Abstract
This research demonstrates the high efficiency of thermal plasma gasification for the treatment of simulated operational radioactive waste (SORW), representative of the low-level radioactive waste (LLW) generated in Argentina. A prototype system with a 4.8 kW plasma torch was used to process SORW composed of nitrile gloves, laboratory paper, and stable non-radioactive elements to simulate 60Co, 90Sr, 137Cs, and 144Ce. The process achieved vast volume reduction (99.6%), converting 5625 mL of waste into minimal volumes of four different solid residues (SR): SR1 (20 mL), SR2 (1 mL), SR3 (1 mL), and SR4 (3 mL), resulting in a volume reduction factor (VRF) of 225. Elemental analysis showed clear differences in retention behavior: excellent retention for Co (96 ± 10% inside the plasma reactor) and Ce (59 ± 6%), while more volatile Sr (39 ± 4%) and Cs (26 ± 3%). The latter were partially captured in downstream components (22.8 ± 1.1% Sr and 2.9 ± 0.15% Cs in quencher). The gases treatment system achieved >97% reduction for most plasma generated pollutants: NOx (98.9 ± 0.6%), CO (98.2 ± 0.8%), H2S (97.6 ± 0.6%), and H2 (98.1 ± 0.9%), with 80.6 ± 2.5% for SO2 and 75.0 ± 1.1% reduction for CO2.
1. Introduction
In Argentina, approximately 90% of the generated radioactive waste is classified as low-level radioactive waste (LLW) [1]. These wastes are generated from routine operation and maintenance activities in nuclear reactors, as well as from various stages of the nuclear fuel cycle [2,3]. LLWs are identified by the presence of radionuclides with half-lives generally shorter than 30 years, and by activity concentrations below 370 MBq/t for alpha emitters and 37 GBq/t for beta and gamma emitters [4]. Typical radionuclides present in LLWs include 60Co, 90Sr, 137Cs, and 144Ce, among other fission and corrosion products associated with nuclear power generation. Given their potential radiological risks, the safe and efficient management of LLW remains a critical priority [1,5].
According to the National Program for Radioactive Waste Management (PNGRR, for its initials in Spanish), the LLWs generated in Argentina consist primarily of compactable solids such as gloves, cloths, and laboratory paper, as well as non-compactable solids including cables, pipes, wood, masonry, equipment components, and tools [1]. Currently, LLWs in Argentina are kept in 200 L drums within facilities specifically designed to ensure their safe storage, with a mandatory holding period of 50 years [1]. Although volume reduction strategies, such as compaction, the low initial density and limited compressibility of these materials significantly constrain their effectiveness. Consequently, even after treatment, LLWs continue to occupy substantial storage space, posing logistical and economic challenges for long-term management.
In this context, thermal plasma gasification arises as a highly promising alternative for the treatment of LLWs. This advanced technology achieves volumetric reductions exceeding 99%, significantly outperforming conventional compaction methods in terms of waste minimization and long-term storage efficiency [6]. In comparison, incineration, although widely implemented, typically operates at 800–1000 °C and relies on complete combustion. This method produces significant volumes of secondary waste, including bottom ash and fly ash, which require further stabilization and proper disposal. Furthermore, the combustion process can generate various undesirable pollutants, such as dioxins, furans, acid gases, and fine particulate matter, especially when chlorine or nitrogen containing waste is present. Incineration also typically requires complex and energy intensive exhaust gas treatment systems to comply with environmental regulations, and the relatively lower temperatures can limit the complete decomposition of hazardous organic compounds. Pyrolysis, on the other hand, occurs under oxygen-deficient conditions at moderate temperatures (400–800 °C), yielding heterogeneous mixtures of gases, tars, and solid char, which often require further treatment, while its lower operating temperatures limit the complete destruction of hazardous organic compounds. In contrast, thermal plasma gasification operates at extremely high temperatures (often above 1000 °C), enabling the near-complete decomposition of organic matter while simultaneously suppressing the formation of persistent toxic by-products, including dioxins, furans, and heavy hydrocarbons [7]. Furthermore, the process produces chemically stable solid wastes with favorable properties for subsequent handling, conditioning, and final disposal, further supporting its potential for integration into advanced radioactive waste management strategies [6].
In plasma gasification processes, the volatilization of metallic species is a critical factor affecting both process efficiency and the distribution of contaminants in downstream components. This phenomenon is well-documented in fundamental research on high-temperature thermal treatment of wastes [8]. The behavior of metals such as Cs, Sr, and Co is governed by principles of high-temperature thermochemistry, where factors such as reactor temperature, residence time, and gas composition (e.g., the presence of chlorine or sulfur) are known to strongly influence their vaporization, transport, and subsequent condensation [9,10]. In particular, the formation of volatile species (e.g., chlorides) is a key mechanism driving the release of these metals [11]. Consequently, low-boiling-point elements (e.g., Cs) tend to migrate from the high-temperature plasma zone to cooler sections of the system, a behavior consistently observed not only in plasma systems but also in incineration and vitrification studies [12,13]. This migration can significantly impact the retention efficiency within the vitrified slag and promote the formation of deposits in heat exchangers or filters, thus complicating the gas cleaning train. Therefore, an understanding of these thermochemical mechanisms is essential for optimizing plasma gasification operations and designing effective off-gas cleaning and retention systems.
Currently, a limited number of industrial-scale facilities have successfully implemented plasma technology for the treatment of radioactive waste (RW), demonstrating its feasibility and effectiveness in real-world applications. Nevertheless, the applicability of plasma systems remains limited due to their high energy consumption, the complexity of off-gas cleaning requirements, the need for specialized operational expertise, and relatively high capital and maintenance costs. As a result, their deployment has so far been restricted mainly to sites with large waste inventories.
The first large-scale plasma gasification plant, ZWILAG in Switzerland, began operations in 2004 [14]. The facility processes up to 200 kg/h of combustible waste and 300 kg/h of fusible waste, organized in two annual campaigns treating approximately 500 containers per campaign. Waste is fed into the reactor via a horizontal feeding system, where organic materials are gasified and inorganic components are melted into a slag at operating temperatures typically ranging from 1200 to 1400 °C. A rotating crucible facilitates slag movement, ensuring efficient extraction, while off-gases undergo secondary oxidation in a post-combustion chamber followed by a two-stage gas cleaning system (physical-wet and chemical-wet stages) and selective catalytic reduction (DENOX) before release.
The PLUTON facility in Russia treats mixed radioactive waste in a plasma furnace using two torches with powers between 100 and 150 kW, achieving melting temperatures in the range of 1500–1800 °C [15]. The off-gas treatment system comprises a high-temperature oxidation chamber, a cyclone for particulate removal, scrubbers for acid gas absorption, and HEPA filtration to ensure compliance with strict emission standards. Operational experience has demonstrated the plant’s ability to volume-reduce waste by a factor of 10–20 while producing a chemically inert final product suitable for long-term disposal. More recently, a similar plasma installation was implemented at the Kozloduy nuclear power plant in Bulgaria, beginning operations in early 2018 [16]. This reactor employs a non-transferred arc plasma torch and a gas system based on conventional incinerators. Waste, introduced in 200 L drums or as loose material, including organics, metals, concrete, and ion-exchange resins, is shredded for continuous feeding. The plant’s design allows treatment of liquids and resins, and due to high operational costs, fixed installations at sites with large waste streams are preferred over mobile units. In all cases, specific operational temperatures are not fully disclosed. These industrial-scale implementations provide valuable operational data, supporting the scalability, reliability, and robustness of plasma-based systems for safe RW processing, and highlight the international interest in advanced thermal technologies.
At the same time, small-scale experimental research has made significant progress in the comprehensive characterization of the by-products generated during plasma treatment. This line of research plays a crucial role in understanding the fundamental mechanisms of waste transformation under plasma gasification and in optimizing process parameters. Particular attention has been devoted to key aspects such as the chemical composition and structural stability of secondary solid waste, the retention behavior of volatile metallic species, and the overall efficiency of the gases purification systems. These parameters are critical not only for ensuring the safety and environmental compatibility of the process, but also for its technical and economic viability [17]. The growing body of experimental evidence has thus contributed to reinforcing the credibility of plasma gasification as a viable and sustainable option within the broader framework of radioactive waste management.
Although plasma treatment has demonstrated clear technical advantages, the economic aspects remain a critical factor influencing its broader implementation. Several studies have highlighted that plasma systems involve significantly higher capital and operational costs compared with conventional thermal treatments, mainly due to the energy demand of plasma torches and the complexity of off-gas cleaning systems. Nevertheless, these costs are partially offset by the high volume reduction ratios (10–20 times), the chemical stability of the vitrified product, and the potential for long-term safety in final disposal. From an industrial perspective, plasma facilities become economically viable primarily at sites with large waste streams or where stringent regulatory requirements demand robust containment of hazardous radionuclides. Thus, while cost remains a limiting factor, ongoing technological advancements and accumulated operational experience continue to improve the economic outlook of plasma-based radioactive waste treatment.
In the present study, the reaction products (both solid and gaseous) obtained from the thermal plasma gasification of simulated operational radioactive waste (SORW) were characterized, analyzed and tracked throughout the process facility. The SORW was formulated using representative components of typical Argentine LLWs, including nitrile gloves, laboratory paper, and stable non-radioactive elements (added in the form of salts and oxides) to simulate the presence of 60Co, 90Sr, 137Cs, and 144Ce. The experimental tests were conducted in a prototype plasma gasification plant developed and built by the Departamento Materiales Nucleares of the National Atomic Energy Commission of Argentina (CNEA, for its initials in Spanish).
2. Materials and Methods
2.1. Experimental Setup
The thermal plasma gasification process implemented in this study consists of three main subsystems: a reaction system, a watercooling system, and a gaseous effluent treatment system (Figure 1).
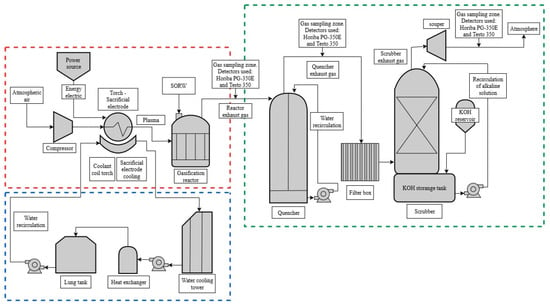
Figure 1.
Experimental setup scheme.
As shown in Figure 1, the reaction system (outlined in red) involves a gasification reactor which allocates a plasma torch (cathode), a sacrificial electrode (anode), a power source and an air compressor. An Hypertherm-brand plasma torch was used. This commercial device consists of a distributor ring, a copper cathode, a copper nozzle, a retaining cap, and a front shield. To ensure adequate cooling, the torch is surrounded by a 0.62 cm diameter copper pipe connected to two 1.27 cm diameter hoses, which allow for the circulation of cooling water in and out of the system [18]. Air ionization is achieved through the electric arc generated between the plasma torch cathode and the disposable electrode, powered by a Hypertherm Powermax105 power supply (Hipertherm Inc., Hanover, NH, USA). This unit applies a voltage of 160 VDC through air, with a current ranging from 30 to 105 A. As a result, the power delivered spans from 4800 to 16,800 W, providing sufficient energy to carry out the gasification process effectively [19,20].
A Kaeser screw compressor, model SX8 (Kaesser Compresores, Neuquén, Argentina) supplies compressed air to the plasma torch. This unit delivers a maximum flow rate of 9 kg/h at a pressure of 7 bar and is equipped with a KA-type filter to remove oil vapors and a KE-type filter to eliminate liquids and solid particles. These filtration systems ensure a consistent air supply with a high level of purity [21].
The disposable anode consists of a copper head attached to a double stainless steel tube through which cooling water circulates [20,22]. The copper head has an outer diameter of 37.3 mm, a length of 43.1 mm, a wall thickness of 6.1 mm, and a mass of 270 g. The double stainless steel tube measures 34.5 cm in length and has an external diameter of 2.37 cm [22,23].
The gasification reactor is a vertical cylinder with an outer diameter of 18.4 cm, a wall thickness of 4 cm, an inner diameter of 11 cm, and a height of 21.5 cm (1.4 L). It is made of Surecast 60 refractory cement (manufactured by S.A.E.M.S.A (Surecast 60 refractory cement, S.A.E.M.S.A., Buenos Aires, Argentina) composed of 60.0–62.0% Al2O3 and 31.0–35.0% SiO2, and capable for operation at temperatures up to 1650 °C. The refractory lid includes an inlet for waste feeding and a gas outlet. Two openings on the lateral walls allow for the insertion of the plasma torch and the sacrificial electrode. The reactor thermal insulation 10.5 cm thick Kaowool 1260 mineral blanket (ThermalCeramics, Augusta, GA, USA), composed of 53.0% SiO2 and 47.0% Al2O3, and housed within a 2 mm thick steel outer casing [20,22].
The cooling system, highlighted in blue in Figure 1, consists of a polypropylene tank containing 1500 L of water, two Avag Pumpen-brand pumps with flow rates ranging from 40 to 180 L/min and corresponding head heights of 27.5 to 80.7 m, respectively, and one Alfa Laval heat exchanger designed for a volumetric flow rate of 7.1 m3/h and an inlet temperature of 40 °C on the hot side, and a volumetric flow rate of 21 m3/h with an outlet temperature of 20 °C on the cold side. In both cases, the outlet temperature is 25 °C, with a maximum exchanged heat of 104.8 Mcal/h. The system also includes a Bio Prisa-brand induced-draft cooling tower, operating with counterflow air-water circulation and a water flow rate of 15 m3/h. System control is achieved through a panel of valves, flow meters, hoses, and thermocouples, all interconnected using fusion-welded piping [20].
For the treatment of gaseous effluents, highlighted in green in Figure 1, originating in the reaction chamber, the system includes one quencher installed downstream of the gasification reactor, a filter box containing a single filter, a scrubber, and one exhaust fan. The quencher is made of AISI 316 stainless steel and is equipped with a 100 L water storage tank. This device uses a fine counterflow rain of water to cool the effluent gases from the gasification reactor, operating with a water flow rate of 1 to 10 L/min, assuming an inlet water temperature below 20 °C and a maximum reservoir temperature of 70 °C. The quencher is designed to operate with a gas flow range of 6 to 30 kg/h and a maximum gas inlet temperature of 1500 °C. This component reduces the gas temperature to below 80 °C, thereby preventing thermal damage to downstream system components.
Downstream of the quencher is a filter box constructed from galvanized steel sheeting, with dimensions of 85 cm in height, 60 cm in width, and 60 cm in depth. Inside the box, a commercial polypropylene microfiber filter (model Soniq 9544228P, manufactured by Microfilter S.A. Filtración de Aire, San Antonio, TX, USA) is installed. This filter has an efficiency rating of 90–95%, corresponding to high-efficiency performance according to ASHRAE Standard 52.1 [24], and measures 60.96 cm × 60.96 cm × 55.88 cm. The filter is secured within the metal housing by a galvanized steel frame fitted with metal guide rails to prevent leakage. The unit is designed to operate at a flow rate of 3400 m3/h with a maximum working temperature of 82 °C [25].
After passing through the filter box, the gas stream goes through an alkaline scrubber tower. This unit is made of stainless steel and has a maximum height of 2850 mm, a length of 2050 mm, and a width of 770 mm [26]. Inside, it contains a packed bed of ceramic rings with an outer diameter of 15 mm, an inner diameter of 8 mm, a height of 15 mm, a total volume of 0.1 m3, and a packing thickness of 1000 mm. A potassium hydroxide (KOH) solution with a concentration of 50 g/L, diluted into 50 L of water, flows counter currently through these rings to neutralize acidic gases such as NOx and SO2 generated during the treatment. This equipment is designed for a maximum gas flow rate of 300 m3/h and achieves an outlet gas temperature ranging from 30 to 75 °C. It provides a purification efficiency exceeding 95% for solid particles and over 70% for acidic gases and aerosols [26].
Temperatures at critical points of the system were monitored using different thermocouples: Type K thermocouples connected to Testo 174H controllers (Testo SE & Co. KGaA, Lenzkirch, Germany) were used to measure the temperature at the quencher outlet and at the scrubber outlet, while Type S thermocouples monitored by a NOVUS LogBox Connect datalogger (NOVUS Produtos Eletrônicos Ltd.a., Canoas, Brazil) recorded the temperature inside the gasification reactor. This arrangement allowed precise characterization of the thermal conditions throughout the process and ensured the safety and efficiency of both the gasification and the treatment of gaseous effluents.
Finally, the gaseous effluent treatment system includes an exhaust fan that generates low pressure at the end of the circuit, driving gases from the reactor through the processing units and releasing the purified gases into the atmosphere. The exhaust unit is a centrifugal fan made of AISI 304 stainless steel, model Series 280—Size 500 SASE, manufactured by Cirigliano (Junín, Peru) [27]. It features an outlet opening of 200 mm × 140 mm and backward-curved blades in a type 4 arrangement. The equipment has an efficiency of 53%, with an absorbed power of 1.37 BHP and an installed power of 2 HP operating at 3000 rpm. The nominal flow rate is 500 m3/h at a static pressure of 400 mm of water column. In the event of a fan malfunction during operation, the plasma torch must be shut down manually [27]. To characterize the process gases (NOx (NO + NO2) CO, H2, H2S, CO2, O2, and SO2), an Horiba PG-350E gas analyzer (Horiba Ltd., Kyoto, Japan) [28] and a Testo 350 analyzer (Testo SE & Co. KGaA., Lenzkirch, Germany) [29] were used. Both instruments enabled the measurement of plasma generated gases concentrations at three key points: the outlet of the gasification reactor, the outlet of the scrubber tower, and the final system outlet. Additionally, to monitor gaseous species in the work area (H2, NO, and CO) an RKI Instruments Eagle gas detector (RKI Instruments, Inc., Union City, CA, USA) was employed [30].
2.2. Sample Preparation
The SORW was prepared to reflect a representative composition of LLW typically generated in Argentina, which predominantly is composed by disposable nitrile gloves and laboratory paper. As detailed in Table 1, the formulation included 63 g of nitrile gloves (corresponding to a volume of 3900 mL) and 7 g of laboratory paper (1725 mL). The total initial mass was 70 g, and an overall volume was 5625 mL.

Table 1.
Composition of the SORW.
Figure 2a illustrates the total volume of the SORW, highlighting the proportional contribution of nitrile gloves and laboratory paper in its formulation.
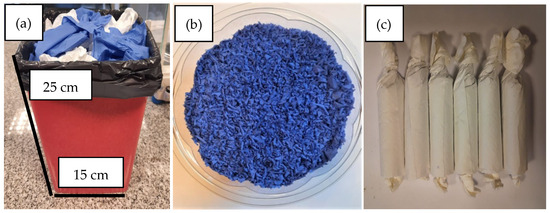
Figure 2.
(a) Materials of the SORW in a container of 5625 mL. (b) Shredded gloves. (c) Shredded gloves packed in paper wrappers.
To ensure compatibility with the feeding system of the gasification facility, the nitrile gloves were subsequently shredded to an average particle size of approximately 0.2 cm using a tungsten carbide blade mill Pulverisette 25 model, Fritsch brand (Fritsch GmbH, Berlin, Germany) [31], as shown in Figure 2b.
With the resulting material, 6 equivalent packs were prepared, using 6 paper sheets measuring 20 × 20 cm each to enable controlled feeding into the reaction chamber (Figure 2c).
Finally, to simulate the presence of the radionuclides 60Co, 90Sr, 137Cs, and 144Ce within the waste matrix, 0.17 g of each corresponding metal (Co, Sr, Cs, and Ce) was introduced into every of the six individual packs as Co3O4 (≥99.5%), Sr(NO3)2 (≥99.0%), CsNO3 (≥99.9%), and CeO2 (≥99.9%) (Sigma-Aldrich, Buenos Aires, Argentina), respectively, in the form of fine solid powders. This procedure resulted in the distribution of approximately 1 g of each metal across the 6 prepared packs (6 × 0.17 g ≈ 1 g). The specific quantities and compounds used for simulation are detailed in Table 2.

Table 2.
Masses of inorganic compounds added to the 70 g of simulated waste to achieve a final content of 1 g of each target metal (Co, Sr, Cs, and Ce).
Figure 3 shows the specific inorganic compounds (Co3O4, Sr(NO3)2, CsNO3 and CeO2) to simulate the behavior of volatile and semi-volatile metals under plasma gasification conditions.
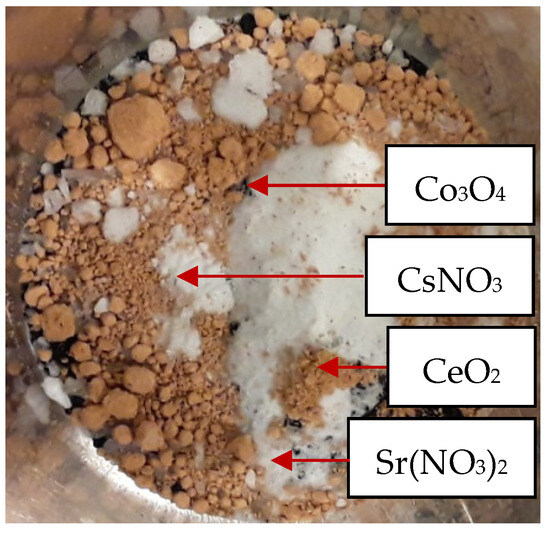
Figure 3.
Added inorganic compounds.
Accordingly, the total mass of the final waste, comprising the 6 prepared units, was 77.97 g. This included 63 g of nitrile gloves, 7 g of laboratory paper (used as wrappers), and 7.97 g of metal compounds. The quantity of simulant metals was deliberately selected to ensure detectable signal levels during analysis, considering the sensitivity and detection limits of the characterization techniques applied in this research.
This consideration was critical to the design of the study, since that incorporating the actual concentrations of metals typically present in a 70 g SORW sample would have resulted in quantities far below the detection limits of conventional analytical techniques. Moreover, such minimal masses would have been impractical to measure with sufficient accuracy, thereby compromising the reliability of the characterization results.
2.3. SORW Treatment Conditions
The gasification of the SORW was performed using a Hypertherm plasma torch (Hypertherm, Inc., Hanover, NH, USA), operated in gouging mode under a direct current of 30 A and a voltage of 160 VDC. Compressed air was employed as plasmatic gas, delivered at the maximum flow rate and pressure conditions (9 kg/h and 7 bar).
To ensure thermal stability and equipment integrity, the cooling circuits for the plasma torch and the disposable electrode were maintained with constant water flow rates of 6 L/min and 8 L/min, respectively.
To ensure controlled and consistent feeding of the SORW, the waste packages were introduced into the reactor at 2 min intervals using a batch-feeding strategy. Simultaneously, the gas cooling unit (quencher) operated with a continuous water flow of 8 L/min to rapidly reduce gas temperatures. The subsequent gas scrubbing stage employed a 0.5 L aliquot of KOH solution at a concentration of 50 g/L (pH = 13.6), diluted into 50 L of water and recirculated at a flow rate of 0.15 m3/h throughout the duration of the treatment process.
Additionally, solid waste produced during the treatment was manually extracted from inside the reactor to ensure complete recovery for subsequent analysis, allowing a comprehensive assessment of the distribution and retention of surrogate elements. Furthermore, liquid effluent samples were collected from both the quencher and the scrubber to characterize their chemical composition, providing information of soluble species.
2.4. Characterization
Prior to the gasification of the SORW, representative fragments of the nitrile gloves and laboratory paper constituting the waste were individually selected and subjected to chemical analysis using energy-dispersive X-ray spectroscopy (EDS) (Oxford Instruments, Abingdon, UK). On the other hand, blank samples were collected from different sectors of the gasification system to evaluate their elemental composition by EDS. These samples were obtained by swab testing selected internal surfaces with clean laboratory-grade paper, with the objective of detecting any pre-existing residues or deposits and establishing a baseline for subsequent comparative analysis. At the end of the experiment, solid samples were manually retrieved from the internal part of the gasification reactor, as well as from deposits attached to various components of the system. All EDS analyses were performed using a Zeiss Crossbeam 340 scanning electron microscope (SEM, Zeiss, Buenos Aires, Argentina), equipped with a field emission electron source, an Oxford Max 80 energy-dispersive X-ray detector (Oxford Instrument, Oxford, UK), and built-in secondary and backscattered electron detectors [32]. Prior to analysis, samples were mounted on graphite carbon tape fixed to aluminum stubs. The semi-quantitative analyses were conducted using sample masses in the milligram range. Each measurement was performed with an accelerating voltage of 25 keV and a dwell time of 3 min per spot.
The mineralogic analyses of the samples were performed by X-ray diffraction (XRD) analyses to identify the crystalline phases present. These analyses were carried out using a Bruker D8 Advance diffractometer (Bruker D8 Advance, Karlsruhe, Germany). The measurements were conducted over a 2θ range of 5° to 85°, with a step size of 0.02° and a counting time of 0.5 s per step. Phase identification was performed using the HighScore Plus software (version 3.0a (3.0.5), PANalytical B.V., Almelo, The Netherlands) in conjunction with the COD2023 crystallographic database. In this work, the analyses were limited to qualitative phase identification, and no quantitative phase analysis was performed.
Instrumental Neutron Activation Analysis (INAA) was used to determine the elemental concentrations in the liquid samples collected from the quencher and scrubber, both prior to and following the gasification experiment. For each analysis, 10 μL of sample were pipetted onto a 1 cm2 section of filter paper, which was subsequently dried, encapsulated, and loaded into the pneumatic irradiation system of the RA-6 nuclear research reactor (National Atomic Energy Commission, CNEA, Autónoma de Buenos Aires, Argentina). The irradiations were carried out under a thermal neutron flux of 2.3 × 1012 n·cm−2·s−1, with a measurement duration of approximately 6 h per sample.
3. Results and Discussions
3.1. Solids Characterization: Samples and Reaction Products
The initial components of the SORW, nitrile gloves and laboratory paper, were chemically characterized by EDS prior to the gasification experiment. For each material, three random samples were selected from different surface zones and analyzed to assess compositional uniformity; the resulting values were averaged. The elemental composition of both materials is presented in Table 3, with concentrations reported as weight percentages (% w/w), providing a representative baseline for subsequent interpretation of the gasification results.

Table 3.
EDS results of the SORW components. N.D.: Not detected.
The results for elements with Z > 5 are presented in Table 3 reveal clearly distinct compositional profiles for the components from the SORW.
The nitrile gloves exhibited a high C content (71.9% w/w), characteristic of their polymeric nature, along with significant amounts of O (13.2% w/w), Cl (6.4% w/w), S (2.5% w/w), trace metals of Al (1.4% w/w), Ti (1.3% w/w), Zn (1.4% w/w), and trace of Ca (1.9% w/w), K (0.5% w/w) and Si (0.1% w/w). These elements are most likely derived from additives and fillers commonly used during glove manufacturing [33]. The chlorine content is primarily derived from the chemical treatment of the gloves with Cl2, applied to improve surface texture, remove manufacturing dust, and reduce residues from sulfur-containing compounds used during vulcanization; in this process, Cl is retained as HCl on the surface and does not incorporate into the acrylonitrile copolymer backbone. The detected S also originates from residual compounds from the vulcanization process. The detected metals in the sample (Al, Ti, Zn, Ca, K, and Si) correspond to trace additives incorporated into the polymer matrix to enhance mechanical and thermal properties.
On the other hand, the notable Cl content is of special importance, since under high-temperature conditions it can favor the formation of volatile metal chlorides, a process that is thermodynamically feasible in systems such as the one employed in this study, potentially enhancing the migration of elements such as Cs and Sr during plasma gasification. Overall, the elemental composition observed is consistent with values reported for nitrile-based materials, confirming both the polymeric carbon backbone and the presence of additives commonly used during glove production.
In contrast, the laboratory paper was composed almost exclusively of C (50.3% w/w) and O (49.6% w/w), consistent with cellulose-based materials, with only minor traces of Na (0.1% w/w) detected [34]. This composition reflects the high purity of the material, characteristic of products manufactured for laboratory use, and confirms its predominantly organic nature with negligible inorganic additives. Such simplicity in elemental composition makes the paper an appropriate reference component for studying gasification behavior, as its degradation pathways are mainly governed by cellulose thermal decomposition mechanisms.
On the other hand, to establish a reference baseline for subsequent analyses, blank samples from the plasma gasification system were also collected and analyzed using EDS prior to the gasification treatment. The samples studied included: dust collected from the refractory brick lining the inner chamber of the gasification reactor (S01); shavings from the inner surface of the reactor’s metal lid (S02); dust from the internal surfaces of the pipe connecting the gasification reactor to the quencher (S03, S04, and S05); scraps from the internal structure of the quencher (S06); scraps from the internal surfaces of the pipe connecting the quencher to the filter box (S07 and S08); a sample of the filter material inside the filter box (S09); and scraps from the inner surface of the pipe connecting the scrubber outlet to the atmosphere (S10). The samples are shown in Figure 4.
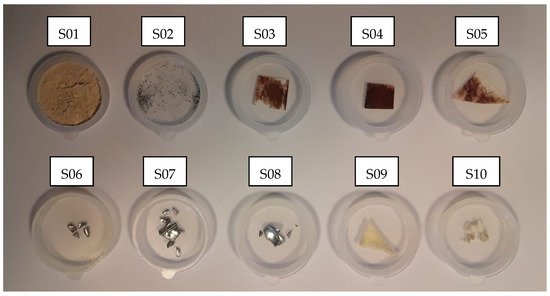
Figure 4.
Blank samples S01–S10 from the plasma gasification system.
The dust samples S03, S04, and S05 were collected by rubbing a piece of paper (previously analyzed and labeled as sample S11) against the inner surface of the connecting pipe. The EDS chemical results of the blank samples are presented in Table 4.

Table 4.
EDS analysis results of the blank samples from the gasification system. N.D.: Not detected.
The EDS results presented in Table 4 highlight the chemical heterogeneity among the blank samples collected prior to the treatment, reflecting the diverse materials and functions of different components within the plasma gasification system. This variability is consistent with the use of alloys, coatings, and sealing compounds that are typically employed in high-temperature reactors, and may introduce background signals in the analysis.
Sample S01 (refractory brick dust) exhibited a composition dominated by Al (22.3% w/w) and O (44.4% w/w), with minor Si (5.4% w/w) and Ca (5.5% w/w) content, characteristic of aluminosilicate-based linings [35]. Minor amounts of C (20.3% w/w), Ti (0.8% w/w), Fe (0.9% w/w) and Cu (0.1% w/w) were also detected, likely originating from trace additives or impurities present in the refractory material, consistent with the typical composition of aluminosilicate-based refractory bricks used in high-temperature applications.
In contrast, S02 (metal lid shavings) exhibited a composition characteristic of stainless steel [36], dominated by Fe (62.7% w/w) with significant alloying elements including Cr (4.3% w/w) and Ni (2.6% w/w). Trace elements included Mn (0.7% w/w), Zn (0.6% w/w) and Cu (0.2% w/w), consistent with secondary alloy components or surface contaminants. The carbonaceous composition observed in samples S03-S05 (C (45.1–46.2% w/w) and O (49.6–50.4% w/w)) reflects the sampling methodology rather than pipe deposits. These samples were collected by swabbing internal pipe surfaces with laboratory-grade filter paper (S11 composition: C (48.5% w/w), O (49.8% w/w)), indicating the measured values primarily represent the paper matrix. The slight compositional variations suggest minimal pipe-derived contamination. Trace element of samples S03-S05 revealed the presence of Ca (2.0–2.9% w/w) and Cl (0.1% w/w) at concentrations exceeding those detected in the blank reference paper S11 (Ca (1.3% w/w); Cl (0.1% w/w)), confirming the existence of surface deposits in the pipes. These deposits account for less than 3% of the total mass fraction, indicating minimal contribution compared to the paper matrix used for sampling. The specific detection of Fe (0.9–1.1% w/w) in the pipe samples, which was absent (<0.1% w/w in the blank paper, provides conclusive evidence of metallic material transfer from the system’s internal surfaces.
Sample S06 (quencher shavings) exhibited a notable Ti content (8.2% w/w), accompanied by significant Si (10.4% w/w) and high C (50.9% w/w) and O (25.2% w/w) levels. This Ti-enriched profile, along with detectable Al (0.6% w/w) and Ca (2.4% w/w), strongly suggests the presence of corrosion-resistant surface treatments [37], potentially titanium-based ceramic coatings or alloys designed for thermal shock resistance.
In contrast, samples S07 and S08 from the filter box piping showed markedly different compositions: S07 displayed Zn dominance (65.5% w/w) with complementary Fe (8.1% w/w), characteristic of galvanized steel components, while S08 presented a ferrous alloy signature with Fe (76.5% w/w) and Cr (14.2% w/w), consistent with stainless steel construction.
The filter medium (S09) composition validated its expected carbonaceous nature, showing C: 87.6% w/w with minimal elements (O: 11.5% w/w; others ≤0.2% w/w), reflecting its design as a high-purity filtration matrix.
The elemental analysis of sample S10, collected from a PVC pipe at the scrubber outlet, demonstrated a characteristic Cl content (11.4% w/w) consistent with the fundamental composition of polyvinyl chloride [38], correlating well with the theoretical formulation (C2H3Cl)n where chlorine represents ≈56.8% at (equivalent to ≈25–30% w/w in industrial-grade plastic compounds with additives). In comparison, sample S11 (filter paper used for residue collection) exhibited a distinct composition dominated by C (48.5% w/w) and O (49.8% w/w) with minimal trace elements (≤1.3% w/w combined), perfectly matching the molecular structure of cellulose (C6H10O5)n.
The comprehensive pre-treatment analysis revealed that all blank samples (S01–S11) exhibited non-detectable levels of Co, Sr, Cs, and Ce below the instrumental detection threshold (<0.1% w/w by EDS analysis), confirming three fundamental experimental conditions:
- (1)
- System cleanliness validation (absence of cross-contamination between operations and validated cleaning protocols (≤0.1% w/w detection limit)).
- (2)
- Analytical methodological reliability (appropriate EDS detection limits and consistent null results for the elements used as simulants across all sampling locations).
- (3)
- Establishment of experimental controls (pre-treatment reference values to differentiate between system materials and process-derived contaminants).
This rigorous baseline characterization the was the previous step for calculating retention efficiencies of surrogate radionuclides (Co, Sr, Cs, and Ce), tracing migration pathways through system components, and verifying that all surrogate elements detected in post-treatment samples originated exclusively from the SORW.
After the gasification experiment, 4 solid residues were identified within the reactor: a solid ashes (SR1) was deposited at the bottom of the gasification chamber (Figure 5a); a black solid layer (SR2) formed on the surface of the sacrificial electrode (Figure 5b); a third residue (SR3) was observed attached to the inner walls of the reactor (Figure 5c); and a fourth deposit (SR4) accumulated on the internal surface of the metallic lid (Figure 5d).

Figure 5.
Solid residues observed within the reactor after the gasification experiment. (a) SR1 accumulated at the bottom of the gasification reactor. (b) SR2 deposited on the lateral surfaces of the sacrificial electrode. (c) SR3 attached to the internal walls of the reactor chamber. (d) SR4 attached to the internal surface of the reactor’s metallic lid.
The solid residues SR1–SR4 were weighed using a high-precision analytical scale Radwag, model AS 220.R2 (Radwag Wagi Elektroniczne, Radom, Poland). Their volumes were determined using glass graduated cylinders Corning PYREX, Class A, to contain 100–500 mL, (Corning Inc., New York, NY, USA) to ensure reliable and reproducible measurements.
This approach minimizes uncertainties associated with manual handling and guarantees that the calculated metrics can be directly compared across different residues without introducing systematic bias.
Table 5 presents the measured volumes and masses of each residue, along with the calculated volume reduction factor (VRF = VInitial/VFinal) and the corresponding percentage of volume reduction relative to the initial volume of the SORW (VInitial = 5625 mL).

Table 5.
Volume, mass, and reduction efficiency of solid residues recovered post treatment.
As shown in Table 5, the plasma gasification process resulted in a substantial reduction in the initial volume of SORW, decreasing from 5625 mL to a final volume of just 25 mL. This corresponds to a volume reduction factor of VRF = 5625 mL/25 mL = 225 and a volume reduction efficiency of 99.6%.
Subsequently, samples were collected from the solid residues SR1–SR4, which were labeled with the letter S followed by the number corresponding to their sampling location (S12, S13, S14, and S15) (Figure 6).

Figure 6.
Samples S12–S23 recovered after the gasification treatment of the SORW.
In addition, after the experiment, samples were taken from powders attached to the inner walls of the pipe connecting the gasification reactor to the quencher (S16, S17, and S18), as well as from deposits on the internal surface of the quencher (S19). Further samples were recovered from the ducts connecting the quencher to the filter box (S20), from inside the filter box (S21), from the inner surface of the pipe between filter box and the scrubber (S22), and from the outlet pipe of the scrubber (S23) (Figure 6). Samples S16–S23 were collected by rubbing a clean piece of paper over the corresponding surfaces.
Samples S12–S23 underwent systematic EDS point analysis to evaluate the distribution of surrogate elements Co, Sr, Cs, and Ce across the gasification system. Measurement areas were strategically selected to ensure representative sampling of each component, with reported values representing the arithmetic mean in Table 6. This approach allowed not only the identification of localized accumulations of specific elements, but also the comparison of retention trends between distinct system zones, thus providing a more comprehensive understanding of the migration of the surrogates.

Table 6.
EDS analysis results for samples S12–S23. N.D.: Not detected.
The EDS results presented in Table 6 reveal a clear preferential retention of surrogate elements Co, Sr, Cs and Ce in the reactor residue samples S12–S15 (S12: Secondary solid residue (SR1); S13: Solid deposited on the surface of the disposable electrode (SR2); S14: Solid deposited on the inner surface of the gasification reactor (SR3); S15: Solid deposited on the surface of the reactor’s metallic lid (SR4), with the highest concentrations observed in the sample S12 (SR1: 9.2% w/w Co, 4.2% w/w Sr, 4.1% w/w Ce, 1.2% w/w Cs).
A progressive decrease in these elements content is evident across subsequent samples: S13 (SR2) retained 3.2% w/w Co, 1.3% w/w Sr, 1.6% w/w Ce, 1.3% w/w Cs, while S14 (SR3) showed intermediate levels (2.2% w/w Co, 1.0% w/w Sr, 0.9% w/w Ce, 0.5% w/w Cs), reflecting a thermal deposition gradient correlated with reactor temperature zones.
Notably, less volatile elements Co and Ce (approximately 1.0 × 10−3 Pa for Co and 1.6 × 10−2 Pa for Ce at 1500 °C)) exhibited stronger retention in high-temperature regions (>1000 °C, with the reactor operating at ~1030 °C), as seen in S12’s dominant Co (9.2% w/w) and Ce (4.1% w/w) concentrations. In contrast, more volatile species (Sr, Cs) migrated toward cooler reactor sections, with S15 (SR4), likely from the reactor lid, showing elevated Sr (1.5% w/w) and Cs (1.7% w/w) despite lower Co and Ce levels (4.3% w/w Co, 3.4% w/w Ce).
This distribution aligns with the observed thermal gradient, where the downstream gas stream underwent rapid quenching from 1030 °C at the reactor outlet to 30 °C in the quencher, and further cooling to 21 °C at the scrubber outlet.
Downstream samples (S16–S23) showed an exponential decrease in the concentrations of the surrogate elements, reflecting progressive deposition and dilution along the connecting pipes. Residual amounts ≤0.5% w/w were detected in samples S16–S18 for certain elements, indicating that only trace quantities of these species remained in the gas stream after passing through the primary reactor components. This trend highlights the effectiveness of the reactor in retaining the majority of non-volatile species and demonstrates the minimal carryover of surrogate elements toward the quenching and gas treatment sections.
Remarkably, from sample S19 onward, all four surrogate elements fell below detection limits (<0.1% w/w), demonstrating complete deposition within upstream reactor components. This abrupt transition confirms >99% retention of these non-volatile species prior to the quench system and underscores the critical role of temperature gradients in governing the partitioning behavior of surrogate elements.
Based on the EDS results for Co, Sr, Cs, and Ce presented in Table 6, together with the experimentally measured masses of the solid residues SR1–SR4 (Table 5), the retained mass of each surrogate element in the corresponding solid waste fraction was determined. These calculations provide a quantitative assessment of element partitioning within the reactor and allow for a detailed evaluation of retention across the different solid residues, as summarized in Table 7.

Table 7.
Masses of Co, Sr, Cs, and Ce retained in the solid residues SR1, SR2, SR3, and SR4.
To further quantify element partitioning, Table 8 presents the volatilized mass and retention percentages of Co, Sr, Cs, and Ce, calculated as the difference between the initial mass (1 g per element) and the total retained mass in SR1–SR4.

Table 8.
Masses of Co, Sr, Cs, and Ce volatilized during plasma gasification, with corresponding SR1–SR4 retention percentages.
The plasma gasification treatment of SORW resulted in different spatial distributions of the surrogate elements, strongly influenced by the measured thermal profile of the system:
- (1)
- Co demonstrated exceptional retention (96 ± 10% of initial mass), with 0.96 ± 0.10 g recovered primarily in the bulk residue (SR1: 0.44 ± 0.04 g) and reactor lid deposits (SR4: 0.46 ± 0.05 g). The minimal volatilization (0.04 ± 0.10 g) can be attributed to the high thermodynamic stability of cobalt oxides, Co3O4 and CoO, which remain stable under the 1030 °C reactor conditions. The absence of Co in downstream zones confirms that rapid cooling in the quencher (to 30 °C) effectively suppressed its transport.
- (2)
- Sr showing 39 ± 4% retention (0.39 ± 0.04 g retained; 0.61 ± 0.04 g volatilized). The Sr(NO3)2 surrogate decomposed completely between 650 and 700 °C, following the reaction: Sr(NO3)2 → SrO(s) + 2NO2(g) + ½O2(g), with the resulting SrO remaining thermally stable up to 2500 °C. Despite this stability, only 39 ± 4% of initial Sr (0.39 ± 0.04 g) was retained in the reactor, primarily as particulated SrO entrained in the residue SR1. The significant volatilized fraction (0.61 ± 0.04 g) is attributed to SrO particles physically carried by the high-velocity plasma stream at 1030 °C, with possible minor vaporization at plasma core temperatures, and subsequent migration toward cooler zones where deposition became limited before the rapid temperature drop in the quencher (30 °C).
- (3)
- Ce exhibited superior retention (59 ± 6%, 0.59 ± 0.06 g) due to the remarkable stability of CeO2, which remains undecomposed under plasma conditions (melting point: 2400 °C). Its retention was favored in the 1030 °C reactor core, as evidenced by significant concentrations in SR1 and SR4. The volatilized fraction (0.41 ± 0.06 g) likely escaped primarily through mechanical entrainment of CeO2 particulates in the high-velocity gas stream.
- (4)
- Cs displayed the most pronounced volatility (74 ± 3% loss, 0.74 ± 0.03 g), with only 26 ± 3% retention. This behavior stems from the thermal decomposition of CsNO3 between 700 and 900 °C (CsNO3 → Cs2O + N2 + O2), followed by the volatilization of Cs2O at temperatures above 490 °C. The 1030 °C reactor temperature ensured extensive volatilization, with deposition occurring preferentially in cooler reactor surfaces (SR4) before the gases entered the quencher. The sudden decrease to 30 °C in the quencher and 21 °C in the scrubber effectively prevented further downstream transport, confirming that most Cs was retained in proximal reactor sections.
Furthermore, to complement the EDS results presented in Table 6, XRD analyses were conducted to identify the crystalline phases present in samples S12–S15, corresponding to the solid residues SR1–SR4, respectively. The obtained diffractograms, along with the identified crystalline phases, are shown in Figure 7a–d.
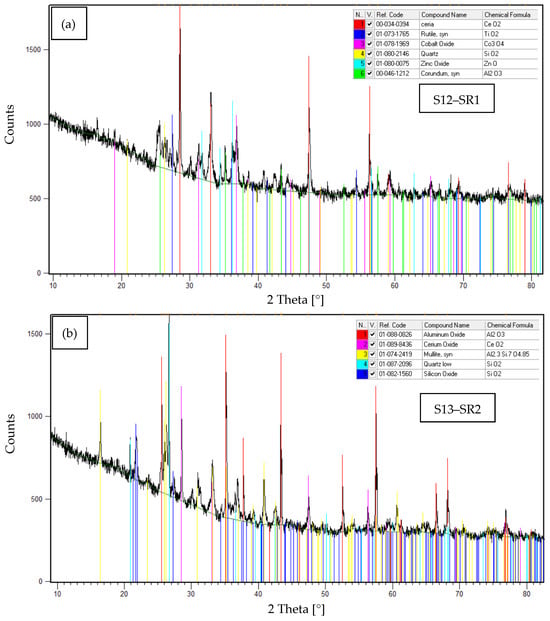
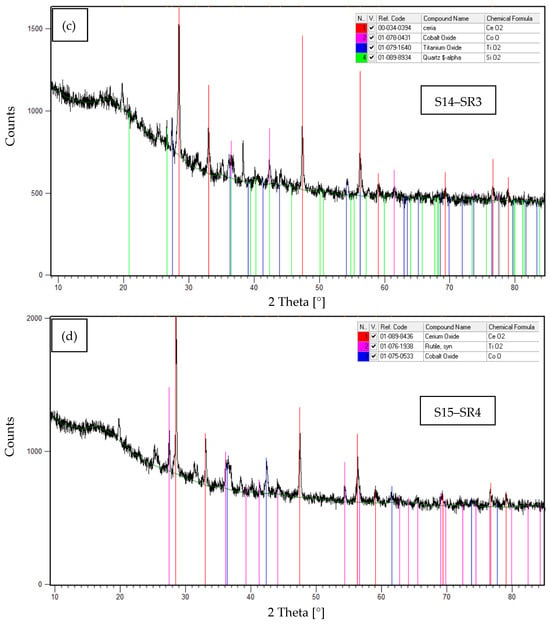
Figure 7.
XRD patterns of samples S12–S15, corresponding to: (a) SR1. (b) SR2. (c) SR3. (d) SR4.
Table 9 summarizes the experimental results obtained from the XRD analyses performed on samples S12–S15. The crystalline phases identification were performed by matching experimental diffraction patterns with reference standards from the International Centre for Diffraction Data (ICDD) database [39].

Table 9.
Results of the XRD analysis of the solid samples S12–S15 (Ok: Present). N.D.: Not detected.
The XRD results presented in Table 9 reveal a marked heterogeneity in the mineralogical composition of the recovered solid residues (S12–S15), reflecting differences such as thermal conditions, and degrees of interaction with the gasification system materials. The identified phases were:
- (1)
- Stable surrogate phases: CeO2 and Co3O4 (originally introduced in the SORW), confirming their exceptional thermal stability in reactor zones with temperatures ˂900 °C during plasma gasification.
- (2)
- Oxidation reaction products: TiO2 and ZnO, formed through high-temperature oxidation of Ti (˃1000 °C) and Zn (˃1400 °C) originating from nitrile glove decomposition (present in the initial waste).
- (3)
- Thermal decomposition products: Co3O4 underwent thermal reduction to CoO in reactor zones ≥900 °C, demonstrating temperature-dependent cobalt speciation (Co3+ → Co2+) under plasma conditions.
- (4)
- Reactor-derived contaminants: SiO2, Al2O3 and Al2.3Si7O4.85, attributable to the refractory lining material within the gasification chamber, which became mechanically entrained (spalling/carry-over) during residues recovery. This observation indicates that only minimal interaction between the molten/slag fraction and the refractory occurred, leading to limited incorporation of lining-derived oxides into the solid residues.
- (5)
- A significant proportion of amorphous material was identified in all analyzed samples (S12–S15), as evidenced by a pronounced low-angle background hump (10–25° 2θ) in XRD difractograms. This feature suggests rapid solidification of vaporized species under extreme plasma conditions, where high quenching rates inhibited crystalline phases formation.
3.2. Liquids Characterization
During the plasma gasification treatment of the SORW, the generated gases sequentially passed through the quencher and the scrubber. To evaluate the potential incorporation of surrogate radionuclides into the aqueous phase, water samples were collected from both units before (S24–S25) and after the treatment (S26–S27). These samples were analyzed to detect the presence of Co, Sr, Cs, and Ce using INAA. The results of these measurements are summarized in Table 10.

Table 10.
Concentrations of surrogate elements in water samples collected before and after the gasification process.
On the other hand, since these concentrations correspond to volumes of 100 L for the quencher and 50 L for the scrubber, the total masses of each element were determined at both stage of the process, before and after the gasification experiment. The results are summarized in Table 11.

Table 11.
Total masses of Co, Sr, Cs, and Ce in the liquid samples collected from the quencher and scrubber before and after the treatment of the SORW.
Results obtained by INAA confirmed that all the surrogate elements (Co, Sr, Cs, and Ce) in pre-treatment aqueous samples from both the quencher (S24) and scrubber (S25), are below the detection limits of the technique (Co: <0.5 ppb, Sr: <2 ppb, Cs: <1 ppb, Ce: <3 ppb) (Table 10).
Consequently, the total mass of these elements in both components was assumed to be zero (Table 11). This interpretation is further substantiated by national sanitary regulations in Argentina, which do not include these elements among those monitored in drinking water [40]; the type of water used in the system.
These results establish no pre-existing contamination in the liquid treatment systems, and that all post-treatment elemental detections (S26–S27) exclusively originated from the plasma process.
Quencher and scrubber water analysis following gasification revealed significant elemental concentration of differences between system components:
- (1)
- The quencher water (S26) contained measurable concentrations of all surrogate elements, with Sr showing the highest accumulation (2275 ± 110 ppb), followed by Co (302 ± 15 ppb), Cs (293 ± 20 ppb), and Ce (28 ± 3 ppb).
- (2)
- Based on the initial 1 g mass of each surrogate element in the SORW, the quencher captured 22.8 ± 1.1% of Sr (0.2275 ± 0.011 g), 3.0 ± 0.15% of Co (0.0302 ± 0.0015 g), 2.9 ± 0.15% of Cs (0.0293 ± 0.0015 g), and 0.3 ± 0.03% of Ce (0.0028 ± 0.0003 g).
- (3)
- The scrubber water (S27) showed <LOD concentrations for all elements (Co: <0.5 ppb, Sr: <2 ppb, Cs: <1 ppb, Ce: <3 ppb); INAA detection limits), indicating >3-log reduction from quencher levels.
- (4)
- Analysis confirmed scrubber retention of <0.001 g per surrogate element (<0.1% of input mass).
On the other hand, based on the surrogate elements masses retained in the quencher (Table 11) and the masses retained in the gasification reactor (Table 7), while considering their absence at all sampling points downstream of the quencher (Table 6), we estimated the residual mass of each element retained in the connecting pipe between the gasification reactor and the quencher.
This analysis allows a comprehensive view of the element partitioning throughout the system, highlighting the intermediate deposition along the gas flow path. The resulting distribution of masses is illustrated in Figure 8.
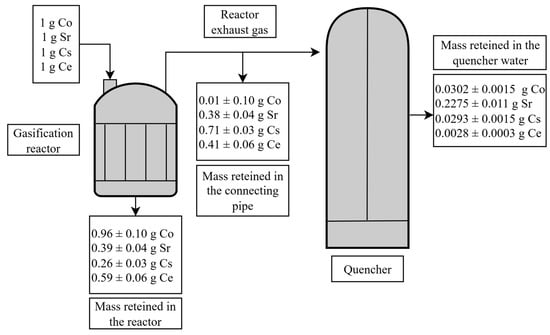
Figure 8.
Mass balance of the simulant elements retained in the system.
Figure 8 illustrates the distribution of Co, Sr, Cs, and Ce across the gasification reactor, the connecting pipe, and the quencher. Most Co and Ce are retained within the reactor, whereas significant fractions of Cs and Sr are captured in the connecting pipe. Only minimal amounts are present in the quencher water, confirming that the majority of the surrogate elements are retained within the system. The values provided in Figure 8 consider both the instrumental uncertainty of the EDS measurements and the variability associated with sampling and residue heterogeneity.
In order to complement these results, Table 12 shows the retention percentage in these three stages of the process. In doing this, we assume that no Co, Sr, Cs and/or Ce are released into the atmosphere by the gas extraction system.

Table 12.
Retention percentage of surrogate elements in the reactor, connecting pipe and quencher.
The mass distribution of surrogate elements across the gasification system reveals different retention patterns:
- (1)
- Co demonstrated near-complete reactor retention (96 ± 10%), with minimal transfer to other system components: pipe deposition was statistically insignificant (1.0 ± 10%) and quencher capture was limited (3.02 ± 0.15%). This distribution reflects the exceptional stability of cobalt oxides under plasma conditions, as evidenced by their high melting point and low volatility. Notably, the vapor pressure of Co at 1500 °C is approximately 10−3 Pa, indicating its strong resistance to vaporization at the operating temperatures of the reactor (1030 °C).
- (2)
- Sr showed similar partitioning between three system components: reactor retention (39 ± 4%) matched pipe deposition (38 ± 4%), while quencher capture accounted for the remaining 22.75 ± 1.1%. This balanced distribution indicates partial volatilization during gasification, with subsequent recondensation of Sr species in the connecting pipe and in the quencher.
- (3)
- Cs exhibited the most different partitioning pattern among all elements, with dominant accumulation in the connecting pipe (71 ± 3%), significantly lower retention in the reactor (26 ± 3%), and moderate capture in the quencher (2.93 ± 0.15%). This three-phase distribution is characteristic of alkali metal behavior in high-temperature processes, where vapor-phase transport of Cs species predominates above 600 °C.
- (4)
- Ce exhibited predominant retention in the reactor (59 ± 6%) and significant deposition in the connecting pipe (41 ± 6%), while quencher capture was minimal (0.28 ± 0.03%). This distribution reflects the thermal stability of CeO2 (melting point: 2400 °C), where one fraction remains in the reactor while another is mechanically transported as fine particles that attach in both the pipe and quencher.
Although no crystalline metal chlorides (e.g., CsCl and SrCl2) were detected in the solid residues analyzed, their possible formation during plasma gasification cannot be excluded and may contribute to the observed migration of volatile species such as Cs and Sr. This highlights the importance of considering Cl chemistry when evaluating the behavior of volatile metallic elements in future experiments.
3.3. Gases Characterization
A systematic chemical characterization of gaseous emissions produced during plasma gasification and downstream was performed by sampling at three key stages of the process: (1) the gasification reactor outlet, (2) the quencher outlet, and (3) the scrubber outlet. The study focused on quantifying the cumulative concentrations (CT) of major gaseous species, including nitrogen oxides (NOx, NO and NO2), CO, CO2, SO2, H2S, H2 and O2. These measurements were conducted to evaluate the evolution of gas composition across successive treatment stages. Table 13 presents the measured concentrations, demonstrating the progressive transformation of the gas stream through the reactor, quencher, and scrubber.

Table 13.
Temporal evolution of cumulative concentrations (CT) for monitored gaseous species. Units: ppm·min (except CO2/O2: %Vol·min).
The derived mass values and their associated percentage reductions are detailed in Table 14. The mass reduction percentage was calculated as the removed fraction of each species (capture, transformation or retention) between successive treatment stages, normalized to its initial mass at the reactor outlet. This metric provides a direct evaluation of the gas treatment system’s removal efficiency, highlighting its performance in remotion of specific pollutants.

Table 14.
Masses of each gaseous species at the outlet of each stage of the gasification system and corresponding percentage reduction.
The stage-resolved concentration of gaseous species (Table 14) provides critical insights into the plasma gasification process efficiency and underlying pollutant removal mechanisms.
- (1)
- Nitrogen oxides (NOx) exhibited exceptional reduction performance, with total mass decreasing from 9.7 ± 0.6 g at the reactor outlet to 0.10 ± 0.01 g after the scrubber, representing a 98.9 ± 0.6% reduction. Detailed analysis revealed different removal pathways for NO and NO2: while NO showed higher reduction efficiency (99.0 ± 0.5%, from 8.9 ± 0.5 g to 0.10 ± 0.01 g) compared to NO2 (97.0 ± 1.2%, from 1.2 ± 0.1 g to 0.03 ± 0.003 g), the intermediate quencher stage showed a 17.8% mass increase for NO2 (1.2 to 2.0 g) concurrent with a 32.6% NO decrease. This behavior suggests: (i) rapid thermal oxidation of NO to NO2 during quenching (NO + ½O2 → NO2, ΔH = −57 kJ/mol) [41], and (ii) subsequent preferential alkaline absorption of NO2 in the scrubber through the reaction NO2 + 2KOH → KNO3 + KNO2 + H2O (k = 3.2 × 104 M−1s−1 at 25 °C) [42].
- (2)
- CO exhibited near-complete elimination (98.2 ± 0.8%), decreasing from 2.4 ± 0.1 g to 0.04 ± 0.004 g across the treatment train. This exceptional reduction efficiency suggests: (i) effective oxidation of CO to CO2 in the high-temperature plasma zone (CO + ½O2 → CO2, ΔH = −283 kJ/mol; from NIST-JANAF thermochemical data) [43], and (ii) subsequent physical absorption in the quencher (Henry’s constant of CO in water at 25 °C is ≈ 9.7 × 10−4 mol/L·atm) [44]. In contrast, CO2 showed a less pronounced but still significant 75.0 ± 1.1% mass reduction (109 ± 2 g to 27 ± 0.4 g), primarily achieved in the alkaline scrubber through carbonation reactions (CO2 + 2KOH → K2CO3 + H2O, k = 1.4 × 103 M−1s−1) [45].
- (3)
- SO2 showed an 80.6 ± 2.5% mass reduction (0.10 ± 0.01 g to 0.02 ± 0.003 g), while H2S exhibited near-complete removal (97.6 ± 0.6%, from 1.0 ± 0.05 g to 0.03 ± 0.002 g). The superior removal efficiency for H2S is mainly due to its rapid acid-base neutralization in the alkaline scrubber (H2S + 2KOH → K2S + 2H2O, pKa = 7.0) [46] and the additional oxidative pathway facilitated by dissolved oxygen (HS− + 4OH− → SO42− + 2H2O + 3e−) [47]. Although SO2 is more soluble than H2S (Henry’s constant = 1.47 mol/L·atm vs. 0.087 mol/L·atm at 25 °C) [44], its removal is slower due to less rapid chemical reaction with KOH (SO2 + 2KOH → K2SO3 + H2O, k = 2.1 × 104 M−1s−1 at 25 °C [48].
- (4)
- H2 exhibited quantitative removal (98.1 ± 0.9%), decreasing from 0.20 ± 0.01 g at the reactor outlet to 0.003 ± 0.0003 g after the scrubber. This reduction efficiency suggests multiple concurrent mechanisms: (i) physical absorption in the quencher (Henry’s constant = 7.8 × 10−4 mol/L·atm at 25 °C) [49], (ii) potential catalytic recombination with oxygen on scrubber packing media (2H2 + O2 → 2H2O, ΔH = −286 kJ/mol) [50], and (iii) dissolution in the aqueous phase as molecular hydrogen [51]. The system’s ability to effectively remove H2 is particularly noteworthy given its low solubility, suggesting optimized gas–liquid contact in the scrubber design.
- (5)
- O2 content increased significantly (21.9 ± 2.9%, from 4170 ± 83 g to 5085 ± 102 g), which may be attributed to: (i) controlled air inlet maintaining oxidative conditions, (ii) incomplete consumption during gasification [52], or (iii) potential release from decomposed oxygen-containing compounds in the feedstock. This oxygen mass balance suggests the process operates under slightly oxygen-rich conditions, which may enhance complete combustion of organic constituents [53].
The gas treatment system demonstrated massive pollutant reduction performance, achieving removal efficiencies exceeding 97% for NOx, CO, H2S, and H2. This high capture efficiency reflects the synergistic effect of thermal quenching and chemical scrubbing, effectively neutralizing both acidic and combustible species.
In contrast to the very high abatement levels obtained, the reduction efficiencies for SO2 (80.6 ± 2.5%) and CO2 (75.0 ± 1.1%) were comparatively lower. This difference is mainly attributed to intrinsic chemical factors rather than to limitations of the scrubber design. Although SO2 is relatively soluble in water (Henry’s constant = 1.47 mol/L·atm at 25 °C) [44], its absorption and subsequent neutralization in alkaline media proceed at slower rates, which restricts its complete removal under the tested conditions [54]. Similarly, CO2 uptake through carbonation (CO2 + 2KOH → K2CO3 + H2O) is kinetically limited and further hindered by the high concentration of CO2 in the gas stream, which reduces the relative capture efficiency [55].
The packed-bed scrubber employed (ceramic rings and 50 g/L KOH solution) achieved removal efficiencies consistent with conventional alkaline wet-scrubbing systems, where SO2 and CO2 typically reach 70–80% [26] abatement but only approach complete elimination under multi-stage or specially optimized configurations. Therefore, the observed values for SO2 and CO2 reflect inherent chemical limitations of their absorption in alkaline media under the present operating conditions rather than a shortcoming of the scrubber design.
The system maintained robust operational stability, as evidenced by the consistent oxygen mass balance (21.9 ± 2.9% increase) and controlled gas-phase reactions. These results confirm the plasma gasification system’s capability to meet stringent emission standards while processing complex waste streams [56,57].
While the current study focused on major gaseous species, future work should address the capture of trace volatile metals, which may have important implications for both environmental safety and regulatory compliance. Promising strategies for this include the use of specialized adsorbents such as functionalized composites [58,59], sulfur-modified biochars, or sorbents specifically designed for high-temperature applications. The development of MOF-activated carbon (or biochar) composites is particularly appealing for this purpose, as they combine high surface area, tunable porosity, and enhanced thermal and chemical stability, properties critically reviewed for environmental applications [60,61].
These advanced materials have demonstrated potential not only for adsorption but also for gas storage and selective separation, making them strong candidates for integration into advanced, multi-stage gas cleaning trains in future plasma gasification systems. In particular, their ability to capture trace radionuclide simulants, could significantly enhance retention efficiency and reduce potential emissions, complementing the bulk retention achieved in the primary reactor and quencher stages. Incorporating these materials into the treatment system could therefore improve overall radiological safety.
4. Conclusions
- (1)
- Thermal plasma gasification proved highly efficient for treating SORW, representative of LLW, achieving a volume reduction factor VRF of 225, corresponding to a 99.6% reduction from the initial 5625 mL waste volume.
- (2)
- The plasma gasification system effectively retained surrogate elements at different stages. The reactor showed high retention of Co (96 ± 10%) and Ce (59 ± 6%), while more volatile elements like Sr (39 ± 4%) and Cs (26 ± 3%) exhibited lower retention. Significant deposition of Cs (71 ± 3%) and Sr (38 ± 4%) was observed in the connecting duct, and the quencher captured substantial fractions of Sr (22.8 ± 1.1%) and Cs (2.9 ± 0.15%), with lower values for Co (3.0 ± 0.15%) and Ce (0.3 ± 0.03%). These findings demonstrate the system’s ability to retain non-volatile elements in the reactor while capturing volatile species in downstream stages.
- (3)
- XRD analysis revealed distinct crystalline phases in the solid residues (SR1–SR4), with stable surrogate phases (CeO2 and Co3O4) and oxidation products (TiO2 and ZnO) from glove decomposition. Temperature-dependent speciation was observed, with Co3O4 reducing to CoO in high-temperature zones (≥900 °C). Reactor-derived contaminants (SiO2, Al2O3, and mullite) were also identified. All samples contained an amorphous fraction (10–25° 2θ).
- (4)
- The gas treatment system achieved the following reduction efficiencies with their associated uncertainties: NOx (98.9 ± 0.6%), NO (99.0 ± 0.5%), NO2 (97.0 ± 1.2%), CO (98.2 ± 0.8%), CO2 (75.0 ± 1.1%), SO2 (80.6 ± 2.5%), H2S (97.6 ± 0.6%), and H2 (98.1 ± 0.9%). These results demonstrate highly effective gas cleaning performance across all measured species.
These results confirm that the plasma gasification process, coupled with the integrated gas cleaning system, meets Argentina’s stringent environmental protection standards for industrial emissions [56].
Perspectives for Further Research
- (1)
- Based on these results, process optimization could include implementing additional filtration stages, such as a combination of activated carbon and ULPA filters, to further minimize potential emissions of radioactive species.
- (2)
- The incorporation of a post-combustion chamber at the reactor outlet could enable complete oxidation of residual CO and H2, improving both safety and environmental compliance.
- (3)
- Studying the influence of process parameters, such as temperature and air excess, on NOx formation. While the present study focused on overall gas cleaning performance, systematic optimization of process parameters could provide strategies to minimize NOx emissions and further improve environmental performance.
- (4)
- Assessing the long-term chemical and physical stability of the solid residues (SR1–SR4), including leaching tests, to evaluate their suitability for final disposal and confirm that the residues meet regulatory requirements for long-term storage.
- (5)
- Although the high temperatures and reducing conditions of plasma gasification inherently suppress the formation of persistent organic pollutants, future work should include a quantitative analysis of dioxins and furans in the gaseous emissions and solid residues. This would provide a comprehensive environmental assessment and confirm the system’s advantage over conventional incineration, ensuring full compliance with international emission standards for these highly toxic compounds.
These perspectives highlight clear pathways for advancing the plasma gasification technology for low-level radioactive waste. Future work focusing on process optimization, emissions control, and long-term residue stability will be essential to fully validate the system’s performance, ensure regulatory compliance, and support its potential scale-up for practical implementation in advanced waste management strategies.
Author Contributions
Conceptualization, J.A.P. and F.E.B.; methodology, J.A.P. and F.E.B.; software, J.A.P. and F.E.B.; formal analysis, J.A.P., F.E.B. and Y.S.V.; investigation, J.A.P., F.E.B., Y.S.V.; J.R.I.R. and L.A.N.P.; data curation, J.A.P. and Y.S.V.; writing—original draft preparation, J.A.P., F.E.B. and Y.S.V.; writing—review and editing, J.A.P., F.E.B., M.O.P. and D.C.L.; visualization, J.A.P.; supervision, J.A.P. and F.E.B.; project administration, F.E.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
We extend our sincere gratitude to Gastón Galo Fouga for conducting the XRD analyses, to Carlos Bertoli and technician Bernardo Pentke for performing the SEM-EDS characterizations, and to Gastón Goldmann for the INAA measurements. We also thank Maximiliano Viqueira and Diego Bagnarol for their support in the preparation of liquid samples, and Facundo Smietuch for his leadership as Head of the Departamento Materiales Nucleares-CNEA during the execution of the experiment. Special thanks go to technicians Gustavo Sepúlveda, Jorge Issa, and Leonardo Neira for their valuable contributions to the construction of the prototype plasma gasification system. Their expertise and dedication were essential to the success of this project.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- National Radioactive Waste Management Program (PNGRR). Eighth National Report to the Joint Convention on the Safety of Spent Fuel Management and on the Safety of Radioactive Waste Management; National Atomic Energy Commission: Buenos Aires, Argentina, 2023; Available online: https://www.argentina.gob.ar/sites/default/files/8_informe_nacional_convencion_conjunta_argentina_2020-2023.pdf (accessed on 10 June 2025).
- International Atomic Energy Agency. Management of Radioactive Waste from Nuclear Power Plants; IAEA Safety Standards Series No. SSR-5; IAEA: Vienna, Austria, 2018. [Google Scholar]
- OECD Nuclear Energy Agency. Radioactive Waste Management Programmes in OECD/NEA Member Countries; OECD: Paris, France, 2020. [Google Scholar]
- International Atomic Energy Agency. Classification of Radioactive Waste; General Safety Guide No. GSG-1; IAEA: Vienna, Austria, 2014. [Google Scholar]
- World Nuclear Association. Radioactive Waste Management: Global Practices; WNA: London, UK, 2022; Available online: https://www.world-nuclear.org (accessed on 10 June 2025).
- Ojovan, M.I. (Ed.) Handbook of Advanced Radioactive Waste Conditioning Technologies; Woodhead Publishing: Cambridge, UK, 2011; pp. 1–512. [Google Scholar]
- Hrabovský, M.; Jeremiáš, M.; van Oost, G. Plasma Gasification and Pyrolysis, 1st ed.; CRC Press: Boca Raton, FL, USA, 2022; pp. 1–350. [Google Scholar] [CrossRef]
- Gomez, E.; Rani, D.A.; Cheeseman, C.R.; Deegan, D.; Wise, M.; Boccaccini, A.R. Thermal plasma technology for the treatment of wastes: A critical review. J. Hazard. Mater. 2009, 161, 614–626. [Google Scholar] [CrossRef]
- Linak, W.P.; Wendt, J.O.L. Toxic metal emissions from incineration: Mechanisms and control. Prog. Energy Combust. Sci. 1993, 19, 145–185. [Google Scholar] [CrossRef]
- Barton, R.G.; Clark, W.D.; Seeker, W.R. Fate of metals in waste combustion systems. Combust. Sci. Technol. 1990, 74, 327–342. [Google Scholar] [CrossRef]
- Senior, C.L.; Helble, J.J.; Sarofim, A.F. Emissions of mercury, trace elements, and fine particles from stationary combustion sources. Fuel Process. Technol. 2000, 65–66, 263–288. [Google Scholar] [CrossRef]
- Vienna, J.D.; Ryan, J.V.; Gin, S.; Inagaki, Y. Current understanding and remaining challenges in modeling long-term degradation of borosilicate nuclear waste glasses. Int. J. Appl. Glass Sci. 2013, 4, 283–294. [Google Scholar] [CrossRef]
- Verhulst, D.; Buekens, A.; Spencer, P.J.; Eriksson, G. Thermodynamic behavior of metal chlorides and sulfates under the conditions of incineration furnaces. Environ. Sci. Technol. 1996, 30, 50–56. [Google Scholar] [CrossRef]
- Heep, B. Plasma Technology for Treatment of Radioactive Waste: Operational Experience at ZWILAG. Nucl. Eng. Des. 2011, 241, 1248–1255. [Google Scholar]
- Polkanov, M.A.; Gorbunov, V.A.; Kadyrov, I.I.; Spirin, N.A.; Kobelev, A.P.; Lifanov, F.A.; Dmitriev, S.A. Technology of Plasma Treating Radioactive Waste: The Step Forward in Comparison with Incineration. In Proceedings of the WM2010 Conference, Phoenix, AZ, USA, 7–11 March 2010; Paper 10166. Available online: https://archivedproceedings.econference.io/wmsym/2010/pdfs/10166.pdf (accessed on 12 June 2025).
- Nuclear Engineering International. Kozloduy’s Plasma Plant; Nuclear Engineering International: London, UK, 2025; Available online: https://www.neimagazine.com/advanced-reactorsfusion/kozloduys-plasma-plant-6230627/ (accessed on 12 June 2025).
- Prado, E.S.P.; Miranda, F.S.; Araujo, L.G.; Petraconi, G.; Baldan, M.R.; Essiptchouk, A.; Potiens, A.J. Experimental Study on Treatment of Simulated Radioactive Waste by Thermal Plasma: Temporal Evaluation of Stable Co and Cs. Ann. Nucl. Energy 2021, 160, 108433. [Google Scholar] [CrossRef]
- Rivero, R.E. Treatment of Radioactive Waste Through Thermal Processes; Technical Report; National Atomic Energy Commission: San Carlos de Bariloche, Argentina, 2019. [Google Scholar]
- Hypertherm Associates. Powermax 105 Plasma System. Available online: https://www.hypertherm.com/es/hypertherm/powermax/powermax105/ (accessed on 17 June 2025).
- Pullao, J.A. Pre-Conditioning Process of Simulated Radioactive Waste by Hot Plasma Gasification; Technical Report; National Atomic Energy Commission: San Carlos de Bariloche, Argentina, 2020. [Google Scholar]
- Kaeser Compressors. Rotary Screw Compressors: SX Series with the World-Renowned SIGMA PROFILE. Available online: https://us.kaeser.com/download.ashx?id=tcm%3A46-37612 (accessed on 17 June 2025).
- Rivero, R.E. Treatment of Radioactive Waste Through Thermal Processes; Technical Report; National Atomic Energy Commission: San Carlos de Bariloche, Argentina, 2018. [Google Scholar]
- Pinelli, B.J.F. Design and Characterization of Consumable Electrodes for a Laboratory-Scale Radioactive Waste Gasification System. Bachelor’s Thesis, Balseiro Institute, National University of Cuyo, San Carlos de Bariloche, Argentina, 2017. [Google Scholar]
- ASHRAE Standard 52.1-1992; Gravimetric and Dust-Spot Procedures for Testing Air-Cleaning Devices Used in General Ventilation for Removing Particulate Matter. ASHRAE: Atlanta, GA, USA, 1992.
- Microfilter SA. Air Filtration. Soniq—Multi-Bag Filters: Technical Sheet. Available online: https://www.microfilter.com.ar/producto/898 (accessed on 20 June 2025).
- FSUE RADON. Packed Scrubber for Exhaust Gases with Irrigation Solution Recirculation: Operating Manual; FSUE RADON: Moscow, Russia, 2016. [Google Scholar]
- Cirigliano/Ventilation Engineering. Centrifugal Fan. Available online: https://ciriglianosa.com/en/home/ (accessed on 20 June 2025).
- Horiba. Portable Gas Analyzer PG-350E—Instruction Manual; Horiba Ltd.: Kyoto, Japan, 2013; Available online: https://www.e3-global.com/wp-content/uploads/2020/12/PG-350-HRE2879A-v5.4-small-res-1.pdf (accessed on 23 June 2025).
- Testo SE & Co. KGaA. Testo 350 Industrial Combustion Gas Analyzer; Testo: Titisee-Neustadt, Germany, 2024; Available online: https://suministrosenmetrologia.com/wp-content/uploads/2019/10/testo-350-Ficha-tcnica.pdf (accessed on 2 July 2025).
- RKI Instruments. One to Six Gas Portable Monitor: Eagle Model; RKI Instruments: Union City, CA, USA, 2024; Available online: https://www.transcat.com/media/pdf/eagle.pdf (accessed on 2 July 2025).
- Fritsch GmbH. Cutting Tools Sets; Fritsch: Idar-Oberstein, Germany, 2021. [Google Scholar]
- Oxford Instruments. AZtec 3.1: User Manual; Oxford Instruments NanoAnalysis: High Wycombe, UK, 2015; Available online: https://utw10193.utweb.utexas.edu/InstrumentManuals/Oxford%20EDS%20AZtec%20User%20Manual.pdf (accessed on 7 July 2025).
- ASTM D3578-19; Standard Specification for Nitrile Rubber Examination Gloves for Medical Application. ASTM: West Conshohocken, PA, USA, 2019.
- TAPPI Press. Paper and Paperboard: Composition, Structure, and Properties; TAPPI Monograph Series No. 45; TAPPI: Atlanta, GA, USA, 2018. [Google Scholar]
- Schneider, H.; Komarneni, S. Mullite Ceramics; Wiley-VCH: Weinheim, Germany, 2005; ISBN 3-527-30974-8. [Google Scholar]
- Davis, J.R. (Ed.) Stainless Steels: Alloying, Properties, and Applications; ASM International: Materials Park, OH, USA, 2020. [Google Scholar]
- Leyens, C.; Peters, M. Titanium and Titanium Alloys: Fundamentals and Applications; Wiley-VCH: Weinheim, Germany, 2003. [Google Scholar]
- PVC Pipe Association. Handbook of PVC Pipe: Design and Construction, 6th ed.; Uni-Bell: Dallas, TX, USA, 2022. [Google Scholar]
- International Center for Diffraction Data. XRD Diffraction Patterns. Available online: https://www.icdd.com/ (accessed on 15 July 2025).
- Ministerio de Salud de la Nación Argentina. Código Alimentario Argentino (CAA). Capítulo XII—Bebidas Hídricas, Agua y Agua Gasificada. Available online: https://w3.fcq.unc.edu.ar/sites/default/files/biblioteca/CAPITULO_XII_Bebidas_Hidricas_Agua_y_agua_gasificada.pdf (accessed on 21 July 2025).
- Zhao, M.; Xue, P.; Liu, J.; Liao, J.; Guo, J. A Review of Removing SO2 and NOx by Wet Scrubbing. Sustain. Energy Technol. Assess. 2021, 47, 101451. [Google Scholar] [CrossRef]
- Li, S.; Medrano, J.A.; Hessel, V.; Gallucci, F. Recent Progress of Plasma-Assisted Nitrogen Fixation Research: A Review. Processes 2018, 6, 248. [Google Scholar] [CrossRef]
- Chase, M.W., Jr. NIST-JANAF Thermochemical Tables. Fourth edition. J. Phys. Chem. Ref. Data 1998, 28, 1951. [Google Scholar]
- Sander, R. Compilation of Henry’s Law Constants (Version 5.0.0) for Water as Solvent. Atmos. Chem. Phys. 2023, 23, 10901–12440. [Google Scholar] [CrossRef]
- Gondal, S.; Asif, N.; Svendsen, H.F.; Knuutila, H.K. Kinetics of the Absorption of Carbon Dioxide into Aqueous Hydroxides of Lithium, Sodium and Potassium and Blends of Hydroxides and Carbonates. Chem. Eng. Sci. 2015, 123, 487–499. [Google Scholar] [CrossRef]
- Millero, F.J. The Thermodynamics and Kinetics of the Hydrogen Sulfide System in Natural Waters. Mar. Chem. 1986, 18, 121–147. [Google Scholar] [CrossRef]
- Yang, J.H. Hydrogen Sulfide Removal Technology: A Focused Review on Adsorption and Catalytic Oxidation. Korean J. Chem. Eng. 2021, 38, 674–691. [Google Scholar] [CrossRef]
- Dou, J.; Zhao, Y.; Duan, X.; Chai, H.; Li, L.; Yu, J. Desulfurization Performance and Kinetics of Potassium Hydroxide-Impregnated Char Sorbents for SO2 Removal from Simulated Flue Gas. ACS Omega 2020, 5, 19194–19201. [Google Scholar] [CrossRef]
- Wilhelm, E.; Battino, R.; Wilcock, R.J. Low-Pressure Solubility of Gases in Liquid Water. Chem. Rev. 1977, 77, 219–262. [Google Scholar] [CrossRef]
- Kozhukhova, A.E.; du Preez, S.P.; Bessarabov, D.G. Catalytic Hydrogen Combustion for Domestic and Safety Applications: A Critical Review of Catalyst Materials and Technologies. Energies 2021, 14, 4897. [Google Scholar] [CrossRef]
- Zhu, Z.; Cao, Y.; Zheng, Z.; Chen, D. An Accurate Model for Estimating H2 Solubility in Pure Water and Aqueous NaCl Solutions. Energies 2022, 15, 5021. [Google Scholar] [CrossRef]
- Park, S.-W.; Lee, S.-Y.; Jeong, Y.-O.; Han, G.-H.; Seo, Y.-C. Effects of Oxygen Enrichment in Air Oxidants on Biomass Gasification Efficiency and the Reduction of Tar Emissions. Energies 2018, 11, 2664. [Google Scholar] [CrossRef]
- Khlifi, S.; Pozzobon, V.; Lajili, M. A Comprehensive Review of Syngas Production, Fuel Properties and Operational Parameters for Biomass Conversion. Energies 2024, 17, 3646. [Google Scholar] [CrossRef]
- Yu, H.; Shan, C.; Li, J.; Hou, X.; Yang, L. Alkaline Absorbents for SO2 and SO3 Removal: A Comprehensive Review. J. Environ. Manag. 2024, 366, 121532. [Google Scholar] [CrossRef] [PubMed]
- Ghavamipour, S.; Vafajoo, L.; Pourhossein, G.; Parthasarathy, P.; McKay, G. Post-Combustion CO2 Capturing by KOH Solution: An Experimental and Statistical Optimization Modeling Study. Energy Environ. 2024, 36, 0958305X241230944. [Google Scholar] [CrossRef]
- República Argentina. Ley N° 20.284: Ley de Preservación de los Recursos del Aire. Boletín Oficial de la República Argentina. 1973. Available online: https://www.argentina.gob.ar/normativa/nacional/ley-20284-40167/texto (accessed on 1 August 2025).
- Parlamento Europeo y Consejo de la Unión Europea. Directiva 2010/75/UE Sobre las Emisiones Industriales (Prevención y Control Integrados de la Contaminación). D. Of. Unión Eur. 2010, L 334, 17–119. Available online: https://eur-lex.europa.eu/legal-content/ES/ALL/?uri=CELEX%3A32010L0075 (accessed on 5 August 2025).
- Zheng, Y.; Cheng, P.; Li, Z.; Fan, C.; Wen, J.; Yu, Y.; Jia, L. Efficient Removal of Gaseous Elemental Mercury by Fe-UiO-66@BC Composite Adsorbent: Performance Evaluation and Mechanistic Elucidation. Sep. Purif. Technol. 2025, 372, 133463. [Google Scholar] [CrossRef]
- Vaz, R.C.A.; Lopez, M.A.R.; Ferreira, G.M.D. Unlocking the Potential of MOF-Biochar Composites: Advanced Functional Materials for Adsorption, Catalysis, and Energy Storage. Mater. Today Chem. 2024, 38, 102383. [Google Scholar] [CrossRef]
- Ghaedi, S.; Rajabi, H.; Hadi Mosleh, M.; Sedighi, M. MOF Biochar Composites for Environmental Protection and Pollution Control. Bioresour. Technol. 2025, 418, 131982. [Google Scholar] [CrossRef]
- Ullah, S.; Rehman, A.u.; Najam, T.; Hossain, I.; Anjum, S.; Ali, R.; Shahid, M.U.; Shah, S.S.A.; Nazir, M.A. Advances in Metal-Organic Framework@Activated Carbon (MOF@AC) Composite Materials: Synthesis, Characteristics and Applications. J. Ind. Eng. Chem. 2024, 137, 87–105. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).