1. Introduction
As a predominantly agricultural country, Botswana produces a lot of keratinous biomass, such as feathers, hooves, horns, and hair, that is difficult to dispose of, thus posing environmental and health hazards [
1,
2]. The accumulation, at or near primary production factories, of disposed of keratinous products as solid waste attracts rodents and flies that may serve as vectors for the spread of human pathogens [
3]. Other methods of keratinous waste disposal include incineration, which can produce greenhouse gases such as carbon monoxide, hydrogen sulfide, nitrous oxide, and carbon dioxide that can also contribute to global warming [
4]. On the other hand, landfilling takes up land space, produces ammonia and hydrogen sulfide, and may also contaminate underground water sources [
5]. Furthermore, the hydrolysis of keratinous waste into feather meal by hydrothermal treatment results in feedstock that is poor in essential amino acids due to the high pressures and temperatures used [
6].
Keratinous biomass is chiefly composed of keratin, a fibrous insoluble protein belonging to the scleroprotein group that consists of the β-hard and soft α-helical types [
7]. Keratins are structurally complex, consisting of hydrophobic interactions and hydrogen bonds, interwoven by disulfide bridges, which especially account for their recalcitrance and high stability [
8]. The disulfide bridges coupled with the high cysteine content impart the keratin molecular structure that is very rigid and fairly resistant to mechanical stress, as well as the action by conventional proteolytic agents such as papain, pepsin, or trypsin [
9]. The recalcitrant nature of keratin and problems associated with the disposal of keratinous waste, therefore, call for cost-effective and ecofriendly strategies for their breakdown and degradation.
In this context, keratinases (E.C.3.4.21/24/99.11) are a group of protein-degrading enzymes that can break down insoluble keratins (feathers, nail, and hair) more efficiently when compared to conventional proteases [
10]. Keratinases occur widely in nature and are produced by bacteria and fungi [
11]. Keratinolytic microbes offer an alternative ecofriendly and economical approach for the hydrolysis of keratinous biomass waste into biotechnologically relevant biomolecules [
12]. Because of this potential, keratinases have emerged as promising tools in industries such as leather processing, biomedicine, animal protein supplements, cosmetics and processing of feather meal for animal feed, and bioremediation of environments polluted by keratinous waste [
12,
13]. Therefore, there is a growing need for the search of keratinolytic microorganisms with a heightened potential to produce keratinases.
Makgadikgadi pans, found in northern Botswana, are considered extremity havens harboring resilient microorganisms that are metabolically diverse and able to produce important byproducts with potent biotechnological applications [
14,
15]. Therefore, we sought to explore these pristine environments for bacterial isolates with the ability to produce keratinolytic enzymes with potential for use in the degradation of feather waste.
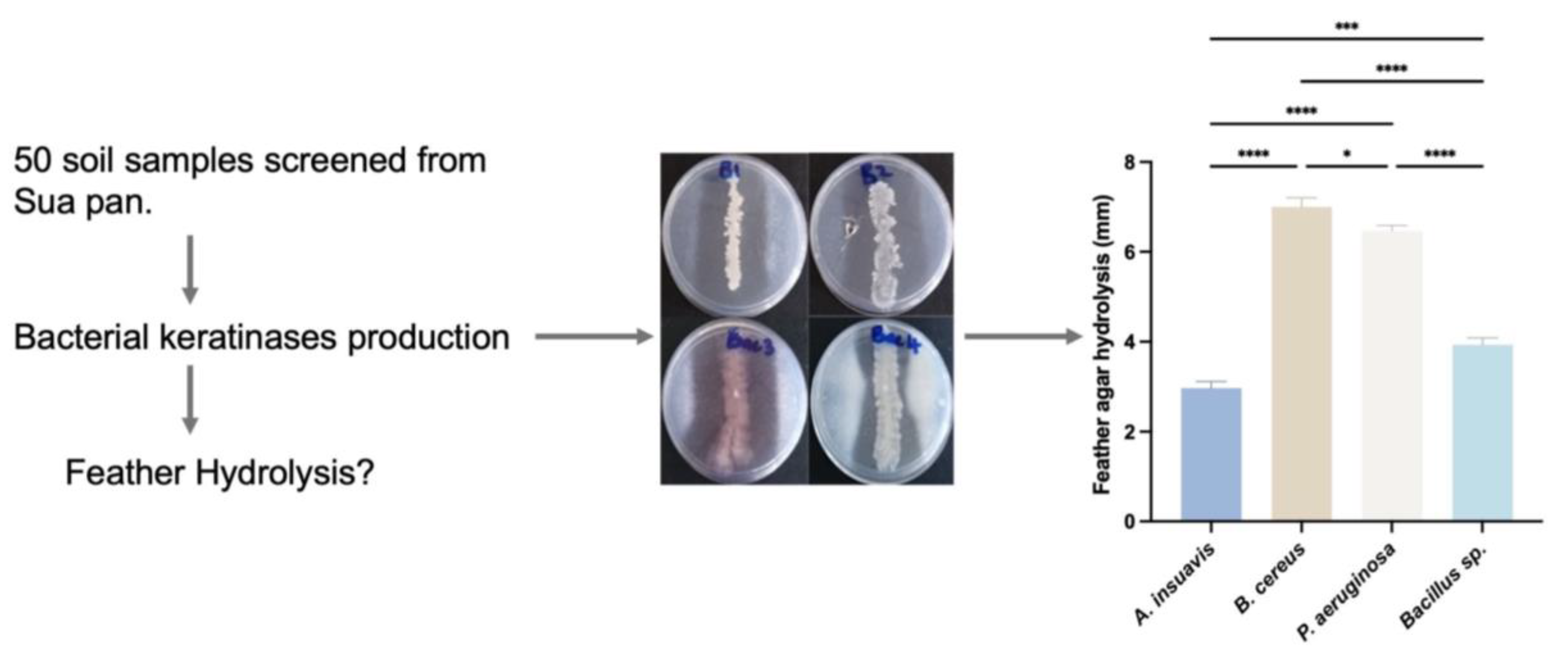
In this work, we report on four bacterial isolates capable of the complete degradation of feather waste within a reasonable time. The four bacteria displayed suitable proteolytic and keratinolytic properties under different environmental conditions (temperature and pH) and were identified and chosen as promising and potential producers of keratinases.
2. Material and Methods
2.1. Collection of Samples
Soil samples utilized for the isolation of keratinolytic bacteria were collected from the Sua Pan area, a part of the Makgadikgadi pans in northeastern Botswana (20.55° S:26.28° E). Spanning approximately 3400 km2, Sua Pan is characterized by alkaline sandy clays and silts. Fifty (50) soil samples were collected from a depth of approximately 10 cm across ten (10) different sites using a soil auger. The samples were promptly placed in sterile polyethylene bags and kept in a cooler box containing ice packs for maintenance. The samples were then transported to the Microbiology Laboratory at the University of Botswana, Gaborone, and were processed within 24 h of collection.
2.2. Preparation of Feather Meal Media
Waste feathers were collected from poultry farms around Gaborone. Feathers were thoroughly washed and put in boiling water to remove any residual waste. The feathers were then placed in a mixture of chloroform (Merck, Darmstadt, Germany) and water (1:1) for 15 min to remove fats and rinsed 3 times in distilled water. The feathers were then completely dried using an oven set at 100 °C for 2 h. One part of the treated feathers was used wholly for liquid media enrichment, and the other part was used to make feather meal agar (FMA).
2.3. Isolation of Keratinase Producing Microorganisms
The isolation of keratinase-producing bacteria was carried out following a previously described method [
1] using the spread plate technique. The soil (5 g) of each sample was weighed into 100 mL of keratin (Sigma-Aldrich, St. Louis, Missouri, USA) containing defined broth (g/L): (NH
4Cl, 0.5; NaCl, 0.5; K
2HPO
4, 0.3; KH
2PO
4, 0.4; MgCl
2·6H
2O, 0.1; yeast extract, 0.1; feather meal powder, 10; pH 7.5), and it was incubated with shaking (150 rpm) at 37 °C for 3 days. All chemicals were obtained from Sigma-Aldrich. After 3 days, 0.1 mL of the culture was transferred onto solid media (FMA) of the same composition and incubated at 37 °C for 48 h. The different colonies observed to hydrolyze keratin (forming zones of hydrolysis when applying mercuric chloride (HgCl
2) (Merck) were selected for further testing to select the best keratinase producers. The selected bacterial strains were transferred into feather broth and incubated with shaking (150 rpm) at 37 °C for 7 days. Thereafter, the strains that showed complete feather hydrolysis after 7 days were selected. Feather meal medium without bacteria was included as a negative control to ensure that the activity observed was due to keratinolytic bacteria.
2.4. Selection of the Best Keratinase-Producing Bacteria
The selected bacterial strains were streaked onto FMA (g/L): (NH4Cl, 0.5; NaCl, 0.5; K2HPO4, 0.3; KH2PO4, 0.4; MgCl2.6H2O, 0.1; yeast extract, 0.1; feather meal powder, 10; agar powder, 20; and pH 7.5) and incubated at 37 °C for 48 h. The keratinase production was observed as a clearing zone, and the enzyme index for the enzyme was calculated as follows:
Enzyme index = Zone of hydrolysis (mm)/Colony diameter (mm)
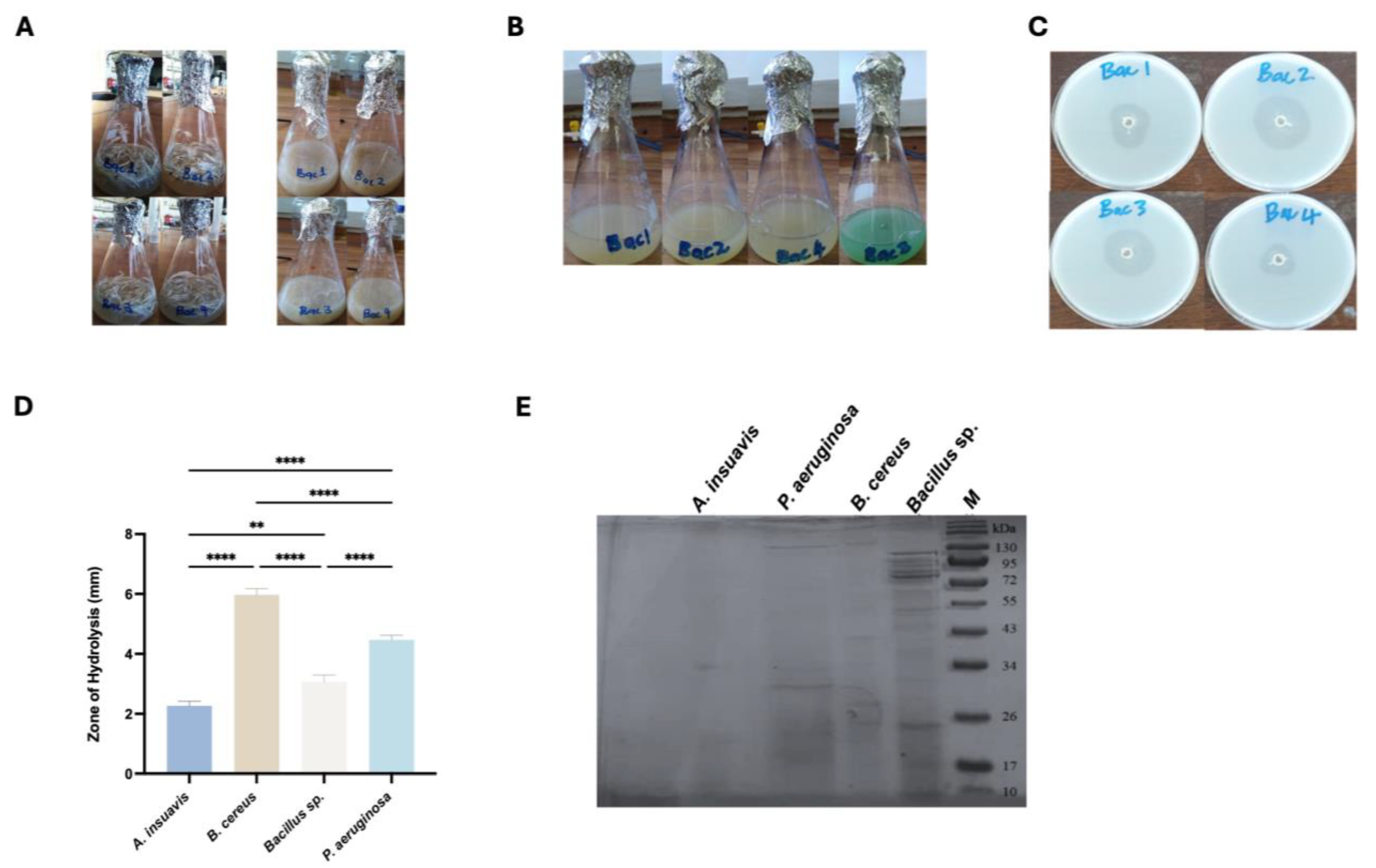
Four keratinase-producing isolates that produced the highest enzyme indices were then selected and transferred to fresh nutrient agar plates and purified by repeated streaking and then stored on agar slants and maintained at 4 °C. In these experiments, un-streaked FMA without test bacteria served as a negative control.
2.5. Purification of Selected Keratinase-Producing Bacteria
The isolates were further purified using the streak plate method and were checked whether they were Gram positive or Gram negative by the Gram stain technique. Single colonies were picked up and streaked onto fresh nutrient agar and incubated at 37 °C for 48 h and thereafter checked for purity microscopically. The pure isolates were streaked onto feather meal agar with the same conditions as above to check for loss of keratinase activity. The four isolates were transferred to a solution containing nutrient broth and 15% glycerol and stored at −20 °C for use in subsequent tests.
2.6. Identification of Keratinolytic Bacteria
The identification of selected bacteria was based on morphological, physiological, and biochemical methods following Bergey’s Manual of Systematic Bacteriology [
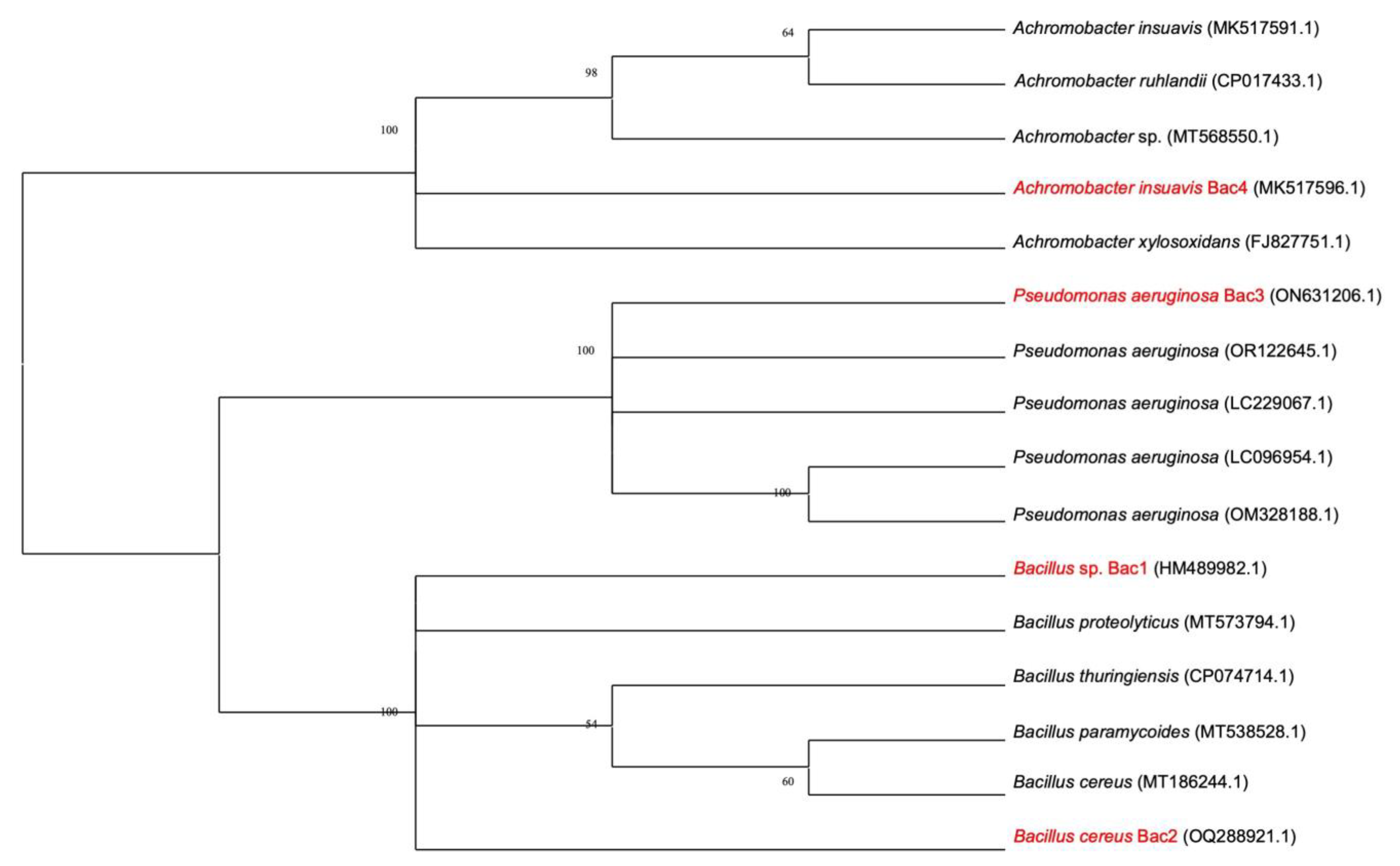
16]. The four best keratinase-producing bacteria were furthermore identified using 16S rRNA sequencing. First, DNA extraction from pure cultures was performed using a Zymo Fungal/Bacterial DNA miniprep kit (Zymo Research, Irvine, CA, USA), followed by amplification of the 16S rRNA gene using universal primers (8F, AGAGTTTGATCCTGGCTCAG and 1541R, AAGGAGGTGATCCAGCCGGA) in a Techne thermocycler (Cole-Palmer, Staffordshire, UK). PCR amplification was performed in a 25 μL reaction mix containing 12.5 μL of 2X Master Mix (New England Biolabs, Ipswich, MA, USA), 20 ng of total DNA, and 0.5 μM of each of the reverse and forward primers, and the mixture was made up to 25 μL with sterile nuclease-free water. Amplification conditions consisted of a denaturation step at 94 °C for 5 min, followed by 35 cycles at 94 °C for 1 min, 50 °C for 2 min and 72 °C for 2 min. A final extension step consisting of 7 min at 72 °C was included. The PCR amplification fragments were resolved on 1.2% agarose gel (Sigma Aldrich, St. Louis, MO, USA) at 75 V for 1 h 30 min. The gels were visualized on a gel documentation system (BIO-RAD, Hercules, CA, USA). The PCR products were purified and sequenced in both directions using an automated ABI 3500XL sequencer (Applied Biosystems, Waltham, MA, USA). The sequence data of 16S rDNA were further aligned using the BioEdit program, and sequence similarity searches were performed using the BLAST (version 2.16.0+) program that is available from the National Centre for Biotechnology Information (NCBI).
2.7. Phylogenetic Analysis
The evolutionary history of the sequences was inferred by using the Maximum Likelihood method and Kimura 2-parameter model [
17]. The percentages of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) were shown next to the branches [
18]. The initial tree(s) for the heuristic search were obtained automatically by applying Neighbor-Join and BioNJ algorithms to a matrix of pairwise distances estimated using the Maximum Composite Likelihood (MCL) approach and then selecting the topology with superior log likelihood value. A discrete Gamma distribution was used to model evolutionary rate differences among sites (5 categories (+G, parameter = 0.768)). This analysis involved 16 nucleotide sequences. There were a total of 1169 positions in the final dataset. Evolutionary analyses were conducted in MEGA11 [
19].
2.8. Enzyme Production Using Defined Feather Meal Liquid Medium
A 16 h culture (1 mL) was inoculated into a feather meal defined broth (g/L): (NH
4Cl, 0.5; NaCl, 0.5; K
2HPO
4, 0.3; KH
2PO
4, 0.4; MgCl
2·6H
2O, 0.1; feather powder, 10.0; pH 7.5), and it was incubated with shaking (150 rpm) at 37 °C for 96 h. After 96 h, cells and insoluble residues in the culture were removed by centrifugation, and the culture supernatant collected was used as the enzyme source [
20].
2.9. Molecular Weight Determination Using Sodium Dodecyl Sulphate Polyacrylamide Gel Electrophoresis (SDS-PAGE)
Determination of keratinase molecular weight was performed using the modified SDS-PAGE method of [
21], and all chemicals were obtained from Sigma-Aldrich. Protein samples were prepared by mixing them with a 4X sample buffer containing Tris-HCl, SDS, glycerol, bromophenol blue, and the reducing agent 2-mercaptoethanol (BME). Samples were heated at 95 °C for 5 min to denature proteins and reduce disulfide bonds prior to loading.
Electrophoresis was performed using a 12% polyacrylamide separating gel with a 4% stacking gel. Forty microliters (40 µL) of each sample and a Takara pre-stained protein molecular weight ladder (Takara Bio Inc., Shiga, Japan) were loaded into the wells. The gel was run in Tris–glycine running buffer (25 mM Tris, 192 mM glycine, 0.1% SDS, pH 8.3) at a constant voltage of 120 V until the tracking dye reached the bottom of the gel. Following electrophoresis, the gels were stained with Coomassie Brilliant Blue R-250 (0.1% in 50% methanol, 10% acetic acid) for 1 h and destained in 40% methanol/10% acetic acid until a clear background was achieved. Protein band sizes were estimated by comparing migration distances to the molecular weight marker (New England Biolabs), and the images were taken using the Chemidoc XRS+ system (Bio-Rad, Hercules, CA, USA).
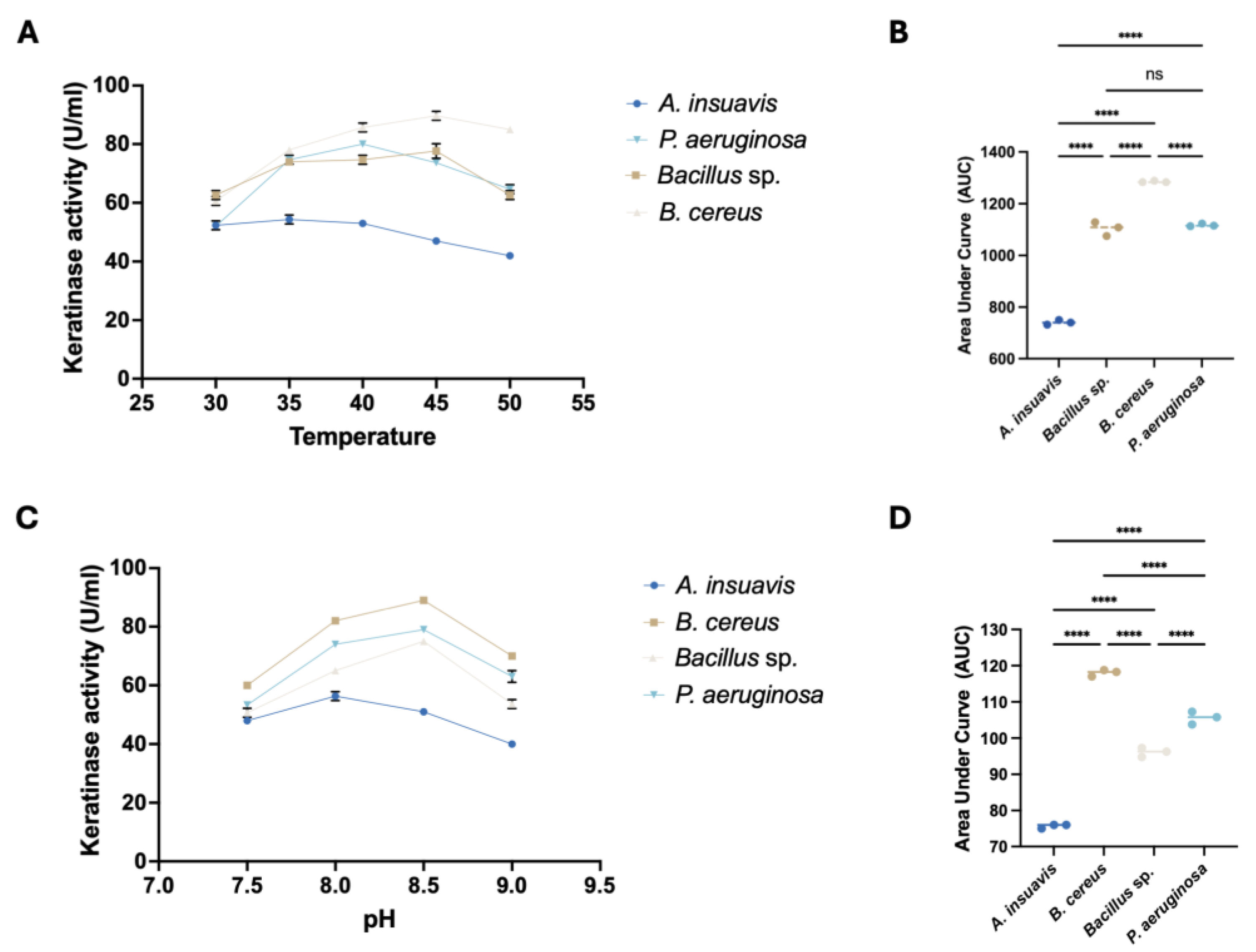
2.10. Effect of Temperature and pH on Keratinase Activity
The effect of temperature on crude keratinases activity was studied over a temperature range of 30–50 °C. All chemicals were obtained from Sigma-Aldrich. The basal medium was prepared by adding (i) salts (g/L): (NH
4Cl, 0.5; NaCl, 0.5; K
2HPO
4, 0.3; KH
2PO
4, 0.4; MgCl
2·6H
2O) and (ii) buffer stock solution (14.2 g NaH
2PO
4) adjusted to a pH of 9.0 with a concentrated solution of KH
2PO
4. Finely chopped keratin azure (4 mg) was added to 1 mL of basal medium. Twenty microliters of the crude keratinase was added to the 1 mL keratin azure mixture and incubated at different temperatures on a shaker set at 150 rpm. For each temperature regime, the incubation period was 1 h, and the reaction was terminated by adding 1 mL of 10% trichloroacetic acid. Keratinase activity was measured using a spectrophotometer (Zeiss, Oberkochen, Baden-Württemberg, Germany) using L-Tyrosine (1 mg/mL) as a standard. The absorbance was measured at 660 nm using the reagent as a blank. One unit of keratinase activity was expressed as the amount of enzyme that releases 1 µg of amino acid equivalent to tyrosine per minute under the standard assay conditions or the amount of enzyme sample that will cause an increase of the absorbance unit [
21]. The effect of pH on the enzyme activity was determined at 37 °C over a pH range of 7.5–9. Twenty microliters of the crude keratinase was added to the 1 mL keratin azure and incubated at 37 °C for 1 h on a shaker set at 150 rpm, and the reaction was terminated by the addition of 10% trichloroacetic aid. The keratinase activity was measured using a spectrophotometer. The absorbance was measured at 660 nm using the reagent as a blank. One unit of keratinase activity was expressed as the amount of enzyme that releases 1 µg of amino acid equivalent to tyrosine per minute under the standard assay conditions or the amount of enzyme sample that will cause an increase of 1 absorbance unit [
22]. In all the experiments, crude keratinase free media were run alongside the treatments as negative controls.
2.11. Statistical Analysis
All experiments in this study were performed in triplicates, and data are presented as mean ± standard deviation (SD). Statistical analyses were carried out using GraphPad Prism Version 9.5.1 unless otherwise stated. To compare feather hydrolysis and keratinase activity among bacterial isolates, a one-way analysis of variance (ANOVA) was used followed by Tukey’s multiple comparison post hoc test to identify significant pairwise differences. A p value of less than 0.05 was considered statistically significant; significance levels are indicated in figures as ns, *, **, ***, **** indicating, respectively, not significant, p < 0.05, p < 0.01, p < 0.001, and p < 0.0001.
4. Discussion
The disposal of feather waste from poultry farms globally presents waste management problems that may also impact public health [
23]. Keratin, the main protein component of chicken feathers, is a hardy substance that is very difficult to degrade under natural conditions. However, keratinolytic microorganisms readily degrade keratin in a cost-effective and ecofriendly manner by elaborating keratinases. The by-products from keratin breakdown include peptides, amino acids, and peptones, and due to their multifarious biotechnological potential and diverse properties, keratinases have garnered interest in industries such as cosmetics, biofertilizer, pharmaceuticals, and animal nutrients, as well as waste management and bioremediation applications [
12].
Microorganisms that inhabit extremely saline and alkaline habitats have been considered useful because of possible biotechnological uses for their enzymes and other bioactive secondary metabolites of industrial importance. Thus, the bioprospecting of keratinase-producing bacteria continues to be necessary to discover new biocatalysts. Previous studies [
14,
24] have isolated haloalkophilic microorganisms from these pristine habitats of Botswana, indicating that they harbor a diversity of microorganisms with unique traits important in biotechnological applications.
In this study, we report, for the first time, the recovery and characterization of four keratinolytic bacteria and their keratinases from the soils of Sua Pan in northern Botswana. Based on phenotypic and physiological characteristics and the phylogenetic relationships of the selected strains, our isolated keratinolytic bacteria were identified as
Bacillus cereus Bac 2,
Pseudomonas aeruginosa Bac 3,
Achromobacter insuavis Bac 4, and
Bacillus sp. Bac 1. All our strains grew well in feather medium and rich nutrient medium (nutrient agar) despite being recovered from soils that are low in nutrients. The findings of this study are different from those by [
25], who reported that their hydrolytic bacterial isolates (
Variovorax sp. and
Bradyrhizobium sp.) from pristine Hawaiian soils were slow growers in nutrient-rich media and, thus, were classified as oligotrophs. Therefore, our bacterial isolates might be described as copiotrophs because of their ability to grow well on rich media.
Bacillus sp. and
P.
aeruginosa have been previously isolated and reported by different researchers as keratinolytic bacteria [
26]. However, there are no reports of
A.
insuavis as a keratinolytic bacterial species. To our knowledge, this is the first study to report this bacterium as a keratinase-producing bacterium.
In our study, the use of feathers as the sole carbon and nitrogen source successfully discriminated all non-keratinolytic bacteria.
B.
cereus Bac 2 exhibited the best hydrolytic activity among the four bacteria, based on the largest zone of hydrolysis produced, followed closely by
Bacillus sp. Bac 3 and
P. aeruginosa. The feather hydrolysis test was followed by the separation of the cells from the culture medium, and the supernatant containing the crude keratinase was used to study keratinolytic activity of the four bacteria, with feather meal as the sole source of energy. Overall, the proteases and keratinases of alkalohalophilic bacteria studied herein ranged from 26 to 129 kDa, which was comparable to previous studies [
27]. While
Bacillus and
Pseudomonas species are well established in keratinase production [
28,
29], the potential of
A. insuavis is preliminary at this stage because only the effects of temperature and pH on keratinase activity were explored in this study. However, keratinase production can be strain-specific and can also be dependent on various chemical formulations of microbiological media [
30]. Due to findings on
A. insuavis Bac 4 being novel, future studies are necessary to classify it as a keratinolytic bacterium.
We also studied keratinase activity of the four bacterial isolates, using 1% chicken feather meal as the sole carbon and nitrogen sources. Our results indicated that keratinase activity for the bacterial strains was optimal between 40 and 45 °C, except for
A. insuavis. Our findings on the temperature functionality of keratinases from both
Bacillus and
Pseudomonas were comparable to previous studies [
12,
31]. However, other studies reported peak keratinase activity at temperatures up to 65 °C [
32].
The pH of the growth medium has influence not only on bacterial growth but also on the enzyme activity, stability, as well production of associated metabolites [
33]. In this study, maximal keratinase activity was found between a pH of 8.0 and 8.5, revealing the alkaliphilic function of the keratinases, thus defining their applicability in alkaline industrial processes. Similar to our temperature studies, results established keratinase from
B. cereus as the most potent across the pH range investigated. Changes in ambient pH can destabilize the enzyme functional structure, impacting the net charge and eventually deactivating the protein [
34]. While our results were in the range of previous studies on the pH stability of
Bacillus [
31] and
Pseudomonas [
6] keratinases, our on-going studies will help establish the parameters required for optimal keratinase production by
A. insuavis.Overall, our work presents a cost-effective method for producing keratinases from feather biomass as the major and cheap energy source, thus lowering the production cost and breaking down the normally difficult-to-digest feather substrate. Although previous studies have explored feather biomass dumping sites for keratinolytic microorganisms, this study explored untapped pristine and extremophilic environments in Botswana for keratinase-producing bacteria. This study has revealed that the keratinase landscape is vast and quite unexplored and that different degradomes will grow in the future, as new enzymes with unique characteristics are identified and characterized; thus, our isolated extremophilic bacteria show promise in the industrial production of keratinases. In addition, the considerably high pH and temperature functionality exhibited by keratinases in this study are in good agreement with the conditions in which these enzymes are used, such as the high temperatures and pH used in detergent industries. Further studies involving enzyme purification, recombineering, and process scale-up are required to unlock the full biotechnological potential of these strains.