Recovery of High-Value Compounds from Yarrowia lipolytica IMUFRJ 50682 Using Autolysis and Acid Hydrolysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Microorganism Maintenance and Biomass Production
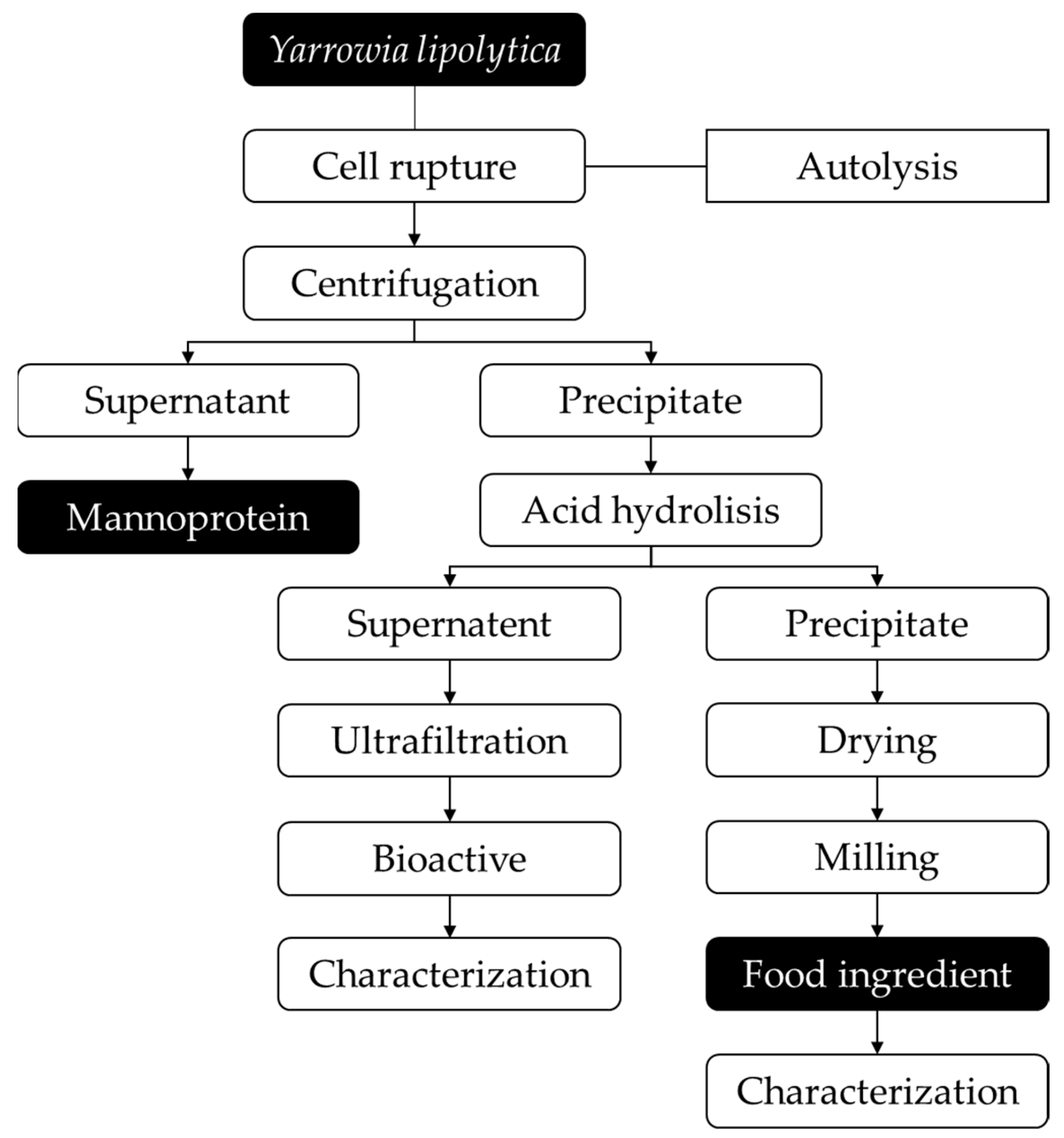
2.3. Two-Step Cell Wall Disruption of Yarrowia lipolytica IMUFRJ 50682
2.4. Mannoprotein Extraction and Characterization
2.4.1. Surface Tension (ST) and Emulsification Index (EI)
2.4.2. Thermogravimetric Analysis (TGA)
2.4.3. Fourier Transform Infrared Spectroscopy (FTIR)
2.4.4. Total Carbohydrate and Protein Determination
2.4.5. Determination of Total Glucan, β-Glucan, and α-Glucan
2.4.6. Optical Microscopy
2.5. Technological Properties
2.5.1. Water Holding Capacity (WHC) and the Index of Water Solubility (IWS)
2.5.2. Oil-Binding Capacity
2.6. Ultrafiltration
2.7. Antioxidant Properties
2.8. Proximal Composition
2.9. Statistical Analyses
3. Results and Discussion
3.1. Obtaining Mannoproteins from Yarrowia lipolytica IMUFRJ 50682 Autolysis
3.2. Characterization of the Soluble Fraction Rich in Mannoproteins
3.2.1. Composition, Surface Tension, and Emulsification Property
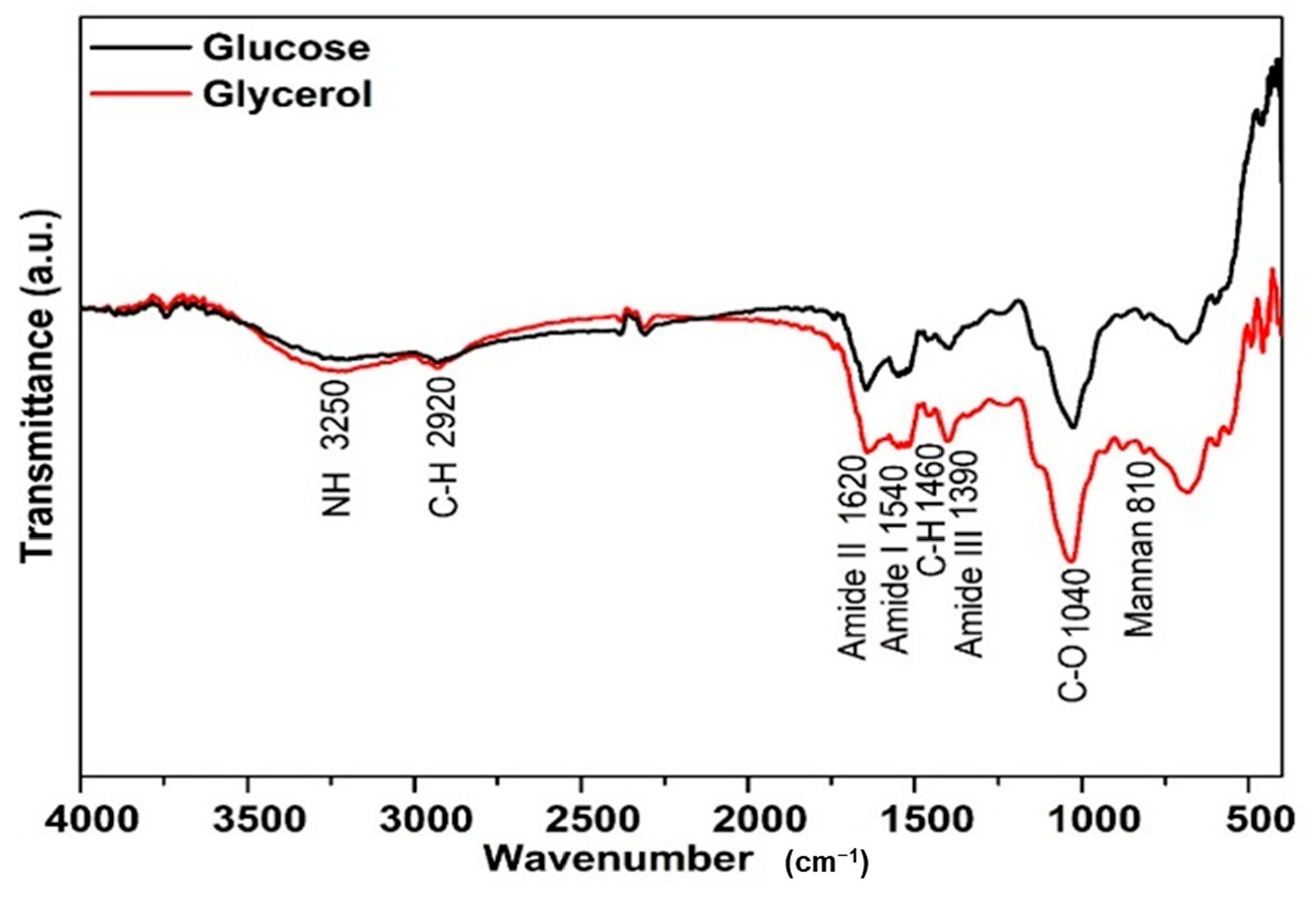
3.2.2. FTIR Structural Characterization of Mannoprotein
3.2.3. Thermogravimetric Characterization of Mannoprotein
3.3. Characterization of Crude Biomass and Food Ingredient
3.3.1. Chemical Composition and Technological Properties of Crude Biomass and Food Ingredient
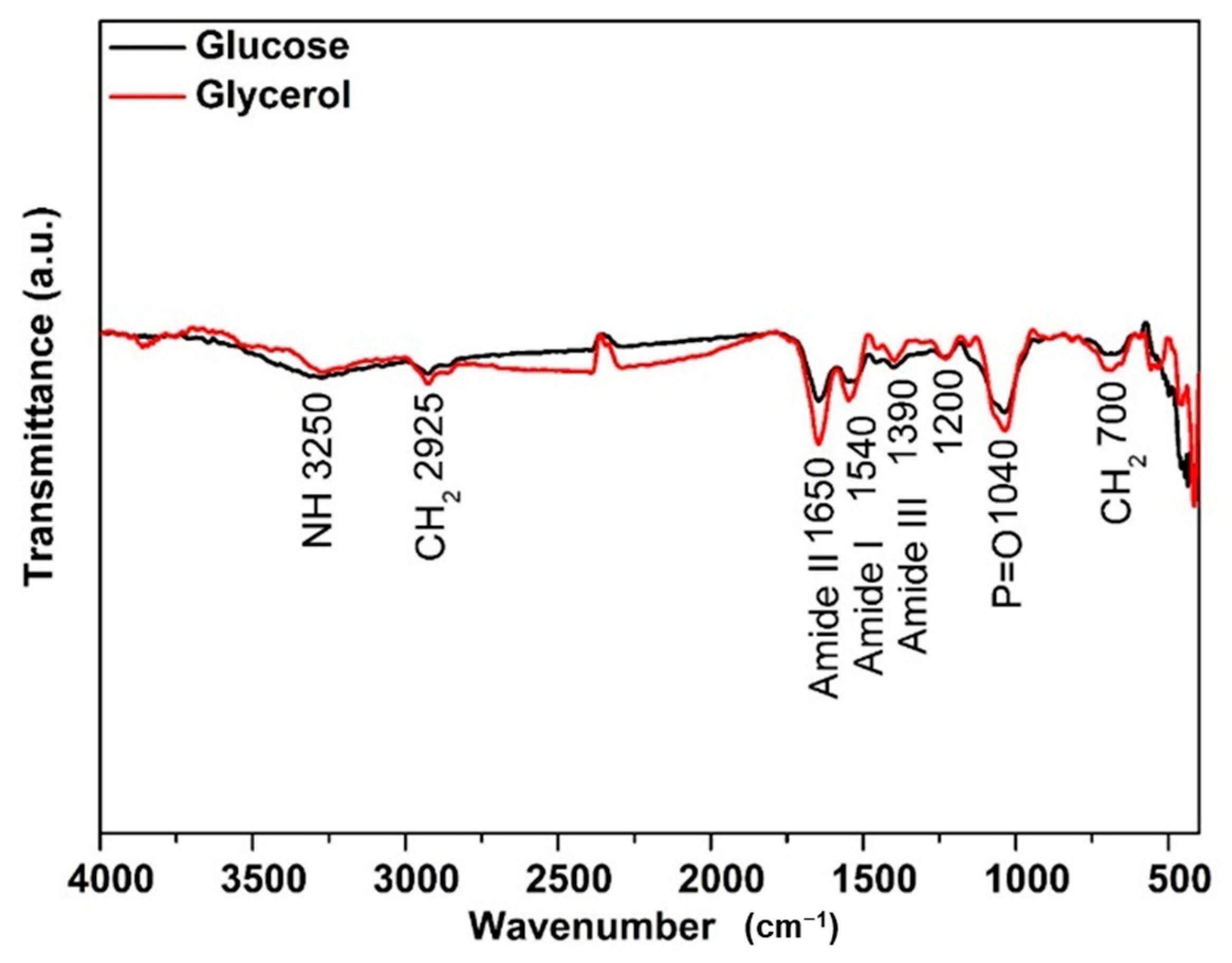
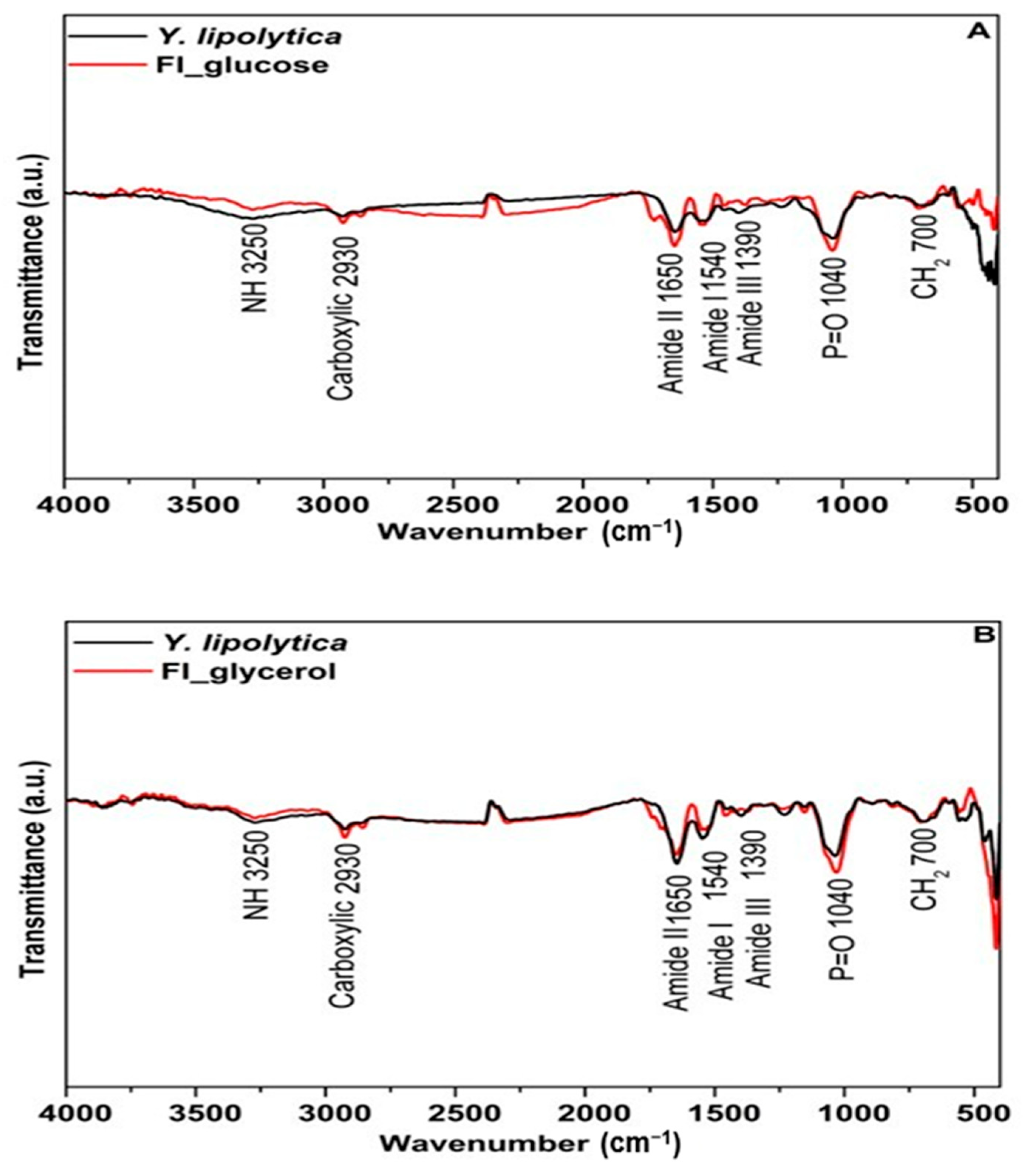
3.3.2. FTIR Structural Characterization of Yarrowia lipolytica Biomass and Its Respective Food Ingredient
3.3.3. Optical Microscopy of Yarrowia lipolytica Biomass and Its Respective Food Ingredient
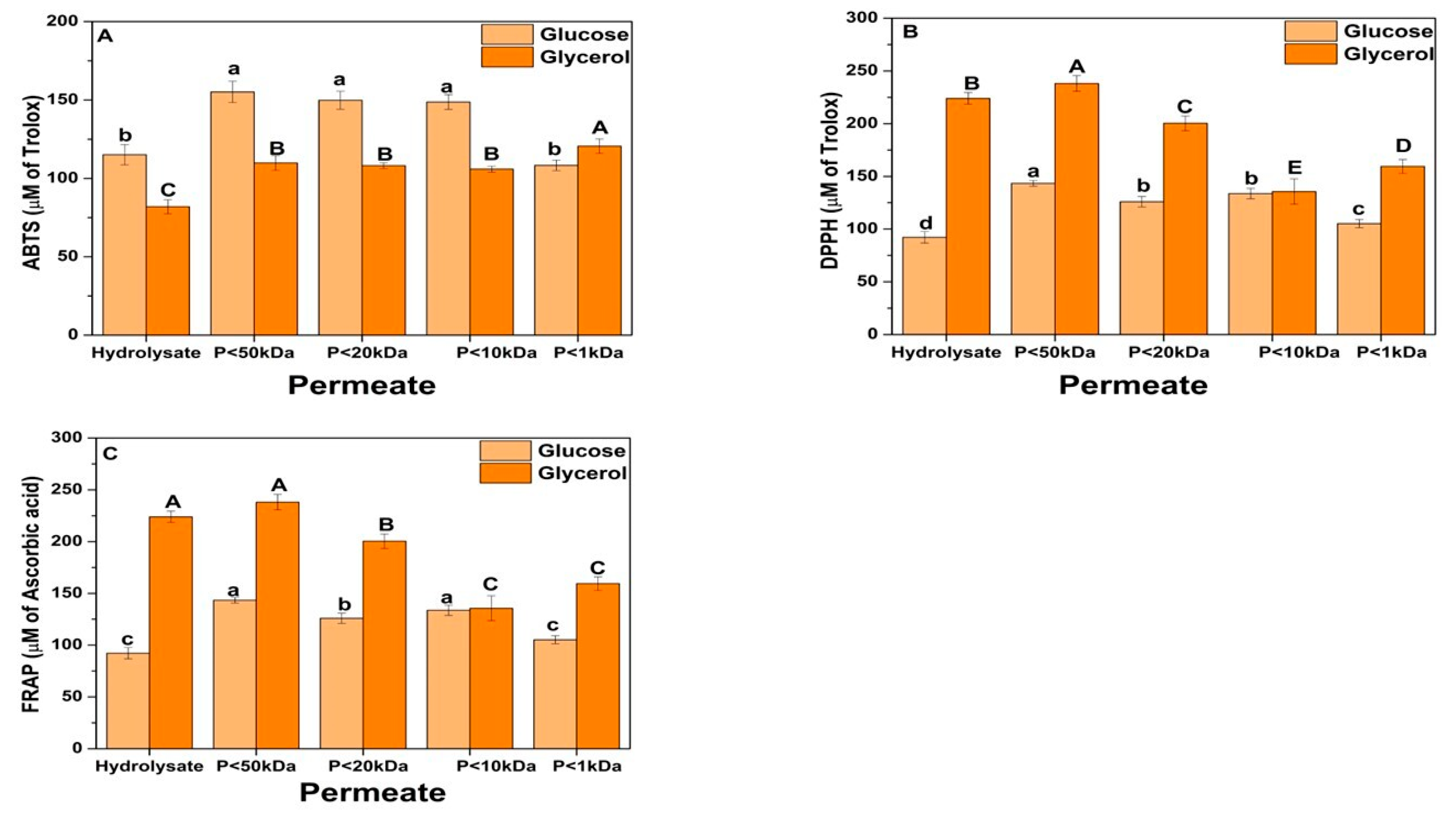
3.4. Ultrafiltration of Y. lipolytica Biomass Subjected to Sequential Hydrolysis
3.4.1. Composition and Antioxidant Activity
3.4.2. Antioxidant Property of Soluble Fraction Separated by Ultrafiltration
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Campos, A.L.B.M.A.; Nascimento, F.V.d.; Secchi, A.R.; Coelho, M.A.Z. Phenomenological modeling of polyols, citric acid and bio-oil concurrent production by Yarrowia lipolytica from glycerol. Clean. Chem. Eng. 2023, 5, 100100. [Google Scholar] [CrossRef]
- Silva, J.R.; Souza, C.E.C.; Valoni, E.; Castro, A.M.; Coelho, M.A.Z.; Ribeiro, B.D.; Henriques, C.A.; Langone, M.A.P. Biocatalytic esterification of fatty acids using a low-cost fermented solid from solid-state fermentation with Yarrowia lipolytica. 3 Biotech 2019, 9, 38. [Google Scholar] [CrossRef] [PubMed]
- Sales, J.C.S.; de Castro, A.M.; Ribeiro, B.D.; Coelho, M.A.Z. Improved production of biocatalysts by Yarrowia lipolytica using natural sources of the biopolyesters cutin and suberin, and their application in hydrolysis of poly (ethylene terephthalate) (PET). Bioprocess Biosyst. Eng. 2021, 44, 2277–2287. [Google Scholar] [CrossRef] [PubMed]
- Fontes, G.C.; Amaral, P.F.; Nele, M.; Coelho, M.A. Factorial design to optimize biosurfactant production by Yarrowia lipolytica. J. Biomed. Biotechnol. 2010, 2010, 821306. [Google Scholar] [CrossRef] [PubMed]
- Sales, J.C.S.; Botelho, A.M.; Carvalho, A.S.S.; Giudicelli, L.; Castro, A.M.; Ribeiro, B.D.; Amaral, P.F.F.; Coelho, M.A.Z. Evaluation of Yarrowia lipolytica potential for the biodegradation of poly(ethylene terephthalate) (PET) from mooring lines of Oil & Gas offshore platforms. Clean. Chem. Eng. 2023, 7, 100109. [Google Scholar] [CrossRef]
- Santos, F.; Freitas, K.; Pereira, A.; Fontes, G.; Rocha-Leão, M.H.; Amaral, P. Butter whey and corn steep liquor as sole raw materials to obtain a bioemulsifier from Yarrowia lipolytica for food oil-in-water emulsions. Ciênc. Rural 2021, 51, e20200323. [Google Scholar] [CrossRef]
- Souza, C.E.C.; Farias, M.A.; Ribeiro, B.D.; Coelho, M.A.Z. Adding Value to Agro-industrial Co-products from Canola and Soybean Oil Extraction through Lipase Production Using Yarrowia lipolytica in Solid-State Fermentation. Waste Biomass Valoriz. 2017, 8, 1163–1176. [Google Scholar] [CrossRef]
- Park, Y.-K.; Ledesma-Amaro, R. What makes Yarrowia lipolytica well suited for industry? Trends Biotechnol. 2022, 41, 242–254. [Google Scholar] [CrossRef]
- Vega, R.; Domínguez, A. Cell wall composition of the yeast and mycelial forms of Yarrowia lipolytica. Arch. Microbiol. 1986, 144, 124–130. [Google Scholar] [CrossRef]
- Zainuddin, M.F.; Fai, C.K.; Ariff, A.B.; Rios-Solis, L.; Halim, M. Current Pretreatment/Cell Disruption and Extraction Methods Used to Improve Intracellular Lipid Recovery from Oleaginous Yeasts. Microorganisms 2021, 9, 251. [Google Scholar] [CrossRef]
- Aguilar-Uscanga, B.; François, J.M. A study of the yeast cell wall composition and structure in response to growth conditions and mode of cultivation. Lett. Appl. Microbiol. 2003, 37, 268–274. [Google Scholar] [CrossRef]
- Ferreira, T.; Andrade, L.; Coelho, M.A.; Rocha-Leão, M.H. A new method to obtain β-glucan from Saccharomyces cerevisiae cells. Catal. Sci. Technol. 2011, 1, 1068–1071. [Google Scholar] [CrossRef]
- Ramos-Viana, V.; Møller-Hansen, I.; Kempen, P.; Borodina, I. Modulation of the cell wall protein Ecm33p in yeast Saccharomyces cerevisiae improves the production of small metabolites. FEMS Yeast Res. 2022, 22, foac037. [Google Scholar] [CrossRef]
- Gautério, G.V.; Silvério, S.I.D.C.; Egea, M.B.; Lemes, A.C. β-glucan from brewer’s spent yeast as a techno-functional food ingredient. Front. Food Sci. Technol. 2022, 2, 1074505. [Google Scholar] [CrossRef]
- Groenewald, M.; Boekhout, T.; Neuvéglise, C.; Gaillardin, C.; van Dijck, P.W.; Wyss, M. Yarrowia lipolytica: Safety assessment of an oleaginous yeast with a great industrial potential. Crit. Rev. Microbiol. 2014, 40, 187–206. [Google Scholar] [CrossRef]
- Madzak, C. Yarrowia lipolytica Strains and Their Biotechnological Applications: How Natural Biodiversity and Metabolic Engineering Could Contribute to Cell Factories Improvement. J. Fungi 2021, 7, 548. [Google Scholar] [CrossRef]
- Turck, D.; Castenmiller, J.; de Henauw, S.; Hirsch-Ernst, K.-I.; Kearney, J.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; Pelaez, C.; et al. Safety of Yarrowia lipolytica yeast biomass as a novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 2019, 17, e05594. [Google Scholar] [CrossRef]
- Czech, A.; Merska-Kazanowska, M.; Katarzyna, O.; Zieba, G. Effect of the Use of Yarrowia lipolytica or Saccharomyces cerevisiae Yeast with a Probiotic in the Diet of Turkey Hens on Growth Performance and Gut Histology. Ann. Anim. Sci. 2020, 20, 1047–1063. [Google Scholar] [CrossRef]
- Qiao, Y.; Xia, C.; Liu, L.; Tang, L.; Wang, J.; Xu, C.; Wang, J.; Zhang, L.; Ye, X.; Huang, Y.; et al. Structural characterization and emulsifier property of yeast mannoprotein enzymatically prepared with a β-1,6-glucanase. LWT 2022, 168, 113898. [Google Scholar] [CrossRef]
- Assunção-Bicca, S.; Poncet-Legrand, C.; Williams, P.; Mekoue Nguela, J.; Doco, T.; Vernhet, A. Structural characteristics of Saccharomyces cerevisiae mannoproteins: Impact of their polysaccharide part. Carbohydr. Polym. 2022, 277, 118758. [Google Scholar] [CrossRef]
- Gonçalves, F.; Heyraud, A.; de Pinho, M.N.; Rinaudo, M. Characterization of White Wine Mannoproteins. J. Agric. Food Chem. 2002, 50, 6097–6101. [Google Scholar] [CrossRef] [PubMed]
- Martínez, J.M.; Cebrián, G.; Álvarez, I.; Raso, J. Release of Mannoproteins during Saccharomyces cerevisiae Autolysis Induced by Pulsed Electric Field. Front. Microbiol. 2016, 7, 1435. [Google Scholar] [CrossRef] [PubMed]
- Berzosa, A.; Delso, C.; Sanz, J.; Sánchez-Gimeno, C.; Raso, J. Sequential extraction of compounds of interest from yeast biomass assisted by pulsed electric fields. Front. Bioeng. Biotechnol. 2023, 11, 1197710. [Google Scholar] [CrossRef] [PubMed]
- Ganeva, V.; Angelova, B.; Galutzov, B.; Goltsev, V.; Zhiponova, M. Extraction of Proteins and Other Intracellular Bioactive Compounds From Baker’s Yeasts by Pulsed Electric Field Treatment. Front. Bioeng. Biotechnol. 2020, 8, 552335. [Google Scholar] [CrossRef] [PubMed]
- Storebakken, T.; Sørensen, M.; Bjerkeng, B.; Harris, J.; Monahan, P.; Hiu, S. Stability of astaxanthin from red yeast, Xanthophyllomyces dendrorhous, during feed processing: Effects of enzymatic cell wall disruption and extrusion temperature. Aquaculture 2004, 231, 489–500. [Google Scholar] [CrossRef]
- Marson, G.V.; Machado, M.T.d.C.; Castro, R.J.S.; Hubinger, M.D. Sequential hydrolysis of spent brewer’s yeast improved its physico-chemical characteristics and antioxidant properties: A strategy to transform waste into added-value biomolecules. Process Biochem. 2019, 84, 91–102. [Google Scholar] [CrossRef]
- Marson, G.V.; de Castro, R.J.S.; Belleville, M.-P.; Hubinger, M.D. Spent brewer’s yeast as a source of high added value molecules: A systematic review on its characteristics, processing and potential applications. World J. Microbiol. Biotechnol. 2020, 36, 95. [Google Scholar] [CrossRef]
- Bertolo, A.P.; Biz, A.P.; Kempka, A.P.; Rigo, E.; Cavalheiro, D. Yeast (Saccharomyces cerevisiae): Evaluation of cellular disruption processes, chemical composition, functional properties and digestibility. J. Food Sci. Technol. 2019, 56, 3697–3706. [Google Scholar] [CrossRef] [PubMed]
- Imatoukene, N.; Koubaa, M.; Perdrix, E.; Benali, M.; Vorobiev, E. Combination of cell disruption technologies for lipid recovery from dry and wet biomass of Yarrowia lipolytica and using green solvents. Process Biochem. 2020, 90, 139–147. [Google Scholar] [CrossRef]
- Kosel, J.; Šuštaršič, M.; Petkovšek, M.; Zupanc, M.; Sežun, M.; Dular, M. Application of (super)cavitation for the recycling of process waters in paper producing industry. Ultrason. Sonochem. 2020, 64, 105002. [Google Scholar] [CrossRef]
- Šarc, A.; Kosel, J.; Stopar, D.; Oder, M.; Dular, M. Removal of bacteria Legionella pneumophila, Escherichia coli, and Bacillus subtilis by (super)cavitation. Ultrason. Sonochem. 2018, 42, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Boutros, J.A.; Magee, A.S.; Cox, D. Comparison of structural differences between yeast β-glucan sourced from different strains of saccharomyces cerevisiae and processed using proprietary manufacturing processes. Food Chem. 2022, 367, 130708. [Google Scholar] [CrossRef]
- Schiavone, M.; Vax, A.; Formosa, C.; Martin-Yken, H.; Dague, E.; François, J.M. A combined chemical and enzymatic method to determine quantitatively the polysaccharide components in the cell wall of yeasts. FEMS Yeast Res. 2014, 14, 933–947. [Google Scholar] [CrossRef]
- Alves, E.M.; Souza, J.F.d.; Oliva Neto, P.d. Advances in yeast autolysis technology—A faster and safer new bioprocess. Braz. J. Food Technol. 2021, 24, 1–14. [Google Scholar] [CrossRef]
- Martínez, J.M.; Delso, C.; Aguilar, D.; Cebrián, G.; Álvarez, I.; Raso, J. Factors influencing autolysis of Saccharomyces cerevisiae cells induced by pulsed electric fields. Food Microbiol. 2018, 73, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Gautério, G.V.; da Silva, R.M.; Karraz, F.C.; Coelho, M.A.Z.; Ribeiro, B.D.; Lemes, A.C. Cell disruption and permeabilization methods for obtaining yeast bioproducts. Clean. Chem. Eng. 2023, 6, 100112. [Google Scholar] [CrossRef]
- San Martin, D.; Ibarruri, J.; Iñarra, B.; Luengo, N.; Ferrer, J.; Alvarez-Ossorio, C.; Bald, C.; Gutierrez, M.; Zufía, J. Valorisation of Brewer’s Spent Yeasts’ Hydrolysates as High-Value Bioactive Molecules. Sustainability 2021, 13, 6520. [Google Scholar] [CrossRef]
- Alves, E.M.; Souza, J.F.; Macieja, S.; Oliva-Neto, P. 5′-Ribonucleotides production using 5′-phosphodiesterase from spent malt roots. Braz. J. Food Technol. 2021, 24, e2020246. [Google Scholar] [CrossRef]
- Marson, G.V.; Pereira, D.T.V.; da Costa Machado, M.T.; Di Luccio, M.; Martínez, J.; Belleville, M.-P.; Hubinger, M.D. Ultrafiltration performance of spent brewer’s yeast protein hydrolysate: Impact of pH and membrane material on fouling. J. Food Eng. 2021, 302, 110569. [Google Scholar] [CrossRef]
- Vollet Marson, G.; Belleville, M.-P.; Lacour, S.; Dupas Hubinger, M. Membrane Fractionation of Protein Hydrolysates from By-Products: Recovery of Valuable Compounds from Spent Yeasts. Membranes 2021, 11, 23. [Google Scholar] [CrossRef]
- Silva, L.V.; Tavares, C.; Amaral, P.; Coelho, M.A. Production of Citric Acid by Yarrowia lipolytica in Different Crude Glycerol Concentrations and in Different Nitrogen Sources. Chem. Eng. Trans. 2012, 27, 199–204. [Google Scholar]
- Faustino, M.; Durão, J.; Pereira, C.F.; Oliveira, A.S.; Pereira, J.O.; Pereira, A.M.; Ferreira, C.; Pintado, M.E.; Carvalho, A.P. Comparative Analysis of Mannans Extraction Processes from Spent Yeast Saccharomyces cerevisiae. Foods 2022, 11, 3753. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- McCleary, B.V.; Draga, A. Measurement of β-Glucan in Mushrooms and Mycelial Products. J. AOAC Int. 2016, 99, 364–373. [Google Scholar] [CrossRef]
- Marson, G.V.; Lacour, S.; Hubinger, M.D.; Belleville, M.-P. Serial fractionation of spent brewer’s yeast protein hydrolysate by ultrafiltration: A peptide-rich product with low RNA content. J. Food Eng. 2022, 312, 110737. [Google Scholar] [CrossRef]
- Lemes, A.C.; Molon, F.d.O.; Fagundes, A.d.S.; Egea, M.B.; Di Luccio, M.; Kalil, S.J. Membrane Technology as a Strategy for Improving β-Galactosidase Concentration Processes: The Influence of the pH, Membrane Molecular Weight, Pressure, and Ionic Strength in the Process. Appl. Sci. 2023, 13, 1626. [Google Scholar] [CrossRef]
- Rufino, M.d.S.M.; Alves, R.E.; de Brito, E.S.; Pérez-Jiménez, J.; Saura-Calixto, F.; Mancini-Filho, J. Bioactive compounds and antioxidant capacities of 18 non-traditional tropical fruits from Brazil. Food Chem. 2010, 121, 996–1002. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International; Association of Official Analytical Chemists: Washington, DC, USA, 1995. [Google Scholar]
- Liu, H.; Li, Y.; Shi, A.; Hu, H.; Sheng, X.; Liu, L.; Wang, Q.; Adhikari, B. Rheological characteristics and chain conformation of mannans obtained from Saccharomyces cerevisiae. Int. J. Biol. Macromol. 2018, 107, 2404–2411. [Google Scholar] [CrossRef]
- Dimopoulos, G.; Limnaios, A.; Aerakis, E.; Andreou, V.; Taoukis, P. Effect of high pressure on the proteolytic activity and autolysis of yeast Saccharomyces cerevisiae. Innov. Food Sci. Emerg. Technol. 2021, 74, 102865. [Google Scholar] [CrossRef]
- Ribeiro, R.A.; Bourbon-Melo, N.; Sá-Correia, I. The cell wall and the response and tolerance to stresses of biotechnological relevance in yeasts. Front. Microbiol. 2022, 13, 953479. [Google Scholar] [CrossRef]
- Klis, F.M.; Mol, P.; Hellingwerf, K.; Brul, S. Dynamics of cell wall structure in Saccharomyces cerevisiae. FEMS Microbiol. Rev. 2002, 26, 239–256. [Google Scholar] [CrossRef]
- Cermeño, M.; Felix, M.; Connolly, A.; Brennan, E.; Coffey, B.; Ryan, E.; FitzGerald, R.J. Role of carbohydrate conjugation on the emulsification and antioxidant properties of intact and hydrolysed whey protein concentrate. Food Hydrocoll. 2019, 88, 170–179. [Google Scholar] [CrossRef]
- Ereifej, K.I.; Rababah, T.M.; Al-Rababah, M.A. Quality Attributes of Halva by Utilization of Proteins, Non-hydrogenated Palm Oil, Emulsifiers, Gum Arabic, Sucrose, and Calcium Chloride. Int. J. Food Prop. 2005, 8, 415–422. [Google Scholar] [CrossRef]
- Herceg, Z.; Režek, A.; Lelas, V.; Krešić, G.; Franetović, M. Effect of carbohydrates on the emulsifying, foaming and freezing properties of whey protein suspensions. J. Food Eng. 2007, 79, 279–286. [Google Scholar] [CrossRef]
- Reis, S.F.; Fernandes, P.A.R.; Martins, V.J.; Gonçalves, S.; Ferreira, L.P.; Gaspar, V.M.; Figueira, D.; Castelo-Branco, D.; Mano, J.F.; Coimbra, M.A.; et al. Brewer’s Spent Yeast Cell Wall Polysaccharides as Vegan and Clean Label Additives for Mayonnaise Formulation. Molecules 2023, 28, 3540. [Google Scholar] [CrossRef]
- Lopes, M.; Miranda, S.M.; Costa, A.R.; Pereira, A.S.; Belo, I. Yarrowia lipolytica as a biorefinery platform for effluents and solid wastes valorization-challenges and opportunities. Crit. Rev. Biotechnol. 2022, 42, 163–183. [Google Scholar] [CrossRef]
- Araújo, V.B.d.S.; Melo, A.N.F.d.; Costa, A.G.; Castro-Gomez, R.H.; Madruga, M.S.; Souza, E.L.d.; Magnani, M. Followed extraction of β-glucan and mannoprotein from spent brewer’s yeast (Saccharomyces uvarum) and application of the obtained mannoprotein as a stabilizer in mayonnaise. Innov. Food Sci. Emerg. Technol. 2014, 23, 164–170. [Google Scholar] [CrossRef]
- Silva, J.F.d.; Silva, L.A.R.d.; Barbosa, M.R.; Houllou, L.M.; Malafaia, C.B. Bioemulsifier produced by Yarrowia lipolytica using residual glycerol as a carbon source. J. Environ. Anal. Prog. 2020, 5, 031–037. [Google Scholar] [CrossRef]
- Sumiardi, A.; Soetarto, E.S.; Susilaningsih, D. Screening and characterization of biosurfactant produced by bacterial consortium in degrading polycyclic aromatic hydrocarbon compound. AIP Conf. Proc. 2002, 2002, 1–9. [Google Scholar] [CrossRef]
- Chmielarz, M.; Sampels, S.; Blomqvist, J.; Brandenburg, J.; Wende, F.; Sandgren, M.; Passoth, V. FT-NIR: A tool for rapid intracellular lipid quantification in oleaginous yeasts. Biotechnol. Biofuels 2019, 12, 169. [Google Scholar] [CrossRef] [PubMed]
- Shapaval, V.; Brandenburg, J.; Blomqvist, J.; Tafintseva, V.; Passoth, V.; Sandgren, M.; Kohler, A. Biochemical profiling, prediction of total lipid content and fatty acid profile in oleaginous yeasts by FTIR spectroscopy. Biotechnol. Biofuels 2019, 12, 140. [Google Scholar] [CrossRef]
- Wan, M.; Wang, M.; Zhao, Y.; Deng, H.; Tan, C.; Lin, S.; Kong, Y.; Tong, Y.; Meng, X. Extraction of mannoprotein from Saccharomyces cerevisiae and analysis of its chemical composition and molecular structure. Int. J. Biol. Macromol. 2021, 193, 2252–2259. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.-W.; Fu, J.-J.; Li, J.-J.; Tang, Y.; Shao, Z.-W.; Zhou, D.-Y.; Song, L. Efficient encapsulation of curcumin into spent brewer’s yeast using a pH-driven method. Food Chem. 2022, 394, 133537. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Tian, J.; Zhou, Z.; Pan, Y.; Li, Z. Antioxidant activity and interactions between whey protein and polysaccharides from different parts of Houttuynia cordata. Front. Nutr. 2023, 10, 1020328. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-H.; Chu, Y.-S.; Liu, J.-L.; Chang, J.-S. Thermal degradation of carbohydrates, proteins and lipids in microalgae analyzed by evolutionary computation. Energy Convers. Manag. 2018, 160, 209–219. [Google Scholar] [CrossRef]
- Erian, A.M.; Egermeier, M.; Marx, H.; Sauer, M. Insights into the glycerol transport of Yarrowia lipolytica. Yeast 2022, 39, 323–336. [Google Scholar] [CrossRef]
- Bzducha-Wróbel, A.; Kieliszek, M.; Błażejak, S. Chemical composition of the cell wall of probiotic and brewer’s yeast in response to cultivation medium with glycerol as a carbon source. Eur. Food Res. Technol. 2013, 237, 489–499. [Google Scholar] [CrossRef]
- Guardiola, F.A.; Esteban, M.; Angulo, C. Yarrowia lipolytica, health benefits for animals. Appl. Microbiol. Biotechnol. 2021, 105, 7577–7592. [Google Scholar] [CrossRef]
- Zlatanović, S.; Kalušević, A.; Micić, D.; Laličić-Petronijević, J.; Tomić, N.; Ostojić, S.; Gorjanović, S. Functionality and Storability of Cookies Fortified at the Industrial Scale with up to 75% of Apple Pomace Flour Produced by Dehydration. Foods 2019, 8, 561. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, Y.; Zhou, R.; Liu, D.; Xu, X.; Zhou, G. Water-soluble myofibrillar proteins prepared by high-pressure homogenisation: A comparison study on the composition and functionality. Int. J. Food Sci. Technol. 2017, 52, 2334–2342. [Google Scholar] [CrossRef]
- Rios, R.V.; Pessanha, M.D.F.; Almeida, P.F.d.; Viana, C.L.; Lannes, S.C.d.S. Application of fats in some food products. Food Sci. Technol. 2014, 34, 3–15. [Google Scholar] [CrossRef]
- Jach, M.E.; Malm, A. Yarrowia lipolytica as an Alternative and Valuable Source of Nutritional and Bioactive Compounds for Humans. Molecules 2022, 27, 2300. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Bassart, Z.; Fabra, M.J.; Martínez-Abad, A.; López-Rubio, A. Compositional differences of β-glucan-rich extracts from three relevant mushrooms obtained through a sequential extraction protocol. Food Chem. 2023, 402, 134207. [Google Scholar] [CrossRef] [PubMed]
- Kurek, M.A.; Wyrwisz, J.; Brzeska, M.; Moczkowska, M.; Karp, S.; Wierzbicka, A. Effect of different beta-glucan preparation pretreatments on fortified bread quality. Food Sci. Technol. 2018, 38, 606–611. [Google Scholar] [CrossRef]
- Szczepańska, P.; Rychlicka, M.; Moroz, P.; Janek, T.; Gliszczyńska, A.; Lazar, Z. Elevating Phospholipids Production Yarrowia lipolytica from Crude Glycerol. Int. J. Mol. Sci. 2022, 23, 10737. [Google Scholar] [CrossRef] [PubMed]
- Chotigavin, N.; Sriphochanart, W.; Yaiyen, S.; Kudan, S. Increasing the Production of β-Glucan from Saccharomyces carlsbergensis RU01 by Using Tannic Acid. Appl. Biochem. Biotechnol. 2021, 193, 2591–2601. [Google Scholar] [CrossRef] [PubMed]
- Yoshimi, A.; Miyazawa, K.; Abe, K. Function and Biosynthesis of Cell Wall α-1,3-Glucan in Fungi. J. Fungi 2017, 3, 63. [Google Scholar] [CrossRef]
- Rakicka, M.; Lazar, Z.; Dulermo, T.; Fickers, P.; Nicaud, J.M. Lipid production by the oleaginous yeast Yarrowia lipolytica using industrial by-products under different culture conditions. Biotechnol. Biofuels 2015, 8, 104. [Google Scholar] [CrossRef]
- Pereyra, C.M.; Gil, S.; Cristofolini, A.; Bonci, M.; Makita, M.; Monge, M.P.; Montenegro, M.A.; Cavaglieri, L.R. The production of yeast cell wall using an agroindustrial waste influences the wall thickness and is implicated on the aflatoxin B1 adsorption process. Food Res. Int. 2018, 111, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Snyman, C.; Mekoue Nguela, J.; Sieczkowski, N.; Marangon, M.; Divol, B. Optimised Extraction and Preliminary Characterisation of Mannoproteins from Non-Saccharomyces Wine Yeasts. Foods 2021, 10, 924. [Google Scholar] [CrossRef] [PubMed]
- Pancrazio, G.; Cunha, S.C.; de Pinho, P.G.; Loureiro, M.; Meireles, S.; Ferreira, I.M.P.L.V.O.; Pinho, O. Spent brewer’s yeast extract as an ingredient in cooked hams. Meat Sci. 2016, 121, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Jach, M.E.; Serefko, A.; Ziaja, M.; Kieliszek, M. Yeast Protein as an Easily Accessible Food Source. Metabolites 2022, 12, 63. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, B.F.; Aranha, J.B.; Vieira, T.M.F.d.S. Replacing synthetic antioxidants in food emulsions with microparticles from green acerola (Malpighia emarginata). Future Foods 2022, 5, 100130. [Google Scholar] [CrossRef]
- Pereira, A.S.; Sant’Ana, G.C.F.; Amaral, P.F.F. Mango agro-industrial wastes for lipase production from Yarrowia lipolytica and the potential of the fermented solid as a biocatalyst. Food Bioprod. Process. 2019, 115, 68–77. [Google Scholar] [CrossRef]
- He, S.; Zhang, Y.; Sun, H.; Du, M.; Qiu, J.; Tang, M.; Sun, X.; Zhu, B. Antioxidative Peptides from Proteolytic Hydrolysates of False Abalone (Volutharpa ampullacea perryi): Characterization, Identification, and Molecular Docking. Mar. Drugs 2019, 17, 116. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Chen, D.; Mao, X.; He, J.; Yu, B.; Cheng, L.; Zeng, D. Purified β-glucans of Different Molecular Weights Enhance Growth Performance of LPS-challenged Piglets via Improved Gut Barrier Function and Microbiota. Animals 2019, 9, 602. [Google Scholar] [CrossRef] [PubMed]
- Vasilieva, T.; Sigarev, A.; Kosyakov, D.; Ul’yanovskii, N.; Anikeenko, E.; Chuhchin, D.; Ladesov, A.; Hein, A.M.; Miasnikov, V. Formation of low molecular weight oligomers from chitin and chitosan stimulated by plasma-assisted processes. Carbohydr. Polym. 2017, 163, 54–61. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef]
- Alcolea, J.F.; Cano, A.; Acosta, M.; Arnao, M.B. Hydrophilic and lipophilic antioxidant activities of grapes. Die Nahr. 2002, 46, 353–356. [Google Scholar] [CrossRef]
- Machová, E.; Bystrický, S. Antioxidant capacities of mannans and glucans are related to their susceptibility of free radical degradation. Int. J. Biol. Macromol. 2013, 61, 308–311. [Google Scholar] [CrossRef] [PubMed]
- Patterson, R.; Rogiewicz, A.; Kiarie, E.G.; Slominski, B.A. Yeast derivatives as a source of bioactive components in animal nutrition: A brief review. Front. Vet. Sci. 2023, 9, 1067383. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wang, Y.; Xu, M.; Ding, L.; Zhang, T.; Liu, J. Antioxidant Synergetic Effect Between the Peptides Derived from the Egg White Pentapeptide Trp-Asn-Trp-Ala-Asp. Int. J. Pept. Res. Ther. 2017, 23, 509–518. [Google Scholar] [CrossRef]
- Jia, X.Y.; Zhu, M.F.; Zhang, L.; Ma, T.X.; Li, Y.H.; Sheng, W.S.; Tu, Z.C. Extraction optimization and screening of antioxidant peptides from grass carp meat and synergistic-antagonistic effect. Food Sci. Nutr. 2022, 10, 1481–1493. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Girgih, A.T.; Malomo, S.A.; Ju, X.; Aluko, R.E. Antioxidant activities of enzymatic rapeseed protein hydrolysates and the membrane ultrafiltration fractions. J. Funct. Foods 2013, 5, 219–227. [Google Scholar] [CrossRef]
- Wang, Y.-Z.; Zhao, Y.-Q.; Wang, Y.-M.; Zhao, W.-H.; Wang, P.; Chi, C.-F.; Wang, B. Antioxidant peptides from Antarctic Krill (Euphausia superba) hydrolysate: Preparation, identification and cytoprotection on H2O2-induced oxidative stress. J. Funct. Foods 2021, 86, 104701. [Google Scholar] [CrossRef]
- Cai, W.W.; Hu, X.M.; Wang, Y.M.; Chi, C.F.; Wang, B. Bioactive Peptides from Skipjack Tuna Cardiac Arterial Bulbs: Preparation, Identification, Antioxidant Activity, and Stability against Thermal, pH, and Simulated Gastrointestinal Digestion Treatments. Mar. Drugs 2022, 20, 626. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-M.; Li, X.-Y.; Wang, J.; He, Y.; Chi, C.-F.; Wang, B. Antioxidant peptides from protein hydrolysate of skipjack tuna milt: Purification, identification, and cytoprotection on H2O2 damaged human umbilical vein endothelial cells. Process Biochem. 2022, 113, 258–269. [Google Scholar] [CrossRef]
- Tadesse, S.A.; Emire, S.A. Production and processing of antioxidant bioactive peptides: A driving force for the functional food market. Heliyon 2020, 6, e04765. [Google Scholar] [CrossRef]
- Fakruddin, M.; Hossain, M.N.; Ahmed, M.M. Antimicrobial and antioxidant activities of Saccharomyces cerevisiae IFST062013, a potential probiotic. BMC Complement. Altern. Med. 2017, 17, 64. [Google Scholar] [CrossRef] [PubMed]



| Substrate | Carbohydrate (g/L) | Protein (g/L) |
|---|---|---|
| Glucose | 15.10 ± 0.34 b | 38.52 ± 0.38 b |
| Glycerol | 23.33 ± 1.70 a | 49.94 ± 0.42 a |
| Glucose | Glycerol | |
|---|---|---|
| Extraction yield (%) | 7.70 ± 0.10 b | 10.13 ± 0.10 a |
| Total Carbohydrate (g/L) | 14.65 ± 0.34 b | 19.63 ± 1.26 a |
| Total Protein (g/L) | 30.97 ± 1.44 a | 23.85 ± 1.62 b |
| Surface tension (mN/m) * | 44.50 ± 1.60 b | 49.21 ± 1.10 a |
| Emulsification index (%) * | 37.2 ± 1.60 b | 47.35 ± 4.73 a |
| Parameters | Glucose | Glycerol | ||
|---|---|---|---|---|
| Biomass (%) | Food Ingredient (%) | Biomass (%) | Food Ingredient (%) | |
| Process yield | 55.58 ± 2.54 a | - | 46.14 ± 2.84 b | - |
| WHC | 7.85 ± 0.30 a | 5.15 ± 0.49 b | 4.78 ± 0.18 b | 5.35 ± 0.18 b |
| IWS | 29.12 ± 1.27 a | 9.55 ± 0.61 c | 20.0 ± 1.37 b | 11.05 ± 0.89 c |
| OHC | 4.07 ± 0.97 a | 3.95 ± 0.89 a | 3.23 ± 0.2 a | 3.85 ± 0.21 a |
| Moisture | 4.33 ± 0.40 b | 5.58 ± 0.57 a | 5.90 ± 0.68 a | 5.30 ± 0.51 a |
| Total Soluble Solids | 95.67 ± 0.40 a | 94.42 ± 0.57 b | 94.19 ± 0.76 b | 94.70 ± 0.51 b |
| α-glucan (%) | 2.30 ± 0.02 b | 0.96 ± 0.02 d | 1.81 ± 0.02 c | 3.31 ± 0.15 a |
| β-glucan (%) | 14.13 ± 0.32 b | 11.96 ± 0.48 c | 19.31 ± 0.68 a | 12.74 ± 0.19 c |
| Total glucan (%) | 16.43 ± 0.30 b | 12.05 ± 0.49 c | 21.11 ± 0.66 a | 16.05 ± 0.09 b |
| Ash (%) | 5.63 ± 0.09 a | 1.12 ± 0.05 c | 5.73 ± 0.01 a | 0.66 ± 0.02 b |
| Carbohydrate (%) | 38.87 ± 1.15 c | 53.79 ± 1.38 b | 32.35 ± 0.15 d | 57.48 ± 0.72 a |
| Protein (%) | 45.70 ± 0.89 b | 37.63 ± 0.75 c | 48.92 ± 1.07 a | 33.60 ± 0.70 d |
| Lipid (%) | 5.47 ± 0.45 b | 2.30 ± 0.08 d | 9.34 ± 0.11 a | 2.96 ± 0.05 c |
| Caloric value (kcal) | 387.48 | 384.68 | 407.81 | 390.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, R.M.; Ribeiro, B.D.; Lemes, A.C.; Coelho, M.A.Z. Recovery of High-Value Compounds from Yarrowia lipolytica IMUFRJ 50682 Using Autolysis and Acid Hydrolysis. Processes 2024, 12, 1132. https://doi.org/10.3390/pr12061132
da Silva RM, Ribeiro BD, Lemes AC, Coelho MAZ. Recovery of High-Value Compounds from Yarrowia lipolytica IMUFRJ 50682 Using Autolysis and Acid Hydrolysis. Processes. 2024; 12(6):1132. https://doi.org/10.3390/pr12061132
Chicago/Turabian Styleda Silva, Rhonyele Maciel, Bernardo Dias Ribeiro, Ailton Cesar Lemes, and Maria Alice Zarur Coelho. 2024. "Recovery of High-Value Compounds from Yarrowia lipolytica IMUFRJ 50682 Using Autolysis and Acid Hydrolysis" Processes 12, no. 6: 1132. https://doi.org/10.3390/pr12061132
APA Styleda Silva, R. M., Ribeiro, B. D., Lemes, A. C., & Coelho, M. A. Z. (2024). Recovery of High-Value Compounds from Yarrowia lipolytica IMUFRJ 50682 Using Autolysis and Acid Hydrolysis. Processes, 12(6), 1132. https://doi.org/10.3390/pr12061132









