Performance and Characteristics of Low-Molecular-Weight Cross-Linked Grafting Terpolymers as Thickening Agents in Reservoir Fracturing Processes
Abstract
1. Introduction
2. Design of Experiments
3. Results and Discussions
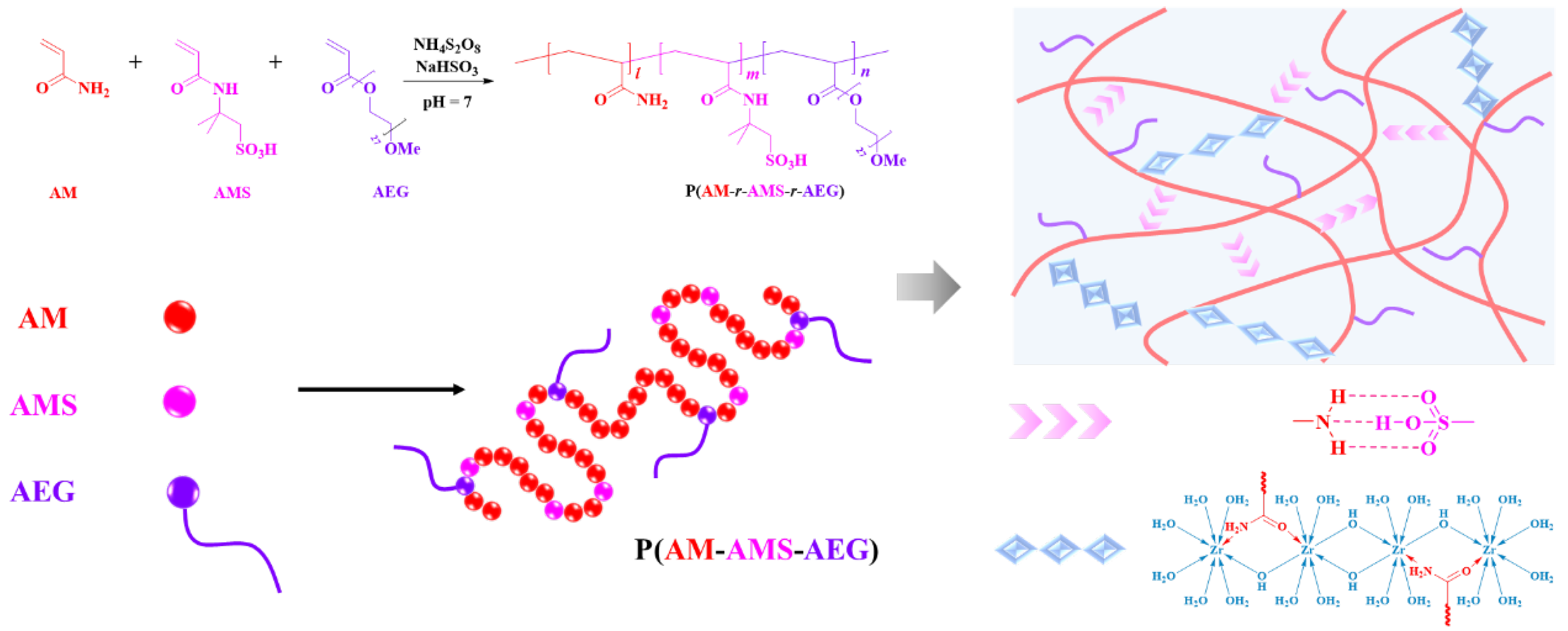
3.1. Synthesis and Chemical Structure of Fracture Fluid Based on Low-Mw Cross-Linking Terpolymer
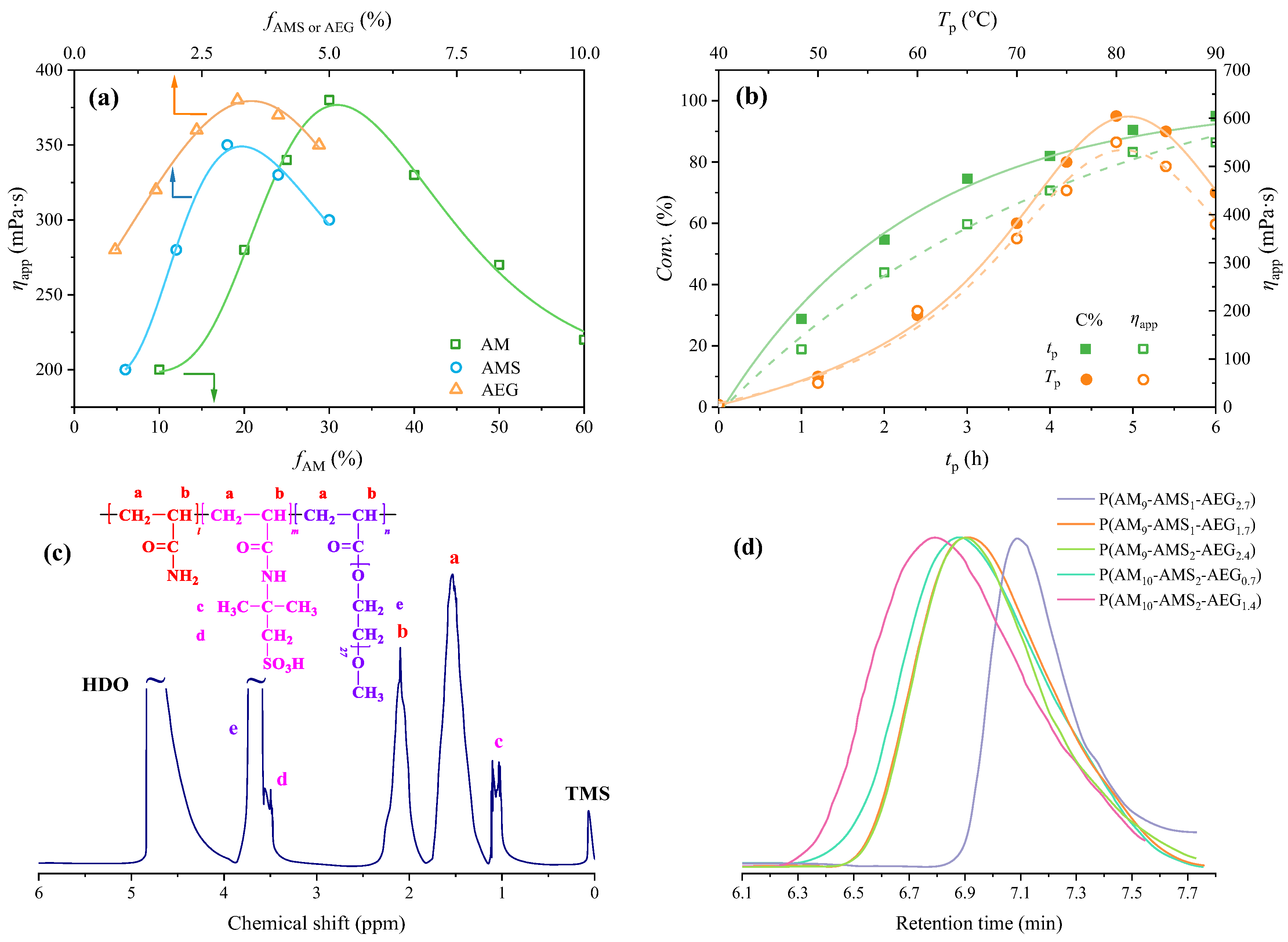
3.1.1. Polymerization of Terpolymer
3.1.2. Cross-Linking Process of Terpolymer Solution
3.1.3. Formula of Fracture Fluid
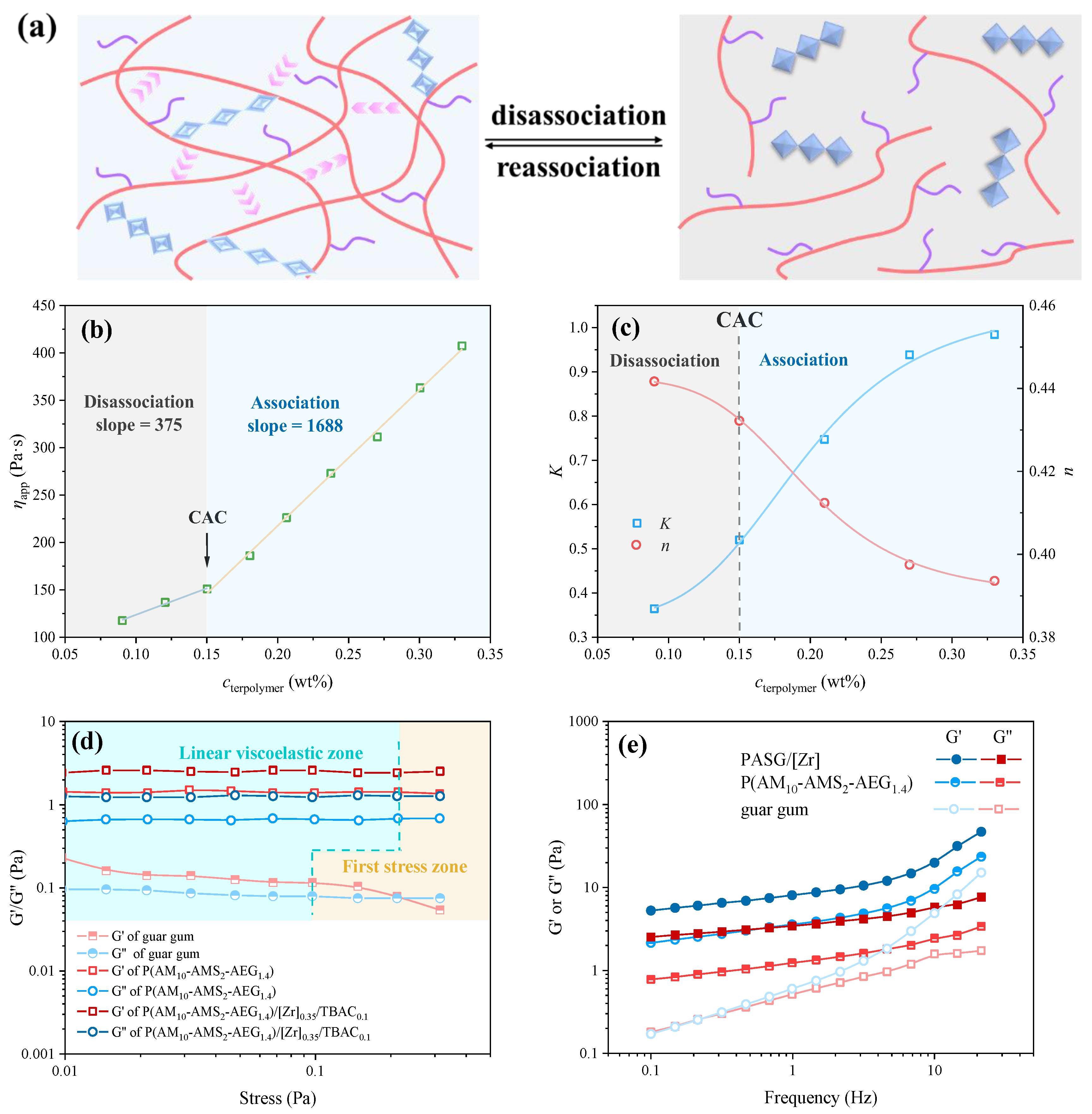
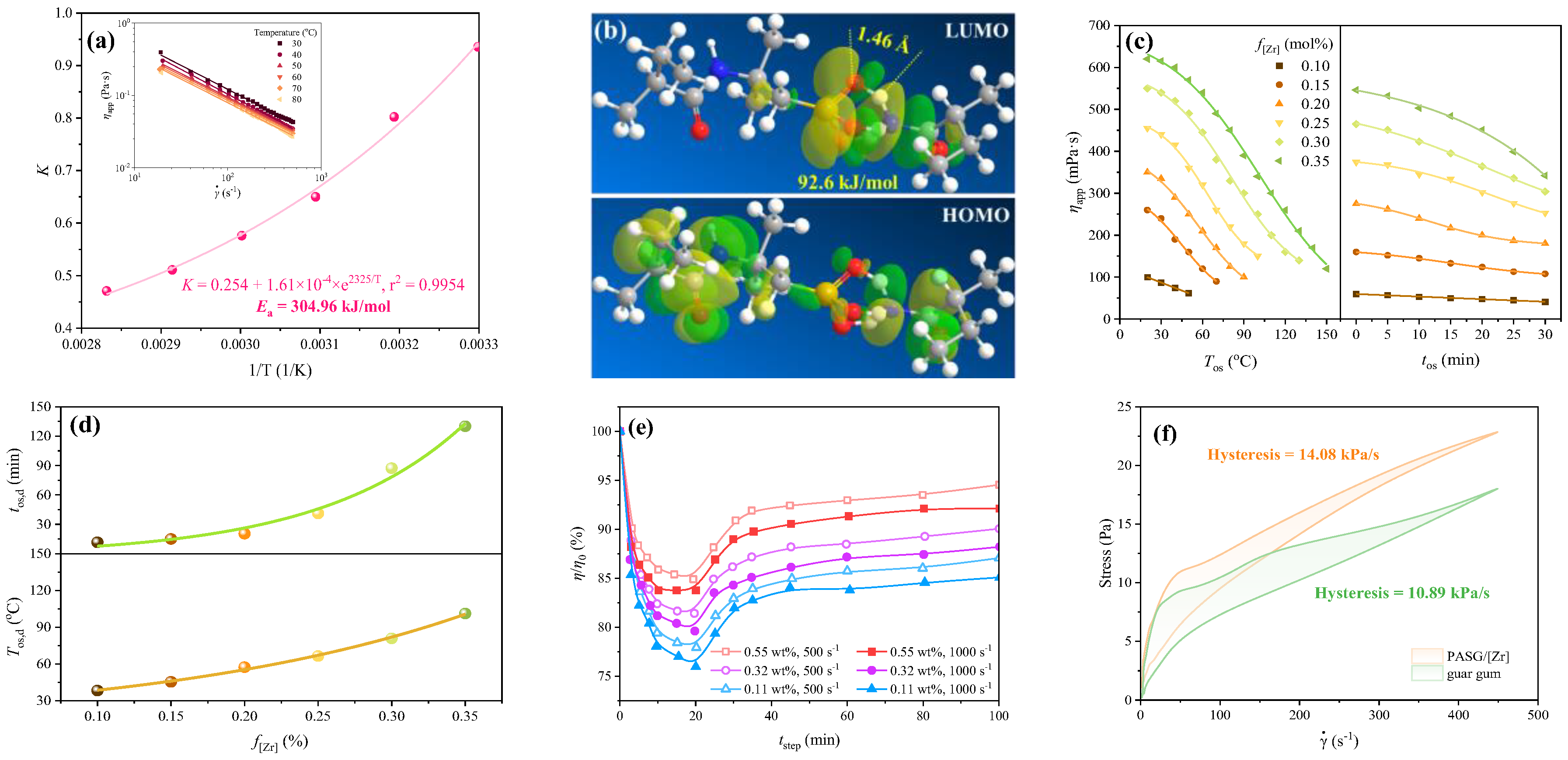
3.2. Rheological Behavior of Fracture Fluid Under Various Conditions
3.2.1. Associating Behavior and Segmental Motion in P(AM10-AMS2-AEG1.4)/[Zr]0.35/TBAC0.1 Aqueous Solution
3.2.2. Effect of Temperature on the Rheological Performance of Fracture Fluid
3.2.3. Thixotropy and Self-Recovery Ability of Fracture Fluid
3.3. Fracturing Fluid as Thickening Agent Protecting Cores
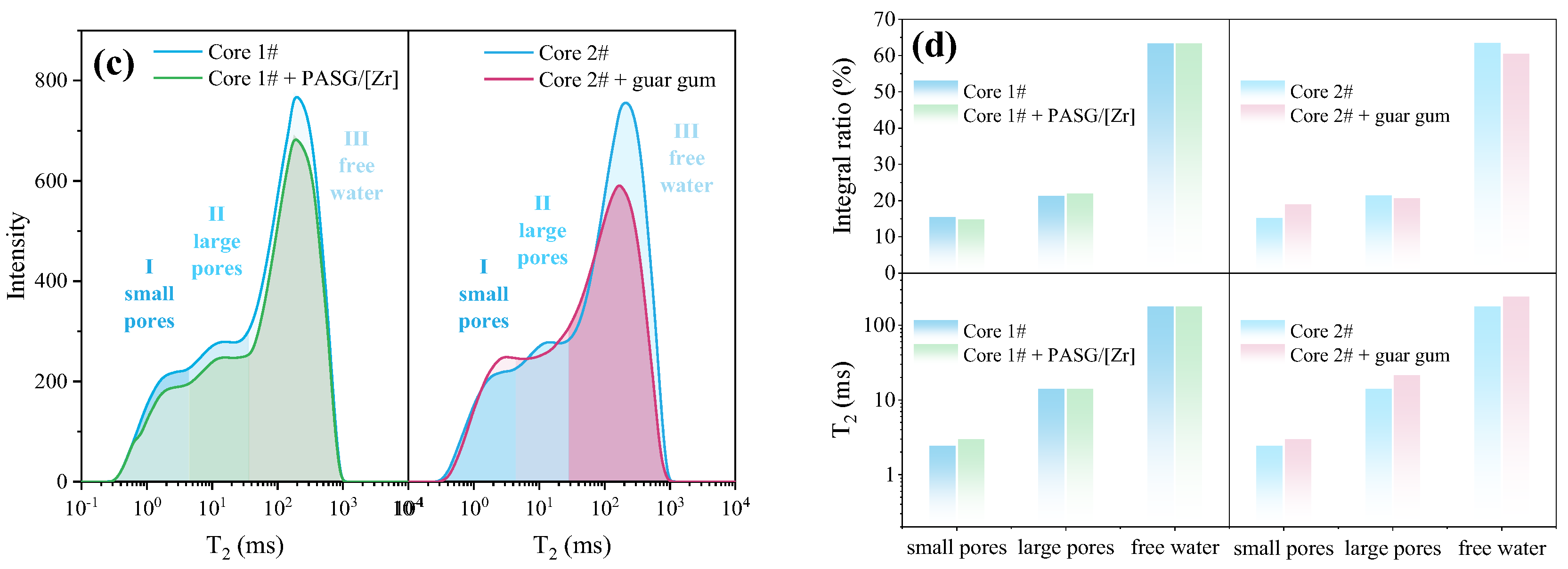
3.3.1. Sand-Carrying Ability of PASG/[Zr]-Based Fracturing Fluid
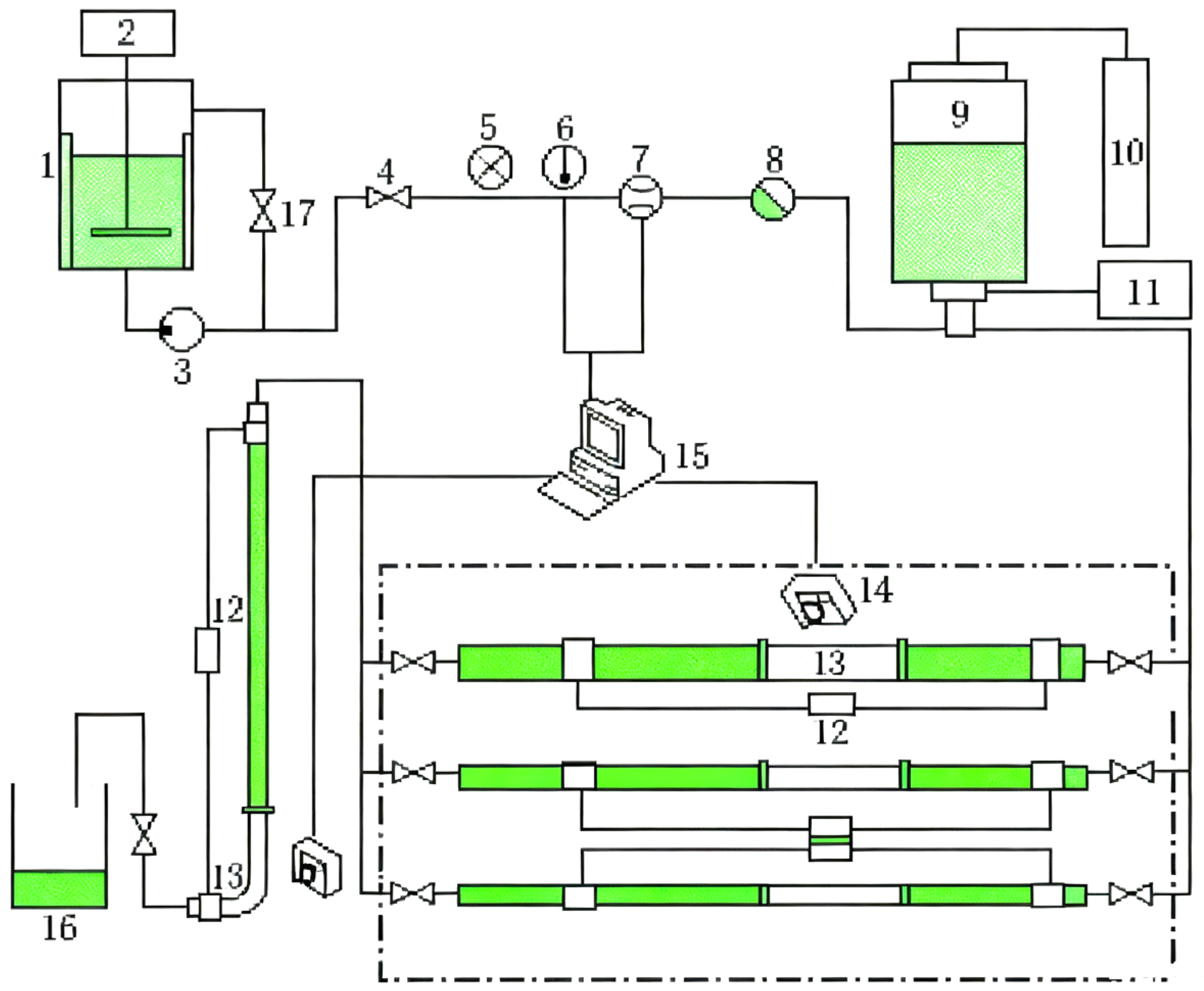
3.3.2. Filtration System Based on PASG/[Zr] Fracturing Fluid
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liang, H.; Qi, Z.; Wang, S.; Huang, X.; Yan, W.; Yuan, Y.; Li, Z. Adsorption Models for Shale Gas: A Mini-Review. Energy Fuels 2022, 36, 12946–12960. [Google Scholar] [CrossRef]
- Striolo, A.; Cole, D.R. Understanding Shale Gas: Recent Progress and Remaining Challenges. Energy Fuels 2017, 31, 10300–10310. [Google Scholar] [CrossRef]
- Sun, C.; Nie, H.; Dang, W.; Chen, Q.; Zhang, G.; Li, W.; Lu, Z. Shale Gas Exploration and Development in China: Current Status, Geological Challenges, and Future Directions. Energy Fuels 2021, 35, 6359–6379. [Google Scholar] [CrossRef]
- You, L.; Zhang, N.; Kang, Y.; Xu, J.; Cheng, Q.; Zhou, Y. Zero Flowback Rate of Hydraulic Fracturing Fluid in Shale Gas Reservoirs: Concept, Feasibility, and Significance. Energy Fuels 2021, 35, 5671–5682. [Google Scholar] [CrossRef]
- Da, Q.; Yao, C.; Zhang, X.; Li, L.; Lei, G. Reservoir Damage Induced by Water-Based Fracturing Fluids in Tight Reservoirs: A Review of Formation Mechanisms and Treatment Methods. Energy Fuels 2024, 38, 18093–18115. [Google Scholar] [CrossRef]
- Yang, J.; Dong, T.; Yi, J.; Jiang, G. Development of Multiple Crosslinked Polymers and Its Application in Synthetic-Based Drilling Fluids. Gels 2024, 10, 120. [Google Scholar] [CrossRef]
- Huang, J.; Chen, J.; Lv, L.; Yu, Z.; Yao, W.; Cheng, H.; Niu, W.; Wang, J.; Zhang, J.; Qi, H. Design and Verification of a Wearable Micro-Capacitance Test System for POC Biosensing. IEEE Trans. Instrum. Meas. 2025, 74, 1–11. [Google Scholar] [CrossRef]
- Li, J.; Wen, M.; Yang, J.; Liu, Y.; Jiang, Z.; Chen, J. Synthesis and analysis of magnetic nanoparticles within foam matrix for foam drainage gas production. Geoenergy Sci. Eng. 2024, 238, 212887. [Google Scholar] [CrossRef]
- He, S.; Ding, L.; Xiong, Z.; Spicer, R.; Farnsworth, A.; Valdes, P.; Wang, C.; Cai, F.; Wang, H.; Sun, Y.; et al. A distinctive Eocene Asian monsoon and modern biodiversity resulted from the rise of eastern Tibet. Sci. Bull. 2022, 21, 2245–2258. [Google Scholar] [CrossRef]
- Al-Hajri, S.; Negash, B.M.; Rahman, M.M.; Haroun, M.; Al-Shami, T.M. Perspective Review of Polymers as Additives in Water-Based Fracturing Fluids. ACS Omega 2022, 7, 7431–7443. [Google Scholar] [CrossRef] [PubMed]
- Davoodi, S.; Al-Shargabi, M.; Wood, D.A.; Rukavishnikov, V.S.; Minaev, K.M. Synthetic polymers: A review of applications in drilling fluids. Pet. Sci. 2024, 21, 475–518. [Google Scholar] [CrossRef]
- Yang, S.; Yu, W.; Zhao, M.; Ding, F.; Zhang, Y. A Review of Weak Gel Fracturing Fluids for Deep Shale Gas Reservoirs. Gels 2024, 10, 345. [Google Scholar] [CrossRef]
- Ahmad, H.M.; Iqbal, T.; Kamal, M.S.; Al-Harthi, M.A. Influence of Hydrophobically Modified Polymer and Titania Nanoparticles on Shale Hydration and Swelling Properties. Energy Fuels 2020, 34, 16456–16468. [Google Scholar] [CrossRef]
- Movahedi, H.; Schiefler, A.A.; Dwivedi, S.; Sørensen, H.O.; Poulsen, H.-F.; Bovet, N. Novel Temperature-Responsive Organic Polymer Gel for Efficient Abandonment of Aged Porous Chalk Formations Characterized by X-ray Computed Tomography. Energy Fuels 2024, 38, 19478–19493. [Google Scholar] [CrossRef]
- Protsak, I.S.; Morozov, Y.M. Fundamentals and advances in stimuli-responsive hydrogels and their applications: A review. Gels 2025, 11, 30. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Bai, B.; Hou, J. Polymer gel systems for water management in high-temperature petroleum reservoirs: A chemical review. Energy Fuels 2017, 17, 13063–13087. [Google Scholar] [CrossRef]
- de Leon, A.C.C.; da Silva, Í.G.M.; Pangilinan, K.D.; Chen, Q.; Caldona, E.B.; Advincula, R.C. High performance polymers for oil and gas applications. React. Funct. Polym. 2021, 162, e104878. [Google Scholar] [CrossRef]
- Liu, X.; Liu, K.; Gou, S.; Liang, L.; Luo, C.; Guo, Q. Water-Soluble Acrylamide Sulfonate Copolymer for Inhibiting Shale Hydration. Ind. Eng. Chem. Res. 2014, 53, 2903–2910. [Google Scholar] [CrossRef]
- Bagaria, H.G.; Yoon, K.Y.; Neilson, B.M.; Cheng, V.; Lee, J.H.; Worthen, A.J.; Xue, Z.; Huh, C.; Bryant, S.L.; Bielawski, C.W.; et al. Stabilization of Iron Oxide Nanoparticles in High Sodium and Calcium Brine at High Temperatures with Adsorbed Sulfonated Copolymers. Langmuir 2013, 29, 3195–3206. [Google Scholar] [CrossRef]
- Wang, J.; Ran, D.; Lu, H.; Zhang, J.; Wu, Y. Realizing the Efficient Dissolution of Drag Reducer upon pH-Induced Demulsification of Inverse Polymer Emulsion. Langmuir 2024, 40, 15869–15876. [Google Scholar] [CrossRef]
- Yang, D.; Liu, C.; Piao, H.; Quan, P.; Fang, L. Enhanced Drug Loading in the Drug-in-Adhesive Transdermal Patch Utilizing a Drug-Ionic Liquid Strategy: Insight into the Role of Ionic Hydrogen Bonding. Mol. Pharm. 2021, 18, 1157–1166. [Google Scholar] [CrossRef]
- Yonamine, Y.; Yoshimatsu, K.; Lee, S.-H.; Hoshino, Y.; Okahata, Y.; Shea, K.J. Polymer Nanoparticle−Protein Interface. Evaluation of the Contribution of Positively Charged Functional Groups to Protein Affinity. ACS Appl. Mater. Interfaces 2013, 5, 374–379. [Google Scholar] [CrossRef]
- Nguyen, C.A.; Argun, A.A.; Hammond, P.T.; Lu, X.; Lee, P.S. Layer-by-Layer Assembled Solid Polymer Electrolyte for Electrochromic Devices. Chem. Mater. 2011, 23, 2142–2149. [Google Scholar] [CrossRef]
- Lei, S.; Sun, J.; Lv, K.; Zhang, Q.; Yang, J. Types and Performances of Polymer Gels for Oil-Gas Drilling and Production: A Review. Gels 2022, 8, 386. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhou, X.; Hu, Q.; Zhou, K.; Zhang, Y.; Dong, S.; Zhao, G.; Zhang, S. Concentration-induced spontaneous polymerization of protic ionic liquids for efficient in situ adhesion. Nat. Comm. 2024, 15, e4265. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Azeim, S. Phase Behavior and Interfacial Properties of Salt-Tolerant Polymers: Insights from Molecular Dynamics Simulations. ACS Appl. Polym. Mater. 2021, 3, 6488–6501. [Google Scholar] [CrossRef]
- Si, S.; Liu, Y.; Dai, C.; Zou, C.; Zhuo, L.; Li, X.; Yang, N.; Ma, D. Salt Resistance Mechanism and Oil Displacement Performance Evaluation of a New Zwitterionic Polymer. Energy Fuels 2025, 39, 13443–13453. [Google Scholar] [CrossRef]
- Wang, S.; Sun, Z.; Zhu, Q.; Cao, X.; Feng, Y.; Yin, H. In Situ Generated Hydrogels Exhibiting Simultaneous High-Temperature and High-Salinity Resistance for Deep Hydrocarbon Reservoir Exploitation. Ind. Eng. Chem. Res. 2024, 63, 18263–18278. [Google Scholar] [CrossRef]
- Chen, Y.; Li, J.; Song, Q.; Bu, C.; Ye, K.; He, F.; Lv, Z.; Chen, X. Research on Flow Patterns of Two-Phase Fracturing Fluid in Hydraulic Fracture Considering the Fluid Leak-off. ACS Omega 2024, 9, 2432–2442. [Google Scholar] [CrossRef]
- Huang, C.; Mu, L.; Gong, X. Development and Characterization of Environmentally Responsive Thickening Agents for Fracturing Fluids in Shale Gas Reservoir Stimulation. Processes 2025, 13, 1253. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, H.; Xu, P.; Zhang, X.; Wang, H.; Hu, M.; Guo, J. Effect of Ultra-High Temperature Degradation on the Physical Properties and Chemical Structure of an AMPS-Based Copolymer Oil-Well Cement Additive PADIM in Aqueous Solution. Polymers 2025, 17, 591. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Mandal, P.; Kumar, R. Solid state thermal degradation behaviour of graft copolymers of carboxymethyl cellulose with vinyl monomers. Int. J. Biol. Macromol. 2016, 87, 357–365. [Google Scholar] [CrossRef]
- Liu, X.; Wang, A.; Wang, C.; Qu, J.; Wen, Y.; Chen, B.; Wang, Z.; Wu, B.; Yuan, Z.; Wei, B. Preparation and Performance of Salt Tolerance and Thermal Stability Cellulose Nanofibril Hydrogels and Their Application in Drilling Engineering. Pap. Biomater. 2019, 4, 10–19. [Google Scholar] [CrossRef]
- Wang, S.; Xiong, P.; Liu, X.; Meng, F.; Ma, Q.; Song, C.; Zhang, Q.; Che, Y. A Hydroxypropyl Methyl Cellulose-Based Graft Copolymer with Excellent Thermothickening and Anti-salt Ability for Enhanced Oil Recovery. Energy Fuels 2022, 36, 2488–2502. [Google Scholar] [CrossRef]
- Balding, P.; Li, M.; Wu, Q.; Volkovinsky, R.; Russo, P. Cellulose Nanocrystal–Polyelectrolyte Hybrids for Bentonite Water-Based Drilling Fluids. ACS Appl. Bio Mater. 2020, 3, 3015–3027. [Google Scholar] [CrossRef]
- Hao, H.; Yao, E.; Pan, L.; Chen, R.; Wang, Y.; Xiao, H. Exploring heterogeneous drivers and barriers in MaaS bundle subscriptions based on the willingness to shift to MaaS in one-trip scenarios. Transp. Res. Part A Policy Pract. 2025, 199, 104525. [Google Scholar] [CrossRef]
- Bahamdan, A.; Daly, W.H. Poly(oxyalkylene) grafts to guar gum with applications in hydraulic fracturing fluids. Polym. Adv. Technol. 2006, 17, 679–681. [Google Scholar] [CrossRef]
- Lu, W.; Wang, J.; Wang, T.; Zhang, K.; Jiang, X.; Zhao, H. Visual style prompt learning using diffusion models for blind face restoration. Pattern Recognit. 2025, 161, 111312. [Google Scholar] [CrossRef]
- Shi, S.L.; Sun, J.S.; Mu, S.B.; Lv, K.H.; Liu, J.P.; Bai, Y.R.; Wang, J.T.; Huang, X.B.; Jin, J.F.; Li, J. Effect of Chemical Composition of Metal–Organic Crosslinker on the Properties of Fracturing Fluid in High-Temperature Reservoir. Molecules 2024, 29, 2798. [Google Scholar] [CrossRef]
- Xu, Z.Z.; Zhao, M.W.; Sun, N.; Meng, X.J.; Yang, Z.T.; Xie, Y.X.; Ding, F.; Dong, Y.B.; Gao, M.W.; Wu, Y.N.; et al. Delayed Crosslinking Gel Fracturing Fluid with Dually Crosslinked Polymer Network for Ultra-Deep Reservoir: Performance and Delayed Crosslinking Mechanism. Colloids Surf. A Physicochem. Eng. Asp. 2025, 708, e135967. [Google Scholar] [CrossRef]
- Zhou, Y.; Xia, H.; Yu, D.; Cheng, J.; Li, J. Outlier detection method based on high-density iteration. Inf. Sci. 2024, 662, 120286. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Zha, H.; Cheng, P.-Y.; Zhang, S.; Liu, X.-W.; Wu, Y.-X. Vanadium(V) Complexes Containing Unsymmetrical N-Heterocyclic Carbene Ligands: Highly Efficient Synthesis and Catalytic Behavior towards Ethylene/Propylene Copolymerization. Chin. J. Polym. Sci. 2024, 42, 32–41. [Google Scholar] [CrossRef]
- Gao, Y.; Li, A.; Chen, J.; Cui, Z.; Wu, Y. Quaternized Sodium Alginate-g-Ethyl-Oxazoline Copolymer Brushes and Their Supramolecular Networks via Hydrogen Bonding. ACS Biomater. Sci. Eng. 2022, 8, 3424–3437. [Google Scholar] [CrossRef]
- Li, A.; Li, J.-L.; Zhang, J.-M.; Ma, J.-Y.; Wu, Y.-X. In-situ Synthesis of Chemically Stable Hybrid Networks of Poly(thioctic acid) with Fe3+ via Controlled/living Cationic Ring-opening Polymerization. Macormol. Rapid. Commun. 2025, 4, e2401115. [Google Scholar] [CrossRef]
- Qi, H.; Hu, Z.; Yang, Z.; Zhang, J.; Wu, J.J.; Cheng, C.; Wang, C.; Zheng, L. Capacitive aptasensor coupled with microfluidic enrichment for real-time detection of trace SARS-CoV-2 nucleocapsid protein. Anal. Chem. 2022, 6, 2812–2819. [Google Scholar] [CrossRef]
- Li, A.; Zhang, J.; Yi, Q.; Wang, Y.; Wu, Y. Optical Performance of Hybrid Co-Networks of Poly(thioctic Acid) Coordinated with Fe3+: Optical Focusing and Photothermal Conversion. Adv. Opt. Mater. 2025, 13, e01148. [Google Scholar] [CrossRef]
- Fang, Y.; Yue, T.; Zhang, S.L.; Liu, J.; Zhang, L. Molecular Dynamics Simulations of Self-Healing Topological Copolymers with a Comblike Structure. Macromolecules 2021, 54, 1095–1105. [Google Scholar] [CrossRef]
- Sun, L.; Shi, W.; Tian, X.; Li, J.; Zhao, B.; Wang, S.; Tan, J. A plane stress measurement method for CFRP material based on array LCR waves. NDT E Int. 2025, 151, 103318. [Google Scholar] [CrossRef]
- Zhang, M.; Li, X. Understanding the relationship between coopetition and startups’ resilience: The role of entrepreneurial ecosystem and dynamic exchange capability. J. Bus. Ind. Mark. 2025, 2, 527–542. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, L.; Wan, X.; Tang, Y.; Liu, Q.; Li, W.; Liao, J. Synthesis and Characterization of a Temperature-Sensitive Microcapsule Gelling Agent for High-Temperature Acid Release. ACS Omega 2024, 9, 20849–20858. [Google Scholar] [CrossRef]



| Sample | FAMS (%) | FAEG (%) | Mn (kg·mol−1) | PDI |
|---|---|---|---|---|
| P(AM10-AMS2-AEG1.4) | 13.22 | 11.09 | 250.2 | 1.71 |
| P(AM10-AMS2-AEG0.7) | 15.03 | 5.84 | 205.3 | 1.88 |
| P(AM9-AMS2-AEG2.4) | 22.42 | 17.96 | 202.1 | 1.92 |
| P(AM9-AMS1-AEG1.7) | 21.27 | 13.65 | 200.7 | 2.07 |
| P(AM9-AMS1-AEG2.7) | 20.80 | 20.31 | 152.6 | 2.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Guo, C.; Gong, Q.; Li, G.; Zhang, C.; Jiang, T. Performance and Characteristics of Low-Molecular-Weight Cross-Linked Grafting Terpolymers as Thickening Agents in Reservoir Fracturing Processes. Processes 2025, 13, 3032. https://doi.org/10.3390/pr13103032
Wang K, Guo C, Gong Q, Li G, Zhang C, Jiang T. Performance and Characteristics of Low-Molecular-Weight Cross-Linked Grafting Terpolymers as Thickening Agents in Reservoir Fracturing Processes. Processes. 2025; 13(10):3032. https://doi.org/10.3390/pr13103032
Chicago/Turabian StyleWang, Kai, Chenye Guo, Qisen Gong, Gen Li, Cuilan Zhang, and Teng Jiang. 2025. "Performance and Characteristics of Low-Molecular-Weight Cross-Linked Grafting Terpolymers as Thickening Agents in Reservoir Fracturing Processes" Processes 13, no. 10: 3032. https://doi.org/10.3390/pr13103032
APA StyleWang, K., Guo, C., Gong, Q., Li, G., Zhang, C., & Jiang, T. (2025). Performance and Characteristics of Low-Molecular-Weight Cross-Linked Grafting Terpolymers as Thickening Agents in Reservoir Fracturing Processes. Processes, 13(10), 3032. https://doi.org/10.3390/pr13103032





