Storage Duration and Added Docosahexaenoic Acid Modify the Rates of Esterified and Free Oxylipin Formation in Infant Milk Formula
Abstract
:1. Introduction
2. Materials and Methods
2.1. Infant Milk Formula Samples
2.2. Oxylipins Quantification
2.2.1. Folch Extraction
2.2.2. Sample Preparation and Hydrolysis
2.2.3. Liquid Chromatography–Mass Spectrometry (LC-MS/MS) Analysis
2.3. Kinetics Calculations
2.4. Statistical Analysis
3. Results and Discussion
3.1. Oxylipin Profile in Infant Milk Formula: Matrix Effect and Recovery
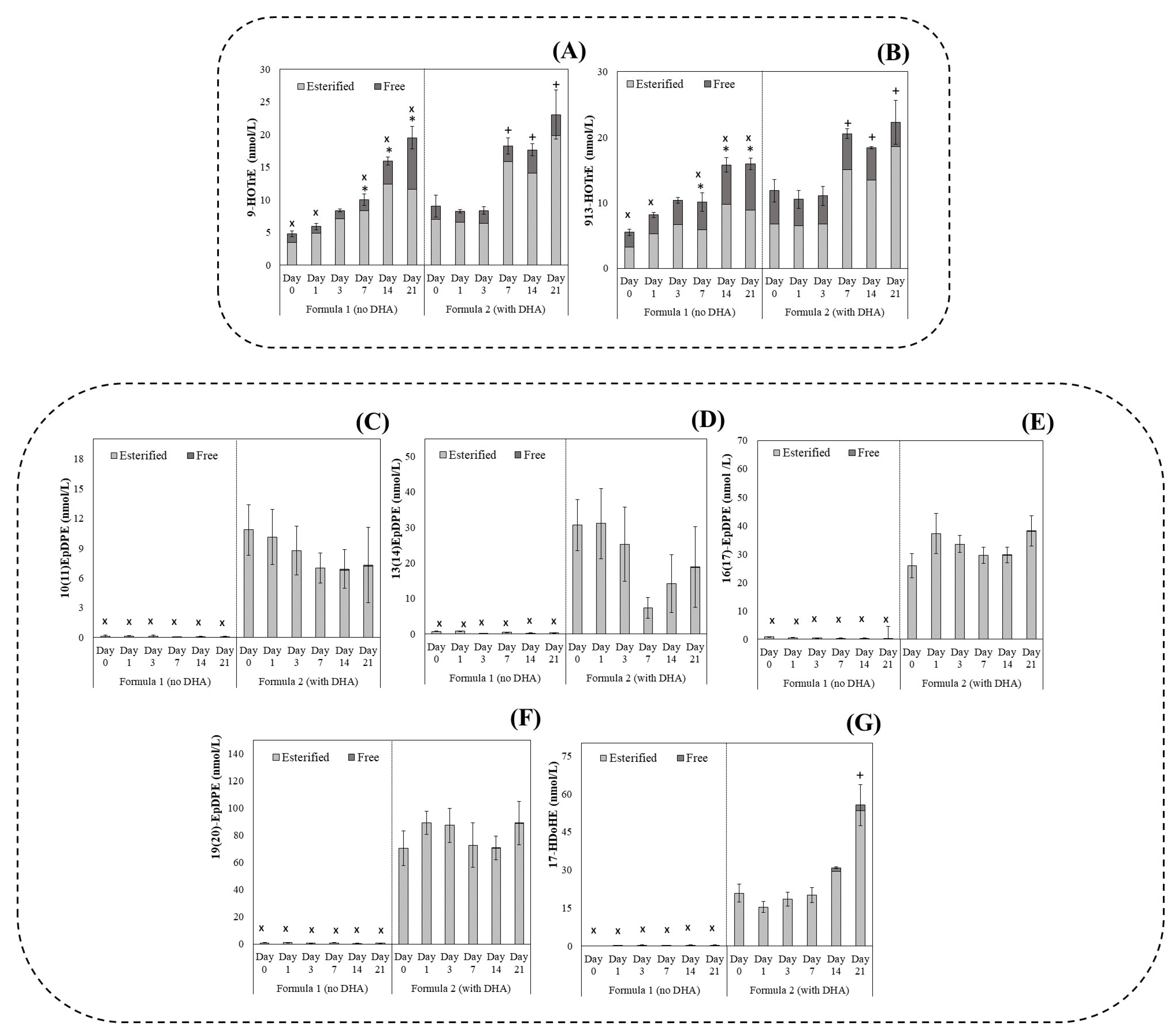
3.2. Oxylipin Profile in Infant Milk Formula: Effect of Lipid Composition and Storage Time under Refrigerated Conditions
3.3. Kinetics of LA-, ALA-, and DHA-Derived Oxylipin Formation in Infant Milk Formula under 21 Days of Cold Storage
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Koletzko, B.; Bergmann, K.; Brenna, J.T.; Calder, P.C.; Campoy, C.; Clandinin, M.T.; Colombo, J.; Daly, M.; Decsi, T.; Demmelmair, H.; et al. Should formula for infants provide arachidonic acid along with DHA? A position paper of the European Academy of Paediatrics and the Child Health Foundation. Am. J. Clin. Nutr. 2020, 111, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Decker, J.E.; Delahanty, M.T.; Davey, A.; Robson, S.M.; Trabulsi, J.C. Human Milk, Infant Formula, and Other Milks Fed to Infants and Toddlers in the United States, NHANES 2007–2018. J. Acad. Nutr. Diet. 2023, 123, 1320–1328.e3. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, B.; Baker, S.; Cleghorn, G.; Neto, U.F.; Gopalan, S.; Hernell, O.; Hock, Q.S.; Jirapinyo, P.; Lonnerdal, B.; Pencharz, P.; et al. Global standard for the composition of infant formula: Recommendations of an ESPGHAN coordinated international expert group. J. Pediatr. Gastroenterol. Nutr. 2005, 41, 584–599. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine (US) Committee on the Evaluation of the Addition of Ingredients New to Infant Formula. Infant Formula: Evaluating the Safety of New Ingredients; National Academies Press: Washington, DC, USA, 2004. [Google Scholar]
- Code of Federal Regulations Title 21. 21CFR106.3. US Food and Drug Administration, Department of Health and Human Services. Subchapter B—Food for Human Consumption. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr¼106.3 (accessed on 15 September 2023).
- Salas Lorenzo, I.; Chisaguano Tonato, A.M.; de la Garza Puentes, A.; Nieto, A.; Herrmann, F.; Dieguez, E.; Castellote, A.I.; López-Sabater, M.C.; Rodríguez-Palmero, M.; Campoy, C. The effect of an infant formula supplemented with AA and DHA on fatty acid levels of infants with different FADS genotypes: The COGNIS study. Nutrients 2019, 11, 602. [Google Scholar] [CrossRef] [PubMed]
- Koch, E.; Löwen, A.; Kampschulte, N.; Plitzko, N.; Wiebel, M.; Rund, K.M.; Willenberg, I.; Schebb, N.H. Beyond Autoxidation and Lipoxygenases: Fatty Acid Oxidation Products in Plant OilsBeyond Autoxidation and Lipoxygenases: Fatty Acid Oxidation Products in Plant Oils. J. Agric. Food Chem. 2023, 71, 13092–13106. [Google Scholar] [CrossRef] [PubMed]
- Angulo, A.J.; Romera, J.M.; Ramírez, M.; Gil, A. Effects of storage conditions on lipid oxidation in infant formulas based on several protein sources. J. Am. Oil Chem. Soc. 1998, 75, 1603–1607. [Google Scholar] [CrossRef]
- German, J.B. Food Processing and Lipid Oxidation. In Advances in Experimental Medicine and Biology; Springer: Boston, MA, USA, 1999; Volume 459, pp. 23–50. ISBN 978-1-4615-4853-9. [Google Scholar] [CrossRef]
- Shen, Q.; Zhang, Z.; Emami, S.; Chen, J.; de Moura Bell, J.M.L.N.; Taha, A.Y. Triacylglycerols are preferentially oxidized over free fatty acids in heated soybean oil. npj Sci. Food 2021, 5, 7. [Google Scholar] [CrossRef] [PubMed]
- Koch, E.; Kampschulte, N.; Schebb, N.H. Comprehensive Analysis of Fatty Acid and Oxylipin Patterns in n3-PUFA Supplements. J. Agric. Food Chem. 2022, 70, 3979–3988. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, B.F.; Dias, F.F.G.; Vieira, T.M.F.S.; de Moura Bell, J.M.L.N.; Taha, A.Y. Method optimization of oxylipin hydrolysis in nonprocessed bovine milk indicates that the majority of oxylipins are esterified. J. Food Sci. 2021, 86, 1791–1801. [Google Scholar] [CrossRef] [PubMed]
- Albert, B.B.; Cameron-Smith, D.; Hofman, P.L.; Cutfield, W.S. Oxidation of Marine Omega-Supplements and Human Health. Biomed Res. Int. 2013, 2013, 464921. [Google Scholar] [CrossRef] [PubMed]
- Daoud, S.; Bou-Maroun, E.; Waschatko, G.; Horemans, B.; Mestdagh, R.; Billecke, N.; Cayot, P. Detection of Lipid Oxidation in Infant Formulas: Application of Infrared Spectroscopy to Complex Food Systems. Foods 2020, 9, 1432. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, B.F.; Dias, F.F.G.; Vieira, T.M.F.S.; Taha, A.Y.; de Moura Bell, J.M.L.N. Early detection of lipid oxidation in infant milk formula by measuring free oxylipins—Comparison with hydroperoxide value and thiobarbituric acid reactive substance methods. J. Food Sci. 2022, 87, 5252–5262. [Google Scholar] [CrossRef] [PubMed]
- Schuchardt, J.P.; Hahn, A. Bioavailability of long-chain omega-3 fatty acids. Prostaglandins Leukot. Essent. Fat. Acids 2013, 89, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Van Eeckhaut, A.; Lanckmans, K.; Sarre, S.; Smolders, I.; Michotte, Y. Validation of bioanalytical LC–MS/MS assays: Evaluation of matrix effects. J. Chromatogr. B 2009, 877, 2198–2207. [Google Scholar] [CrossRef] [PubMed]
- Bioanalytical Method Validation, Office of Clinical Pharmacology in the Center for Drug Evaluation and Research and the Center for Veterinary Medicine at the Food and Drug Administration. 2018. Available online: fda.gov/media/70858/download (accessed on 15 September 2023).
- Zhou, W.; Yang, S.; Wang, P.G. Matrix effects and application of matrix effect factor. Bioanalysis 2017, 9, 1839–1844. [Google Scholar] [CrossRef] [PubMed]
- Amate, F.C.; Unterluggauer, H.; Fischer, R.J.; Fernández-Alba, A.R.; Masselter, S. Development and validation of a LC–MS/MS method for the simultaneous determination of aflatoxins, dyes and pesticides in spices. Anal. Bioanal. Chem. 2010, 397, 93–107. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.; Zu, H.; Wang, X. Effect of storage on n-3 PUFA-enriched eggs. CyTA—J. Food 2020, 18, 102–107. [Google Scholar] [CrossRef]


| Composition (%) | |||||
|---|---|---|---|---|---|
| Ingredient (%) | Water | Lipid | Maltodextrin | Milk Protein Concentrate | Lecithin |
| Formula (1) | 86.69 | 4 (100% canola oil) | 8 | 1.3 | 0.01 |
| Formula (2) | 86.69 | 4 (99.7% canola and 0.3% DHA ethyl ester | 8 | 1.3 | 0.01 |
| Compounds | Ion Suppression (%) | |
|---|---|---|
| LA-derived | Formula (1) | Formula (2) |
| 9-HODE | 92.6 | 88.7 |
| 13-HODE | 91.8 | 89.9 |
| 9-oxo-ODE | 87.5 | 93.9 |
| 13-oxo-ODE | 91.1 | 102.9 |
| 9(10)-EpOME | 89.8 | 93.6 |
| 12(13)-EpOME | 88.7 | 88.2 |
| 9,10-DiHOME | 98.3 | 94.8 |
| 12,13-DiHOME | 87.9 | 86.3 |
| 9,10,13-TriHOME | 91.4 | 91.4 |
| 9,12,13-TriHOME | 92.4 | 92.8 |
| ALA-derived | ||
| 9-HOTrE | 94.3 | 94.3 |
| 13-HOTrE | 93.3 | 89.4 |
| DHA-derived | ||
| 10(11)EpDPE | 94.0 | 93.7 |
| 13(14)EpDPE | 95.6 | 92.9 |
| 16(17)-EpDPE | 103.6 | 91 |
| 19(20)-EpDPE | 91.7 | 93.3 |
| 17-HDoHE | 90.5 | 101.7 |
| Compounds | Day 0 | Day 01 | Day 03 | Day 07 | Day 14 | Day 21 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LA-derived | F1 | F2 | F1 | F2 | F1 | F2 | F1 | F2 | F1 | F2 | F1 | F2 |
| 9-HODE | 88.95 ± 2.69 | 80.58 ± 6.73 | 83.17 ± 5.45 | 79.23 ± 3.86 | 85.28 ± 8.65 | 78.77 ± 9.16 | 84.91 ± 2.65 | 86.35 ± 0.55 | 86.2 ± 4.81 | 77.37 ± 13.44 | 88.33 ± 2.06 | 80.1 ± 4.92 |
| 13-HODE | 86.95 ± 2.69 | 81.58 ± 3.73 | 83.17 ± 5.45 | 79.23 ± 3.86 | 85.28 ± 8.65 | 78.77 ± 10.16 | 84.91 ± 2.65 | 86.35 ± 0.55 | 86.2 ± 4.81 | 78.37 ± 9.44 | 88.33 ± 2.06 | 80.29 ± 4.92 |
| 9-oxo-ODE | 82.16 ± 3.48 | 76.11 ± 6.3 | 76.89 ± 4.33 | 79.16 ± 5.33 | 82.94 ± 4.80 | 73.8 ± 8.28 | 85.09 ± 6.72 | 85.05 ± 4.85 | 83.37 ± 2.61 | 78.73 ± 8.02 | 85.77 ± 0.65 | 84.38 ± 4.12 |
| 13-oxo-ODE | 84.91 ± 8.23 | 78.35 ± 6.15 | 76.89 ± 4.33 | 77.33 ± 5.2 | 89.24 ± 16.91 | 75.13 ± 8.09 | 85.09 ± 6.72 | 83.08 ± 4.73 | 85.75 ± 2.49 | 77.04 ± 7.6 | 85.77 ± 0.65 | 82.43 ± 4.02 |
| 12(13)-EpOME | 76.6 ± 7.020 | 75.91 ± 4.56 | 77.48 ± 6.90 | 79.42 ± 7.27 | 75.66 ± 7.28 | 72.21 ± 4.21 | 78.97 ± 1.35 | 80.08 ± 0.46 | 82.7 ± 4.50 | 78.87 ± 8.07 | 83.9 ± 6.62 | 78.02 ± 2.84 |
| 9(10)-EpOME | 76.51 ± 9.11 | 75.3 ± 4.65 | 75.92 ± 7.04 | 75.77 ± 7.41 | 77.12 ± 7.42 | 77.97 ± 5.01 | 80.5 ± 1.38 | 81.63 ± 0.47 | 84.3 ± 4.59 | 78.2 ± 8.42 | 85.52 ± 6.75 | 79.67 ± 3.14 |
| 9,10-DiHOME | 94.44 ± 4.29 | 88.8 ± 6.09 | 85.21 ± 3.46 | 82.23 ± 3.58 | 88.8 ± 10.08 | 80.48 ± 9.6 | 90.51 ± 0.91 | 83.69 ± 4.93 | 88.03 ± 4.39 | 78.54 ± 7.75 | 89.79 ± 1.78 | 82.02 ± 4.89 |
| 12,13-DiHOME | 92.54 ± 4.21 | 90.62 ± 6.21 | 87.5 ± 3.39 | 83.92 ± 3.65 | 87.02 ± 9.88 | 82.13 ± 9.8 | 88.69 ± 0.89 | 85.41 ± 5.03 | 86.26 ± 4.3 | 80.15 ± 7.91 | 84.07 ± 3.76 | 83.7 ± 4.99 |
| 9,10,13-TriHOME | 85.99 ± 9.26 | 75.58 ± 5.99 | 83.14 ± 3.56 | 76.04 ± 4.52 | 78.03 ± 6.97 | 74.6 ± 5.67 | 78.14 ± 5.46 | 77.03 ± 5.52 | 78.64 ± 3.10 | 77.49 ± 5.29 | 74.87 ± 1.56 | 86.05 ± 4.69 |
| 9,12,13-TriHOME | 78.53 ± 3.56 | 74.52 ± 4.15 | 78.16 ± 7.38 | 79.1 ± 3.27 | 77.36 ± 6.52 | 79.99 ± 4.9 | 78.95 ± 4.12 | 77.72 ± 7.08 | 77.23 ± 1.32 | 75.04 ± 9.23 | 71.13 ± 1.96 | 78.55 ± 3.32 |
| ALA-derived | ||||||||||||
| 9-HOTrE | 88.95 ± 2.69 | 81.8 ± 3.93 | 83.17 ± 5.45 | 83.53 ± 4.07 | 85.28 ± 8.65 | 83.05 ± 13.87 | 85.4 ± 2.55 | 91.05 ± 6.58 | 86.2 ± 4.81 | 76.31 ± 14.17 | 88.33 ± 2.06 | 80.44 ± 4.14 |
| 13-HOTrE | 93.78 ± 2.84 | 77.58 ± 3.73 | 87.69 ± 5.75 | 79.23 ± 3.86 | 89.92 ± 9.12 | 78.77 ± 13.16 | 90.04 ± 2.69 | 86.35 ± 0.55 | 90.88 ± 5.07 | 78.37 ± 13.44 | 93.13 ± 2.17 | 82.37 ± 7.22 |
| DHA-derived | ||||||||||||
| 10(11)EpDPE | 83.69 ± 7.03 | 83.49 ± 5.73 | 85.08 ± 7.89 | 75.49 ± 5.49 | 85.18 ± 6.45 | 72.74 ± 13.26 | 90.21 ± 1.54 | 74.37 ± 8.17 | 94.48 ± 5.15 | 74.85 ± 7.29 | 95.84 ± 7.57 | 76.91 ± 3.27 |
| 13(14)EpDPE | 79.72 ± 6.7 | 78.26 ± 4.96 | 81.05 ± 7.51 | 73.91 ± 10.67 | 81.14 ± 6.14 | 75.77 ± 12.02 | 85.94 ± 1.47 | 80.26 ± 6.13 | 90.00 ± 4.90 | 74.95 ± 9.66 | 91.30 ± 7.21 | 85.05 ± 3.35 |
| 16(17)-EpDPE | 79.72 ± 6.7 | 75.54 ± 2.69 | 81.05 ± 7.51 | 72.52 ± 13.04 | 81.14 ± 6.14 | 75.00 ± 12.99 | 85.94 ± 1.47 | 77.65 ± 9.61 | 90.00 ± 4.90 | 71.72 ± 12.1 | 91.30 ± 7.21 | 83.68 ± 4.78 |
| 19(20)-EpDPE | 89.3 ± 7.9 | 79.3 ± 2.82 | 78.51 ± 3.19 | 76.13 ± 13.69 | 78.89 ± 6.73 | 80.38 ± 11.46 | 80.29 ± 5.26 | 84.58 ± 6.11 | 81.1 ± 4.05 | 75.29 ± 13.1 | 79.04 ± 0.71 | 87.84 ± 5.02 |
| 17-HDoHE | 91.81 ± 2.78 | 80.08 ± 3.85 | 85.85 ± 5.62 | 81.78 ± 3.99 | 88.03 ± 8.92 | 81.31 ± 13.58 | 87.64 ± 2.74 | 89.13 ± 0.57 | 88.97 ± 4.97 | 74.7 ± 13.87 | 91.17 ± 2.13 | 82.67 ± 5.08 |
| Formula (1) | Formula (2) | |||||
|---|---|---|---|---|---|---|
| Oxylipins | Total | Esterified | Free | Total | Esterified | Free |
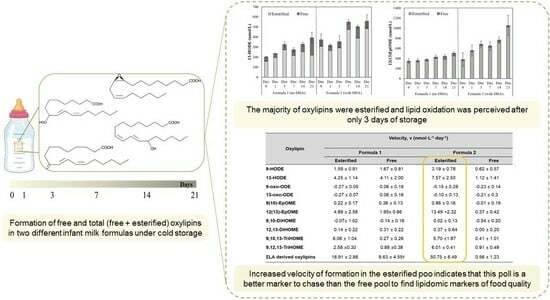
| 9-HODE | 3.83 ± 0.41 | 1.59 ± 0.81 | 1.67 ± 0.81 | 5.29 ± 0.98 | 3.19 ± 0.78 *ϯ | 0.62 ± 0.57 |
| 13-HODE | 7.24 ± 1.85 | 4.25 ± 1.14 | 4.11 ± 2.00 | 10.28 ± 3.03 | 7.57 ± 2.50 *ϯ | 1.12 ± 1.41 |
| 9-oxo-ODE | −0.09 ± 0.05 | −0.27 ± 0.05 | 0.06 ± 0.18 | −0.07 ± 0.22 | −0.15 ± 0.28 | −0.23 ± 0.14 |
| 13-oxo-ODE | −0.10 ± 0.04 | −0.27 ± 0.07 | 0.06 ± 0.16 | −0.07 ± 0.17 | −0.10 ± 0.13 | −0.21 ± 0.3 |
| 9(10)-EpOME | 0.59 ± 0.03 | 0.22 ± 0.17 | 0.36 ± 0.13¥ | 1.54 ± 0.42 | 0.86 ± 0.16 *ϯ | −0.01 ± 0.19 |
| 12(13)-EpOME | 7.21 ± 2.14 | 4.89 ± 2.58 | 1.95± 0.66¥ | 25.22 ± 5.95 | 13.49 + 2.32 *ϯ | 0.37 ± 0.42 |
| 9,10-DiHOME | −0.37 ± 0.02 | −0.07± 1.02 | −0.14 ± 0.16 | −0.37 ± 0.11 | 0.02 ± 0.13 | −0.54 ± 0.20 |
| 12,13-DiHOME | 0.47 ± 0.06 | 0.14 ± 0.22 | 0.31 ± 0.22 | 0.70 ± 0.17 | 0.37 ± 0.64 | 0.00 ± 0.20 |
| 9,10,13-TriHOME | 6.33 ± 1.08 | 6.06 ± 1.04 * | 0.27 ± 0.26 | 6.32 ± 1.53 | 5.70 ± 1.87 * | 0.41 ± 1.01 |
| 9,12,13-TriHOME | 3.76 ± 0.77 | 2.58 ± 0.30 * | 0.88 ± 0.38 | 6.77 ± 1.70 | 6.01 ± 0.41 *ϯ | 0.91 ± 0.49 |
| ΣLA-derived oxylipins | 27.85 ± 1.90 | 18.91 ± 2.88 * | 8.63 ± 4.55 ϯ | 59.99 ± 7.89 | 50.75 ± 6.49 *ϯ | 0.56 ± 1.23 |
| 9-HOTrE | 0.70 ± 0.08 | 0.52 ± 0.12 | 0.30 ± 0.10 ¥ | 0.71 ± 0.16 | 0.68 ± 0.16 * | 0.07 ± 0.03 |
| 13-HOTrE | 0.47 ± 0.20 | 0.25 ± 0.11 | 0.19 ± 0.10 ¥ | 0.55 ± 0.10 | 0.44 ± 0.15 *ϯ | −0.01 ± 0.05 |
| ΣALA-derived oxylipins | 1.17 ± 0.08 | 0.62 ± 0.18 | 0.54 ± 0.24 ¥ | 0.71 ± 0.15 | 0.77 ± 0.17 * | 0.05 ± 0.04 |
| 10(11)-EpDPE | ND | ND | ND | −0.19 ± 0.17 | −0.20 ± 0.17 | 0.01 ± 0.00 |
| 13(14)-EpDPE | ND | ND | ND | −0.65 ± 0.48 | −0.65 ± 0.48 | 0.01 ± 0.00 |
| 16(17)-EpDPE | ND | ND | ND | −1.07 ± 0.75 | −1.07 ± 0.75 | 0.01 ± 0.00 |
| 19(20)-EpDPE | ND | ND | ND | −1.15 ± 1.63 | −1.16 ± 1.63 | 0.02 ± 0.00 |
| 17-HDoHE | −0.03 ± 0.02 | −0.03 ± 0.02 | ND | 1.68 ± 0.36 | 1.57 ± 0.38 *ϯ | 0.14 ± 0.06 |
| ΣDHA-derived oxylipins | −0.03 ± 0.02 | −0.03 ± 0.02 | ND | −1.38 ± 3.33 | −1.51 ± 3.36 | 0.16 ± 0.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furlan Gonçalves Dias, F.; Teixeira, B.F.; Vieira, T.M.F.d.S.; de Moura Bell, J.M.L.N.; Taha, A.Y. Storage Duration and Added Docosahexaenoic Acid Modify the Rates of Esterified and Free Oxylipin Formation in Infant Milk Formula. Processes 2023, 11, 3045. https://doi.org/10.3390/pr11103045
Furlan Gonçalves Dias F, Teixeira BF, Vieira TMFdS, de Moura Bell JMLN, Taha AY. Storage Duration and Added Docosahexaenoic Acid Modify the Rates of Esterified and Free Oxylipin Formation in Infant Milk Formula. Processes. 2023; 11(10):3045. https://doi.org/10.3390/pr11103045
Chicago/Turabian StyleFurlan Gonçalves Dias, Fernanda, Bianca Ferraz Teixeira, Thais Maria Ferreira de Souza Vieira, Juliana Maria Leite Nobrega de Moura Bell, and Ameer Y. Taha. 2023. "Storage Duration and Added Docosahexaenoic Acid Modify the Rates of Esterified and Free Oxylipin Formation in Infant Milk Formula" Processes 11, no. 10: 3045. https://doi.org/10.3390/pr11103045
APA StyleFurlan Gonçalves Dias, F., Teixeira, B. F., Vieira, T. M. F. d. S., de Moura Bell, J. M. L. N., & Taha, A. Y. (2023). Storage Duration and Added Docosahexaenoic Acid Modify the Rates of Esterified and Free Oxylipin Formation in Infant Milk Formula. Processes, 11(10), 3045. https://doi.org/10.3390/pr11103045