Abstract
Plant-based residues generated within the agri-food system represent an abundant resource with significant potential for sustainable valorization. However, they are still underutilized and place a substantial burden on the environment and climate. This review discusses research trends over the past decade, combining bibliometric analysis with an overview of emerging technologies applied to the processing of residues generated from conventional crops and medicinal and aromatic plants. The bibliometric analysis reveals main valorization pathways, ranging from energy production to recovery of high-value bioactive compounds. Recent advances in this field are discussed in detail, with emphasis on low-energy and non-thermal processing (ultrasound, microwave, cold plasma), green solvents (natural deep eutectic solvents, bio-based solvents), biological pretreatments (with ligninolytic microorganisms and enzymes), thermochemical technologies (hydrothermal carbonization, pyrolysis), and emerging cascade strategies applied for multi-product recovery. Published research proves that these approaches have a great potential for sustainable valorization, while process optimization and economic feasibility remain a challenge at industrial scales for wider adoption. By providing an integrated perspective on diverse types of plant-based residues, this review highlights the importance of developing cascade and circular processing strategies, which align with global sustainability goals and encourage innovation in bio-based industries. New knowledge and advances in this field are highly required and will further help the transition of the current agri-food system towards greater circularity and sustainability.
1. Introduction
The agri-food sector is essential for producing sufficient food, fiber, and related agricultural products for the growing human population, while it also places a substantial burden on the environment and climate through the loss of biodiversity, water overuse, and greenhouse gas (GHG) emissions. The global agri-food system is expanding significantly in response to increasing demand for food, driven by the continuous growth of the world’s population, which is projected to exceed 9 billion by 2050. To satisfy the growing nutritional needs, it has been estimated that global food production must increase by 70% [1]. Currently, one-third of all anthropogenic GHG emissions originates from food and agricultural activities across the entire value chain [2]. In recent decades, emissions from crop residues and food waste have increased significantly, which is directly linked to the expansion of production capacity and ineffective or unavailable residue and waste management strategies. Many agricultural residues and waste streams are still discarded in open dumps, left in the field, or openly burned, becoming non-point sources of air pollution and GHG emissions. In 2022, emissions from the decomposition and burning of crop residues, along with the disposal of agri-food waste, amounted to 1.51 Gt CO2 equivalent [3], representing 2.6% of total global greenhouse gas emissions [4].
The global annual production of residues from major crops, such as barley, maize, rice, soybean, sugarcane, and wheat, was estimated at 3.7 Gt of dry matter [5]. At larger geographical scales, the availability of agricultural residues is usually estimated using standard residue-to-product ratios that approximate how much residue is produced in relation to the main product of the crop [6]. For example, the ratio of straw to rice grain (paddy) varies between 0.7 and 1.4, depending on cultivation conditions and specific rice variety [7], indicating that for each kg of paddy harvested, 0.7 kg to 1.4 kg of straw remains as residue. Similarly, harvesting and processing of each kg of sugarcane generate approximately 0.27–0.28 kg of tops and leaves, which are frequently burned in the field, while bagasse accounts for 0.28–0.3 kg, representing a substantial secondary biomass stream [8]. Such volumes of crop residues, together with current management practices, not only impose environmental and economic burdens but also waste valuable biomass that could be valorized into a wide range of products within a circular bioeconomy. This has emerged as an important research topic over the past decade.
Besides residues from conventional crops, including food, fiber, forage, and industrial plants, valorization research also focuses on residues derived from medicinal and aromatic plants (MAPs). Production of MAPs is increasingly integrated into the agri-food system through their expanded use in functional foods, pharmaceuticals, botanical extracts, and other herbal products. They are also considered industrial crops, since their products can be used across multiple industrial sectors beyond food and agriculture [9]. When cultivated as agricultural crops, their production and processing generate residues and waste streams (e.g., stems, leaves, roots, extraction by-products), which are comparable to those from conventional crops and must be managed sustainably.
Data on waste generation and residue availability in the global MAPs sector are scarce and vary widely. It is estimated that processing of aromatic plants alone generates around 20 Mt of dry biomass annually [10]. The estimates of residue availability within the MAPs sector differ because a substantial part of MAPs are harvested from the wild and therefore are not systematically reported in national statistics. In countries that traditionally rely on herbal medicine, such as China and India, the quantities of residual biomass derived from MAPs are exceptionally high. For example, traditional Chinese medicine relies on over 11,000 plant species [11], generating an estimated 35 to even 70 Mt [12] of herbal processing residues annually. Such large amounts of residual biomass often result from the utilization of only small portions of MAPs, typically valorized as extracts or even specific compounds. For example, in the case of the isabgol plant, only the seed and husk are commercially used for fiber recovery, while >95% of the total biomass is considered to be waste [13].
This is particularly evident for aromatic plants used in the production of essential oils via steam or hydrodistillation. Hydrodistillation of spike lavender results in essential oil yields of 1.75–4.58% (dry weight basis) [14], meaning that approximately 95–98% of the harvested biomass is left as processing residue.
Figure 1 presents the approximate residue generation potential across major crop categories, with the global production of corresponding crops. The residue production potential for each crop category was calculated based on the average residue-to-product ratio for specific crops belonging to each category, which were found in the literature [15,16,17,18]. The data on global crop production for each crop category were obtained from the FAOSTAT database. All data used for this visualization can be found in Table S1.
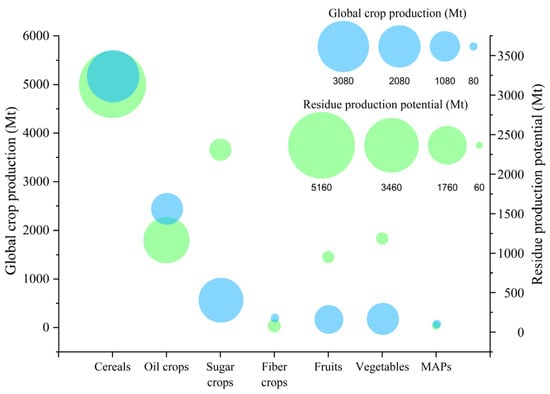
Figure 1.
Approximate estimates of residue production across major crop categories based on residue-to-product ratio and global crop production. Circle sizes are scaled proportionally to residue production potential and global crop production (according to FAOSTAT 2023), with a uniform scaling factor applied for visualization purposes. Estimates for MAPs are based only on available FAOSTAT subcategories (e.g., cinnamon, peppermint, tea leaves, etc.).
Emerging trends in the agri-food system, including the global shift towards plant-based diets, are driving an increase in plant production [19], resulting in larger amounts of residues and by-products generated during plant harvesting and processing. The valorization of such side streams is closely aligned with the global sustainability agendas and is recognized as a strategy to minimize resource losses, while simultaneously supporting innovation in bio-based industries and contributing to more sustainable agri-food systems [20]. This review aims to map the research trends of the past decade, focusing on valorization of plant-based residues and processing technologies, identifying strategies and their potential role in moving the current agri-food system towards greater circularity and sustainability. In addition, by merging conventional crop residues with those derived from MAPs into a single framework, this review provides an integrated perspective on valorization pathways that is often missing from studies focused only on conventional crops or mixed plant–animal waste. This approach brings an overview of how knowledge on valorization of plant-based residues has evolved, highlights synergies across different research areas, and discovers the gaps that require further attention to support the transition towards sustainable agri-food system.
2. Bibliometric Analysis of Plant-Based Residues from the Agri-Food Sector
In this review, terms plant-based residues, waste, and by-products refer to biomass of plant origin, including primary residues left in the field as harvesting waste (e.g., straw, leaves, stalks) and secondary residues generated during processing of food, fiber, industrial, and forage crops, as well as medicinal and aromatic plants (e.g., peels, cobs, husks). For clarity and consistency, these categories will be uniformly referred to as plant-based residues throughout this review. Animal-derived residues and waste are beyond the scope of this research. The bibliometric analysis was performed to gain knowledge on the research progress in processing and valorization of plant-based residues and to identify the main researched areas and countries leading research efforts on this topic. The analysis was conducted by searching the Scopus database, selecting research articles in English published from 2015 to 2025, and identifying terms that appeared in the title, abstract, or keywords. The search included combinations of terms related to residues, waste, and by-products of crops, fruits, vegetables, herbs, medicinal and aromatic plants (e.g., “crop residue”, “fruit waste”, “vegetable waste”, “herbal by-product”, “aromatic plant residue”, “herbal dust”, etc.). The exact search string applied in Scopus is provided in the Supplementary Information. The total number of selected publications was 13178. The network of all keywords extracted from the articles published within the search timeframe was visualized using VOSviewer software (version 1.6.20).
The lack of standardized terminology in scientific writing usually poses a huge challenge for bibliometric analyses. Variations in the terms used to describe different types of plant-based residues and the lack of precise classification can affect the identification and quantification of relevant publications in this field. To address this challenge, different terms related to residues of plant origin and their combinations were included in the search string, ensuring that this review covers all relevant articles in the field. Figure 2 illustrates the progress of research related to different categories of plant-based residues over the past decade. Crop residues were the most prevalent in the literature, with a consistently high number of publications each year, reaching a maximum around 2019 and 2021, followed by a slight decline. This may indicate both the global scale of crop production and availability of plant-based residues, and also the importance of the research subject and the necessity for development of sustainable management and valorization strategies.
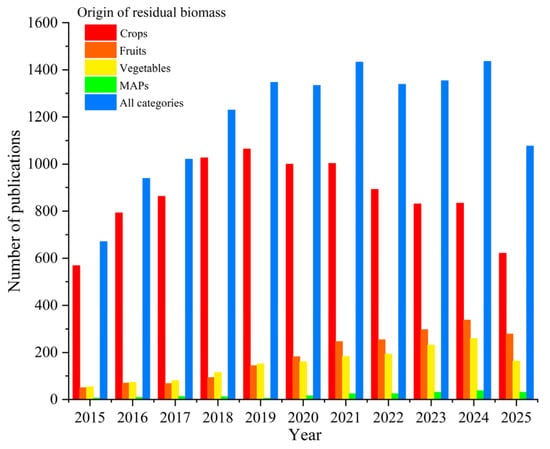
Figure 2.
Progress of research on different categories of plant-based residues over the past decade, based on a keyword search in the Scopus database.
The number of publications addressing fruit and vegetable side streams has gradually increased over the past decade, reflecting growing interest in their utilization as sources of bioactive compounds. However, it can be seen that compared to other categories, research on residual biomass derived from MAPs remains significantly underrepresented. This gap highlights a critical knowledge deficit, despite the pharmaceutical, economic, and cultural value of MAPs and substantial amounts of side streams within the MAPs sector due to low biomass utilization rates (Figure 1). Generally, the overall growth trend in all categories of plant-based residues indicates the rising attention of the global scientific community to circular bioeconomy strategies, resource recovery, and sustainability within the agri-food system. In addition, disparity between categories of plant-based residues demonstrates where research has concentrated (crops) and where opportunities for further investigation exist (particularly MAPs). According to the Scopus database, the five top countries dominating this research topic are China, India, the United States, Brazil, and Italy (Figure 3).
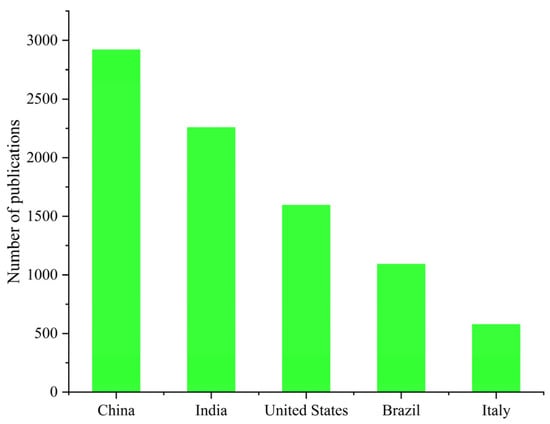
Figure 3.
Top-performing countries in research on plant-based residues over the past decade, based on a keyword search in the Scopus database.
The publications dealing with plant-based residues are classified into research fields, as shown in Figure 4. The dominant fields were agricultural and biological sciences, environmental science, biochemistry, genetics and molecular biology, energy, and chemical engineering. This distribution reflects the growing need to develop sustainable management strategies and processing technologies for plant-based residues and highlights the importance of implementing circular bioeconomy practices in the agri-food system.
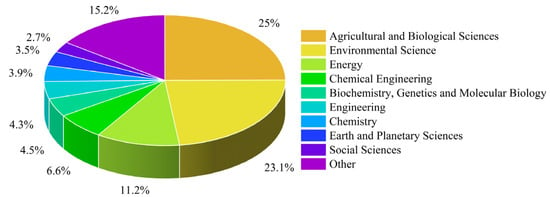
Figure 4.
Research fields dealing with plant-based residues, based on a keyword search in the Scopus database.
Published research available in Scopus and other databases covers different aspects of plant-based residues, their production, environmental impact, processing, valorization, etc. Figure 5 displays the analysis of the most prevalent keywords connected with research articles published during the past 10 years. The keyword co-occurrence network highlights the main research clusters and interconnections in the field. Distinct clusters indicated by different colors correspond to specific research topics. The red cluster includes keywords related to conservation agriculture and soil management, reflecting the importance of agronomic practices and environmental sustainability. The yellow cluster covers the studies on biochar and adsorption technologies and indicates an applied research focus on transforming plant-based residues into sorbent materials for pollution control. The blue cluster covers the studies related to biorefinery processes and lignocellulosic valorization, including the conversion of plant-based residues into biofuels and biochemicals. The green cluster embraces the keywords referring to anaerobic digestion and strategies for bioenergy production. The purple cluster is oriented to the extraction of polyphenols and other bioactive compounds, reflecting a growing interest in using plant-based residues as a source of high-value compounds for food, pharmaceutical, cosmetic, and other applications. Overall, the keyword co-occurrence network shows two research directions. One reflects agronomic and environmental management of plant-based residues, and the other valorization pathways, ranging from energy production to the extraction of bioactive compounds. The connections between clusters indicate a shift from considering the management of residues and waste streams only as a disposal problem to integrating them into bioeconomy strategies and sustainability goals. In this context, residual biomass and waste are seen as resources for obtaining new products while simultaneously reducing environmental impacts.
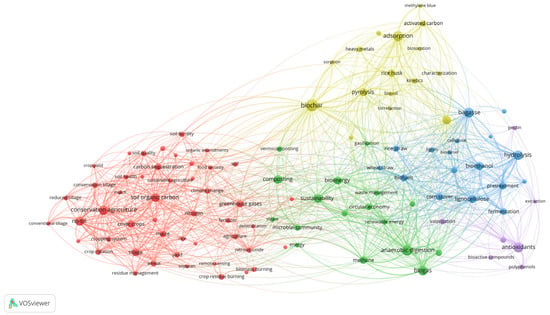
Figure 5.
Co-occurrence network map of keywords from research articles published between 2015 and 2025.
The evolution of research topics focused on plant-based residues over the past decade is shown in Figure 6. The purple cluster, referring to the extraction of bioactive compounds, though comparatively small, has recently gained increasing attention from the scientific community, indicating current research priorities. This is followed by the yellow cluster, which reflects growing interest in biochar and adsorption technologies, and the green cluster, focusing on anaerobic digestion and bioenergy production. In contrast, studies within the blue cluster, addressing biorefinery processes and the production of biochemicals and biofuels, appear as more established and pioneering areas of research in the valorization of plant-based residues. The most prominent valorization strategies and processing technologies that emerged from the clustering in bibliometric map (Figure 5) are discussed in more detail in the next sections.
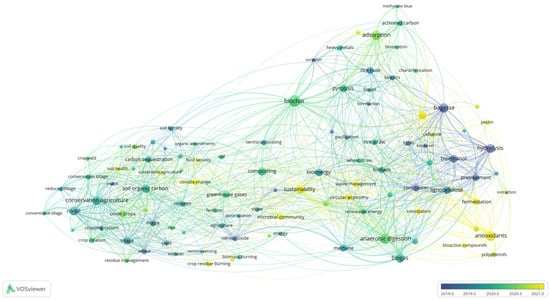
Figure 6.
Timeline network map of keywords from research articles published between 2015 and 2025.
3. Biorefinery Strategies and Processing Technologies for the Valorization of Plant-Based Residues
3.1. Low-Energy and Non-Thermal Technologies for Biomass Fractionation
Low-energy and non-thermal technologies, such as ultrasound, microwave, and cold plasma, have been increasingly studied for the processing and valorization of different agri-food residues. Applied alone or in combination with other methods, they demonstrate strong potential in this field. These technologies provide energy-efficient and environmentally friendly alternatives to conventional chemical and thermal methods. They enable process intensification by improving yields and product characteristics, shortening processing time, preserving the biological activity of bioactive compounds, and overall supporting the development of more sustainable biorefinery approaches [21,22,23]. In this section, the application of low-energy technologies is analyzed from the perspective of biomass fractionation, corresponding to the blue cluster in the keyword co-occurrence network (Figure 5). Additionally, low-energy processing such as ultrasound and microwave for the recovery of bioactive compounds, related to the purple cluster, is extensively discussed in Section 3.2.
Ultrasound-assisted processes are based on implementing sound waves with frequencies within a range of 10–100 kHz, which produce cavitation zones. The cavitation zones resemble bubbles that grow until they reach a critical size and burst. This bursting increases the temperature and pressure locally, from 500 to 15,000 K and 100 to 5000 atm, respectively [24]. Although lasting for a relatively short time, these harsh conditions can cause severe deformation of cell walls, inducing morphological and structural changes and enhancing the extraction of bioactive compounds from the cells [25]. Another important effect of ultrasound is the formation of oxidizing radicals of H· and OH· due to the decomposition of water molecules, which can further contribute to the breakdown of complex biomaterials and facilitate the release of valuable compounds. These radicals are highly reactive and, therefore, can induce glycosidic bond cleavage, causing ruptures in complex lignocellulose structure [26]. Moreover, ultrasound causes lignin detachment from the carbohydrate part of lignocellulose and its further disintegration by disrupting α-O-4 and β-O-4 linkages in lignin [22]. Some of the advantages of ultrasound processing are high activation energy, sufficient mass transfer, less solvent requirements, and the possibility of inducing a broad variety of reactions occurring in a shorter time [26]. Furthermore, applying ultrasound irradiation increases temperature and pressure locally, causing localized pasteurization. This allows for skipping conventional sterilization steps that are usually performed at elevated temperatures throughout the entire sample [27]. This is especially important to prevent sugar or thermosensitive polyphenols degradation, but also to reduce the overall cost of the process. These are all reasons why ultrasound-assisted processing has received considerable attention in the field of processing of plant-based residues over the last decades. Ma et al. [28] implemented ultrasound irradiation, followed by ternary deep eutectic solvents (DESs) to enhance the saccharification of corncob. DESs are mixtures of two or more components in specific molar ratios that interact through hydrogen bonding. Under specific conditions, such as mixing time and temperature, these interactions result in a homogeneous mixture with a melting point significantly lower than that of the individual components. Under optimal conditions using a DES composed of choline chloride (ChCl), glycerol, and lactic acid in a 1:1:2 ratio at 120 °C, 88% of lignin and 67% of hemicellulose were successfully removed. This process also allowed the recovery of 82% of cellulose and finally led to 62% and 27% higher glucose and xylose yields, respectively, compared to untreated corncob [28]. In addition, ultrasound can be applied for sugar extraction. Alyammahi et al. [29] optimized ultrasound-assisted sugar extraction from date fruit powder. They achieved a maximal carbohydrate extraction yield of 81% with 30 min extraction at 60 °C, with a liquid-to-solid ratio of 7.6 mL of water per g of date fruit powder [29]. Although ultrasound-assisted processing proved to be suitable for treating both lignocellulose and food waste, drawbacks regarding the process scaling up to the industrial level must be considered. Cano et al. [30] suggested that ultrasound implementation on an industrial scale can be feasible if commercial and patented technologies are implemented, such as Sonix (Sonico, UK), Biosonator (Ultrawaves, Germany), Sonolyzer (Ovivo), etc.
Microwave irradiation can also be used as a controlled, moderate heating technique to efficiently disrupt the recalcitrant lignocellulose structure. When applied, this irradiation induces an explosion of plant cells by evaporating the present moisture, which creates immense pressure on cell walls [31]. Additionally, Zhu et al. [32] confirmed that heating induced by microwave irradiation could cleave Cα–Cβ bonds for efficient degradation of lignin and lignin-like structures. Some of the factors affecting the overall efficiency of microwave pretreatment are the dielectric properties of the pretreated material, chemical composition of biomass and its size, pretreatment duration and heating rate, microwave power, moisture content, etc. [33]. To ensure enough moisture in the substrate before processing, microwave irradiation is often combined with different acids, alkalis, or organic solvents. Mladenović et al. [34] studied pretreatment of corncob using a microwave reactor and alkaline hydrogen peroxide, with reference to key process parameters, such as microwave power, pretreatment duration, hydrogen peroxide dosage, and biomass-to-alkali ratio. Under optimal conditions, around 75% of lignin was removed within a 1 min pretreatment. As a result, hexose and pentose yields obtained after enzymatic hydrolysis were 2.7- and 5.6-fold higher compared to the untreated corncob. In another study, a significant delignification rate of 82% was achieved when microwave-assisted alkaline hydrogen peroxide pretreatment was conducted for 2 min at 100 °C, whereas 70% of lignin was removed by applying conventional heating instead [35]. Besides alkalis, microwave irradiation could be combined with DESs. Gan et al. [36] performed microwave-assisted DES pretreatment of sugarcane bagasse at 135 °C for 20 min. When applying DESs composed of ChCl and lactic acid in a 1:13 molar ratio, up to 88% of lignin was removed, compared to the raw sugarcane bagasse, while 95% of cellulose was preserved. As a result, 70.6% of initially pretreated sugarcane bagasse was utilized through the co-production of paper, lignin microspheres, and furfural [36]. Microwave processing is considered as energy-efficient, cost-effective, and eco-friendly, allowing for uniform heating in a short reaction time with minimal generation of side products [21]. Yet, some difficulties must be overcome when shifting to industrial scale-up, such as high capital investment, high operating expenses at high loads and high pressure, as well as providing enough moisture within the biomass sample [22].
Cold plasma is an emerging non-thermal technology that produces reactive oxygen and/or nitrogen species, depending on the feed gas used to generate plasma. The underlying principle of cold plasma is based on using mostly electrical or electromagnetic fields to produce various ions, radicals, free electrons, and molecules in their basic or excited forms in the interaction of feed gas and media. The temperature of heavier species ranges from 300 to 1000 K, while electrons can reach up to several 10,000 K [37]. This non-equilibrium state and generated UV radiation efficiently induce diverse thermodynamically unfavorable physicochemical reactions in the pretreated sample. Moreover, the elevated temperatures of the generated species are localized, preventing heating of the entire sample. This is especially important for thermosensitive materials [23], such as those of biological origin, including plant-based residues. Depending on the desired outcome, cold plasma pretreatment can be performed as a dry or wet technique. Anari et al. [38] used dry dielectric barrier discharge (DBD) plasma pretreatment to extract micro- and nano-cellulose fibers from walnut shells. The process included three main steps: dewaxing, hemicellulose removal, and bleaching. Cold plasma was employed in different stages of the process. After the analysis of the obtained products, it was confirmed that plasma increased the cellulose purity, making it more suitable for further processing. Moreover, when implemented before the hemicellulose removal and bleaching process, plasma pretreatment contributed to the conversion of microfibrils to nanofibrils. Zhu et al. [39] applied a similar approach for isolating cellulose from sugarcane bagasse first and then subjecting it to DBD pretreatment. However, in this case, cellulose was mixed with water before DBD plasma pretreatment. Overall results of that study showed that DBD-pretreated cellulose had better dissolving capacity than pure cellulose, which was used as a control. On the other side, Grbić et al. [40,41] combined a cold plasma jet with an alkaline hydrogen peroxide and Fenton reagent for efficient corn stalk delignification. When applying combined cold plasma/Fenton pretreatment under acidic conditions, approximately 53% of lignin was removed after 30 min, resulting in almost doubled glucose content after enzymatic hydrolysis compared to the raw corn stalk [41]. However, up to 86% of lignin was removed after 10 min at 80 °C when alkaline hydrogen peroxide was used as a solvent. This notable delignification resulted in a 3.5 times higher conversion rate of the pretreated sample compared to the raw corn stalk [40]. Table 1 summarizes the main findings of recent studies on the application of ultrasound, microwave, and cold plasma processes in biomass fractionation.

Table 1.
Application of low-energy and non-thermal technologies in biomass fractionation.
Beyond its role in biomass decomposition, cold plasma also exhibits antimicrobial properties, making it a promising alternative to conventional sterilization methods. A 30 min cold plasma pretreatment of distillery stillage enabled performing open lactic acid fermentation, which resulted in significantly higher lactic acid yield (0.82 g/g) compared to ultrasound pretreatment (0.69 g/g) and conventional sterilization methods (0.66 g/g) [27]. Estimated energy requirements showed that cold plasma pretreatment was the most cost-effective compared with sterilization by autoclave or ultrasound. Since the cost of feed gas plays a significant role in the overall cost of the process [27], depending on the purpose, different gases can be used to improve economic viability.
Recent studies have shown that ultrasound, microwave, and cold plasma technologies are promising alternatives to conventional pretreatment and extraction methods. They provide advantages in process efficiency, reduced processing times, and better preservation of heat-sensitive compounds. Moreover, their potential to achieve higher product yields while reducing energy demand makes them highly promising for further development of sustainable valorization strategies for a wide range of plant-based residues. However, challenges related to scaling up, process standardization, and economic feasibility remain to be further addressed and proven for wider industrial implementation of these technologies.
3.2. Green Solvents for the Extraction of Bioactive Compounds from Plant-Based Residues
The extraction of bioactive compounds from plant-based residues is becoming crucial within the framework of sustainable management and waste minimization. The conventional extraction methodologies primarily rely on organic solvents, which are often toxic and flammable, and at the end of the process, they require specific management. However, green extraction processes have been developed based on the 12 principles of green chemistry and green engineering [42]. They aim to address specific goals, such as enhancing process efficiency, reducing energy consumption, promoting the use of renewable resources and alternative solvents, and ensuring a safe and high-quality products. Among the key principles reported by Chemat et al. [42], the second highlights the use of alternative solvents, primarily water, plant-derived solvents, or solvents produced sustainably. Choosing the right solvent is crucial for developing a green extraction process, directly affecting both sustainability and efficiency. Some examples of green solvents that have been used for extraction of bioactive compounds are natural deep eutectic solvents (NADESs), other bio-based solvents derived from renewable resources (e.g., glycerol, limonene), as well as supercritical fluids. Recently, the use of supercritical fluids for extracting bioactive compounds from different types of agri-food residues has been extensively reviewed [43,44,45]. The following section discusses recent advances in the field of green extraction of plant-based residues using NADESs and other solvents derived from renewable resources.
3.2.1. Natural Deep Eutectic Solvents (NADESs)
NADESs, as a type of DES, are composed of natural products, mainly primary metabolites such as sugars, amino acids, organic acids, polyols, etc., which act as hydrogen bond donors and acceptors. NADESs are characterized by low toxicity and biodegradability, while their extensive hydrogen bonding network provides excellent solubilization and stabilization capabilities for a wide variety of bioactive compounds, such as polyphenols, carbohydrates, proteins, and alkaloids [46,47,48]. Table 2 summarizes the main findings of recent studies on the application of NADESs for extracting bioactive compounds from plant-based residues.
ChCl is emerging as the most common compound of NADESs for the extraction of antioxidants from various plant-based residues. However, a deeper analysis of the literature reveals the importance of tunability of NADES properties for achieving high recovery yields of bioactive compounds from chemically complex biomass [49,50,51]. The selection of proper NADESs is very sensitive to compounds being extracted and the complex matrix of plant-based residues. Ozturk et al. [52] studied the extraction of polyphenolic compounds from orange peels using ChCl-based DESs and solid–liquid extraction, assessing the impact of solvent structure and operating parameters on extraction efficiency. Under optimal conditions (10 wt.% DES, 333.15 K, 1:10 solid/liquid ratio, 100 min), the ChCl/ethylene glycol (1:4) NADES provided an extract with the highest total phenolic content and antioxidant activity, demonstrating superiority compared to the hydroethanolic extracts [52]. The recovery of polyphenols from olive leaves and red grape pomace was also investigated using a food-grade ternary NADESs composed of glycerol, citric acid, and L-proline [53]. NADES-based extracts of red grape pomace showed higher antioxidant activity compared to aqueous and hydroethanolic extracts, while in the case of olive leaves, their performance was comparable to aqueous ethanol. Grisales-Mejía et al. [54] studied the use of NADESs for the recovery of phenolic compounds from Hass avocado residues (epicarp and seed). According to their findings, ChCl-based NADESs were the most effective extraction solvents, especially in the case of the avocado epicarp, which showed greater antioxidant capacity. Similarly, the extraction of anthocyanins and polyphenols from blueberry pomace using NADESs was investigated by Zhang et al. [55]. The NADESs composed of ChCl/1,4-butanediol demonstrated higher extraction efficiency and selectivity due to its low viscosity and broader polarity range compared with the other tested NADESs [55].
The extraction combining NADESs with microwave or ultrasound has been recognized as a promising strategy for process intensification, as it couples the green and tunable solvent properties of NADESs with the enhanced mass and heat transfer provided by these two methods. NADESs derived from ChCl and L(+)-tartaric acid were combined with ultrasound and microwave for the extraction of carotenoids from apricot waste, demonstrating significantly higher yields using either microwave or ultrasound compared with conventional extraction methods [56]. Similarly, the microwave-assisted extraction using NADESs was investigated for the recovery of bioactive compounds from olive oil processing waste [57]. All the extracts were characterized by performing HPLC analysis, and the results were found to be superior compared to those obtained using water as an extraction solvent. The development and optimization of the process for recovery of bioactive compounds from spent coffee grounds using NADESs combined with microwave and ultrasound was reported by Tzani et al. [58]. The optimum NADES for this process according to the total phenolic content and antioxidant activity of the extracts was found to be betaine/glycerol (1:3). That study demonstrated that betaine-based NADESs are more effective than those based on ChCl or traditional hydroethanolic solutions [58]. The recovery of anthocyanins from blueberry peels using sugar-based NADESs and microwave was studied by Alchera et al. [59], achieving significantly higher yields compared with conventional solvent extraction. A combined NADES and ultrasound-assisted extraction was also studied by De Lima et al. [60] for the extraction of phenolic compounds from the by-product of purple araçá (Psidium myrtoides). Among the studied NADESs, ChCl/glycerol (1:2) was found to be the most efficient extraction medium, yielding extracts with higher antioxidant and antidiabetic activity compared to a hydroethanolic solvent [60]. Hamieau et al. [61] used various NADESs with ultrasound-assisted extraction to recover antioxidants from buckwheat husk and chokeberry pomace. In that study, betaine/urea (1:1) was the most effective for chokeberry pomace extraction, while glucose/urea (1:1) with added water achieved the best yields from buckwheat husk [61].
This review discusses the potential of NADESs for resource recovery from plant-based residues; however, NADESs are also increasingly recognized as important in the food and pharmaceutical industries, which introduces additional regulatory considerations. Further information on the applications of bioactive compounds extracted using DESs in the food industry, as well as regulatory and safety aspects, can be found in the review of Mavai et al. [62].

Table 2.
Application of NADESs for the extraction of bioactive compounds from plant-based residues.
Table 2.
Application of NADESs for the extraction of bioactive compounds from plant-based residues.
| Biomass | Type of NADES | Target Compounds | Main Findings | Ref. |
|---|---|---|---|---|
| Orange peel waste | ChCl-based NADES composed of glycerol or ethylene glycol as hydrogen bond donors. | Polyphenols |
| [52] |
| Red grape pomace and olive leaves | Tailor-made ternary NADES composed of glycerol, citric acid, and L-proline | Polyphenols |
| [53] |
| Avocado residues | ChCl and betaine-based NADES paired with glycerol and organic acids/sugars | Phenolics |
| [54] |
| Blueberry pomace | ChCl-based NADES | Polyphenols, antho-cyanins |
| [55] |
| Apricot waste | ChCl/tartaric acid NADES | Phenolics, carotenoids |
| [56] |
| Olive oil processing waste | Sugar- and acid-based NADES | Phenolics |
| [57] |
| Spent coffee grounds | NADES composed of betaine/glycerol (1:3) (with water as a co-solvent) | Phenolic compounds and flavonoids |
| [58] |
| Blueberry peels | Sugar-based NADES | Polyphenols, anthocyanins |
| [59] |
| Purple araçá by-product | ChCl-based NADES | Phenolics |
| [60] |
| Herbs and agri-food wastes | Various NADESs | Phenolics |
| [61] |
3.2.2. Other Solvents Derived from Renewable Resources
Characteristic examples of solvents derived from renewable resources, which are also considered and recognized as more sustainable and environmentally friendly, are D-limonene and glycerol. Limonene is a monoterpene commonly found in the peel of citrus fruits and is considered a bio-based, sustainable, and non-toxic solvent. D-limonene is being widely used as a flavoring agent and cosmetic ingredient [63]. The use of D-limonene as an industrial solvent is not new. It dates back to the early 1990s, when it was used as a replacement for highly toxic trichloroethylene in microelectronics as a degreasing agent, and as a bioremediation aid in oil spill accidents. However, since then, D-limonene has been increasingly used in extraction processes as a green solvent [64]. Sahad et al. [65] used D-limonene as a green alternative to n-hexane for the extraction of residual crude palm oil from oil palm decanter cake. According to that study, both n-hexane and D-limonene were recovered completely after extraction; however, the recyclability of D-limonene was higher compared with n-hexane (90% vs. 70%). Overall, the fatty acid composition of the extracted residual crude palm oil remained mostly unchanged [65]. Similarly, Akretche-Kelfat et al. [66] used D-limonene for the extraction of vegetable oils from olive pomace, demonstrating its superiority compared to n-hexane as a conventional extraction solvent. A recent study by Xu et al. [67] reported the use of D-limonene as an alternative solvent for extracting the alkaloid nuciferine from lotus leaves, which can be considered a food processing by-product.
Glycerol, a byproduct of the biodiesel industry, is considered as a green non-conventional solvent and has been extensively explored for extraction of valuable compounds from plants; however, its use in the valorization of agri-food processing residues and by-products is less explored. Mourtzinos et al. [68] studied the recovery of polyphenols from olive leaves using a glycerol/water system along with 2-hydroxypropyl-β-cyclodextrin as an extraction enhancer. Similarly, Makris et al. [69] studied the optimization of polyphenol extraction from red grape pomace using aqueous glycerol/tartaric acid mixtures. The results revealed that 20% (w/v) glycerol in water was the most effective extraction mixture for obtaining polyphenols, flavonoids, and pigments from grape pomace, whereas tartaric acid had a negative impact on this process when tested at concentrations up to 2% (w/v) [69]. The extraction of polyphenols and anthocyanins from red grape pomace was also studied by Trasanidou et al. [70] using ultrasound-assisted extraction and glycerol/water as a solvent. Similarly, hot pressurized liquid extraction of grape pomace from the Carménère variety was studied using glycerol/water mixtures [71]. In that study, the highest recovery of phenolic acids, flavanols, and stilbenes was obtained at 150 °C with 50% glycerol, while the higher recovery of flavonols was achieved with 32.5% glycerol. An organosolv thermal extraction of polyphenols from orange peel waste using glycerol/water mixtures was studied by Abdoun et al. [72], showing that the highest yields were obtained with 90% (w/w) glycerol/water mixture. Recent studies on the applications of these two alternative green solvents for extracting bioactive compounds from plant-based residues, along with their main findings, are summarized in Table 3.

Table 3.
Application of green solvents for the extraction of bioactive compounds from plant-based residues.
Green solvents have proven their potential in the recovery of bioactive compounds from a variety of feedstocks and offer a more sustainable alternative to conventional solvents. Variability in the chemical composition of residues imposes a need for thorough optimization of solvent composition and the extraction process, sometimes even between batches of feedstock. This can be costly and time-consuming, hindering scale-up and wider adoption at the industrial scale. However, it also opens new opportunities for cascade and zero-waste processing, with a good hierarchy of recovered products as an important criterion, which is discussed more in Section 3.5.
3.3. Enzymatic and Microbial Processes for Lignin Degradation
Biological processes have emerged as another environmentally friendly alternative to conventional, energy-intensive technologies applied for biomass valorization. These processes involve microorganisms, commonly fungi and bacteria, or their enzymes, which act under mild conditions and show high substrate selectivity, enabling bioconversion of agri-food residues into diverse high-value products.
When processing lignocellulosic residues into valuable products, pretreatment is usually applied as an initial step, which should depolymerize or remove lignin and enable better accessibility of cellulose and hemicellulose in subsequent enzymatic hydrolysis. The use of microorganisms and enzymes for biomass hydrolysis has been extensively studied over the years, and the following section discusses only their potential to degrade lignin during the pretreatment of plant-based residues. Lignin degradation by fungi and bacteria, including lignin-degrading enzymes produced by various microbial species, has gained increasing attention [73,74,75,76,77]. White-rot fungi and their ligninolytic oxidative enzymes (including peroxidases and laccases) showed a great capability of lignin depolymerization and are considered the most efficient for biological treatment of lignocellulosic biomass. For example, a 30-day solid-state pretreatment of corn stover with several white-rot fungi significantly improved enzymatic hydrolysis, resulting in the highest sugar yield of 394 mg/g of pretreated stover [78]. However, in addition to lignin degradation, this pretreatment also resulted in carbohydrate loss [78]. To shorten pretreatment time and avoid carbohydrate degradation, pretreatment by directly applying ligninolytic enzymes is usually proposed as a possible strategy. In this context, a 2-day pretreatment of corn stover with crude peroxidases and laccase extracted from white-rot fungi Phanerochete chrysosporium and Coriolus versicolor increased the sugar yield by 50.2% [79]. Similarly, pretreatment of sugarcane tops by laccase produced from Pleurotus djamor was optimized, achieving maximum delignification of 79.1% in a 6 h pretreatment, which resulted in 3.3-fold higher sugar yield in subsequent enzymatic hydrolysis [80]. In addition to conventional aqueous systems applied for laccase-mediated lignin depolymerization, the use of laccase in green solvents such as DESs has emerged as a promising approach, which can improve lignin solubilization and product selectivity [81]. Despite challenges related to enzyme stability and process optimization, research in this area is rapidly advancing, and further insights into the laccase–DES interactions are expected in the near future.
Recent studies have also shown that delignification efficiency of lignocellulosic biomass can be further improved by the addition of certain inducers, which are essential for fungal metabolism or for stimulating the production and activity of ligninolytic enzymes. The study by Knežević et al. [82] demonstrated that delignification of wheat straw by different Trametes species can be significantly enhanced with the addition of low-molecular-weight inducers, such as p-anisidine and veratryl alcohol. These inducers intensified the production and activity of manganese peroxidases and laccases [82]. In another study, supplementing solid-state fermentation of wheat straw with calcium ions significantly increased activity of Trametes gibbosa manganese peroxidase and resulted in improved lignin degradation of this crop residue [83]. In addition to these approaches, using appropriate redox mediators with laccase, such as 4-hydroxybenzoic acid, significantly enhanced delignification of sugarcane bagasse and rice husks [84]. This mediator extended the oxidative capacity of laccases to the non-phenolic subunits of lignin, thus enabling higher sugar yields during hydrolysis [84].
Besides white-rot fungi, other fungal species were also shown to be promising in lignin degradation, while some species were capable of simultaneously producing laccase, lignin peroxidase, and manganese peroxidase. In the study by Su et al. [85], Myrothecium verrucaria simultaneously produced all three lignin-degrading enzymes during the pretreatment of corn stover. This led to substantial lignin removal (42.3%) and significantly increased the sugar conversion rate during saccharification [85]. Although fungal pretreatments have shown promising results in biomass delignification, their application at a biorefinery scale remains economically unfeasible. Significant improvements are still required to shorten pretreatment times, increase sugar yields and biomass density, and eliminate the need for sterilization [86].
Unlike fungi, bacteria usually have higher growth rates, show greater environmental tolerance, and can be easily modified using genetic engineering techniques, which makes them attractive candidates for industrial delignification. The recently isolated Bacillus amyloliquefaciens SL-7 strain has shown strong lignin-degrading ability, producing laccase, lignin peroxidase, and manganese peroxidase, and achieving almost 29% lignin removal from straw within 15 days [87]. Similarly, a 7-day pretreatment of rice straw with ligninolytic Bacillus strains, capable of producing lignin peroxidase and laccase, led to significant lignin degradation and structural modifications [88]. These changes resulted in increased biogas yields during anaerobic digestion [88]. The study by Chen et al. [89] showed that constructing a synthetic microbial community tailored for lignocellulose degradation can significantly enhance the enzymatic pretreatment of wheat straw, yielding cellulose, hemicellulose, and lignin degradation rates of 39.85, 36.99, and 19.21%, respectively, which ultimately improved biogas production. Stenotrophomonas sp. CFB-09 was also proved efficient in delignification of various plant-based residues, including coconut shell and husk, palm kernel shell, oil palm empty fruit bunch, and sawdust, showing the capability of producing thermotolerant ligninolytic enzymes [86]. Applying a 9-day pretreatment at 80 °C and pH 8.0, this strain achieved lignin degradation of 41–55% [90], demonstrating the potential for biorefinery applications.
Recent studies have shown that combining biological and chemical pretreatments can further intensify lignocellulose degradation and enhance sugar yields, proving the superiority of combined pretreatments to single approaches. Thus, acid and alkali treatments were combined with ligninolytic (Pandoraea sp. B-6) and saccharolytic (Acinetobacter sp. B-2) bacterial strains, which effectively reduced lignin content and enhanced sugar yields by 40.9% and 31.8%, respectively [91]. This way, the combined approach highlighted the potential for more efficient rice straw valorization [91]. In the study by Shen et al. [92], Cupriavidus basilensis B-8 was applied after sodium carbonate pretreatment to further degrade residual lignin and hemicellulose in rice straw, showing that a 2-day pretreatment significantly enhanced enzymatic hydrolysis.
Despite important advances in this field, the large-scale application of enzymatic and microbial delignification is difficult due to challenges related to biomass heterogeneity, enzyme cost, long pretreatment times, and process scalability. However, future research focused on strain engineering, discovery of novel microbial strains and oxidative enzymes, enzyme immobilization, and process intensification can address these limitations.
3.4. Thermochemical Processing of Plant-Based Residues and By-Products
In recent years, thermochemical treatments of residual biomass and organic waste have been studied as a possible solution for obtaining energy or products with exchangeable characteristics that can be used for various purposes, such as wastewater and soil treatment, as well as for improving soil quality. Important thermochemical technologies in this field are hydrothermal carbonation (HTC) and pyrolysis. HTC has the advantage of processing wet raw materials and thus avoids the highly energy-intensive drying step. On the other hand, pyrolysis is usually applied to the raw materials with low moisture content. In both cases, the raw material undergoes significant physical and chemical changes, yielding a product with modified composition and higher carbon content. HTC is a process that takes place in a humid environment, usually a mixture of solid raw materials and water, at elevated temperature (180–250 °C) under pressure. Pressure is usually generated by increasing the temperature in a closed vessel (reactor). HTC is an accelerated process of natural coal formation, which leads to an increasing content of carbon, up to 70%. The product of this process is called hydrochar. HTC is a method of choice to recycle materials from organic residues when valorization is complicated and expensive. In contrast, pyrolysis is a thermal treatment that is performed on dry raw materials at 300–900 °C under partial presence or complete absence of oxygen [93,94]. Both processes can be applied to residues from the agri-food sector. From an economic point of view, moisture content is the main criterion when selecting HTC or pyrolysis. The products generated by these two thermochemical treatments usually require additional treatments and tailoring of properties for desired industrial applications. Nevertheless, they can contribute to the substitution of fossil coal, reduce CO2 emissions, and foster a circular bioeconomy [94,95].
Chemical transformations of biomass by HTC involve hydrolysis, dehydration, decarboxylation, aromatization, and condensation, which may occur simultaneously due to differing reaction kinetics [96]. Hydrolysis, with the lowest activation energy, initiates biomass breakdown by cleaving ester and ether bonds, forming oligosaccharides and lignin fragments [97]. Dehydration and decarboxylation remove hydroxyl and carboxyl groups, producing intermediates like 5-hydroxymethylfurfural. Reactive intermediates generated in these reactions form hydrochar polymerization and condensation [98]. Plant-based residues are composed of complex biopolymers (including lipids, proteins, and carbohydrates), and each of them undergoes distinct transformation pathways during HTC [96]. In subcritical water, HTC initiates hydrolysis, breaking down polysaccharides into sugars, proteins into peptides and amino acids, and lipids into glycerol and fatty acids. Carbohydrates primarily drive hydrochar formation through dehydration, decarboxylation, and condensation, producing furanic and aromatic structures. Proteins contribute nitrogen-containing compounds via deamination and decarboxylation, although their breakdown is slower due to stable peptide bonds. Lipids do not directly carbonize into hydrochar but influence its properties by adsorbing onto the hydrochar surface and reacting with proteins via amidation, forming fatty acid amides and reducing Maillard reactions. Additionally, HTC improves dechlorination, reduces ash content through acetic acid-driven solubilization, and enhances hydrochar energy content due to lipid-derived long-chain hydrocarbons [99].
The HTC process is influenced by factors like temperature, time, pH, and water content. Temperature is a crucial parameter in the HTC process. The temperature during the process defines the yield of hydrochar and its physical and chemical characteristics. The maximum yield of hydrochar is achieved at lower temperatures up to 200 °C [100]. With increasing temperature, the yield of hydrochar decreases, and the yield of the liquid phase and gases increases, and in terms of chemical composition, hydrochar is closer to lignite. Another factor that affects the yield and composition of hydrochar is the duration of the process. It can last from a few minutes to several hours. The economic viability of the process depends on its duration. Pavkov et al. [100] used the HTC process on various plant-based residues, including wheat straw, soybean stalks, corncobs, corn stalks, sunflower stalks, hazelnut shells, and walnuts. The process was conducted at 200 and 250 °C under a pressure of 8.0 MPa for 120 min. Among the tested residues, walnut and hazelnut shells resulted in higher yields of hydrochar. A similar conclusion was reported by Nizamuddin et al. [101] using comparable residues. The reason for this behavior is the higher proportion of lignin, which is less degradable in HTC. In hydrochar samples treated at 250 °C, the amount of carbon reached more than 70% in some samples. These samples were positioned on the van Krevelen diagram in the lignite region, while the samples treated at 200 °C were in the peat region [100]. Azaare et al. [102] investigated how temperature, residence time, and mixing ratio affect hydrochar yield and energy content during HTC of pineapple and watermelon peels. They found that higher temperatures and longer residence times reduced hydrochar yield but increased its energy and carbon content.
Energy valorization of hydrochar is also an important aspect to be considered. Mannarino et al. [103] concluded that the temperature of carbonization had an important effect on ash content, mass yield, volatile matter, carbon content, heating value, and energy densification ratio of hydrochar obtained from fruit and vegetable residues. The same conclusion was reported by Pavkov et al. [100] for experiments conducted on crop residues. All these physico-chemical properties of produced hydrochar affect its subsequent application. Also, Gupta et al. [104] concluded that raising the HTC temperature from 160 to 180 °C enhanced both carbon and energy densification. However, further temperature increases had no additional effect on energy densification, despite continued improvement in carbon content [104]. Therefore, optimization and deep understanding of HTC processing conditions enables valorization of different residues into hydrochar with fine-tuned characteristics.
HTC provides several advantages over other processing techniques for converting residues into energy or new commercial product, including better volume reduction, smaller treatment footprints, and no process odors [105]. Although the process is energy-intensive, HTC can be an adequate solution for the treatment of organic residues when environmental impact, greenhouse gas emissions, carbon sequestration, and the added value of the final product are taken into account.
Numerous studies have focused on the pyrolysis of plant-based residues to produce value-added products such as biochar. Residue type, composition, and production conditions affect mostly the yield and physico-chemical properties of the resulting biochars [106,107]. The application of biochar derived from biomass pyrolysis to soil is increasingly recognized as an effective method for enhancing water and nutrient retention [108]. This practice also holds great potential for supporting food security, particularly in hyper-arid regions [109]. Pyrolysis has attracted significant interest for producing fuel bio-oils from a wide range of biomass sources, including microalgae [110], lignocellulosic wastes [108], woody biomass [111], and certain types of food waste [112]. The group of authors suspended biochar particles obtained from plant-based residues in a liquid to enhance their thermophysical properties [113,114]. Tang et al. [115] examined the co-pyrolysis of soybean-based food waste and plastic waste to evaluate their synergistic effects on thermal degradation. Using a fixed-bed reactor, the study analyzed the yields and characteristics of tar and biochar at temperatures of 400 °C, 500 °C, and 600 °C. The results indicated that the tar yield initially increased with temperature, reaching a peak before declining at higher temperatures [115]. Zhang et al. [116] produced biochar from four types of residues (peanut shell, pine, oak, and sugarcane) using slow pyrolysis. This method was chosen due to its higher biochar yield compared to fast pyrolysis. The biomass samples were subjected to pyrolysis at twelve different temperatures ranging from 350 to 900 °C, with a residence time of one hour at each targeted peak temperature. The highest biochar yield from peanut shell pyrolysis (43.16%) was obtained at 350 °C. This yield decreased steadily to 29.48% at 850 °C, before slightly increasing to 30.14% at 900 °C. Among the four types of residues studied, the peanut shell consistently produced the highest biochar yield. Furthermore, the biochars derived from peanut shells also exhibited the highest carbon content across all measured mineral elements [116]. The pyrolysis of potato peel waste was investigated for the production of bio-oils and biochars using a laboratory-scale auger pyrolizer operated at 450 °C [117]. The process resulted in a biochar yield of 30.5% and a bio-oil yield of 22.7%. However, the study noted the formation of a significant amount of unrecovered tar residue accumulating on the transfer tube condenser, which likely contributed to the reduced bio-oil yield [117].
Pyrolysis is widely regarded as the most practical and economical method for large-scale production of carbonaceous materials from various plant-based residues [94]. It enables the formation of unique structures characterized by high specific surface areas and porosity. This opens possibilities for many applications, from use as adsorbents in wastewater treatment [93] to the use of biochar as a storage material for H2 [118].
3.5. Multi-Product Recovery and Cascade Processing of Plant-Based Residues and By-Products
The challenge in valorizing plant-based residues generated within the agri-food sector is that their diverse chemical composition and high volumes are usually greatly underutilized when they are directed to a low-value and/or single-product recovery pathways. The cascade approach tackles this issue and focuses on successive recovery of multiple products from the same biomass, giving a priority to high-value applications. In this way, cascade processing enhances the overall value extracted from residues, while simultaneously reducing waste, improving process efficiency, and supporting both economic feasibility and environmental sustainability [119].
A recently published study by Durán-Aranguren et al. [120] provides an example of cascade valorization of orange residue, obtained from juice processing, in which approximately 85% of the initial orange biomass was sequentially extracted/transformed into valuable products. They first applied steam distillation to recover essential oils, followed by solid–liquid extraction with a water–ethanol solution to obtain free sugars and phenolic compounds. Subsequent citric acid hydrolysis enabled the recovery of pectin, while a final sulfuric acid hydrolysis released fermentable sugars, which were converted into ethanol, xylitol, and single-cell protein [120]. A similar cascade approach was recently applied to valorize Glycyrrhiza uralensis residue for the coproduction of flavonoids, cellulase, and bioethanol [121]. In that study, mechanical extrusion was initially applied to disrupt the complex structure of plant residues, followed by biological pretreatment with Penicillium oxalicum G2 for the production of cellulases, and in situ enzymatic hydrolysis for the production of reducing sugars. Obtained sugars were finally converted into bioethanol by a pentose-consuming strain, Pichia stipitis G32, while the solid biomass remaining after enzymatic hydrolysis was re-extracted using ultrasonication in 70% (w/w) ethanol to recover flavonoids. In total, the cascade processing of 100 g of Glycyrrhiza uralensis residue in that study resulted in 1.49 g of flavonoids, 294.36 U of cellulase, and 14.13 g of ethanol [121], demonstrating efficient conversion of plant-based residue into multiple value-added products. Battista et al. [122] investigated the cascade processing of spent coffee grounds, demonstrating the potential of multi-product recovery from this abundant residue. The first step included extraction with a 50:50 (v/v) ethanol–isopropanol mixture, yielding approximately 16% w/w coffee oil rich in tocopherols. Afterwards, hydrolysis of the carbohydrate fraction followed by bioethanol fermentation produced a final ethanol concentration of 50 g/L. Finally, anaerobic digestion of the remaining solids generated approximately 250 NL CH4/kg VS [122], ensuring almost complete utilization of spent coffee grounds. A cascade process for brewer’s spent grain was recently developed, combining dilute acid pretreatment and enzymatic hydrolysis to obtain protein- and fiber-enriched fractions along with released carbohydrates for food applications [123]. This process yielded three solid fractions: the first rich in protein, polyphenols, and microcrystalline cellulose; the second rich in protein and fiber, suitable for animal feed or further processing; and a third obtained by alkaline washing of the second fraction, with higher fiber content and improved homogeneity [123].
Such real examples confirm the potential of the cascade approach to move the biomass valorization pathway from a single application to a multi-product model. In this way, multi-product recovery from plant-based residues applying a cascade processing aligns well with the principles of the circular bioeconomy, establishing the groundwork to integrate waste reduction with adding value and consequently contributing to a more sustainable and resilient agri-food system. When implementing cascading use strategies, it remains critically important to prioritize the “food first” principle, thereby supporting food security and sustainable resource management [124].
Detailed analysis and environmental performance assessment through life cycle assessment (LCA) should be performed for all newly proposed biotechnological processes for plant-based residues, ideally with clear benchmarking against petroleum-based counterparts. Numerous studies have analyzed the environmental performance of complex processes and compared different scenarios. For example, the fermentative production of lactic acid, a well-established bio-based platform chemical, has been extensively assessed through techno-economic analysis and LCA, depending on the type of plant-based residues used, such as sugarcane in the multiproduct biorefinery studied by Brobbey et al. [125]. More recently, rigorous calculations of GHG emissions and other environmental indicators have further advanced these assessments [126]. Similar analyses are emerging for less developed bioprocesses, such as the production of bio-succinic acid [127] or bio-based aromatics like aniline [128], which remain at earlier stages of industrial maturity.
4. Future Perspectives
Future progress in these areas will depend on advances in science, industry investment, and regulatory support. Usually, critical hotspots include the selection of the feedstock and waste stream management. Additionally, most bioprocesses, including biorefineries, operate in batch or fed-batch mode, with high product concentrations but relatively low space-time yields. Moving towards continuous processing in biorefineries, while addressing existing bottlenecks, will be critical for future development. These challenges could be particularly emphasized in the case of plant-based residues and 2nd generation feedstocks such as lignocellulose. Potential problems include maintaining contamination control in continuous systems with complex substrates, the extraction of bio-based products present at lower concentrations compared to batch processes, and the presence of inhibitors. These are important to be faced and hopefully solved in the future, contributing significantly towards large-scale, sustainable valorization of agri-food residues.
Different tools, approaches, and assessment frameworks are available to support the evaluation and development of bio-based chemicals. Among them, the safe-and-sustainable-by-design (SSbD) framework [129] is emerging as particularly relevant for circular bioeconomy processes and products. SSbD integrates safety, environmental sustainability, and socio-economic dimensions from the earliest stages of product development, facilitating the design of new bio-based chemicals (e.g., organic acids) and materials (e.g., biochar). It enables moving towards zero-pollution for air, soil, water, and biota, which is an ultimate goal. Importantly, this framework is open for new improvements and identification of data gaps [130] from both the scientific community and industry.
Programs supporting circular bioeconomy solutions and flagship projects at national and global levels promote the sustainability of the agri-food system and put this approach in the spotlight to enable wider adoption. Dedicated support for innovation actions, high-TRL technologies, and flagship processing plants and biorefining facilities in the EU (e.g., CBE Joint Undertaking), India (e.g., BioE3 Policy), China, and other countries [131,132,133,134] reinforces the circular bioeconomy roadmap, guiding current and future research, innovations, and green transition.
5. Conclusions
The growing production and availability of residues generated during the harvesting and processing of crops (including food, fiber, industrial, forage, and medicinal and aromatic plants) requires innovative strategies that will reduce environmental and climate burdens. A good strategy for the processing of plant-based residues can simultaneously generate new products within the agri-food system. Low-energy and green processing technologies, such as biological delignification/biotransformation and green extraction, can enable the production of high-value bioactive extracts and biogas from MAP residues, or lignin, biochar, and fermentable sugars for bio-based chemicals from crop residues when combined with thermochemical conversion or anaerobic digestion. Challenges related to scalability, costs, and process optimization remain, but dedicated support for flagship projects in the circular bioeconomy (e.g., at EU or national levels) supports wider adoption and improves confidence in a successful green transition in the agri-food sector. The cascade approach emerges as a highly efficient strategy for valorizing plant-based residues, enabling multi-product recovery according to the principles of the circular bioeconomy. Future efforts should focus on bridging knowledge gaps in underexplored residues, particularly those of complex composition such as MAP residues, advancing process intensification and integration and addressing regulatory and economic barriers. New results in each of these areas will facilitate the conversion of underutilized resources into high-value bio-based products and accelerate the industrial adoption of the proposed valorization strategies.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/pr13103179/s1: Table S1: Crop categories, associated crops, and corresponding residues with their residue-to-product ratios, used to create Figure 1.
Author Contributions
Conceptualization, D.M., M.R. and A.D.-V.; methodology, D.M. and A.D.-V.; writing—original draft preparation, D.M., J.G., A.T., M.B., A.D., M.R. and A.D.-V.; writing—review and editing, D.M., J.G., A.T., M.B., A.D., M.R. and A.D.-V.; visualization, D.M.; funding acquisition, A.D.-V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Alliance of International Science Organizations, project SparkGREEN (ANSO-CR-PP 2022-08), and the Ministry of Science, Technological Development and Innovation of the Republic of Serbia (Contract No. 451-03-136/2025-03/200135, 451-03-136/2025-03/200117 and 451-03-136/2025-03/200287).
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials; further inquiries can be directed to the corresponding author.
Conflicts of Interest
The funding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| MAPs | Medicinal and aromatic plants |
| HTC | Hydrothermal carbonation |
| DBD | Dielectric barrier discharge |
| DES | Deep eutectic solvent |
| NADES | Natural deep eutectic solvent |
| ChCl | Choline chloride |
| GHG | Greenhouse gas |
| LCA | Life cycle assessment |
| SSbD | Safe-and-sustainable-by-design |
References
- Falcon, W.P.; Naylor, R.L.; Shankar, N.D. Rethinking global food demand for 2050. Popul. Dev. Rev. 2022, 48, 921–957. [Google Scholar] [CrossRef]
- Tubiello, F.N.; Karl, K.; Flammini, A.; Gütschow, J.; Obli-Laryea, G.; Conchedda, G.; Pan, X.; Qi, S.Y.; Halldórudóttir Heiðarsdóttir, H.; Wanner, N.; et al. Pre- and Post-Production Processes Increasingly Dominate Greenhouse Gas Emissions from Agri-Food Systems. Earth Syst. Sci. Data 2022, 14, 1795–1809. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/faostat/en/#data/GT (accessed on 16 August 2025).
- Emissions Gap Report 2023: Broken Record|UNEP—UN Environment Programme. Available online: https://www.unep.org/interactives/emissions-gap-report/2023/ (accessed on 16 August 2025).
- Bentsen, N.; Felby, C.; Thorsen, B.J. Agricultural Residue Production and Potentials for Energy and Materials Services. Prog. Energy Combust. Sci. 2014, 40, 59–73. [Google Scholar] [CrossRef]
- Karan, S.K.; Hamelin, L. Crop Residues May Be a Key Feedstock to Bioeconomy but How Reliable Are Current Estimation Methods? Resour. Conserv. Recycl. 2021, 164, 105211. [Google Scholar] [CrossRef]
- Morya, R.; Andrianantenaina, F.H.; Singh, S.; Pandey, A.K.; Kim, G.B.; Verma, J.P.; Kumar, G.; Raj, T.; Kim, S.H. Exploring Rice Straw as Substrate for Hydrogen Production: Critical Challenges and Opportunities. Environ. Technol. Innov. 2023, 31, 103153. [Google Scholar] [CrossRef]
- Ungureanu, N.; Vlăduț, V.; Biriș, S. Ștefan Sustainable Valorization of Waste and By-Products from Sugarcane Processing. Sustainability 2022, 14, 11089. [Google Scholar] [CrossRef]
- Lubbe, A.; Verpoorte, R. Cultivation of Medicinal and Aromatic Plants for Specialty Industrial Materials. Ind. Crops Prod. 2011, 34, 785–801. [Google Scholar] [CrossRef]
- Yarin, T.; Dutta, B.; Murmu, D.; Medda, P.; Das, S. Valorization of Medicinal and Aromatic Plants Waste. J. Pharm. Innov. 2022, 11, 532–537. Available online: https://www.thepharmajournal.com/archives/?year=2022&vol=11&issue=1&ArticleId=10030 (accessed on 17 August 2025).
- Chen, S.L.; Yu, H.; Luo, H.M.; Wu, Q.; Li, C.F.; Steinmetz, A. Conservation and Sustainable Use of Medicinal Plants: Problems, Progress, and Prospects. Chin. Med. 2016, 11, 37. [Google Scholar] [CrossRef]
- Tao, W.; Jin, J.; Zheng, Y.; Li, S. Current Advances of Resource Utilization of Herbal Extraction Residues in China. Waste Biomass Valorization 2021, 12, 5853–5868. [Google Scholar] [CrossRef]
- Saha, A.; Basak, B.B. Scope of Value Addition and Utilization of Residual Biomass from Medicinal and Aromatic Plants. Ind. Crops Prod. 2020, 145, 111979. [Google Scholar] [CrossRef]
- Fernández-Sestelo, M.; Carrillo, J.M. Environmental Effects on Yield and Composition of Essential Oil in Wild Populations of Spike Lavender (Lavandula latifolia Medik.). Agriculture 2020, 10, 626. [Google Scholar] [CrossRef]
- Okello, C.; Pindozzi, S.; Faugno, S.; Boccia, L. Bioenergy Potential of Agricultural and Forest Residues in Uganda. Biomass Bioenergy 2013, 56, 515–525. [Google Scholar] [CrossRef]
- Ramesh, D.; Muniraj, I.K.; Thangavelu, K.; Karthikeyan, S. Chemicals and Fuels Production from Agro Residues: A Biorefinery Approach. In Sustainable Approaches for Biofuels Production Technologies. Biofuel and Biorefinery Technologies; Srivastava, N., Srivastava, M., Mishra, P., Upadhyay, S., Ramteke, P., Gupta, V., Eds.; Springer: Cham, Switzerland, 2018; Volume 7, pp. 47–71. [Google Scholar] [CrossRef]
- Tesfay, A.G.; Tesfay, A.H.; Adaramola, M.S. Quantifying Agricultural Residues Biomass Resources and the Energy Potentials with Characterization of Their Nature and Ethiopian Case Consumption Inference. Energies 2024, 17, 4736. [Google Scholar] [CrossRef]
- Bedoić, R.; Ćosić, B.; Duić, N. Technical Potential and Geographic Distribution of Agricultural Residues, Co-Products and by-Products in the European Union. Sci. Total Environ. 2019, 686, 568–579. [Google Scholar] [CrossRef]
- Hassoun, A.; Boukid, F.; Pasqualone, A.; Bryant, C.J.; García, G.G.; Parra-López, C.; Jagtap, S.; Trollman, H.; Cropotova, J.; Barba, F.J. Emerging Trends in the Agri-Food Sector: Digitalisation and Shift to Plant-Based Diets. Curr. Res. Food Sci. 2022, 5, 2261–2269. [Google Scholar] [CrossRef]
- EU Green Deal Guidance Note: Sustainable Agri-Food Systems|Knowledge for Policy. Available online: https://knowledge4policy.ec.europa.eu/publication/eu-green-deal-guidance-note-sustainable-agri-food-systems_en (accessed on 19 August 2025).
- Li, H.; Qu, Y.; Yang, Y.; Chang, S.; Xu, J. Microwave Irradiation—A Green and Efficient Way to Pretreat Biomass. Bioresour. Technol. 2016, 199, 34–41. [Google Scholar] [CrossRef]
- Mankar, A.R.; Pandey, A.; Modak, A.; Pant, K.K. Pretreatment of Lignocellulosic Biomass: A Review on Recent Advances. Bioresour. Technol. 2021, 334, 125235. [Google Scholar] [CrossRef]
- Abolore, R.S.; Jaiswal, S.; Jaiswal, A.K. Green and Sustainable Pretreatment Methods for Cellulose Extraction from Lignocellulosic Biomass and Its Applications: A Review. Carbohydr. Polym. Technol. Appl. 2024, 7, 100396. [Google Scholar] [CrossRef]
- More, T.T.; Ghangrekar, M.M. Improving Performance of Microbial Fuel Cell with Ultrasonication Pre-Treatment of Mixed Anaerobic Inoculum Sludge. Bioresour. Technol. 2010, 101, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, G.; Chen, X.; Yang, Y.; Liew, R.K.; Abo-Dief, H.M.; Lam, S.S.; Sellami, R.; Peng, W.; Li, H. Extraction Strategies for Lignin, Cellulose, and Hemicellulose to Obtain Valuable Products from Biomass. Adv. Compos. Hybrid. Mater. 2024, 7, 219. [Google Scholar] [CrossRef]
- Luo, J.; Fang, Z.; Smith, R.L. Ultrasound-Enhanced Conversion of Biomass to Biofuels. Prog. Energy Combust. Sci. 2014, 41, 56–93. [Google Scholar] [CrossRef]
- Djukić-Vuković, A.; Lazović, S.; Mladenović, D.; Knežević-Jugović, Z.; Pejin, J.; Mojović, L. Non-Thermal Plasma and Ultrasound-Assisted Open Lactic Acid Fermentation of Distillery Stillage. Environ. Sci. Pollut. Res. 2019, 26, 35543–35554. [Google Scholar] [CrossRef]
- Ma, Q.; Ji, Q.; Chen, L.; Zhu, Z.; Tu, S.; Okonkwo, C.E.; Out, P.; Zhou, C. Multimode Ultrasound and Ternary Deep Eutectic Solvent Sequential Pretreatments Enhanced the Enzymatic Saccharification of Corncob Biomass. Ind. Crops Prod. 2022, 188, 115574. [Google Scholar] [CrossRef]
- AlYammahi, J.; Hai, A.; Krishnamoorthy, R.; Arumugham, T.; Hasan, S.W.; Banat, F. Ultrasound-Assisted Extraction of Highly Nutritious Date Sugar from Date palm (Phoenix dactylifera) Fruit Powder: Parametric Optimization and Kinetic Modeling. Ultrason. Sonochem. 2022, 88, 106107. [Google Scholar] [CrossRef]
- Cano, R.; Pérez-Elvira, S.I.; Fdz-Polanco, F. Energy Feasibility Study of Sludge Pretreatments: A Review. Appl. Energy 2015, 149, 176–185. [Google Scholar] [CrossRef]
- Bagade, S.B.; Patil, M. Recent Advances in Microwave Assisted Extraction of Bioactive Compounds from Complex Herbal Samples: A Review. Crit. Rev. Anal. Chem. 2021, 51, 138–149. [Google Scholar] [CrossRef]
- Zhu, G.; Jin, D.; Zhao, L.; Ouyang, X.; Chen, C.; Qiu, X. Microwave-Assisted Selective Cleavage of CαCβ Bond for Lignin Depolymerization. Fuel Process. Technol. 2017, 161, 155–161. [Google Scholar] [CrossRef]
- Kumar, B.; Bhardwaj, N.; Agrawal, K.; Chaturvedi, V.; Verma, P. Current Perspective on Pretreatment Technologies Using Lignocellulosic Biomass: An Emerging Biorefinery Concept. Fuel Process. Technol. 2020, 199, 106244. [Google Scholar] [CrossRef]
- Mladenović, D.; Djukić-Vuković, A.; Veselinović, L.; Mijin, D.; Kocić-Tanackov, S.; Mojović, L. Short-Term Microwave-Assisted Peroxide Treatment for Intensified Lignin and Sugar Recovery from Corncob. Waste Biomass Valorization 2025, 16, 3909–3923. [Google Scholar] [CrossRef]
- Mladenović, D.; Grbić, J.; Đukić-Vuković, A.; Mijin, D.; Mojović, L. Lignin Removal from Corncob by Microwave-Coupled Peroxide Treatment. J. Process. Energy Agric. 2024, 28, 1–6. [Google Scholar] [CrossRef]
- Gan, P.; Zhang, K.; Xu, Q.; Zhao, Y.; Li, J.; Yang, G.; Zhang, Y.; Wang, Y.; Zhang, L.; Wang, B.; et al. Microwave-Assisted Choline Chloride/Lactic Acid Pretreatment for High-Efficiency Separation and Value-Added Utilization of Sugarcane Bagasse Components. Ind. Crops Prod. 2024, 220, 119251. [Google Scholar] [CrossRef]
- Kumar, A.; Škoro, N.; Gernjak, W.; Puač, N. Cold Atmospheric Plasma Technology for Removal of Organic Micropollutants from Wastewater—A Review. Eur. Phys. J. D 2021, 75, 283. [Google Scholar] [CrossRef]
- Anari, E.S.; Soltanizadeh, N.; Fathi, M. The Potential of DBD Plasma Pretreatment for the Isolation of Micro- and Nano-Cellulose Fibers from the Walnut Shells. Carbohydr. Polym. 2024, 327, 121692. [Google Scholar] [CrossRef]
- Zhu, H.; Han, Z.; Cheng, J.H.; Sun, D.W. Modification of Cellulose from Sugarcane (Saccharum officinarum) Bagasse Pulp by Cold Plasma: Dissolution, Structure and Surface Chemistry Analysis. Food Chem. 2022, 374, 131675. [Google Scholar] [CrossRef]
- Grbić, J.Z.; Mladenović, D.D.; Veljković, M.B.; Lazarević, S.S.; Lević, S.M.; Lazović, S.S.; Djukić-Vuković, A.P. Cold Plasma/Alkaline Pretreatment Facilitates Corn Stalk Fractionation and Valorization towards Zero-Waste Approach. Ind. Crops Prod. 2024, 222, 119498. [Google Scholar] [CrossRef]
- Grbić, J.; Mladenović, D.; Pavlović, S.; Lazović, S.; Mojović, L.; Djukić-Vuković, A. Advanced Oxidation Processes in the Treatment of Corn Stalks. Sustain. Chem. Pharm. 2023, 32, 100962. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green Extraction of Natural Products: Concept and Principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef]
- Zhou, J.; Gullón, B.; Wang, M.; Gullón, P.; Lorenzo, J.M.; Barba, F.J. The Application of Supercritical Fluids Technology to Recover Healthy Valuable Compounds from Marine and Agricultural Food Processing By-Products: A Review. Processes 2021, 9, 357. [Google Scholar] [CrossRef]
- Fraguela-Meissimilly, H.; Bastías-Monte, J.M.; Vergara, C.; Ortiz-Viedma, J.; Lemus-Mondaca, R.; Flores, M.; Toledo-Merma, P.; Alcázar-Alay, S.; Gallón-Bedoya, M. New Trends in Supercritical Fluid Technology and Pressurized Liquids for the Extraction and Recovery of Bioactive Compounds from Agro-Industrial and Marine Food Waste. Molecules 2023, 28, 4421. [Google Scholar] [CrossRef] [PubMed]
- Herzyk, F.; Piłakowska-Pietras, D.; Korzeniowska, M. Supercritical Extraction Techniques for Obtaining Biologically Active Substances from a Variety of Plant Byproducts. Foods 2024, 13, 1713. [Google Scholar] [CrossRef] [PubMed]
- Ristivojević, P.; Krstić Ristivojević, M.; Stanković, D.; Cvijetić, I. Advances in Extracting Bioactive Compounds from Food and Agricultural Waste and By-Products Using Natural Deep Eutectic Solvents: A Circular Economy Perspective. Molecules 2024, 29, 4717. [Google Scholar] [CrossRef] [PubMed]
- Palos-Hernández, A.; Gutiérrez Fernández, M.Y.; Escuadra Burrieza, J.; Pérez-Iglesias, J.L.; González-Paramás, A.M. Obtaining Green Extracts Rich in Phenolic Compounds from Underexploited Food By-Products Using Natural Deep Eutectic Solvents. Opportunities and Challenges. Sustain. Chem. Pharm. 2022, 29, 100773. [Google Scholar] [CrossRef]
- Molnar, M.; Gašo-Sokač, D.; Komar, M.; Jakovljević Kovač, M.; Bušić, V. Potential of Deep Eutectic Solvents in the Extraction of Organic Compounds from Food Industry By-Products and Agro-Industrial Waste. Separations 2024, 11, 35. [Google Scholar] [CrossRef]
- Schuh, L.; Reginato, M.; Florêncio, I.; Falcao, L.; Boron, L.; Gris, E.F.; Mello, V.; Báo, S.N. From Nature to Innovation: The Uncharted Potential of Natural Deep Eutectic Solvents. Molecules 2023, 28, 7653. [Google Scholar] [CrossRef]
- Cannavacciuolo, C.; Pagliari, S.; Frigerio, J.; Giustra, C.M.; Labra, M.; Campone, L. Natural Deep Eutectic Solvents (NADESs) Combined with Sustainable Extraction Techniques: A Review of the Green Chemistry Approach in Food Analysis. Foods 2023, 12, 56. [Google Scholar] [CrossRef]
- Benítez-Correa, E.; Bastías-Montes, J.M.; Acuña-Nelson, S.; Muñoz-Fariña, O. Effect of Choline Chloride-Based Deep Eutectic Solvents on Polyphenols Extraction from Cocoa (Theobroma cacao L.) Bean Shells and Antioxidant Activity of Extracts. Curr. Res. Food Sci. 2023, 7, 100614. [Google Scholar] [CrossRef]
- Ozturk, B.; Parkinson, C.; Gonzalez-Miquel, M. Extraction of Polyphenolic Antioxidants from Orange Peel Waste Using Deep Eutectic Solvents. Sep. Purif. Technol. 2018, 206, 1–13. [Google Scholar] [CrossRef]
- Athanasiadis, V.; Palaiogiannis, D.; Poulianiti, K.; Bozinou, E.; Lalas, S.I.; Makris, D.P. Extraction of Polyphenolic Antioxidants from Red Grape Pomace and Olive Leaves: Process Optimization Using a Tailor-Made Tertiary Deep Eutectic Solvent. Sustainability 2022, 14, 6864. [Google Scholar] [CrossRef]
- Grisales-Mejía, J.F.; Cedeño-Fierro, V.; Ortega, J.P.; Torres-Castañeda, H.G.; Andrade-Mahecha, M.M.; Martínez-Correa, H.A.; Álvarez-Rivera, G.; Mendiola, J.A.; Cifuentes, A.; Ibañez, E. Advanced NADES-Based Extraction Processes for the Recovery of Phenolic Compounds from Hass Avocado Residues: A Sustainable Valorization Strategy. Sep. Purif. Technol. 2024, 351, 128104. [Google Scholar] [CrossRef]
- Zhang, X.J.; Liu, Z.T.; Chen, X.Q.; Zhang, T.T.; Zhang, Y. Deep Eutectic Solvent Combined with Ultrasound Technology: A Promising Integrated Extraction Strategy for Anthocyanins and Polyphenols from Blueberry Pomace. Food Chem. 2023, 422, 136224. [Google Scholar] [CrossRef] [PubMed]
- Koutsoukos, S.; Tsiaka, T.; Tzani, A.; Zoumpoulakis, P.; Detsi, A. Choline Chloride and Tartaric Acid, a Natural Deep Eutectic Solvent for the Efficient Extraction of Phenolic and Carotenoid Compounds. J. Clean. Prod. 2019, 241, 118384. [Google Scholar] [CrossRef]
- Bonacci, S.; Di Gioia, M.L.; Costanzo, P.; Maiuolo, L.; Tallarico, S.; Nardi, M. Natural Deep Eutectic Solvent as Extraction Media for the Main Phenolic Compounds from Olive Oil Processing Wastes. Antioxidants 2020, 9, 513. [Google Scholar] [CrossRef] [PubMed]
- Tzani, A.; Lymperopoulou, T.; Pitterou, I.; Karetta, I.; Belfquih, F.; Detsi, A. Development and Optimization of Green Extraction Process of Spent Coffee Grounds Using Natural Deep Eutectic Solvents. Sustain. Chem. Pharm. 2023, 34, 101144. [Google Scholar] [CrossRef]
- Alchera, F.; Ginepro, M.; Giacalone, G. Microwave-Assisted Extraction (MAE) of Bioactive Compounds from Blueberry by-Products Using a Sugar-Based NADES: A Novelty in Green Chemistry. LWT 2024, 192, 115642. [Google Scholar] [CrossRef]
- de Lima, N.D.; da Silva Monteiro Wanderley, B.R.; Andrade Ferreira, A.L.; Pereira-Coelho, M.; da Silva Haas, I.C.; Vitali, L.; dos Santos Madureira, L.A.; Müller, J.M.; Fritzen-Freire, C.B.; de Mello Castanho Amboni, R.D. Green Extraction of Phenolic Compounds from the By-Product of Purple Araçá (Psidium myrtoides) with Natural Deep Eutectic Solvents Assisted by Ultrasound: Optimization, Comparison, and Bioactivity. Food Res. Int. 2024, 191, 114731. [Google Scholar] [CrossRef]
- Hamieau, M.; Loulergue, P.; Szydłowska-Czerniak, A. Green Solvent Extraction of Antioxidants from Herbs and Agro-Food Wastes: Optimization and Capacity Determination. Appl. Sci. 2024, 14, 2936. [Google Scholar] [CrossRef]
- Mavai, S.; Bains, A.; Sridhar, K.; Chawla, P.; Sharma, M. Emerging Deep Eutectic Solvents for Food Waste Valorization to Achieve Sustainable Development Goals: Bioactive Extractions and Food Applications. Food Chem. 2025, 462, 141000. [Google Scholar] [CrossRef]
- Ciriminna, R.; Lomeli Rodriguez, M.; Demma Carà, P.; Lopez Sanchez, J.A.; Pagliaro, M. Limonene: A Versatile Chemical of the Bioeconomy. Chem. Commun. 2014, 50, 15288–15296. [Google Scholar] [CrossRef]
- Pagliaro, M.; Fabiano-Tixier, A.S.; Ciriminna, R. Limonene as a Natural Product Extraction Solvent. Green. Chem. 2023, 25, 6108–6119. [Google Scholar] [CrossRef]
- Sahad, N.; Som, A.M.; Baharuddin, A.S.; Mokhtar, M.N.; Busu, Z.; Sulaiman, A. Recovery of Residual Crude Palm Oil (RCPO) from Oil Palm Decanter Cake (OPDC) Using D-Limonene. Adv. Mat. Res. 2015, 1113, 405–410. [Google Scholar] [CrossRef]
- Akretche-K, S.; Ferhat, Z.; Amiali, M. Vegetable and Nut Oils Extraction by D-Limonene as Alternative Solvent. Int. J. Agric. Res. 2017, 12, 82–87. [Google Scholar] [CrossRef]
- Xu, J.; Peng, L.; Chu, J.; Shi, J.; Cui, Q.; Shi, Q. D-Limonene as an Alternative for the Extraction and Purification of Nuciferine from Lotus Leaf via Multi-Stage Vortex Assisted Two-Phase Solvent Extraction Integrated with Solid Phase Extraction Using Mesoporous Material SBA-15 as Adsorbent. Sustain. Chem. Pharm. 2023, 32, 100997. [Google Scholar] [CrossRef]
- Mourtzinos, I.; Anastasopoulou, E.; Petrou, A.; Grigorakis, S.; Makris, D.; Biliaderis, C.G. Optimization of a Green Extraction Method for the Recovery of Polyphenols from Olive Leaf Using Cyclodextrins and Glycerin as Co-Solvents. J. Food Sci. Technol. 2016, 53, 3939–3947. [Google Scholar] [CrossRef] [PubMed]
- Makris, D.P.; Passalidi, V.; Kallithraka, S.; Mourtzinos, I. Optimization of Polyphenol Extraction from Red Grape Pomace Using Aqueous Glycerol/Tartaric Acid Mixtures and Response Surface Methodology. Prep. Biochem. Biotechnol. 2016, 46, 176–182. [Google Scholar] [CrossRef]
- Trasanidou, D.; Apostolakis, A.; Makris, D.P. Development of a Green Process for the Preparation of Antioxidant and Pigment-Enriched Extracts from Winery Solid Wastes Using Response Surface Methodology and Kinetics. Chem. Eng. Commun. 2016, 203, 1317–1325. [Google Scholar] [CrossRef]
- Huamán-Castilla, N.L.; Mariotti-Celis, M.S.; Martínez-Cifuentes, M.; Pérez-Correa, J.R. Glycerol as Alternative Co-Solvent for Water Extraction of Polyphenols from Carménère Pomace: Hot Pressurized Liquid Extraction and Computational Chemistry Calculations. Biomolecules 2020, 10, 474. [Google Scholar] [CrossRef]
- Abdoun, R.; Grigorakis, S.; Kellil, A.; Loupassaki, S.; Makris, D.P. Process Optimization and Stability of Waste Orange Peel Polyphenols in Extracts Obtained with Organosolv Thermal Treatment Using Glycerol-Based Solvents. ChemEngineering 2022, 6, 35. [Google Scholar] [CrossRef]
- Shin, S.K.; Ko, Y.J.; Hyeon, J.E.; Han, S.O. Studies of Advanced Lignin Valorization Based on Various Types of Lignolytic Enzymes and Microbes. Bioresour. Technol. 2019, 289, 121728. [Google Scholar] [CrossRef]
- Grgas, D.; Rukavina, M.; Bešlo, D.; Štefanac, T.; Crnek, V.; Šikić, T.; Habuda-Stanić, M.; Landeka Dragičević, T. The Bacterial Degradation of Lignin—A Review. Water 2023, 15, 1272. [Google Scholar] [CrossRef]
- Andlar, M.; Rezić, T.; Marđetko, N.; Kracher, D.; Ludwig, R.; Šantek, B. Lignocellulose Degradation: An Overview of Fungi and Fungal Enzymes Involved in Lignocellulose Degradation. Eng. Life Sci. 2018, 18, 768. [Google Scholar] [CrossRef]
- Atiwesh, G.; Parrish, C.C.; Banoub, J.; Le, T.A.T. Lignin Degradation by Microorganisms: A Review. Biotechnol. Prog. 2022, 38, e3226. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Li, Q.; Liu, C.; Meng, E.; Zhang, B. Microbial Degradation of Lignocellulose for Sustainable Biomass Utilization and Future Research Perspectives. Sustainability 2025, 17, 4223. [Google Scholar] [CrossRef]
- Saha, B.C.; Qureshi, N.; Kennedy, G.J.; Cotta, M.A. Biological Pretreatment of Corn Stover with White-Rot Fungus for Improved Enzymatic Hydrolysis. Int. Biodeterior. Biodegrad. 2016, 109, 29–35. [Google Scholar] [CrossRef]
- Wang, F.Q.; Xie, H.; Chen, W.; Wang, E.T.; Du, F.G.; Song, A.D. Biological Pretreatment of Corn Stover with Ligninolytic Enzyme for High Efficient Enzymatic Hydrolysis. Bioresour. Technol. 2013, 144, 572–578. [Google Scholar] [CrossRef]
- Sherpa, K.C.; Ghangrekar, M.M.; Banerjee, R. A Green and Sustainable Approach on Statistical Optimization of Laccase Mediated Delignification of Sugarcane Tops for Enhanced Saccharification. J. Environ. Manag. 2018, 217, 700–709. [Google Scholar] [CrossRef]
- Zhou, M.; Fakayode, O.A.; Ren, M.; Li, H.; Liang, J.; Yagoub, A.E.G.A.; Fan, Z.; Zhou, C. Laccase-Catalyzed Lignin Depolymerization in Deep Eutectic Solvents: Challenges and Prospects. Bioresour. Bioprocess. 2023, 10, 21. [Google Scholar] [CrossRef]
- Knežević, A.; Stajić, M.; Jovanović, V.M.; Kovačević, V.; Ćilerdžić, J.; Milovanović, I.; Vukojević, J. Induction of Wheat Straw Delignification by Trametes Species. Sci. Rep. 2016, 6, 26529. [Google Scholar] [CrossRef] [PubMed]
- Knežević, A.; Stajić, M.; Milovanović, I.; Vukojević, J. Wheat Straw Degradation by Trametes Gibbosa: The Effect of Calcium Ions. Waste Biomass Valorization 2018, 9, 1903–1908. [Google Scholar] [CrossRef]
- Matei, J.C.; Soares, M.; Bonato, A.C.H.; de Freitas, M.P.A.; Helm, C.V.; Maroldi, W.V.; Magalhães, W.L.E.; Haminiuk, C.W.I.; Maciel, G.M. Enzymatic Delignification of Sugar Cane Bagasse and Rice Husks and Its Effect in Saccharification. Renew. Energy 2020, 157, 987–997. [Google Scholar] [CrossRef]
- Su, Y.; Yu, X.; Sun, Y.; Wang, G.; Chen, H.; Chen, G. Evaluation of Screened Lignin-Degrading Fungi for the Biological Pretreatment of Corn Stover. Sci. Rep. 2018, 8, 5385. [Google Scholar] [CrossRef]
- Vasco-Correa, J.; Shah, A. Techno-Economic Bottlenecks of the Fungal Pretreatment of Lignocellulosic Biomass. Fermentation 2019, 5, 30. [Google Scholar] [CrossRef]
- Mei, J.; Shen, X.; Gang, L.; Xu, H.; Wu, F.; Sheng, L. A Novel Lignin Degradation Bacteria-Bacillus Amyloliquefaciens SL-7 Used to Degrade Straw Lignin Efficiently. Bioresour. Technol. 2020, 310, 123445. [Google Scholar] [CrossRef] [PubMed]
- Shah, T.A.; Lee, C.C.; Orts, W.J.; Tabassum, R. Biological Pretreatment of Rice Straw by Ligninolytic Bacillus Sp. Strains for Enhancing Biogas Production. Environ. Prog. Sustain. Energy 2019, 38, e13036. [Google Scholar] [CrossRef]
- Chen, J.; Cai, Y.; Wang, Z.; Wang, S.; Li, J.; Song, C.; Zhuang, W.; Liu, D.; Wang, S.; Song, A.; et al. Construction of a Synthetic Microbial Community for Enzymatic Pretreatment of Wheat Straw for Biogas Production via Anaerobic Digestion. Environ. Sci. Technol. 2024, 58, 9446–9455. [Google Scholar] [CrossRef]
- Olajuyigbe, F.M.; Fatokun, C.O.; Oyelere, O.M. Biodelignification of Some Agro-Residues by Stenotrophomonas Sp. CFB-09 and Enhanced Production of Ligninolytic Enzymes. Biocatal. Agric. Biotechnol. 2018, 15, 120–130. [Google Scholar] [CrossRef]
- Si, M.; Liu, D.; Liu, M.; Yan, X.; Gao, C.; Chai, L.; Shi, Y. Complementary Effect of Combined Bacterial-Chemical Pretreatment to Promote Enzymatic Digestibility of Lignocellulose biomass. Bioresour. Technol. 2019, 272, 275–280. [Google Scholar] [CrossRef]
- Shen, Z.; Zhang, K.; Si, M.; Liu, M.; Zhuo, S.; Liu, D.; Ren, L.; Yan, X.; Shi, Y. Synergy of Lignocelluloses Pretreatment by Sodium Carbonate and Bacterium to Enhance Enzymatic Hydrolysis of Rice Straw. Bioresour. Technol. 2018, 249, 154–160. [Google Scholar] [CrossRef]
- Amalina, F.; Razak, A.S.A.; Krishnan, S.; Sulaiman, H.; Zularisam, A.W.; Nasrullah, M. Biochar Production Techniques Utilizing Biomass Waste-Derived Materials and Environmental Applications—A Review. J. Hazard. Mater. Adv. 2022, 7, 100134. [Google Scholar] [CrossRef]
- Berenguer, C.V.; Perestrelo, R.; Pereira, J.A.M.; Câmara, J.S.; Kijevcanin, M.; Šoštari’c, T.Š.; Berenguer, C.V.; Perestrelo, R.; Pereira, J.A.M.; Câmara, J.S. Management of Agri-Food Waste Based on Thermochemical Processes towards a Circular Bioeconomy Concept: The Case Study of the Portuguese Industry. Processes 2023, 11, 2870. [Google Scholar] [CrossRef]
- Bioenergy—International Collaboration in Bioenergy. Available online: https://www.ieabioenergy.com/ (accessed on 24 August 2025).
- Funke, A.; Ziegler, F. Hydrothermal Carbonization of Biomass: A Summary and Discussion of Chemical Mechanisms for Process Engineering. Biofuels Bioprod. Biorefining 2010, 4, 160–177. [Google Scholar] [CrossRef]
- Reza, M.T.; Andert, J.; Wirth, B.; Busch, D.; Pielert, J.; Lynam, J.G.; Mumme, J. Hydrothermal Carbonization of Biomass for Energy and Crop Production. Appl. Bioenergy 2014, 1, 11–29. [Google Scholar] [CrossRef]
- Fang, J.; Zhan, L.; Ok, Y.S.; Gao, B. Minireview of Potential Applications of Hydrochar Derived from Hydrothermal Carbonization of Biomass. J. Ind. Eng. Chem. 2018, 57, 15–21. [Google Scholar] [CrossRef]
- Le, H.S.; Chen, W.-H.; Ahmed, S.F.; Said, Z.; Rafa, N.; Le, A.T.; Ağbulut, Ü.; Veza, I.; Nguyen, X.P.; Duong, X.Q.; et al. Hydrothermal Carbonization of Food Waste as Sustainable Energy Conversion Path. Bioresour. Technol. 2022, 363, 127958. [Google Scholar] [CrossRef] [PubMed]
- Pavkov, I.; Radojčin, M.; Stamenković, Z.; Bikić, S.; Tomić, M.; Bukurov, M.; Despotović, B. Hydrothermal Carbonization of Agricultural Biomass: Characterization of Hydrochar for Energy Production. Solid. Fuel Chem. 2022, 56, 225–235. [Google Scholar] [CrossRef]
- Nizamuddin, S.; Baloch, H.A.; Griffin, G.J.; Mubarak, N.M.; Bhutto, A.W.; Abro, R.; Mazari, S.A.; Ali, B.S. An Overview of Effect of Process Parameters on Hydrothermal Carbonization of Biomass. Renew. Sustain. Energy Rev. 2017, 73, 1289–1299. [Google Scholar] [CrossRef]
- Azaare, L.; Commeh, M.K.; Smith, A.M.; Kemausuor, F. Co-Hydrothermal Carbonization of Pineapple and Watermelon Peels: Effects of Process Parameters on Hydrochar Yield and Energy Content. Bioresour. Technol. Rep. 2021, 15, 100720. [Google Scholar] [CrossRef]
- Mannarino, G.; Sarrion, A.; Diaz, E.; Gori, R.; De la Rubia, M.A.; Mohedano, A.F. Improved Energy Recovery from Food Waste through Hydrothermal Carbonization and Anaerobic Digestion. Waste Manag. 2022, 142, 9–18. [Google Scholar] [CrossRef]
- Gupta, D.; Mahajani, S.M.; Garg, A. Investigation on Hydrochar and Macromolecules Recovery Opportunities from Food Waste after Hydrothermal Carbonization. Sci. Total Environ. 2020, 749, 142294. [Google Scholar] [CrossRef]
- Tran, T.H.; Le, H.H.; Pham, T.H.; Nguyen, D.T.; La, D.D.; Chang, S.W.; Lee, S.M.; Chung, W.J.; Nguyen, D.D. Comparative Study on Methylene Blue Adsorption Behavior of Coffee Husk-Derived Activated Carbon Materials Prepared Using Hydrothermal and Soaking Methods. J. Environ. Chem. Eng. 2021, 9, 105362. [Google Scholar] [CrossRef]
- Oh, J.I.; Lee, J.; Lee, T.; Ok, Y.S.; Lee, S.R.; Kwon, E.E. Strategic CO2 Utilization for Shifting Carbon Distribution from Pyrolytic Oil to Syngas in Pyrolysis of Food Waste. J. CO2 Util. 2017, 20, 150–155. [Google Scholar] [CrossRef]
- Carmona-Cabello, M.; Garcia, I.L.; Leiva-Candia, D.; Dorado, M.P. Valorization of Food Waste Based on Its Composition through the Concept of Biorefinery. Curr. Opin. Green. Sustain. Chem. 2018, 14, 67–79. [Google Scholar] [CrossRef]
- Kan, T.; Strezov, V.; Evans, T.J. Lignocellulosic Biomass Pyrolysis: A Review of Product Properties and Effects of Pyrolysis Parameters. Renew. Sustain. Energy Rev. 2016, 57, 1126–1140. [Google Scholar] [CrossRef]
- Singh, T.; Hussien, M.A.A.; Al-Ansari, T.; Saoud, K.; McKay, G. Critical Review of Solar Thermal Resources in GCC and Application of Nanofluids for Development of Efficient and Cost Effective CSP Technologies. Renew. Sustain. Energy Rev. 2018, 91, 708–719. [Google Scholar] [CrossRef]
- Azizi, K.; Keshavarz Moraveji, M.; Abedini Najafabadi, H. A Review on Bio-Fuel Production from Microalgal Biomass by Using Pyrolysis Method. Renew. Sustain. Energy Rev. 2018, 82, 3046–3059. [Google Scholar] [CrossRef]
- Papari, S.; Hawboldt, K. A Review on the Pyrolysis of Woody Biomass to Bio-Oil: Focus on Kinetic Models. Renew. Sustain. Energy Rev. 2015, 52, 1580–1595. [Google Scholar] [CrossRef]
- Aboagye, D.; Banadda, N.; Kiggundu, N.; Kabenge, I. Assessment of Orange Peel Waste Availability in Ghana and Potential Bio-Oil Yield Using Fast Pyrolysis. Renew. Sustain. Energy Rev. 2017, 70, 814–821. [Google Scholar] [CrossRef]
- Radojčin, M.; Bikić, S.; Pavkov, I.; Bukurov, M.; Despotović, B.; Stamenković, Z.; Oluški, N. Experimental Investigation on Thermophysical Properties of Iobiofluids. Adv. Mech. Eng. 2022, 14, 16878140221075457. [Google Scholar] [CrossRef]
- Bikić, S.; Radojčin, M.; Pavkov, I.; Bukurov, M.; Despotović, B.; Stamenković, Z.; Oluški, N.; Afif, R. Al Thermophysical Dispersion Properties of Agricultural Biomass Particles in Ethylene Glycol. Int. J. Thermofluids 2022, 16, 100226. [Google Scholar] [CrossRef]
- Tang, Y.; Huang, Q.; Sun, K.; Chi, Y.; Yan, J. Co-Pyrolysis Characteristics and Kinetic Analysis of Organic Food Waste and Plastic. Bioresour. Technol. 2018, 249, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, C.; Gray, E.M.; Boyd, S.E. Effect of Feedstock and Pyrolysis Temperature on Properties of Biochar Governing End Use Efficacy. Biomass Bioenergy 2017, 105, 136–146. [Google Scholar] [CrossRef]
- Liang, S.; Han, Y.; Wei, L.; McDonald, A.G. Production and Characterization of Bio-Oil and Bio-Char from Pyrolysis of Potato Peel Wastes. Biomass Convers. Biorefin 2015, 5, 237–246. [Google Scholar] [CrossRef]
- Rawat, S.; Wang, C.T.; Lay, C.H.; Hotha, S.; Bhaskar, T. Sustainable Biochar for Advanced Electrochemical/Energy Storage Applications. J. Energy Storage 2023, 63, 107115. [Google Scholar] [CrossRef]
- Aguado-González, L.; Sierra-Pérez, J.; Forte, C.; López-Forniés, I.; Blanc, S. Cascade Valorization of Hazelnut Industry By-Products for Industrial Use through Co-Creative Processes. J. Clean. Prod. 2025, 494, 144989. [Google Scholar] [CrossRef]
- Durán-Aranguren, D.D.; Yamakawa, C.K.; Ordeñana, J.; Sierra, R.; Posada, J.A.; Mussatto, S.I. An Experimental Cascade Biorefinery from Orange Residues: Sequential Recovery of Bioactive Compounds, Pectin, and Fermentation of Sugar-Rich Side Streams Using Conventional and Non-Conventional Yeasts. Biomass Bioenergy 2025, 193, 107514. [Google Scholar] [CrossRef]
- Chang, S.; Yun, C.; Yang, B.; Duan, J.; Chen, T.; Liu, L.; Li, B.; Guo, S.; Zhang, S. Comprehensive Reutilization of Glycyrrhiza uralensis Residue by Extrusion-Biological Pretreatment for Coproduction of Flavonoids, Cellulase, and Ethanol. Bioresour. Technol. 2024, 406, 131002. [Google Scholar] [CrossRef]
- Battista, F.; Zanzoni, S.; Strazzera, G.; Andreolli, M.; Bolzonella, D. The Cascade Biorefinery Approach for the Valorization of the Spent Coffee Grounds. Renew. Energy 2020, 157, 1203–1211. [Google Scholar] [CrossRef]
- Castilla-Archilla, J.; Cermeño, M.; Tuohy, M.; FitzGerald, R.J.; Lens, P.N.L. Fractionation of Brewer’s Spent Grain Using a Cascade Process for Carbohydrate Release and the Simultaneous Production of Protein and Fiber Fractions Targeting the Food Industry. ACS Sustain. Resour. Manag. 2024, 1, 2350–2360. [Google Scholar] [CrossRef]
- Muscat, A.; de Olde, E.M.; de Boer, I.J.M.; Ripoll-Bosch, R. The Battle for Biomass: A Systematic Review of Food-Feed-Fuel Competition. Glob. Food Secur. 2020, 25, 100330. [Google Scholar] [CrossRef]
- Brobbey, M.S.; Louw, J.; Görgens, J.F. A Techno-Economic and Life Cycle Assessment of Multiproduct Sugarcane Biorefinery: Lactic Acid as a Platform Chemical. Biomass Bioenergy 2025, 193, 107605. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, B.; Bao, J. Rigorous Calculation of Greenhouse Gases (GHG) in Sustainable l-Lactic Acid Production from Lignocellulosic Biomass Based on Advanced Biorefinery Processing Technology. ACS Sustain. Chem. Eng. 2025, 13, 6186–6196. [Google Scholar] [CrossRef]
- Kosamia, N.; Sanchez, A.; Rakshit, S. Scenario-Based Life Cycle Assessment and Environmental Monetary Valuation of Biosuccinic Acid Production from Lignocellulosic Biomass. Ind. Crops Prod. 2023, 202, 117101. [Google Scholar] [CrossRef]
- Winter, B.; Meys, R.; Bardow, A. Towards Aromatics from Biomass: Prospective Life Cycle Assessment of Bio-Based Aniline. J. Clean. Prod. 2021, 290, 125818. [Google Scholar] [CrossRef]
- Caldeira, C.; Garmendia, A.I.; Tosches, D.; Mancini, L.; Abbate, E.; Farcal, R.; Lipsa, D.; Rasmussen, K.; Rauscher, H.; Riego, S.J.; et al. Safe and Sustainable by Design Chemicals and Materials—Application of the SSbD Framework to Case Studies. European Commission. JRC Tech. Rep. 2023, JRC131878. [Google Scholar] [CrossRef]
- Fantke, P. Safe and Sustainable-by-Design (SSbD): Calling for Efficient Metrics, Biophysical Benchmarks, and Broader Application. Sustain. Chem. Pharm. 2025, 45, 101986. [Google Scholar] [CrossRef]
- Circular Bio-Based Europe-Joint Undertaking (CBE-JU), Why CBE-JU Matters? Available online: https://www.cbe.europa.eu/why-does-cbe-ju-matter (accessed on 31 August 2025).
- Ma, W.; Hoppe, T.; de Jong, M. Policy accumulation in China: A longitudinal analysis of circular economy initiatives. Sustain. Prod. Consum. 2022, 34, 490–504. [Google Scholar] [CrossRef]
- Santa Rita, L.P.; Ramos Filho, J.R.B.; da Silva, E.M.; da Silva, B.A.; Balliano, T.L.; de Oliveira, W.S.; de Oliveira, R.; Silva, R.J.; Lima, C.P. Strategies for Bioeconomy Development: Insights into Brazil’s New Industrial Policy. In Intangibles in the Knowledge Economy; Tomé, E.L.S., Ed.; Springer Proceedings in Business and Economics; Springer Nature: Cham, Switzerland, 2024; pp. 111–121. [Google Scholar] [CrossRef]
- BioE3 Policy: Accelerating Sustainable Biomanufacturing Initiatives and Bioeconomy Growth in Delhi, India. Available online: https://bmi.dbtindia.gov.in/ (accessed on 26 September 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).