Abstract
Tire recycling is becoming an increasingly important problem due to the growing number of end-of-life tires (ELTs). World-wide, ELTs account for more than 80 million tons. ELTs contribute to environmental pollution in the long term. They are flammable, toxic and non-biodegradable. At the same time, ELTs contain rubber, metal and textile cord, which are valuable raw materials. ELTs are buried in landfills, burned, crushed and restored. Most of these methods have a negative impact on the environment. From an environmental point of view, the most preferred ways to recycle tires are retreading and shredding. Rubber powder (RP) or crumb is mainly used for rubber pavers production, waterproofing, curbs, road slabs and various surfaces. An alternative method for RP processing, eliminating the disadvantages of the above approaches, is plasma gasification and pyrolysis. The paper presents a thermodynamic and kinetic analysis and an experiment on plasma processing of RP from worn tires to produce flammable gas. At a mass-average temperature of 1750 K, the yield of synthesis gas from plasma-air gasification of RP was 44.6% (hydrogen—19.1, carbon monoxide—25.5), and 95.6% of carbon was gasified. The experimental and calculated results satisfactorily agreed. It was found that plasma products from RP did not contain harmful impurities, either in calculations or experiments. Plasma gasification allows for recycling ELTs in an environmentally friendly way while also generating flammable gases that are valuable commodities. In this research, plasma technology was demonstrated to be effective for gasifying RP to produce flammable gas.
1. Introduction
For both environmental and economic reasons, there is a continuing wide interest in the processing and disposal of industrial and domestic waste [1], including rubber waste [2], and the development of recycling technologies [3,4,5,6]. Tires make up the bulk of rubber waste [7]. Waste tires (WTs) disposal is one of the world’s most pressing problems. Global tire sales amounted to 2.388 billion units in 2023 and will reach 3.012 billion units in 2032 [8]. The volume of WTs accumulated in the world is around 100 million tons [9]. In 2019, the total amount of end-of-life tires has been around 30.9 million tons worldwide and only 59% of them are correctly disposed of (including export to other countries, burning for energy and mechanical grinding) while 41% are landfilled, stockpiled or lost [10]. Approximately one billion WTs are released into the environment each year, and forecasts indicate that this number will increase by more than 20% by 2030 [11]. WTs are a source of long-term environmental pollution. In addition, rubber is flammable, toxic and non-biodegradable. For example, rubber covered with earth decomposes for more than 100 years. At the same time, tires contain valuable raw materials, namely, rubber, metal and textile cords. A cost–benefit analysis of the economy of waste tire recycling is presented in [12]. The paper illustrates an innovative technology for recycling textile fiber from end-of-life tires, which allows it to be converted into a useful secondary raw material for different applications.
WTs are buried in landfills, burned, crushed and restored. Most of these methods are environmentally harmful. To select the most effective waste disposal method, a life cycle assessment methodology can be used for the cleanup of contaminated sites, formulated and analyzed in [13,14,15]. Biorefineries can be used as an environmentally friendly option for waste disposal and the production of a wide range of commercial bioproducts and bioenergy [16]. Unfortunately, the rubber in end-of-life tires is not biorecyclable. As a result of burials, land is additionally alienated, including forest and agricultural lands, fire risks at the disposal site are more prominent and toxic organic compounds are washed out of tires by contact with leachate or simply precipitation. Dioxins, furans and benzo (a) pyrene are among the toxic substances released during tire burning [17]. The recycling of tires is most environmentally friendly through retreading and shredding. Rubber powder (RP) or crumb is mainly used for the production of rubber pavers, waterproofing, curbs, road slabs and various surfaces, including sports. Plasma pyrolysis or gasification at high temperatures are two alternative methods for recycling RP [5,18]. When RP is plasma gasified, carbon monoxide (CO) is produced along with hydrogen (H2), as well as hydrogen sulfide (H2S). As opposed to sulfur oxides, hydrogen sulfide can be purified on an industrial scale using the same technology used to purify natural gas from mercaptans. In organic synthesis, synthesis gas (CO + H2) obtained from gasification is a valuable chemical raw material. High process temperatures accelerate chemical reactions significantly with plasma processing. Automation of process control is another advantage of plasma processing. It has an increase in energy efficiency and a reduction in noncombustible components that dilute the synthesis gas (CO2, N2, H2O). The produced synthesis gas can be used to produce methanol, ethanol, bioplastics, biofuels and other valuable products [19,20,21,22]. The resulting synthesis gas can also be used as the working fluid of a new generation of highly efficient electric generators, including solid oxide fuel cells [23].
There have been very few publications on the plasma processing of WTs, compared to municipal solid waste and solid fuels. Publications on plasma processing of WT relate mainly to thermodynamic modeling or experimental studies of pyrolysis and gasification processes. In the work carried out by authors in [24], using Chemical WorkBench ver.3.5, thermodynamic calculations were performed for plasma–steam catalytic gasification of waste tires to produce synthesis gas. The composition of the synthesis gas was studied depending on the temperature in the range of 650–1500 K and the steam/WT mass ratio in the range of 0.8–3.1 kg/kg, at a pressure of 1–7 atm. It has been shown that plasma gasification of 1 kg of waste tires in an environment of 1.2 kg of steam at a temperature of 1350 K can produce 4.2 Nm3 of synthesis gas (H2—60.3 and CO—39.0 vol.%). In [25], the process of producing synthesis gas from WT by plasma gasification is studied using a thermodynamic method. The influence of various gasifying agents (air, steam, oxygen) on plasma gasification efficiency was also studied. During steam–air gasification, hydrogen and carbon monoxide concentrations increase, whereas condensed carbon concentrations decrease. Article [26] discusses a high-voltage alternating current (AC) plasma torch operating on a mixture of steam and air with a power of up to 90 kW. A thermodynamic calculation of combined plasma steam–air gasification of waste tires and brown coal is presented. The possibility of obtaining synthesis gas with carbon monoxide and hydrogen contents of 44.3 and 29.5 vol.%, respectively, has been shown. The calculated specific energy consumption for the process was 1.34 kWh/kg. Paper [27] presents the results of an experimental investigation of crumb rubber processing from WTs by plasma-thermal methods. An induction plasma torch is used with a frequency of 2 MHz, and a power of 50 kW. The purpose of the experiments was to obtain carbon black. Experiments have shown the possibility of its production in the particle size range from 50 to 300 nm. It was noted that when rubber powder is processed by combining plasma with induction heating, it can be obtained with a high proportion of nanostructures in the resulting carbon black. In [28], the thermal plasma pyrolysis of WT was tested in a direct current (DC) plasma reactor with an electric power of 35.2 kW using plasma-forming gas nitrogen. The combustion heat value of the produced gas ranged from 5.3 to 8.96 MJ/Nm−3 (with water gas shift reaction). WT pyrolysis products underwent steam treatment, and after the water gas shift reaction, synthesis gas was obtained with CO and H2 contents of 14.2 and 24.1%, respectively. The solid product contained more than 80% carbon black. Thermal plasma pyrolysis is a potentially useful way of treating WT for resource recovery. The authors [29] studied plasma–steam gasification of used tires in a reactor with an AC plasma torch with a power of 6.7 kW. In the experiments, a synthesis gas was obtained containing 30.6 vol.% H2 and 24 vol.% CO. This gas had a heat of combustion of 5.9 MJ/Nm3 and an output of 5 Nm3/kg. The work of [30] employed graphite electrodes and a DC arc plasma system for plasma gasification and plasma pyrolysis of WTs. The process temperature was maintained at 700–800 °C and the material was fed at a constant rate of 10 kg/h. The two processes are compared based on gas composition, syngas yield, carbon black yield and process efficiency. Results indicated a 4% increase in syngas yield in plasma gasification than in plasma pyrolysis. Analysis of synthesis gas showed that plasma gasification produced more CO and H2, and its higher calorific value increased by 12.9% compared to plasma pyrolysis. The authors believe that plasma gasification is a better alternative to plasma pyrolysis. The authors of [31] proposed a method for producing ultrafine carbon black from rubber powder, as well as a mixture of methane and synthesis gas, based on the application of electric arc plasma at atmospheric pressure. As a result of a series of experiments, an ultrafine carbon powder was obtained containing micro- and nano-sized objects with a graphite-like structure, as well as a mixture of gases containing up to 27.5 vol.% methane, 14.7 vol.% hydrogen, 3 vol.% carbon monoxide and 6 vol.% carbon dioxide. The specific energy consumption for the process was 24 kWh/kg WT, which is quite acceptable for a research plasma reactor.
The above papers investigate the processes of RP plasma gasification and plasma pyrolysis through various approaches, including thermodynamic analysis and experimental methods. These studies aim to analyze the yield and composition of both condensed and gaseous products produced during these processes. Unfortunately, there are no works devoted to numerical and experimental research with a comparison of their results and verification of the numerical models used. To date, there is a lack of research on the kinetic calculations of plasma pyrolysis and gasification of RP. This information is crucial for determining the optimal geometry and configuration of plasma devices, as well as the necessary reagent residence times, their consumptions and power required to complete these processes effectively. The objective of this study was to demonstrate the fundamental feasibility of combustible gases being obtained from used tires in a combined plasma reactor. In this regard, this article focuses on thermodynamic, kinetic and experimental studies of plasma processing of WTs in the form of RP, with a comparison of the results of calculations and experiments and a verification of the thermodynamic and kinetic models used. Unlike well-known experimental works [27,28,29,30,31], this work uses a combined plasma reactor of flow type with the combination in one reaction volume of zones of heat release from an electric arc and heat absorption by reacting agents. The combination of heat generation and heat absorption zones increases the efficiency of plasma reactors, reduces energy costs, and intensifies the ongoing processes. The proposed technology for processing used tires in a combined plasma reactor is environmentally effective, since it does not lead to the formation of strong carcinogens (dioxins, furans and benzo(a)pyrene), as well as the main greenhouse gas—carbon dioxide. By combining heat release and absorption zones, the combined plasma reactor offers improved thermal efficiency, reducing RP processing energy consumption. Increasing the efficiency of the reactor ultimately leads to an increase in the energy and financial efficiency of the plasma gasification technology of RP.
2. Materials and Methods
2.1. Materials
Recycling WTs involves separating the rubber from the cord frame, crushing it into the powder—RP. 73.5% rubber (C5H8) together with 25% carbon (C) and 1.5% sulfur (S), which are the main ingredients in automobile tire production, was used as the RP [32]. C—89.8%, H—8.7%, S—1.5% make up the RP composition. The amount of air necessary to gasify 1 kg of RP in air plasma is 5 kg; to pyrolyze 1 kg of RP, 1 kg of plasma-forming nitrogen is required. Rubber powder was obtained by cryogenic milling. The size of the particles was less than 100 microns.
2.2. Numerical Modeling Methods
2.2.1. Thermodynamic Modeling
To perform the thermodynamic analysis of plasma gasification and pyrolysis of RP, the universal program for calculating multicomponent heterogeneous systems TERRA (abbreviation for Russian spelling “thermodynamic calculations”) was used. TERRA has an extensive database of approximately 3000 individual substances, covering a wide temperature range from 300 to 6000 K [33]. The system was developed to compute high-temperature processes and contrasts with equilibrium computation methods based on Gibbs energy, equilibrium constants and Guldberg and Vaage law of acting mass, which are used in traditional thermochemical calculations. TERRA is based on the concept of maximizing entropy in equilibrium isolated thermodynamic systems. Calculations for plasma processing of RP were conducted at a pressure of 0.1 MPa at temperatures ranging from 300 to 3000 K.
2.2.2. Kinetic Modeling
Kinetic simulation of plasma processing of RP in the flow of oxidizer in a cylindrical channel having an internal source of arc plasma, in contrast to thermodynamic modeling, makes it possible to consider the process in time. According to the finite time of chemical reactions, the geometric dimensions of the plasma reactor can be determined based on its performance in addition to temperatures, velocities and concentrations of plasma gasification products. The “Plasma-RP” computer program was used to calculate the plasma gasification kinetics in a combined plasma reactor (Figure 1). The program was created using the “Plasma-Coal” program [33,34,35]. A pulverized PR and a gaseous oxidant are fed into the electric arc area of such a gasifier through pipes on its lid. An electric arc rotates in an electromagnetic field thanks to coil 4 and forms a heat release zone. The electric arc releases heat, and PR and gas absorb heat in the same space. The direct current (DC) arc establishes as sketched between graphite rod cathode 2 and the graphite ring anode 5. Due to a copper water-cooled electromagnetic coil 4, arc movement is limited to a 0.07 m wide strip inside the ring electrode. The gasification process completes mainly within the reactor volume. The reaction products come out through the orifice 6 with a diameter of 0.04 m and pass through a tube with an internal diameter of 0.15 m. It is obvious that such design presents an entrained flow gasifier with plug flow.
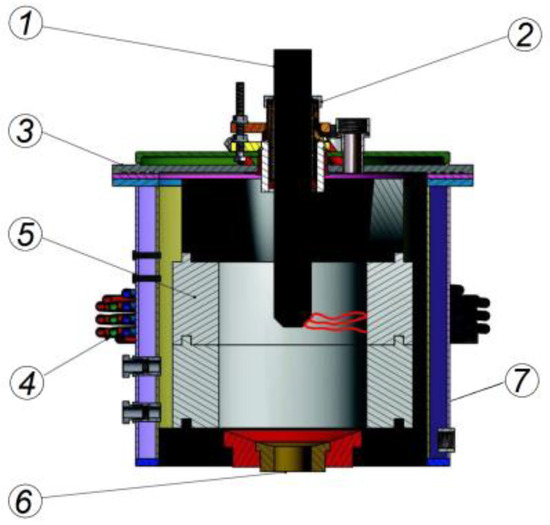
Figure 1.
Layout of a combined cylindrical electric arc reactor: 1—graphite rod cathode; 2—cathode insulator; 3—water-cooled lid; 4—electromagnetic coil; 5—graphite ring anode; 6—graphite orifice; 7—water-cooled wall.
The “Plasma-RP” code is based on a one-dimensional model that describes a chemically reacting flow consisting of two phases (RP and a gaseous oxidizer) in a reactor with an internal electric arc heat source. Rubber particles and gas are fed into the reactor at the same temperature. Heat and mass transfer between particles, gas and particles and the electric arc and gas is taken into account. In addition, the model provides for the transfer of heat and momentum between the flow and the reactor wall. Also considered are some chemical transformations of fuel. They describe the formation of primary volatile products, the transfer of released volatile products into the gas phase, and gasification reactions of residual carbon.
Rubber-dust gasification was mathematically described under the following assumptions: it is a one-dimensional, steady-state process; the ideal gas equation of state applies; a homogeneous mixture of gases and particles is at the reactor’s inlet; in the case of gas and solids, local heat transfer takes place through convection and conductivity; in the case of particles, temperature gradients are negligible; there is no significant temperature gradient in particles; as a result of a solid–gas reaction, only the solid’s temperature is affected, but for a gas-phase reaction, only the gas-phase temperature is affected; particle–particle interactions and solid wall friction are neglected; viscosity effects only apply when the solid–gas phase is in contact with the gas phase.
As a result, ordinary differential Equations (1)–(10) can be used to simplify the computations regarding heat exchange and hydrodynamics, while still allowing a detailed description of the chemical reactions involved. In order to obtain a precise mathematical model of the gasification of polydisperse rubber particles, the equations must include chemical kinetics equations (component concentration equations) along with gas and particle velocity equations and temperature equations. Using an empirically assigned distribution of heat evolution along the plasma reactor axis, the conservation of energy equation has taken into account an electric arc as an internal heat source.
The set of components can be numbered as follows without losing generality:
i = 1,…, n are components of gas phase;
i = n + 1,…, N are components of solid phase;
j = 1,…, m are heterogeneous reactions;
j = m + 1,…, M are reactions in gas phase;
l = 1,…, L are the numbers of the particle fractions.
As a result, we have the following set of equations:
- The equation for the conservation of the gaseous components is
- The equation for the conservation of the solid components is
- The equation for the number of particles’ conservation is
- The momentum equation is
Based on the Blasius empirical formula, fg = 0.316/(Rew0.25), the friction force on the wall is normalized to the reactor volume as . This is correct within the range of Reynolds number () Rew < 100,000. An empirical value for the gas dynamics viscosity coefficient is η = ρg ∙ (1.49 ∙ 10−10T2g − 1.16 ∙ 10−8Tg + 1.42 ∙ 10−4).
For the present case, the Knudsen flow has been assumed to be negligible. Also, it is important to note that the motion of the particles is not significantly affected by the evolution of volatile products.
- The equation for energy is
In this equation, qw is the loss of power from convection and radiation through the surface πDdx. Experimentally, the thermal contribution of the arc was determined by the calorimetric method from the difference between the measured electrical power generated by the arc Parc and the heat flux P2 through the wall of the water-cooled sectional reactor as follows: (Parc − P2)/V = ξqarc, qarc represents the distributed power in the cylindrical volume V = Sdx and ξ = (1 − P2/Parc)—thermal efficiency of the plasma reactor. The relative error in measuring the electrical power of the Parc reactor was 2%, and the heat flux P2 was 10%. As a function of heat loss, arc electric power is expressed as .
A dimensional coefficient of proportionality between qarc(x) and qw(x) is ψ = qarc(x)/qw(x), [m−1], giving the following expression: .
It would be more convenient to calculate contributed power using the non-dimensional coefficient, , then the formula for the efficiency degree will be the following: , or .
- The equation of the particles’ motion is
Under the action of Stokes force, the drag coefficient of a particle is calculated as follows:
where .
- The equation for the particles’ temperature is
The following equations complete the equation system:
- The equation of the state of ideal gas is
- The gas residence time is
- The solids’ residence time is
The model differs from others not just because it accounts for the arc’s energy contribution, but also because it describes the kinetics of chemical reactions in detail. Table 1 presents the kinetic scheme of RP devolatilization, the process of carbon gasification and the evolved volatile products conversion [35]. The Arrhenius’ equation governs how rate constants depend on temperature, as follows:
where n is the temperature coefficient, A is the pre-exponential coefficient, and index j is the reaction number.

Table 1.
Kinetic scheme of RP gasification.
The RP is specified by carbon and a set of functional groups (H2, CH4, and tar). RP thermal destruction has been assumed to be the first step (reactions 1–3 in Table 1), generating volatile and tar components consisting of benzene (C6H6). The reactions between carbon and steam and carbon dioxide and oxygen (reactions 4–7) are the limiting process reactions. Each of the above reactions is a complex process and includes elementary reactions (reagent adsorption on the particle surface, dissociation, gaseous reactions, desorption, etc.). In the model, only the general transformation, in accordance with gross reactions 4–7, was considered, not the exact mechanism of these reactions.
For the equation sets (1–10), initial conditions must be assigned for gas and particle velocities, initial temperatures, pressures, temperatures of the reactor wall, compositions of RP and gas as well as their consumptions. Due to the fact that the equation set (1–10) has a relatively large dimension and is appreciably non-linear, it can only be solved numerically. This model was implemented in a program called “Plasma-RP”, which solves stiff systems of chemical kinetics equations using Gear’s method.
2.3. Experimental Setup
In order to conduct experiments on RP plasma gasification, the universal plasma setup for the processing of fuel (Figure 2) was used. It is a cylindrical reactor with a supply system for electric, steam, gas and powder [23]. The main technological components of the installation are DC plasma reactor 1, gas and particle separation chamber 3, solid residue collector 4, chambers for cooling and sampling gas 6, and RP feeding systems 9 and 10. The combined type reactor is a water-cooled cylinder with a lid. Its power can be adjusted in the range of 30–100 kW. The lid is equipped with graphite rod electrode 11 and branch pipes for feeding RP and plasma forming gas (an oxidizer). The inside of the reactor is lined with 0.02 m thick graphite rings, which act as an anode 5 (Figure 1). The reactor has an internal diameter of 0.15 m and a height of 0.3 m. An electromagnetic coil 4 surrounds the reactor, and a graphite diaphragm 6 is mounted below. The DC electric arc is generated between graphite rod and ring electrodes with diameters of 0.04 m and 0.15 m, respectively. The rod electrode and ring electrode are 0.055 m apart. The arc, rotating in the magnetic field created by the electromagnetic coil, is held at a distance of 0.15 m from the reactor lid, and forms the electric arc zone of the reactor. The RP feeding system consists of RP bunkers 10 linked to RP feeders 9, from which through quartz pipes, RP is fed into reactor 1 under its own weight (Figure 2). The solid residue obtained after plasma processing of the RP enters the collector 4, installed on a trolley with a lift 5. After the reactor is shut down and the installation is cooled, the solid residue is manually removed from the collector. The collector is a water-cooled cylinder lined from the inside with graphite with an internal diameter of 0.22 m. Its height is 0.56 m. Graphite cooling and gas extraction chambers in the form of separate sections 6 and gas exhaust systems 7 and 8 form the flammable gas removal system. Gas extraction and cooling section 6 is a stainless steel cylinder, also water-cooled and lined from the inside with graphite. Plasma-forming air is supplied to the reactor from compressors. Figure 2b shows a photograph of the experimental installation for the plasma processing of RP from used tires.
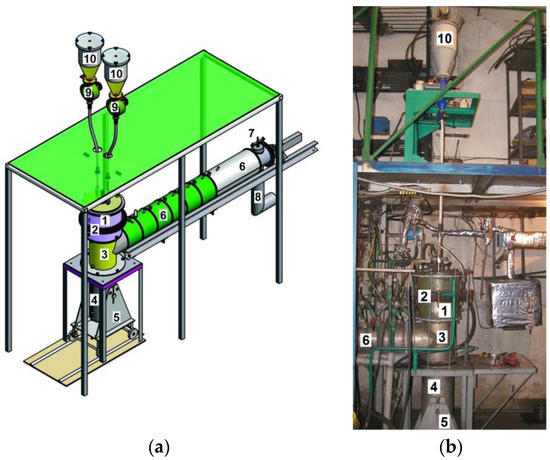
Figure 2.
A schematic showing (a) and a photo (b) of a plasma installation with a direct current arc reactor for RP processing: 1—DC plasma reactor, 2—electromagnetic coil, 3—section for separating solid residues and gas; 4—solid residue collector; 5—solid residue collector elevator; 6—gas extraction and cooling section; 7—safety valve; 8—exhaust gas outlet section; 9—RP feeder; 10—RP bunker.
2.4. Measurements
Experiments with plasma gasification of RP were conducted according to the following technique. After the reactor’s start up, the RP is fed from the bunkers via branch pipes mounted on the lid of the reactor, and then air is blown to the reactor, spraying the RP into the arc zone. A two-phase plasma flow is formed in the reactor as RP is heated to high temperatures. This is where RP gasification primarily occurs. In a solid residue collector, solid residue is collected during gasification. Gaseous products flow through the section for separating solid residues and gas to the gas extraction and cooling section, and then the gases are vented.
The main purpose of the experiments was to determine the efficiency of RP plasma processing in order to obtain synthesis gas. The integral indicators of the process, the degree of carbon gasification of the RP (XC), specific energy consumption (QSP) and mass average temperature (TAV), determine its efficiency. For material and heat balance, chemical and spectral analysis of the condensed phase of RP products are required to calculate these indices [18,36]. As per Equation (12), XC is determined by the carbon content of solid products resulting from gasification.
XC = (Cin − Cfin)/Cin · 100%,
Cin is the initial carbon content of RP, and Cfin is the carbon content of the solid residue at the current temperature. Carbon from ferric and silicon carbides is considered when estimating the carbon content in solid residues. To find XC, the initial and final concentrations of carbon in RP and in the solid residue of RP gasification products were determined by the absorption-weight method. According to this method, dried oxygen oxidizes the carbon present in the solid residue sample heated to a temperature of 850–950 °C to carbon dioxide. Ascarite (KOH or NaOH) absorbs carbon dioxide produced during sample incineration. CO2 + 2NaOH = Na2CO3 + H2O is the reaction that causes carbon dioxide to be absorbed. The same absorbent absorbs water formed during solid residue incineration. Then, the combustion products enter a quartz tube filled with dry chromic anhydride (CrO3). Chromic anhydride is needed to further oxidize traces of SO2 to SO3, which is then dissolved with concentrated sulfuric acid. The carbon dioxide dried in this way enters the absorption cartridge, which is weighed on an analytical balance. A cartridge’s weight gain corresponds to the carbon dioxide content absorbed by the cartridge. It is converted into carbon content using the formula C = 27.27 A/m, where A refers to the weight gain of the cartridge, and M refers to the weight of the sample. Formula (12) substitutes the carbon concentration obtained this way.
Temperatures inside the reactor were measured with pyrometers. Temperatures in the interval 600–3000 °C (873–3273 K) were measured using an Ircon Ultrimax Plus UX10P pyrometer (USA). The relative error of its measurement is ±0.5% of the measured value for the temperature interval 600–1500 °C (873–1773 K), ±1% for the temperature interval 1500–2000 °C (1773–2273 K) and ± 2% for temperature interval 2000–3000 °C (2273–3273 K). The device allows for measuring temperature with a resolution of 1°. The universal program for thermodynamic calculations TERRA was used to calculate the average mass temperature in the reactor according to the method [36]. Technological integral indicators were determined based on material and heat balance data; the method for finding it is described in [18]. The material and heat balance components were measured every 5 min during the experiments. The experiment lasted 60 min.
The concentrations of gaseous products of RP processing were measured using a gas chromatograph “Khromatek-Gazochrom-2000” (Russia). Helium or argon was used as a carrier gas, and thermal conductivity sensors were the detectors. Light gases (H2, CO, O2, N2, CH4) were analyzed on CaX columns filled with molecular sieves, and CO2 was analyzed on a silica gel column. The absolute calibration method was used when processing the data obtained in the experiment. Samples for analysis were taken in the gas extraction and cooling section 6 (Figure 2). Chemical analysis was used to determine the composition of the solid residue.
3. Results and Discussion
3.1. Thermodynamic Analysis
The calculation results are shown in Figure 3 and Figure 4. By air gasification of RP, complete gasification of carbon can be achieved at a temperature of 1200 K (Figure 3a). However, during the pyrolysis of RP, the main part of carbon remains in the condensed phase up to a temperature of 1800 K. At a temperature of 2600 K, a 100% degree of carbon gasification is achieved. Figure 3b shows the dependence on temperature of the specific energy consumption reduced to one kilogram of the working fluid for plasma-air gasification (curve 1) and pyrolysis (curve 2) of RP. At a temperature of 1200 K, the specific energy consumption for gasification and pyrolysis is 0.74 and 0.8 kWh/kg, respectively. At this temperature, which also ensures 100% gasification of the RP, the following gas composition was found: H2—21.2 vol.%, CO—26.9 vol.%, CO2—0.02 vol.%, N2—51.3 vol.%, H2O—0.2 vol.%, H2S—0.2 vol.% (Figure 3a). At temperatures above 2000 K, hydrogen sulfide (H2S) partially decomposes into sulfanyl (SH) and volatile sulfur (S), which are not shown in the figure since they have a concentration below 0.1%. At the same RP pyrolysis temperature, which ensures the maximum yield of hydrogen, the following combustible gas composition was obtained: vol.%: H2—60.5, CH4—0.6, N2—38.4, H2S—0.5 (Figure 4b). Calculations have shown that the plasma-air gasification of RP produces a combustible gas with a synthesis gas yield of 48.1%. Plasma pyrolysis of RP produces pure condensed carbon and combustible gas with a hydrogen concentration of 60.5%. The gas phase in both processes contains hydrogen sulfide with a concentration not exceeding 0.5%, the purification of which has been worked out on an industrial scale. At the same time, no other harmful impurities were found in the products of RP plasma processing.
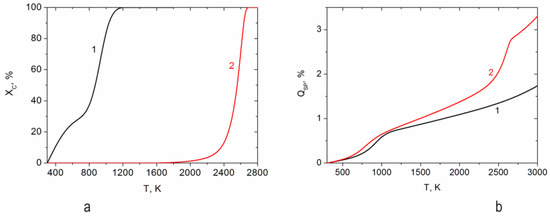
Figure 3.
Dependence of the degree of carbon gasification (a) and specific energy consumption (b) for plasma-air gasification (1) and pyrolysis (2) of RP depending on the process temperature.
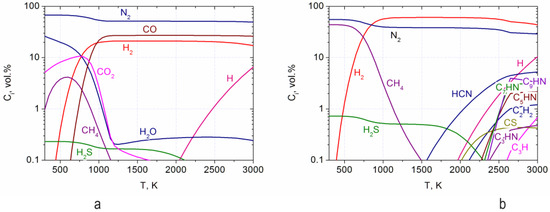
Figure 4.
Dependence of the composition of the gas phase on the temperature of plasma-air gasification of RP (a) and plasma pyrolysis of RP in nitrogen plasma (b).
Comparison of the compositions of gaseous products and the degree of gasification of RP carbon in the processes of plasma-air gasification and pyrolysis showed the following. Due to the absence of oxygen during plasma pyrolysis, there is no CO in the composition of gaseous products, and the main part of carbon remains in the condensed phase up to a temperature of 1800 K, while during plasma-air gasification complete gasification is already achieved at 1200 K. In this regard, kinetic calculations and experiments on the plasma pyrolysis of RP have not been carried out.
3.2. Kinetic Analysis
For kinetic calculations and experimental studies, the following initial data and technical characteristics of a plasma reactor for RP gasification, found through thermodynamic calculations, were used. The plasma reactor’s power was 40.5 kW, the RP’s initial temperature was 300 K and the RP and air consumption were 4.6 and 23 kg/h. The length of the plasma reactor is 0.4 m and its diameter is 0.15 m. The reactor efficiency is 75% [37]. The specific energy consumption for gasification of the RP, calculated with the help of the TERRA program, made it possible to determine reactor power. Electric arc plasma produces a heat release zone of 0.3 m in length. The RP composition is specified in the Plasma-RP program, wt. %: C—45, CH4—42, C6H6—13. RP density is 920 kg/m3, and the average particle size is 100 µm.
Figure 5 shows the changes in the concentrations of the components of the RP air gasification products along the plasma reactor for two values of its power. The gas phase of the gasification products is represented mainly by synthesis gas diluted with oxidizers (H2O и CO2) and nitrogen (N2). The concentration of combustible gases (CO + H2 + CH4 + CH3) at the outlet of the reactor (0.4 m) reaches 46.18%. Moreover, carbon monoxide concentrations reach 26.7% and hydrogen concentrations reach 19.3%. The concentrations of methane (CH4) and methyl radical (CH2) are much lower and amount to 0.9 and 0.2%, respectively. The nitrogen concentration at the outlet of the reactor is 52.3%. The concentrations of water vapor (H2O) and carbon dioxide (CO2) at the exit from the reactor decrease to 0.4 and 0.06, respectively, due to carbon gasification reactions (H2O + C = CO + H2, CO2 + C = 2CO). The maximum concentration of benzene (C6H6) in the initial section of the reactor (0.1 m) is only 0.8% and tends to be zero at the reactor outlet (0.4 m). As a result of plasma-air gasification of RP at its consumption of 4.6 kg/h, 27.6 kg/h of combustible gas with a calorific value of 5660 kJ/kg is formed at the reactor outlet. The residence time of powder particles in the reactor during plasma-air gasification of RP is 0.38 s.
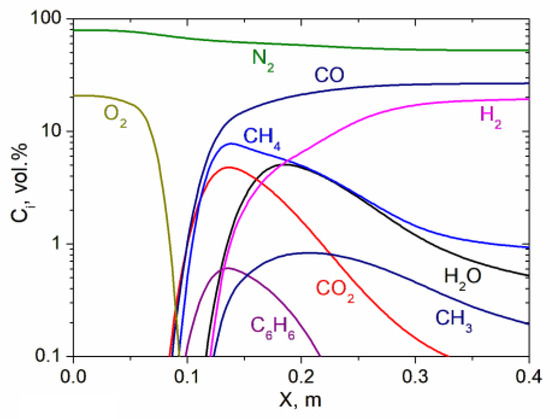
Figure 5.
Change in the concentration of components of gaseous products of RP gasification along the length of the reactor.
Figure 6a shows the dependence of the temperature of the gas and particles on the length of the reactor during air gasification of the RP. It can be seen that the gas temperature exceeds the particle temperature starting from the reactor length of 0.15 m. In this case, the maximum temperature difference, as well as the maximum temperature, is observed at 0.3 m, the end of the heat release zone from the plasma source. The temperature of the gas at this point reaches 1883 K, and that of the particles reaches 1826 K. With an increase in the length of the reactor (X > 0.3 m), the temperatures of the gas and particles begin to decrease slightly, tending to thermal equilibrium. Figure 6b shows the change in the speed of gas and particles along the length of the reactor. With a slight excess of gas over particle velocity, they practically coincide along the entire reactor length. Once the heat release zone (X = 0.3 m) is passed, the gas and particles move at equal speeds, and eventually, the particles begin to move faster than the gas, which is a consequence of particle inertia and temperature decrease. A distance of 0.3 m results in maximum velocities for gas and particles of 2.6 m/s. Figure 6c shows the change in the degree of RP carbon gasification along the length of the reactor. XC increases along the length of the reactor, reaching 100% already at a distance of 0.2 m. Thus, in the plasma reactor, RP gasification is completed at a length of 0.2 m, after which only gas-phase reactions proceed with the formation of target components CO and H2. Figure 6d shows the change in the residence time of the reagents along the length of the reactor. It follows from the figure that for the almost complete completion of the RP gasification process (Figure 5), the achievement of thermal (Figure 6a) and dynamic (Figure 6b) equilibrium, the required residence time of the reagents is 0.3–0.4 s.
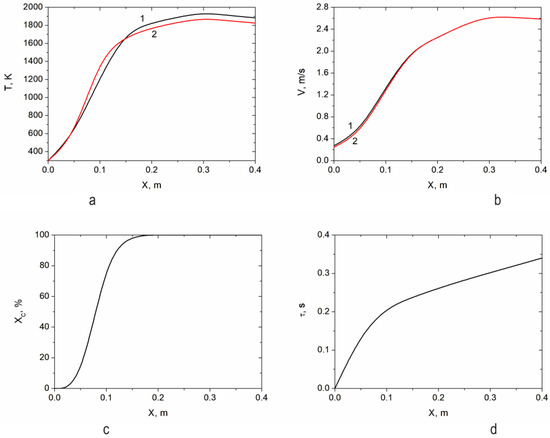
Figure 6.
Change in the temperature (a) and speed (b) of gas (1) and RP particles (2), degree of RP carbon gasification (c), and the residence time of the reagents (d) along the length of the reactor.
3.3. Experiment
The initial data of two experiments for reactor powers of 40.5 and 40.8 kW are presented in Table 2. Table 3 summarizes the concentrations and degrees of RP carbon gasification, as well as the average temperatures of the gases produced by plasma-air gasification. During RP gasification, as can be seen from the table, carbon monoxide represents 25.5–26.4% of the gas phase, hydrogen represents 18.8–19.1% of the gas phase and nitrogen represents 55.2–55.5% of the gas phase, resulting in an impressive gas concentration synthesis of 44.2–44.6%. An experiment on plasma-air gasification of RP showed that oxygen (O2) concentrations at the reactor outlet were no higher than 0.3%, indicating that the weight ratio of RP to air was selected correctly. With a mass-average temperature of 1700–1750 K in the reactor, 93.7–95.6% of RP gasification degree was reached. The tables indicate that higher specific energy consumption (QSP) corresponds to a higher average mass temperature and, therefore, to a higher degree of RP carbon gasification.

Table 2.
The initial metrics of RP plasma gasification experiments.

Table 3.
Results of experimental studies of RP plasma gasification.
3.4. Comparison of Experimental and Calculated Data
Before moving on to comparing the calculation results with the experiment, we note that a water-cooled flow plasma reactor is an open system in which energy is exchanged with the external environment. It is nonetheless possible to model the plasma processing of RP thermodynamically, since actual heat losses with cooling water are included in material and thermal balances for plasma reactors, as well as the average mass temperature of reagents is defined based on this. The validity of using a thermodynamic model to calculate the process of plasma gasification of RP is also justified by the fact that, according to kinetic calculations, the residence time of the reagents in the reactor reaches a significant value of about 0.35 s. This residence time at the high temperature characteristic of plasma processes ensures the achievement of local thermodynamic equilibrium in the reaction zone.
A comparison of the calculation results with experimental data (Table 4) enabled the thermodynamic and kinetic mathematical models to be evaluated. When calculating thermodynamically, a temperature value equal to the experimental value (1750 K) is used, while a kinetic calculation uses the energy equation for gases and particles to calculate the temperatures of the reagents at given power values and heat losses from the reactor walls. Temperatures of the reagents averaged 1873 K under these conditions, with a 7% difference between experiment and thermodynamic calculation. Discrepancies between calculations and experiments for synthesis gas yields did not exceed 8% (thermodynamic calculation) and 3% (kinetic calculation). The discrepancy in terms of inert nitrogen (N2) in the calculations and experiment did not exceed 7%. There was no discrepancy greater than 5% between the calculations and experiments regarding the degree of gasification. The discrepancy between the specific energy consumption for the process in the calculations and experiment was 9.5%. The calculated and experimental data matched well for plasma processes, and their agreement was acceptable. There was no evidence of harmful impurities in the gasification products of RP in either the calculations or experiments.

Table 4.
Comparison of calculation results with experimental data.
4. Conclusions
Within the framework of this article, a comprehensive study of the plasma-chemical processing of RP from waste tires was carried out, including thermodynamic and kinetic calculations, and experiments in a combined flow-type plasma reactor with the combination of heat release zones from an electric arc and heat absorption by reacting agents in one reaction volume.
Thermodynamic analysis of plasma-air gasification of the RP showed that complete gasification of the RP carbon is achieved at a temperature of 1200 K. In this case, the following composition of the combustible gas was obtained: vol.%: H2—21.2, CO—26.9, N2—51.3. At the same temperature of RP pyrolysis, the composition of the combustible gas included the following: H2—60.5, CH4—0.6, N2—38.4 vol.%. However, during pyrolysis, complete gasification of RP carbon occurs only at a temperature of 2600 K, which leads to a significant increase in specific energy consumption for the process from 0.8 to 3 kWh/kg.
Kinetic calculations have shown that with a plasma reactor power of 40.5 kW, over a length of 0.2 m (the residence time of the reactants in the reactor is 0.25 s), the average mass temperature of the reactants reaches 1800 K, practically unchanged until leaving the reactor at a length of 0.4 m (time residence time of the reagents in the reactor is 0.34 s). At the outlet of the reactor, the concentrations of CO, H2 and N2 are 26.7, 19.3 and 52.3 vol.%, respectively. As a result of plasma-air gasification of RP at its consumption of 4.6 kg/h, 27.6 kg/h of combustible gas with a calorific value of 5660 kJ/kg is formed at the reactor outlet.
A plasma-air gasification of RP in a combined reactor produced CO concentrations between 25.5% and 26.4%, H2 concentrations between 18.8% and 19.1%, and N2 concentrations between 55.2 and 55.5%, with significant synthesis gas yields of 44.2–44.6% as a result. It was found that mass-average temperatures of 1700–1750 K in the reactor contributed to high RP gasification degrees of 93.7–95.6%.
There was satisfactory agreement between calculated and experimental data regarding RP plasma processing. The discrepancy between the specific energy consumption for the process in the calculations and the experiment did not exceed 9.5%; in terms of synthesis gas yield, it was 8% (thermodynamic calculation) and 3% (kinetic calculation), and in terms of the degree of carbon RP gasification, it was 5%. The verification of the TERRA and Plasma-RP programs confirmed the legality of their use for calculations of plasma processing of RP.
There was no evidence of harmful impurities in the gasification products of RP either in calculations or in experiments. The study has shown the promise of using plasma technology for end-of-life tires processing to produce fuel gas.
The research results showed that the proposed technology for the worn tires process in a combined plasma reactor can become promising for industry due to its high environmental and energy efficiency.
The objective of further research includes the development of an environmental and economic assessment of the effectiveness of the proposed technology for recycling used tires in a combined plasma reactor.
Author Contributions
Conceptualization, V.M. and A.U.; methodology, V.M. and A.U.; software, V.M. and A.U.; validation, V.M. and A.U.; resources, V.M. and A.U.; data curation, V.M. and A.U.; writing—original draft preparation, V.M. and A.U.; writing—review and editing, V.M. and A.U.; visualization, A.U.; supervision, V.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science and Higher Education of the Republic of Kazakhstan [Grants No. BR18574084 and AP19674754].
Data Availability Statement
All data are reported in the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- An’shakov, A.S.; Faleev, V.A.; Danilenko, A.A.; Urbakh, E.K.; Urbakh, A.E. Investigation of plasma gasification of carbonaceous technogeneous wastes. Thermophys. Aeromechanics 2007, 14, 607–616. [Google Scholar] [CrossRef]
- Miller, C. Scrap Tires. Scrap Tire Stockpiles Have Been Reduced by 87 Percent since 1990. Waste 360. 2010. Available online: https://www.waste360.com/Recycling_And_Processing/scrap-tires-201003 (accessed on 26 March 2024).
- Davidson, G. Waste Management Practices: Literature Review. Dalhousie University–Office of Sustainability. 2011, 54p. Available online: https://studylib.net/doc/18527000/waste-management-practices--literature-review (accessed on 26 March 2024).
- Heberlein, J.; Murphy, A.B. Topical review: Thermal plasma waste treatment. J. Phys. D Appl. Phys. 2008, 41, 053001. [Google Scholar] [CrossRef]
- Messerle, V.E.; Mosse, A.L.; Ustimenko, A.B. Processing of biomedical waste in plasma gasifier. Waste Manag. 2018, 79, 791–799. [Google Scholar] [CrossRef]
- Surov, A.V.; Popov, S.D.; Popov, V.E.; Subbotin, D.I.; Serba, E.O.; Spodobin, V.A.; Nakonechny, G.V.; Pavlov, A.V. Multi-gas AC plasma torches for gasification of organic substances. Fuel 2017, 203, 1007–1014. [Google Scholar] [CrossRef]
- Tire Market: Global Industry Trends, Share, Size, Growth, Opportunity and Forecast 2023–2028. Report. January 2023. ID: 5732907, 2005, 165p. Available online: https://www.researchandmarkets.com/report/automotive-tires (accessed on 17 April 2024).
- Market Research Report. Tier Market Report by Design (Radial Market, Bias Market), End-Use (OEM Market, Replacement Market), Vehicle Type (Passenger Cars, Light Commercial Vehicles, Medium and Heavy Commercial Vehicles, Two Wheelers, Three Wheelers, Off-The-Road (OTR)), Distribution Channel (Offline, Online), Season (All Season Tires, Winter Tires, Summer Tiers), and Region 2023-2028. Report ID: SR112023A575. 2023. Available online: https://www.imarcgroup.com/tyre-manufacturing-plant (accessed on 26 March 2024).
- Ćetković, J.; Lakić, S.; Žarković, M.; Vujadinović, R.; Knežević, M.; Živković, A.; Cvijović, J. Environmental Benefits of Air Emission Reduction in the Waste Tire Management Practice. Processes 2022, 10, 787. [Google Scholar] [CrossRef]
- Valentini, F.; Pegoretti, A. End-of-life options of tyres. A review. Adv. Ind. Eng. Polym. Res. 2022, 5, 203–213. [Google Scholar] [CrossRef]
- Dabic-Miletic, S.; Simic, V. Smart and Sustainable Waste Tire Management: Decision-Making Challenges and Future Directions. Decis. Mak. Adv. 2023, 1, 10–16. [Google Scholar] [CrossRef]
- Gigli, S.; Landi, D.; Germani, M. Cost-benefit analysis of a circular economy project: A study on a recycling system for end-of-life tyres. J. Clean. Prod. 2019, 229, 680–694. [Google Scholar] [CrossRef]
- Castagnoli, A.; Pasciucco, F.; Iannelli, R.; Meoni, C.; Pecorini, I. Keu Contamination in Tuscany: The Life Cycle Assessment of Remediation Project as a Decision Support Tool for Local Administration. Sustainability 2022, 14, 14828. [Google Scholar] [CrossRef]
- Astrup, T.F.; Tonini, D.; Turconi, R.; Boldrin, A. Life cycle assessment of thermal Waste-to-Energy technologies: Review and recommendations. Waste Manag. 2015, 37, 104–115. [Google Scholar] [CrossRef]
- Khandelwal, H.; Dhar, H.; Thalla, A.K.; Kumar, S. Application of life cycle assessment in municipal solid waste management: A worldwide critical review. J. Clean. Prod. 2019, 209, 630–654. [Google Scholar] [CrossRef]
- Mohan, S.V.; Nikhil, G.N.; Chiranjeevi, P.; Reddy, C.N.; Rohit, M.V.; Kumar, A.N.; Sarkar, O. Waste biorefinery models towards sustainable circular bioeconomy: Critical review and future perspectives. Bioresour. Technol. 2016, 215, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Lönnermark, A.; Blomqvist, P. Emissions from Tyre Fires. SP Swedish National Testing and Research Institute. SP Report 2005, 43. Available online: https://www.academia.edu/84151122/Emissions_from_Tyre_Fires (accessed on 26 March 2024).
- Messerle, V.E.; Ustimenko, A.B.; Lavrichshev, O.A. Plasma coal conversion including mineral mass utilization. Fuel 2017, 203, 877–883. [Google Scholar] [CrossRef]
- Iaquaniello, G.; Centi, G.; Salladini, A.; Palo, E.; Perathoner, S.; Spadaccinid, L. Waste-to-Methanol: Process and Economics Assessmen. Bioresour. Technol. 2017, 243, 611–619. [Google Scholar] [CrossRef]
- Beneroso, D.; Bermúdez, J.M.; Arenillas, A.; Menéndez, J.A. Comparing the composition of the synthesis-gas obtained from the pyrolysis of different organic residues for a potential use in the synthesis of bioplastics. J. Anal. Appl. Pyrolysis 2015, 111, 55–63. [Google Scholar] [CrossRef]
- Borgogna, A.; Salladini, A.; Spadacini, L.; Pitrelli, A.; Annesini, M.C.; Iaquaniello, G. Methanol production from Refuse Derived Fuel: Influence of feedstock composition on process yield through gasification analysis. J. Clean. Prod. 2019, 235, 1080–1089. [Google Scholar] [CrossRef]
- Yasin, M.; Cha, M.; Chang, I.S.; Atiyeh, H.K.; Munasinghe, P.; Khanal, S.K. Chapter 13-Syngas Fermentation Into Biofuels and Biochemicals. In Biomass, Biofuels, Biochemicals, Biofuels: Alternative Feedstocks and Conversion Processes for the Production of Liquid and Gaseous Biofuels, 2nd ed.; Pandey, A., Larroche, C., Dussap, C.-G., Gnansounou, E., Khanal, S.K., Ricke, S., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 301–327. [Google Scholar] [CrossRef]
- Galvita, V.; Messerle, V.E.; Ustimenko, A.B. Hydrogen production by coal plasma gasification for fuel cell technology. Int. J. Hydrogen Energy 2007, 32, 3899–3906. [Google Scholar] [CrossRef]
- Kuznetsov, V.A.; Kumkova, I.I.; Lerner, A.S.; Popov, V.E. Equilibrium analysis of hydrogen production using the steam-plasma gasification process of the used car tires. J. Phys. Conf. Ser. 2012, 406, 012023. [Google Scholar] [CrossRef]
- Ma, Y.; Qi, H.; Zhang, J.; Cui, P.; Zhu, Z.; Wang, Y. Thermodynamic analysis of a carbon capture hydrogen production process for end-of-life tires using plasma gasification. J. Clean. Prod. 2023, 384, 135662. [Google Scholar] [CrossRef]
- Popov, V.E.; Subbotin, D.I.; Surov, A.V.; Popov, S.D.; Serba, E.O.; Godina, E.P.; Kiselev, A.A. Co-gasification of lignite and used car tires by H2O/air thermal plasma. J. Phys. Conf. Ser. 2019, 1243, 012010. [Google Scholar] [CrossRef]
- Paskalov, G.; Gafarov, I.; Mong, T.H.; Jarvis, R.; Abdullin, I.; Hubathuzin, A.; Musharatskiy, L. Plasma-thermal processing of rubber crumb from waste tires. In Proceedings of the 22nd International Symposium on Plasma Chemistry, Antwerp, Belgium, 5–10 July 2015; Available online: http://www.ispc-conference.org/ispcproc/ispc22/P-III-9-9.pdf (accessed on 26 March 2024).
- Huang, H.; Tang, L.; Wu, C.Z. Characterization of gaseous and solid product from thermal plasma pyrolysis of waste rubber. Environ. Sci. Technol. 2003, 37, 4463–4467. [Google Scholar] [CrossRef] [PubMed]
- Tendler, M.; Rutberg, P.; Van Oost, G. Plasma based waste treatment and energy production. Plasma Phys. Control. Fusion 2005, 47, A219–A230. [Google Scholar] [CrossRef]
- James, G.S.; Nema, S.K.; Murugan, P.; Deendayal, P. A Comparative Analysis of Pyrolysis and Gasification of WT by Thermal Plasma Technology for Environmentally Sound Waste Disposal. CorpusID:204856961. 2019. Available online: https://api.semanticscholar.org/CorpusID:204856961 (accessed on 26 March 2024).
- Pak, A.Y.; Larionov, K.B.; Kolobova, E.N.; Slyusarskiy, K.V.; Bolatova, J.; Yankovsky, S.A.; Stoyanovskii, V.O.; Vassilyeva, Y.Z.; Gubin, V.E. A novel approach of waste tires rubber utilization via ambient air direct current arc discharge plasma. Fuel Process. Technol. 2022, 227, 107111. [Google Scholar] [CrossRef]
- Markl, E.; Lackner, M. Devulcanization Technologies for Recycling of Tire-Derived Rubber: A Review. Materials 2020, 13, 1246. [Google Scholar] [CrossRef] [PubMed]
- Gorokhovski, M.; Karpenko, E.I.; Lockwood, F.C.; Messerle, V.E.; Trusov, B.G.; Ustimenko, A.B. Plasma Technologies for Solid Fuels: Experiment and Theory. J. Energy Inst. 2005, 78, 157–171. [Google Scholar] [CrossRef]
- Kalinenko, R.A.; Kuznetsov, A.P.; Levitsky, A.A.; Messerle, V.E.; Mirokhin, Y.A.; Polak, L.S.; Sakipov, Z.B.; Ustimenko, A.B. Pulverized Coal Plasma Gasification. Plasma Chem. Plasma Process 1993, 13, 141–167. [Google Scholar] [CrossRef]
- Askarova, A.S.; Karpenko, E.I.; Lavrishcheva, Y.I.; Messerle, V.E.; Ustimenko, A.B. Plasma-Supported Coal Combustion in Boiler Furnace. IEEE Trans. Plasma Sci. 2007, 35, 1607–1616. [Google Scholar] [CrossRef]
- Messerle, V.E.; Ustimenko, A.B.; Lavrichshev, O.A. Comparative study of coal plasma gasification: Simulation and experiment. Fuel 2016, 164, 172–179. [Google Scholar] [CrossRef]
- Messerle, V.E.; Ustimenko, A.B. Hydrogen Production by Thermal Plasma Pyrolysis of Hydrocarbon Gases. IEEE Trans. Plasma Sci. 2023, 1–5. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).