Construction of Honeycomb-like ZnO/g-C3N5 Heterojunction for MB Photocatalytic Degradation
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
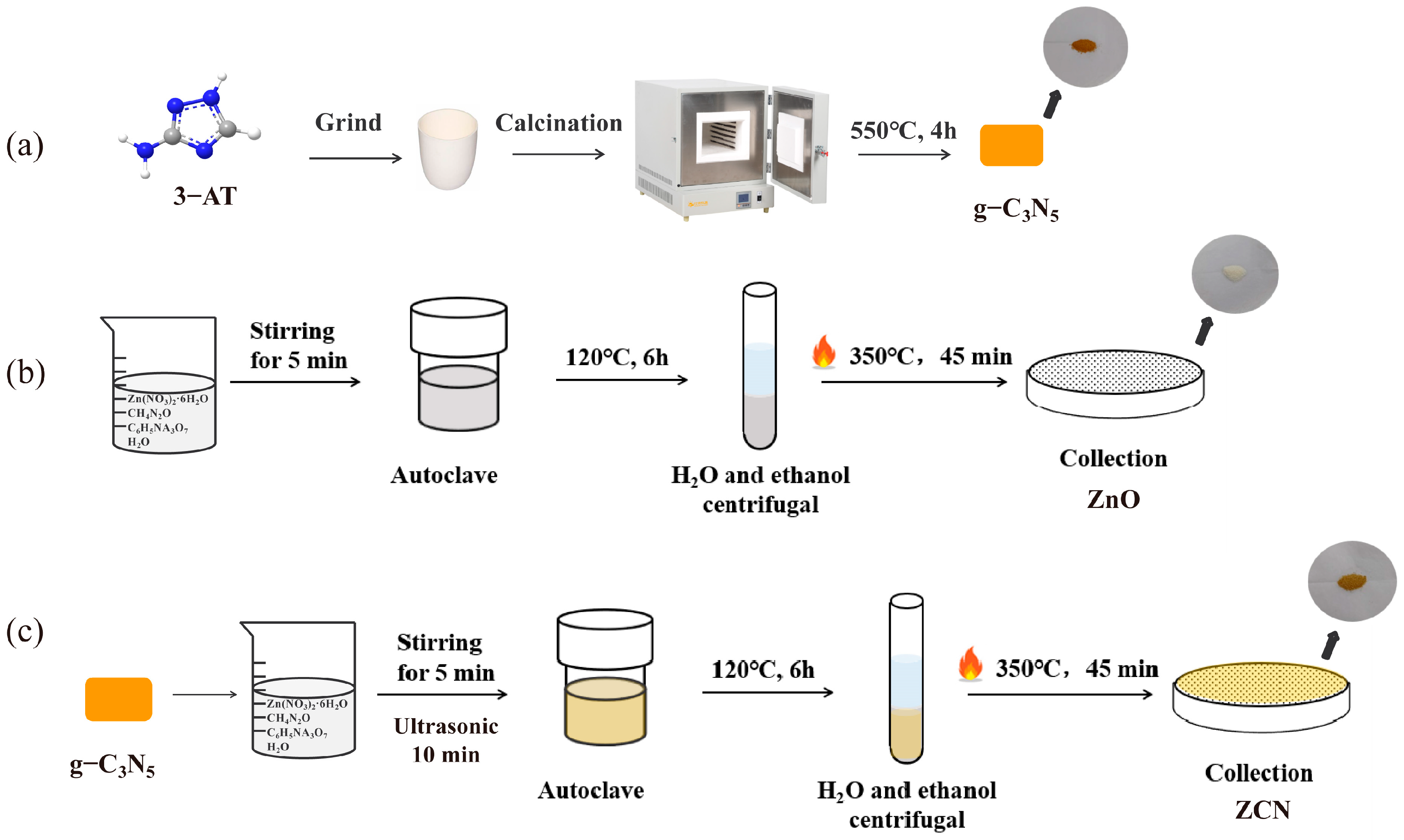
2.2. Synthesis of Catalysts
2.2.1. Synthesis of g-C3N5
2.2.2. Synthesis of ZnO and ZnO/g-C3N5
2.3. Characterization of Samples
2.4. Degradation Experiments
2.5. Density Functional Theory (DFT) Calculations
3. Results and Discussion
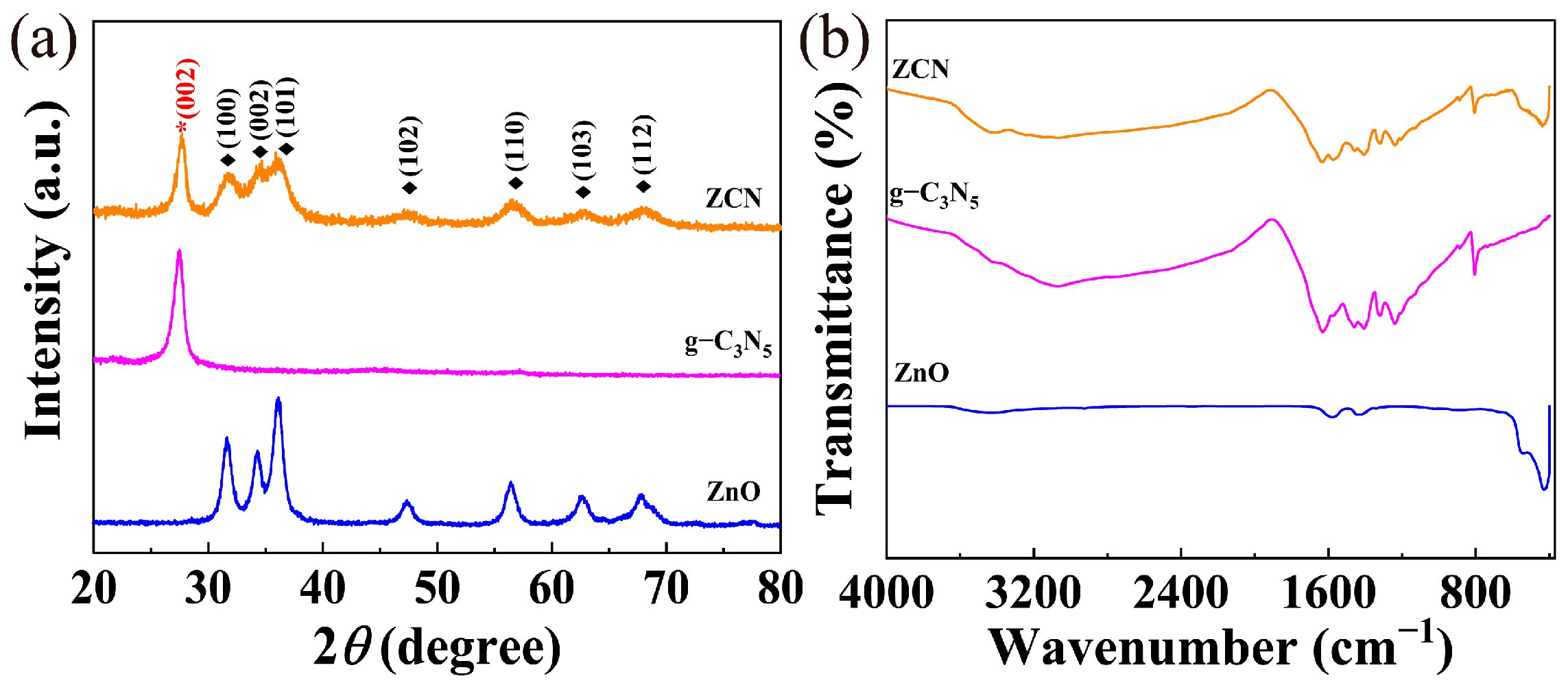
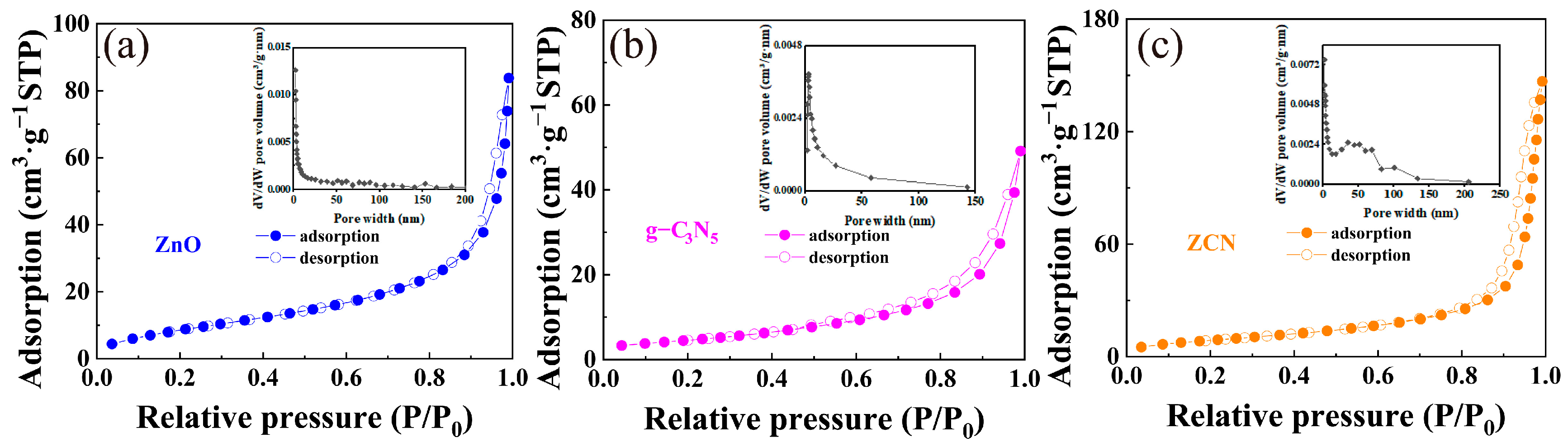
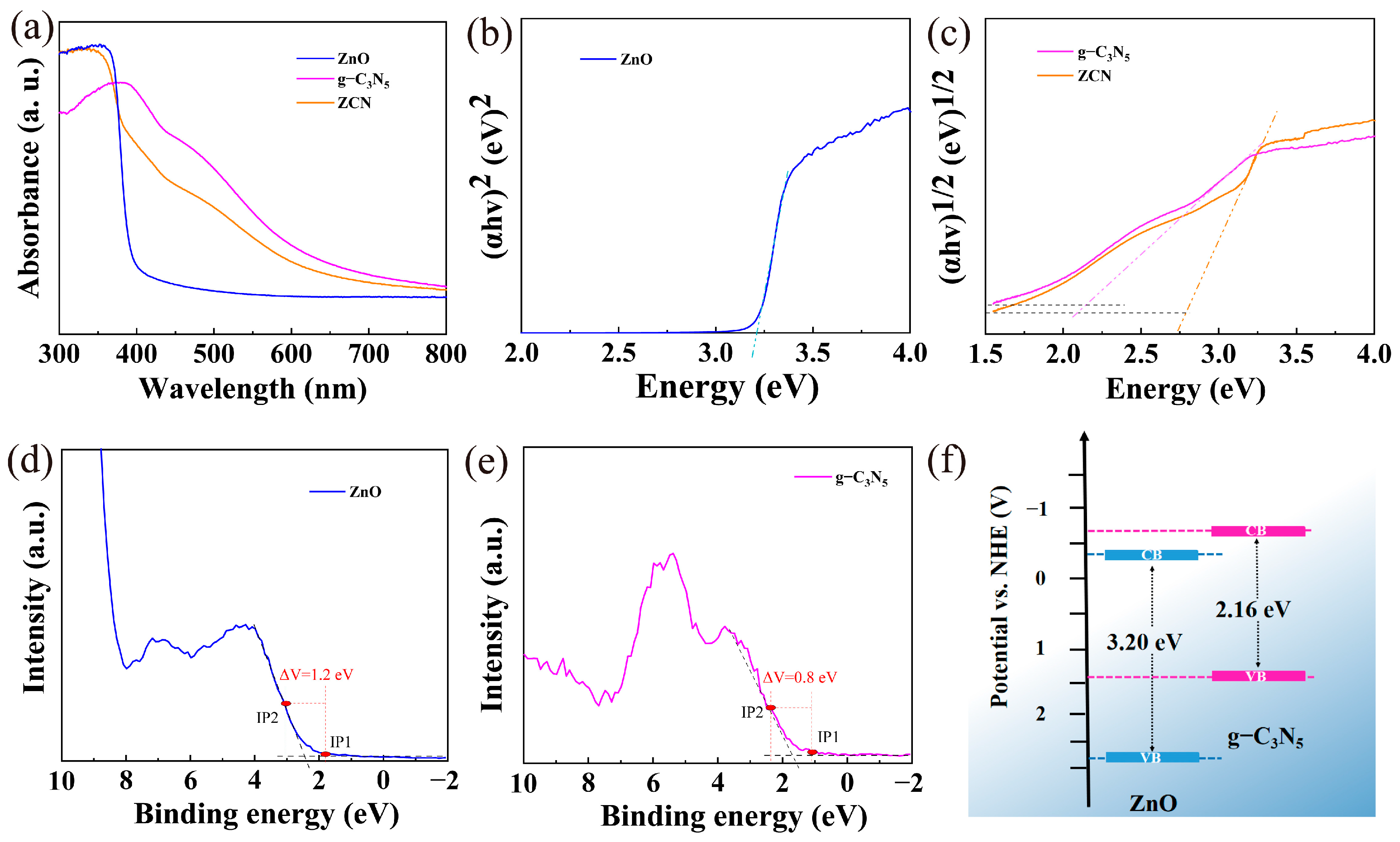
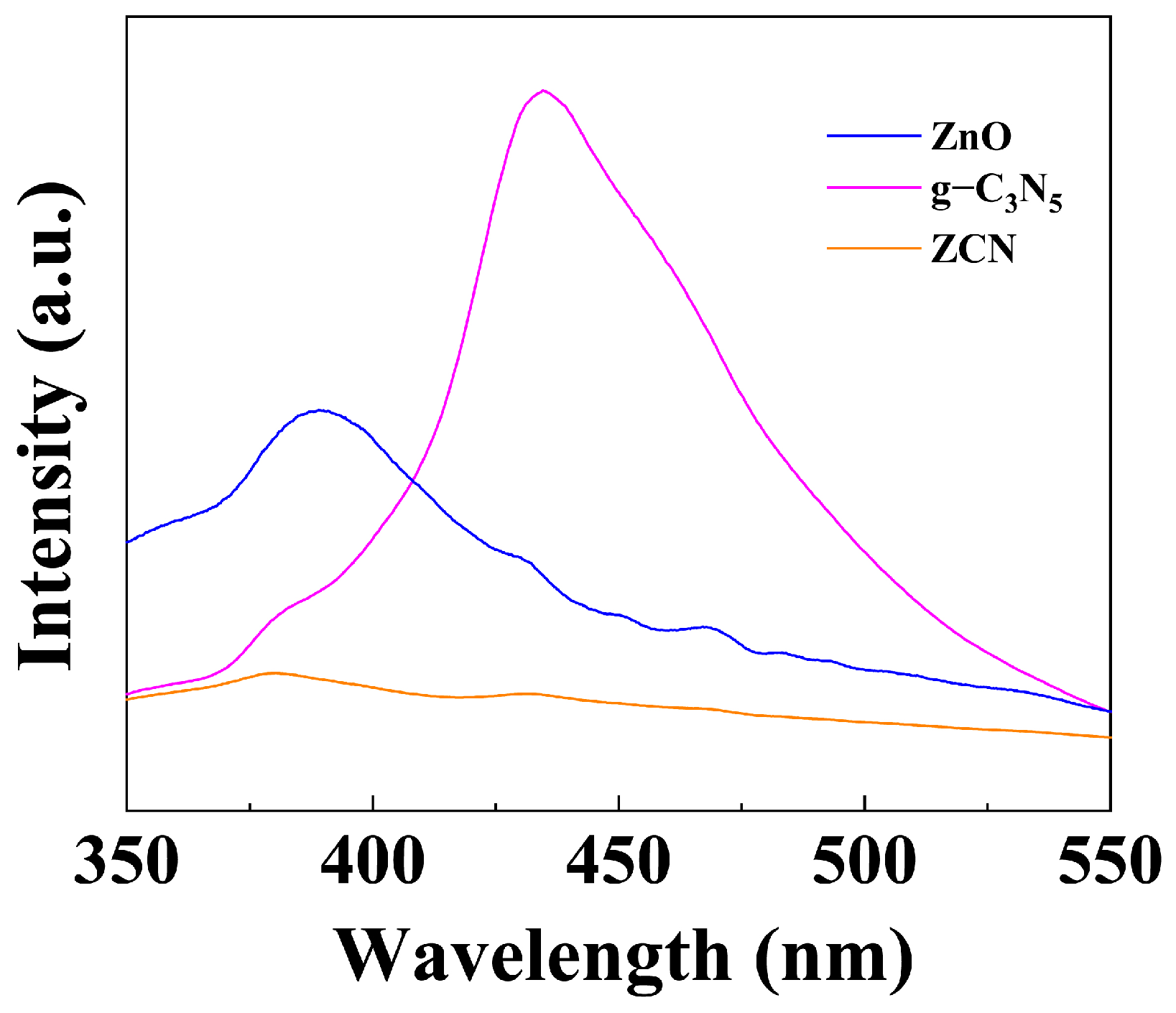
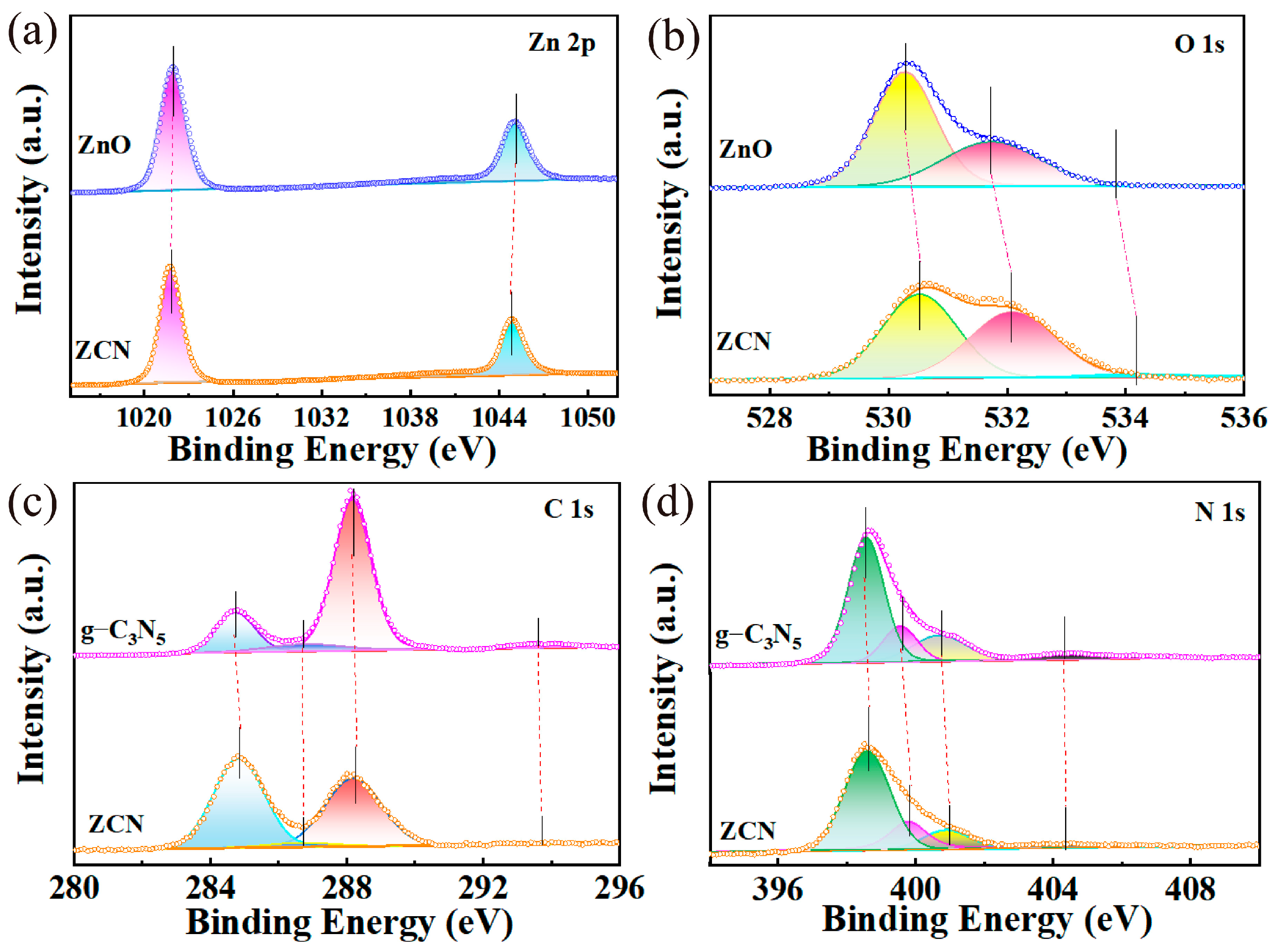
3.1. Characterization of Catalysts
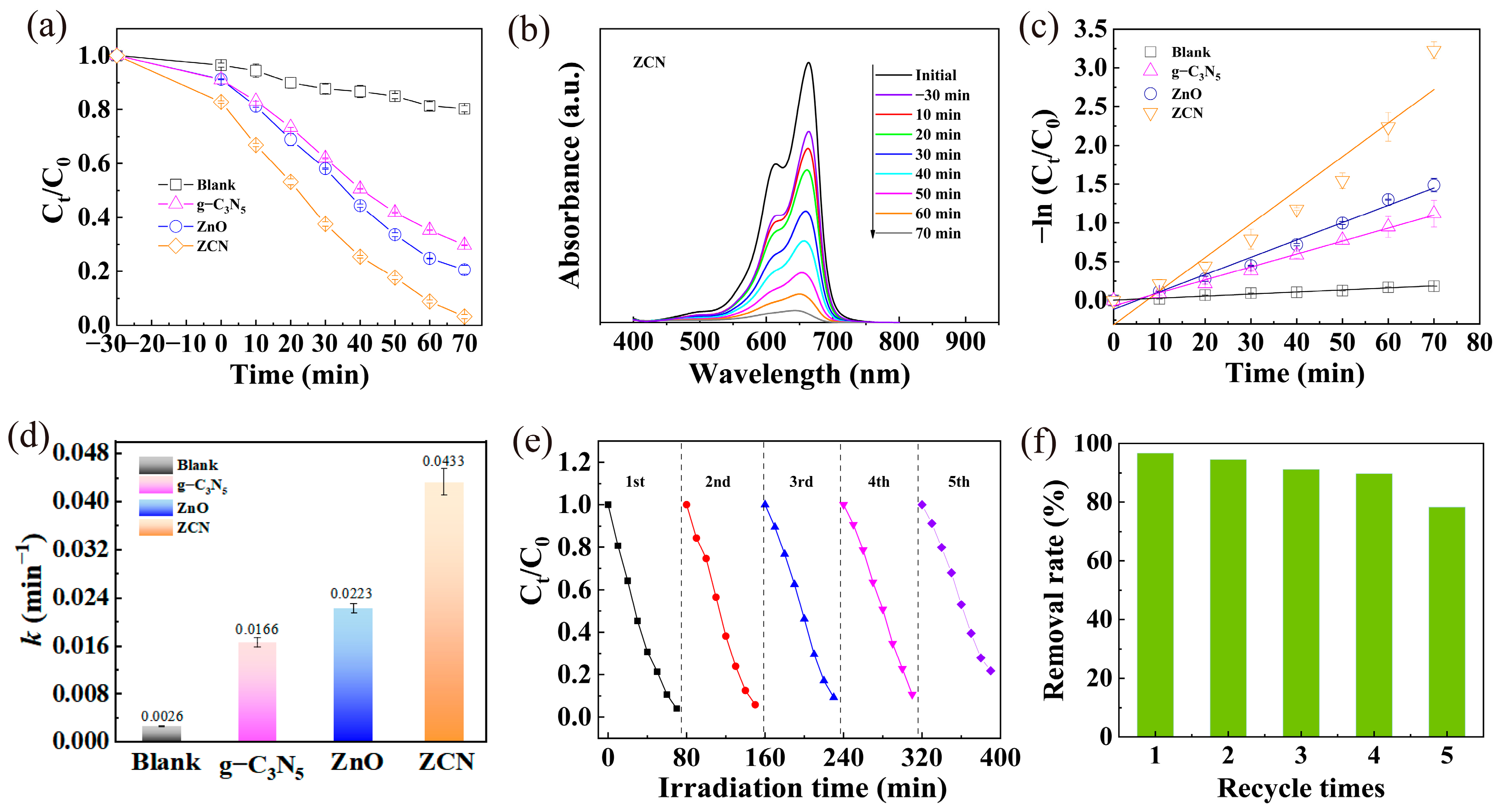
3.2. Photocatalytic Performance
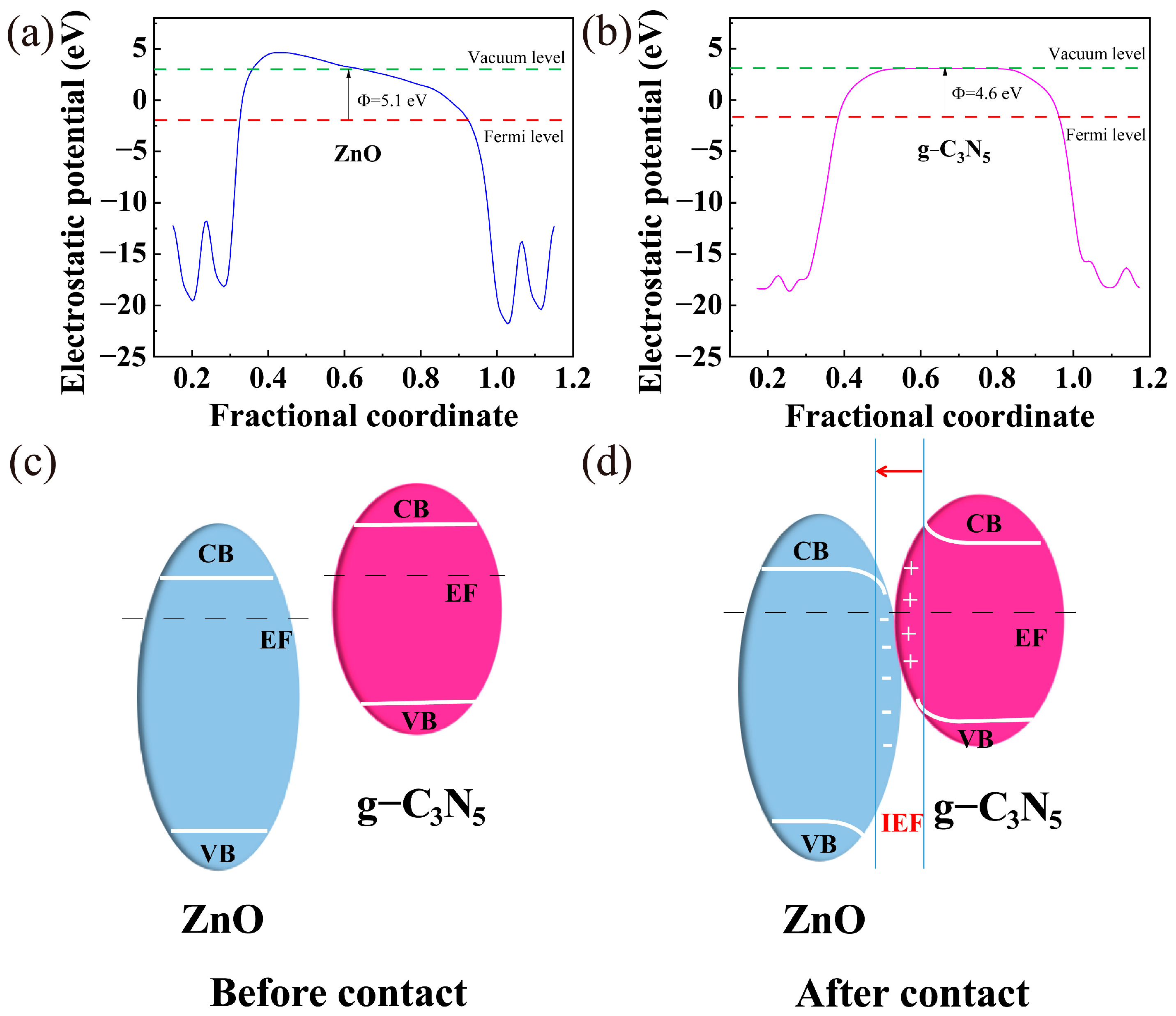
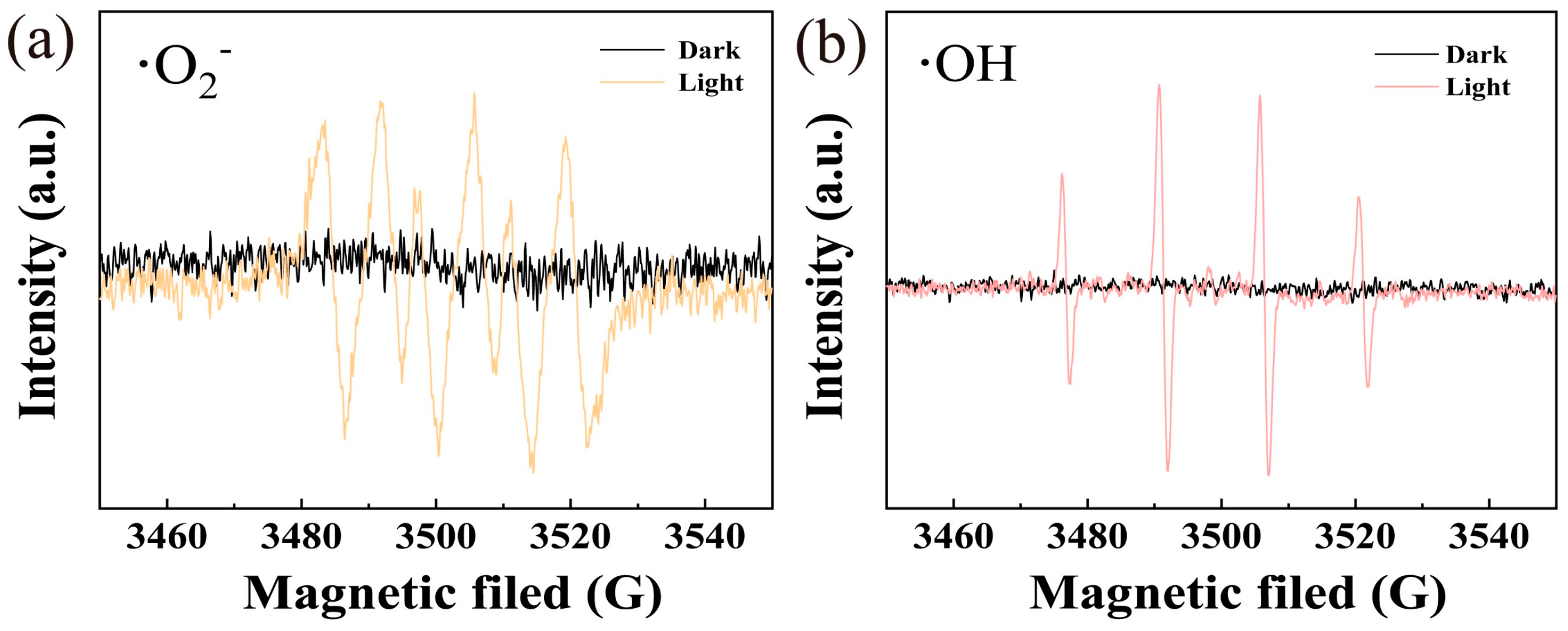
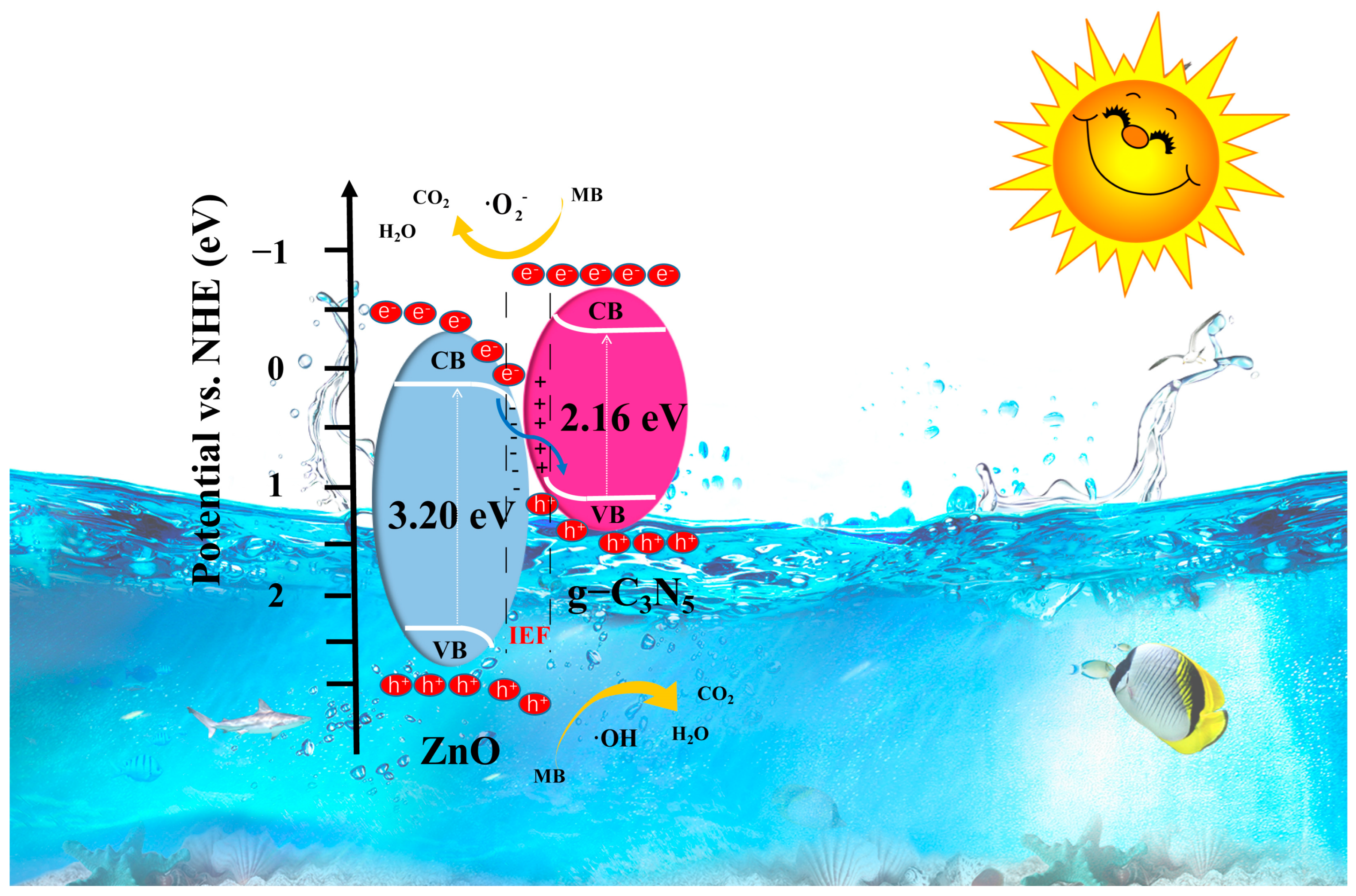
3.3. Photocatalytic Mechanism Exploration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dihom, H.R.; Al-Shaibani, M.M.; Radin Mohamed, R.M.S.; Al-Gheethi, A.A.; Sharma, A.; Khamidun, M.H.B. Photocatalytic degradation of disperse azo dyes in textile wastewater using green zinc oxide nanoparticles synthesized in plant extract: A critical review. J. Water Process Eng. 2022, 47, 102705. [Google Scholar] [CrossRef]
- Medina-Acosta, M.; Chinchillas-Chinchillas, M.J.; Garrafa-Gálvez, H.E.; Garcia-Maro, C.A.; Rosas-Casarez, C.A.; Lugo-Medina, E.; Luque-Morales, P.A.; Soto-Robles, C.A. Photocatalytic Degradation of Four Organic Dyes Present in Water Using ZnO Nanoparticles Synthesized with Green Synthesis Using Ambrosia ambrosioides Leaf and Root Extract. Processes 2024, 12, 2456. [Google Scholar] [CrossRef]
- Chakraborty, S.; De, S.; Basu, J.K.; DasGupta, S. Treatment of a textile effluent: Application of a combination method involving adsorption and nanofiltration. Desalination 2005, 174, 73–85. [Google Scholar] [CrossRef]
- Mohammed, H.A.; Khaleefa Ali, S.A.; Basheer, M.I. Heavy metal ions removal using advanced oxidation (UV/H2O2) technique. IOP Conf. Ser. Mater. Sci. Eng. 2020, 870, 012026. [Google Scholar] [CrossRef]
- Ihaddaden, S.; Aberkane, D.; Boukerroui, A.; Robert, D. Removal of methylene blue (basic dye) by coagulation-flocculation with biomaterials (bentonite and Opuntia ficus indica). J. Water Process Eng. 2022, 49, 102952. [Google Scholar] [CrossRef]
- Rafatullah, M.; Sulaiman, O.; Hashim, R.; Ahmad, A. Adsorption of methylene blue on low-cost adsorbents: A review. J. Hazard. Mater. 2010, 177, 70–80. [Google Scholar] [CrossRef]
- Rashid, M.; Al-Zaqri, N.; Guerrero-Barajas, C.; Hussain, F.; Ibrahim, M.N. Waste Derived Graphene Oxide-ZnO: An Efficient Photocatalyst for Rhodamine 6G. Processes 2022, 10, 2266. [Google Scholar] [CrossRef]
- Nguyen, C.H.; Tran, M.L.; Tran, T.T.V.; Juang, R.-S. Enhanced removal of various dyes from aqueous solutions by UV and simulated solar photocatalysis over TiO2/ZnO/rGO composites. Sep. Purif. Technol. 2020, 232, 115962. [Google Scholar] [CrossRef]
- Tichapondwa, S.M.; Newman, J.P.; Kubheka, O. Effect of TiO2 phase on the photocatalytic degradation of methylene blue dye. Phys. Chem. Earth Parts A/B/C 2020, 118–119, 102900. [Google Scholar] [CrossRef]
- Cheng, T.; Gao, H.; Liu, G.; Pu, Z.; Wang, S.; Yi, Z.; Wu, X.; Yang, H. Preparation of core-shell heterojunction photocatalysts by coating CdS nanoparticles onto Bi4Ti3O12 hierarchical microspheres and their photocatalytic removal of organic pollutants and Cr(VI) ions. Colloids Surf. A Physicochem. Eng. Asp. 2022, 633, 127918. [Google Scholar] [CrossRef]
- Kheirabadi, M.; Samadi, M.; Asadian, E.; Zhou, Y.; Dong, C.; Zhang, J.; Moshfegh, A.Z. Well-designed Ag/ZnO/3D graphene structure for dye removal: Adsorption, photocatalysis and physical separation capabilities. J. Colloid Interface Sci. 2019, 537, 66–78. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, A.; Farhan, A.; Maqbool, F.; Ahmad, H.; Qayyum, W.; Ghazy, E.; Rahdar, A.; Díez-Pascual, A.M.; Fathi-karkan, S. Zinc oxide nanoparticles: Pathways to micropollutant adsorption, dye removal, and antibacterial actions—A study of mechanisms, challenges, and future prospects. J. Mol. Struct. 2024, 1312, 138545. [Google Scholar] [CrossRef]
- Yashni, G.; Al-Gheethi, A.; Radin Mohamed, R.M.S.; Dai-Viet, N.V.; Al-Kahtani, A.A.; Al-Sahari, M.; Nor Hazhar, N.J.; Noman, E.; Alkhadher, S. Bio-inspired ZnO NPs synthesized from Citrus sinensis peels extract for Congo red removal from textile wastewater via photocatalysis: Optimization, mechanisms, techno-economic analysis. Chemosphere 2021, 281, 130661. [Google Scholar] [CrossRef]
- Sahu, S.K.; Palai, A.; Sahu, D. Photocatalytic applications of metal oxide-based nanocomposites for sustainable environmental remediation. Sustain. Chem. Environ. 2024, 8, 100162. [Google Scholar] [CrossRef]
- Trandafilović, L.V.; Jovanović, D.J.; Zhang, X.; Ptasińska, S.; Dramićanin, M.D. Enhanced photocatalytic degradation of methylene blue and methyl orange by ZnO:Eu nanoparticles. Appl. Catal. B Environ. 2017, 203, 740–752. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, Q.; Chen, C.; Zhang, Z.; Fang, X. Theoretical design and experimental study a novel direct Z-scheme V2O5/C3N5 heterojunction for efficient photocatalytic hydrogen production. Sol. Energy Mater. Sol. Cells 2023, 257, 112385. [Google Scholar] [CrossRef]
- Liu, D.; Yao, J.; Chen, S.; Zhang, J.; Li, R.; Peng, T. Construction of rGO-coupled C3N4/C3N5 2D/2D Z-scheme heterojunction to accelerate charge separation for efficient visible light H2 evolution. Appl. Catal. B Environ. 2022, 318, 121822. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, Z.; Jiao, Y.; Chen, W.; Liu, L.; Wang, X. Experimental Investigation of Vitrification Process for the Disposal of Hazardous Solid Waste Containing Chlorides. Processes 2022, 10, 526. [Google Scholar] [CrossRef]
- Chen, S.; Wang, H.; Mo, Z.; Li, S.; Yang, J.; Li, M.; Zhang, H.; Wei, L.; Yang, X. Highly efficient g-C3N5/Bi2MoO6 heterojunction for aflatoxin B1 photocatalytic degradation. Inorg. Chem. Commun. 2024, 170, 113156. [Google Scholar] [CrossRef]
- Fan, W.; Chang, H.; Zheng, X.; Lu, J.; Yin, G.; Jiang, Z. Elaborate construction of a MOF-derived novel morphology Z-scheme ZnO/ZnCdS heterojunction for enhancing photocatalytic H2 evolution and tetracycline degradation. Sep. Purif. Technol. 2025, 357, 130068. [Google Scholar] [CrossRef]
- Wang, L.; Chen, R.; Zhang, Z.; Chen, X.; Ding, J.; Zhang, J.; Wan, H.; Guan, G. Constructing direct Z-scheme heterojunction g-C3N5/BiOBr for efficient photocatalytic CO2 reduction with H2O. J. Environ. Chem. Eng. 2023, 11, 109345. [Google Scholar] [CrossRef]
- Liu, S.; Wang, W.; Cheng, Y.; Yao, L.; Han, H.; Zhu, T.; Liang, Y.; Fu, J. Methyl orange adsorption from aqueous solutions on 3D hierarchical PbS/ZnO microspheres. J. Colloid Interface Sci. 2020, 574, 410–420. [Google Scholar] [CrossRef]
- Dong, Y.; Mushtaq, N.; Almutairi, B.S.; Shah, M.A.K.Y.; Lu, Y.; Deng, C. Highly improved ionic conduction in reverse spinel structured CoAl2O4 and ZnO heterostructure through interfacial disordering. Ceram. Int. 2024, 50, 28113–28122. [Google Scholar] [CrossRef]
- El Golli, A.; Fendrich, M.; Bazzanella, N.; Dridi, C.; Miotello, A.; Orlandi, M. Wastewater remediation with ZnO photocatalysts: Green synthesis and solar concentration as an economically and environmentally viable route to application. J. Environ. Manag. 2021, 286, 112226. [Google Scholar] [CrossRef]
- Liu, S.; Liu, S.; Wang, W.; Zhang, C.; Wang, L.; Liang, Y.; Fu, J.; Zhou, Q. Mn-periodate complex in situ formed at Mn-N coordinate site in Mn-doped g-C3N5 for efficient and rapid water purification. Chem. Eng. J. 2024, 498, 155546. [Google Scholar] [CrossRef]
- Liang, M.-K.; Limo, M.J.; Sola-Rabada, A.; Roe, M.J.; Perry, C.C. New Insights into the Mechanism of ZnO Formation from Aqueous Solutions of Zinc Acetate and Zinc Nitrate. Chem. Mater. 2014, 26, 4119–4129. [Google Scholar] [CrossRef]
- Yu, F.; Zhang, J.; Ren, J.; Chen, H.; Tian, X.; Feng, C.; Li, C.; Zhang, J.; Tang, X.; Hou, X. ZnO@g-C3N4 photocatalyst with switchable carrier transfer mechanism between type-Ⅱ and S-scheme through S doping. J. Alloys Compd. 2024, 982, 173756. [Google Scholar] [CrossRef]
- Kim, I.Y.; Kim, S.; Jin, X.; Premkumar, S.; Chandra, G.; Lee, N.-S.; Mane, G.P.; Hwang, S.-J.; Umapathy, S.; Vinu, A. Ordered Mesoporous C3N5 with a Combined Triazole and Triazine Framework and Its Graphene Hybrids for the Oxygen Reduction Reaction (ORR). Angew. Chem. Int. Ed. 2018, 57, 17135–17140. [Google Scholar] [CrossRef]
- Zhang, Z.; Ji, R.; Sun, Q.; He, J.; Chen, D.; Li, N.; Li, H.; Marcomini, A.; Xu, Q.; Lu, J. Enhanced photocatalytic degradation of 2-chlorophenol over Z-scheme heterojunction of CdS-decorated oxygen-doped g-C3N4 under visible-light. Appl. Catal. B Environ. 2023, 324, 122276. [Google Scholar] [CrossRef]
- Li, F.; Huang, T.; Sun, F.; Chen, L.; Li, P.; Shao, F.; Yang, X.; Liu, W. Ferric oxide nanoclusters with low-spin FeIII anchored g-C3N4 rod for boosting photocatalytic activity and degradation of diclofenac in water under solar light. Appl. Catal. B Environ. 2022, 317, 121725. [Google Scholar] [CrossRef]
- Liang, H.; Wang, H.; Wang, A.; Cheng, R.; Jing, S.; Chen, F.; Kannan, P.; Balkourani, G.; Tsiakaras, P. Efficient photocatalytic hydrogen peroxide production over S-scheme In2S3/molten salt modified C3N5 heterojunction. J. Colloid Interface Sci. 2024, 669, 506–517. [Google Scholar] [CrossRef] [PubMed]
- Qi, K.; Xing, X.; Zada, A.; Li, M.; Wang, Q.; Liu, S.; Lin, H.; Wang, G. Transition metal doped ZnO nanoparticles with enhanced photocatalytic and antibacterial performances: Experimental and DFT studies. Ceram. Int. 2020, 46, 1494–1502. [Google Scholar] [CrossRef]
- Sajjadi, S.M.; Hossinzadeh, G. Textile dyes removing from the wastewater by green synthesized Cu-doped ZnO photocatalysts under the simulated sunlight illumination. Ceram. Int. 2024, 50, 36271–36285. [Google Scholar] [CrossRef]
- de Moraes, N.P.; Pereira, R.A.; da Silva, T.V.C.; da Silva, B.H.B.; de Assis, G.P.; Campos, T.M.B.; Thim, G.P.; de Vasconcelos Lanza, M.R.; de Freitas, L.; Rodrigues, L.A. Cross-linked cellulose beads as an eco-friendly support for ZnO/SnO2/carbon xerogel hybrid photocatalyst: Exploring the synergy between adsorption and photocatalysis under simulated sunlight. Int. J. Biol. Macromol. 2024, 254, 127826. [Google Scholar] [CrossRef]
- Abbasi, E.; Haghighi, M.; Shabani, M.; Niyati, A.; Mahboob, S. Boosted ultrasound-sunlight-driven removal of organic pollutant over double Z-scheme plasmonic ZnO–Cu–CuO/g-C3N4 nanophotocatalyst: Design via in-situ co-precipitation hybrid with various sonication powers. Mater. Today Sustain. 2023, 24, 100580. [Google Scholar] [CrossRef]
- Girish, Y.R.; Udayabhanu; Byrappa, N.M.; Alnaggar, G.; Hezam, A.; Nagaraju, G.; Pramoda, K.; Byrappa, K. Rapid and facile synthesis of Z-scheme ZnO/g-C3N4 heterostructure as efficient visible light-driven photocatalysts for dye degradation and hydrogen evolution reaction. J. Hazard. Mater. Adv. 2023, 9, 100230. [Google Scholar] [CrossRef]
- Liu, S.; Bu, Y.; Cheng, S.; Tao, R. Synthesis of TiO2/g-C3N5 heterojunction for photocatalytic degradation of methylene blue wastewater under visible light irradiation: Mechanism analysis. Diam. Relat. Mater. 2023, 136, 110062. [Google Scholar] [CrossRef]
- Chellapandi, T.; Madhumitha, G.; Roopan, S.M.; Manjupriya, R.; Arunachalapandi, M.; Pouthika, K.; Elamathi, M. Facile synthesis route for visible active g-C3N5/MK30 nanocomposite and its computationally guided photocatalytic degradation of organic pollutants. Sep. Purif. Technol. 2023, 307, 122865. [Google Scholar] [CrossRef]
- Tuama, A.N.; Alzubaidi, L.H.; Jameel, M.H.; Abass, K.H.; bin Mayzan, M.Z.H.; Salman, Z.N. Impact of electron–hole recombination mechanism on the photocatalytic performance of ZnO in water treatment: A review. J. Sol-Gel Sci. Technol. 2024, 110, 792–806. [Google Scholar] [CrossRef]
- Jiang, T.; Lin, M.; He, S.; Xu, T.; Fu, J.; Sun, J.; Zhang, S.; Liang, Y.; Wang, L.; Wang, W. Non-metallic plasmonic effect and step-like charge transport channel synergistically accelerate surface reaction kinetics and charge separation of TiN/CdS-sensitized ZnO photoanode for efficient photoelectrochemical hydrogen evolution. Appl. Catal. B Environ. Energy 2025, 361, 124631. [Google Scholar] [CrossRef]
- Bahiraei, H.; Azarakhsh, S.; Ghasemi, S. Ternary CoFe2O4/g-C3N4/ZnO heterostructure as an efficient and magnetically separable visible-light photocatalyst: Characterization, dye purification, and mechanism. Ceram. Int. 2023, 49, 21050–21059. [Google Scholar] [CrossRef]
- Wang, X.; Yang, S.; Jin, P.; Peng, A.; Zhao, X.; Yang, K.; He, J. Highly efficient photocatalytic degradation of microcystin-LR with permonosulfate activated by visible-light over Z-scheme ZnO/g-C3N4: Degradation pathways and mechanism. J. Environ. Chem. Eng. 2023, 11, 111088. [Google Scholar] [CrossRef]
- Li, K.; Cai, W.; Zhang, Z.; Xie, H.; Zhong, Q.; Qu, H. Boron doped C3N5 for photocatalytic nitrogen fixation to ammonia: The key role of boron in nitrogen activation and mechanism. Chem. Eng. J. 2022, 435, 135017. [Google Scholar] [CrossRef]
- Li, M.; Lu, Q.; Liu, M.; Yin, P.; Wu, C.; Li, H.; Zhang, Y.; Yao, S. Photoinduced Charge Separation via the Double-Electron Transfer Mechanism in Nitrogen Vacancies g-C3N5/BiOBr for the Photoelectrochemical Nitrogen Reduction. ACS Appl. Mater. Interfaces 2020, 12, 38266–38274. [Google Scholar] [CrossRef]
- Barman, D.; Borah, J.; Deb, S.; Sarma, B.K. Design strategy for CuO-ZnO S-scheme heterojunction photocatalysts in the presence of plasmonic Ag and insights into photoexcited carrier generation and interfacial transfer in diverse structural configurations of the heterostructure system. Colloids Surf. A Physicochem. Eng. Asp. 2023, 663, 131077. [Google Scholar] [CrossRef]
- Fang, J.; Fan, H.; Ma, Y.; Wang, Z.; Chang, Q. Surface defects control for ZnO nanorods synthesized by quenching and their anti-recombination in photocatalysis. Appl. Surf. Sci. 2015, 332, 47–54. [Google Scholar] [CrossRef]
- Bao, C.; Chen, M.; Jin, X.; Hu, D.; Huang, Q. Efficient and stable photocatalytic reduction of aqueous hexavalent chromium ions by polyaniline surface-hybridized ZnO nanosheets. J. Mol. Liq. 2019, 279, 133–145. [Google Scholar] [CrossRef]
- Hu, J.; Chen, C.; Yang, H.; Yang, F.; Qu, J.; Yang, X.; Sun, W.; Dai, L.; Li, C.M. Tailoring well-ordered, highly crystalline carbon nitride nanoarrays via molecular engineering for efficient photosynthesis of H2O2. Appl. Catal. B Environ. 2022, 317, 121723. [Google Scholar] [CrossRef]
- Yin, H.; Cao, Y.; Fan, T.; Zhang, M.; Yao, J.; Li, P.; Chen, S.; Liu, X. In situ synthesis of Ag3PO4/C3N5 Z-scheme heterojunctions with enhanced visible-light-responsive photocatalytic performance for antibiotics removal. Sci. Total Environ. 2021, 754, 141926. [Google Scholar] [CrossRef] [PubMed]
- Ai, Y.; Hu, J.; Xiong, X.; Carabineiro, S.A.C.; Li, Y.; Sirotkin, N.; Agafonov, A.; Lv, K. Synergistic interfacial engineering of a S-scheme ZnO/In2S3 photocatalyst with S−O covalent bonds: A dual-functional advancement for tetracycline hydrochloride degradation and H2 evolution. Appl. Catal. B Environ. Energy 2024, 353, 124098. [Google Scholar] [CrossRef]
- Xiong, J.; Li, X.; Huang, J.; Gao, X.; Chen, Z.; Liu, J.; Li, H.; Kang, B.; Yao, W.; Zhu, Y. CN/rGO@BPQDs high-low junctions with stretching spatial charge separation ability for photocatalytic degradation and H2O2 production. Appl. Catal. B Environ. 2020, 266, 118602. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A.Y. Generation and Detection of Reactive Oxygen Species in Photocatalysis. Chem. Rev. 2017, 117, 11302–11336. [Google Scholar] [CrossRef] [PubMed]

| System | Condition | Time | Degradation Percentage | Ref. |
|---|---|---|---|---|
| ZCN | 300 W Xe lamp, [MB]0 = 30 mg·L−1, [Catalyst]0 = 0.2 g·L−1 | 70 min | 97% | This work |
| TiO2/ZnO/rGO | 300 W Xe lamp, [MB]0 = 20 mg·L−1, [Catalyst]0 = 0.5 g·L−1 | 180 min | ~80% | [8] |
| Cu-ZnO | 300 W Xe lamp, [MB]0 = 5 mg·L−1, [Catalyst]0 = 0.5 g·L−1 | 90 min | ~81% | [32] |
| ZnO: Eu (10%)-M | 300 W Xe lamp, [MB]0 = 10 mg·L−1, [Catalyst]0 = 1 g·L−1 | 150 min | ~90% | [15] |
| 3wt % Cu-doped ZnO (CuZn3) | 300 W Xe lamp, [MB]0 = 10 mg·L−1, [Catalyst]0 = 0.1 g·L−1 | 180 min | 92% | [33] |
| ZnO/SnO2/carbon | 300 W Xe lamp, [MB]0 = 25 mg·L−1, [Catalyst]0 = 3.6 g·L−1 | 300 min | ~50% | [34] |
| ZnO-Cu-CuO/C3N4 | 300 W Xe lamp, [MB]0 = 30 mg·L−1, [Catalyst]0 = 0.2 g·L−1 | 120 min | ~75% | [35] |
| ZnO/g-C3N4 | 300 W Xe lamp, [MB]0 = 5 mg·L−1, [Catalyst]0 = 0.4 g·L−1 | 100 min | ~80% | [36] |
| TiO2/g-C3N5 | 500 W Xe lamp, [MB]0 = 20 mg·L−1, [Catalyst]0 = 2.5 g·L−1 | 180 min | 97% | [37] |
| g-C3N5/MK30 | 300 W Xe lamp, [MB]0 = 10 mg·L−1, [Catalyst]0 = 0.2 g·L−1 | 40 min | 90% | [38] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Liu, S.; Li, L.; Yang, L.; Wu, X.; Si, Z.; Ran, R.; Wu, H. Construction of Honeycomb-like ZnO/g-C3N5 Heterojunction for MB Photocatalytic Degradation. Processes 2025, 13, 253. https://doi.org/10.3390/pr13010253
Liu S, Liu S, Li L, Yang L, Wu X, Si Z, Ran R, Wu H. Construction of Honeycomb-like ZnO/g-C3N5 Heterojunction for MB Photocatalytic Degradation. Processes. 2025; 13(1):253. https://doi.org/10.3390/pr13010253
Chicago/Turabian StyleLiu, Sitong, Shicheng Liu, Letao Li, Letong Yang, Xiaodong Wu, Zhichun Si, Rui Ran, and Hui Wu. 2025. "Construction of Honeycomb-like ZnO/g-C3N5 Heterojunction for MB Photocatalytic Degradation" Processes 13, no. 1: 253. https://doi.org/10.3390/pr13010253
APA StyleLiu, S., Liu, S., Li, L., Yang, L., Wu, X., Si, Z., Ran, R., & Wu, H. (2025). Construction of Honeycomb-like ZnO/g-C3N5 Heterojunction for MB Photocatalytic Degradation. Processes, 13(1), 253. https://doi.org/10.3390/pr13010253









