Functional and Bioactive Characterization of Hemp Cake Proteins and Polyphenols from Non-Psychoactive Cannabis sativa
Abstract
1. Introduction
2. Materials and Methods
2.1. Obtaining the Non-Psychoactive Cannabis Flowers
2.2. Reagents
2.3. Obtaining the Cannabis Hemp CakeReagents
2.4. Preparation of Cannabis Protein Concentrates
2.5. Replication and Experimental Design
2.6. Quantification of Protein Content
2.6.1. Duma’s Method
2.6.2. Bradford Method
2.6.3. Bicinchoninic Acid (BCA) Method
2.6.4. Protein Recovery Yield
2.7. Determination of Functional Properties
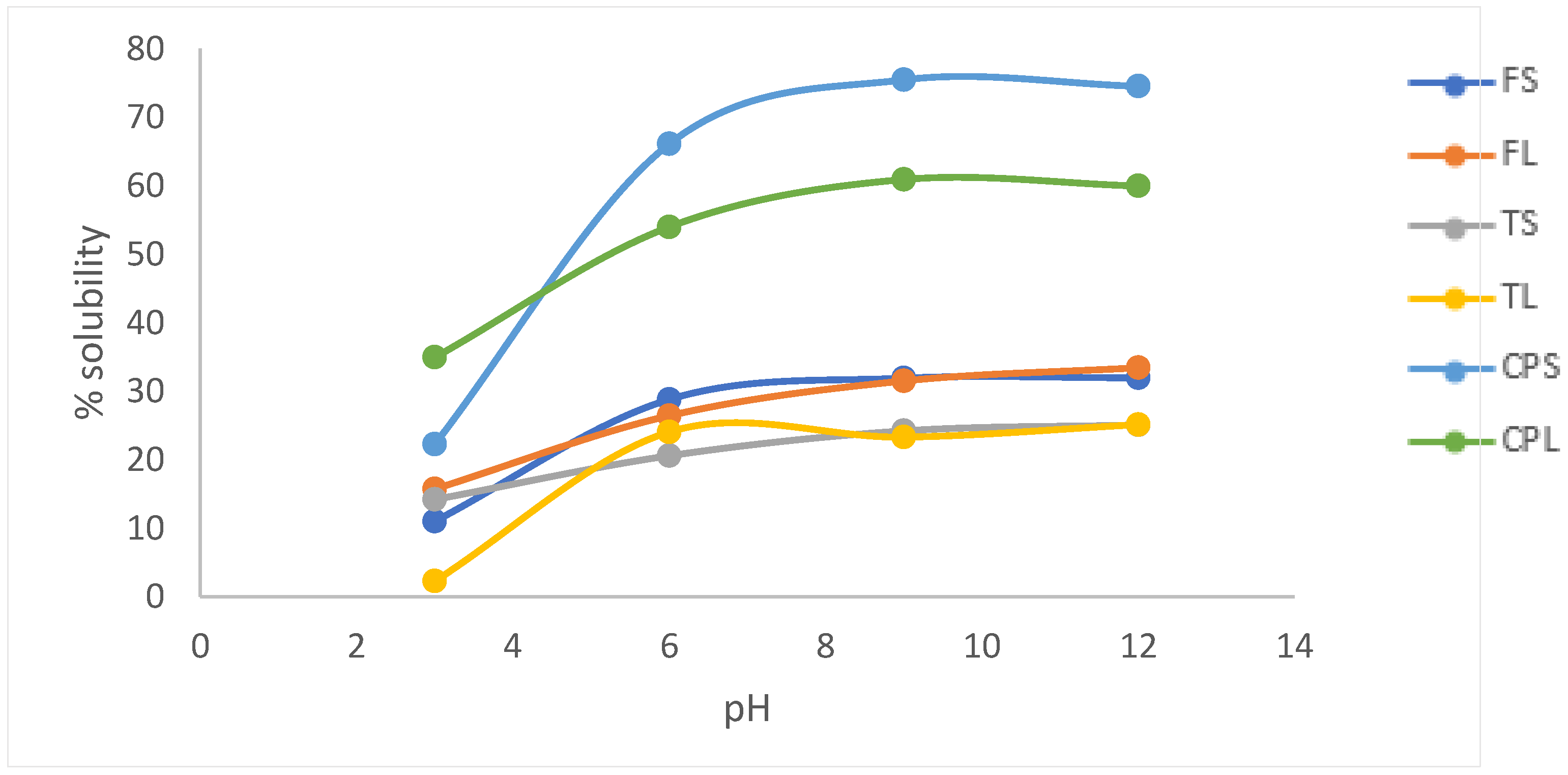
2.7.1. Protein Solubility
2.7.2. Water Absorption Capacity
2.7.3. Oil Absorption Capacity
2.7.4. Foaming Capacity and Foam Stability
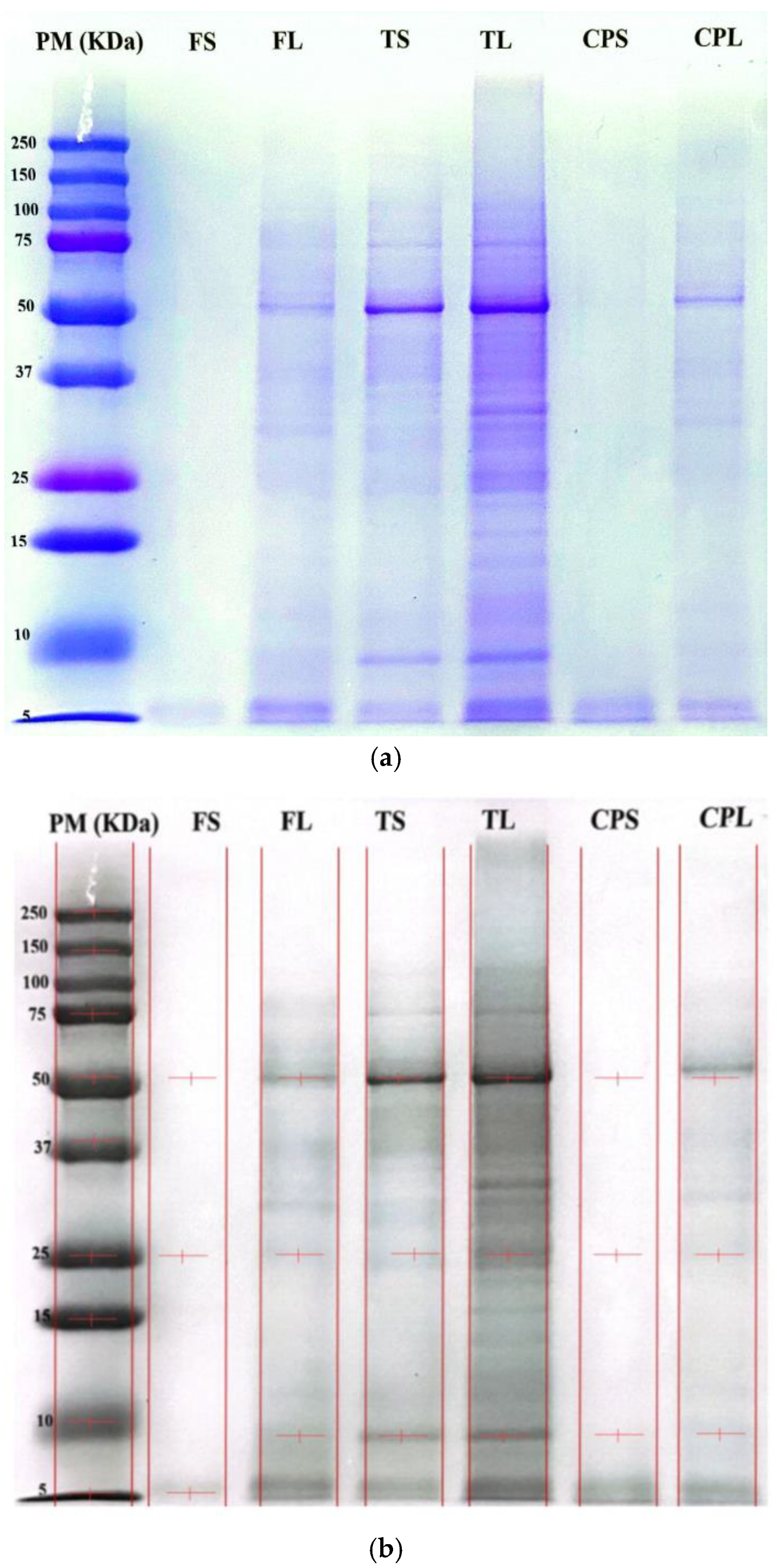
2.8. Protein Characterization by Electrophoresis
2.9. Determination of Phenolic Compounds and Antioxidants
2.9.1. Obtaining Extracts
2.9.2. Total Polyphenols
2.9.3. Total Flavonoids
2.9.4. Antioxidant Activity by ABTS Assay
2.9.5. Antioxidant Activity by DPPH Assay
2.9.6. Antioxidant Activity by FRAP Assay
2.10. In Vitro Anti-Inflammatory Activity of Protein Concentrates
2.11. Statistical Analysis
3. Results and Discussion
3.1. Protein Content
3.2. Functional Properties
3.3. Protein Profile of Cannabis Concentrates Using SDS-PAGE Electrophoresis
3.4. Polyphenols and Flavonoids in Non-Psychoactive Cannabis Samples
3.5. Antioxidant Activity
3.6. In Vitro Anti-Inflammatory Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Brkljača, N.; Đurović, S.; Milošević, S.; Gašić, U.; Panković, D.; Zeković, Z.; Pavlić, B. Sequential extraction approach for sustainable recovery of various hemp (Cannabis sativa L.) bioactive compounds. Sustain. Chem. Pharm. 2023, 35, 101213. Available online: https://www.sciencedirect.com/science/article/pii/S2352554123002474 (accessed on 1 September 2024). [CrossRef]
- Lazo, X. Acuerdo Ministerial No. 109; Quito: Quito, Ecuador, 2020; p. 48. [Google Scholar]
- Leonard, W.; Zhang, P.; Ying, D.; Xiong, Y.; Fang, Z. Extrusion improves the phenolic profile and biological activities of hempseed (Cannabis sativa L.) hull. Food Chem. 2021, 346, 128606. Available online: https://www.sciencedirect.com/science/article/pii/S0308814620324687 (accessed on 1 July 2024). [CrossRef]
- Chandra, S.; Lata, H.; ElSohly, M.A. Cannabis sativa L.—Botany and Biotechnology; Springer: Cham, Switzerland, 2017; pp. 1–474. [Google Scholar] [CrossRef]
- Dos Santos, N.A.; Romão, W. Cannabis—A state of the art about the millenary plant: Part I. Forensic Chem. 2023, 32, 100470. Available online: https://www.sciencedirect.com/science/article/pii/S2468170923000061 (accessed on 1 September 2024). [CrossRef]
- Julakanti, S.; Charles, A.P.R.; Zhao, J.; Bullock, F.; Syed, R.; Myles, Y.; Wu, Y. Hempseed protein (Cannabis sativa L.): Influence of extraction pH and ball milling on physicochemical and functional properties. Food Hydrocoll. 2023, 143, 108835. Available online: https://www.sciencedirect.com/science/article/pii/S0268005X23003818 (accessed on 1 September 2024). [CrossRef]
- Callaway, J.C. Hempseed as a nutritional resource: An overview. Euphytica 2004, 140, 65–72. [Google Scholar] [CrossRef]
- Karabulut, G.; Kahraman, O.; Pandalaneni, K.; Kapoor, R.; Feng, H. A comprehensive review on hempseed protein: Production, functional and nutritional properties, novel modification methods, applications, and limitations. Int. J. Biol. Macromol. 2023, 253, 127240. [Google Scholar] [CrossRef]
- Leonard, W.; Zhang, P.; Ying, D.; Nie, S.; Tindal, E.; Fang, Z. Transformation of hempseed (Cannabis sativa L.) oil cake proteome, structure and functionality after extrusion. Food Chem. 2022, 384, 132499. Available online: https://www.sciencedirect.com/science/article/pii/S0308814622004617 (accessed on 1 August 2024). [CrossRef]
- Leonard, W.; Zhang, P.; Ying, D.; Nie, S.; Liu, S.; Fang, Z. Post-extrusion physical properties, techno-functionality and microbiota-modulating potential of hempseed (Cannabis sativa L.) hull fiber. Food Hydrocoll. 2022, 131, 107836. Available online: https://www.sciencedirect.com/science/article/pii/S0268005X22003563 (accessed on 15 August 2024). [CrossRef]
- Mamone, G.; Picariello, G.; Ramondo, A.; Nicolai, M.A.; Ferranti, P. Production, digestibility and allergenicity of hemp (Cannabis sativa L.) protein isolates. Food Res. Int. 2019, 115, 562–571. Available online: https://www.sciencedirect.com/science/article/pii/S0963996918307427 (accessed on 1 May 2024). [CrossRef]
- Karabulut, G.; Feng, H.; Yemiş, O. Physicochemical and Antioxidant Properties of Industrial Hemp Seed Protein Isolate Treated by High-Intensity Ultrasound. Plant Foods Hum. Nutr. 2022, 77, 577–583. [Google Scholar] [CrossRef]
- Vilcacundo, E.; García, A.; Vilcacundo, M.; Morán, R.; Samaniego, I.; Carrillo, W. Antioxidant Purple Corn Protein Concentrate from Germinated Andean Purple Corn Seeds. Agronomy 2020, 10, 1282. Available online: https://www.mdpi.com/2073-4395/10/9/1282 (accessed on 1 May 2024). [CrossRef]
- Serrano, S.; Rincón, F.; García-Olmo, J. Cereal protein analysis via Dumas method: Standardization of a micro-method using the EuroVector Elemental Analyser. J. Cereal Sci. 2013, 58, 31–36. Available online: https://www.sciencedirect.com/science/article/pii/S0733521013000787 (accessed on 15 May 2024). [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. Available online: https://www.sciencedirect.com/science/article/pii/0003269776905273 (accessed on 10 August 2024). [CrossRef] [PubMed]
- Krieg, R.C.; Dong, Y.; Schwamborn, K.; Knuechel, R. Protein quantification and its tolerance for different interfering reagents using the BCA-method with regard to 2D SDS PAGE. J. Biochem. Biophys. Methods 2005, 65, 13–19. [Google Scholar] [CrossRef]
- Quinteros, M.F.; Martínez, J.; Barrionuevo, A.; Rojas, M.; Carrillo, W. Functional, Antioxidant, and Anti-Inflammatory Properties of Cricket Protein Concentrate (Gryllus assimilis). Biology 2022, 11, 776. Available online: https://www.mdpi.com/2079-7737/11/5/776 (accessed on 12 August 2024).
- Thirumuruga, T.; Kanchana, S.; Hemalatha, C.; Vellaikumar, S.; Kalpana, K. Functional and microstructural properties of Pleurotus florida and Calocybe indica flour and protein concentrate. J. Anim. Plant Sci. 2022, 32, 1430–1439. [Google Scholar] [CrossRef]
- Rosero, S.; Del Pozo, F.; Simbaña, W.; Álvarez, M.; Quinteros, M.F.; Carrillo, W.; Morales, D. Polyphenols and Flavonoids Composition, Anti-Inflammatory and Antioxidant Properties of Andean Baccharis macrantha Extracts. Plants 2022, 11, 1555. Available online: https://www.mdpi.com/2223-7747/11/12/1555 (accessed on 15 August 2024). [CrossRef]
- Piñuel, L.; Edgar, V.; Boeri, P.; Barrio, D.; Morales, D.; Pinto, A.; Moran, R.; Samaniego, I.; Carrillo, W. Extraction of protein concentrate from red bean (Phaseolus vulgaris L.): Antioxidant activity and inhibition of lipid peroxidation. J. Appl. Pharm. Sci. 2019, 9, 1–14. [Google Scholar] [CrossRef]
- Ramos-Escudero, F.; Muñoz, A.M.; Alvarado-Ortíz, C.; Alvarado, Á.; Yáñez, J.A. Purple corn (Zea mays L.) phenolic compounds profile and its assessment as an agent against oxidative stress in isolated mouse organs. J. Med. Food 2012, 15, 206–215. [Google Scholar] [CrossRef]
- Vilcacundo, E.; Montalvo, V.; Sanaguano, H.; Moran, R.; Carrillo, W.; García, A. Identification of Phytochemical Compounds, Functional Properties and Antioxidant Activity of Germinated Purple Corn Protein Concentrate and Its Gastrointestinal Hydrolysates. Agronomy 2022, 12, 2217. Available online: https://www.mdpi.com/2073-4395/12/9/2217 (accessed on 1 June 2024). [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. Available online: https://www.sciencedirect.com/science/article/pii/S0023643895800085 (accessed on 1 June 2024). [CrossRef]
- Samaniego, I.; Brito, B.; Viera, W.; Cabrera, A.; Llerena, W.; Kannangara, T.; Vilcacundo, R.; Angós, I.; Carrillo, W. Influence of the Maturity Stage on the Phytochemical Composition and the Antioxidant Activity of Four Andean Blackberry Cultivars (Rubus glaucus Benth) from Ecuador. Plants 2020, 9, 1027. Available online: https://www.mdpi.com/2223-7747/9/8/1027 (accessed on 1 June 2024). [CrossRef]
- Hueso, S.; Fontecha, J.; Gómez-Cortés, P. Comparative study of the most commonly used methods for total protein determination in milk of different species and their ultrafiltration products. Food Chem. 2022, 381, 132237. [Google Scholar] [CrossRef]
- Stoscheck, C.M. Quantitation of protein. Methods Enzymol. 1990, 182, 50–68. [Google Scholar] [CrossRef]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.H.; Ten, Z.; Wang, X.S.; Yang, X.Q. Physicochemical and functional properties of hemp (Cannabis sativa L.) protein isolate. J. Agric. Food Chem. 2006, 54, 8945–8950. [Google Scholar] [CrossRef] [PubMed]
- Roland, I.S.; Aguilera-Toro, M.; Nielsen, S.D.H.; Poulsen, N.A.; Larsen, L.B. Processing-induced markers in proteins of commercial plant-based drinks in relation to compositional aspects. Foods 2023, 12, 3282. [Google Scholar] [CrossRef] [PubMed]
- Manzanilla-Valdez, M.L.; Ma, Z.; Mondor, M.; Hernández-Álvarez, A.J. Decoding the duality of antinutrients: Assessing the impact of protein extraction methods on plant-based protein sources. J. Agric. Food Chem. 2024, 72, 12319–12339. [Google Scholar] [CrossRef]
- Ajibola, C.F.; Aluko, R.E. Physicochemical and Functional Properties of 2S, 7S, and 11S Enriched Hemp Seed Protein Fractions. Molecules 2022, 27, 1059. [Google Scholar] [CrossRef]
- Ajibola, C.F.; Malomo, S.A.; Fagbemi, T.N.; Aluko, R.E. Polypeptide composition and functional properties of African yam bean seed (Sphenostylis stenocarpa) albumin, globulin and protein concentrate. Food Hydrocoll. 2016, 56, 189–200. Available online: https://www.sciencedirect.com/science/article/pii/S0268005X15301818 (accessed on 15 June 2024). [CrossRef]
- Ma, Z.; Jiang, F.; Liu, K.; Gong, F.; Liu, Y.; Zheng, Z.; Xu, Y.J. A comparison with soy protein isolate and wheat protein: Water absorption capacity of PsmPs (4.4–4.8 g water/g protein). LWT 2024, 197, 115920. [Google Scholar] [CrossRef]
- de Paiva Gouvêa, L.; Caldeira, R.; de Lima Azevedo, T.; Galdeano, M.C.; Felberg, I.; Lima, J.R.; Mellinger, C.G. Physical and techno-functional properties of a common bean protein concentrate compared to commercial legume ingredients for the plant-based market. Food Hydrocoll. 2023, 137, 108351. [Google Scholar] [CrossRef]
- Karabulut, G. A comprehensive review on hempseed protein: Functional properties including foaming, water binding. Int. J. Biol. Macromol. 2023, 253, 127240. [Google Scholar]
- Ding, Z.; Jiang, F.; Liu, K.; Gong, F.; Liu, Y.; Zheng, Z.; Xu, Y.J. Structural and Functional Characteristics of Hemp Protein Isolate–Pullulan Polysaccharide Glycosylation Conjugate. Foods 2023, 12, 1416. [Google Scholar] [CrossRef]
- Kahraman, O.; Petersen, G.E.; Fields, C. Physicochemical and Functional Modifications of Hemp. Food 2022, 11, 587. [Google Scholar] [CrossRef]
- Dapčević-Hadnađev, T.; Hadnađev, M.; Lazaridou, A.; Moschakis, T.; Biliaderis, C.G. Hempseed meal protein isolates prepared by different isolation techniques. Part II. gelation properties at different ionic strengths. Food Hydrocoll. 2018, 81, 481–489. Available online: https://www.sciencedirect.com/science/article/pii/S0268005X17320672 (accessed on 1 May 2024). [CrossRef]
- Dapčević-Hadnađev, T.; Dizdar, M.; Pojić, M.; Krstonošić, V.; Zychowski, L.M.; Hadnađev, M. Emulsifying properties of hemp proteins: Effect of isolation technique. Food Hydrocoll. 2019, 89, 912–920. Available online: https://www.sciencedirect.com/science/article/pii/S0268005X1831083X (accessed on 1 May 2024). [CrossRef]
- Fang, B.; Chang, L.; Ohm, J.B.; Chen, B.; Rao, J. Structural, functional properties, and volatile profile of hemp protein isolate as affected by extraction method: Alkaline extraction–isoelectric precipitation vs salt extraction. Food Chem. 2023, 405, 135001. Available online: https://www.sciencedirect.com/science/article/pii/S0308814622029636 (accessed on 15 May 2024). [CrossRef] [PubMed]
- Hadnađev, M.; Dapčević-Hadnađev, T.; Lazaridou, A.; Moschakis, T.; Michaelidou, A.M.; Popović, S.; Biliaderis, C.G. Hempseed meal protein isolates prepared by different isolation techniques. Part I. physicochemical properties. Food Hydrocoll. 2018, 79, 526–533. Available online: https://www.sciencedirect.com/science/article/pii/S0268005X17315151 (accessed on 1 June 2024). [CrossRef]
- Tanger, C.; Engel, J.; Kulozik, U. Influence of extraction conditions on the conformational alteration of pea protein extracted from pea flour. Food Hydrocoll. 2020, 107, 105949. Available online: https://www.sciencedirect.com/science/article/pii/S0268005X1932819X (accessed on 15 June 2024). [CrossRef]
- Pacifico, S.; Galasso, S.; Piccolella, S.; Kretschmer, N.; Pan, S.P.; Marciano, S.; Bauer, R.; Monaco, P. Seasonal variation in phenolic composition and antioxidant and anti-inflammatory activities of Calamintha nepeta (L.) Savi. Food Res Int. 2015, 69, 121–132. Available online: https://www.sciencedirect.com/science/article/pii/S0963996914007893 (accessed on 1 July 2024). [CrossRef]
- Siger, A.; Nogala-Kalucka, M.; Lampart-Szczapa, E. The content and antioxidant activity of phenolic compounds in cold-pressed plant oils. J. Food Lipids 2008, 15, 137–149. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1745-4522.2007.00107.x (accessed on 1 July 2024). [CrossRef]
- Smeriglio, A.; Galati, E.M.; Monforte, M.T.; Lanuzza, F.; D’Angelo, V.; Circosta, C. Polyphenolic Compounds and Antioxidant Activity of Cold-Pressed Seed Oil from Finola Cultivar of Cannabis sativa L. Phyther. Res. 2016, 30, 1298–1307. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/ptr.5623 (accessed on 1 July 2024).
- Faugno, S.; Piccolella, S.; Sannino, M.; Principio, L.; Crescente, G.; Baldi, G.M.; Baldi, G.M.; Fiorentino, N.; Pacifico, S. Can agronomic practices and cold-pressing extraction parameters affect phenols and polyphenols content in hempseed oils? Ind. Crops Prod. 2019, 130, 511–519. Available online: https://www.sciencedirect.com/science/article/pii/S0926669018311488 (accessed on 1 July 2024).
- Gao, J.; Li, T.; Chen, D.; Gu, H.; Mao, X. Identification and molecular docking of antioxidant peptides from hemp seed protein hydrolysates. LWT 2021, 147, 111453. Available online: https://www.sciencedirect.com/science/article/pii/S002364382100606X (accessed on 15 July 2024). [CrossRef]
- Cotabarren, J.; Rosso, A.M.; Tellechea, M.; García-Pardo, J.; Rivera, J.L.; Obregón, W.D.; Parisi, M.G. Adding value to the chia (Salvia hispanica L.) expeller: Production of bioactive peptides with antioxidant properties by enzymatic hydrolysis with Papain. Food Chem. 2019, 274, 848–856. Available online: https://www.sciencedirect.com/science/article/pii/S0308814618316327 (accessed on 1 August 2024). [CrossRef]
- Rodriguez-Martin, N.M.; la Paz, S.; Toscano, R.; Grao-Cruces, E.; Villanueva, A.; Pedroche, J.; Millan, F.; Millan-Linares, M.C. Hemp (Cannabis sativa L.) Protein Hydrolysates Promote Anti-Inflammatory Response in Primary Human Monocytes. Biomolecules 2020, 10, 803. Available online: https://www.mdpi.com/2218-273X/10/5/803 (accessed on 1 August 2024). [CrossRef]
- Andre, C.M.; Hausman, J.F.; Guerriero, G. Cannabis sativa: The Plant of the Thousand and One Molecules. Front. Plant Sci. 2016, 7, 19. [Google Scholar] [CrossRef]
- Izzo, A.A.; Borrelli, F.; Capasso, R.; Di Marzo, V.; Mechoulam, R. Non-psychotropic plant cannabinoids: New therapeutic opportunities from an ancient herb. Trends Pharmacol. Sci. 2009, 30, 515–527. Available online: https://www.sciencedirect.com/science/article/pii/S016561470900128X (accessed on 1 September 2024). [CrossRef]
- Sangiovanni, E.; Fumagalli, M.; Pacchetti, B.; Piazza, S.; Magnavacca, A.; Khalilpour, S.; Melzi, G.; Martinelli, G.; Dell’AGli, M. Cannabis sativa L. extract and cannabidiol inhibit in vitro mediators of skin inflammation and wound injury. Phytother. Res. 2019, 33, 2083–2093. [Google Scholar] [CrossRef]
- Shebaby, W.; Saliba, J.; Faour, W.H.; Ismail, J.; El Hage, M.; Daher, C.F.; Taleb, R.I.; Nehmeh, B.; Dagher, C.; Chrabieh, E.; et al. In vivo and in vitro anti-inflammatory activity evaluation of Lebanese Cannabis sativa L. ssp. indica (Lam.). J. Ethnopharmacol. 2021, 270, 113743. Available online: https://www.sciencedirect.com/science/article/pii/S037887412033631X (accessed on 1 September 2024). [CrossRef]
| Samples | BCA | Bradford % Protein | Dumas |
|---|---|---|---|
| FS | 56.00 ± 0.00 f | 5.75 ± 0.08 c | 21.83 ± 0.14 e |
| FL | 83.55 ± 0.00 d | 4.15 ± 0.03 d | 18.67 ± 0.31 f |
| TS | 77.54 ± 1.54 c | 1.67 ± 0.08 e | 27.08 ± 0.10 c |
| TL | 84.73 ± 2.11 c | 0.34 ± 0.04 f | 24.17 ± 0.45 d |
| CPS | 90.42 ± 1.02 a | 17.23 ± 0.08 a | 32.27 ± 0.85 b |
| CPL | 87.33± 1.00 b | 8.68 ± 0.23 b | 42.67 ± 0.47 a |
| Samples | % Water Absorption Capacity | % Oil Absorption Capacity | % Foaming Capacity Dumas | % Foam Stability |
|---|---|---|---|---|
| FS | 36.63 ± 0.04 d | 22.53 ± 0.07 e | 0.00 ± 0.00 c | 0.00 ± 0.00 c |
| FL | 45.53 ± 0.95 c | 38.52 ± 0.30 c | 0.00 ± 0.00 c | 0.00 ± 0.00 c |
| TS | 51.07 ± 0.52 b | 28.53 ± 0.95 d | 20.00 ± 0.00 b | 20.00 ± 0.00 b |
| TL | 54.20 ± 0.92 a | 42.79 ± 0.37 b | 40.00 ± 0.00 a | 40.00 ± 0.00 a |
| CPS | 20.44 ± 0.16 f | 44.84 ± 0.44 b | 40.00 ± 0.00 a | 20.00 ± 0.00 b |
| CPL | 27.42 ± 0.09 e | 53.95 ± 0.64 a | 20.00 ± 0.00 b | 0.00 ± 0.00 c |
| Samples | mg GAE/g Samples | mg QE/g Samples |
|---|---|---|
| FS | 38.64 ± 0.34 b | 1.23 ± 0.04 e |
| FL | 31.39 ± 0.69 c | 2.07 ± 0.02 d |
| TS | 25.19 ± 0.35 d | 1.31 ± 0.07 e |
| TL | 15.49 ± 0.60 e | 2.38 ± 0.04 c |
| CPS | 51.11 ± 0.35 a | 2.68 ± 0.09 b |
| CPL | 24.87 ± 0.37 d | 4.94 ± 0.02 a |
| Samples | FRAP | ABTS | DPPH |
|---|---|---|---|
| µmol ET/g Samples | |||
| FS | 39.78 ± 0.95 e | 2773.31 ± 0.01 b | 72.11 ± 2.41 b |
| FL | 55.37 ± 0.55 d | 2221.79 ± 0.00 d | 74.27 ± 2.28 b |
| TS | 40.61 ± 0.56 e | 2435.24 ± 0.01 c | 64.59 ± 2.86 c |
| TL | 76.31 ± 0.00 b | 1490.90 ± 0.02 f | 72.27 ± 3.44 b |
| CPS | 72.40 ± 2.22 c | 3034.71 ± 0.02 a | 82.80 ± 2.24 a |
| CPL | 90.31 ± 0.59 a | 1848.68 ± 0.02 e | 86.66 ± 0.79 a |
| Samples | % Protection |
|---|---|
| FS | 70.76 ± 0.77 f |
| FL | 75.45 ± 0.39 e |
| TS | 82.59 ± 0.67 d |
| TL | 95.54 ± 0.77 a |
| CPS | 87.28 ± 0.67 c |
| CPL | 91.07 ± 0.38 b |
| Diclofenac | 96.88 ± 1.02 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quinteros, M.; Wilcaso, P.; Ribadeneira, C.; Vilcacundo, E. Functional and Bioactive Characterization of Hemp Cake Proteins and Polyphenols from Non-Psychoactive Cannabis sativa. Processes 2025, 13, 3184. https://doi.org/10.3390/pr13103184
Quinteros M, Wilcaso P, Ribadeneira C, Vilcacundo E. Functional and Bioactive Characterization of Hemp Cake Proteins and Polyphenols from Non-Psychoactive Cannabis sativa. Processes. 2025; 13(10):3184. https://doi.org/10.3390/pr13103184
Chicago/Turabian StyleQuinteros, María, Paola Wilcaso, Carlos Ribadeneira, and Edgar Vilcacundo. 2025. "Functional and Bioactive Characterization of Hemp Cake Proteins and Polyphenols from Non-Psychoactive Cannabis sativa" Processes 13, no. 10: 3184. https://doi.org/10.3390/pr13103184
APA StyleQuinteros, M., Wilcaso, P., Ribadeneira, C., & Vilcacundo, E. (2025). Functional and Bioactive Characterization of Hemp Cake Proteins and Polyphenols from Non-Psychoactive Cannabis sativa. Processes, 13(10), 3184. https://doi.org/10.3390/pr13103184






