience 2024, 59, 1114–1126. [Google Scholar] [CrossRef]
- Soppelsa, S.; Kelderer, M.; Casera, C.; Bassi, M.; Robatscher, P.; Matteazzi, A.; Andreotti, C. Foliar applications of biostimulants promote growth, yield and fruit quality of strawberry plants grown under nutrient limitation. Agronomy 2019, 9, 483. [Google Scholar] [CrossRef]
- Cipriani, M.D.; De Nardi, F.S.; Trentin, T.S.; Silveira, D.C.; Calvete, E.O.; Chiomento, J.L.T. Morpho-horticultural potential of seven strawberry cultivars for two consecutive cycles. Revista Brasileira de Ciências Agrárias 2023, 18, 2753. [Google Scholar] [CrossRef]
- Chiomento, J.L.T.; De Nardi, F.S.; Grando, L.A.; Trentin, T.S.; Anzolin, J.; Albrecht, G.E.; Huzar-Novakowiski, J.; Basílio, L.S.P.; Monteiro, G.C.; Lima, G.P.P. Circular bioeconomy in strawberry cultivation: Phytochemicals in fruits and leaves, and yield potential of seven genotypes. Bragantia 2024, 83, 20230219. [Google Scholar] [CrossRef]
- Villamil-Galindo, E.; Van De Velde, F.; Piagentini, A. Strawberry agro-industrial by-products as a source of bioactive compounds: Effect of cultivar on the phenolic profile and the antioxidant capacity. Bioresour. Bioprocess. 2021, 8, 61. [Google Scholar] [CrossRef] [PubMed]
- Dorosh, O.; Fernandes, V.C.; Delerue-Matos, C.; Moreira, M.M. Blueberry pruning wastes: From an undervalued agricultural residue to a safe and valuable source of antioxidant compounds for the food industry. Foods 2024, 13, 317. [Google Scholar] [CrossRef]
- Ladika, G.; Christodoulou, P.; Kritsi, E.; Tsiaka, T.; Sotiroudis, G.; Cavouras, D.; Sinanoglou, V.J. Exploring postharvest metabolic shifts and NOX2 inhibitory potential in strawberry fruits and leaves via untargeted LC-MS/MS and chemometric analysis. Metabolites 2025, 15, 321. [Google Scholar] [CrossRef]
- Tse, T.J.; Wiens, D.J.; Reaney, M.J.T. Production of bioethanol—A review of factors affecting ethanol yield. Fermentation 2021, 7, 268. [Google Scholar] [CrossRef]
- Sica, P.; Prado, L.M.L.M.; Granja, P.; Carvalho, E.M.D.; Mattos, E.D.C.; Calegari, R.P.; Silverio, M.; Martins, B.C.; Baptista, A.S. Effects of energy cane (Saccharum spp.) juice on corn ethanol (Zea mays) fermentation efficiency: Integration towards a more sustainable production. Fermentation 2021, 7, 30. [Google Scholar] [CrossRef]
- Kazmi, A.; Sultana, T.; Ali, A.; Nijabat, A.; Li, G.; Hou, H. Innovations in bioethanol production: A comprehensive review of feedstock generations and technology advances. Energy Strategy Rev. 2025, 57, 101634. [Google Scholar] [CrossRef]
- Colussi, J.; Paulson, N.; Schnitkey, G.; Baltz, J. Brazil emerges as corn-ethanol producer with expansion of second crop corn. Farmdoc Dly. 2023, 13, 120. [Google Scholar]
- Raj, T.; Chandrasekhar, K.; Kumar, A.N.; Banu, J.R.; Yoon, J.J.; Bhatia, S.K.; Yang, Y.H.; Varjani, S.; Kim, S.H. Recent advances in commercial biorefineries for lignocellulosic ethanol production: Current status, challenges and future perspectives. Bioresour. Technol. 2022, 344, 126292. [Google Scholar] [CrossRef]
- Jayakumar, M.; Gindaba, G.T.; Gebeyehu, K.B.; Periyasamy, S.; Jabesa, A.; Baskar, G.; John, B.I.; Pugazhendhi, A. Bioethanol production from agricultural residues as lignocellulosic biomass feedstock′s waste valorization approach: A comprehensive review. Sci. Total Environ. 2023, 879, 163158. [Google Scholar] [CrossRef] [PubMed]
- Chiomento, J.L.T.; Stürmer, S.L.; Carrenho, R.; Costa, R.C.; Scheffer-Basso, S.M.; Antunes, L.E.C.; Nienow, A.A.; Calvete, E.O. Composition of arbuscular mycorrhizal fungi communities signals generalist species in soils cultivated with strawberry. Sci. Hortic. 2019, 253, 286–294. [Google Scholar] [CrossRef]
- Redecker, D.; Schubler, A.; Stockinger, H.; Stürmer, S.L.; Morton, J.B.; Walker, C. An evidence based consensus for the classification of arbuscular mycorrhizal fungi (Glomeromycota). Mycorrhiza 2013, 23, 515–531. [Google Scholar] [CrossRef] [PubMed]
- De Nardi, F.S.; Trentin, T.S.; Trentin, N.S.; Costa, R.C.; Calvete, E.O.; Palencia, P.; Chiomento, J.L.T. Mycorrhizal biotechnology reduce phosphorus in the nutrient solution of strawberry soilless cultivation systems. Agronomy 2024, 14, 355. [Google Scholar] [CrossRef]
- Kim, H.; Choi, Y.; Verpoorte, R. NMR-based metabolomic analysis of plants. Nat. Protoc. 2010, 5, 536–549. [Google Scholar] [CrossRef]
- White Jr, J.W. Spectrophotometric method for hydroxymethylfurfural in honey. J. Assoc. Off. Anal. Chem. 1979, 62, 509–514. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. J. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Caputi, B. Compêndio Brasileiro de Alimentação Animal; Sindirações: São Paulo, Brazil, 2017; p. 840. [Google Scholar]
- Van Soest, P.J.; Wine, R.H. Use of detergents in the analysis of fibrous feeds. IV. Determination of plant cell-wall constituents. J. Assoc. Off. Anal. Chem. 1967, 50, 50–55. [Google Scholar]
- Salik, F.L.; Povh, N.P. Método espectrofotométrico para determinação de teores alcoólicos em misturas hidroalcoólicas. Congresso Nacional STAB 1993, 5, 262–266. [Google Scholar]
- Stirk, W.A.; Rengasamy, K.R.R.; Kulkarni, M.G.; Van Staden, J. Plant biostimulants from seaweed. In The Chemical Biology of Plant Biostimulants; Geelen, D., Xu, L., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2020; pp. 31–55. [Google Scholar]
- Tajdinian, S.; Rahmati-Joneidabad, M.; Ghodoum Parizipour, M.H. Macroalgal treatment to alleviate the strawberry yield loss caused by Macrophomina phaseolina (Tassi) Goid. in greenhouse cultivation system. Front. Sustain. Food Syst. 2022, 6, 1089553. [Google Scholar] [CrossRef]
- De Saeger, J.; Van Praet, S.; Vereecke, D.; Park, J.; Jacques, S.; Han, T.; Depuydt, S. Toward the molecular understanding of the action mechanism of Ascophyllum nodosum extracts on plants. J. Appl. Phycol. 2020, 32, 573–597. [Google Scholar] [CrossRef]
- Brummer, V.; Skryja, P.; Jurena, T.; Hlavacek, V.; Stehlik, P. Suitable technological conditions for enzymatic hydrolysis of waste paper by Novozymes® enzymes NS50013 and NS50010. Appl. Biochem. Biotechnol. 2014, 174, 1299–1308. [Google Scholar] [CrossRef]
- Maitan-Alfenas, G.P.; Visser, E.M.; Guimarães, V.M. Enzymatic hydrolysis of lignocellulosic biomass: Converting food waste in valuable products. Curr. Opin. Food Sci. 2015, 1, 44–49. [Google Scholar] [CrossRef]
- Sato, T.; Ikeya, Y.; Adachi, S.; Yagasaki, K.; Nihei, K.; Itoh, N. Extraction of strawberry leaves with supercritical carbon dioxide and entrainers: Antioxidant capacity, total phenolic content, and inhibitory effect on uric acid production of the extract. Food Bioprod. Process. 2019, 117, 160–169. [Google Scholar] [CrossRef]
- Terpinc, P.; Dobroslavić, E.; Garofulić, I.E.; Repajić, M.; Cegledi, E.; Dobrinčić, A.; Pedisić, S.; Levaj, B. Maximizing the recovery of phenolic antioxidants from wild strawberry (Fragaria vesca) leaves using microwave-assisted extraction and accelerated solvent extraction. Processes 2023, 11, 3378. [Google Scholar] [CrossRef]
- Das, N.; Jena, P.K.; Padhi, D.; Kumar Mohanty, M.; Sahoo, G. A comprehensive review of characterization, pretreatment and its applications on different lignocellulosic biomass for bioethanol production. Biomass Convers. Biorefinery 2023, 13, 1503–1527. [Google Scholar] [CrossRef]
- Devos, R.J.B.; Colla, L.M. Simultaneous saccharification and fermentation to obtain bioethanol: A bibliometric and systematic study. Bioresour. Technol. Rep. 2022, 17, 100924. [Google Scholar] [CrossRef]
- Rastogi, M.; Shrivastava, S. Recent advances in second generation bioethanol production: An insight to pretreatment, saccharification and fermentation processes. Renew. Sustain. Energy Rev. 2017, 80, 330–340. [Google Scholar] [CrossRef]
- Bender, L.E.; Colvero, G.L.; Da Luz Monteiro, E.; Rempel, A.; Colla, L.M. Utilization of food waste for bioethanol production in a circular bioeconomy approach. Biomass Convers. Biorefinery 2025, 15, 8525–8541. [Google Scholar] [CrossRef]
- Simon, V.; Freitag, J.F.; Da Silva, J.L.; Colla, L.M. Optimizing the enzymatic hydrolysis of bioflocculated microalgae for bioethanol production. Processes 2025, 13, 364. [Google Scholar] [CrossRef]
- Santos, R.B.; Lee, J.M.; Jameel, H.; Chang, H.M.; Lucia, L.A. Effects of hardwood structural and chemical characteristics on enzymatic hydrolysis for biofuel production. Bioresour. Technol. 2012, 110, 232–238. [Google Scholar] [CrossRef]
- Adeyemi, M.M.; Olatubosun, R.S.; Babarinde, O.A.; Omale, P.E. Comparative study of bioethanol production from wheat straw and rice straw. J. Chem. Soc. Nigeria 2019, 44, 301–309. [Google Scholar]
- Corbin, K.R.; Byrt, C.S.; Bauer, S.; DeBolt, S.; Chambers, D.; Holtum, J.A.M.; Karem, G.; Henderson, M.; Lahnstein, J.; Beahan, C.T.; et al. Prospecting for energy-rich renewable raw materials: Agave leaf case study. PLoS ONE 2015, 10, 0135382. [Google Scholar] [CrossRef]
- Nwakaire, J.N.; Ezeoha, S.L.; Ugwuishiwu, B.O. Production of cellulosic ethanol from wood sawdust. Agric. Eng. Int. CIGR J. 2013, 15, 136–140. [Google Scholar]
- El-Hawary, S.S.; Mohammed, R.; El-Din, M.E.; Hassan, H.M.; Ali, Z.Y.; Rateb, M.E.; El-Naggar, E.M.B.; Othman, E.M.; Abdelmohsen, U.R. Comparative phytochemical analysis of five Egyptian strawberry cultivars (Fragaria × ananassa Duch.) and antidiabetic potential of Festival and Red Merlin cultivars. RSC Adv. 2021, 11, 16755–16767. [Google Scholar] [CrossRef]
- Aremu, A.O.; Makhaye, G.; Tesfay, S.Z.; Gerrano, A.S.; Du Plooy, C.P.; Amoo, S.O. Influence of commercial seaweed extract and microbial biostimulant on growth, yield, phytochemical content, and nutritional quality of five Abelmoschus esculentus genotypes. Agronomy 2022, 12, 428. [Google Scholar] [CrossRef]
- Lombardi, N.; Caira, S.; Troise, A.D.; Scaloni, A.; Vitaglione, P.; Vinale, F.; Marra, R.; Salzano, A.M.; Lorito, M.; Woo, S.L. Trichoderma applications on strawberry plants modulate the physiological processes positively affecting fruit production and quality. Front. Microbiol. 2020, 11, 1364. [Google Scholar] [CrossRef]
- Pereira, S.; Rodrigues, J.; Sujeeth, N.; Guinan, K.J.; Gonçalves, B. Optimizing strawberry growth: Impact of irrigation and biostimulant application on physiology and fruit quality. Plant Stress 2025, 15, 100715. [Google Scholar] [CrossRef]
- Zhao, Y.; Cartabia, A.; Lalaymia, I.; Declerck, S. Arbuscular mycorrhizal fungi and production of secondary metabolites in medicinal plants. Mycorrhiza 2022, 32, 221–256. [Google Scholar] [CrossRef]
- Erb, M.; Kliebenstein, D.J. Plant secondary metabolites as defenses, regulators, and primary metabolites: The blurred functional trichotomy. Plant Physiol. 2020, 184, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.Q.; Zhu, H.H.; Zhao, H.Q.; Yao, Q. Arbuscular mycorrhizal fungal inoculation increases phenolic synthesis in clover roots via hydrogen peroxide, salicylic acid and nitric oxide signaling pathways. J. Plant Physiol. 2013, 170, 74–79. [Google Scholar] [CrossRef]
- Devi, A.; Bajar, S.; Kour, H.; Kothari, R.; Pant, D.; Singh, A. Lignocellulosic biomass valorization for bioethanol production: A circular bioeconomy approach. Bioenergy Res. 2022, 15, 1820–1841. [Google Scholar] [CrossRef]
- Nandabalan, Y.K.; Usman, M.; Kavitha, S.; Sachdeva, S.; Thakur, S.; Somanathan, A.K.; Banu, J.R. Lignocellulosic biorefinery technologies: A perception into recent advances in biomass fractionation, biorefineries, economic hurdles and market outlook. Fermentation 2023, 9, 238. [Google Scholar] [CrossRef]
- Herrero, M.; Ibañez, E. Green extraction processes, biorefineries and sustainability: Recovery of high added-value products from natural sources. J. Supercrit. Fluids 2018, 134, 252–259. [Google Scholar] [CrossRef]
- Mantuano, P.; Boccanegra, B.; Bianchini, G.; Conte, E.; De Bellis, M.; Sanarica, F.; Camerino, G.M.; Pierno, S.; Cappellari, O.; Allegretti, M.; et al. BCAAs and Di-Alanine supplementation in the prevention of skeletal muscle atrophy: Preclinical evaluation in a murine model of hind limb unloading. Pharmacol. Res. 2021, 171, 105798. [Google Scholar] [CrossRef]
- Olloquequi, J.; Cano, A.; Sanchez-López, E.; Carrasco, M.; Verdaguer, E.; Fortuna, A.; Folch, J.; Bulló, M.; Auladell, C.; Camins, A.; et al. Protein tyrosine phosphatase 1B (PTP1B) as a potential therapeutic target for neurological disorders. Biomed. Pharmacother. 2022, 155, 113709. [Google Scholar] [CrossRef]
- Fashogbon, R.O.; Samson, O.J.; Awotundun, T.A.; Olanbiwoninu, A.A.; Adebayo-Tayo, B.C. Microbial gamma-aminobutyric acid synthesis: A promising approach for functional food and pharmaceutical applications. Lett. Appl. Microbiol. 2024, 77, 122. [Google Scholar] [CrossRef]
- Thorpe, J.; Shum, B.; Moore, R.A.; Wiffen, P.J.; Gilron, I. Combination pharmacotherapy for the treatment of fibromyalgia in adults. Cochrane Database Syst. Rev. 2018, 2, CD010585. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, M.; Zhu, L.; Zhao, X.; Chen, J.; Chen, W.; Chang, C. Hydrolysis of lignocellulose to succinic acid: A review of treatment methods and succinic acid applications. Biotechnol. Biofuels Bioprod. 2023, 16, 1. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, F.W.; Peters, S. Carbohydrates as green raw materials for the chemical industry. Comptes Rendus Chim. 2004, 7, 65–90. [Google Scholar] [CrossRef]
- Broda, M.; Yelle, D.J.; Serwańska, K. Bioethanol production from lignocellulosic biomass—Challenges and solutions. Molecules 2022, 27, 8717. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Jiang, B.; Chen, H.; Wu, W.; Wu, S.; Jin, Y.; Xiao, H. Recent advances in understanding the effects of lignin structural characteristics on enzymatic hydrolysis. Biotechnol. Biofuels 2021, 14, 205. [Google Scholar] [CrossRef]
- Ubando, A.T.; Felix, C.B.; Chen, W.H. Biorefineries in circular bioeconomy: A comprehensive review. Bioresour. Technol. 2020, 299, 122585. [Google Scholar] [CrossRef]


| Bromatological Analyses 1 | |||||||
|---|---|---|---|---|---|---|---|
| Humidity | Protein | Lipids | Ash | Crude Fiber | NDF | ADF | Total Carbohydrates |
| % | |||||||
| 4.14 | 8.99 | 3.86 | 12.87 | 24.79 | 26.4 | 25.91 | 70.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klein, A.P.; Mulinari, J.; Reichert Junior, F.W.; Trentin, T.d.S.; Aguilar, M.G.d.; Machado, A.R.T.; Oliveira, D.F.d.; Colla, L.M.; Chiomento, J.L.T. Potential of Strawberry Leaves with Biostimulants: Repository of Metabolites and Bioethanol Production. Processes 2025, 13, 3244. https://doi.org/10.3390/pr13103244
Klein AP, Mulinari J, Reichert Junior FW, Trentin TdS, Aguilar MGd, Machado ART, Oliveira DFd, Colla LM, Chiomento JLT. Potential of Strawberry Leaves with Biostimulants: Repository of Metabolites and Bioethanol Production. Processes. 2025; 13(10):3244. https://doi.org/10.3390/pr13103244
Chicago/Turabian StyleKlein, Arthur Pegoraro, Jéssica Mulinari, Francisco Wilson Reichert Junior, Thomas dos Santos Trentin, Mariana Guerra de Aguilar, Alan Rodrigues Teixeira Machado, Denilson Ferreira de Oliveira, Luciane Maria Colla, and José Luís Trevizan Chiomento. 2025. "Potential of Strawberry Leaves with Biostimulants: Repository of Metabolites and Bioethanol Production" Processes 13, no. 10: 3244. https://doi.org/10.3390/pr13103244
APA StyleKlein, A. P., Mulinari, J., Reichert Junior, F. W., Trentin, T. d. S., Aguilar, M. G. d., Machado, A. R. T., Oliveira, D. F. d., Colla, L. M., & Chiomento, J. L. T. (2025). Potential of Strawberry Leaves with Biostimulants: Repository of Metabolites and Bioethanol Production. Processes, 13(10), 3244. https://doi.org/10.3390/pr13103244









