Functional, Antioxidant, and Anti-Inflammatory Properties of Cricket Protein Concentrate (Gryllus assimilis)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal and Plant Materials
2.2. Materials
2.3. Proximal Analysis
2.4. Preparation of Cricket Protein Concentrate (CPC)
2.5. Quantification of Protein Content of CPC
2.6. Functionals Properties of CPC
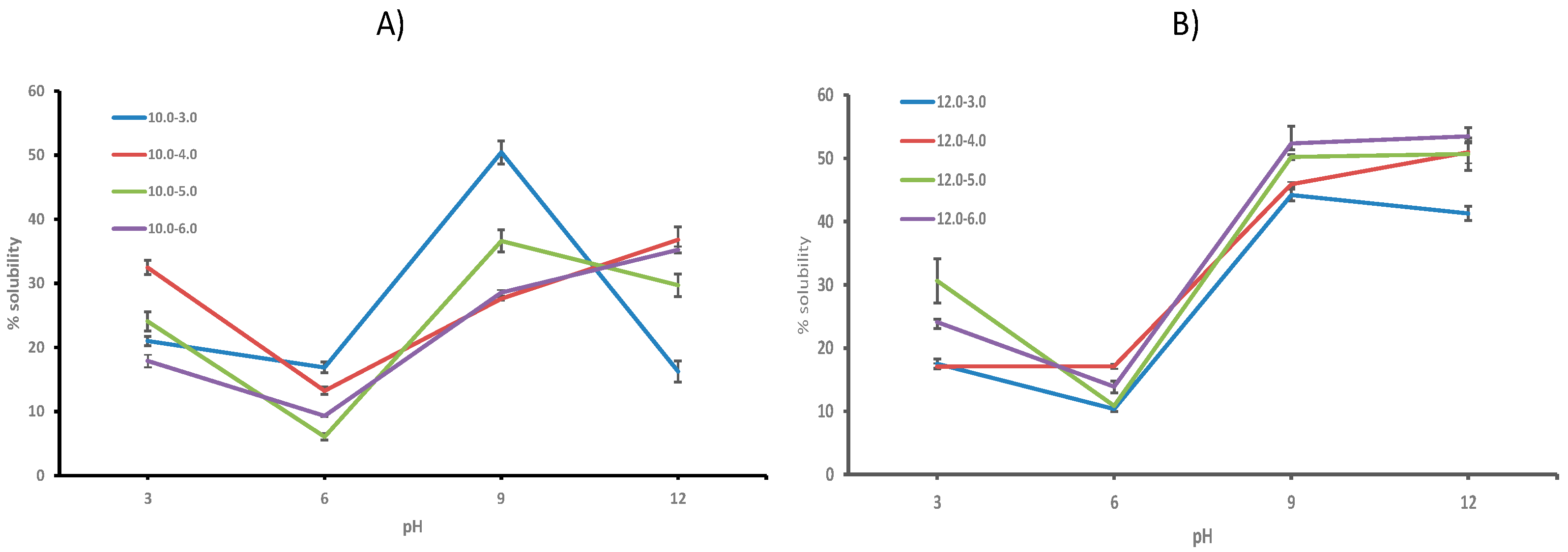
2.6.1. Protein Solubility
2.6.2. Oil Absorption Capacity (OAC)
2.6.3. Water Absorption Capacity (WAC)
2.7. Simulation of The Digestion Gastrointestinal in Vitro of CPCs
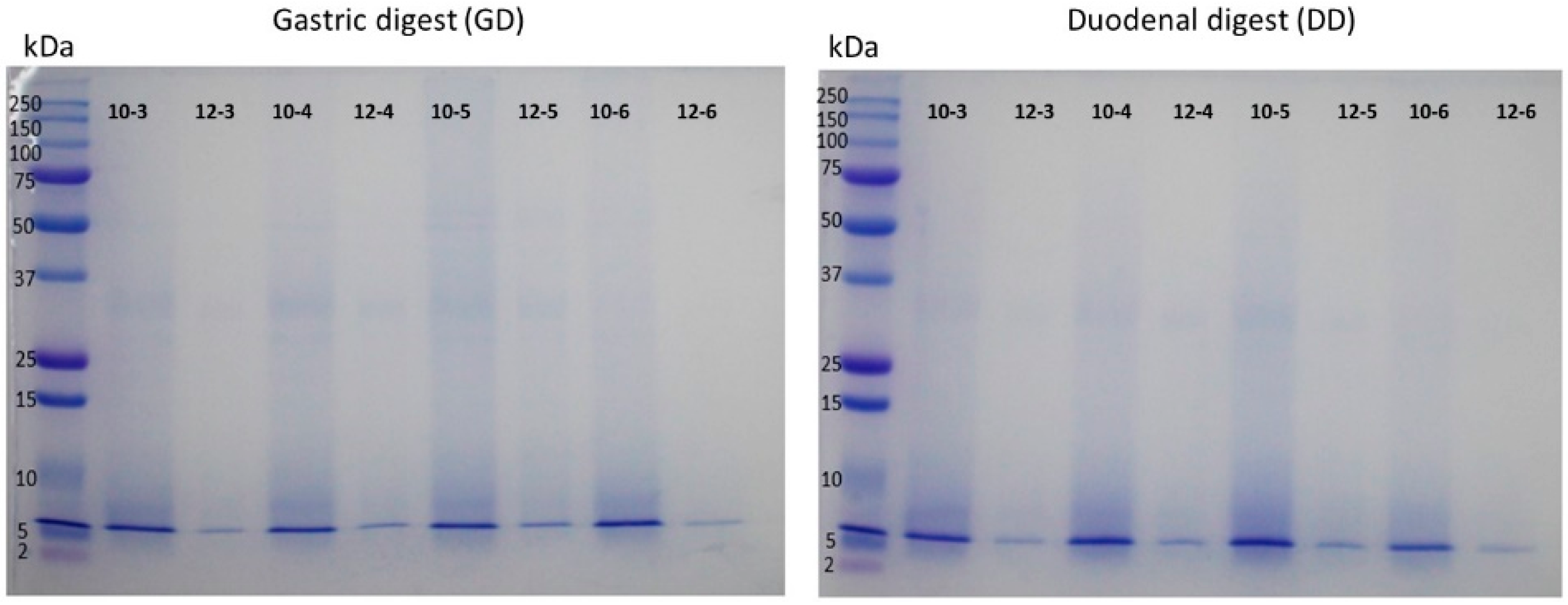
2.8. Analysis by Electrophoresis of CPCs and Hydrolysates
2.9. Antioxidant Activity In Vitro of CPCs
2.9.1. Quantification of Total Polyphenol Content (TPC)
2.9.2. Ferric-Reducing Antioxidant Power (FRAP) Method
2.9.3. 2-Azinobis (3-Ethyl-Benzothiazoline-6-Sulfonic Acid) Cation Bleaching ABTS Method
2.9.4. 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Radical Scavenging Assay
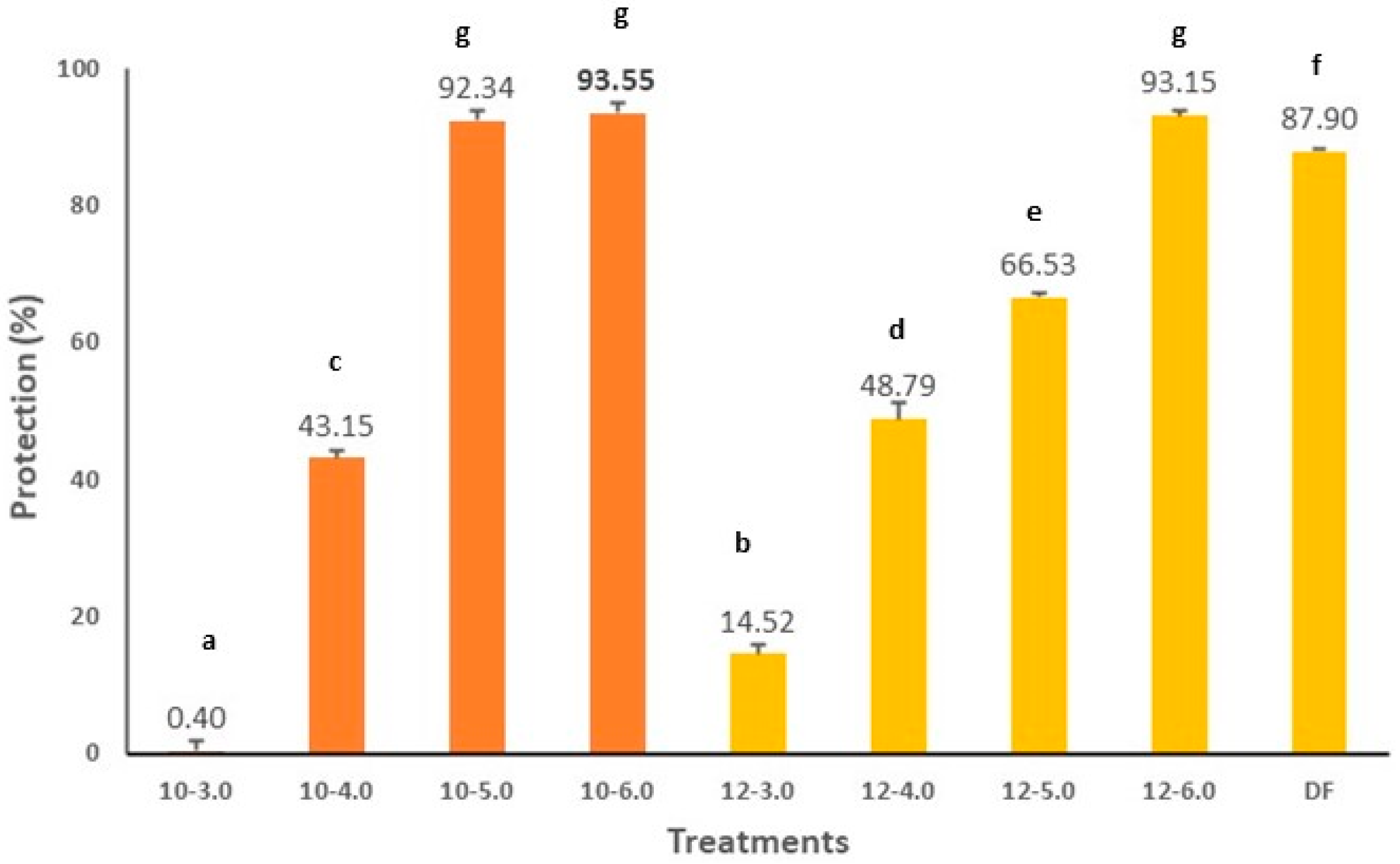
2.10. Anti-Inflammatory Activity In Vitro of CPC
2.11. Statistical Analysis
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Department of Economic and Social Affairs, Population Division, United Nations. World Population Prospects: The 2017 Revision, Key Findings and Advance Tables; Working Paper No. ESA/P/WP/248 ed; United Nations: New York, NY, USA, 2017. [Google Scholar]
- FAO. How to Feed the World in 2050; FAO: Rome, Italy, 2009; Available online: http://www.fao.org/wsfs/forum2050/wsfs-forum/en/ (accessed on 5 May 2021).
- Da Rosa Machado, C.; Thys, R.C.S. Cricket powder (Gryllus assimilis) as a new alternative protein source for gluten-free breads. Innov. Food Sci. Emerg. Technol. 2019, 56, 102180. [Google Scholar] [CrossRef]
- Kiiru, S.M.; Kinyuru, J.N.; Kiage, B.N.; Martin, A.; Marel, A.K.; Osen, R. Extrusion texturization of cricket flour and soy protein isolate: Influence of insect content, extrusion temperature, and moisture-level variation on textural properties. Food Sci. Nutr. 2020, 8, 4112–4120. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Elorduy, J. The importance of edible insects in the nutrition and economy of people of the rural areas of Mexico. Ecol. Food Nutr. 1997, 36, 347–366. [Google Scholar] [CrossRef]
- Ramos-Elorduy, J.; Pino Moreno, J.M. Edible insects of Chiapas, Mexico. Ecol. Food Nutr. 2002, 41, 271–299. [Google Scholar] [CrossRef]
- Santiago, L.A.; Fadel, O.M.; Tavares, G.M. How does the thermal-aggregation behavior of black cricket protein isolate affect its foaming and gelling properties? Food Hydrocol. 2021, 110, 106169. [Google Scholar] [CrossRef]
- Bußler, S.; Rumpold, B.A.; Jander, E.; Rawel, H.M.; Schlüter, O.K. Recovery and techno-functionality of flours and proteins from two edible insect species: Meal worm (Tenebrio molitor) and black soldier fly (Hermetia illucens) larvae. Heliyon 2016, 2, e00218. [Google Scholar] [CrossRef]
- Kooij, P.W.; Dentinger, B.M.; Donoso, D.A.; Shik, J.Z.; Gaya, E. Cryptic diversity in Colombian edible leaf-cutting ants (Hymenoptera: Formicidae). Insects 2018, 9, 191. [Google Scholar] [CrossRef] [Green Version]
- Dufour, D.L. Insects as food: A case study from the Northwest Amazon. Am. Anthropol. 1987, 89, 383–397. [Google Scholar] [CrossRef] [Green Version]
- FAO. Edible Insects Future Prospects for Food and Feed Security; FAO Forestry Paper; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013; Volume 171. [Google Scholar]
- House, J. Insects as food in the Netherlands: Production networks and the geographies of edibility. Geoforum 2018, 94, 82–93. [Google Scholar] [CrossRef]
- EFSA Scientific Committee. Risk profile related to production and consumption of insects as food and feed. EFSA J. 2015, 13, 4257. [Google Scholar] [CrossRef] [Green Version]
- Hirsch, A.; Cho, Y.H.; Kim, Y.H.B.; Jones, O.G. Contributions of protein and milled chitin extracted from domestic cricket powder to emulsion stabilization. Curr. Res. Food Sci. 2019, 1, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.X.; Chong, G.H.; Hamzah, H.; Ghazali, H.M.; Xuan, T.C.; Hean, C.G. Comparison of subcritical CO2 and ultrasound-assisted aqueous methods with the conventional solvent method in the extraction of avocado oil. J. Supercrit. Fluids 2018, 135, 45–51. [Google Scholar] [CrossRef]
- Tzompa-Sosa, D.A.; Yi, L.; Van Valenberg, H.J.; Van Boekel, M.A.; Lakemond, C.M. Insect lipid profile: Aqueous versus organic solvent-based extraction methods. Food Res. Int. 2014, 62, 1087–1094. [Google Scholar] [CrossRef]
- Rumpold, B.A.; Schlüter, O.K. Nutritional composition and safety aspects of edible insects. Mol. Nutr. Food Res. 2013, 57, 802–823. [Google Scholar] [CrossRef]
- Barton, A.; Richardson, C.D.; McSweeney, M.B. Consumer attitudes toward entomophagy before and after evaluating cricket (Acheta domesticus) based protein powders. J. Food Sci. 2020, 85, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Lakemond, C.M.; Sagis, L.M.; Eisner-Schadler, V.; van Huis, A.; van Boekel, M.A. Extraction and characterization of protein fractions from five insect species. Food Chem. 2013, 141, 3341–3348. [Google Scholar] [CrossRef] [PubMed]
- Stone, A.K.; Tanaka, T.; Nickerson, M.T. Protein quality and physicochemical properties of commercial cricket and mealworm powders. J. Food Sci. Technol. 2019, 56, 3355–3363. [Google Scholar] [CrossRef]
- Ndiritu, A.K.; Kinyuru, J.N.; Gichuhi, P.N.; Kenji, G.M. Effects of NaCl and pH on the functional properties of edible crickets (Acheta domesticus) protein concentrate. J. Food Meas. Charact. 2019, 13, 1788–1796. [Google Scholar] [CrossRef]
- AOAC—Association of Official Analytical Chemists. Official Methods of Analysis of AOAC International, 19th ed.; AOAC: Washington, DC, USA, 2012; pp. 121–130. [Google Scholar]
- Janssen, R.H.; Vincken, J.P.; van den Broek, L.A.; Fogliano, V.; Lakemond, C.M. Nitrogen-to-protein conversion factors for three edible insects: Tenebrio molitor, Alphitobius diaperinus, and Hermetia illucens. J. Agric. Food Chem. 2017, 65, 2275–2278. [Google Scholar] [CrossRef]
- Vilcacundo, R.; Barrio, D.; Carpio, C.; García-Ruiz, A.; Rúales, J.; Hernández-Ledesma, B.; Carrillo, W. Digestibility of quinoa (Chenopodium quinoa Willd) protein concentrate and its potential to inhibit lipid peroxidation in the Zebrafish larvae model. Plant Foods Hum. Nutr. 2017, 72, 294–300. [Google Scholar] [CrossRef]
- Pazmiño, A.; Vásquez, G.; Carrillo, W. Pigeon pea protein concentrate (Cajanus cajan) seeds grown in Ecuador functional properties. Asian J. Pharm. Clin. Res. 2018, 11, 430–435. [Google Scholar]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardized static in vitro digestion method suitable for food an international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piñuel, L.; Vilcacundo, E.; Boeri, P.; Barrio, D.A.; Morales, D.; Pinto, A.; Moran, R.; Samaniego, I.; Carrillo, W. Extraction of protein concentrate from red bean (Phaseolus vulgaris L.): Antioxidant activity and inhibition of lipid peroxidation. J. Appl. Pharm. Sci. 2019, 9, 45–58. [Google Scholar]
- Samaniego, I.; Espin, S.; Cuesta, X.; Arias, V.; Rubio, A.; Llerena, W.; Angos, I.; Carrillo, W. Analysis of environmental conditions effect in the phytochemical composition of potato (Solanum tuberosum) cultivars. Plants 2020, 9, 815. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, V.; Zhong, B.; Nawaz, M.A.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A. Screening of phenolic compounds in Australian grown berries by LC-ESI-QTOF-MS/MS and determination of their antioxidant potential. Antioxidants 2020, 10, 26. [Google Scholar] [CrossRef] [PubMed]
- Boeri, P.; Piñuel, L.; Dalzotto, D.; Monasterio, R.; Fontana, A.; Sharry, S.; Bario, D.A.; Carrillo, W. Argentine Patagonia barberry chemical composition and evaluation of its antioxidant capacity. J. Food Biochem. 2020, 44, e13254. [Google Scholar] [CrossRef] [PubMed]
- Piñuel, L.; Boeri, P.; Zubillaga, F.; Barrio, D.A.; Torreta, J.; Cruz, A.; Vasquez, G.; Pinto, A.; Carrillo, W. Production of white, red and black quinoa (Chenopodium quinoa Willd Var. Real) protein isolates and its hydrolysates in germinated and non-germinated quinoa samples and antioxidant activity evaluation. Plants 2019, 8, 257. [Google Scholar] [CrossRef] [Green Version]
- Bouhlali, E.; Sellam, K.; Bammou, M.; Alem, C.; Filali-Zehzouti, Y. In vitro Antioxidant and anti-inflammatory properties of selected Moroccan medicinal plants. J. Appl. Pharm. Sci. 2016, 6, 156–162. [Google Scholar] [CrossRef] [Green Version]
- Calzada Luna, G.; Martin-Gonzalez, F.S.; Mauer, L.J.; Liceaga, A.M. Cricket (Acheta domesticus) protein hydrolysates’ impact on the physicochemical, structural and sensory properties of tortillas and tortilla chips. J. Insects Food Feed 2021, 7, 109–120. [Google Scholar] [CrossRef]
- Adámková, A.; Mlček, J.; Kouřimská, L.; Borkovcová, M.; Bušina, T.; Adámek, M.; Bednářová, M.; Krajsa, J. Nutritional potential of selected insect species reared on the island of Sumatra. Int. J. Environ. Res. Public Health 2017, 14, 521. [Google Scholar] [CrossRef] [Green Version]
- Laroche, M.; Perreault, V.; Marciniak, A.; Gravel, A.; Chamberland, J.; Doyen, A. Comparison of conventional and sustainable lipid extraction methods for the production of oil and protein isolate from edible insect meal. Foods 2019, 8, 572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nongonierma, A.B.; Lamoureux, C.; FitzGerald, R.J. Generation of dipeptidyl peptidase IV (DPP-IV) inhibitory peptides during the enzymatic hydrolysis of tropical banded cricket (Gryllodes sigillatus) proteins. Food Funct. 2018, 9, 407–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foo, C.; Bini, E.; Hensman, J.; Knight, D.P.; Lewis, R.V.; Kaplan, D.L. Role of pH and charge on silk protein assembly in insects and spiders. Appl. Phys. A 2006, 82, 223–233. [Google Scholar] [CrossRef]
- Omotoso, O.T.; Adedire, C.O. Nutrient composition, mineral content and the solubility of the proteins of palm weevil, Rhynchophorus phoenicis f. (Coleoptera: Curculionidae). J. Zhejiang Univ. Sci. B 2007, 8, 318–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omotoso, O.T. Nutritional quality, functional properties and anti-nutrient compositions of the larva of Cirina forda (Westwood) (Lepidoptera: Saturniidae). J. Zhejiang Univ. Sci. B 2006, 7, 51–55. [Google Scholar] [CrossRef] [Green Version]
- Hall, F.G.; Jones, O.G.; O’Haire, M.E.; Liceaga, A.M. Functional properties of tropical banded cricket (Gryllodes sigillatus) protein hydrolysates. Food Chem. 2017, 224, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Hall, F.; Liceaga, A. Effect of microwave-assisted enzymatic hydrolysis of cricket (Gryllodes sigillatus) protein on ACE and DPP-IV inhibition and tropomyosin-IgG binding. J. Funct. Foods 2020, 64, 103634. [Google Scholar] [CrossRef]
- Andersen, S.O.; Rafn, K.; Krogh, T.N.; Hojrup, P.; Roepstorff, P. Comparison of larval and pupal cuticular proteins in Tenebrio molitor. Insect Biochem. Mol. Biol. 1992, 25, 177–187. [Google Scholar] [CrossRef]
- Elpidina, E.N.; Tsybina, T.A.; Dunaevsky, Y.E.; Belozersky, M.A.; Zhuzhikov, D.P.; Oppert, B. A chymotrypsin-like proteinase from the midgut of Tenebrio molitor larvae. Biochimie 2005, 87, 771–779. [Google Scholar] [CrossRef]
- Cho, M.Y.; Choi, H.W.; Moon, G.Y.; Kim, M.H.; Kwon, T.H.; Homma, K.; Natori, S.; Lee, B.L. An 86 kDa diapause protein 1-like protein is a component of early-staged encapsulation-relating proteins in coleopteran insect, Tenebrio molitor larvae. FEBS Lett. 1999, 451, 303–307. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, A.H.P.; Marana, S.R.; Terra, W.R.; Clélia, F. Purification molecular cloning, and properties of a β-glycosidase isolated from midgut lumen of Tenebrio molitor (Coleoptera) larvae. Insect Biochem. Mol. Biol. 2001, 31, 1065–1076. [Google Scholar] [CrossRef]
- Prabhakar, S.; Chen, M.S.; Elpidina, E.N.; Vinokurov, K.S.; Smith, C.M.; Marshall, J.; Oppert, B. Sequence analysis and molecular characterization of larval midgut cDNA transcripts encoding peptidases from the yellow mealworm, Tenebrio molitor L. Insect Mol. Biol. 2007, 16, 455–468. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Söderhäll, I.; Park, J.W.; Ma, Y.G.; Osaki, T.; Ha, N.C.; Wu, C.F.; Söderhäll, K.; Lee, B.L. A novel 43-kDa protein as a negative regulatory component of phenol oxidase induced melanin synthesis. J. Biol. Chem. 2005, 280, 24744–24751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.M.; Lee, K.Y.; Choi, H.W.; Cho, M.Y.; Kwon, T.H.; Kawabata, S.I.; Lee, B.L. Activated phenol oxidase from Tenebrio molitor larvae enhances the synthesis of melanin by using a vitellogenin-like protein in the presence of dopamine. Eur. J. Biochem. 2000, 267, 3695–3703. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kim, Y.; Han, J.S. Antioxidant activities and nutritional components of cricket (Gryllus bimaculatus) powder and protein extract. Asian J. Beauty Cosmetol. 2020, 18, 163–172. [Google Scholar] [CrossRef]
- Musundire, R. Bio-active compounds composition in edible stinkbugs consumed in South-Eastern districts of Zimbabwe. Int. J. Biol. 2014, 6, 36–45. [Google Scholar] [CrossRef]
- Musundire, R.; Zvidzai, C.J.; Chidewe, C.; Samende, B.K.; Manditsera, F.A. Nutrient and anti-nutrient composition of Henicus whellani (Orthoptera: Stenopelmatidae), an edible ground cricket, in south-eastern Zimbabwe. Int. J. Trop. Insect Sci. 2014, 34, 223–231. [Google Scholar] [CrossRef]
- Del Hierro, J.N.; Gutiérrez-Docio, A.; Otero, P.; Reglero, G.; Martin, D. Characterization, antioxidant activity, and inhibitory effect on pancreatic lipase of extracts from the edible insects Acheta domesticus and Tenebrio molitor. Food Chem. 2020, 309, 125742. [Google Scholar] [CrossRef]
- De Matos, F.M.; Novelli, P.K.; de Castro, R.J.S. Enzymatic hydrolysis of black cricket (Gryllus assimilis) proteins positively affects their antioxidant properties. J. Food Sci. 2021, 86, 571–578. [Google Scholar] [CrossRef]
- Nino, M.C.; Reddivari, L.; Osorio, C.; Kaplan, I.; Liceaga, A.M. Insects as a source of phenolic compounds and potential health benefits. J. Insects Food Feed 2021, 7, 1077–1087. [Google Scholar] [CrossRef]
- Nino, M.C.; Reddivari, L.; Ferruzzi, M.G.; Liceaga, A.M. Targeted Phenolic Characterization and Antioxidant Bioactivity of Extracts from Edible Acheta domesticus. Foods 2021, 10, 2295. [Google Scholar] [CrossRef] [PubMed]
- Burghardt, F.; Fiedlert, K.; Proksch, P. Uptake of flavonoids from Vicia villosa (Fabaceae) by the lycaenid butterfly, Polyommatus icarus (Lepidoptera: Lycaenidae). Biochem. Syst. Ecol. 1997, 25, 527–536. [Google Scholar] [CrossRef]
- Wiesen, B.; Krug, E.; Fiedler, K.; Wray, V.; Proksch, P. Sequestration of host-plant-derived flavonoids by lycaenid butterfly Polyommatus icarus. J. Chem. Ecol. 1994, 20, 2523–2538. [Google Scholar] [CrossRef] [PubMed]
- Schittko, U.; Burghardt, F.; Fiedler, K.; Wray, V.; Proksch, P. Sequestration and distribution of flavonoids in the common blue butterfly Polyommatus icarus reared on Trifolium repens. Phytochemistry 1999, 51, 609–614. [Google Scholar] [CrossRef]
- Geuder, M.; Wray, V.; Fiedler, K.; Proksch, P. Sequestration and metabolism of host-plant flavonoids by the lycaenid butterfly Polyommatus bellargus. J. Chem. Ecol. 1997, 23, 1361–1372. [Google Scholar] [CrossRef]
- Ferreres, F.; Valentão, P.; Pereira, J.A.; Bento, A.; Noites, A.; Seabra, R.M.; Andrade, P.B. HPLC-DAD-MS/MS-ESI screening of phenolic compounds in Pieris brassicae L. reared on Brassica rapa var. rapa L. J. Agric. Food Chem. 2008, 56, 844–853. [Google Scholar]
- Singh, P.; Kumar, R.; Sabapathy, S.N.; Bawa, A.S. Functional and edible uses of soy protein products. Compr. Rev. Food Sci. Food Saf. 2008, 7, 14–28. [Google Scholar] [CrossRef]
- Jooyandeh, H. Soy products as healthy and functional foods. Middle East J. Sci. Res. 2011, 7, 71–80. [Google Scholar]

| Assay | Cricket | Quinoa |
|---|---|---|
| Protein | 45.75 ± 2.25 | 13.63 ± 1.75 |
| Fat | 20.00 ± 1.94 | 4.20 ± 0.22 |
| Fiber | 5.01 ± 0.07 | 0.76 ± 0.81 |
| Ashes | 4.94 ± 0.18 | 2.08 ± 0.14 |
| Moisture | 3.50 ± 0.05 | 10.02 ± 1.30 |
| Carbohydrates | 20.80 ± 0.06 | 69.31 ± 0.07 |
| CPC | % Yield for 20 g Sample | % Protein |
|---|---|---|
| 10.0–3.0 | 33.92 ± 1.53 b | 66.86 ± 0.68 b,c |
| 10.0–4.0 | 36.18 ± 2.68 b | 69.37 ± 0.92 d,e,f |
| 10.0–5.0 | 32.28 ± 6.05 b | 69.10 ± 0.59 d,e |
| 10.0–6.0 | 19.07 ± 4.90 a | 65.74 ± 0.20 b,c |
| 12.0–3.0 | 67.05 ± 3.32 c | 68.06 ± 0.17 c,d |
| 12.0–4.0 | 72.75 ± 6.18 c | 70.80 ± 1.19 e,f |
| 12.0–5.0 | 67.77 ± 2.39 c | 71.16 ± 0.51 f |
| 12.0–6.0 | 11.56 ± 4.90 a | 63.60 ± 0.40 a |
| CPC | % Water-Binding Capacity (WBC) | % Oil-Binding Capacity (OBC) |
|---|---|---|
| 10.0–3.0 | 29.76 ± 0.49 b | 67.55 ± 2.28 d |
| 10.0–4.0 | 25.11 ± 0.95 a,b | 51.70 ± 1.62 b |
| 10.0–5.0 | 25.29 ± 3.23 a,b | 51.90 ± 1.72 b |
| 10.0–6.0 | 23.40 ± 1.87 a | 27.10 ± 0.09 a |
| 12.0–3.0 | 41.25 ± 0.98 c | 59.03 ± 1.83 c |
| 12.0–4.0 | 28.92 ± 1.58 a,b | 56.67 ± 2.57 b,c |
| 12.0–5.0 | 28.18 ± 3.73 a,b | 59.36 ± 2.04 c |
| 12.0–6.0 | 27.66 ± 0.28 a,b | 72.93 ± 2.04 d |
| Cricket flour | 29.76 ± 0.49 b | 67.55 ± 2.28 d |
| CPC. | TPC mg GAE/100 g Sample | FRAP umol TE/g CPC | ABTS umol TE/g CPC | DPPH umol TE/g CPC |
|---|---|---|---|---|
| 10.0–3.0 | 972 ± 21.54 c | 65154 ± 863 d | 100336 ± 3665 f | 68009 ± 1979 c |
| 10.0–4.0 | 909 ± 43.00 c | 70034 ± 2586 e | 85533 ± 1015 e | 63913 ± 2615 c |
| 10.0–5.0 | 606 ± 21.54 b | 39825 ± 431 c | 61351 ± 508 b | 27446 ± 5942 a |
| 10.0–6.0 | 557 ± 0.00 a | 39776 ± 1790 c | 58059 ± 0.00 a | 21699 ± 7112 a |
| 12.0–3.0 | 924 ± 21.48 c | 31252 ± 745 a,b | 68487 ± 507 c | 22233 ± 1710 a |
| 12.0–4.0 | 969 ± 56.81 c | 34219 ± 745 b | 69038 ± 506 c | 31908 ± 3558 a |
| 12.0–5.0 | 940 ± 37.26 c | 28062 ± 431 a | 63605 ± 1759 b | 31401 ± 2616 a |
| 12.0–6.0 | 1546 ± 38.49 d | 35063 ± 1543 b | 124300 ± 1817 g | 46992 ± 1769 b |
| Cricket flour | 942 ± 37.33 c | 35603 ± 1230 b | 34897 ± 881 h | 69020 ± 990 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quinteros, M.F.; Martínez, J.; Barrionuevo, A.; Rojas, M.; Carrillo, W. Functional, Antioxidant, and Anti-Inflammatory Properties of Cricket Protein Concentrate (Gryllus assimilis). Biology 2022, 11, 776. https://doi.org/10.3390/biology11050776
Quinteros MF, Martínez J, Barrionuevo A, Rojas M, Carrillo W. Functional, Antioxidant, and Anti-Inflammatory Properties of Cricket Protein Concentrate (Gryllus assimilis). Biology. 2022; 11(5):776. https://doi.org/10.3390/biology11050776
Chicago/Turabian StyleQuinteros, María Fernanda, Jenny Martínez, Alejandra Barrionuevo, Marcelo Rojas, and Wilman Carrillo. 2022. "Functional, Antioxidant, and Anti-Inflammatory Properties of Cricket Protein Concentrate (Gryllus assimilis)" Biology 11, no. 5: 776. https://doi.org/10.3390/biology11050776
APA StyleQuinteros, M. F., Martínez, J., Barrionuevo, A., Rojas, M., & Carrillo, W. (2022). Functional, Antioxidant, and Anti-Inflammatory Properties of Cricket Protein Concentrate (Gryllus assimilis). Biology, 11(5), 776. https://doi.org/10.3390/biology11050776







