Microscopic Mechanism of Moisture Affecting Methane Adsorption and Desorption in Coal by Low-Field NMR Relaxation
Abstract
1. Introduction
- 1.
- The adsorption and desorption results acquired by LF-NMR and gas adsorption analysis can be mutually verified. LF-NMR can achieve a more accurate quantification of methane in coal compared with the 3H-2000PHD analyzer using the volumetric method and is nondestructive, fast, and accurate.
- 2.
- Many existing studies mainly involve experiments on coal powder or briquettes. Although micropores are barely affected, macrostructure destruction results in deviation from the actual situation. Therefore, samples are from raw coal and are not pulverized in this work.
- 3.
- Analyzing gas adsorption and desorption in moist coal samples with LF-NMR technology has not been conducted in other studies before, and this kind of sampling and method is in line with the on-site conditions. In addition to the desorption rate and quantity, the methane distribution variation in coal during desorption can be determined, contributing to revealing the moisture mechanism from the microscopic perspective.
2. Materials and Methods
2.1. Sample Preparation
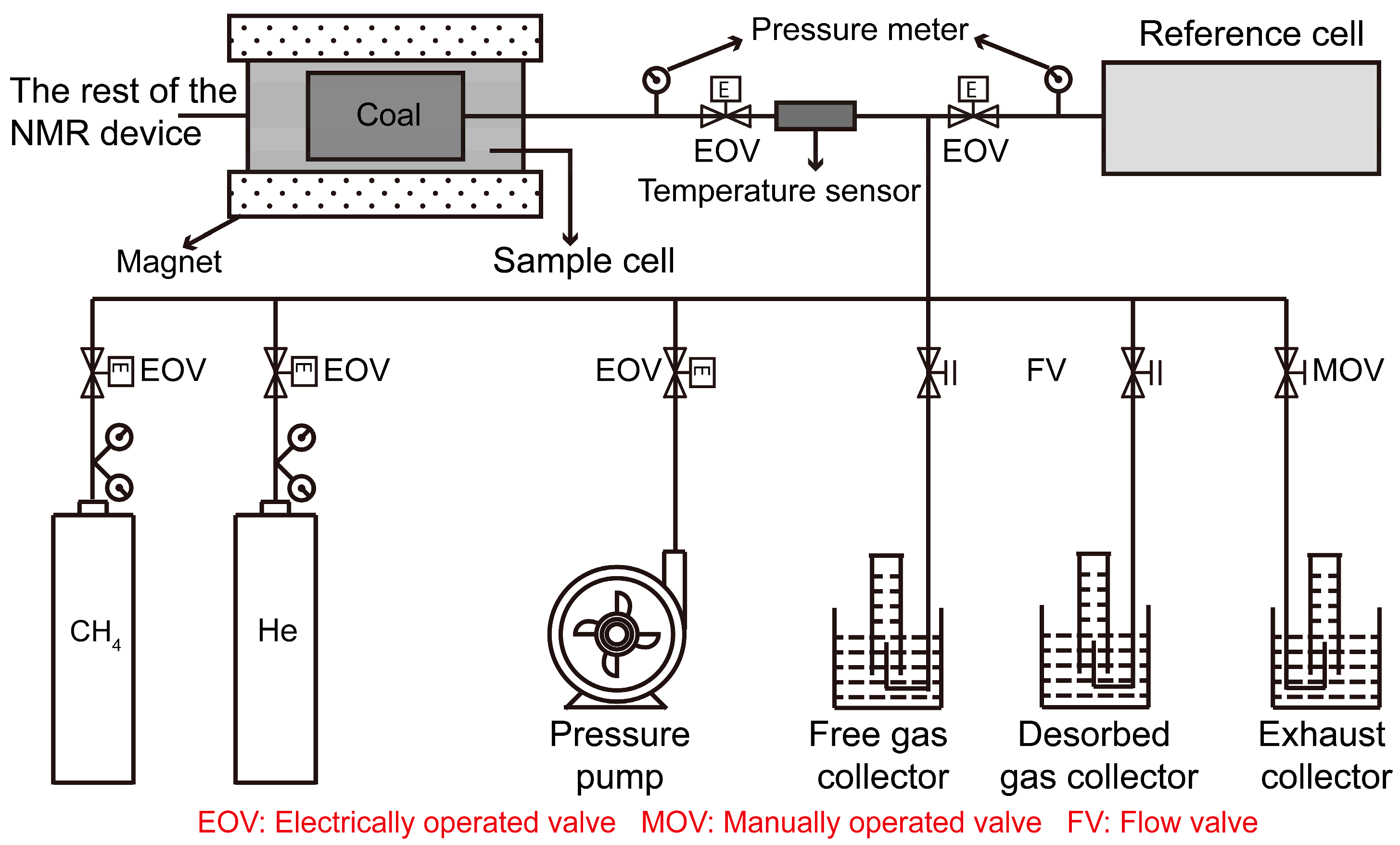
2.2. Experimental Setup
- ASAP 2460 surface area system from Micromeritics Instrument Corporation, Shanghai, China, and
- MacroMR12-150H-I LF-NMR system from Niumag Corporation, Suzhou City of China, and
- 3H-2000PHD automatic high-pressure adsorption and desorption analyzer from Beishide Instrument Corporation in Beijing, China.
2.3. Experimental Methods
- Low-temperature nitrogen measurements were conducted twice to ensure consistency. Samples A and B are powdered samples from the same raw coal, with a mass of about 0.1 g and particle size less than 80 mesh.
- The theoretical basis and steps of volumetric analysis have previously been discussed by many other researchers [17,18,19]. After the pressure tightness was tested, helium was used to assess the gas tightness and measure the reference and sample cell and void volumes. The experiment was then automatically conducted by the built-in program, and the amount of methane adsorption can be calculated under different equilibrium pressures. A flow meter was used to measure the desorption rate and amount. Desorption was considered to be terminated if the desorption amount was smaller than 1 mL within 10 min.
- The T2 spectra before methane adsorption are the base signals. Sample 1 (dry) was used to acquire the linear relation between the LF-NMR and volumetric results. Samples 2 (dry) and 3 (water-saturated) were used for methane adsorption under pressures of 0.29, 0.62, 0.89, 1.22, 1.53, and 1.83 MPa. For samples 2 and 3, LF-NMR measurements were conducted before and during adsorption and after reaching adsorption equilibrium pressures of 0.29, 0.62, 0.89, 1.22, 1.53, and 1.83 MPa. For samples 2–6, the LF-NMR results were recorded at 1 and 60 min after desorption began.
2.4. Basic Principle of LF-NMR
3. Results
3.1. Results Obtained with the ASAP 2460
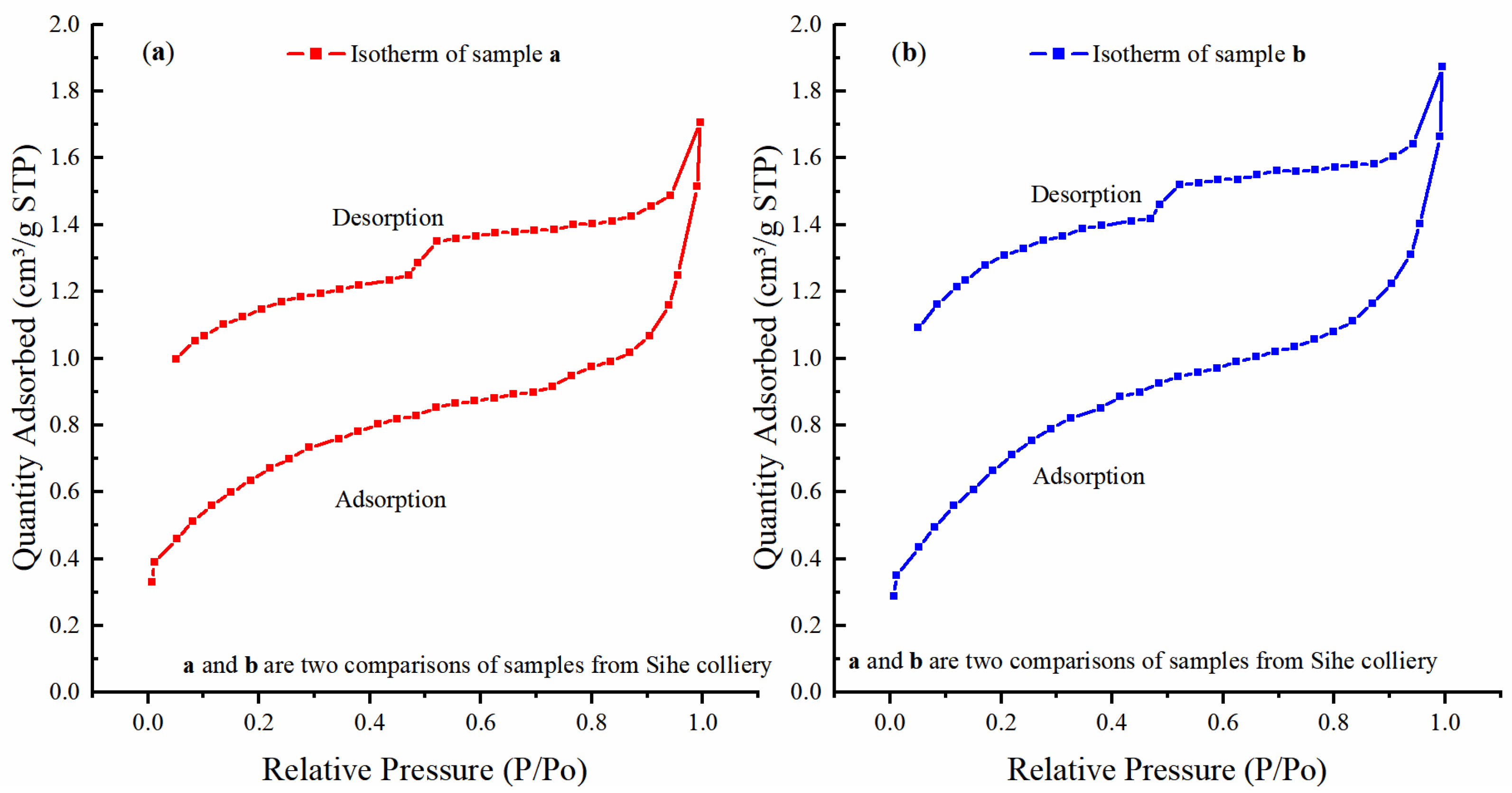
3.1.1. Analysis of Low-Temperature Nitrogen Isotherms
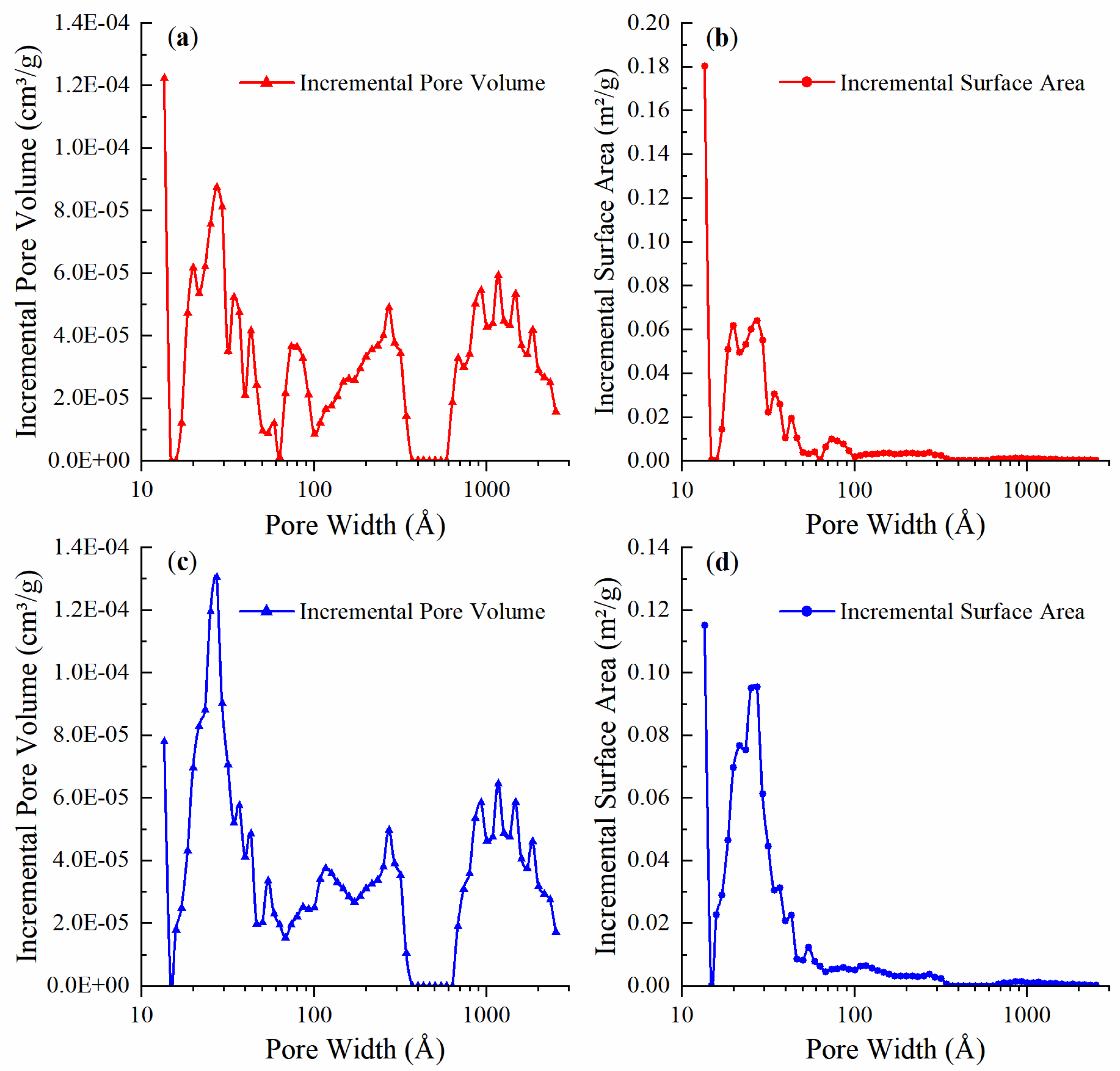
3.1.2. Analysis of the Pore Structure
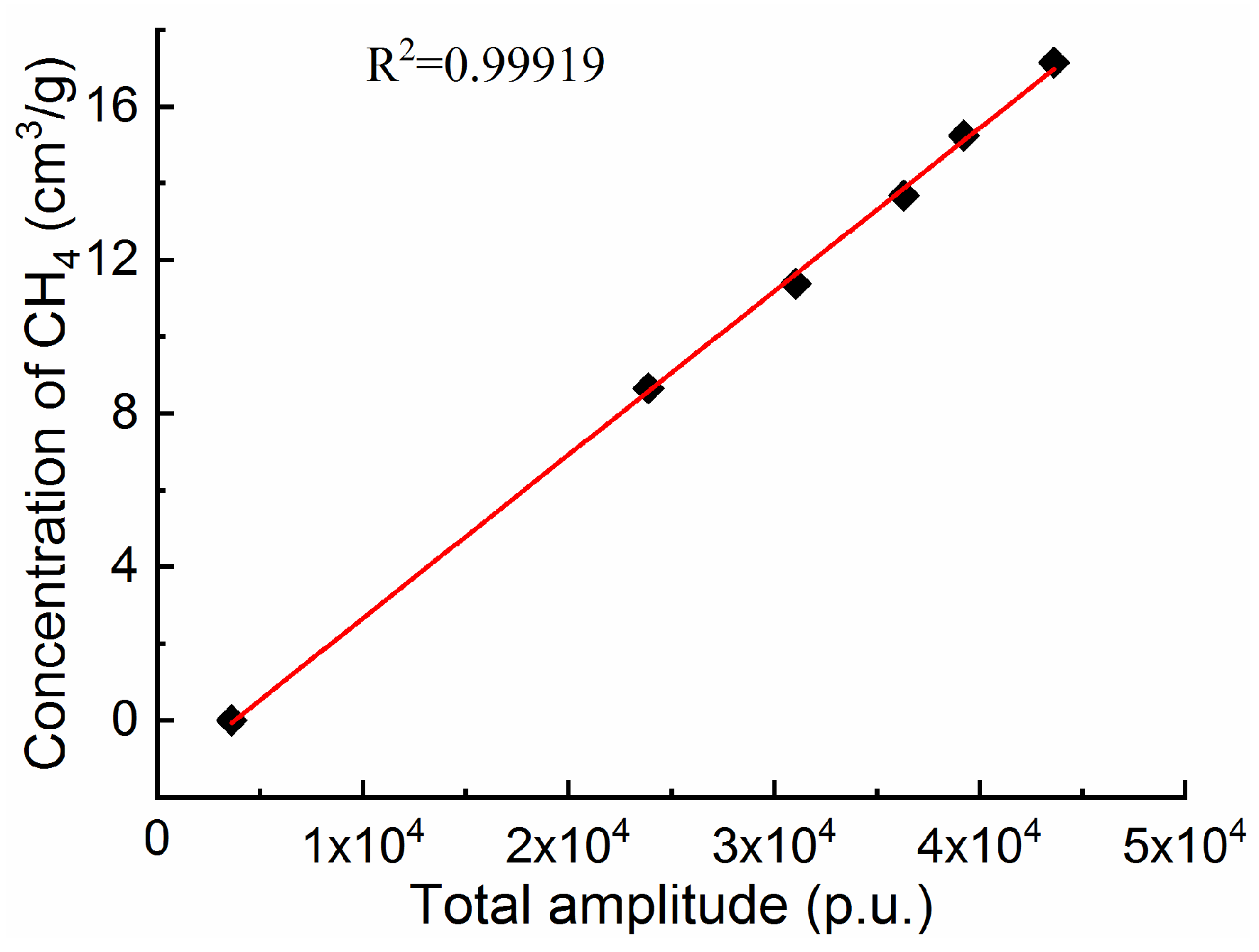
3.2. Relationship Between the Amplitude and Methane Quantity
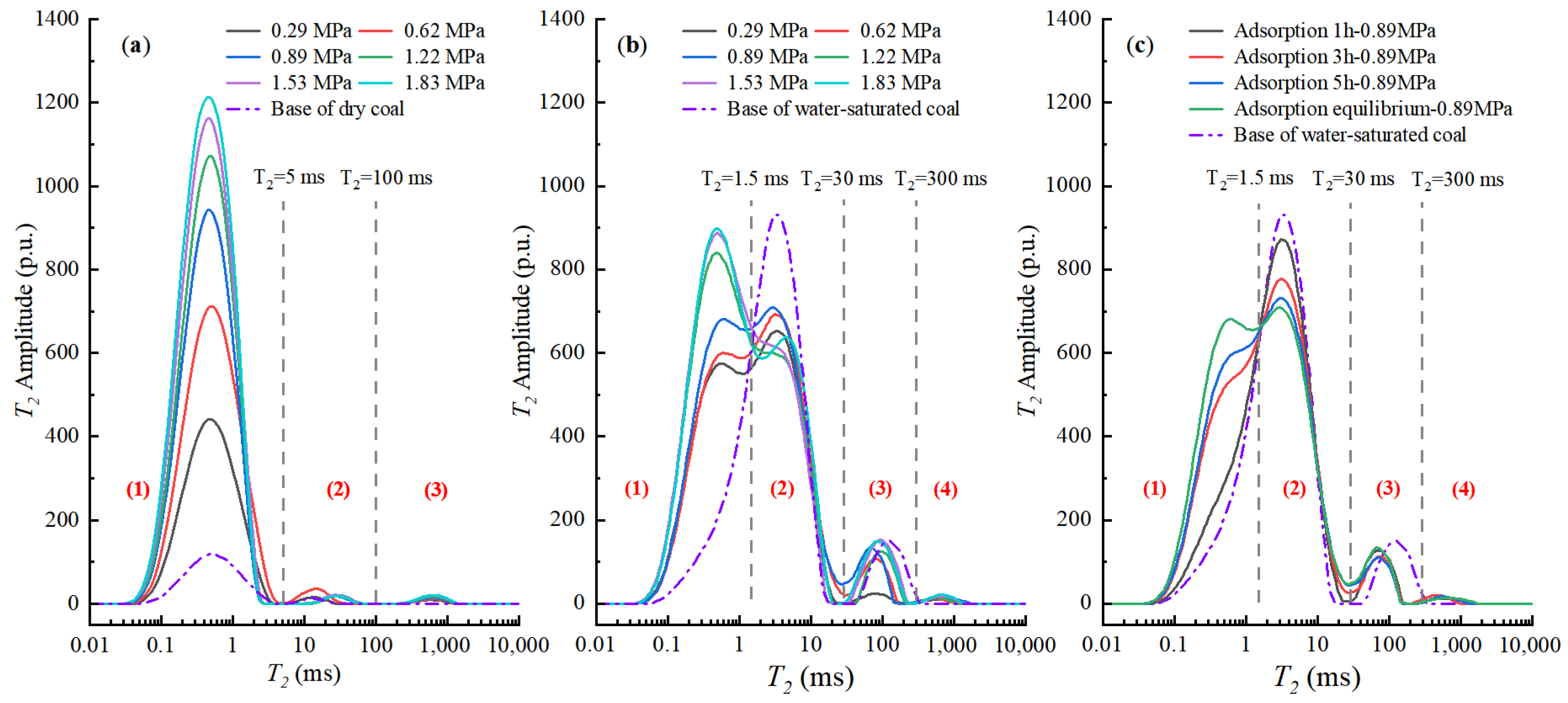
3.3. Methane Adsorption for Dry and Water-Saturated Coals
- The signal amplitude of the wet sample is much lower than that of the dry sample, reflecting the reduction in adsorption capacity.
3.4. Methane Desorption for Moist Coals
4. Discussion
4.1. Moisture Effect
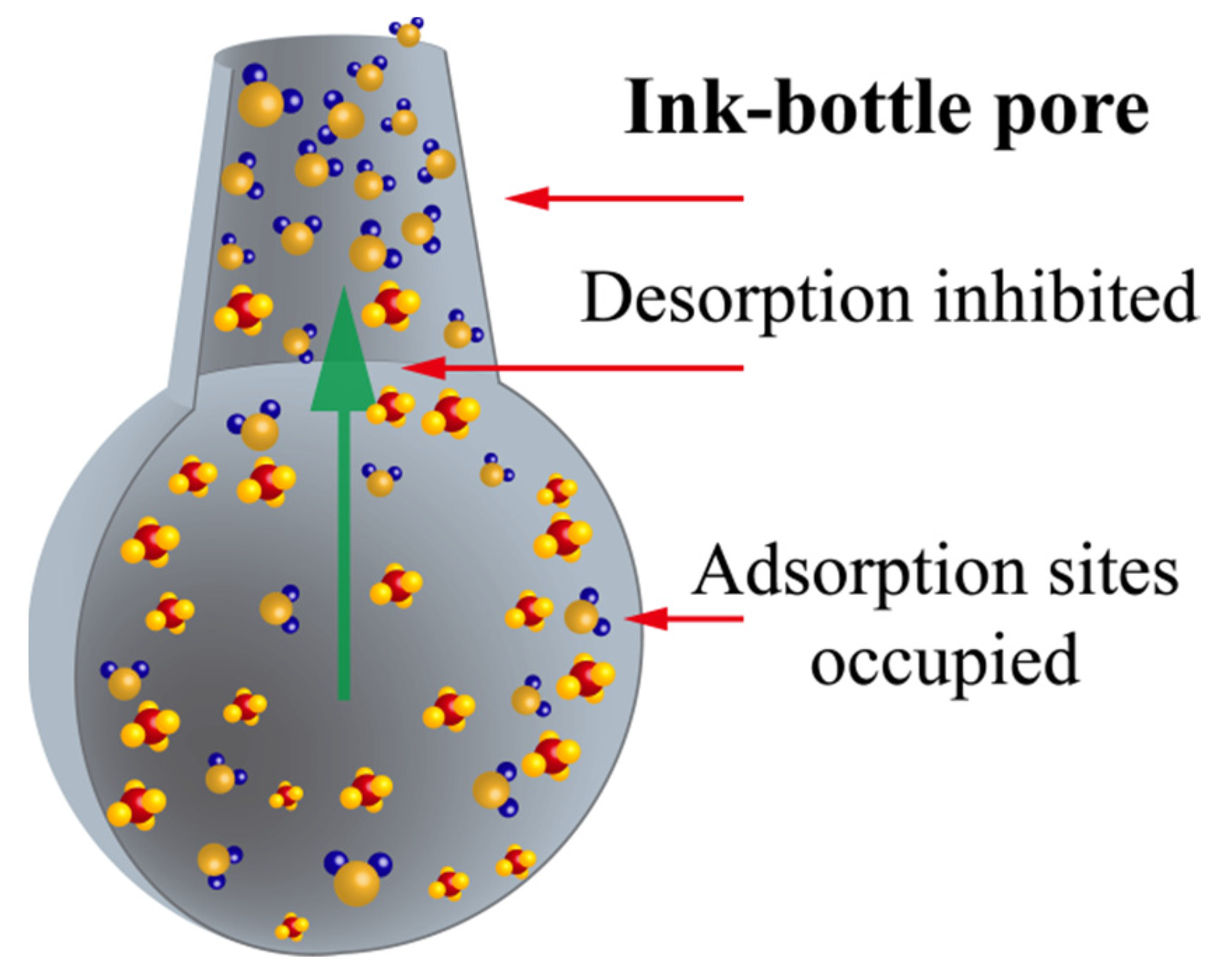
- As previously mentioned and as shown in Figure 10, a large number of ink bottle pores are present in coal. Considering their particular shape, the micropores that are essential for both adsorption and desorption can easily be blocked by water molecules.
- The adsorption capacity can be notably reduced by water, owing to the adsorption competition effect (mainly in micropores) and capillary forces (in transition pores and mesopores) caused by water. Then, the methane amount involved in desorption will be reduced.
- Pores that are suitable for methane migration and diffusion in dry coals can retain methane in moist coals, meaning less space and therefore greater difficulty for methane migration and diffusion.
4.2. Field Application
- 1.
- CBM recovery
- 2.
- Outburst prevention
- 3.
- CMM emission
4.3. Practical Integration of LF-NMR Monitoring in CBM Operations
- 1.
- Downhole NMR Logging Tools: Compact, robust LF-NMR probes can be deployed in boreholes for in situ assessment of coal seam moisture and gas content. This can complement existing well-logging suites (e.g., resistivity, acoustic) to provide direct indicators of gas saturation and mobility.
- 2.
- Laboratory-to-Field Calibration: Establish correlation models between LF-NMR responses and core-scale gas/water content measurements. These models can then be used to interpret field NMR data, reducing reliance on invasive coring.
- 3.
- Monitoring Enhanced CBM Recovery: During enhanced recovery operations (e.g., CO2 injection, nitrogen flushing), LF-NMR can be used to track fluid displacement, methane desorption efficiency, and moisture redistribution, providing real-time feedback for process optimization.
4.4. Potential for Numerical Modeling Integration
5. Conclusions
- This paper verifies both the qualitative and quantitative reliability of LF-NMR technology in obtaining the methane state and amount in coal. The T2 amplitude exhibits a notable linear correlation with the methane quantity, and the errors compared with the results from the 3H-2000PHD analyzer are negligible.
- The SSA, RSSA, and T2 spectra of the dry sample reveal highly developed micropores in the coal, and the significance of micropores in methane adsorption and desorption is proven. Moisture in coal can expand the pore size range for methane adsorption. The adsorption amount in meso- and macropores is quite limited, but their role in methane diffusion cannot be neglected, which facilitates adsorption and desorption. Pores in all pore size ranges are indispensable for adsorption and desorption.
- The moisture effect in inhibiting methane desorption consists of reducing the methane amount and desorption rate. Moisture can not only inhibit methane migration but can also change the function of pores. The ratio of the desorption amount in 1 min vs. that in 60 min can be adopted as an indicator in evaluating the effect of moisture.
- An accurate and reliable understanding of the moisture mechanism will have a profound impact on CMM utilization, greenhouse gas emission reduction, and underground mining safety enhancement.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, F.; Liang, Y.; Li, X.; Li, L.; Li, J.; Chen, Y. Orthogonal Experimental Study on Multifactor Conditions for Gas Desorption in Coal. Adv. Civ. Eng. 2019, 2019, 3028721. [Google Scholar] [CrossRef]
- Li, Y.; Zhai, C.; Xu, J.; Yu, X.; Sun, Y.; Cong, Y.; Tang, W.; Zheng, Y. Effects of steam treatment on the internal moisture and physicochemical structure of coal and their implications for coalbed methane recovery. Energy 2023, 270, 126866. [Google Scholar] [CrossRef]
- Jiang, J.; Peng, H.; Cheng, Y.; Wang, L.; Wang, C.; Ju, S. Effect of moisture on time-varying diffusion properties of methane in low-rank coal. Transp. Porous Media 2023, 146, 617–638. [Google Scholar] [CrossRef]
- Miao, Y.; Luan, G.; Zhao, C.; Li, Y. Molecular Simulation of Methane Adsorption Behavior on Coal: Effects of Maturity and Moisture Content. J. Energy Resour. Technol 2023, 145, 052602. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, B.; Hu, Y. Recent progress in improving the yield of microbially enhanced coalbed methane production. Energy Rep. 2023, 9, 2810–2819. [Google Scholar] [CrossRef]
- Dreger, M.; Celary, P. The outburst probability index (Ww) as a new tool in the coal seam outburst hazard forecasting. J. Sustain. Min. 2024, 23, 55–60. [Google Scholar] [CrossRef]
- Zhang, L.; Ren, T.; Aziz, N. Influences of temperature and moisture on coal sorption characteristics of a bituminous coal from the Sydney Basin, Australia. Int. J. Oil Gas Coal Technol. 2014, 8, 62–78. [Google Scholar] [CrossRef]
- Wang, L.; Liu, M.; Li, J.; Sun, Y.; Lv, B.; Ma, X.; Zhou, M.; Zhang, J. Investigation of methane adsorption mechanism in Low-Rank bituminous coal considering internal water Distribution: A molecular simulation and experimental study. Chem. Eng. J. 2024, 495, 153562. [Google Scholar] [CrossRef]
- Sun, W.; Liang, Y.; Li, Q.; Li, Z.; Zhao, Z.; Zheng, X.; Wang, M.; Liu, S.; Wu, Z. Experimental study on effects of tetrahydrofuran soaking on pore structure and gas adsorption and desorption characteristics of coal. Powder Technol. 2024, 445, 120117. [Google Scholar] [CrossRef]
- Zhao, L.; Ni, G.; Sun, L.; Qian, S.; Shang, L.; Kai, D.; Xie, J.; Gang, W. Effect of ionic liquid treatment on pore structure and fractal characteristics of low rank coal. Fuel 2020, 262, 116513. [Google Scholar] [CrossRef]
- Jiang, X.; Miao, B.; Zhang, J.; Xi, D.; Qin, Z.; Vandeginste, V. Quantitative Characterization of Pore-Fracture Structures in Coal Reservoirs by Using Mercury Injection-Removal Curves and Permeability Variation under Their Constraints. Processes 2024, 12, 1434. [Google Scholar] [CrossRef]
- Zhang, P.; Lu, S.; Li, J.; Chen, C.; Xue, H.; Zhang, J. Petrophysical characterization of oil-bearing shales by low-field nuclear magnetic resonance (NMR). Mar. Petrol. Geol. 2018, 89, 775–785. [Google Scholar] [CrossRef]
- Cai, Y.; Zhai, C.; Yu, X.; Sun, Y.; Xu, J.; Zheng, Y.; Cong, Y.; Li, Y.; Chen, A.; Xu, H.; et al. Quantitative characterization of water transport and wetting patterns in coal using LF-NMR and FTIR techniques. Fuel 2023, 350, 128790. [Google Scholar] [CrossRef]
- GB/T 212-2008; Proximate Analysis of Coal. Standards Press of China: Beijing, China, 2008.
- GB/T 19560-2008; Experimental Method of High-Pressure Isothermal Adsorption to Coal. Standards Press of China: Beijing, China, 2008.
- GB/T 8899-2013; Determination of Maceral Group Composition and Minerals in Coal. Standards Press of China: Beijing, China, 2013.
- Busch, A.; Gensterblum, Y. CBM and CO2-ECBM related sorption processes in coal: A review. Int. J. Coal Geol. 2011, 87, 49–71. [Google Scholar] [CrossRef]
- Krooss, B.V.; Van Bergen, F.; Gensterblum, Y.; Siemons, N.; Pagnier, H.J.M.; David, P. High-pressure methane and carbon dioxide adsorption on dry and moisture-equilibrated Pennsylvanian coals. Int. J. Coal Geol. 2002, 51, 69–92. [Google Scholar] [CrossRef]
- Siemons, N.; Wolf, K.-H.A.A.; Bruining, J. Interpretation of carbon dioxide diffusion behavior in coals. Int. J. Coal Geol. 2007, 72, 315–324. [Google Scholar] [CrossRef]
- Zheng, S.; Yao, Y.; Elsworth, D.; Wang, B.; Liu, Y. A novel pore size classification method of coals: Investigation based on NMR relaxation. J. Nat. Gas Sci. Eng. 2020, 81, 103466. [Google Scholar] [CrossRef]
- Shan, C.; Zhang, T.; Guo, J.; Zhang, Z.; Yang, Y. Characterization of the micropore systems in high-rank coal reservoirs of the southern Sichuan Basin, China. AAPG Bull. 2015, 99, 2099–2119. [Google Scholar] [CrossRef]
- Qin, W.; Xu, J. Horizontal Subzone Characteristics and Methane Seepage Properties of the Gas Flowing Fracture Zone above the Gob. Adv. Civ. Eng. 2018, 2018, 9071578. [Google Scholar] [CrossRef]
- Li, J.; Li, B.; Ren, C.; Zhang, Y.; Wang, B. An adsorption model for evaluating methane adsorption capacity in shale under various pressures and moisture. J. Nat. Gas Sci. Eng. 2020, 81, 103426. [Google Scholar] [CrossRef]
- He, X.; Cheng, Y.; Hu, B.; Wang, Z.; Wang, C.; Yi, M.; Wang, L. Effects of coal pore structure on methane-coal sorption hysteresis: An experimental investigation based on fractal analysis and hysteresis evaluation. Fuel 2020, 269, 117438. [Google Scholar] [CrossRef]
- Gensterblum, Y.; Busch, A.; Krooss, B.M. Molecular concept and experimental evidence of competitive adsorption of H2O, CO2 and CH4 on organic material. Fuel 2014, 115, 581–588. [Google Scholar] [CrossRef]
- Talapatra, A.; Karim, M.M. The influence of moisture content on coal deformation and coal permeability during coalbed methane (CBM) production in wet reservoirs. J. Pet. Explor. Prod. Technol. 2020, 10, 1907–1920. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, D. Influences of moisture on adsorption and desorption of methane on gas shales. Energ. Sources Part A 2020, 46, 12556–12574. [Google Scholar] [CrossRef]
- Zhou, D.; Liu, Z.; Feng, Z.; Shen, Y.; Wu, Y. The study of the local area density homogenization effect of meso-structures in coal during methane adsorption. J. Pet. Sci. Eng. 2020, 191, 107141. [Google Scholar] [CrossRef]
- Cheewaphongphan, P.; Chatani, S.; Saigusa, N. Exploring gaps between bottom-up and top-down emission estimates based on uncertainties in multiple emission inventories: A case study on CH4 emissions in China. Sustainability 2019, 11, 2054. [Google Scholar] [CrossRef]




| Parameters | Values |
|---|---|
| Moisture content | 0.69% |
| Ash content | 15.70% |
| Volatile matter content | 5.68% |
| Fixed carbon | 79.87% |
| Apparent density | 1.48 g/cm3 |
| Samples | Items | Diameter (<10 nm) | Diameter (10–100 nm) | Diameter (100–300 nm) |
|---|---|---|---|---|
| Sample a | Pore volume (cm3) | 0.0010 | 0.00068 | 0.00050 |
| SSA (m2) | 0.75566 | 0.05300 | 0.00691 | |
| Pore volume ratio (%) | 45.87 | 31.19 | 22.94 | |
| SSA ratio (%) | 92.65 | 6.5 | 0.85 | |
| RSSA (m2/cm3) | 755.66 | 77.94 | 13.82 | |
| Sample b | Pore volume (cm3) | 0.00124 | 0.00075 | 0.00054 |
| SSA (m2) | 0.90377 | 0.0679 | 0.00755 | |
| Pore volume ratio (%) | 49.01 | 29.64 | 21.34 | |
| SSA ratio (%) | 92.29 | 6.93 | 0.77 | |
| RSSA (m2/cm3) | 728.85 | 90.53 | 13.98 |
| P (MPa) | Dry Sample | Wet Sample | ||||
|---|---|---|---|---|---|---|
| Cv1 (cm3/g) | T1 | Cn1 (cm3/g) | Cv2 (cm3/g) | T2 | Cn2 (cm3/g) | |
| 0.29 | 4.33 | 13,710.392 | 4.237 | 1.24 | 6596.615 | 1.208 |
| 0.62 | 8.12 | 22,925.595 | 8.161 | 2.68 | 9963.101 | 2.642 |
| 0.89 | 10.21 | 27,508.516 | 10.113 | 3.88 | 12,762.265 | 3.834 |
| 1.22 | 11.92 | 31,439.285 | 11.786 | 4.93 | 15,238.342 | 4.888 |
| 1.53 | 13.52 | 34,949.527 | 13.281 | 5.78 | 17,070.758 | 5.668 |
| 1.83 | 14.41 | 37,029.011 | 14.166 | 6.23 | 18,173.07 | 6.138 |
| ηa = Cn2/Cn1 (%) | D = (Cn − Cv)/Cv (%) | |
|---|---|---|
| D1 | D2 | |
| 28.51 | −2.148 | −2.58 |
| 32.37 | 0.505 | −1.42 |
| 37.91 | −0.95 | −1.19 |
| 41.47 | −1.124 | −0.85 |
| 42.68 | −1.768 | −1.94 |
| 43.33 | −1.693 | −1.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Zhang, L.; Cui, J.; Feng, G.; Zhai, Z.; Li, Z. Microscopic Mechanism of Moisture Affecting Methane Adsorption and Desorption in Coal by Low-Field NMR Relaxation. Processes 2025, 13, 3113. https://doi.org/10.3390/pr13103113
Li Q, Zhang L, Cui J, Feng G, Zhai Z, Li Z. Microscopic Mechanism of Moisture Affecting Methane Adsorption and Desorption in Coal by Low-Field NMR Relaxation. Processes. 2025; 13(10):3113. https://doi.org/10.3390/pr13103113
Chicago/Turabian StyleLi, Qi, Lingyun Zhang, Jiaqing Cui, Guorui Feng, Zhiwei Zhai, and Zhen Li. 2025. "Microscopic Mechanism of Moisture Affecting Methane Adsorption and Desorption in Coal by Low-Field NMR Relaxation" Processes 13, no. 10: 3113. https://doi.org/10.3390/pr13103113
APA StyleLi, Q., Zhang, L., Cui, J., Feng, G., Zhai, Z., & Li, Z. (2025). Microscopic Mechanism of Moisture Affecting Methane Adsorption and Desorption in Coal by Low-Field NMR Relaxation. Processes, 13(10), 3113. https://doi.org/10.3390/pr13103113







