Abstract
The effects of the La2O3/Al2O3 mass ratio (L/A-ratio) in electroslag on La yield and the cleanliness of La-containing H13 steel, as well as slag properties were investigated. The slag–steel interaction experiments between slags bearing varying L/A-ratios based on 50wt%CaF2-20wt%CaO-Al2O3-La2O3 slag and La-containing H13 steel were conducted. The molecular dynamics (MD) simulation was used to estimate the viscosity, electrical conductivity, and structure of the slag. The results show that La yield increases from 5.3% to 63.2% with L/A-ratio increasing from 0.2 to 5.0 due to the increasing La2O3 activity and decreasing Al2O3 activity of slag. Thermodynamic analysis indicates that La2O3 content should be higher than 23.9 wt% to ensure desirable La treatment effect. The desulfurization and deoxidation ability of slag are enhanced with increasing L/A-ratio. In addition, the phosphorus and arsenic content in steel decrease when the L/A-ratio reaches 5.0, and the removal rates are 20.0% and 33.3%, respectively. This is attributed to the formation of La-O-As-S-P inclusions. The MD simulation results indicate that the increasing L/A-ratio leads to a decrease in viscosity and increase in electrical conductivity. The mechanism is that the increasing concentration of free oxygen simplifies the aluminate network and increases the mobility of ions in slag.
1. Introduction
Industrial production relies on tools like die-casting dies, forging dies, stamping dies, and injection molds [1,2,3]. These tools normally work under extreme conditions, which place high quality demands on their raw materials—the die steels. H13 steel, a type of hot-working die steel, is widely used in a variety of industrial applications for its good strength and thermal stability [4,5,6]. The repeated thermal loading makes hot-working die steel sensitive to the thermal fatigue [4,7]. The primary carbide, inhomogeneous chemical composition, and anisotropic microstructure (all caused by segregation during solidification), as well as the inclusions, are the main factors leading to thermal fatigue [8,9,10,11,12,13]. Electroslag remelting (ESR), a secondary refining process, has strong capabilities in the desulfurization, inclusion removal, and improvement of solidification structures [14,15,16,17]. However, the suppression effect on segregation is limited as the scale of remelted ingots becomes larger.
The application of rare earth (RE) treatment to steel can purify the liquid steel and provide a method to further suppress segregation [18,19,20,21]. Although RE treatment significantly enhances the properties of steel and attracts extensive research focus, its industrial application is limited due to severe RE oxidation at elevated temperatures during the ESR process. Studies indicate that the yields of RE are only about 10% under air atmosphere during the ESR process [22,23]. Under argon gas protection, the RE yield only rises to approximately 25% [24]. Al2O3 in typical CaF2-CaO-Al2O3 electroslag can react with RE in liquid steel leading to the oxidation of RE in steel [24]. Thermodynamically, decreasing Al2O3 activity while increasing RE oxide activity by adjusting the slag composition will suppress the driving force of this oxidation reaction [25,26]. However, the composition of electroslag not only affects the RE yield and cleanliness of the steel, but also determines its physical properties, such as viscosity and electrical conductivity related to the metallurgy performance.
Rare earth element Lanthanum is commonly used to improve solidification structures and modify inclusions in steel. The influence of La2O3 in the refining slag on the steel and the properties of slag attracted attention in previous studies. Luo et al. found that La2O3 in the CaO-Al2O3-SiO2-La2O3 could react with Al in steel, leading to the transfer of minor La from slag into the steel and the modification of inclusions [27]. They also found that the increase in La2O3 content promoted the reaction due to the increasing La2O3 activity and decreasing Al2O3 activity. Upolovnikova et al. found that an increase in slag basicity in CaO–SiO2-La2O3-Al2O3-MgO slag also increased the equilibrium La content in the inclusions [28]. Li et al. found that the increasing La2O3 content in CaF2-CaO-Al2O3-(La2O3) slag decreased Al2O3 and CaF2 activity, which contributed to the suppression of the fluoride vaporization reaction [29]. In addition, they found that the viscosity of the molten slag decreased with the increasing La2O3 content. However, the influence of La2O3 in CaF2-CaO-Al2O3-based electroslag remelting slag on La-bearing steel has received less attention. Thus, the present work aims to clarify the effects and mechanism of Al2O3 and La2O3 content in electroslag on La yield and the cleanliness of La-containing H13 steel and figure out its influences on the properties of slag. For this purpose, the interaction between La-containing H13 steel and CaF2-CaO-Al2O3-La2O3 slag with varying La2O3/Al2O3 mass ratios (L/A-ratio) were investigated. La yields and the cleanliness of the H13 steel were analyzed. Molecular dynamics (MD) simulation, widely used to estimate the transport properties of molten slag [30,31,32,33], was employed to clarify effects of L/A-ratio on structures, viscosity, and electrical conductivity of slag.
2. Materials and Methods
2.1. Materials Preparation
Three types of slags with L/A-ratios of 0.2, 1.0, and 5.0 based on 50wt%CaF2-20wt%CaO-Al2O3-La2O3 slag were prepared. The chemical compositions of designed slag1–3 are shown in Table 1. The raw materials of the slag were reagent-grade powder of CaF2 (≥98.5%), Al2O3(≥99.9%), CaO (≥98.0%), and La2O3(≥98.0%), purchased from Sinopharm Chemical Reagent Co., Ltd. To remove the moisture in the powders, CaF2 was calcined at 300 °C for 6 h. Al2O3 and CaO were calcined at 800 °C for 6 h. After mixing in the proportion, the slags were pre-melted using a high-frequency induction furnace and graphite crucibles as the heat source and containers. The pre-melted slags were heated at 800 °C for 6 h to eliminate the carbon introduced by the graphite crucibles. The chemical composition of La-containing H13 steel is listed in Table 2. The steel samples were polished and cleaned to remove the oxidation layer before subsequent experiments.

Table 1.
Chemical composition of designed slag (wt%).

Table 2.
Chemical composition of raw La-containing H13 steel (wt%).
2.2. Slag–Steel Interaction and Examinations
Approximately 100 g of La-containing H13 steel and 25 g of pre-melted slag were charged into the magnesia crucible (inner diameter of 30 mm, height of 90 mm), followed by the insertion of the magnesia crucible into the graphite crucibles. The graphite crucibles were then placed into the chamber of the high-frequency induction furnace. Before the heating process, Ar gas (99.999 pct purity) was blown to eliminate the residual air in the chamber. Subsequently, the furnace was heated to 1873 K. The heating process was observed via the observation window at the top of the furnace. After both steel and slag were melted, the furnace was maintained at 1873 K for 5 min and then cooled. Then, samples were cut from the remelted steel. The samples with a size of 4 mm × 4 mm × 4 mm were cleaned for determining the chemical composition, and those with a size of 4 mm × 4 mm × 10 mm were polished and ground for inclusion observation. The chemical compositions of the remelted H13 steel were determined by inductively coupled plasma-atomic emission spectroscopy (ICP-OES, Agilent-5800, Agilent Technologies Inc., Santa Clara, CA, USA), a C-S analyzer (CS600, LECO Corporation, St. Joseph, MO, USA), and an O-N-H analyzer (ONH836, LECO Corporation, St. Joseph, USA). The inclusions were analyzed by scanning electron microscopy (SEM, Nova 400 Nano, FEI Company, Hillsboro, OR, USA) equipped with energy dispersive spectroscopy (EDS, Le350 PentaFETx-3, Oxford Instruments, Abingdon, UK).
2.3. Molecular Dynamics Simulation
The MD simulation is used to estimate the structure, viscosity, and electrical conductivity of the slag with L/A-ratios of 0.1, 0.2, 0.5, 1.0, 2.0, and 5.0. The Large-scale Atomic/Molecular Massively Parallel Simulator (LAMMPS) package was employed for MD simulation. Buckingham potential was applied for the description of atomic interactions as shown in Equation (1) [30,31,32,33,34,35,36]:
where U(rij) is the interatomic pair potential, qi and qj are the electric charges, rij is the interatomic distance between ion i and j, Aij and ρij are repulsive potential parameters, and Cij is the Van der Waals force parameter. The parameters used in the current work are shown in Table 3 [34,35,36].

Table 3.
Buckingham potential parameters used in the current work.
The electric charges of Ca, Al, La, O, and F were set as +2, +3, +3, −2, and −1, respectively. The liquid slag has an amorphous structure. Thus, the heating–quenching method is used to build the model, namely, putting about 8000 atoms (the number of each kind of atoms decided by the chemical composition of the slag) randomly in a cubic box with a periodic boundary condition. The size of the box is decided by the total mass of the simulated atoms and the density of the slags. The initial density was set as 2.7 g/cm3 due to insufficient data of the La2O3-bearing slag. The timestep was set as 0.001 ps. The Long-range Columb interactions were calculated by the Ewald method. Firstly, under the NPT ensemble, the box was equilibrated at 5000 K for 50 ps, then cooled to 1873 K within 50 ps, and followed by equilibration at 1873 K for 50 ps. Before collecting the data, the system was equilibrated at 1873 K for 50 ps under the NVT ensemble. The atomic trajectories data during modeling time of 1000 ps under the NVT ensemble were collected and used to analyze the transport properties and structures of slag.
3. Results and Discussion
3.1. Effects of La2O3/Al2O3 Ratio on the La Yield
The mass concentrations of La and Al in the steel are shown in Table 4. The La content in the remelted samples decreases compared with the raw materials, whereas the Al content increases after remelting. It implies that La in the steel might react with Al2O3 in the slag, resulting in the Al pickup and La loss. The La content increases with the increase in the L/A-ratio in slag, and the Al content shows an opposite trend. The La yield of T1 to T3 are 5.3%, 10.5%, and 63.2%, respectively. It can be guessed that a higher L/A-ratio in slag contributes to the higher La yield.

Table 4.
Concentration of studied elements in steel after remelting (wt%).
Thermodynamic analysis was carried out to figure out the mechanism of L/A-ratio effects on La yield. The reaction between La in liquid steel and Al2O3 in molten slag can be expressed as Equation (2) [37]. The relationship between the equilibrious La and Al content can be driven as Equation (3). [i]% stands for the weight percent of element i in steel. f[i] represents the activity coefficient of element i. The activity coefficient f[i] can be decided by Equation (4). is interaction coefficient between dissolved elements i and j and that at 1873 K shown in Table 5 [38,39]. As Equation (3) shows, the equilibrious ratio of Al to La in liquid steel is dominated by the activity of Al2O3 and La2O3 in molten slag.

Table 5.
Interaction coefficients of dissolved elements in liquid iron at 1873 K.
In the current work, the activities of Al2O3 and La2O3 are estimated by a mass action concentration model based on the ion and molecule coexistence theory (IMCT). This theory assumes that the thermodynamic equilibrium between the structural units of ion, simple molecules, and complex molecules exists in the molten slag [40]. The possibly formed units in the molten slag are shown in Table 6. The total equilibrium mole number nT can be expressed as follows:
where ni stands for the mole number of each possible formed unit. According to the mass action concentration model, the mass action concentration (MAC) of units i can be described as Ni = ni/nT. The complex molecules keep equilibrium with ions and simple molecules, and their MACs are decided by the equilibrium constant Ki and MASs of the ions and simple molecules. The formation reactions of each possible formed complex molecule and corresponding standard Gibbs free energy change are shown in Table 7 [40,41,42]. In the calculation model, the initial mole numbers of CaF2, CaO, La2O3, and Al2O3 are expressed as m1, m2, m3, and m4, respectively. Based on mass conservation, Equations (6)–(10) can be obtained as follows:

Table 6.
Possible formed units in investigated slag, corresponding mole number, and expression of mass action concentration.

Table 7.
Reactions of possible formed complex molecules in molten slag and corresponding standard Gibbs free energy change.
By solving Equations (6)–(10), the MACs of the slag components will be obtained, which are treated as the activities of slag components in this model. Figure 1a shows the variation in calculated activities of Al2O3 and La2O3 with L/A-ratio. The activities of Al2O3 in slag decrease with increasing L/A-ratio. On the contrary, the activities of La2O3 increase with increasing L/A-ratio. By substituting the activities of La2O3 and Al2O3 into Equation (3), La-Al equilibrium curves can be depicted as shown in Figure 1b. It indicates that the equilibrious La at a given Al content also increases with increasing L/A-ratio. This means that less dissolved La in liquid steel would be oxidized by Al2O3 in molten slag. At the same time, the Al and La contents in samples T1 to T3 are also plotted in Figure 1b. The experimental results show the same tendency that a higher L/A-ratio is beneficial for preventing La loss and the picking up of Al caused by slag–steel interaction.
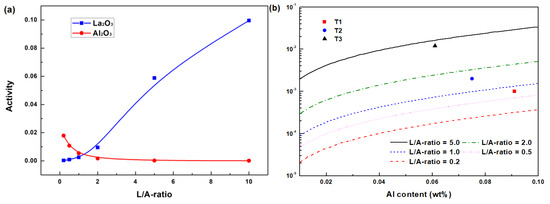
Figure 1.
Thermodynamic calculation of the slag–steel interaction: (a) variation in slag component activity with L/A-ratio and (b) Al-La equilibrium curves varying with L/A-ratio.
In addition, our previous work shows that the primary carbides are refined and the homogeneity of structures as well as precipitates is improved as the RE content becomes larger than 0.013 wt% [19]. As Equation (3) shows, the equilibrious relationship between Al content and activity ratio of Al2O3 to La2O3 (decided by the L/A-ratio) is certain at a given La content, namely, when the initial Al content is known and the target La content is given, the composition of slag can be clarified. Figure 2 shows the relationship between the L/A-ratio in slag and Al content in steel at the target La content of 0.013 wt%. It can be seen from Figure 2 that a high L/A-ratio is required when the Al content in steel is low. According to the thermodynamic analysis, the L/A-ratio should be higher than 3.89. It means that the La2O3 content in the 50wt%CaF2-20wt%CaO-Al2O3-La2O3 slag should be about 23.9 wt% to ensure the La treatment effect on H13 steel.
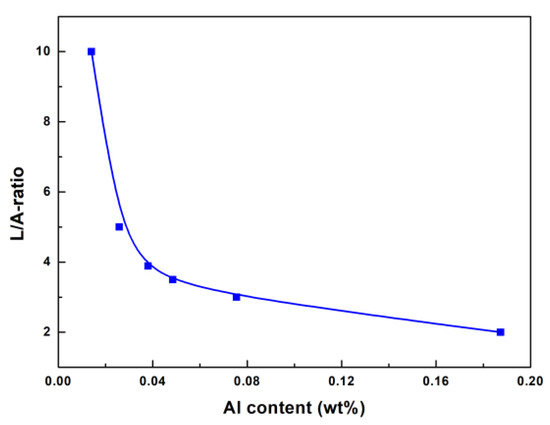
Figure 2.
Relationship between L/A-ratio and Al content in steel at the target La content of 0.013 wt%.
3.2. Effects of La2O3/Al2O3 Ratio on the Cleanliness of the H13 Steel
A critical role of electroslag is to remove impurities (mainly oxygen and sulfur) and control inclusions. The sulfur and oxygen content in remelted steel are shown in Table 4. In comparison with the raw steel, the desulfurization rate of T1 to T3 are 40%, 63%, and 66%, respectively. It means that a rare earth oxide could promote the desulfurization effect of slag, which is also found in previous research [43]. Rare earth oxides are widely reported to reduce viscosity of slag, which may provide better kinetic conditions for desulfurization. In addition, higher optical basicity enhances the sulfur capacity of slag. The optical basicity of multicomponent slag is expressed as Equation (11) [44,45]:
where xi is the mole fraction of components in slag, Oi is oxygen atom number of oxide (two fluorine atoms are regarded as one oxygen atom), and Λi is optical basicity of a slag component. The optical basicity of CaF2, CaO, Al2O3, and La2O3 are 1.20 [45], 1.00 [45], 0.600 [45], and 1.07 [46], respectively. The optical basicity of slag1–3 is calculated as 0.923, 0.979, and 1.011, respectively. It implies that replacement of Al2O3 by La2O3 also provides better thermodynamic conditions for desulfurization. Additionally, the oxygen content also decreases with increasing L/A-ratio as Table 4 shows. The decrease in oxygen content is mainly contributed by the removal of inclusions during slag–steel interaction. Related studies show that rare earth oxides would enhance the ability in inclusion removal of CaO-Al2O3-SiO2-based slag [43], and the reason is believed to be the decreasing viscosity induced by the addition rare earth oxides.
Residual elements phosphorus and arsenic are easy to segregate at phase- and grain-boundaries of steel, and detrimental to mechanical properties [47,48,49]. Removal of phosphorus requires low refining temperature, high oxygen potential, and high slag basicity [47]. The refining temperature of the ESR is relatively high, and the oxygen potential of slag needs to be low to achieve low-oxygen ingot. Arsenic in steel has weak reactivity with oxygen, which means that it cannot be removed by slag through forming an oxide [48]. Thus, phosphorus and arsenic are hardly removed during the ESR process. As shown in Table 4, the phosphorus and arsenic content in T1 and T2 stay unchanged, which means the slag–steel interaction cannot remove phosphorus and arsenic in these conditions. What interest is that the phosphorus and arsenic content in T3 decrease about 20.0% and 33.3%, respectively. A previous study found that a La-S-As or La-O-As inclusion could be generated in deep-deoxidation and deep-desulfurization steel. Based on that, it is proposed that arsenic could be fixed in the inclusions and removed, which has proved to be feasible but the removal effect significantly relies on the lanthanum content in steel [48]. Similarly, phosphorus in steel is also reported to be fixed by La [50].
To further explain the mechanism of phosphorus and arsenic removal in T3, the evolution of inclusions with L/A-ratio in slag is studied. Figure 3a,b shows the SEM image and corresponding EDS point scanning results of a typical inclusion in the raw La-containing H13 steel. The typical inclusion is recognized as La2O2S as shown in Figure 3b, which normally exists in La-bearing steel. After being remelted with slags, inclusions in T1 and T2 become a complex La-Mg-Al-O-S inclusion, as shown in Figure 4. These complex inclusions consist of bright and dark phases under SEM observation with BSE mode, where the bright phases contain higher La. Compared with the complex inclusion in T1 (Figure 4a,b), that in T2 (Figure 4d,e) contains more La-rich phases, which is resulted from the higher La content in T2. In addition, the Mg content in both phases decreases, whereas the La and Al contents increase. It needs to be noticed that the dark phases bear high Mg contents, which are generated from the corrosion effect of high CaF2-containing electroslag on a MgO crucible. MgO dissolves in the molten slag and may react with liquid steel to form MgO phases in complex inclusions. The dissolution rate of MgO increases with increasing Al2O3 content in slag [51]. With the L/A-ratio in slag1 of 0.2 increasing to that in slag2 of 1.0, the dissolution of MgO is suppressed. It further limits the formation of Mg-rich phases. In addition, it should be noted that the MgO dissolution will change the activity of components in slag, which could also account for the deviation between the experimental results and the theoretical calculations of T1 and T2 as shown in Figure 1b.
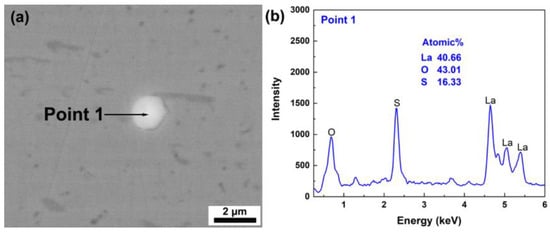
Figure 3.
Typical inclusion in raw La-containing H13 steel: (a) SEM image and (b) point-scanning results of EDS.
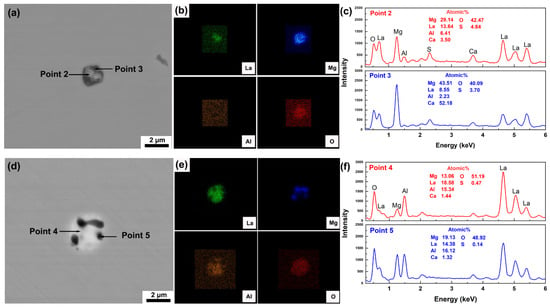
Figure 4.
La-Mg-Al-O-S inclusions in steel after slag–steel interaction: (a–c) SEM image and EDS scanning of inclusion in T1; (d–f) SEM image and EDS scanning of inclusion in T2.
As shown in Figure 5, the inclusions in T3 are La-O-As-S-P inclusions. There is no appearance of Mg-rich phases, which might be because of the low Al2O3 content in slag3 causing less MgO dissolution. The possible formed phase in La-O-As-S-P inclusions includes La2O3, La2O2S, LaS, LaP, LaS‧LaAs, and LaAsO4 [48,49,50,51,52,53]. The thermodynamic data are shown in Table 8 [39,52,53,54]. The Gibbs free energy changes of La2O3, La2O2S, and LaS are negative, and that of La2O3 and La2O2S are less than LaS. It indicates that the oxide and oxysulfide of lanthanum would be formed first, and then the sulfide. The Gibbs free energy changes in LaAs, LaP, LaS‧LaAs, and LaAsO4 are larger than 0, which means the thermodynamic conditions cannot satisfy their formation. However, phosphorus and arsenic are easy to segregate in the liquid phase during solidification, which provides an opportunity for generating the phosphorus- and arsenic-containing inclusions. The concentration of elements in the liquid phase of the solidification front (CL) can be described as Equation (12) [55]:
where C0 is the initial concentration, fs is the solid fraction, and k is segregation coefficient. The segregation coefficient of sulfur, phosphorus, and arsenic between γ-Fe and the liquid phase are 0.05, 0.06, and 0.33, respectively [49]. The segregation of lanthanum is not considered due to the lack of relevant data. The temperature in the solidification front (Tf) can be estimated by Equation (13):
where T0 is melting point of pure iron (1809 K), TL is liquidus temperature, and TS is solidus temperature. The liquidus and solidus temperature of H13 steel are estimated at 1749 K and 1613 K [56], respectively. Taking the pure solid of LaAs, LaP, LaS‧LaAs, and LaAsO4 as the standard state, their activities are 1. The equilibrium activity products can be described as Equation (14). The phosphorus and arsenic containing inclusions will be formed when the actual activity products (considering the segregation) are larger than the equilibrium activity products.
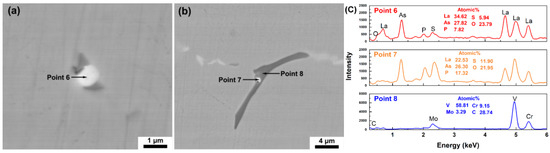
Figure 5.
La-O-As-S-P inclusions in T3: (a) SEM image of a single La-O-As-S-P inclusion; (b) the La-O-As-S-P inclusion located in the V-rich primary carbide; and (c) EDS scanning results.

Table 8.
Standard Gibbs energy of possible formed phases in the La-O-As-S-P inclusion and formation Gibbs free energy of T3 at 1873 K.
Figure 6a–d shows the relationship between the equilibrium activity products and actual activity products of LaAs, LaP, LaS‧LaAs, and LaAsO4, respectively. The calculation results indicate that LaAs, LaP, LaS‧LaAs, and LaAsO4 will be formed when the solid fraction becomes 0.994, 0.773, 0.770, and 0.993, respectively. LaAs and LaAsO4 only appear in the end-stage of the solidification process, which means that they are hardly removed in the form of inclusions. LaP and LaS‧LaAs might be formed in the early stage of solidification, making them possibly removed. It is also found that the La-O-As-S-P inclusions could exist in the core of the V-rich primary carbide (Figure 4b) that would precipitate in the solid fraction around 0.955 [56]. It indicates that the phosphorus and arsenic containing phases appear before the V-rich primary carbide. It also implies that the La-O-As-S-P inclusions may act as heterogeneous nucleation sites and help to refine the primary carbides.
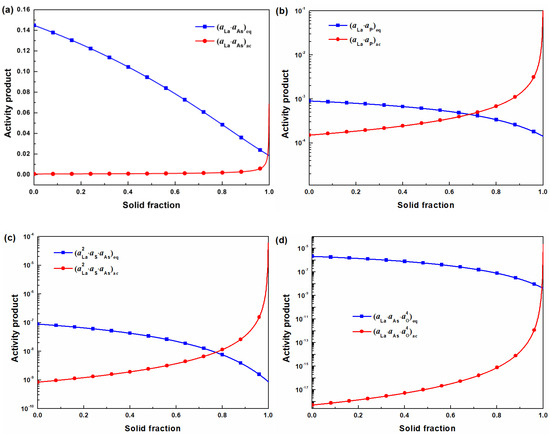
Figure 6.
Equilibrium activity products and actual activity products of (a) LaAs, (b) LaP, (c) LaS‧LaAs, and (d) LaAsO4.
Therefore, LaAs and LaAsO4 might not be phosphorus- and arsenic-containing phases in the present study. On the contrary, LaP and LaS‧LaAs may be components in the La-O-As-S-P inclusions. From the formation mechanism of La-O-As-S-P inclusions, it can be guessed that La2O3 and La2O2S will be formed first before solidification starts, and then LaS, after the enriched arsenic in residual liquid steel replaces sulfur in the La-O-S inclusion to form LaS‧LaAs. Finally, enriched phosphorus combines with lanthanum to form LaP, which is believed to have the tendency to precipitate with heterogeneous nucleation sites of oxide and oxysulfide of lanthanum [51]. La-O-As-S-P inclusions are formed in the early stage of the solidification process, which creates opportunities for them to be removed. This might contribute to the decreasing phosphorus and arsenic content in T3.
3.3. Effects of La2O3/Al2O3 Ratio on the Viscosity, Electrical Conductivity, and Structure of the Slag
Mean square displacement (MSD) indicates the average displacement of a particle during a fixed time, which reflects the transport properties of the particles and can be expressed as follows [31,57]:
where ri(t) is the position of ion i at time t, N is the number of ions, and the angular brackets indicate an ensemble average. Figure 7a shows the MSD curves of the ions in slag with L/A-ratios of 0 and 5.0 at 1873 K, calculated based on MD simulation. For a given ion species, its self-diffusion coefficient (Ds) is calculated via Equation (16) based on the obtained MSD as follows [31,57]:
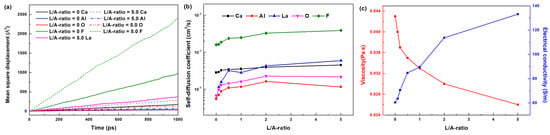
Figure 7.
Transport properties of slags: (a) mean square displacement of ions in slag with L/A-ratios of 0 and 5.0 at 1873 K, (b) the self-diffusion coefficient of ions at 1873 K with varying L/A-ratios, and (c) the estimated viscosity and electrical conductivity of slag.
Figure 7b shows the variations in Ds for each atom species with varying L/A-ratio at 1873 K. It indicates that self-diffusion coefficients increase with the increasing L/A-ratio of slags. The viscosity and electrical conductivity of slag are important properties determining the metallurgy performance of ESR. The viscosity can be estimated by the Stokes–Einstein equation given in Equation (17) [31]:
where kB is Boltzmann’s constant, and λ is jumping distance during diffusion of particles. Diffusion of Ca2+ and O2− are often used to estimate the viscosity of refining slag. In the present work, the jumping distance is taken as the diameter of Ca2+(2.0 Å). The estimated results, as shown in Figure 7c, indicate that the viscosity of slag decreases with increasing L/A-ratio. Lower viscosity contributes to better kinetic conditions for desulfurization and inclusions removal. Thus, higher L/A-ratios lead to better cleanliness improvement effects on the remelted steel. This might be one of the factors that slag3 have better performance on cleanliness improvement during slag–steel interaction than slag1 and slag2. In addition, the electrical conductivity can be estimated based on Nernst-Einstein equations as follows [57]:
where e is the elementary charge, V is the simulation box volume, and zi is the charge of ion i. As depicted in Figure 7c, electrical conductivity increases with increasing L/A-ratio. The reason for the increasing electrical conductivity is that the increase in self-diffusion promotes ion mobility. The increase in electrical conductivity would reduce the Joule heat and lowering the remleting rate of ESR process. It is beneficial for the removal inclusions and suppressing macrosegregation [58]. However, it also increases energy consumption. This phenomenon was observed in our previous industry ESR experiments using rare earth oxide containing slag [59].
The macroscopic transport properties, viscosity, and electrical conductivity of slag are governed by the structure, namely the melt framework. In the electroslag, the dominant network former is aluminum. In the molten slag, the oxygen connecting two network formers is called a bridge oxygen (Ob), oxygen connecting a network former and network modifier is a non-bridge oxygen (Onb), and that connecting no network formers is a free oxygen (Of), while oxygen tri-clusters (OT) appear to balance the charge [33]. Ob in the CaF2-CaO-Al2O3-based slag connects aluminum to generate a [AlO4]−5 tetrahedron complex network structure. According to the number of Ob in [AlO4]−5, the structural units are divided into Q0, Q1, Q2, Q3, and Q4, which demonstrate the degree of polymerization (DOP) of slag [33,57]. Q0, Q1, and Q2 are the structure units with low DOPs, whereas Q3 and Q4 are high-DOP structure units. Figure 8a shows the concentration of each oxygen type varying with the L/A-ratio. It indicates that the concentration of Ob decreases and that of Of increases with an increasing L/A-ratio. As shown in Figure 8b, the concentrations of the high-DOP structure units Q3 and Q4 decrease with increasing L/A-ratio, and almost disappear as the L/A-ratio reaches 5.0. The low-DOP structure unit Q0 increases sharply with increasing L/A-ratio due to a large number of free oxygen. Namely, the aluminate network is depolymerized due to the replacement of Al2O3 by La2O3, which enhances the mobility of ions in slag and results in the decreasing viscosity as well as the increasing ionic conductivity.
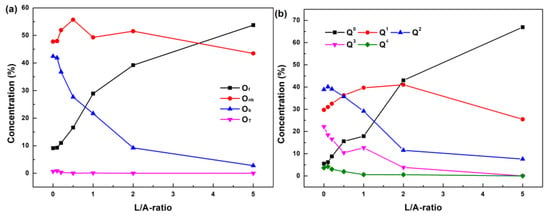
Figure 8.
Structure evolutions of slags with varying L/A-ratio: (a) distribution of oxygen type and (b) distributions of structure unit Qn.
4. Conclusions
- The increase in the L/A-ratio in slag suppresses La oxidation in steel during slag–steel interactions. When L/A-ratio increases from 0.2 to 5.0, La yield increases from 5.3% to 63.2%. Thermodynamic calculation indicates that the activities of La2O3 increases with increasing L/A-ratio, whereas that of Al2O3 decreases. This further increases the equilibrium La content at a given Al content in steel and finally contributes to the increase in La yield. The La2O3 content in the 50wt%CaF2-20wt%CaO-Al2O3-La2O3 slag should be higher than 23.9 wt% to realize a desirable La-treatment effect for the investigated H13 steel.
- The desulfurization and deoxidation effect of slag are enhanced with increasing L/A-ratio. In addition, phosphorus and arsenic decrease when the L/A-ratio reaches 5.0. The removal rates of phosphorus and arsenic are 20.0% and 33.3%, respectively. The inclusion of La2O2S in the original H13 steel transforms into La-Mg-Al-O-S inclusions at L/A-ratios of 0.2 and 1, and changes to La-O-As-S-P inclusions when the L/A-ratio increases to 5.0. Thermodynamic calculation shows that LaP and LaS‧LaAs could be formed in the early stage of solidification as the L/A-ratio is 5.0. It provides an opportunity for the removal of phosphorus and arsenic in steel.
- Molecular dynamics simulation indicates that increasing L/A-ratio enhances the mobility of ions in molten slag and further decreases the viscosity as well as increases the electrical conductivity of slag. The concentration of free oxygen in slag sharply increases with the increasing L/A-ratio. This simplifies the aluminate network structure. The increase in the L/A-ratio provides better kinetic conditions for cleanliness improvement but also brings the risk of higher energy consumption.
Author Contributions
Conceptualization, X.W.; methodology, X.W.; software, H.Y. and S.H.; validation, X.W. and S.H.; formal analysis, X.W., H.Y. and S.H.; investigation, H.Y. and S.H.; resources, X.W.; data curation, X.W.; writing—original draft preparation, X.W.; writing—review and editing, X.W.; visualization, X.W., H.Y. and S.H.; supervision, X.W.; project administration, X.W.; funding acquisition, X.W. All authors have read and agreed to the published version of the manuscript.
Funding
Funding: This research was funded by the project of the Hubei Provincial Department of Science and Technology (No. 2023AFB430), Open Foundation of Key Laboratory for Ferrous Metallurgy and Resources Utilization of Ministry of Education (FMRUlab-22-3) and Scientific Research Foundation of Wuhan Institute of Technology (No. K2023022).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed at the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Altan, T.; Lilly, B.; Yen, Y.C. Manufacturing of dies and molds. CiRP Ann. 2001, 50, 404–422. [Google Scholar] [CrossRef]
- Krugljakow, A.; Rogachev, S.; Lebedeva, N.; Sokolov, P.Y.; Arsenkin, A.; Khatkevich, V. On the nature of hot work hardening phenomenon in die steel with regulated austenitic transformation during exploitation. Mater. Sci. Eng. A 2022, 833, 142548. [Google Scholar] [CrossRef]
- Liu, T.; Pei, Z.; Barton, D.; Thompson, G.B.; Brewer, L.N. Characterization of nanostructures in a high pressure die cast Al-Si-Cu alloy. Acta. Mater. 2022, 224, 117500. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, Z.; Xie, J. Improving strength and ductility of H13 die steel by pre-tempering treatment and its mechanism. Mater. Sci. Eng. A 2019, 752, 101–114. [Google Scholar] [CrossRef]
- Chen, H.; Li, S.; Ren, Y.; Hou, X.; Yang, H.; Zhang, S. Thermo-mechanical fatigue behavior and microstructure evolution of 4Cr5Mo3V hot work die steel. Int. J. Fatigue 2024, 183, 108263. [Google Scholar] [CrossRef]
- Wen, T.; Yang, F.; Wang, J.; Yang, H.; Fu, J.; Ji, S. Ultrastrong and ductile synergy of additively manufactured H13 steel by tuning cellular structure and nano-carbides through tempering treatment. J. Mater. Res. Technol. 2023, 22, 157–168. [Google Scholar] [CrossRef]
- Wang, Y.L.; Song, K.X.; Zhang, Y.M.; Wang, G.X. Microstructure evolution and fracture mechanism of H13 steel during high temperature tensile deformation. Mater. Sci. Eng. A 2019, 746, 127–133. [Google Scholar] [CrossRef]
- Ma, D.; Zhou, J.; Chen, Z.; Zhang, Z.; Chen, Q.; Li, D. Influence of thermal homogenization treatment on structure and impact toughness of H13 ESR steel. J. Iron Steel Res. Int. 2009, 16, 56–60. [Google Scholar] [CrossRef]
- Ma, C.; Zhang, M.; Zhang, Y.; Sun, Z.; Li, Q.; Zheng, T.; Zhou, B.; Shen, Z.; Ding, B.; Liu, C. Unraveling the influence of static magnetic field on carbide precipitation and mechanical properties in large-scale h13 hot work die steel. Metall. Mater. Trans. B 2024, 55, 3058–3071. [Google Scholar] [CrossRef]
- Zerbst, U.; Madia, M.; Klinger, C.; Bettge, D.; Murakami, Y. Defects as a root cause of fatigue failure of metallic components. II: Non-metallic inclusions. Eng. Fail. Anal. 2019, 98, 228–239. [Google Scholar] [CrossRef]
- Yao, D.; Li, J.; Li, J.; Zhu, Q. Effect of cold rolling on morphology of carbides and properties of 7Cr17MoV stainless steel. Mater. Manuf. Process. 2015, 30, 111–115. [Google Scholar] [CrossRef]
- Medvedeva, A.; Bergström, J.; Gunnarsson, S. Inclusions, stress concentrations and surface condition in bending fatigue of an H13 tool steel. Steel Res. Int. 2008, 79, 376–381. [Google Scholar] [CrossRef]
- Li, X.; Jiang, Z.; Geng, X.; Chen, M.; Peng, L. Evolution mechanism of inclusions in h13 steel with rare earth magnesium alloy addition. ISIJ Int. 2019, 59, 1552–1561. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Li, G.; Wang, Q.; Zhang, Z.; Li, B. Role of vacuum on cleanliness improvement of steel during electroslag remelting. Vacuum 2018, 154, 351–358. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Li, G.; Huang, X.; Wang, Q.; Li, B. Cleanliness improvement and microstructure refinement of ingot processed by vacuum electroslag remelting. J. Mater. Res. Technol. 2020, 9, 1619–1630. [Google Scholar] [CrossRef]
- Qi, Y.; Li, J.; Shi, C.; Geng, R.; Zhang, J. Effect of directional solidification in electroslag remelting on the microstructure and cleanliness of an austenitic hot-work die steel. ISIJ Int. 2018, 58, 1275–1284. [Google Scholar] [CrossRef]
- Li, Z.B. Electroslag Metallurgy Theory and Practice; Metallurgical Industry Press: Beijing, China, 2010. [Google Scholar]
- Jiang, X.; Song, S.H. Enhanced hot ductility of a Cr–Mo low alloy steel by rare earth cerium. Mater. Sci. Eng. A 2014, 613, 171–177. [Google Scholar] [CrossRef]
- Wang, X.; Li, G.; Liu, Y.; Wang, F.; Wang, Q. Cerium addition effect on modification of inclusions, primary carbides and microstructure refinement of H13 die steel. ISIJ Int. 2021, 61, 1850–1859. [Google Scholar] [CrossRef]
- Okabe, T.H.; Taninouchi, Y.K.; Zheng, C. Thermodynamic analysis of deoxidation of titanium through the formation of rare-earth oxyfluorides. Metall. Mater. Trans. B 2018, 49, 3107–3117. [Google Scholar] [CrossRef]
- Cao, Y.; Miao, Y.; Li, D.; Chen, Y.; Fu, P.; Liu, H.; Kang, X.; Liu, H.; Sun, C. On the mechanism of steel homogenization via rare earth addition: Experimental characterization and numerical simulation. Metall. Mater. Trans. B 2022, 53, 1858–1874. [Google Scholar] [CrossRef]
- Ren, Y.; Zhang, L.; Yu, L.; Fang, W. Yield of Y, La, Ce in high temperature alloy during electroslag remelting process. Metall. Res. Technol. 2016, 113, 405. [Google Scholar] [CrossRef]
- Weng, J.; Shi, W.; Xie, H. Effect of Ce on stainless steel performance during electroslag remelting (esr). Metalurgija 2018, 57, 67–70. [Google Scholar]
- Zhao, Y.; Shi, C.-b.; Wang, S.-j.; Ren, P.; Li, J. Reoxidation of liquid steel and evolution of inclusions during protective atmosphere electroslag remelting of Ce-containing heat-resistant stainless steel. J. Iron Steel Res. Int. 2024, 31, 1923–1935. [Google Scholar] [CrossRef]
- Duan, S.C.; Park, J.H. Comparison of oxidation behavior of various reactive elements in alloys during electroslag remelting (ESR) process: An overview. ISIJ Int. 2022, 62, 1561–1572. [Google Scholar] [CrossRef]
- Hou, D.; Jiang, Z.H.; Dong, Y.W.; Gong, W.; Cao, Y.L.; Cao, H. Effect of slag composition on the oxidation kinetics of alloying elements during electroslag remelting of stainless steel: Part-1 mass-transfer model. ISIJ Int. 2017, 57, 1400–1409. [Google Scholar] [CrossRef]
- Luo, Y.; Wu, M.; Yang, W.; Zhang, L. Effect of the La2O3 content in slag on inclusions in al-killed steels. Metall. Mater. Trans. B 2022, 53, 2088–2103. [Google Scholar] [CrossRef]
- Upolovnikova, A.; Babenko, A.; Smirnov, L. Equilibrium content of lanthanum in metal under the slag of CaO–SiO2–La2O3–15% Al2O3–8% MgO system. Steel Transl. 2020, 50, 865–869. [Google Scholar] [CrossRef]
- Li, T.; Li, G.; Zhang, Z.; Liu, Y.; Wang, X. Fluoride vaporization and crystallization of CaF2–CaO–Al2O3–(La2O3) slag for vacuum electroslag remelting. Vacuum 2022, 196, 110807. [Google Scholar] [CrossRef]
- Zhu, M.; Wu, G.; Azarov, A.; Monakhov, E.; Tang, K.; Müller, M.; Safarian, J. Effects of La2O3 addition into CaO-SiO2 slag: Structural evolution and impurity separation from Si-Sn alloy. Metall. Mater. Trans. B 2021, 52, 3045–3063. [Google Scholar] [CrossRef]
- Kim, Y.; Park, H. Estimation of TiO2-FeO-Na2O slag viscosity through molecular dynamics simulations for an energy efficient ilmenite smelting process. Sci. Rep. 2019, 9, 17338. [Google Scholar] [CrossRef]
- Wu, T.; He, S.; Liang, Y.; Wang, Q. Molecular dynamics simulation of the structure and properties for the CaO–SiO2 and CaO–Al2O3 systems. J. Non-Cryst. Solids 2015, 411, 145–151. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, C.; Jiang, M. Effect of fluorine on melt structure for CaO–SiO2–CaF2 and CaO–Al2O3–CaF2 by molecular dynamics simulations. ISIJ Int. 2020, 60, 2176–2182. [Google Scholar] [CrossRef]
- Park, B.; Li, H.; Corrales, L.R. Molecular dynamics simulation of La2O3–Na2O–SiO2 glasses. I. The structural role of La3+ cations. J. Non-Cryst. Solids 2002, 297, 220–238. [Google Scholar] [CrossRef]
- Rammutla, K.; Comins, J.; Erasmus, R.; Netshisaulu, T.; Ngoepe, P.; Chadwick, A. Light scattering and computer simulation studies of superionic pure and La-doped BaF2. Chem. Phys. 2016, 467, 6–12. [Google Scholar] [CrossRef]
- Gao, L.W.; Xia, X.B.; Xu, X.Q.; Chen, C.Q. Immobilization of radioactive fluoride waste in aluminophosphate glass: A molecular dynamics simulation. Nucl. Sci. Tech. 2018, 29, 92. [Google Scholar] [CrossRef]
- Hino, M.; Ito, K. Thermodynamic Data for Steelmaking; Tohoku University Press: Sendai, Japan, 2010. [Google Scholar]
- Huang, H.X. Iron and Steel Metallurgy Principles; Metallurgical Industry Press: Beijing, China, 2013. [Google Scholar]
- Chen, J.X. Handbook of Chart Data About Steelmaking, 2nd ed.; Beijing Metallurgical Industry Press: Beijing, China, 2010. [Google Scholar]
- Wu, C.; Cheng, G.; Tian, J. A thermodynamic model for evaluation of mass action concentrations of La2O3-Al2O3-CaF2-CaO-MgO slags for electroslag remelting based on the ion and molecule coexistence theory. High Temp. Mat. Pr. 2013, 32, 541–550. [Google Scholar] [CrossRef]
- Jiang, Z.H.; Hou, D.; Dong, Y.W.; Cao, Y.L.; Cao, H.B.; Gong, W. Effect of slag on titanium, silicon, and aluminum contents in superalloy during electroslag remelting. Metall. Mater. Trans. B 2016, 47, 1465–1474. [Google Scholar] [CrossRef]
- Yang, X.M.; Shi, C.B.; Zhang, M.; Zhang, J. A thermodynamic model for prediction of iron oxide activity in some FeO-containing slag systems. Steel Res. Int. 2012, 83, 244–258. [Google Scholar] [CrossRef]
- Yang, X.; Long, H.; Wu, C.; Wu, B. Effect of refining slag containing Ce2O3 on steel cleanliness. J. Rare Earths 2011, 29, 1079–1083. [Google Scholar] [CrossRef]
- Mills, K.; Yuan, L.; Jones, R. Estimating the physical properties of slags. J. S. Afr. I. Min. Metall. 2011, 111, 649–658. [Google Scholar]
- Mills, K.; Sridhar, S. Viscosities of ironmaking and steelmaking slags. Ironmak. Steelmak. 1999, 26, 262–268. [Google Scholar] [CrossRef]
- Honma, T.; Benino, Y.; Fujiwara, T.; Komatsu, T.; Sato, R.; Dimitrov, V. Electronic polarizability, optical basicity, and interaction parameter of La2O3 and related glasses. J. Appl. Phys. 2002, 91, 2942–2950. [Google Scholar] [CrossRef]
- Li, S.; Cheng, G.; Huang, Y.; Dai, W.; Miao, Z. Kinetics of phosphorus transfer during industrial electroslag remelting of G20CrNi2Mo bearing steel. Metals 2019, 9, 467. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, S.; Yu, P.; Sun, L.; Wang, Y. Effect of steel-refractory reactions on removal of arsenic from molten steel with lanthanum additions. ISIJ Int. 2020, 60, 2316–2324. [Google Scholar] [CrossRef]
- Wang, H.; Xiong, L.; Zhang, L.; Wang, Y.; Shu, Y.; Zhou, Y. Investigation of Re-O-S-As inclusions in high carbon steels. Metall. Mater. Trans. B 2017, 48, 2849–2858. [Google Scholar] [CrossRef]
- Gong, W.; Wang, C.; Wang, P.F.; Jiang, Z.H.; Wang, R.; Li, H.B. Effect of La on inclusions and fracture toughness of low-alloy ultra-high-strength 40CrNi2Si2MoVA steel. Journal of Iron and Steel Res. Int. 2021, 28, 1408–1416. [Google Scholar] [CrossRef]
- Kim, Y.; Kashiwaya, Y.; Chung, Y. Effect of varying Al2O3 contents of CaO–Al2O3–SiO2 slags on lumped MgO dissolution. Ceram. Int. 2020, 46, 6205–6211. [Google Scholar] [CrossRef]
- Zheng, H.; Duan, S.; Zhang, L. Investigation of lanthanum content on nonmetallic inclusions and mechanical properties of low alloy wear-resistant steel containing P and As elements through in situ tensile test. Metall. Mater. Trans. B 2025, 56, 120–140. [Google Scholar] [CrossRef]
- Wang, H.; Bai, B.; Jiang, S.; Sun, L.; Wang, Y. An in situ study of the formation of rare earth inclusions in arsenic high carbon steels. ISIJ Int. 2019, 59, 1259–1265. [Google Scholar] [CrossRef]
- Barin, I. Thermochemical Data of Pure Substances; VCH Verlag GmbH: Weinheim, Germany, 1995. [Google Scholar]
- Clyne, T.; Kurz, W. Solute redistribution during solidification with rapid solid state diffusion. Metall. Trans. A 1981, 12, 965–971. [Google Scholar] [CrossRef]
- Wang, X.; Li, G.; Liu, Y.; Wang, Q.; Zhang, Z. Role of slag on inclusions control and its effect on primary carbides in H13 steel. Metall. Res. Technol. 2020, 117, 111. [Google Scholar] [CrossRef]
- Mongalo, L.; Lopis, A.S.; Venter, G.A. Molecular dynamics simulations of the structural properties and electrical conductivities of CaO–MgO–Al2O3–SiO2 melts. J. Non-Cryst. Solids 2016, 452, 194–202. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Z.; Li, G.; Wang, Q.; Li, B. Effect of current on segregation and inclusions characteristics of dual alloy ingot processed by electroslag remelting. High Temp. Mat. Pr. 2019, 38, 207–218. [Google Scholar] [CrossRef]
- Wang, X.; Li, G.; Chen, Y.; Wang, Q.; Liu, Y. Industrial trials on preparation of cerium-treated H13 steel by electroslag remelting with cerium-oxide containing slag. Steel Res. Int. 2023, 94, 2200786. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).