Numerical Investigation of Combustion and Nitric Oxide Formation in a 130 t/h Pulverized-Coal Boiler Under Lignite–Bituminous Coal Blending
Abstract
1. Introduction
2. Physical and Mathematical Model
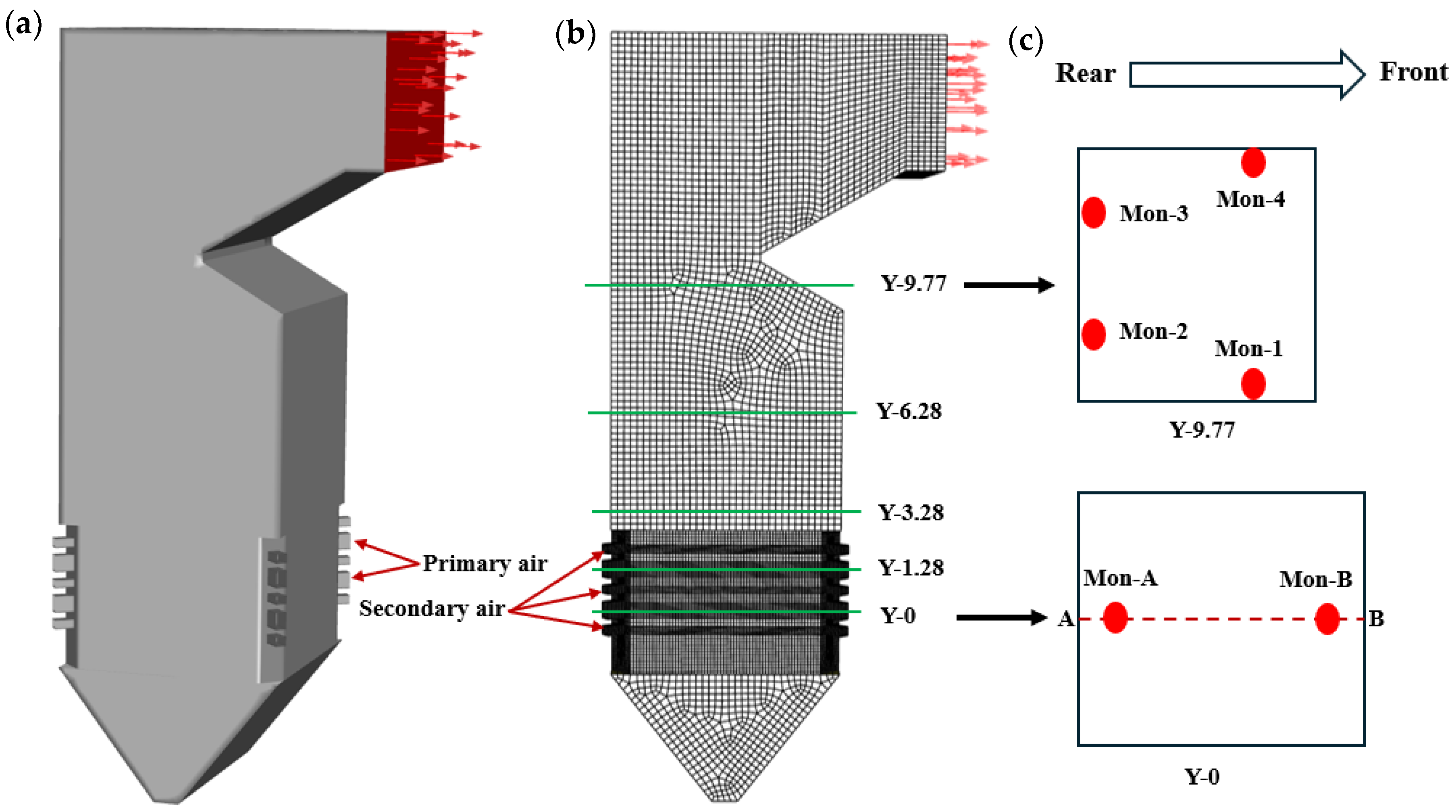
2.1. Physical Model
2.2. Mathematical Model
- (1)
- Basic Equations:
- (2)
- Turbulence Model:
- (3)
- Combustion Reaction Model
- (4)
- Radiation Model
- (5)
- NOx Formation Model
3. Numerical Method and Validation
3.1. Numerical Method
- (1)
- Inlet boundary conditions
- (2)
- Outlet boundary conditions
- (3)
- Wall boundary conditions
3.2. Validation of Numerical Method
4. Results
4.1. The Original Operating Condition Before Coal Blending
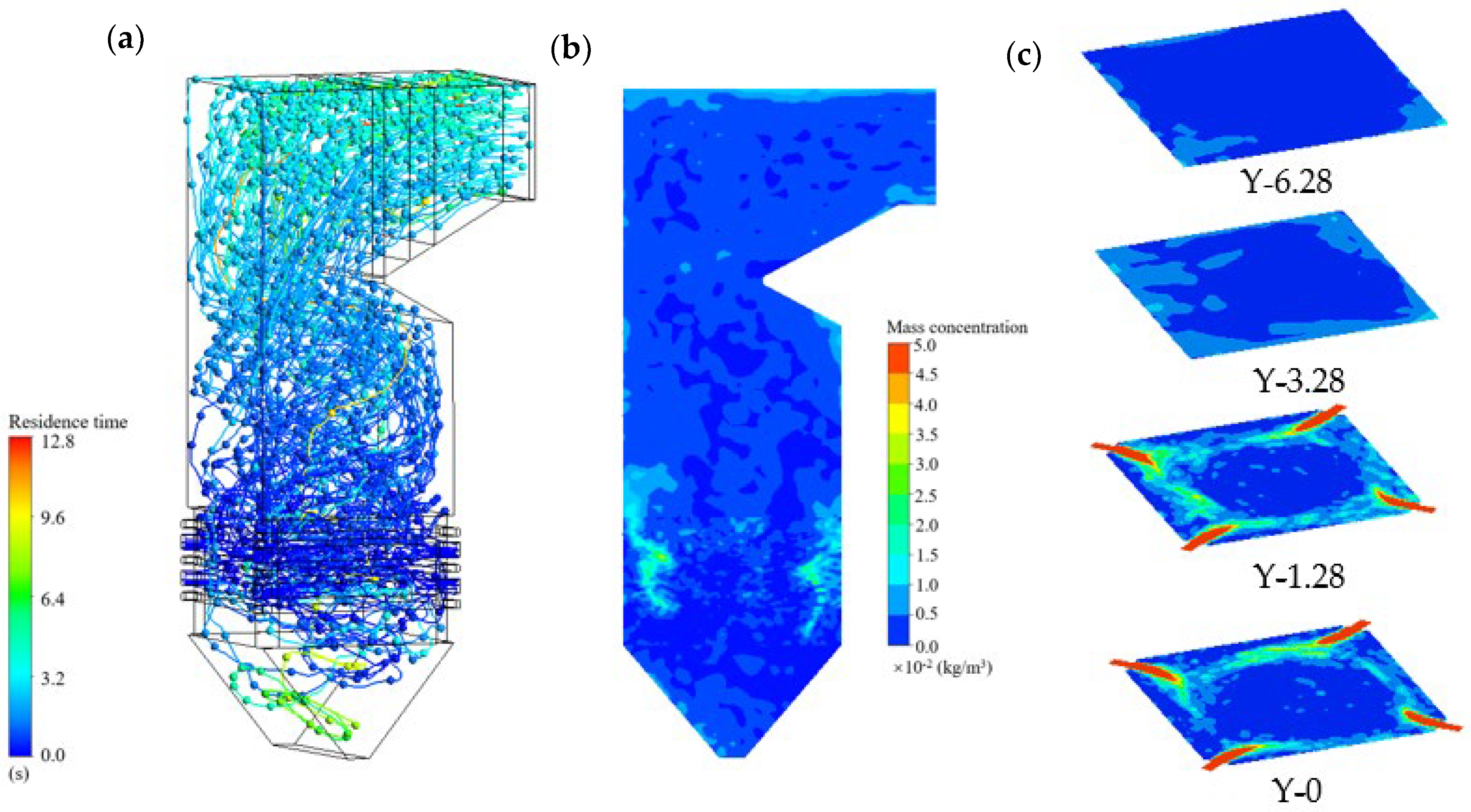
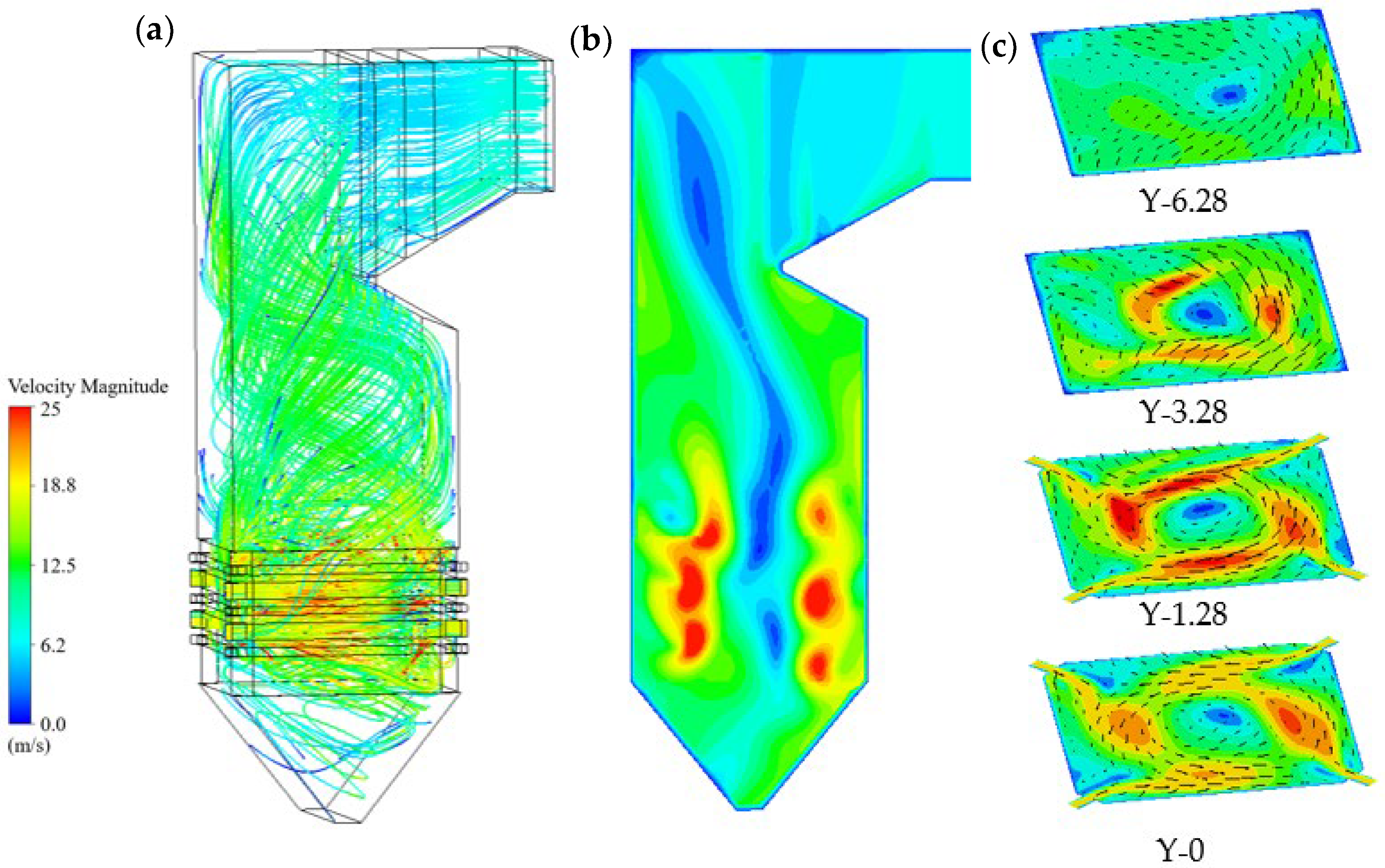
- (1)
- Fluid flow and particle motion
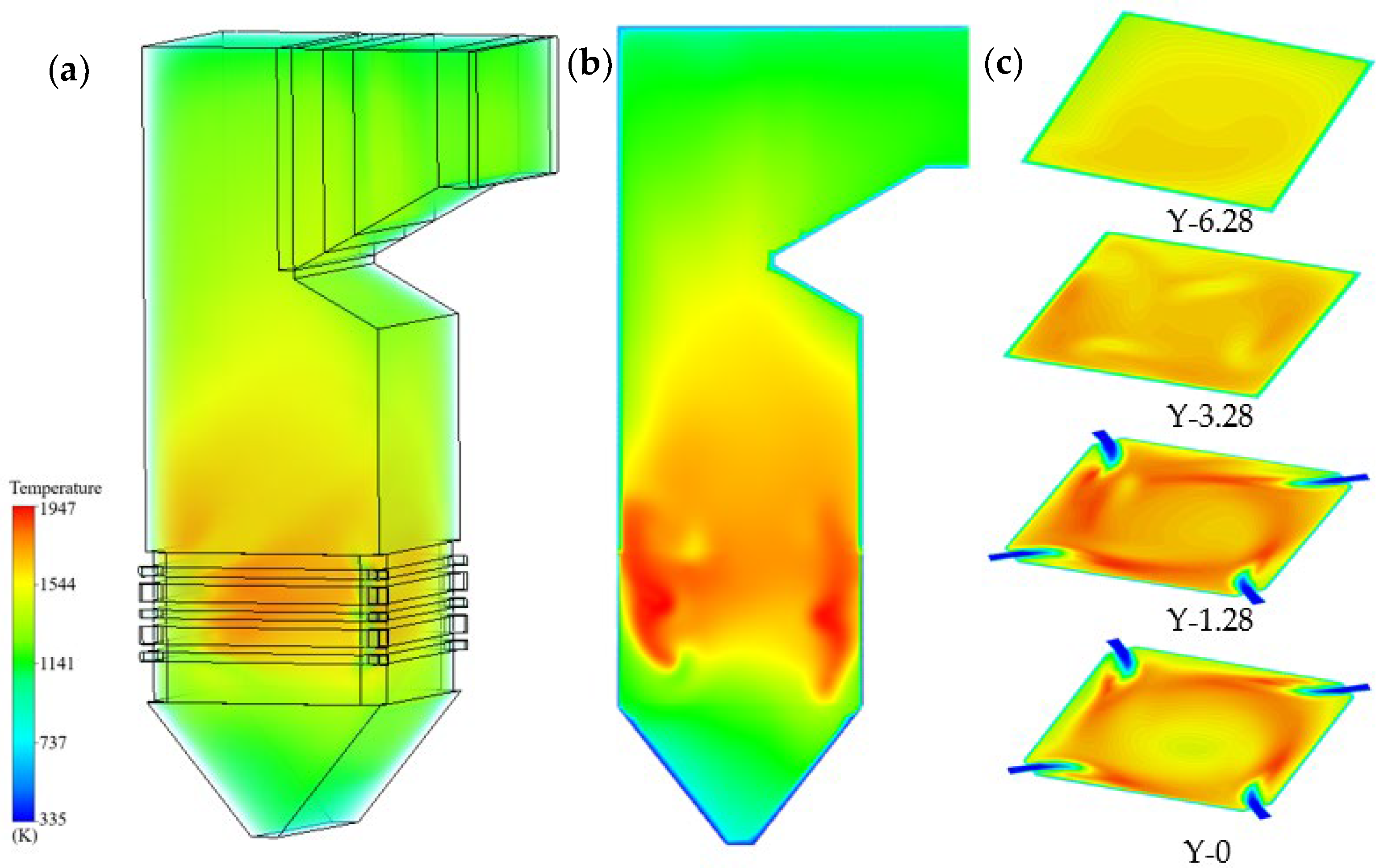
- (2)
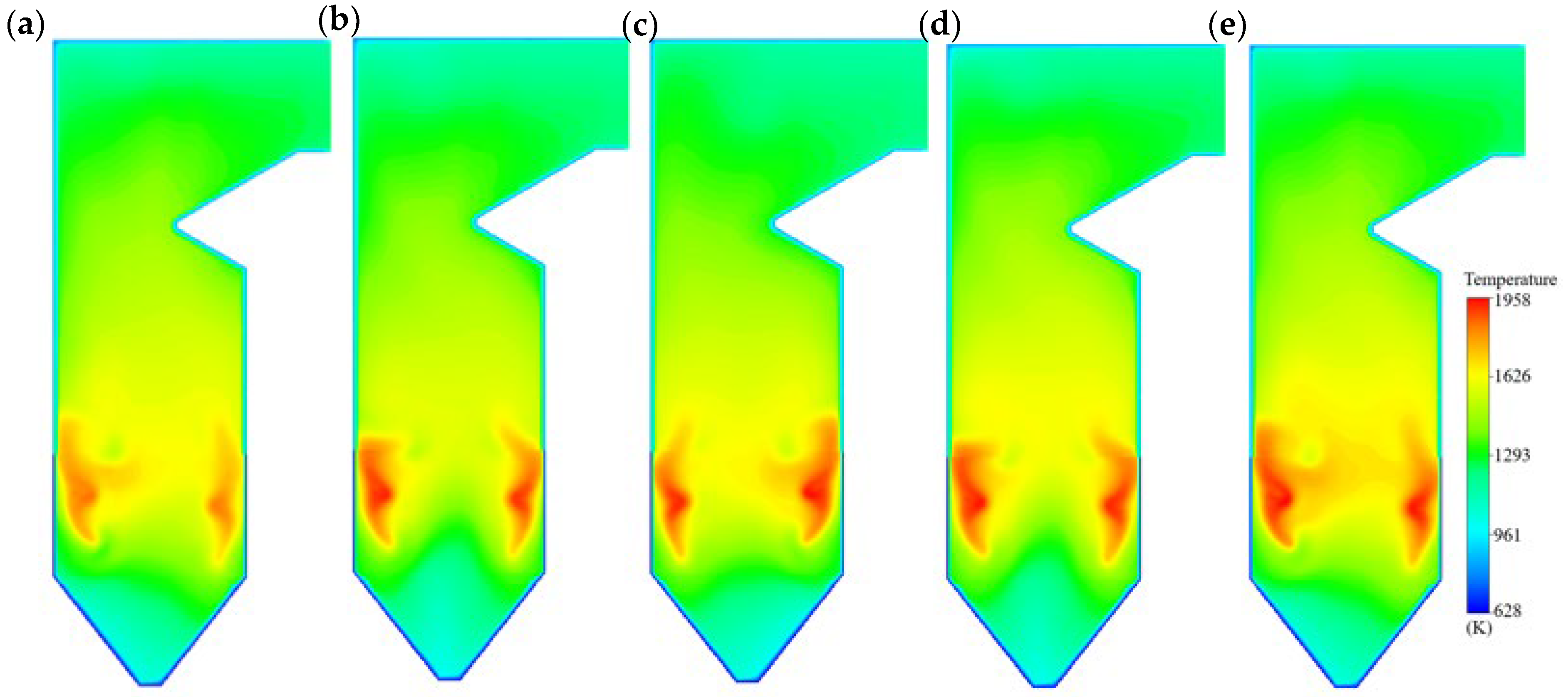
- Temperature fields
- (3)
- Species concentration fields
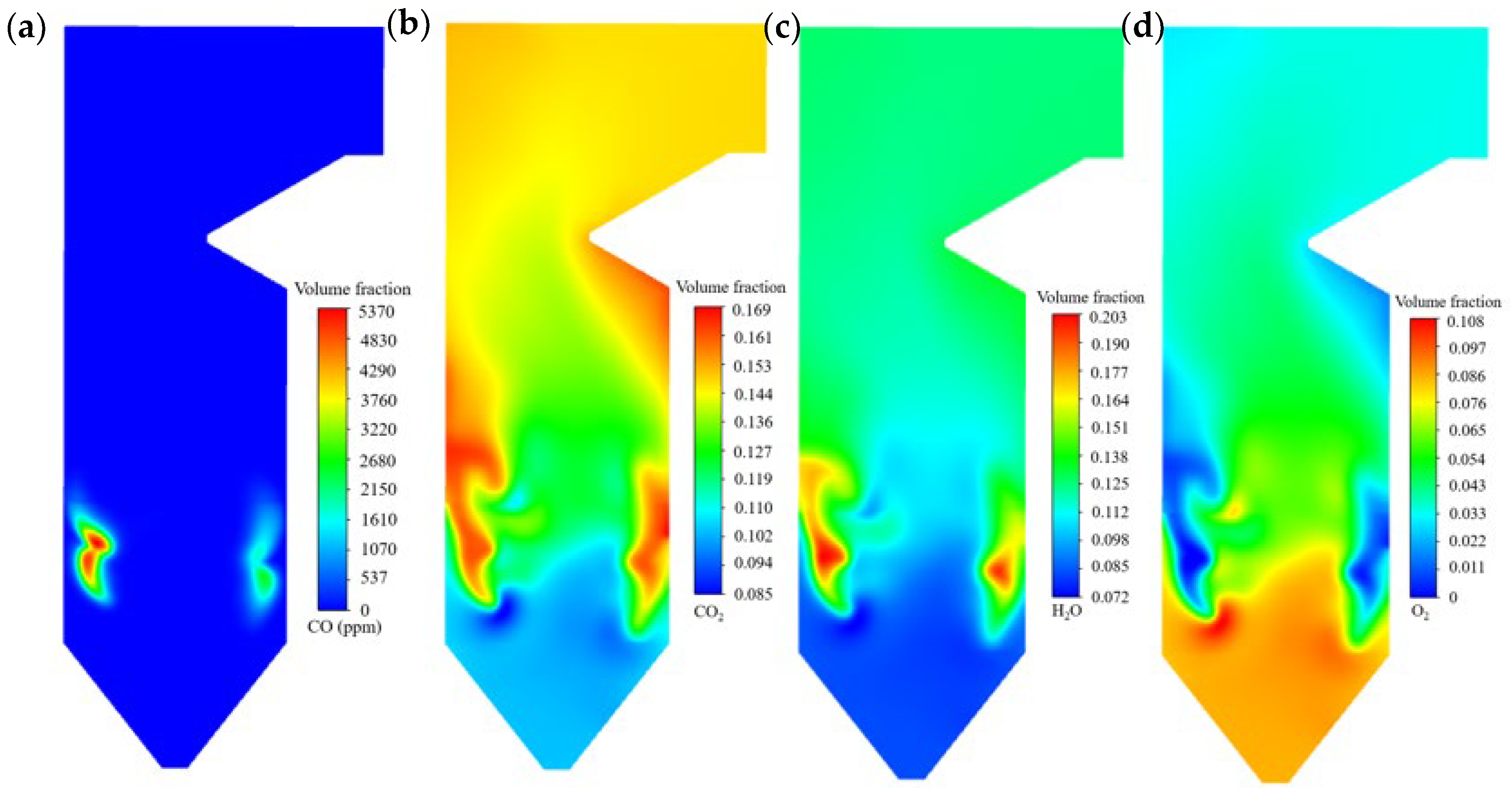
- (4)
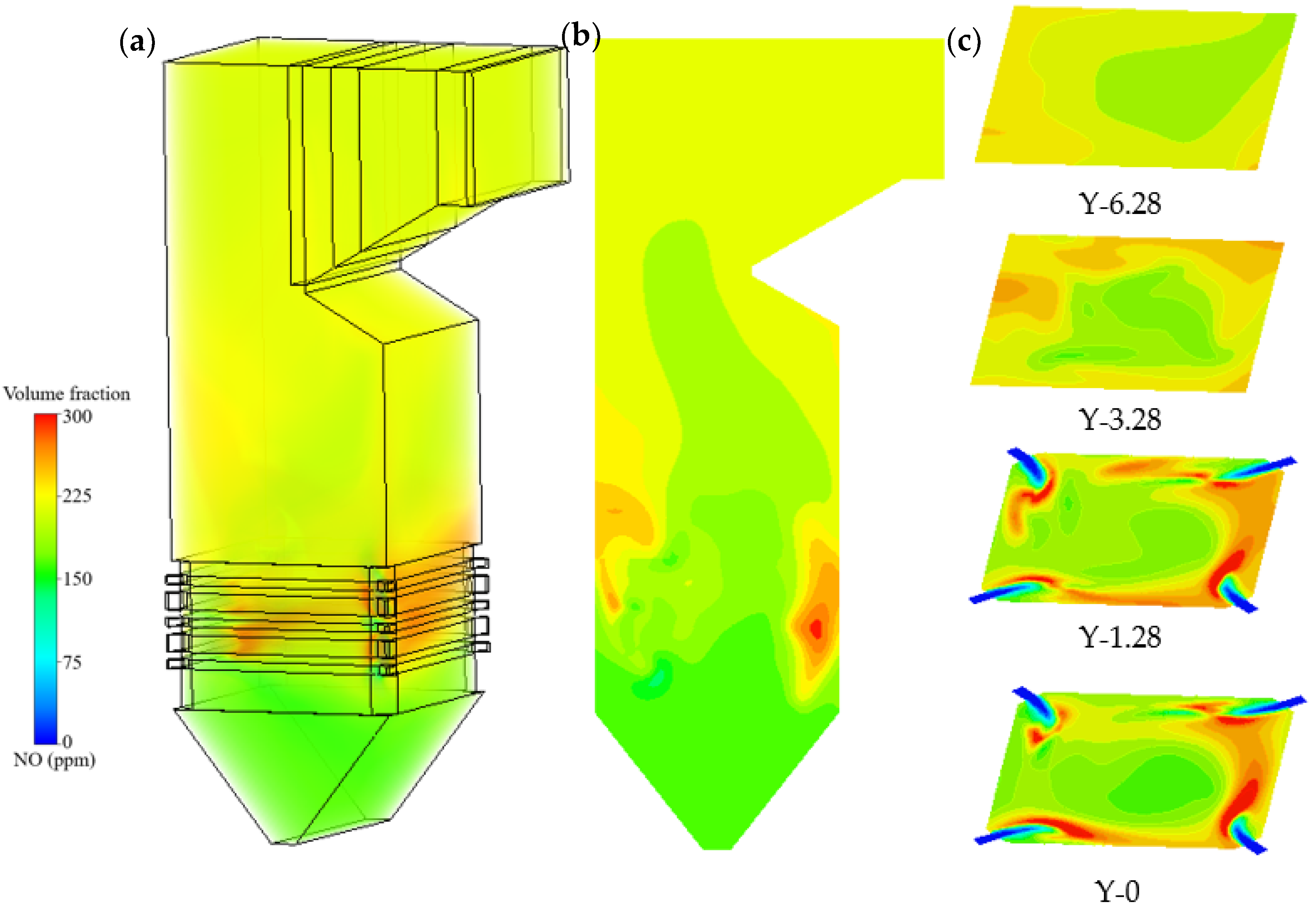
- Nitrogen oxides concentration fields.
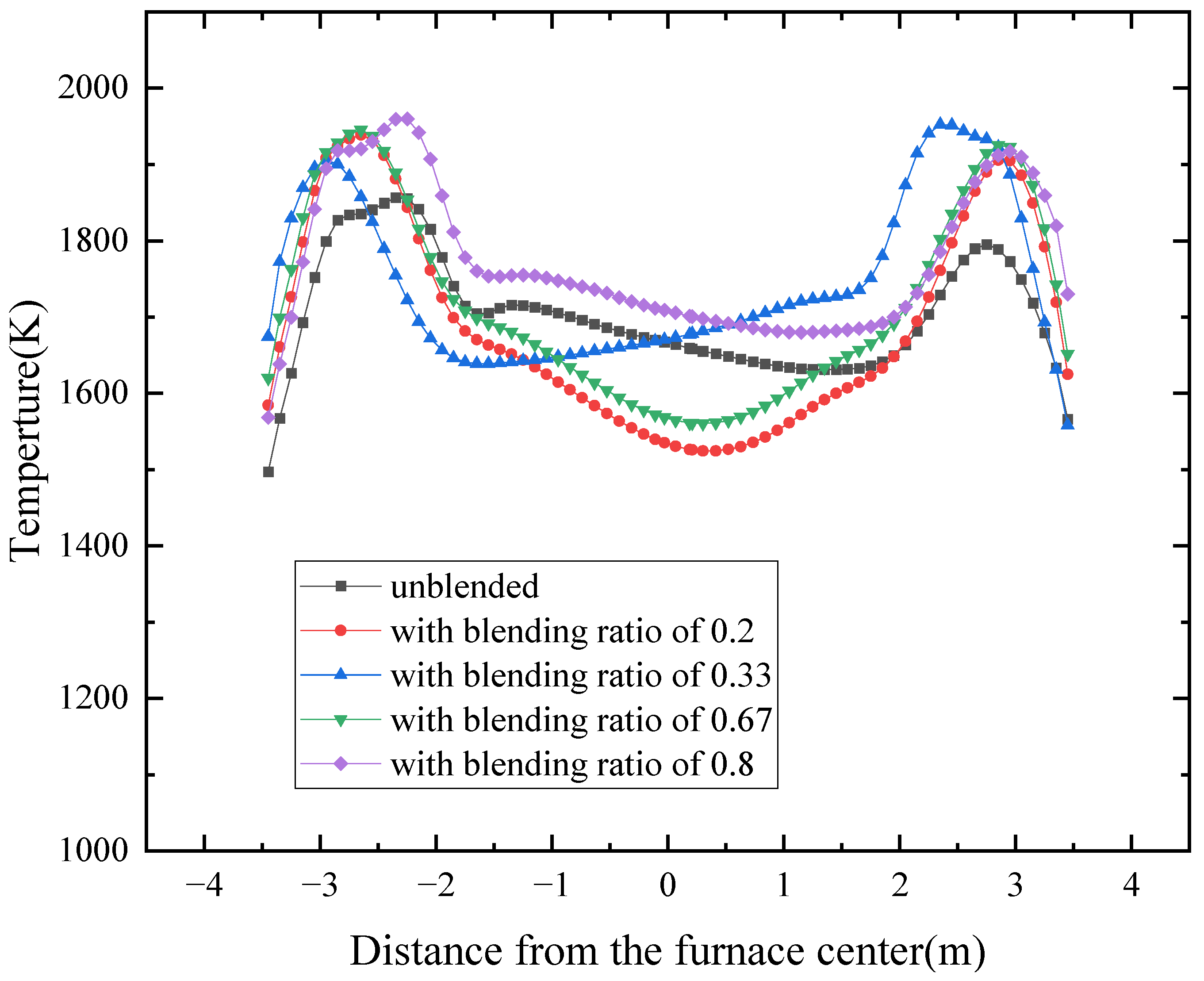
4.2. Influence of the Coal Blending Ratio on Temperature
4.3. Influence of the Coal Blending Ratio on Species Concentration
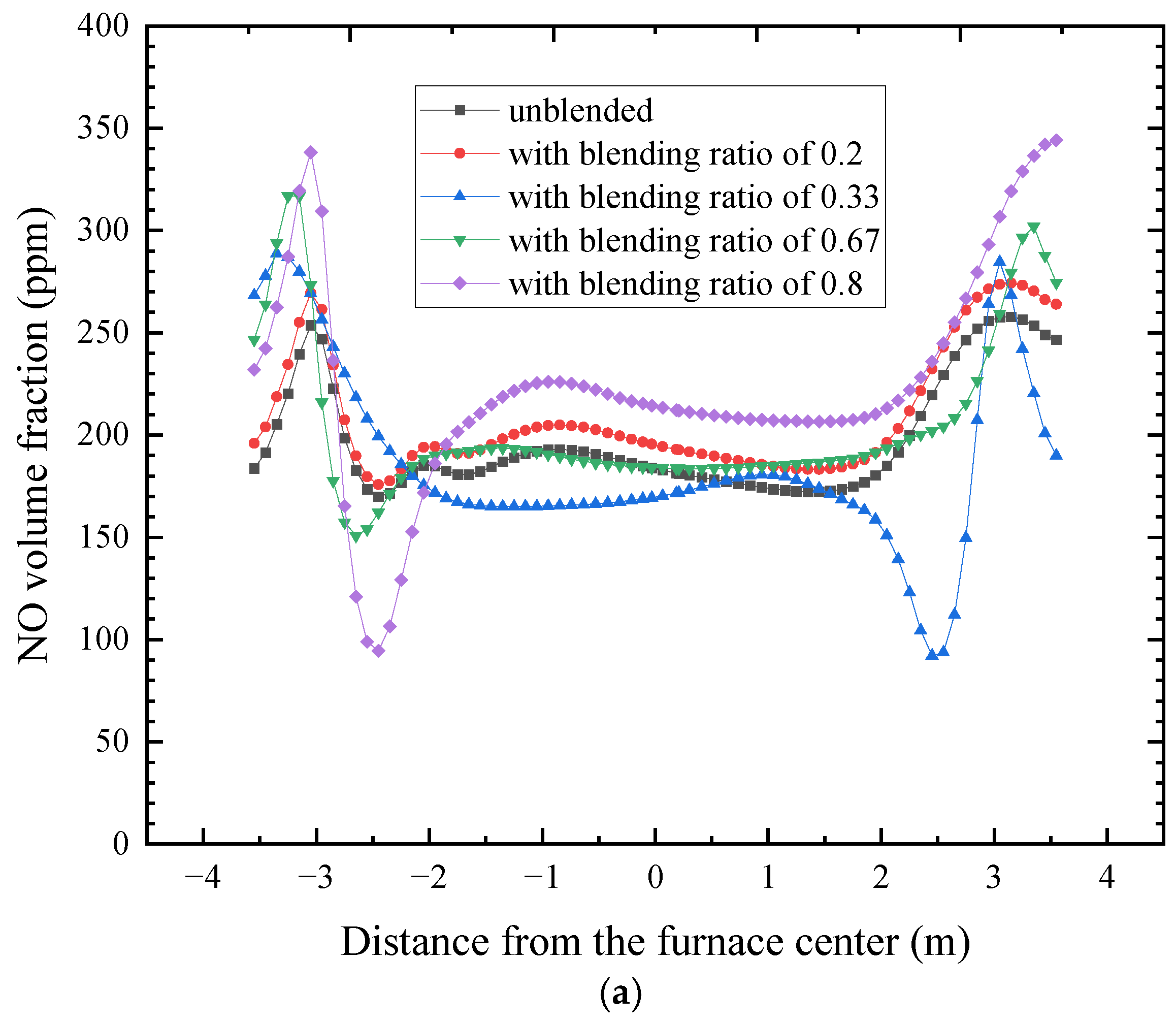
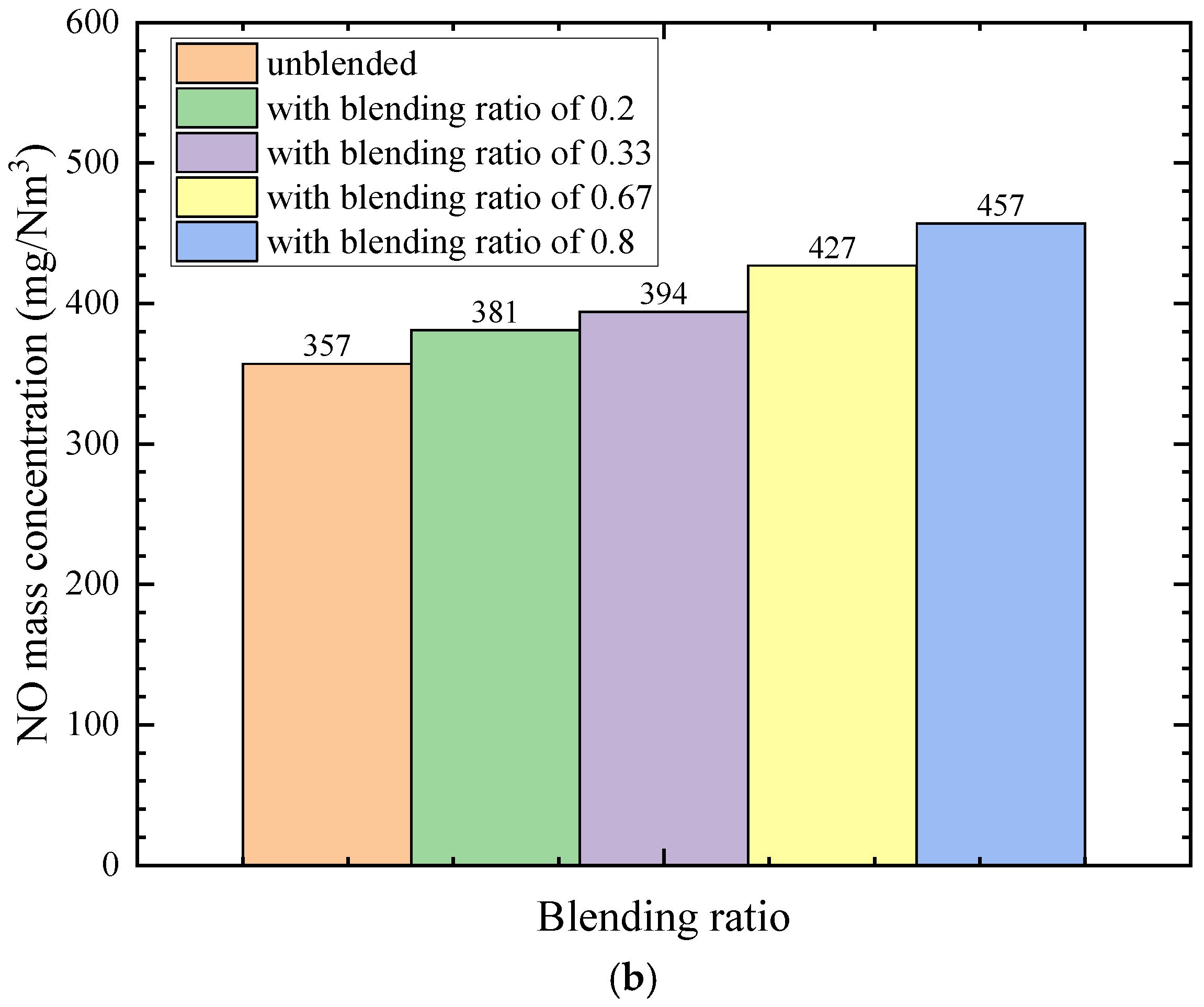
4.4. Influence of the Coal Blending Ratio on Nitric Oxide Concentration
5. Conclusions
- (1)
- Coal particles injected by primary air follow spiral trajectories along an imaginary tangent circle, with residence times on the order of tens of seconds. This circulation ensures sufficient burnout before the particles escape from the furnace outlet. The tangentially arranged air inlets establish a circular airflow, which gradually weakens with increasing height.
- (2)
- Combustion is concentrated below the nose arch, producing a maximum temperature of 1856 K with the designed coal. Above the arch, flue gas mixing leads to more uniform temperature fields, while a ring-shaped high-temperature zone typical of tangential firing gradually disappears with height. Coal blending increases the peak furnace temperature to above 1900 K and shifts the maximum temperature location toward the wall, suggesting a stronger tendency for wall-adjacent combustion.
- (3)
- CO is generally low under over-oxygen conditions, but blending promotes localized CO enrichment near the wall due to deteriorated flame diffusion and intensified oxygen consumption adjacent to the wall. In contrast, CO2 and H2O concentrations decrease with blending compared to unblended coal firing. O2 consumption is most intense near the primary air inlets, resulting in a distinct low-O2 zone, and its concentration falls below 4% at the outlet.
- (4)
- Nitric oxide is dominated by thermal and fuel mechanisms, generated mainly in wall-adjacent high-temperature regions. The outlet NO concentration increases progressively with blending ratio, from 357 mg/m3 in the unblended case to 457 mg/m3 at a blending ratio of 0.8.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, X.; Chen, Z.; Qi, R.; Li, L. Green supply chain for coal: Models and optimization. Clean. Logist. Supply Chain 2025, 14, 100206. [Google Scholar] [CrossRef]
- Dechamps, P. The IEA World Energy Outlook 2022—A brief analysis and implications. Eur. Energy Clim. J. 2023, 11, 100–103. [Google Scholar] [CrossRef]
- Tong, C.; Yang, X.; Chen, G.; Zhang, Y.; Chen, L.; Zhou, Y.; He, T.; Jin, B. Experimental investigation for the combustion characteristics of blends of three kinds of coal. Fuel 2021, 300, 120937. [Google Scholar] [CrossRef]
- Wang, C.; Fan, G.; Sun, R.; Wang, C.; Chen, W.; Che, D. Effects of coal blending on transformation of alkali and alkaline-earth metals and iron during oxy-fuel co-combustion of Zhundong coal and high-Si/Al coal. J. Energy Inst. 2021, 94, 96–106. [Google Scholar] [CrossRef]
- Amini, S.H.; Vass, C.; Shahabi, M.; Noble, A. Optimization of coal blending operations under uncertainty–robust optimization approach. Int. J. Coal Prep. Util. 2022, 42, 30–50. [Google Scholar] [CrossRef]
- Hariana, P.A.; Ahmadi, G.A.; Darmawan, A. Ash Evaluation of Indonesian Coal Blending for Pulverized Coal-Fired Boilers. J. Combust. 2021, 2021, 15. [Google Scholar] [CrossRef]
- Wang, C.a.; Liu, Y.; Zhang, X.; Che, D. A study on coal properties and combustion characteristics of blended coals in northwestern China. Energy Fuels 2011, 25, 3634–3645. [Google Scholar] [CrossRef]
- Zaid, M.Z.S.M.; Wahid, M.A.; Mailah, M.; Mazlan, M.A.; Saat, A. Coal fired power plant: A review on coal blending and emission issues. In AIP Conference Proceedings; American Institute of Physics: College Park, MD, USA, 2019. [Google Scholar]
- Haas, J.; Tamura, M.; Weber, R. Characterisation of coal blends for pulverised fuel combustion. Fuel 2001, 80, 1317–1323. [Google Scholar] [CrossRef]
- Szufa, S.; Piersa, P.; Junga, R.; Błaszczuk, A.; Modliński, N.; Sobek, S.; Marczak-Grzesik, M.; Dzikuć, M. Numerical modeling of the co-firing process of an in situ steam-torrefied biomass with coal in a 230 MW industrial-scale boiler. Energy 2023, 263, 125918. [Google Scholar] [CrossRef]
- Qiao, Y.; Fan, S.; Wu, X.; Chen, Z.; Guan, S.; Li, J.; Yuan, Z.; Hou, J.; Li, Z. Numerical analysis on effect of blend ratio on co-combustion characteristics of semi-coke and bituminous coal in swirl burner. Combust. Sci. Technol. 2024, 196, 504–523. [Google Scholar] [CrossRef]
- Bian, L.; Li, Z.; Lu, Y.; Chen, Z.; Li, Z.; Sun, D. Numerical Simulation on Combustion and Slagging Characteristics of a Down-Fired Boiler Under Different Blending Ratios of Anthracite/Bituminous Coal. Combust. Sci. Technol. 2025, 1–29. [Google Scholar] [CrossRef]
- Fakourian, S.; Roberts, M.; Dai, J. Numerical prediction of ash deposit growth burning pure coal and its blends with woody biomass in a 1.5 MWTH combustor. Appl. Therm. Eng. 2023, 224, 120110. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, E.; Chionoso, O.P. Numerical simulation of the synergistic effect of combustion for the hydrochar/coal blends in a blast furnace. Energy 2022, 238, 121722. [Google Scholar] [CrossRef]
- Glarborg, P.; Jensen, A.; Johnsson, J.E. Fuel nitrogen conversion in solid fuel fired systems. Prog. Energy Combust. Sci. 2003, 29, 89–113. [Google Scholar] [CrossRef]
- Fenimore, C. Formation of NO in premixed hydrocarbon flames. In Proceedings of the 13th Symposium (International) on Combustion, Salt Lake City, UT, USA, 23–29 August 1970; pp. 373–382. [Google Scholar]
- Miller, J.A.; Bowman, C.T. Mechanism and modeling of nitrogen chemistry in combustion. Prog. Energy Combust. Sci. 1989, 15, 287–338. [Google Scholar] [CrossRef]
- Maroa, S.; Inambao, F. The NOx formation routes. In Biodiesel, Combustion, Performance and Emissions Characteristics; Springer: Berlin/Heidelberg, Germany, 2020; pp. 7–15. [Google Scholar]
- Wang, C.a.; Chen, M.; Zhao, P.; Zhou, L.; Hou, Y.; Zhang, J.; Lyu, Q.; Che, D. Investigation on Co-combustion characteristics and NOx emissions of coal and municipal sludge in a tangentially fired boiler. Fuel 2023, 340, 127608. [Google Scholar] [CrossRef]
- Wang, C.a.; Wang, C.; Jia, X.; Zhao, L.; Wang, P.; Che, D. NO formation characteristics and fuel-nitrogen transformation mechanism during co-firing of low-volatile carbon-based solid fuels with bituminous coal. Fuel 2021, 291, 120134. [Google Scholar] [CrossRef]
- Lin, J. Experimental Study on the Oxy-Fuel Combustion Characteristics of Lignite. Master’s Thesis, Inner Mongolia University of Technology, Hohhot, China, 2010. [Google Scholar]
- Ashgriz, N.; Mostaghimi, J. An introduction to computational fluid dynamics. Fluid Flow Handb. 2002, 1, 1–49. [Google Scholar]
- Patankar, S. Numerical Heat Transfer and Fluid Flow; CRC press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Markatos, N. The mathematical modelling of turbulent flows. Appl. Math. Model. 1986, 10, 190–220. [Google Scholar] [CrossRef]
- Launder, B.E.; Spalding, D.B. The numerical computation of turbulent flows. In Numerical Prediction of Flow, Heat Transfer, Turbulence and Combustion; Elsevier: Amsterdam, The Netherlands, 1983; pp. 96–116. [Google Scholar]
- Magnussen, B. On the structure of turbulence and a generalized eddy dissipation concept for chemical reaction in turbulent flow. In Proceedings of the 19th Aerospace Sciences Meeting, St. Louis, MO, USA, 12–15 January 1981. [Google Scholar]
- Gran, I.R.; Magnussen, B.F. A numerical study of a bluff-body stabilized diffusion flame. Part 2. Influence of combustion modeling and finite-rate chemistry. Combust. Sci. Technol. 1996, 119, 191–217. [Google Scholar] [CrossRef]
- Chandrasekhar, S. Radiative Transfer; Courier Corporation: Chelmsford, MA, USA, 2013. [Google Scholar]
- Howell, J.R.; Mengüç, M.P.; Daun, K.; Siegel, R. Thermal Radiation Heat Transfer; CRC press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Blauvens, J.; Smets, B.; Peters, J. The 16th Symposium (International) on Combustion; The Combustion Institute: Cambridge, MA, USA, 1977; Volume 1055. [Google Scholar]
- Baulch, D.L. Evaluated Kinetic Data for High Temperature Reactions; Butterworths: London, UK, 1972. [Google Scholar]

| Component | Unit | Original Coal (Lignite) | Blended Coal (Bituminous Coal) |
|---|---|---|---|
| C_ar | % | 49.3 | 52.4 |
| H_ar | % | 2.0 | 3.7 |
| O_ar | % | 12.7 | 16.5 |
| N_ar | % | 0.5 | 0.7 |
| S_ar | % | 0.2 | 0.4 |
| W_ar | % | 18.2 | 9.7 |
| A_ar | % | 17.0 | 16.0 |
| V_daf | % | 33.7 | 39.4 |
| Q_net,v,ar | kJ/kg | 10,460.0 | 13,397 |
| Forward Constants | Values | Reverse Constants | Values |
|---|---|---|---|
| Number of Mesh Cells | Monitoring Location (Mon-A) | Monitoring Location (Mon-B) | ||||
|---|---|---|---|---|---|---|
| Temperature (K) | Volume Fraction of O2 | Volume Fraction of NO (ppm) | Temperature (K) | Volume Fraction of O2 | Volume Fraction of NO (ppm) | |
| 872,292 | 1792 | 0.00310 | 162 | 1771 | 0.0418 | 205 |
| 1,210,086 | 1849 | 0.00328 | 169 | 1753 | 0.0435 | 219 |
| 1,520,502 | 1856 | 0.00324 | 173 | 1761 | 0.0434 | 223 |
| Monitoring Locations | Measured Temperature (K) | Simulated Temperature (K) | Measured Nitric Oxide Concentration (mg/Nm3) | Simulated Nitric Oxide Concentration (mg/Nm3) |
|---|---|---|---|---|
| Mon-1 | 1392 | 1446 | 340 | 357 |
| Mon-2 | 1298 | 1332 | ||
| Mon-3 | 1288 | 1300 | ||
| Mon-4 | 1349 | 1332 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, C.; Zhang, W.; Wu, B.; Liu, Z. Numerical Investigation of Combustion and Nitric Oxide Formation in a 130 t/h Pulverized-Coal Boiler Under Lignite–Bituminous Coal Blending. Processes 2025, 13, 3187. https://doi.org/10.3390/pr13103187
Zhou C, Zhang W, Wu B, Liu Z. Numerical Investigation of Combustion and Nitric Oxide Formation in a 130 t/h Pulverized-Coal Boiler Under Lignite–Bituminous Coal Blending. Processes. 2025; 13(10):3187. https://doi.org/10.3390/pr13103187
Chicago/Turabian StyleZhou, Chuan, Wei Zhang, Binqian Wu, and Zihan Liu. 2025. "Numerical Investigation of Combustion and Nitric Oxide Formation in a 130 t/h Pulverized-Coal Boiler Under Lignite–Bituminous Coal Blending" Processes 13, no. 10: 3187. https://doi.org/10.3390/pr13103187
APA StyleZhou, C., Zhang, W., Wu, B., & Liu, Z. (2025). Numerical Investigation of Combustion and Nitric Oxide Formation in a 130 t/h Pulverized-Coal Boiler Under Lignite–Bituminous Coal Blending. Processes, 13(10), 3187. https://doi.org/10.3390/pr13103187






