Hybrid Solar Photoelectro-Fenton and Ozone Processes for the Sustainable Removal of COVID-19 Pharmaceutical Contaminants
Abstract
1. Introduction
2. Experimental Section
2.1. Chemicals
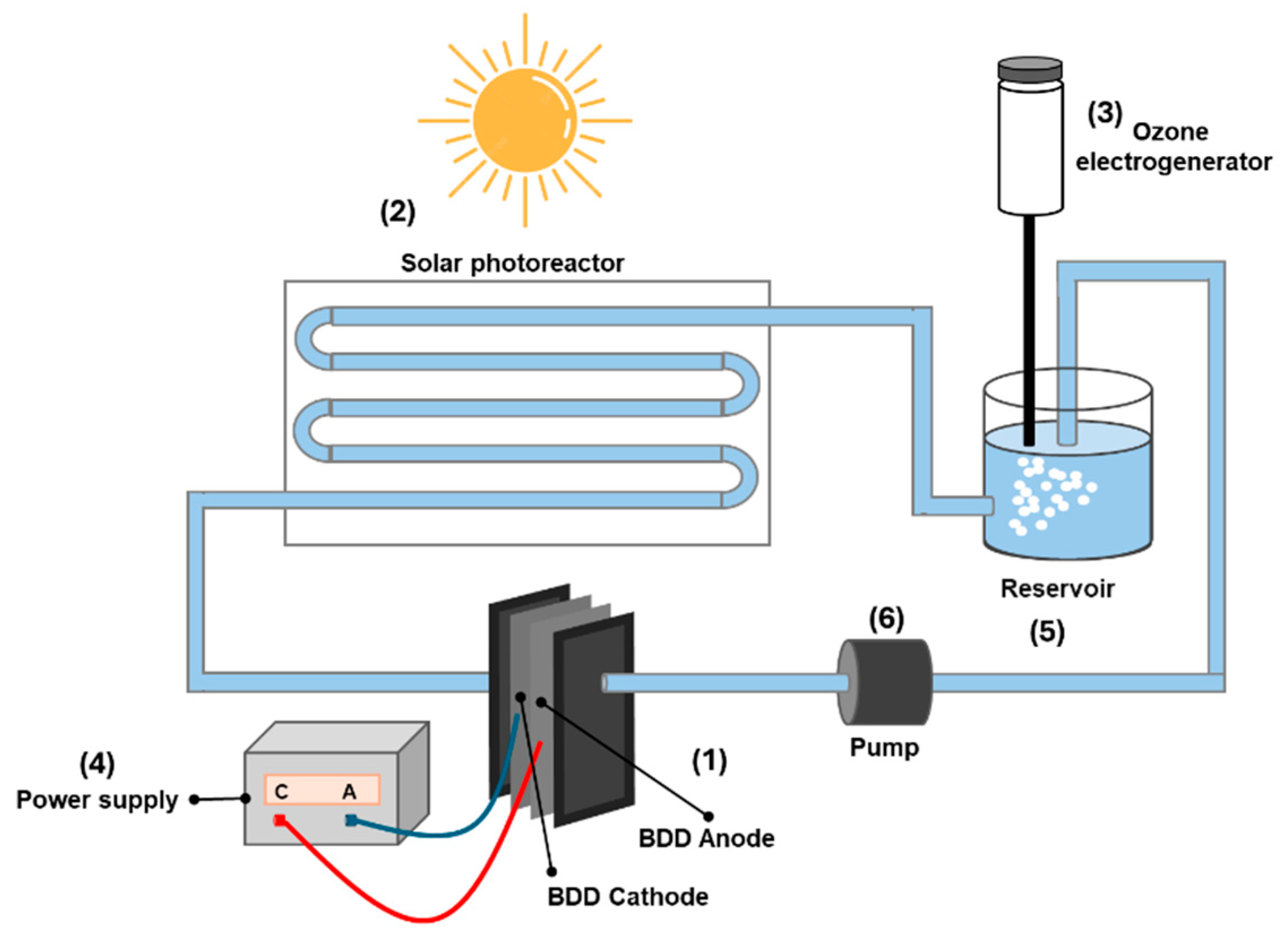
2.2. Electrochemical Set-Up
2.3. Analytical Measurements
2.3.1. High-Performance Liquid Chromatography (HPLC) Methodology
2.3.2. Hydrogen Peroxide (H2O2) Electrogeneration/Accumulation
2.3.3. Chemical Oxygen Demand (COD) Analysis
3. Results and Discussion
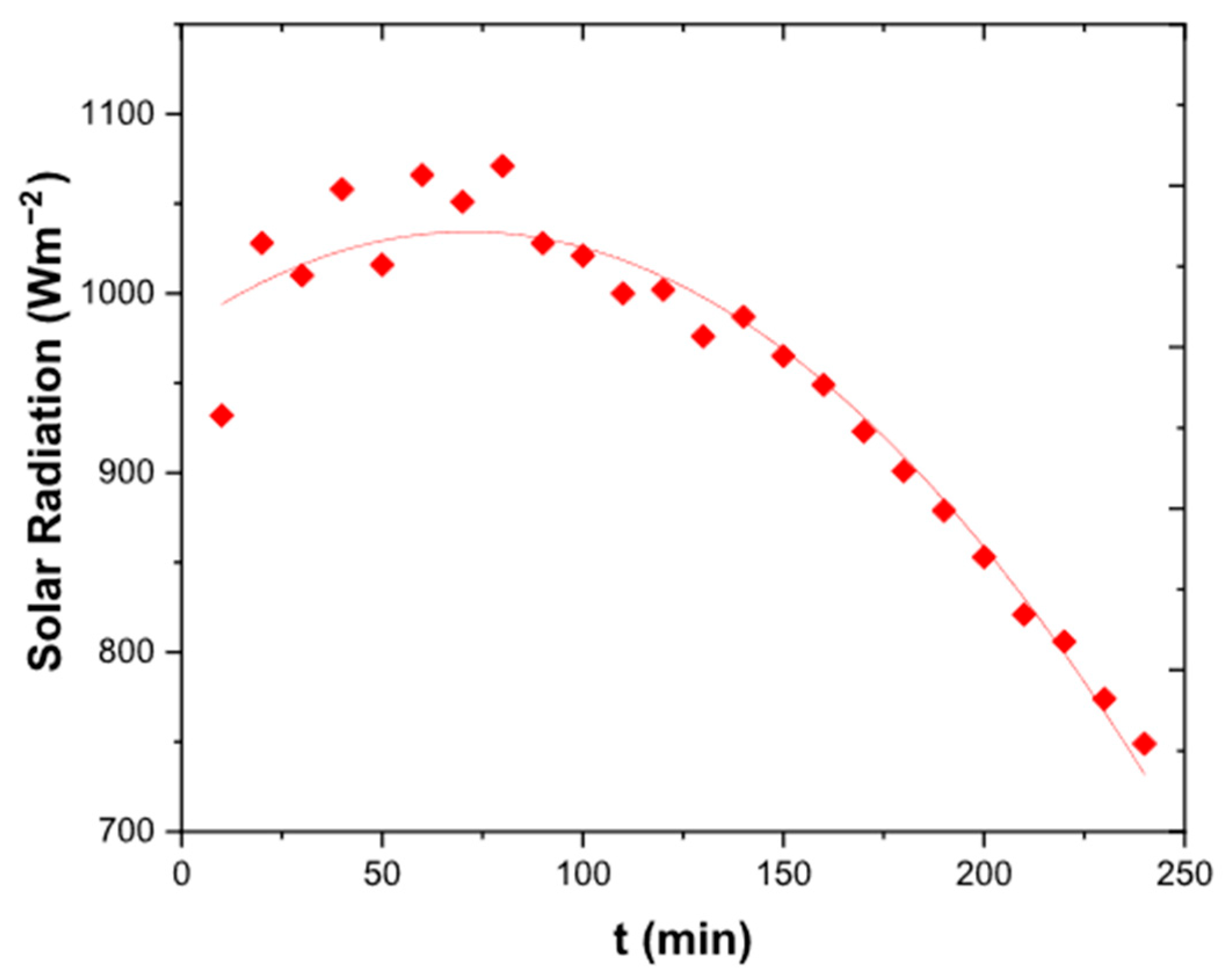
3.1. Solar Radiation
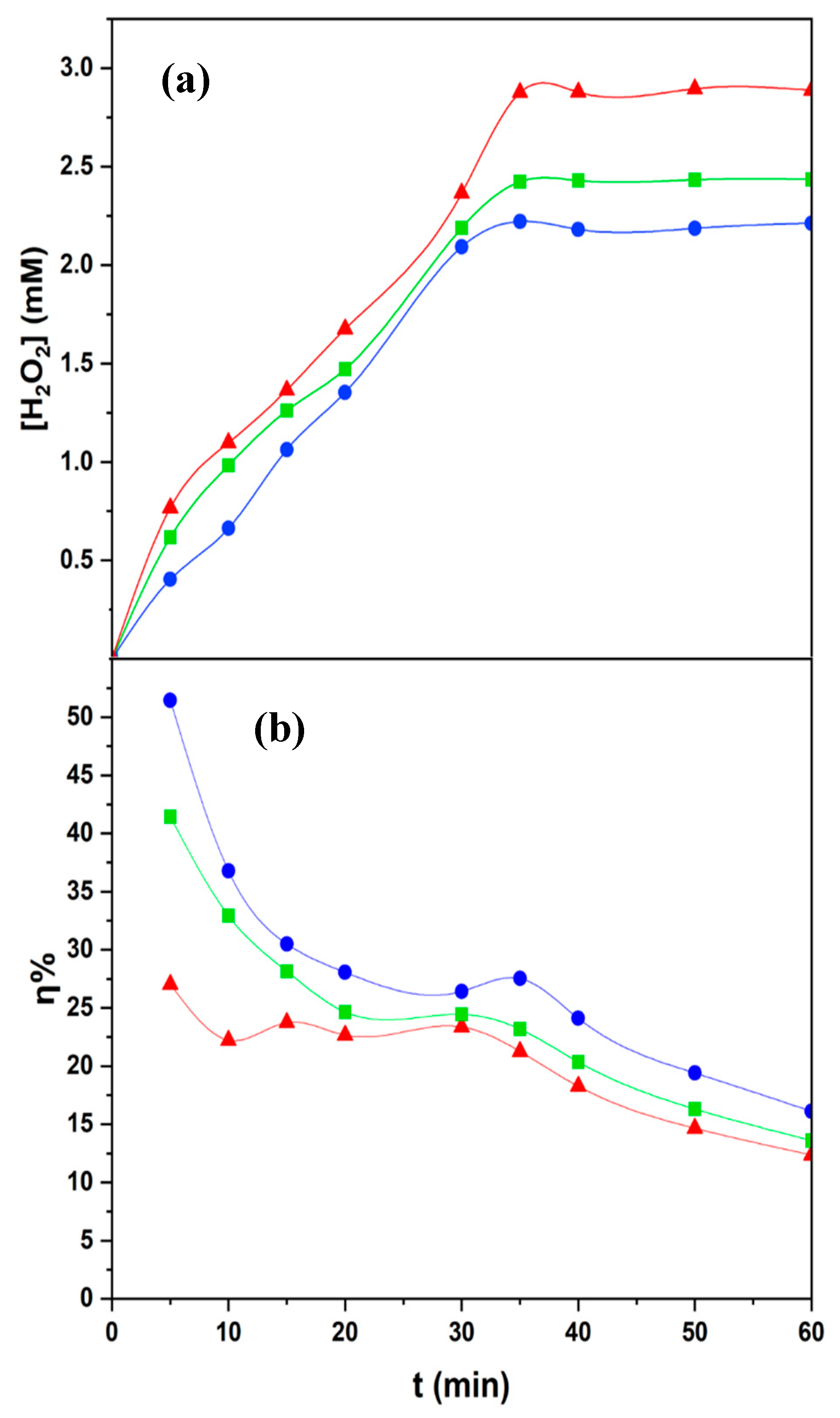
3.2. Electrogeneration of Hydrogen Peroxide at Solar CPC Photoreactor
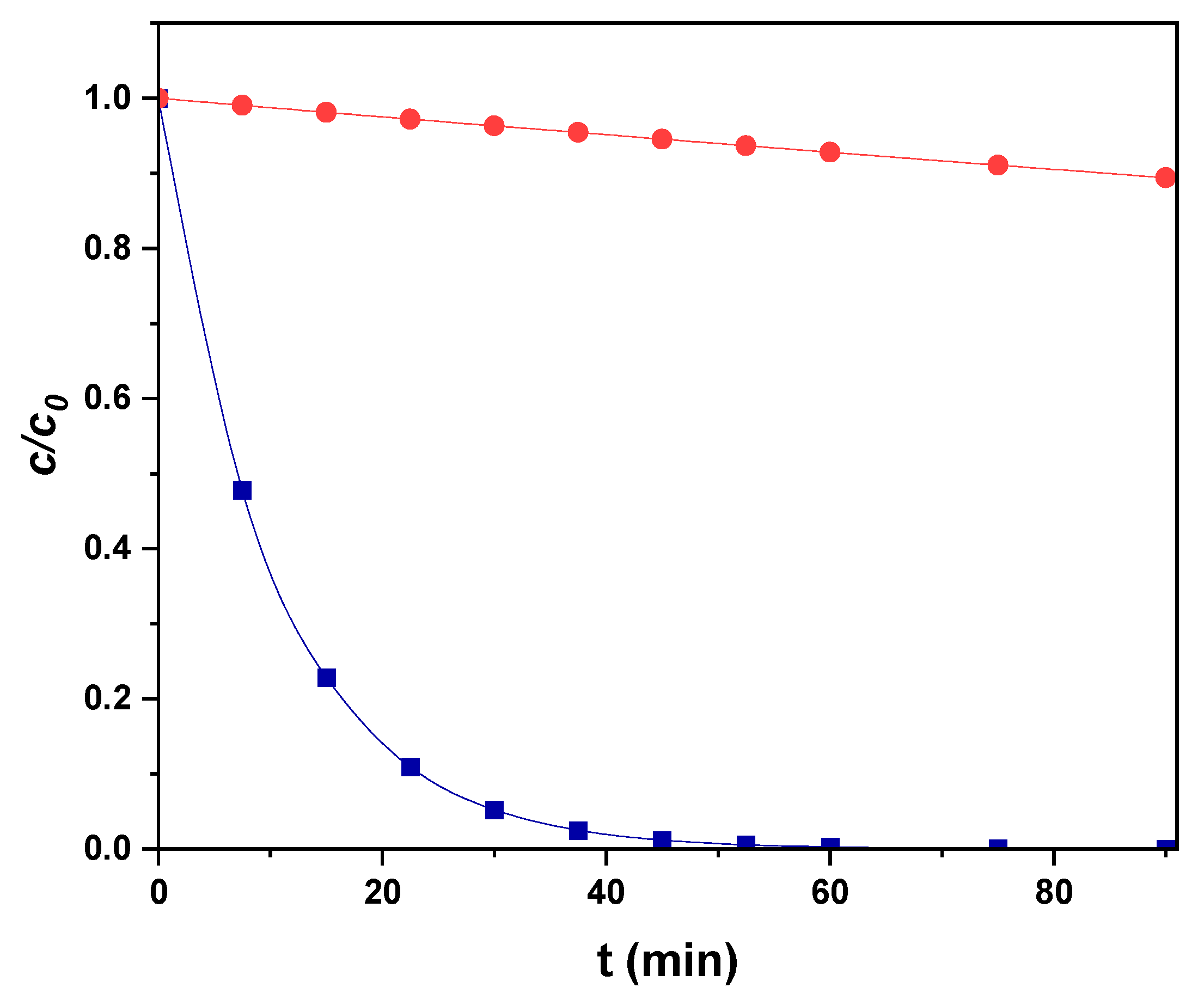
3.3. Degradation of Paracetamol with CPC-Type Photoreactor by SPEF Process
3.4. Identification of Intermediates
3.5. Cyclic Voltammetric Analysis
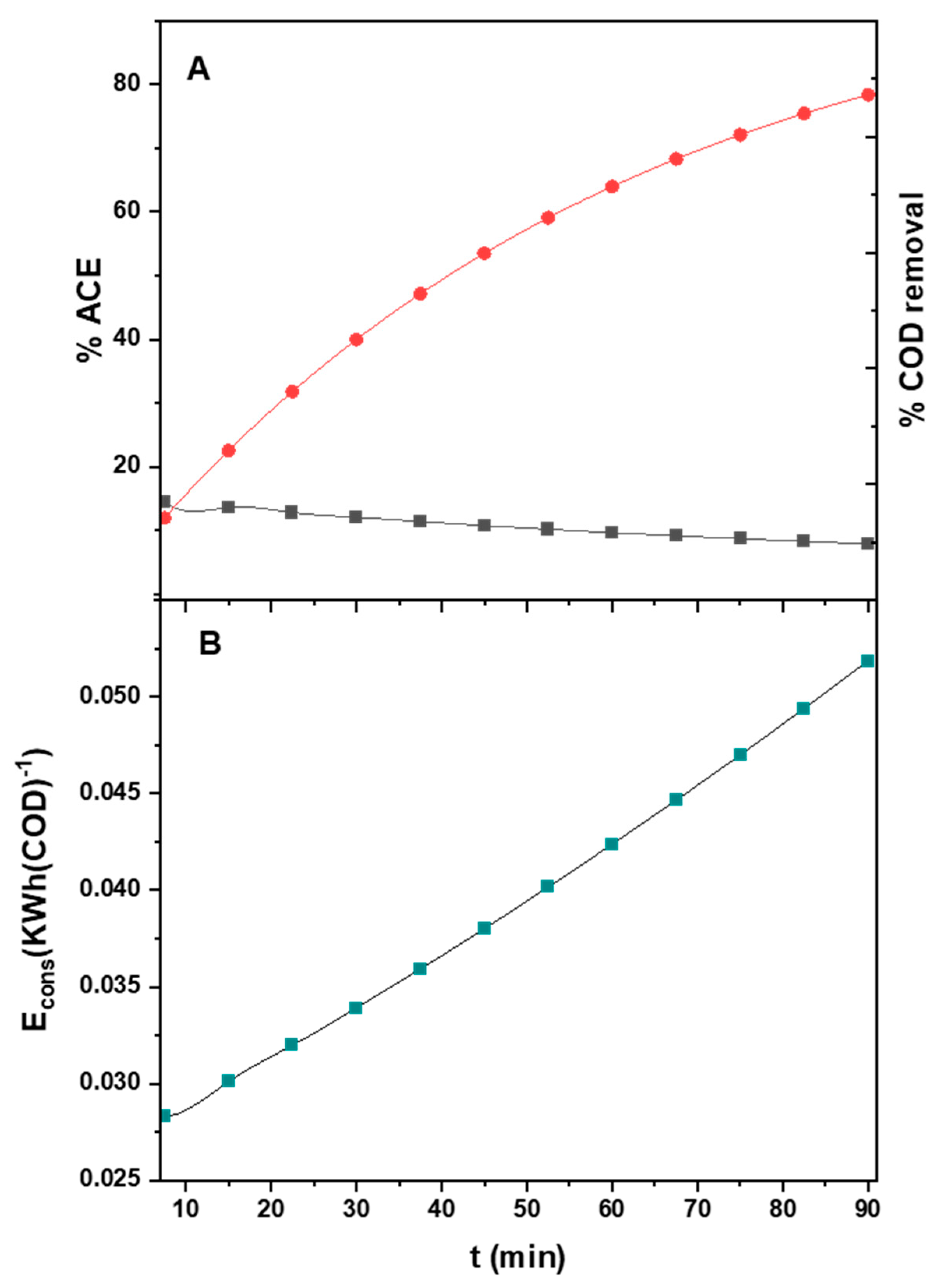
3.6. COD Removal, ACE Evaluation, and Energy Consumption
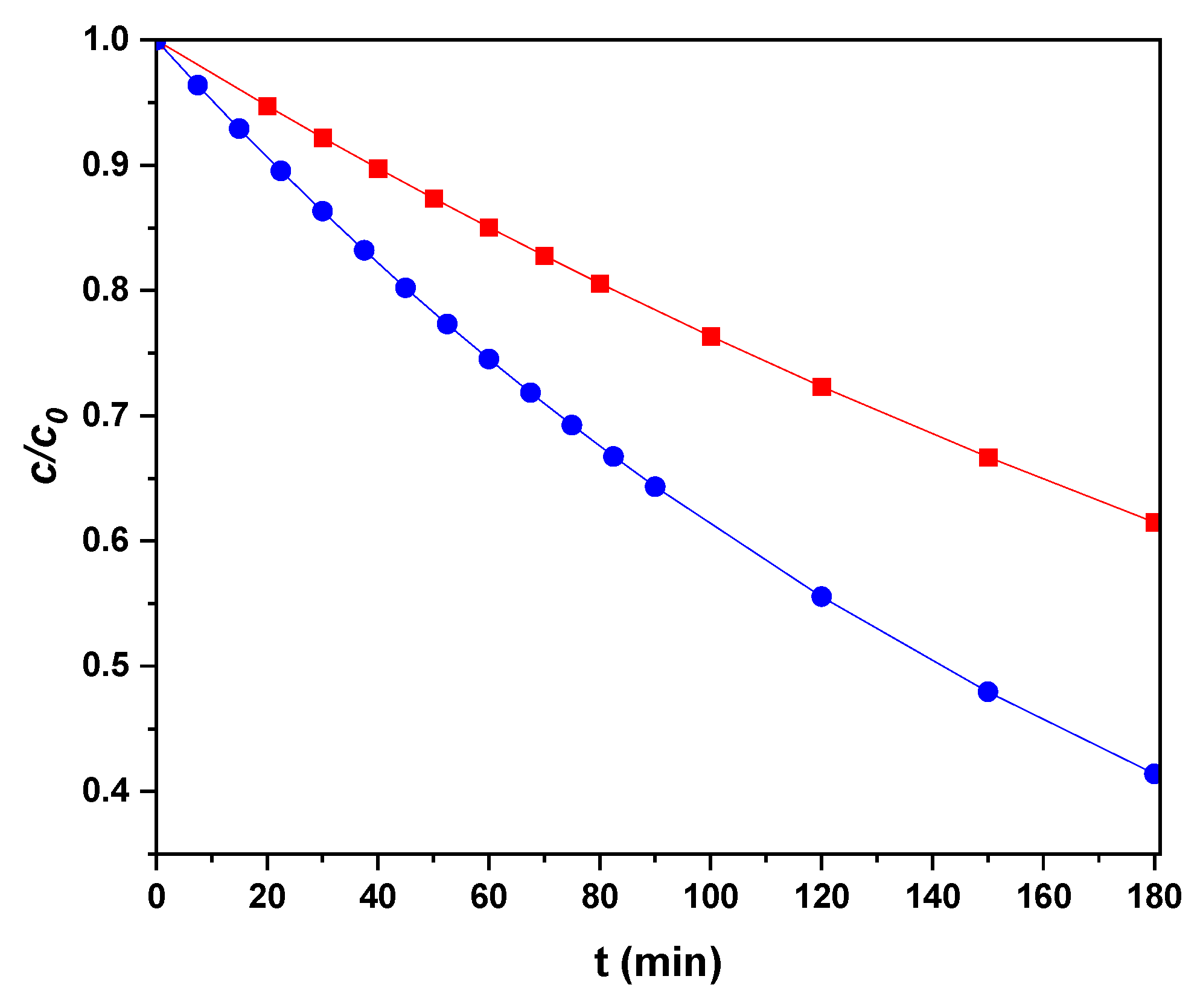
3.7. Treatment of a Mixture of Drugs with the Coupled SPEF/Ozone Process
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tortonese, S.; Scriabine, I.; Anjou, L.; Loens, C.; Michon, A.; Benabdelhak, M.; Ouali, S.; Morin, G.; Laifi, M.; Dobosziewicz, H.; et al. COVID-19 in patients on maintenance dialysis in the Paris region. Kidney Int. Rep. 2020, 5, 1535–1544. [Google Scholar] [CrossRef]
- Noreen, S.; Maqbool, I.; Madni, A. Dexamethasone: Therapeutic potential, risks, and future projection during COVID-19 pandemic. Eur. J. Pharmacol. 2021, 894, 173854. [Google Scholar] [CrossRef]
- Miah, M.S.; Mamun, M.R.; Saif-Ur-Rahman, K.M.; Rabby, A.A.; Zakaria, A.F.M. A qualitative exploration of purchasing, stockpiling, and use of drugs during the COVID-19 pandemic in an urban city of Bangladesh. Public Health Pract. 2024, 7, 100477. [Google Scholar] [CrossRef]
- Mostafa, E.M.A.; Tawfik, A.M.; Abd-Elrahman, K.M. Egyptian perspectives on potential risk of paracetamol/acetaminophen-induced toxicities: Lessons learnt during COVID-19 pandemic. Toxicol. Rep. 2022, 9, 541–548. [Google Scholar] [CrossRef]
- Watanabe, Y.; Nakamura, I.; Sato, S.; Fujita, H.; Kobayashi, T.; Watanabe, H. Comparison of methylprednisolone pulse vs conventional dexamethasone for adult cases of COVID-19 requiring oxygen; a Japanese retrospective cohort study. J. Infect. Chemother. 2023, 29, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Manjani, L.; Desai, N.; Kohli, A.; Arya, R.; Woods, C.; Desale, S. Effects of acetaminophen on outcomes in patients hospitalized with COVID-19. Chest 2021, 160, A1072. [Google Scholar] [CrossRef]
- Albornoz, L.L.; Soroka, V.D.; Silva, M.C.A. Photo-mediated and advanced oxidative processes applied for the treatment of effluents with drugs used for the treatment of early COVID-19: Review. Environ. Adv. 2021, 6, 100140. [Google Scholar] [CrossRef]
- Domingo-Echaburu, S.; Irazola, M.; Prieto, A.; Rocano, B.; Lopez de Torre-Querejazu, A.; Quintana, A.; Orive, G.; Lertxundi, U. Drugs used during the COVID-19 first wave in Vitoria-Gasteiz (Spain) and their presence in the environment. Sci. Total Environ. 2022, 820, 153122. [Google Scholar] [CrossRef]
- Melo, J.R.R.; Duarte, E.C.; de Moraes, M.V.; Fleck, K. Automedicação e uso indiscriminado de medicamentos durante a pandemia da COVID-19. Cad. Saúde Pública 2021, 37, e00053221. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, N. Paracetamol—A contaminant of high concern: Existence in environment and adverse effect. Pharm. Drug Regul. Aff. J. 2022, 5, 128. [Google Scholar] [CrossRef]
- Koagouw, W.; Arifin, Z.; Olivier, G.W.J.; Ciocan, C. High concentrations of paracetamol in effluent dominated waters of Jakarta Bay, Indonesia. Mar. Pollut. Bull. 2021, 169, 112558. [Google Scholar] [CrossRef]
- Miglione, A.; Raucci, A.; Cristiano, F.; Mancini, M.; Gioia, V.; Frugis, A.; Cinti, S. Paper-based 2D configuration for the electrochemical and facile detection of paracetamol in wastewaters. Electrochim. Acta 2024, 488, 144255. [Google Scholar] [CrossRef]
- Garcia-Segura, S.; Brillas, E. Redesigning electrochemical-based Fenton processes: An updated review exploring advances and innovative strategies using phenol degradation as key performance indicator. Appl. Catal. B Environ. 2024, 194, 206980. [Google Scholar] [CrossRef]
- Wang, L.; Jiang, J.; Ma, J.; Pang, S.; Zhang, T. A review on advanced oxidation processes homogeneously initiated by copper (II). Chem. Eng. J. 2022, 427, 131721. [Google Scholar] [CrossRef]
- Jiang, H.; Chen, H.; Duan, Z.; Huang, Z.; Wei, K. Research progress and trends of biochar in the field of wastewater treatment by electrochemical advanced oxidation processes (EAOPs): A bibliometric analysis. J. Hazard. Mater. Adv. 2023, 10, 100305. [Google Scholar] [CrossRef]
- Brillas, E.; Peralta-Hernandez, J.M. The recent development of innovative photoelectro-Fenton processes for the effective and cost-effective remediation of organic pollutants in waters. Chemosphere 2024, 366, 143465. [Google Scholar] [CrossRef]
- Poza-Nogueiras, V.; Arellano, M.; Rosales, E.; Pazos, M.; González-Romero, E.; Sanromán, M.A. Heterogeneous electro-Fenton as plausible technology for the degradation of imidazolinium-based ionic liquids. Chemosphere 2018, 199, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Poza-Nogueiras, V.; Moratalla, A.; Pazos, M.; Sanromán, A.; Sáez, C.; Rodrigo, M.A. Exploring the pressurized heterogeneous electro-Fenton process and modelling the system. Chem. Eng. J. 2022, 431, 133280. [Google Scholar] [CrossRef]
- Nguyen, D.D.D.; Quang, H.H.P.; Nguyen, X.H.; Nguyen, T.P. The treatment of real dyeing wastewater by the electro-Fenton process using drinking water treatment sludge as a catalyst. RSC Adv. 2021, 11, 27443–27452. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, R.; Latha, A.; Venkatesan, G.; Krishnakumari, B. Treatment of textile dyes with steel flakes via Fenton and electro-Fenton processes: An experimental analysis. Desalination Water Treat. 2024, 320, 100837. [Google Scholar] [CrossRef]
- He, Y.; Sutton, N.B.; Rijnaarts, H.H.M.; Langenhoff, A.A.M. Degradation of Paracetamol and Its Oxidation Products in Surface Water by Electrochemical Oxidation. Environ. Eng. Sci. 2018, 35, 1022–1031. [Google Scholar] [CrossRef]
- Xia, Y.; Dai, J.; Yan, Y.; Ma, X.; Feng, H.; Ding, Y. Energy-efficient electrochemical treatment of paracetamol using a PbO2 anode based on pulse electrodeposition strategy: Kinetics, energy consumption and mechanism. Environ. Res. 2022, 216, 114673. [Google Scholar] [CrossRef]
- Zhang, F.; Li, J.; He, Z. Bio-Electrochemical Degradation of Paracetamol in a Microbial Fuel Cell-Fenton System. Chem. Eng. J. 2015, 276, 185–192. [Google Scholar] [CrossRef]
- Tang, J.; Yao, S.; Xiao, F.; Xia, J.; Xing, X. Electrochemical Oxidation of Landfill Leachate after Biological Treatment by Electro-Fenton System with Corroding Electrode of Iron. Int. J. Environ. Res. Public Health 2022, 19, 7745. [Google Scholar] [CrossRef]
- Moreira, F.C.; Boaventura, R.A.R.; Brillas, E.; Vilar, V.J.P. Electrochemical advanced oxidation processes: A review on their application to synthetic and real wastewaters. Appl. Catal. B Environ. 2017, 202, 217–261. [Google Scholar] [CrossRef]
- Guineo-Salinas, R.; Cotillas, S.; Cañizares, P.; Rodrigo, M.A.; Brillas, E.; Sirés, I. Solar photoelectro-Fenton degradation of nalidixic acid in a pilot plant using a BDD/air-diffusion cell coupled with a CPC photoreactor. Chem. Eng. J. 2018, 333, 444–453. [Google Scholar] [CrossRef]
- Salmerón, I.; García-Gómez, J.; López, F.; López-González, J.A.; Arrebola, F.J.; Quiroga, J.M.; Quiroga, J.M. Treatment of winery wastewater by solar photoelectro-Fenton at a pilot plant. Catal. Today 2013, 209, 199–204. [Google Scholar] [CrossRef]
- Prieto-Rodríguez, L.; Oller, I.; Fernández-Ibáñez, P.; Agüera, A.; Cabrera Reina, A.; Méndez-Arriaga, F.; Maldonado, M.I.; Malato, S. Application of solar photocatalytic ozonation for micropollutant removal in real municipal wastewater effluents. Appl. Catal. B Environ. 2013, 142–143, 561–569. [Google Scholar] [CrossRef]
- Moreira, F.C.; Sousa, J.M.; Macedo, G.; Ribeiro, A.R.; Barreiros, L.; Pedrosa, M.; Faria, J.L.; Gomes, H.T.; Nunes, O.C.; Manaia, C.M.; et al. Photocatalytic ozonation of urban wastewater and surface water pollutants. Sci. Total Environ. 2019, 646, 734–744. [Google Scholar] [CrossRef]
- Kuznetsov, V.V.; Ivantsova, N.A.; Kuzin, E.N.; Pirogov, A.V.; Mezhuev, Y.O.; Filatova, E.A.; Averina, Y.M. Study of the Process of Electrochemical Oxidation of Active Pharmaceutical Substances on the Example of Nitrofurazone ((2E)-2-[(5-Nitro-2-furyl)methylene]hydrazine Carboxamide). Processes 2023, 11, 3370. [Google Scholar] [CrossRef]
- Oturan, N.; Oturan, M.A.; Trellu, C. Comparative Performance of Ten Electrodes in Electro-Fenton Process for Removal of Organic Pollutants from Water. ChemElectroChem 2021, 8, 3294–3303. [Google Scholar] [CrossRef]
- Montenegro-Ayo, R.; Morales-Gomero, J.C.; Alarcón, H.; Corzo, A.; Westerhoff, P.; Garcia-Segura, S. Photoelectrocatalytic Degradation of 2,4-Dichlorophenol in a TiO2 Nanotube-Coated Disc Flow Reactor. Chemosphere 2021, 268, 129320. [Google Scholar] [CrossRef]
- Martínez-Huitle, C.A.; Panizza, M. Electrochemical oxidation of organic pollutants for wastewater treatment. Curr. Opin. Electrochem. 2018, 11, 62–71. [Google Scholar] [CrossRef]
- Ganiyu, S.O.; Martínez-Huitle, C.A.; Oturan, M.A. Electrochemical advanced oxidation processes for wastewater treatment: Advances in formation and detection of reactive species and mechanisms. Curr. Opin. Electrochem. 2021, 27, 100678. [Google Scholar] [CrossRef]
- Chaplin, B.P. The prospect of electrochemical technologies advancing worldwide water treatment. Acc. Chem. Res. 2019, 52, 596–604. [Google Scholar] [CrossRef]
- Brillas, E. A review on the photoelectro-Fenton process as efficient electrochemical advanced oxidation for wastewater remediation. In Treatment of Wastewater Using Biological and Chemical Processes; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Brillas, E.; Oturan, M.A. Electro-Fenton process and related electrochemical technologies based on Fenton’s reaction chemistry. Chem. Rev. 2014, 114, 2313–2381. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Huitle, C.A.; Ferro, S. Electrochemical oxidation of organic pollutants for the wastewater treatment: Direct and indirect processes. Chem. Soc. Rev. 2006, 35, 1324–1340. [Google Scholar] [CrossRef]
- Rodrigo, M.A.; Cañizares, P.; Sánchez-Carretero, A.; Sáez, C. Electrochemical technologies for the regeneration of urban wastewaters. Electrochim. Acta 2010, 55, 8160–8164. [Google Scholar] [CrossRef]
- Anglada, Á.; Urtiaga, A.; Ortiz, I. Contributions of electrochemical oxidation to waste-water treatment: Fundamentals and review of applications. J. Chem. Technol. Biotechnol. 2009, 84, 1747–1755. [Google Scholar] [CrossRef]
- Cañizares, P.; Lobato, J.; Paz, R.; Rodrigo, M.A.; Sáez, C. Electrochemical oxidation of phenolic wastes with boron-doped diamond anodes. Water Res. 2005, 39, 2687–2703. [Google Scholar] [CrossRef]
- Peralta-Hernández, J.M.; Meas-Vong, Y.; Rodríguez, F.J.; Chapman, T.W.; Maldonado, M.I.; Godínez, L.A. In situ electrochemical treatment of wastewater polluted with different azo dyes. J. Hazard. Mater. 2006, 138, 173–181. [Google Scholar] [CrossRef]
- Amado-Pina, D.; Roa-Morales, G.; Barrera-Diaz, C.; Balderas-Hernandez, P.; Romero, R.; Martin del Campo, E.; Natividad, R. Synergic effect of ozonation and electrochemical methods on oxidation and toxicity reduction: Phenol degradation. Fuel 2017, 198, 82–90. [Google Scholar] [CrossRef]
- Mecha, A.C.; Onyango, M.S.; Ochieng, A.; Fourie, C.J.S.; Momba, M.N.B. Synergistic effect of UV–vis and solar photocatalytic ozonation on the degradation of phenol in municipal wastewater: A comparative study. J. Catal. 2016, 341, 116–125. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, X.; Wang, Y.; Wang, X.; Wu, D.; Chen, C. Enhanced photocatalytic ozonation of phenol by Ag/ZnO: Activity, mineralization, and stability. Catalysts 2019, 9, 1006. [Google Scholar] [CrossRef]
- Mecha, A.C.; Onyango, M.S.; Ochieng, A.; Momba, M.N.B. Ultraviolet and solar photocatalytic ozonation of municipal wastewater: Catalyst reuse, energy requirements and toxicity assessment. Chemosphere 2017, 186, 669–676. [Google Scholar] [CrossRef]
- Callaway, K.K.; Tadrous, M.; Kane-Gill, S.L.; Barbash, I.; Rothenberger, S.D.; Suda, K. Changes in Purchases for Intensive Care Medicines during the COVID-19 Pandemic. Chest 2021, 160, 2123–2134. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Deng, J.; Heybati, K.; Zuo, Q.K.; Ali, S.; Hou, W.; Wong, C.Y.; Ramaraju, H.B.; Chang, O.; Dhivagaran, T.; et al. Efficacy and Safety of Corticosteroid Regimens for the Treatment of Hospitalized COVID-19 Patients: A Meta-Analysis. Future Virol. 2022, 17, 463–489. [Google Scholar] [CrossRef] [PubMed]
- Van Paassen, J.; Vos, J.S.; Hoekstra, E.M.; Neumann, K.M.; Boot, K.M.; Arbous, S.M. Corticosteroid Use in COVID-19 Patients: A Systematic Review and Meta-Analysis on Clinical Outcomes. Crit. Care 2020, 24, 696. [Google Scholar] [CrossRef] [PubMed]
- Nandi, A.; Pecetta, S.; Bloom, D. Global Antibiotic Use during the COVID-19 Pandemic: Analysis of Pharmaceutical Sales Data from 71 Countries, 2020–2022. EClinicalMedicine 2023, 57, 101848. [Google Scholar] [CrossRef]
- Tanty, A.; Vitale, E.; Lombardo, D.; Grévy, A.; Gibert, P.; Chapuis, C.; Chevallier Brilloit, C.; Allenet, B.; Bedouch, P.; Chanoine, S. Impact des Soins Pharmaceutiques dans la Prise en Charge des Patients Atteints de COVID-19 en Période de Crise Sanitaire. Le Pharm. Clin. 2022, 57, 101377. [Google Scholar] [CrossRef]



| Drug | CAS | Molecular Formula | Molecular Weight | Solubility in Water (mg (100 mL)−1) |
|---|---|---|---|---|
| Paracetamol | 103-90-2 | C8H9NO2 | 151.16 g mol−1 | 1.4 |
| Dexamethasone | 50-02-2 | C22H29FO5 | 392.46 g mol−1 | 1000 |
| Azithromycin | 83905-01-05 | C38H72N2O12 | 749 g mol−1 | 514 |
| Amoxicillin | 26787-78-0 | C16H19N3O5S | 365.4 gmol−1 | 10.7 |
| Parameter | Value |
|---|---|
| Total dissolved solids (TDS) | 232 mg L−1 |
| Temperature | 23–25.5 °C |
| Salinity | 0.042% |
| Specific density | 1.100 |
| pH | 7.1 |
| Oxidation-Reduction Potential (ORP) | 143–154 mV |
| Electrical conductivity (EC) | 480 µS cm−1 |
| Total hardness | 78.05 mg L−1 |
| Hardness to Mg | 19.5 mg L−1 |
| Hardness to Ca | 58.1 mg L−1 |
| Column | Supelco C18 (25 cm × 4.6 mm), 5 μm |
|---|---|
| Wavelength | 245 nm |
| Mobile phase | Water: Acetonitrile |
| Retention time | 5.258 min |
| Flow rate | 1 mL min−1 |
| Temperature | 25 °C |
| Injection volume | 10 μL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrera-Chávez, S.; Pacheco-Álvarez, M.; Godínez, L.A.; Brillas, E.; Peralta-Hernández, J.M. Hybrid Solar Photoelectro-Fenton and Ozone Processes for the Sustainable Removal of COVID-19 Pharmaceutical Contaminants. Processes 2025, 13, 3234. https://doi.org/10.3390/pr13103234
Herrera-Chávez S, Pacheco-Álvarez M, Godínez LA, Brillas E, Peralta-Hernández JM. Hybrid Solar Photoelectro-Fenton and Ozone Processes for the Sustainable Removal of COVID-19 Pharmaceutical Contaminants. Processes. 2025; 13(10):3234. https://doi.org/10.3390/pr13103234
Chicago/Turabian StyleHerrera-Chávez, Sonia, Martin Pacheco-Álvarez, Luis A. Godínez, Enric Brillas, and Juan M. Peralta-Hernández. 2025. "Hybrid Solar Photoelectro-Fenton and Ozone Processes for the Sustainable Removal of COVID-19 Pharmaceutical Contaminants" Processes 13, no. 10: 3234. https://doi.org/10.3390/pr13103234
APA StyleHerrera-Chávez, S., Pacheco-Álvarez, M., Godínez, L. A., Brillas, E., & Peralta-Hernández, J. M. (2025). Hybrid Solar Photoelectro-Fenton and Ozone Processes for the Sustainable Removal of COVID-19 Pharmaceutical Contaminants. Processes, 13(10), 3234. https://doi.org/10.3390/pr13103234







