Performance of CO2 Adsorption on Modified Activated Carbons Derived from Okara Powder Waste: Impacts of Ammonia Impregnation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
2.2. Modification of ACs
2.3. SEM-EDX
2.4. Fourier Transform Infrared Spectroscopy (FTIR)
2.5. Temperature Programmed Desorption of CO2 on ACs
2.6. Thermogravimetric Analysis/Mass Spectrometry (TGA-MS)
3. Results and Discussions
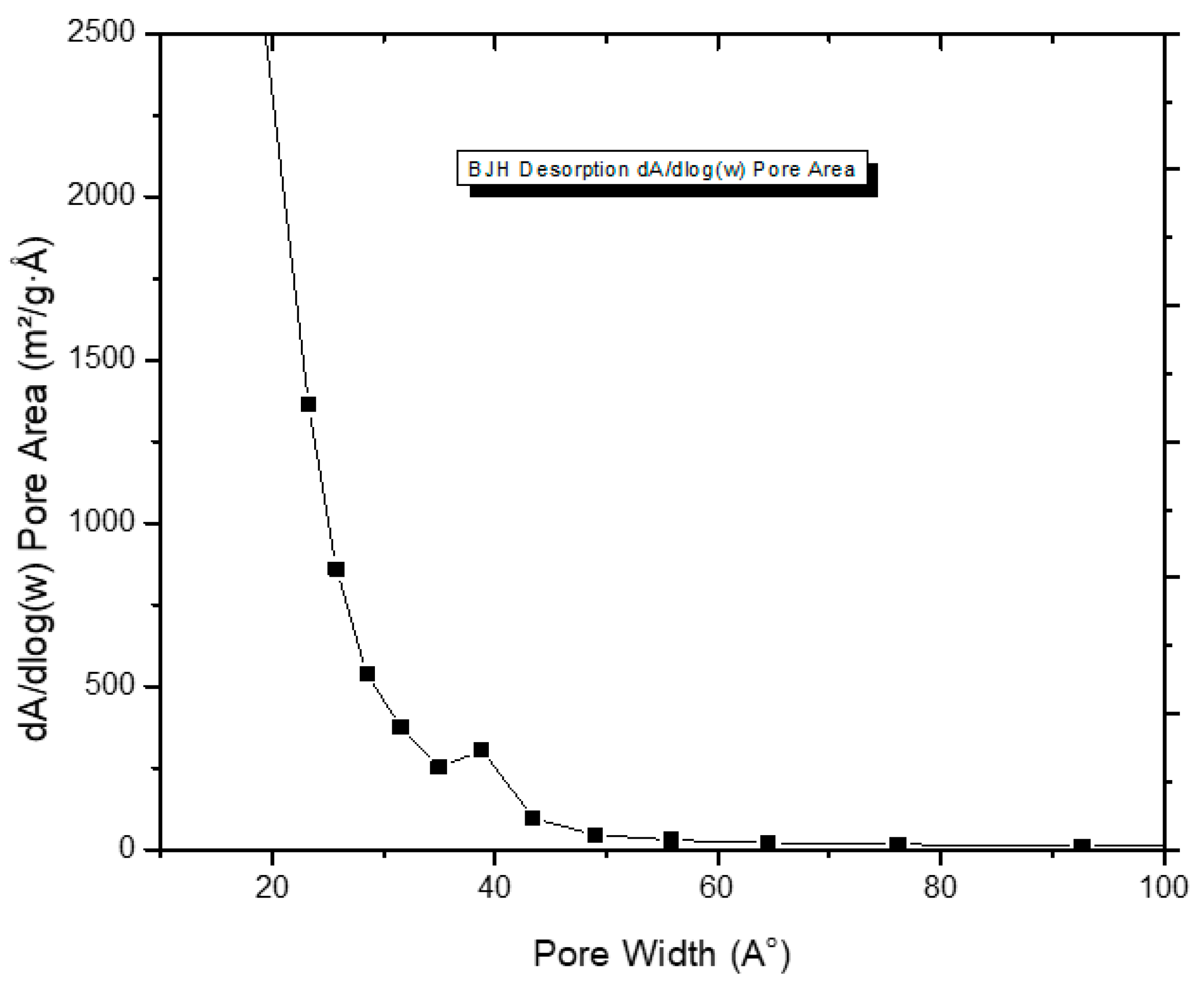
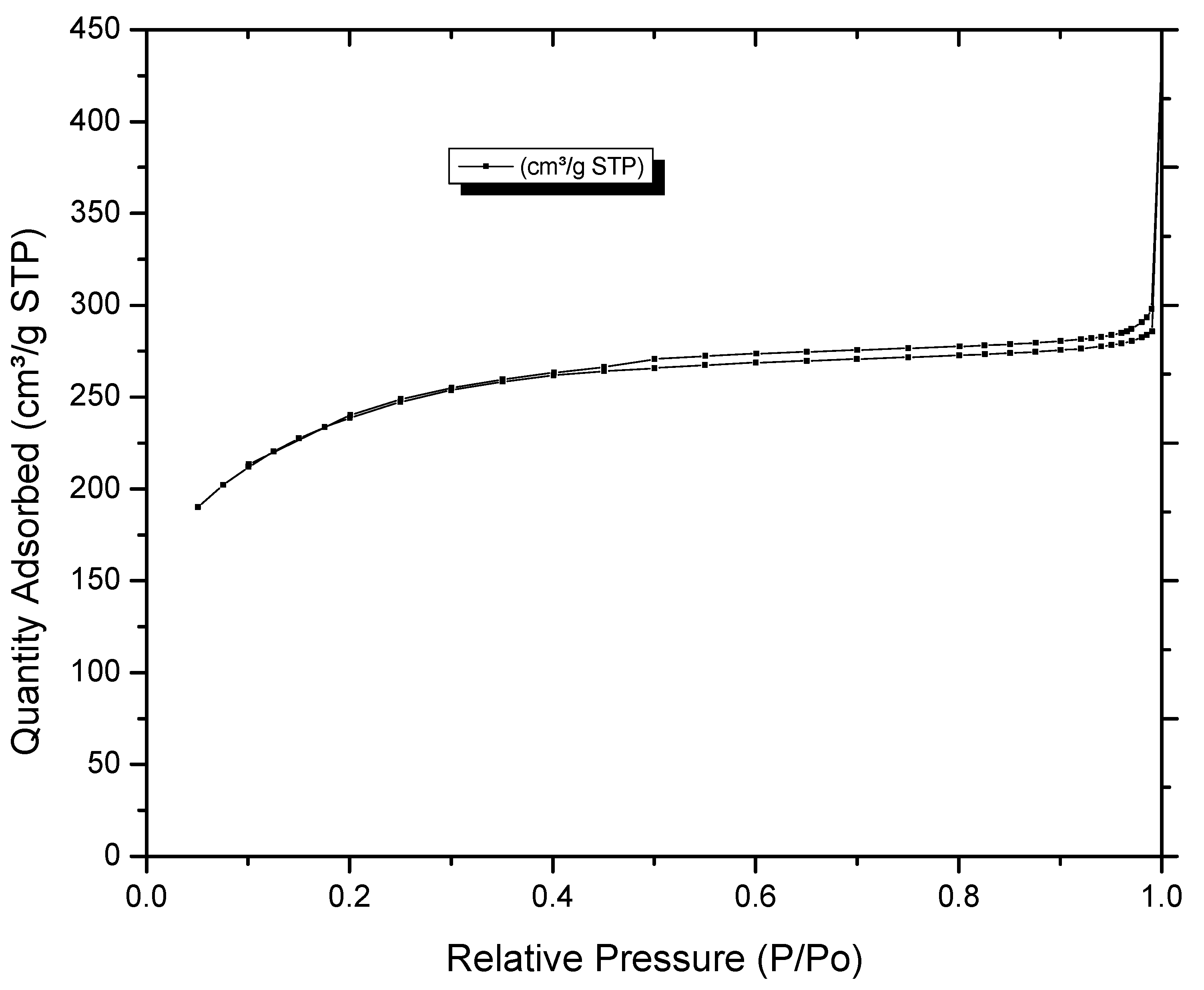
3.1. Impact on Morphology and Composition of Activated Carbons
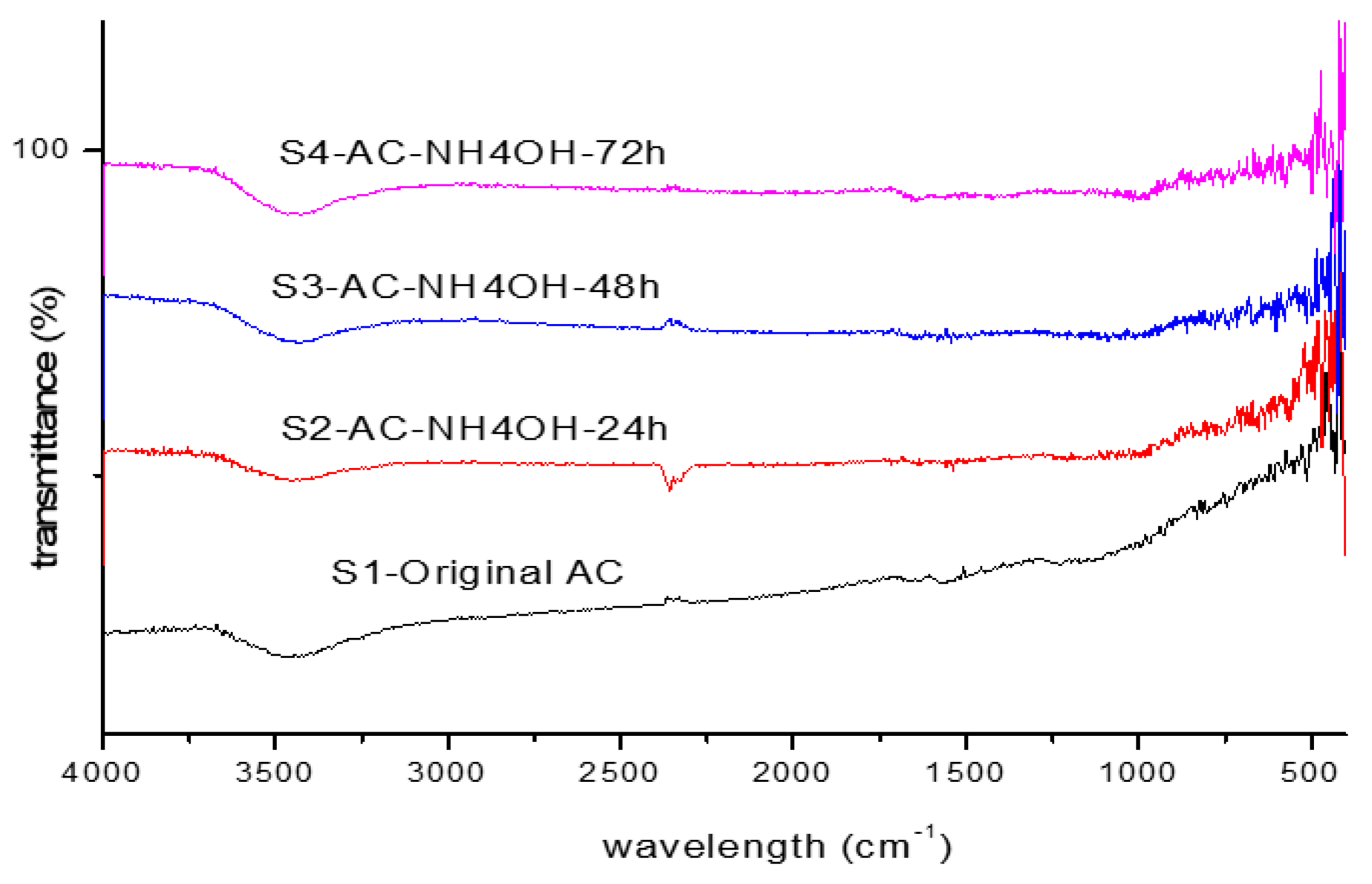
3.2. Impact on Functional Groups of Activated Carbons
3.3. Impact on CO2 Adsorption Ability
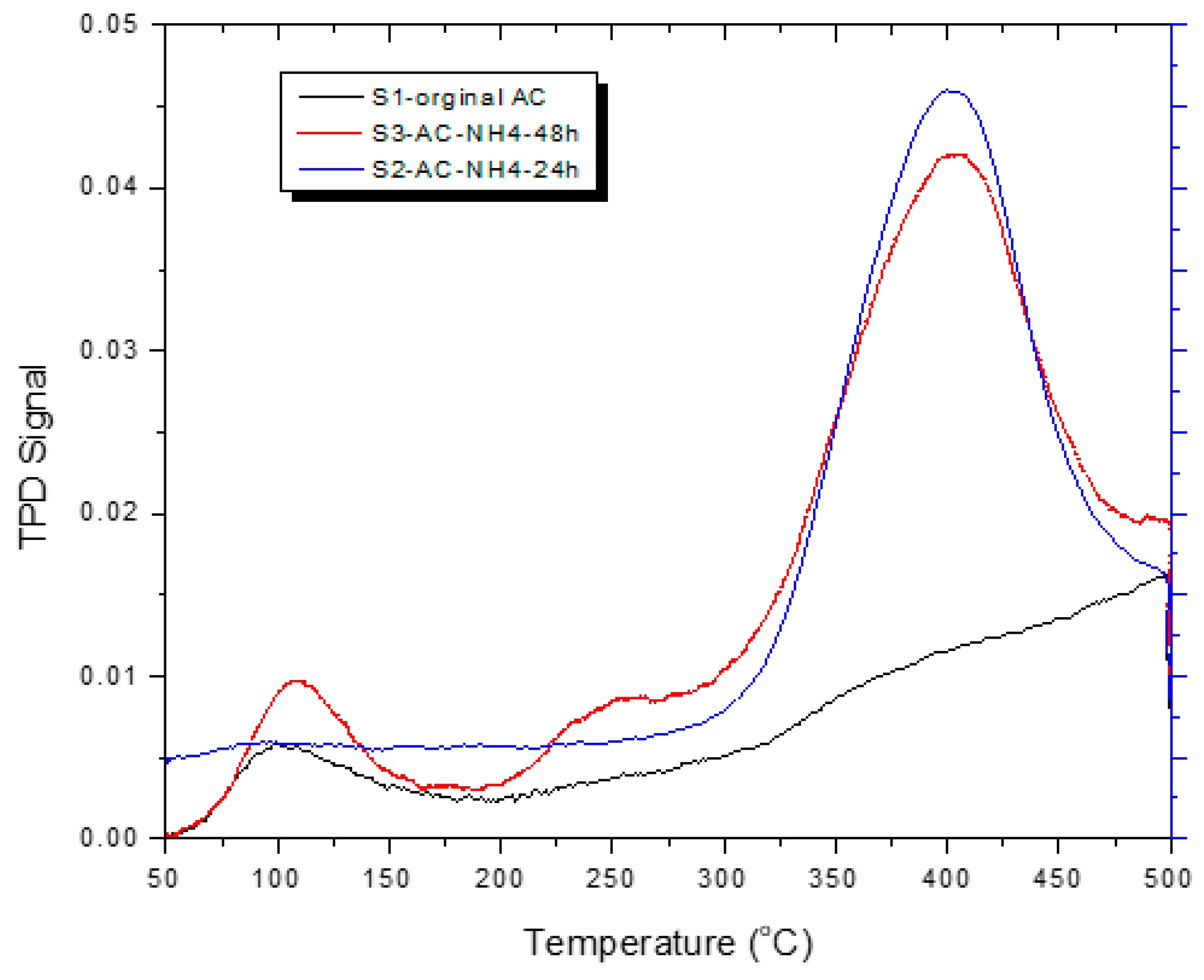
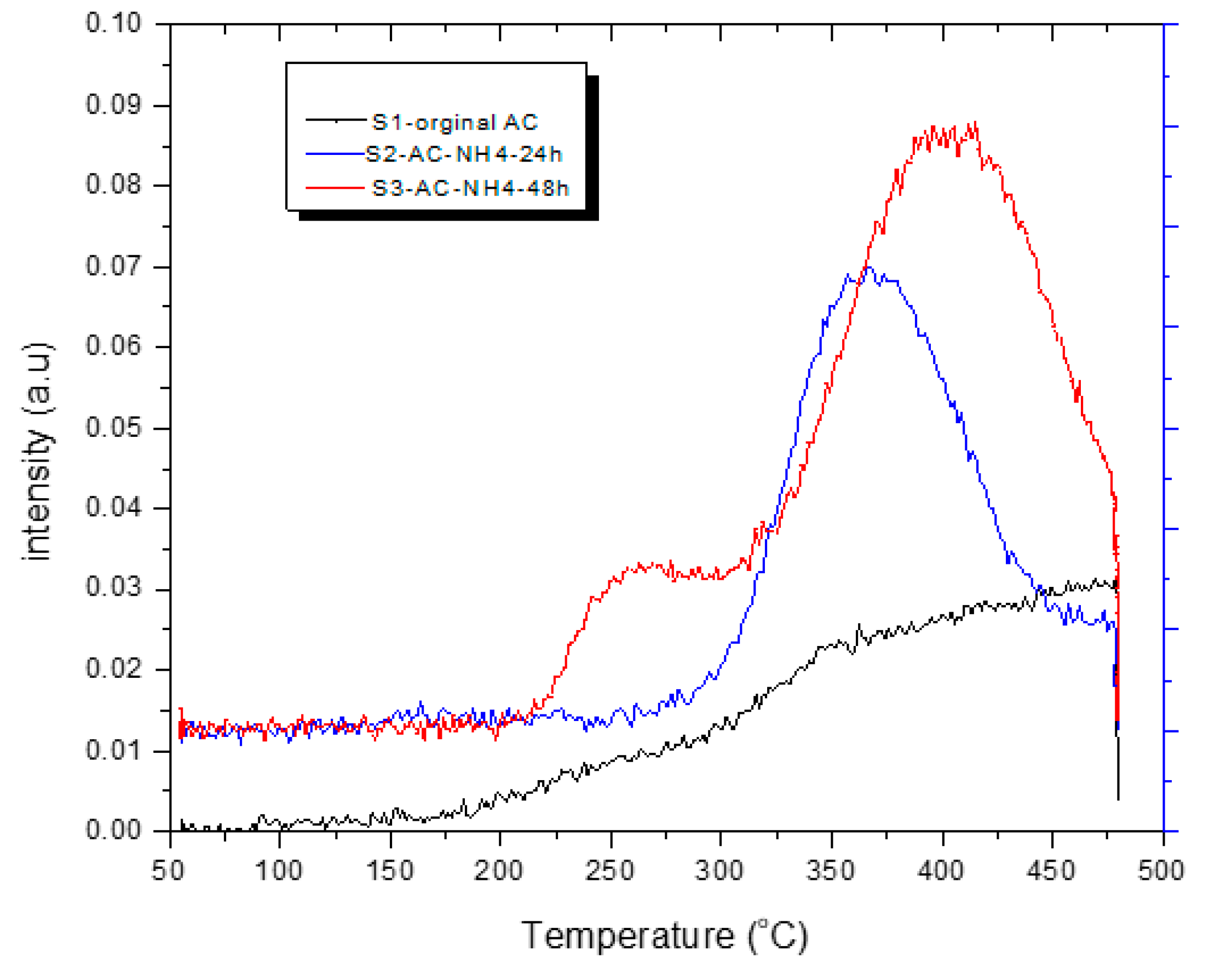
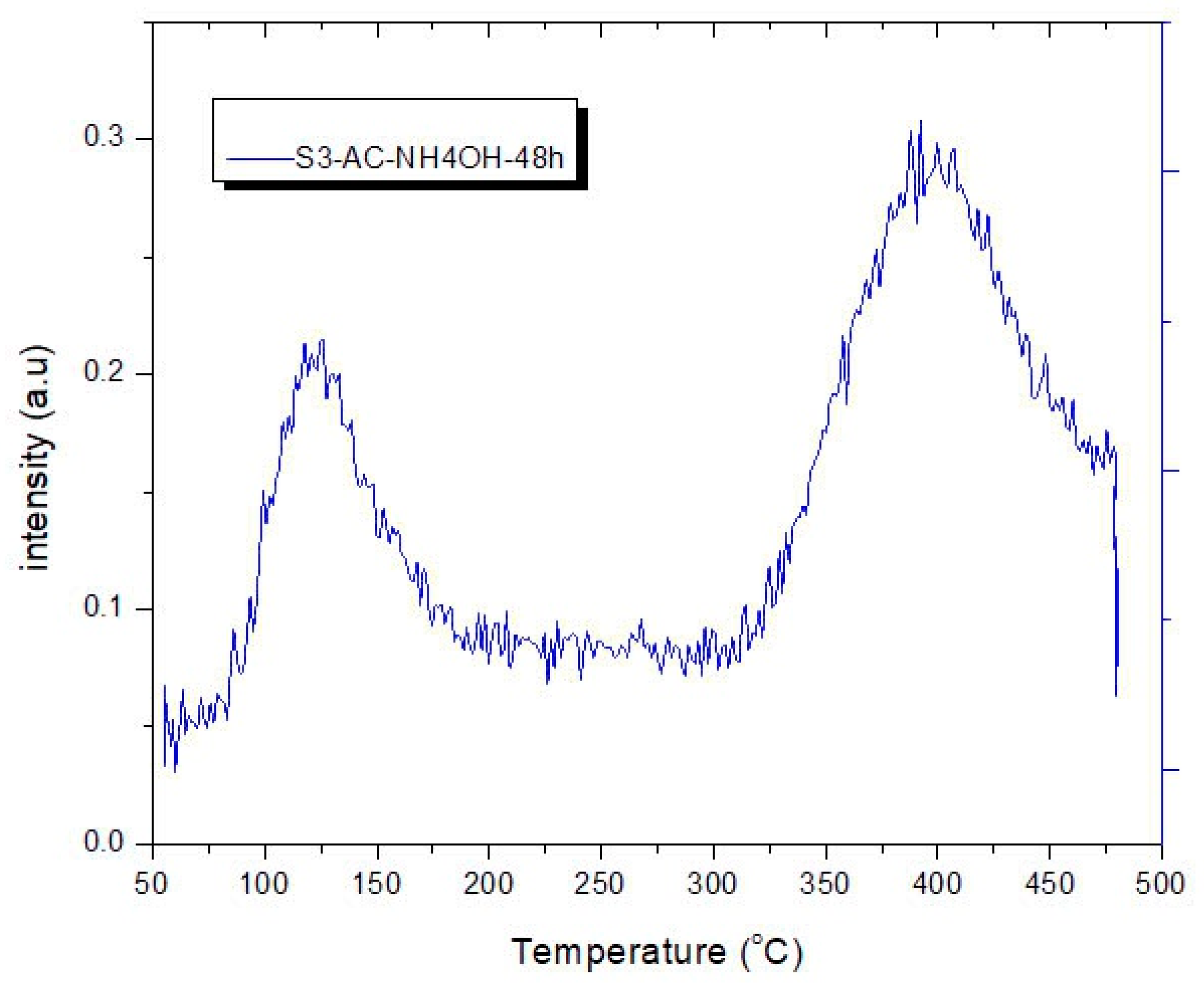
3.4. CO2 Temperature-Programmed Desorption (CO2-TPD) Experimental Results
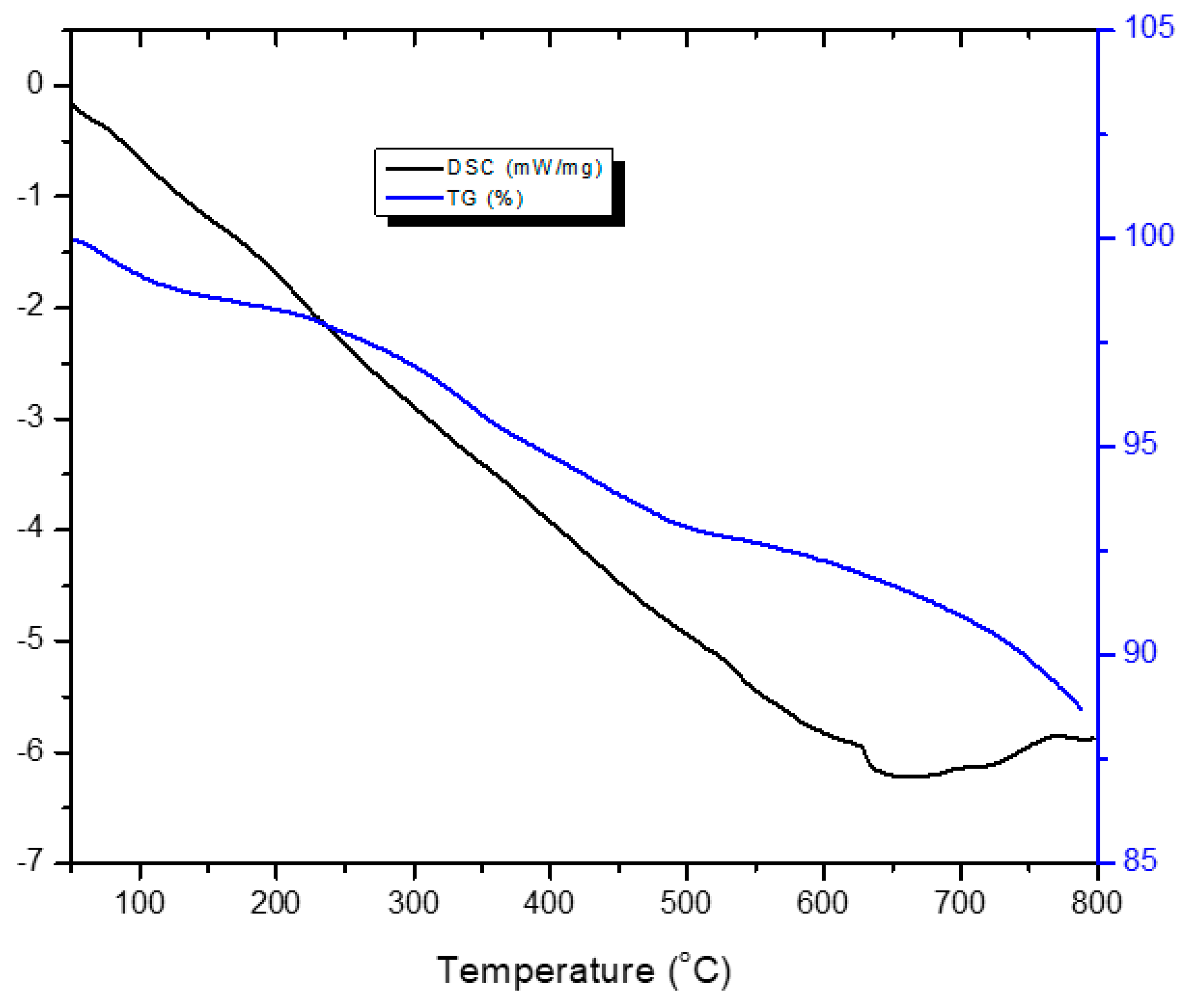
3.5. TG–DSC Analysis
4. Conclusions and Outlooks
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mac Dowell, N.; Fennell, P.S.; Shah, N.; Maitland, G.C. The role of CO2 capture and utilization in mitigating climate change. Nat. Clim. Change 2017, 7, 243–249. [Google Scholar] [CrossRef]
- Bandh, S.A.; Malla, F.A.; Hoang, T.D.; Qayoom, I.; Mohi-Ud-Din, H.; Bashir, S.; Betts, R.; Le, T.T.; Nguyen Le, D.T.; Linh Le, N.V.; et al. Track to reach net-zero: Progress and pitfalls. Energy Environ. 2024. [Google Scholar] [CrossRef]
- Hoang, T.D.; Nghiem, N. Recent developments and current status of commercial production of fuel ethanol. Fermentation 2021, 7, 314. [Google Scholar] [CrossRef]
- Sezali, N.A.A.; Ong, H.L.; Villagracia, A.R.; Hoang, T.D. Bio-based nanomaterials for energy application: A review. Vietnam J. Chem. 2024, 62, 1–12. [Google Scholar] [CrossRef]
- Malla, F.A.; Dung, T.; Bandh, S.A.; Malla, A.A.; Wani, S.A. Circular Economy to Decarbonize Electricity. In Renewable Energy in Circular Economy, 1st ed.; Bandh, S.A., Malla, F.A., Hoang, A.T., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 71–87. [Google Scholar]
- Nobre, L.C.; Teixeira, P.; Pinheiro, C.I.; Palavra, A.M.; Calvete, M.J.; Nieto de Castro, C.A.; Nobre, B.P. The Treatment of Natural Calcium Materials Using the Supercritical Antisolvent Method for CO2 Capture Applications. Processes 2024, 12, 425. [Google Scholar] [CrossRef]
- Hoang, T.D.; Bandh, S.A.; Malla, F.A.; Qayoom, I.; Bashir, S.; Peer, S.B.; Halog, A. Carbon-Based Synthesized Materials for CO2 Adsorption and Conversion: Its Potential for Carbon Recycling. Recycling 2023, 8, 53. [Google Scholar] [CrossRef]
- Azmi, N.Z.M.; Buthiyappan, A.; Raman, A.A.A.; Patah, M.F.A.; Sufian, S. Recent advances in biomass based activated carbon for carbon dioxide capture–A review. J. Ind. Eng. Chem. 2022, 116, 1–20. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, M.; Wang, H.; Li, Z. Enhancement of CO2 adsorption on high surface area activated carbon modified by N2, H2 and ammonia. J. Chem. Eng. 2010, 160, 571–577. [Google Scholar] [CrossRef]
- Pevida, C.; Plaza, M.G.; Arias, B.; Fermoso, J.; Rubiera, F.; Pis, J.J. Surface modification of activated carbons for CO2 capture. Appl. Surf. Sci. 2008, 254, 7165–7172. [Google Scholar] [CrossRef]
- Qasem, N.A.; Ben-Mansour, R. Adsorption breakthrough and cycling stability of carbon dioxide separation from CO2/N2/H2O mixture under ambient conditions using 13X and Mg-MOF-74. Appl. Energy 2018, 230, 1093–1107. [Google Scholar] [CrossRef]
- Abuelnoor, N.; AlHajaj, A.; Khaleel, M.; Vega, L.F.; Abu-Zahra, M.R. Activated carbons from biomass-based sources for CO2 capture applications. Chemosphere 2021, 282, 131111. [Google Scholar] [CrossRef] [PubMed]
- Heidari, A.; Younesi, H.; Rashidi, A.; Ghoreyshi, A.A. Evaluation of CO2 adsorption with eucalyptus wood based activated carbon modified by ammonia solution through heat treatment. J. Chem. Eng. 2014, 254, 503–513. [Google Scholar] [CrossRef]
- Gholidoust, A.; Atkinson, J.D.; Hashisho, Z. Enhancing CO2 adsorption via amine-impregnated activated carbon from oil sands coke. Energy Fuels 2017, 31, 1756–1763. [Google Scholar] [CrossRef]
- Abd, A.A.; Othman, M.R.; Kim, J. A review on application of activated carbons for carbon dioxide capture: Present performance, preparation, and surface modification for further improvement. Environ. Sci. Pollut. Res. 2021, 28, 43329–43364. [Google Scholar] [CrossRef] [PubMed]
- Przepiórski, J.; Skrodzewicz, M.; Morawski, A.W. High temperature ammonia treatment of activated carbon for enhancement of CO2 adsorption. Appl. Surf. Sci. 2004, 225, 235–242. [Google Scholar] [CrossRef]
- Feng, J.Y.; Wang, R.; Thakur, K.; Ni, Z.J.; Zhu, Y.Y.; Hu, F.; Zhang, J.G.; Wei, Z.J. Evolution of okara from waste to value added food ingredient: An account of its bio-valorization for improved nutritional and functional effects. Trends Food Sci. Technol. 2021, 116, 669–680. [Google Scholar] [CrossRef]
- Hoang, T.D.; Liu, Y.; Le, M.T. Synthesis and Characterization of Biochars and Activated Carbons Derived from Various Biomasses. Sustainability 2024, 16, 5495. [Google Scholar] [CrossRef]
- Abi Bianasari, A.; Khaled, M.S.; Hoang, T.D.; Reza, M.S.; Bakar, M.S.A.; Azad, A.K. Influence of combined catalysts on the catalytic pyrolysis process of biomass: A systematic literature review. Energy Convers. Manag. 2024, 309, 118437. [Google Scholar] [CrossRef]
- Tran, T.K.; Huynh, L.; Nguyen, H.L.; Nguyen, M.K.; Lin, C.; Hoang, T.D.; Hung, N.T.Q.; Nguyen, X.H.; Chang, S.W.; Nguyen, D.D. Applications of engineered biochar in remediation of heavy metal (loid) s pollution from wastewater: Current perspectives toward sustainable development goals. Sci. Total Environ. 2024, 926, 171859. [Google Scholar] [CrossRef]
- Adame-Pereira, M.; Durán-Valle, C.J.; Fernández-González, C. Hydrothermal Carbon Coating of an Activated Carbon—A New Adsorbent. Molecules 2023, 28, 4769. [Google Scholar] [CrossRef]
- Gupta, V.K.; Singh, L.P.; Chaudhary, M.; Kushwaha, S. A novel approach to develop activated carbon by an ingenious hydrothermal treatment methodology using Phyllanthus emblica fruit stone. J. Clean. Prod. 2021, 288, 125643. [Google Scholar]
- Hoang, T.D.; Van Anh, N.; Yusuf, M.; Ali SA, M.; Subramanian, Y.; Hoang Nam, N.; Minh Ky, N.; Le, V.G.; Thi Thanh Huyen, N.; Abi Bianasari, A.; et al. Valorization of Agriculture Residues into Value-Added Products: A Comprehensive Review of Recent Studies. Chem. Rec. 2024, 24, e202300333. [Google Scholar] [CrossRef]
- Kourtelesis, M.; Kousi, K.; Kondarides, D.I. CO2 hydrogenation to methanol over La2O3-promoted CuO/ZnO/Al2O3 catalysts: A kinetic and mechanistic study. Catalysts 2020, 10, 183. [Google Scholar] [CrossRef]
- Ghimbeu, C.M.; Gadiou, R.; Dentzer, J.; Schwartz, D.; Vix-Guterl, C. Influence of surface chemistry on the adsorption of oxygenated hydrocarbons on activated carbons. Langmuir 2010, 26, 18824–18833. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.H.; Ruckenstein, E. Temperature-programmed desorption of CO adsorbed on NiO/MgO. J. Catal. 1996, 163, 306–311. [Google Scholar] [CrossRef]
- Liu, C.J.; Lobban, L.L.; Mallinson, R.G. (Eds.) Experimental investigations on the interaction between plasmas and catalyst for plasma catalytic methane conversion (PCMC) over zeolites. In Studies in Surface Science and Catalysis, 2nd ed.; Elsevier: Louvain-la-Neuve, Belgium, 1998; Volume 119, pp. 361–366. [Google Scholar]
- Jayaraman, K.; Kok, M.V.; Gokalp, I. Thermogravimetric and mass spectrometric (TG-MS) analysis and kinetics of coal-biomass blends. Renew. Energy 2017, 101, 293–300. [Google Scholar] [CrossRef]
- Almoneef, M.M.; Jedli, H.; Mbarek, M. Experimental study of CO2 adsorption using activated carbon. Mater. Res. Express 2021, 8, 065602. [Google Scholar] [CrossRef]
- Ghouma, I.; Jeguirim, M.; Sager, U.; Limousy, L.; Bennici, S.; Däuber, E.; Asbach, C.; Ligotski, R.; Schmidt, F.; Ouederni, A. The potential of activated carbon made of agro-industrial residues in NOx immissions abatement. Energies 2017, 10, 1508. [Google Scholar] [CrossRef]
- Wang, T.; Zhai, Y.; Zhu, Y.; Wang, Z.; Xiao, H.; Peng, C.; Wang, B.; Li, C. What is the influence of the nitrogen-containing composition during hydrothermal carbonization of biomass? A new perspective from mimic feedstock. Bioresour. Technol. Rep. 2019, 5, 343–350. [Google Scholar] [CrossRef]
- Leng, L.; Yang, L.; Leng, S.; Zhang, W.; Zhou, Y.; Peng, H.; Li, H.; Hu, Y.; Jiang, S.; Li, H. A review on nitrogen transformation in hydrochar during hydrothermal carbonization of biomass containing nitrogen. Sci. Total Environ. 2021, 756, 143679. [Google Scholar] [CrossRef]
- Xiong, J.; Chen, S.; Wang, J.; Wang, Y.; Fang, X.; Huang, H. Speciation of main nutrients (N/P/K) in hydrochars produced from the hydrothermal carbonization of swine manure under different reaction temperatures. Materials 2021, 14, 4114. [Google Scholar] [CrossRef] [PubMed]
- Amalina, F.; Abd Razak, A.S.; Krishnan, S.; Sulaiman, H.; Zularisam, A.W.; Nasrullah, M. Biochar production techniques utilizing biomass waste-derived materials and environmental applications–A review. Hazard. Mater. 2022, 7, 100134. [Google Scholar] [CrossRef]
- Abdullah, A.H.D.; Chalimah, S.; Primadona, I.; Hanantyo, M.H.G. (Eds.) Physical and chemical properties of corn, cassava, and potato starchs. In IOP Conference Series: Earth and Environmental Science, 2nd ed.; IOP Publishing: Briston, UK, 2018; Volume 160, p. 012003. [Google Scholar]
- Li, J.; Wang, Z.; Yang, X.; Hu, L.; Liu, Y.; Wang, C. Evaluate the pyrolysis pathway of glycine and glycylglycine by TG–FTIR. J. Anal. Appl. Pyrol. 2007, 80, 247–253. [Google Scholar] [CrossRef]
- Jiménez-García, E.A.; Pérez-Huertas, S.; Pérez, A.; Calero, M.; Blázquez, G. Recycling PVC Waste into CO2 Adsorbents: Optimizing Pyrolysis Valorization with Neuro-Fuzzy Models. Processes 2024, 12, 431. [Google Scholar] [CrossRef]
- Moyo, M.; Nyamhere, G.; Sebata, E.; Guyo, U. Kinetic and equilibrium modelling of lead sorption from aqueous solution by activated carbon from goat dung. Desalin Water Treat. 2016, 57, 765–775.38. [Google Scholar] [CrossRef]
- Petrovic, B.; Gorbounov, M.; Soltani, S.M. Influence of surface modification on selective CO2 adsorption: A technical review on mechanisms and methods. Micropor Mesopor Mat. 2021, 312, 110751. [Google Scholar] [CrossRef]
- Wang, B.; Côté, A.P.; Furukawa, H.; O’Keeffe, M.; Yaghi, O.M. Colossal cages in zeolitic imidazolate frameworks as selective carbon dioxide reservoirs. Nature 2008, 453, 207–211. [Google Scholar] [CrossRef]
- Goetz, V.; Pupier, O.; Guillot, A. Carbon dioxide-methane mixture adsorption on activated carbon. Adsorption 2006, 12, 55–63. [Google Scholar] [CrossRef]
- González Plaza, M.; González García, A.S.; Pevida García, C.; Pis Martínez, J.J.; Rubiera González, F. Valorisation of spent coffee grounds as CO2 adsorbents for postcombustion capture applications. Appl. Energy 2012, 99, 272–279. [Google Scholar] [CrossRef]
- Li, D.; Ma, T.; Zhang, R.; Tian, Y.; Qiao, Y. Preparation of porous carbons with high low-pressure CO2 uptake by KOH activation of rice husk char. Fuel 2015, 139, 68–70. [Google Scholar] [CrossRef]
- Serafin, J.; Narkiewicz, U.; Morawski, A.W.; Wróbel, R.J.; Michalkiewicz, B. Highly microporous activated carbons from biomass for CO2 capture and effective micropores at different conditions. J. CO2 Util. 2017, 18, 73–79. [Google Scholar] [CrossRef]
- Wang, R.; Wang, P.; Yan, X.; Lang, J.; Peng, C.; Xue, Q. Promising porous carbon derived from celtuce leaves with outstanding supercapacitance and CO2 capture performance. ACS Appl. Mater. Interfaces 2012, 4, 5800–5806. [Google Scholar] [CrossRef]
- Li, D.; Tian, Y.; Li, L.; Li, J.; Zhang, H. Production of highly microporous carbons with large CO2 uptakes at atmospheric pressure by KOH activation of peanut shell char. J. Porous Mater. 2015, 22, 1581–1588. [Google Scholar] [CrossRef]
- Wang, T.; Wu, L.; Xu, X.; Sun, Y.; Wang, Y.; Zhong, W.; Du, Y. An efficient Co3S4/CoP hybrid catalyst for electrocatalytic hydrogen evolution. Sci. Rep. 2017, 7, 11891. [Google Scholar] [CrossRef] [PubMed]
- Kiełbasa, K.; Kamińska, A.; Niedoba, O.; Michalkiewicz, B. CO2 adsorption on activated carbons prepared from molasses: A comparison of two and three parametric models. Materials 2021, 14, 7458. [Google Scholar] [CrossRef] [PubMed]
- Asadi-Sangachini, Z.; Galangash, M.M.; Younesi, H.; Nowrouzi, M. The feasibility of cost-effective manufacturing activated carbon derived from walnut shells for large-scale CO2 capture. Environ. Sci. Pollut. Res. 2019, 26, 26542–26552. [Google Scholar] [CrossRef] [PubMed]
- Lima, I.M.; McAloon, A.; Boateng, A.A. Activated carbon from broiler litter: Process description and cost of production. Biomass Bioenerg. 2008, 32, 568–572. [Google Scholar] [CrossRef]
- Fakeeha, A.H.; Kasim, S.O.; Ibrahim, A.A.; Abasaeed, A.E.; Al-Fatesh, A.S. Influence of nature support on methane and CO2 conversion in a dry reforming reaction over nickel-supported catalysts. Materials 2019, 12, 1777. [Google Scholar] [CrossRef]
- Zhao, B.; Wang, J.; Zhu, D.; Song, G.; Yang, H.; Chen, L.; Sun, L.; Yang, S.; Guan, H.; Xie, X. Adsorption characteristics of gas molecules (H2O, CO2, CO, CH4, and H2) on CaO-based catalysts during biomass thermal conversion with in situ CO2 capture. Catalysts 2019, 9, 757. [Google Scholar] [CrossRef]
- Li, N.; Ma, X.; Zha, Q.; Kim, K.; Chen, Y.; Song, C. Maximizing the number of oxygen-containing functional groups on activated carbon by using ammonium persulfate and improving the temperature-programmed desorption characterization of carbon surface chemistry. Carbon 2011, 49, 5002–5013. [Google Scholar] [CrossRef]
- Thommes, M.; Morlay, C.; Ahmad, R.; Joly, J.P. Assessing surface chemistry and pore structure of active carbons by a combination of physisorption (H2O, Ar, N2, CO2), XPS and TPD-MS. Adsorption 2011, 17, 653–661. [Google Scholar] [CrossRef]
- Lu, Z.; Sun, K.; Wang, J.; Zhang, Z.; Liu, C. A highly active Au/In2O3-ZrO2 catalyst for selective hydrogenation of CO2 to methanol. Catalysts 2020, 10, 1360. [Google Scholar] [CrossRef]
- Hoang, T.D.; Ky, N.M.; Thuong, N.T.N.; Nhan, H.Q.; Ngan, N.V.C. Artificial intelligence in pollution control and management: Status and future prospects. In Artificial Intelligence and Environmental Sustainability, 1st ed.; Algorithms for Intelligent Systems, Ong, H.L., Doong, R., Naguib, R., Lim, C.P., Nagar, A.K., Eds.; Springer: Singapore, 2022; pp. 23–43. [Google Scholar]
- Gan, Y.X. Activated carbon from biomass sustainable sources. C 2021, 7, 39. [Google Scholar] [CrossRef]



| Elements | S1 (Original AC) | S2 (AC−NH4OH−24h) | S3 (AC−NH4OH−48h) | S4 (AC−NH4OH−72h) | ||||
|---|---|---|---|---|---|---|---|---|
| Mass% | Atom% | Mass% | Atom% | Mass% | Atom% | Mass% | Atom% | |
| C | 88.39 | 95.63 | 66.64 | 74.57 | 71.25 | 78.29 | 71.30 | 87.74 |
| Al | 1.16 | 0.56 | 2.08 | 1.04 | 0.97 | 0.47 | 2.06 | 1.13 |
| Si | 1.81 | 0.84 | 3.23 | 1.55 | ||||
| Cl | 3.05 | 1.12 | ||||||
| K | 5.59 | 1.86 | 2.48 | 0.85 | 4.90 | 1.65 | 25.07 | 9.48 |
| N | 4.34 | 4.17 | 6.05 | 5.70 | 1.58 | 1.16 | ||
| O | 21.22 | 17.83 | 16.82 | 13.88 | ||||
| Total | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| C/N atom ratio | - | 17.88 | 13.74 | 75.63 | ||||
| Adsorbents | Sample Code | Temperature (K) | Impregnation Method and Agent | CO2 Uptake (mmol/g) | References |
|---|---|---|---|---|---|
| Okara | S1-original/raw or unmodified AC | 298 | Chemical, wet, NH4OH | 0.24 | This work |
| Crystallized materials | ZIF−100 | 298 | - | 1.05 | [40] |
| Okara | S4−AC−NH4OH−72h | 298 | Chemical, wet, NH4OH | 1.26 | This work |
| Commercial AC | Norit RB2 | 298 | - | 1.5 | [41] |
| Okara | S2−AC−NH4OH−24h | 298 | Chemical, wet, NH4OH | 1.78 | This work |
| Okara | S3−AC−NH4OH−48h | 298 | Chemical, wet, NH4OH | 2.24 | This work |
| Zeolite-based adsorbents | 13x | 298 | - | 2.27 | [11] |
| Commercial ACs | Commercial ACs | 298 | Chemical, wet, NH4OH | 2.92 | [9] |
| Coffee grounds | - | 298 | Chemical, wet, KOH | 3.00 | [42] |
| Eucalyptus wood | - | 298 | Chemical, H3PO4 | 3.22 | [13] |
| Rice husk char | - | 298 | Chemical, wet, KOH | 3.71 | [43] |
| Carrot peels | - | 298 | Chemical, wet, KOH | 4.18 | [44] |
| Celtuce leaves | - | 298 | Chemical, wet, KOH | 4.36 | [45] |
| Peanut shell char | - | 298 | Chemical, wet, KOH | 4.41 | [46] |
| MOF | Mg−MOF−74 | 298 | - | 5.77 | [11] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoang, T.-D.; Liu, Y.; Le, M.T. Performance of CO2 Adsorption on Modified Activated Carbons Derived from Okara Powder Waste: Impacts of Ammonia Impregnation. Processes 2024, 12, 2024. https://doi.org/10.3390/pr12092024
Hoang T-D, Liu Y, Le MT. Performance of CO2 Adsorption on Modified Activated Carbons Derived from Okara Powder Waste: Impacts of Ammonia Impregnation. Processes. 2024; 12(9):2024. https://doi.org/10.3390/pr12092024
Chicago/Turabian StyleHoang, Tuan-Dung, Yan Liu, and Minh Thang Le. 2024. "Performance of CO2 Adsorption on Modified Activated Carbons Derived from Okara Powder Waste: Impacts of Ammonia Impregnation" Processes 12, no. 9: 2024. https://doi.org/10.3390/pr12092024
APA StyleHoang, T.-D., Liu, Y., & Le, M. T. (2024). Performance of CO2 Adsorption on Modified Activated Carbons Derived from Okara Powder Waste: Impacts of Ammonia Impregnation. Processes, 12(9), 2024. https://doi.org/10.3390/pr12092024






