Hydrophobization of Natural Polymers by Enzymatic Grafting of Hydrophobic Polysaccharides, Partially 2-Deoxygenated Amyloses
Abstract
1. Introduction
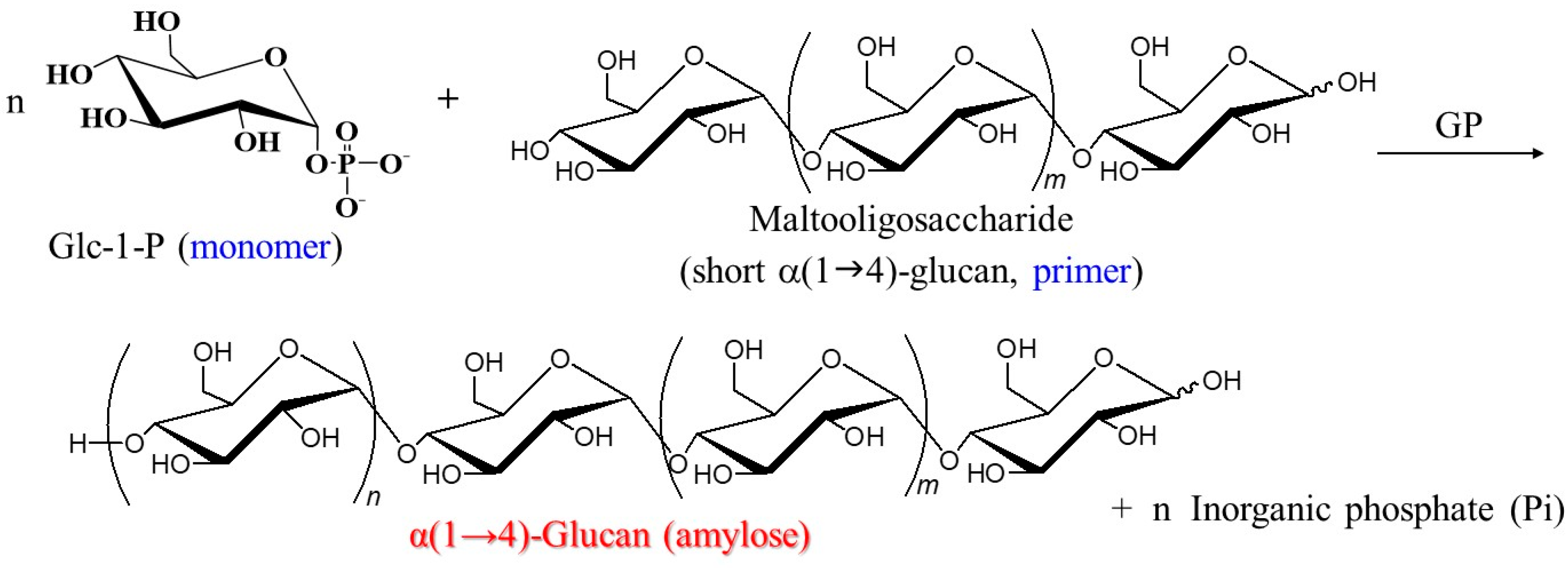
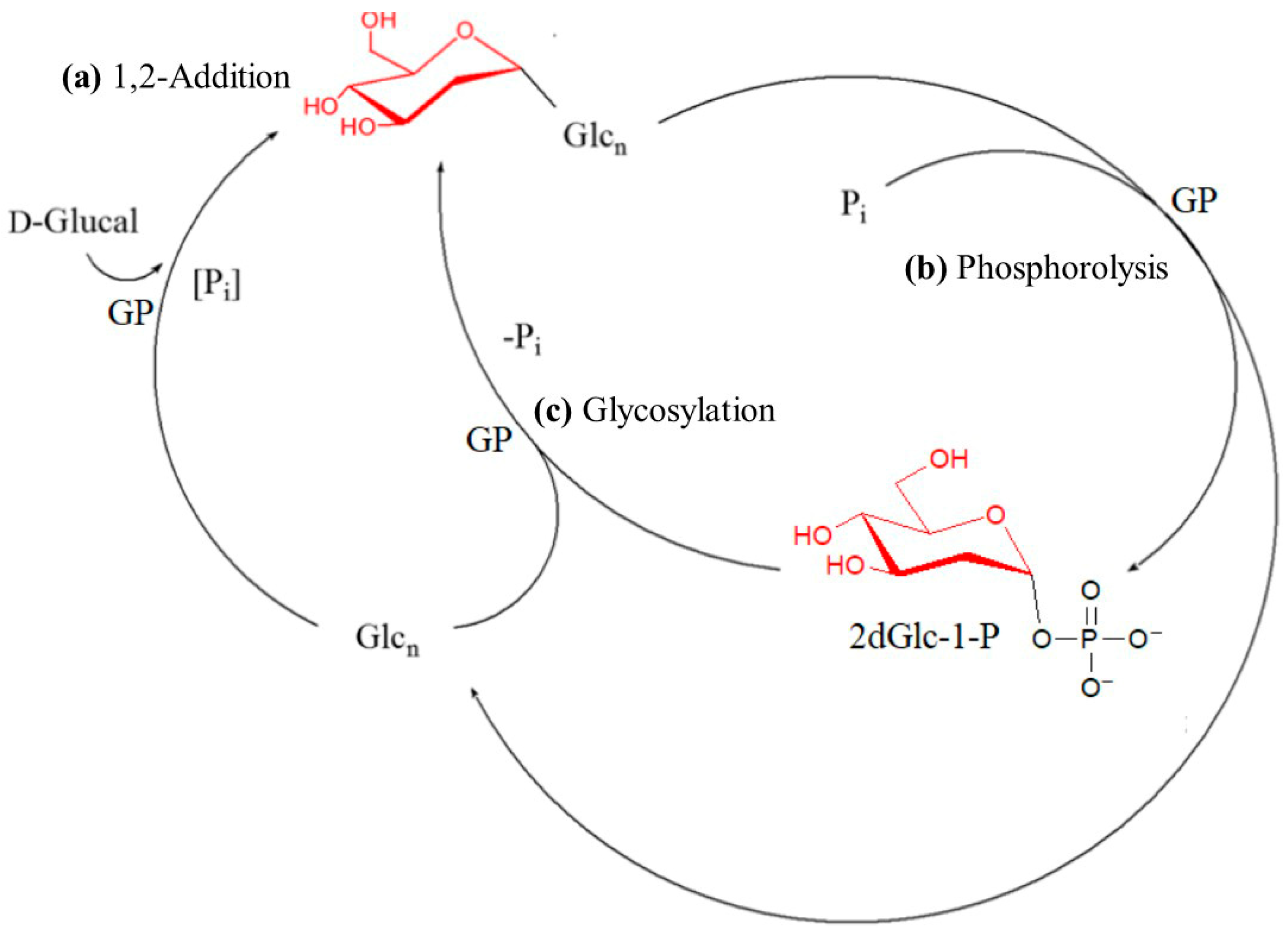
2. Characteristic Features of GP-Induced Enzymatic Polymerization
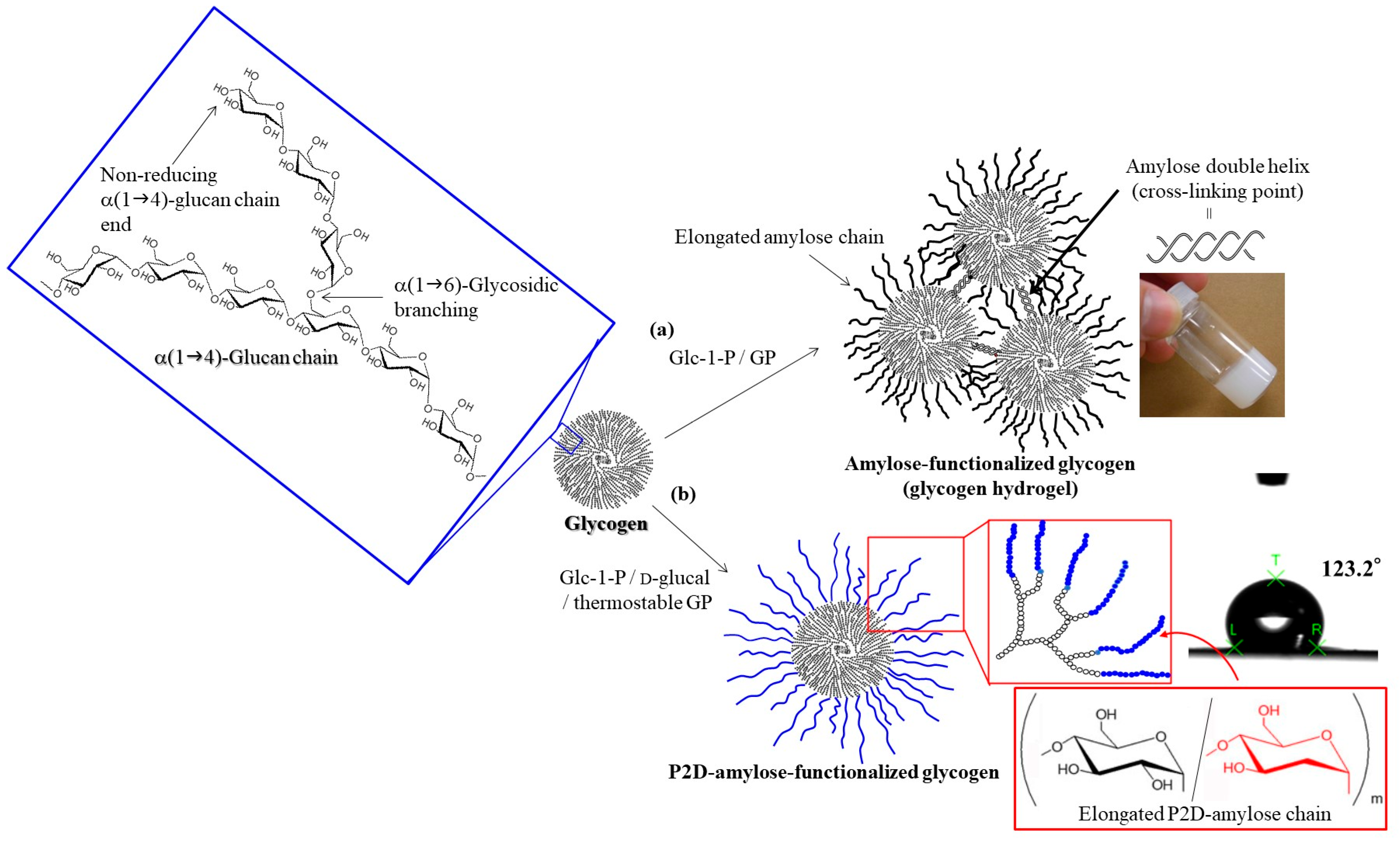
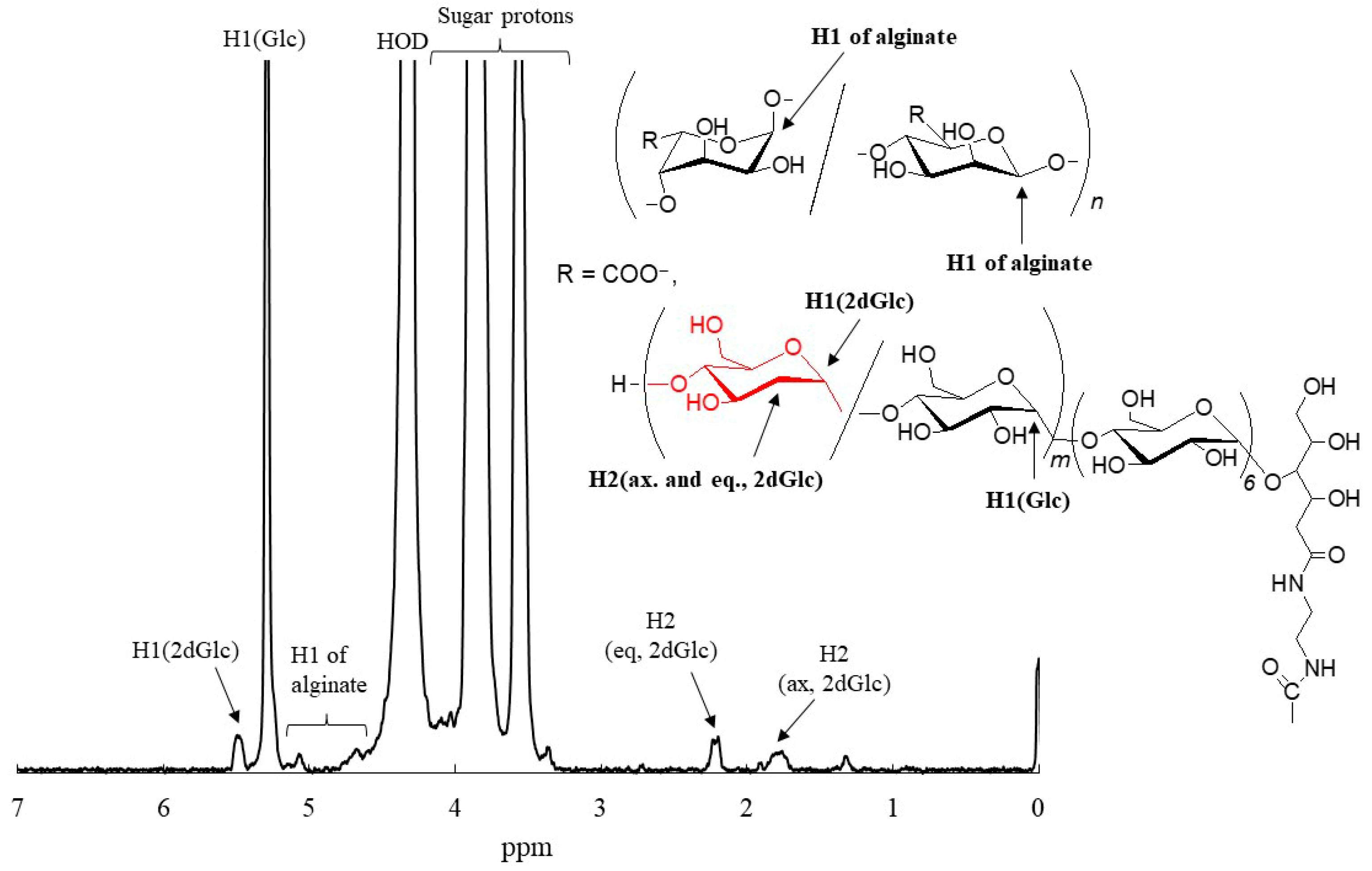
3. Enzymatic Synthesis and Specific Characters of P2D-Amylose
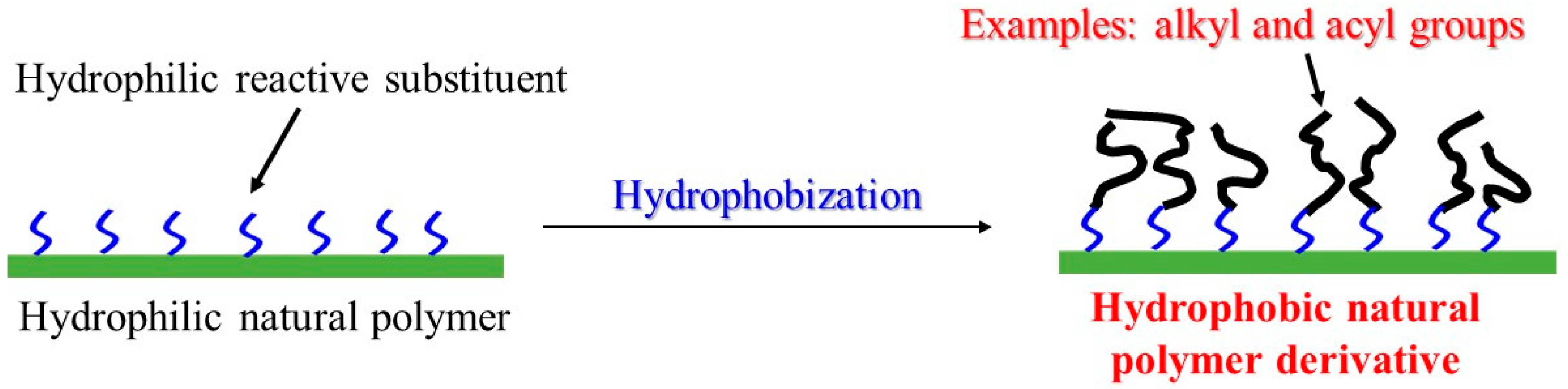
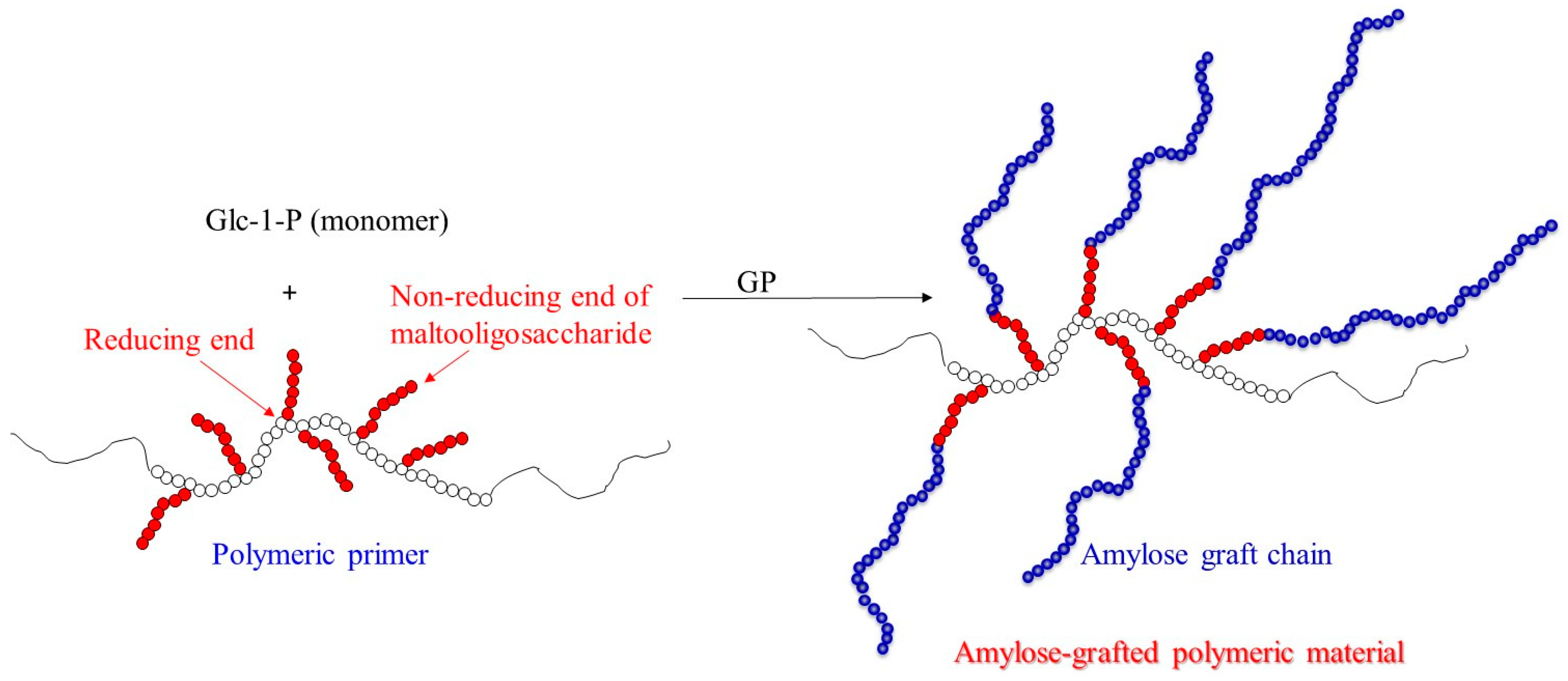
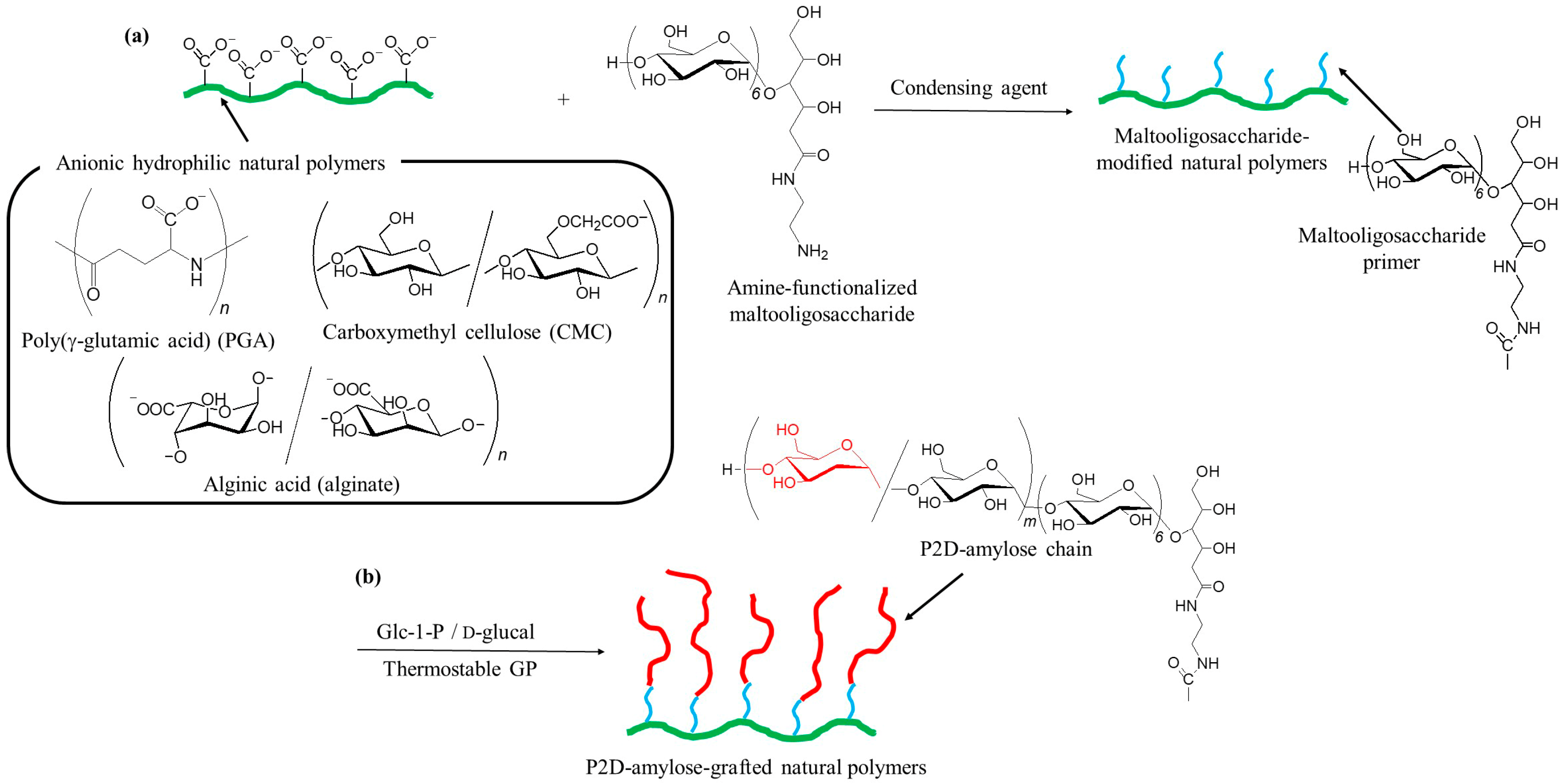
4. Chemoenzymatic Approach for Hydrophobization of Hydrophilic Natural Polymers
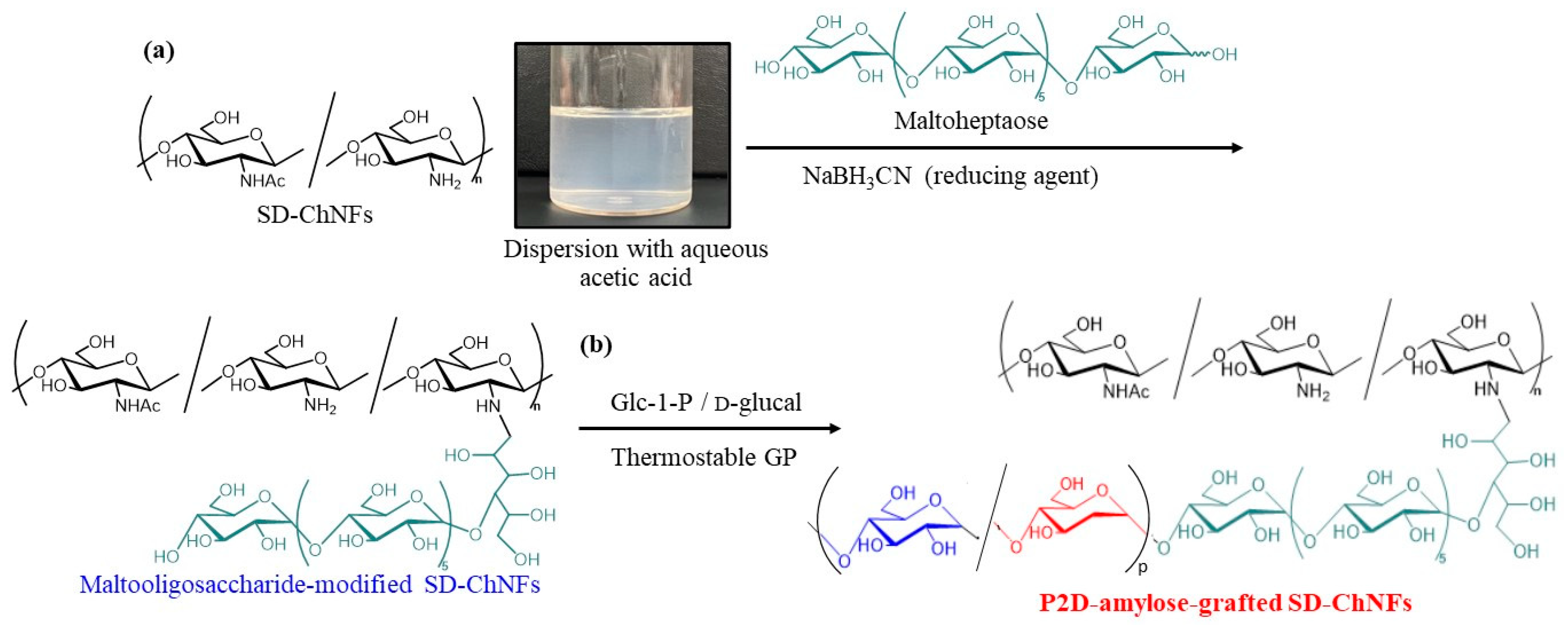
5. Hydrophobization of Natural Polysaccharide Nanofibers by Enzymatic Grafting of P2D-Amylose Chains
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kasapis, S.; Norton, I.T.; Ubbink, J.B. Modern Biopolymer Science: Bridging the Divide between Fundamental Treatise and Industrial Application; Academic Press: San Diego, CA, USA, 2009. [Google Scholar]
- Song, E.H.; Shang, J.; Ratner, D.M. Polysaccharides. In Polymer Science: A Comprehensive Reference; Matyjaszewski, K., Möller, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 137–155. [Google Scholar]
- Pina, S.; Reis, R.L.; Oliveira, J.M. Natural polymeric biomaterials for tissue engineering. In Tissue Engineering Using Ceramics and Polymers, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 75–110. [Google Scholar]
- Stephen, A.M.; Phillips, G.O.; Williams, P.A. Food Polysaccharides and Their Applications, 2nd ed.; CRC/Taylor & Francis: Boca Raton, FL, USA, 2006; 733p. [Google Scholar]
- Schmitt, C.; Turgeon, S.L. Protein/polysaccharide complexes and coacervates in food systems. Adv. Colloid Interface Sci. 2011, 167, 63–70. [Google Scholar] [CrossRef]
- Ustunol, Z. Overview of food proteins. In Applied Food Protein Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2014; Volume 9781119944492, pp. 5–9. [Google Scholar]
- He, M.; Lu, A.; Zhang, L. Advances in cellulose hydrophobicity improvement. In Food Additives and Packaging; Komolprasert, V., Turowsk, P., Eds.; ACS Symposium Series 1162; American Chemical Society: Washington, DC, USA, 2014; Volume 1162, pp. 241–274. [Google Scholar]
- Hojo, H. A strategy for the synthesis of hydrophobic proteins and glycoproteins. Org. Biomol. Chem. 2016, 14, 6368–6374. [Google Scholar] [CrossRef]
- Cunha, A.G.; Gandini, A. Turning polysaccharides into hydrophobic materials: A critical review. Part 1. Cellulose. Cellulose 2010, 17, 875–889. [Google Scholar] [CrossRef]
- Cunha, A.G.; Gandini, A. Turning polysaccharides into hydrophobic materials: A critical review. Part 2. Hemicelluloses, chitin/chitosan, starch, pectin and alginates. Cellulose 2010, 17, 1045–1065. [Google Scholar] [CrossRef]
- Edgar, K.J.; Buchanan, C.M.; Debenham, J.S.; Rundquist, P.A.; Seiler, B.D.; Shelton, M.C.; Tindall, D. Advances in cellulose ester performance and application. Prog. Polym. Sci. 2001, 26, 1605–1688. [Google Scholar] [CrossRef]
- Kostag, M.; Gericke, M.; Heinze, T.; El Seoud, O.A. Twenty-five years of cellulose chemistry: Innovations in the dissolution of the biopolymer and its transformation into esters and ethers. Cellulose 2019, 26, 139–184. [Google Scholar] [CrossRef]
- Winkler, H.; Vorwerg, W.; Rihm, R. Thermal and mechanical properties of fatty acid starch esters. Carbohydr. Polym. 2014, 102, 941–949. [Google Scholar] [CrossRef]
- Terrié, C.; Mahieu, A.; Lequart, V.; Martin, P.; Leblanc, N.; Joly, N. Towards the hydrophobization of thermoplastic starch using fatty acid starch ester as additive. Molecules 2022, 27, 6739. [Google Scholar] [CrossRef] [PubMed]
- Abe, S.; Yamamoto, K.; Kadokawa, J. Hydrophobic polysaccharides: Partially 2-deoxygenated amyloses. Asian J. Org. Chem. 2022, 11, e202100763. [Google Scholar] [CrossRef]
- Kadokawa, J.; Nakamura, S.; Yamamoto, K. Thermostable α-glucan phosphorylase-catalyzed enzymatic copolymerization to produce partially 2-deoxygenated amyloses. Processes 2020, 8, 1070. [Google Scholar] [CrossRef]
- Uto, T.; Nakamura, S.; Yamamoto, K.; Kadokawa, J. Evaluation of artificial crystalline structure from amylose analog polysaccharide without hydroxy groups at C-2 position. Carbohydr. Polym. 2020, 240, 116347. [Google Scholar] [CrossRef]
- Kadokawa, J. Glucan phosphorylase-catalyzed enzymatic synthesis of unnatural oligosaccharides and polysaccharides using nonnative substrates. Polym. J. 2022, 54, 413–426. [Google Scholar] [CrossRef]
- Kadokawa, J. Glucan phosphorylase-catalyzed enzymatic polymerization to synthesize amylose and its analogs: An efficient approach for hydrophobization of hydrophilic natural polymers by enzymatic modification of partially 2-deoxygenated amyloses. In Sustainable Green Chemistry in Polymer Research. Volume 1. Biocatalysis and Biobased Materials; Cheng, H.N., Gross, R.A., Eds.; ACS Symposium Series 1450; American Chemical Society: Washington, DC, USA, 2023; Volume 1450, pp. 39–55. [Google Scholar]
- Kobayashi, S.; Makino, A. Enzymatic polymer synthesis: An opportunity for green polymer chemistry. Chem. Rev. 2009, 109, 5288–5353. [Google Scholar] [CrossRef] [PubMed]
- Kadokawa, J. Precision polysaccharide synthesis catalyzed by enzymes. Chem. Rev. 2011, 111, 4308–4345. [Google Scholar] [CrossRef] [PubMed]
- Shoda, S.; Uyama, H.; Kadokawa, J.; Kimura, S.; Kobayashi, S. Enzymes as green catalysts for precision macromolecular synthesis. Chem. Rev. 2016, 116, 2307–2413. [Google Scholar] [CrossRef]
- Shoda, S. Development of chemical and chemo-enzymatic glycosylations. Proc. Jpn. Acad. Ser. B 2017, 93, 125–145. [Google Scholar] [CrossRef]
- Ziegast, G.; Pfannemüller, B. Linear and star-shaped hybrid polymers. Phosphorolytic syntheses with di-functional, oligo-functional and multifunctional primers. Carbohydr. Res. 1987, 160, 185–204. [Google Scholar] [CrossRef]
- Fujii, K.; Takata, H.; Yanase, M.; Terada, Y.; Ohdan, K.; Takaha, T.; Okada, S.; Kuriki, T. Bioengineering and application of novel glucose polymers. Biocatal. Biotransform. 2003, 21, 167–172. [Google Scholar] [CrossRef]
- Seibel, J.; Beine, R.; Moraru, R.; Behringer, C.; Buchholz, K. A new pathway for the synthesis of oligosaccharides by the use of non-Leloir glycosyltransferases. Biocatal. Biotransform. 2006, 24, 157–165. [Google Scholar] [CrossRef]
- Seibel, J.; Jordening, H.J.; Buchholz, K. Glycosylation with activated sugars using glycosyltransferases and transglycosidases. Biocatal. Biotransform. 2006, 24, 311–342. [Google Scholar] [CrossRef]
- Yanase, M.; Takaha, T.; Kuriki, T. α-Glucan phosphorylase and its use in carbohydrate engineering. J. Sci. Food Agric. 2006, 86, 1631–1635. [Google Scholar] [CrossRef]
- Nakai, H.; Kitaoka, M.; Svensson, B.; Ohtsubo, K. Recent development of phosphorylases possessing large potential for oligosaccharide synthesis. Curr. Opin. Chem. Biol. 2013, 17, 301–309. [Google Scholar] [CrossRef]
- O’Neill, E.C.; Field, R.A. Enzymatic synthesis using glycoside phosphorylases. Carbohydr. Res. 2015, 403, 23–37. [Google Scholar] [CrossRef]
- Hinrichs, W.; Buttner, G.; Steifa, M.; Betzel, C.; Zabel, V.; Pfannemuller, B.; Saenger, W. An amylose antiparallel double helix at atomic resolution. Science 1987, 238, 205–208. [Google Scholar] [CrossRef]
- Imberty, A.; Chanzy, H.; Perez, S.; Buleon, A.; Tran, V. The double-helical nature of the crystalline part of A-starch. J. Mol. Biol. 1988, 201, 365–378. [Google Scholar] [CrossRef]
- Imberty, A.; Perez, S. A revisit to the three-dimensional structure of B-type starch. Biopolymers 1988, 27, 1205–1221. [Google Scholar] [CrossRef]
- Schulz, W.; Sklenar, H.; Hinrichs, W.; Saenger, W. The structure of the left-handed antiparallel amylose double helix: Theoretical studies. Biopolymers 1993, 33, 363–375. [Google Scholar] [CrossRef]
- Kadokawa, J. Synthesis of amylose-grafted polysaccharide materials by phosphorylase-catalyzed enzymatic polymerization. In Biobased Monomers, Polymers, and Materials; Smith, P.B., Gross, R.A., Eds.; ACS Symposium Series 1105; American Chemical Society: Washington, DC, USA, 2012; pp. 237–255. [Google Scholar]
- Kadokawa, J. Synthesis of new polysaccharide materials by phosphorylase-catalyzed enzymatic α-glycosylations using polymeric glycosyl acceptors. In Green Polymer Chemistry: Biocatalysis and Materials II; Cheng, H.N., Gross, R.A., Smith, P.B., Eds.; ACS Symposium Series 1144; American Chemical Society: Washington, DC, USA, 2013; pp. 141–161. [Google Scholar]
- Kadokawa, J. Chemoenzymatic synthesis of functional amylosic materials. Pure Appl. Chem. 2014, 86, 701–709. [Google Scholar] [CrossRef]
- Izawa, H.; Nawaji, M.; Kaneko, Y.; Kadokawa, J. Preparation of glycogen-based polysaccharide materials by phosphorylase-catalyzed chain elongation of glycogen. Macromol. Biosci. 2009, 9, 1098–1104. [Google Scholar] [CrossRef]
- Kadokawa, J. Synthesis of non-natural oligosaccharides by α-glucan phosphorylase-catalyzed enzymatic glycosylations using analogue substrates of α-D-glucose 1-phosphate. Trends Glycosci. Glycotechnol. 2013, 25, 57–69. [Google Scholar] [CrossRef]
- Kadoakwa, J. Enzymatic synthesis of non-natural oligo- and polysaccharides by phosphorylase-catalyzed glycosylations using analogue substrates. In Green Polymer Chemistry: Biobased Materials and Biocatalysis; Cheng, H.N., Gross, R.A., Smith, P.B., Eds.; ACS Symposium Series 1192; American Chemical Society: Washington, DC, USA, 2015; pp. 87–99. [Google Scholar]
- Kadokawa, J. α-Glucan phosphorylase: A useful catalyst for precision enzymatic synthesis of oligo- and polysaccharides. Curr. Org. Chem. 2017, 21, 1192–1204. [Google Scholar] [CrossRef]
- Kadokawa, J. α-Glucan Phosphorylase-Catalyzed Enzymatic Reactions to Precisely Synthesize Non-Natural Polysaccharides. In Sustainability & Green Polymer Chemistry Volume 2: Biocatalysis and Biobased Polymers; Cheng, H.N., Gross, R.A., Eds.; ACS Symposium Series 1373; American Chemical Society: Washington, DC, USA, 2020; Volume 1373, pp. 31–46. [Google Scholar]
- Evers, B.; Mischnick, P.; Thiem, J. Synthesis of 2-deoxy-α-D-arabino-hexopyranosyl phosphate and 2-deoxy-maltooligosaccharides with phosphorylase. Carbohydr. Res. 1994, 262, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Klein, H.W.; Palm, D.; Helmreich, E.J.M. General acid-base catalysis of α-glucan phosphorylases: Stereospecific glucosyl transfer from D-glucal is a pyridoxal 5′-phosphate and orthophosphate (arsenate) dependent reaction. Biochemistry 1982, 21, 6675–6684. [Google Scholar] [CrossRef]
- Nawaji, M.; Izawa, H.; Kaneko, Y.; Kadokawa, J. Enzymatic α-glucosaminylation of maltooligosaccharides catalyzed by phosphorylase. Carbohydr. Res. 2008, 343, 2692–2696. [Google Scholar] [CrossRef] [PubMed]
- Kadokawa, J.; Shimohigoshi, R.; Yamashita, K.; Yamamoto, K. Synthesis of chitin and chitosan stereoisomers by thermostable α-glucan phosphorylase-catalyzed enzymatic polymerization of α-D-glucosamine 1-phosphate. Org. Bimol. Chem. 2015, 13, 4336–4343. [Google Scholar] [CrossRef]
- Yamashita, K.; Yamamoto, K.; Kadoakwa, J. Synthesis of non-natural heteroaminopolysaccharides by α-glucan phosphorylase-catalyzed enzymatic copolymerization: α(1→4)-linked glucosaminoglucans. Biomacromolecules 2015, 16, 3989–3994. [Google Scholar] [CrossRef]
- Baba, R.; Yamamoto, K.; Kadokawa, J. Synthesis of α(1→4)-linked non-natural mannoglucans by α-glucan phosphorylase-catalyzed enzymatic copolymerization. Carbohydr. Polym. 2016, 151, 1034–1039. [Google Scholar] [CrossRef]
- Miyahara, Y.; Takagagki, T.; Totani, M.; Kadokawa, J. Glucan phosphorylase-catalyzed enzymatic synthesis of a new unnatural heteroaminopolysaccharide, glucosamino-2-deoxyglucan, and its N-benzylation for pH responsive property. Macromol. Chem. Phys. 2025, 226, 2500052. [Google Scholar] [CrossRef]
- Omagari, Y.; Kaneko, Y.; Kadokawa, J. Chemoenzymatic synthesis of amylose-grafted alginate and its formation of enzymatic disintegratable beads. Carbohydr. Polym. 2010, 82, 394–400. [Google Scholar] [CrossRef]
- Kadokawa, J.; Arimura, T.; Takemoto, Y.; Yamamoto, K. Self-assembly of amylose-grafted carboxymethyl cellulose. Carbohydr. Polym. 2012, 90, 1371–1377. [Google Scholar] [CrossRef] [PubMed]
- Shouji, T.; Yamamoto, K.; Kadokawa, J. Chemoenzyamtic synthesis and self-assembling gelation behavior of amylose-grafted poly(γ-glutamic acid). Int. J. Biol. Macromol. 2017, 97, 99–105. [Google Scholar] [CrossRef]
- Anai, T.; Abe, S.; Shobu, K.; Kadokawa, J. Synthesis of hydrophobic poly(γ-glutamic acid) derivatives by enzymatic grafting of partially 2-deoxygenated amyloses. Appl. Sci. 2023, 13, 489. [Google Scholar] [CrossRef]
- Kadokawa, J.; Abe, S.; Yamamoto, K. Hydrophobization of carboxymethyl cellulose by enzymatic grafting of partially 2-deoxygenated amyloses. Chem. Lett. 2022, 51, 646–649. [Google Scholar] [CrossRef]
- Isogai, A. TEMPO-catalyzed oxidation of polysaccharides. Polym. J. 2022, 54, 387–402. [Google Scholar] [CrossRef]
- Totani, M.; Anai, T.; Kadokawa, J. Hydrophobization of surfaces on cellulose nanofibers by enzymatic grafting of partially 2-deoxygenated amylose. Carbohydr. Polym. 2024, 335, 122086. [Google Scholar] [CrossRef]
- Prasad, K.; Murakami, M.; Kaneko, Y.; Takada, A.; Nakamura, Y.; Kadokawa, J. Weak gel of chitin with ionic liquid, 1-allyl-3-methylimidazolium bromide. Int. J. Biol. Macromol. 2009, 45, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Kadokawa, J.; Takegawa, A.; Mine, S.; Prasad, K. Preparation of chitin nanowhiskers using an ionic liquid and their composite materials with poly(vinyl alcohol). Carbohydr. Polym. 2011, 84, 1408–1412. [Google Scholar] [CrossRef]
- Hashiguchi, T.; Yamamoto, K.; Kadokawa, J. Fabrication of highly flexible nanochitin film and its composite film with anionic polysaccharide. Carbohydr. Polym. 2021, 270, 118369. [Google Scholar] [CrossRef] [PubMed]
- Kadokawa, J.; Egashira, N.; Yamamoto, K. Chemoenzymatic preparation of amylose-grafted chitin nanofiber network materials. Biomacromolecules 2018, 19, 3013–3019. [Google Scholar] [CrossRef]
- Watanabe, R.; Yamamoto, K.; Kadokawa, J. Hydrogelation from scaled-down chitin nanofibers by reductive amination of monosaccharide residues. J. Fiber Sci. Technol. 2022, 78, 10–17. [Google Scholar] [CrossRef]
- Kadokawa, J. Hydrogelation from self-assembled and scaled-down chitin nanofibers by the modification of highly polar substituents. Gels 2023, 9, 432. [Google Scholar] [CrossRef]
- Yamamoto, N.; Totani, M.; Kadokawa, J. Hydrophobization of chitin nanofibers by grafting of partially 2-deoxygenated amyloses through enzymatic approach. Molecules 2025, 30, 16. [Google Scholar] [CrossRef]
- Evers, B.; Thiem, J. Synthesis of 2-Deoxy-Maltooligosaccharides with Phosphorylase and Their Degradation with Amylases. Starch-Stärke 1995, 47, 434–439. [Google Scholar] [CrossRef]






| Entry | Feed Ratio (Primer/Glc-1-P/d-glucal) | Unit Ratio (Glc/2dGlc) (b) | ||
|---|---|---|---|---|
| 1 | 1/75/25 | 0.98 | / | 0.02 |
| 2 | 1/50/50 | 0.97 | / | 0.03 |
| 3 | 1/25/75 | 0.93 | / | 0.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Totani, M.; Kadokawa, J.-i. Hydrophobization of Natural Polymers by Enzymatic Grafting of Hydrophobic Polysaccharides, Partially 2-Deoxygenated Amyloses. Processes 2025, 13, 3042. https://doi.org/10.3390/pr13103042
Totani M, Kadokawa J-i. Hydrophobization of Natural Polymers by Enzymatic Grafting of Hydrophobic Polysaccharides, Partially 2-Deoxygenated Amyloses. Processes. 2025; 13(10):3042. https://doi.org/10.3390/pr13103042
Chicago/Turabian StyleTotani, Masayasu, and Jun-ichi Kadokawa. 2025. "Hydrophobization of Natural Polymers by Enzymatic Grafting of Hydrophobic Polysaccharides, Partially 2-Deoxygenated Amyloses" Processes 13, no. 10: 3042. https://doi.org/10.3390/pr13103042
APA StyleTotani, M., & Kadokawa, J.-i. (2025). Hydrophobization of Natural Polymers by Enzymatic Grafting of Hydrophobic Polysaccharides, Partially 2-Deoxygenated Amyloses. Processes, 13(10), 3042. https://doi.org/10.3390/pr13103042









