Improving the Functionality of Yogurt after Fortification with a Synbiotic Combination of a Potential Probiotic and Bacteriocin-Producing Bacteria and Hydnora abyssinica Phytosomes
Abstract
1. Introduction
2. Materials and Methods
2.1. Hydnora Sample and Preparation of Extract
2.2. Bacterial Strain Isolation and Culture Conditions
2.3. Molecular Identification of Isolate GA5
2.4. Antimicrobial Spectrum of Strain GA5
2.5. Effect of Enzymes on Antimicrobial Activity of Strain GA5
2.6. Safety Assessment of Strain GA5
2.6.1. Blood Hemolysis Activity
2.6.2. Antibiotic Sensitivity of Strain GA5
2.6.3. Histidine Decarboxylase Activity of Strain GA5
2.7. Probiotic Characteristics of Strain GA5
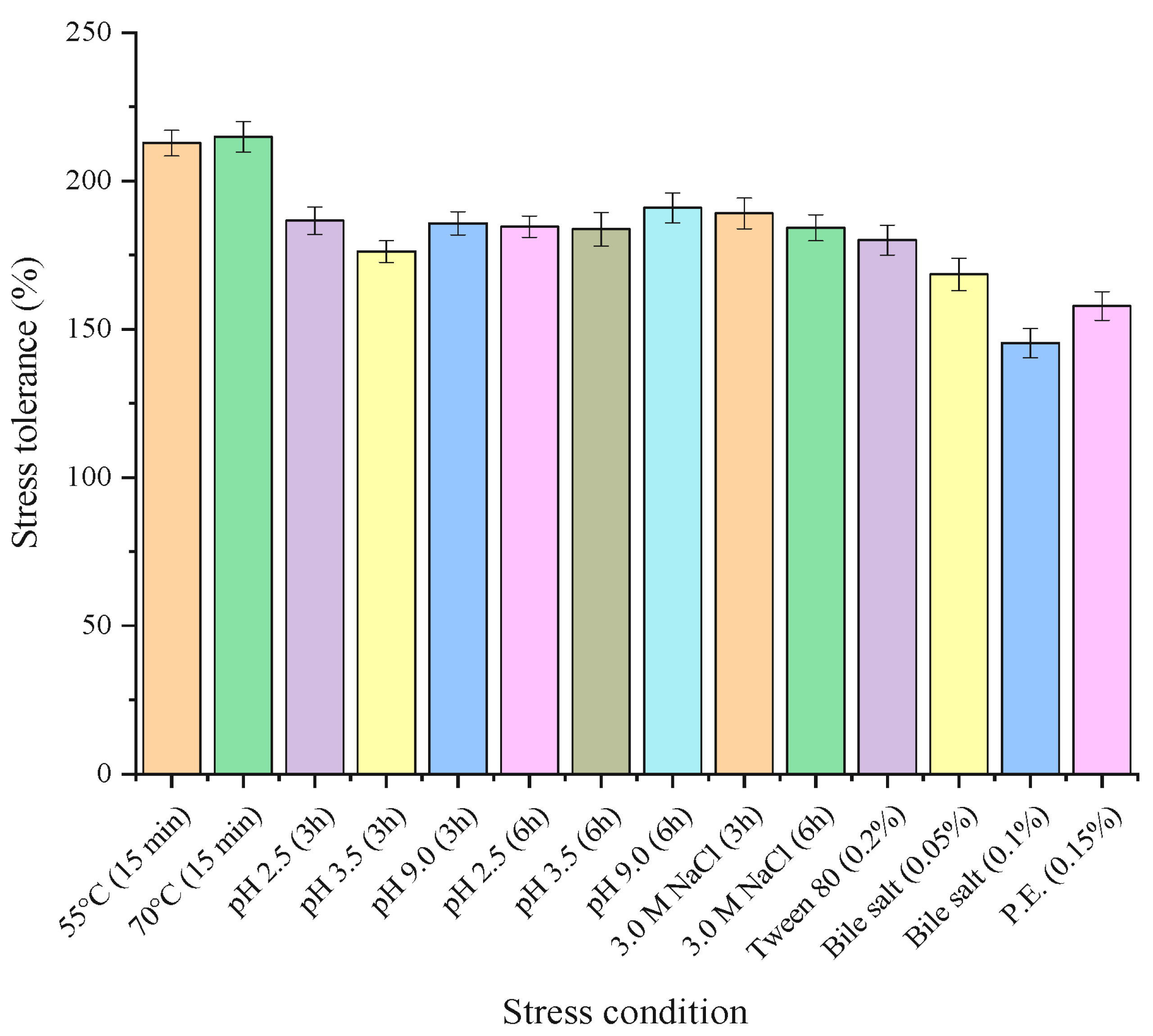
2.7.1. Stress Tolerance of Strain GA5
2.7.2. Cell Surface Hydrophobicity of Strain GA5
2.8. Prebiotic Properties of Hydnora abyssinica Extract
2.9. Quantification of the Polyphenols in H. abyssinica Extract
2.10. Phytosome Preparation
2.10.1. Physical and Morphological Characterization of Phytosomes
2.10.2. Determination of Encapsulation Efficiency
2.11. Preparation of Yogurt and Its Analyses
2.11.1. Preparation of Set Yogurt
2.11.2. Compositional and pH Analysis of Yogurt
2.11.3. Sensory Evaluation
2.11.4. Texture Profile Analysis (TPA)
2.11.5. Viscosity Measurement
2.11.6. Viability of Probiotic Strains in Yogurt Samples
2.12. Evaluation of Total Polyphenols in Yogurt
2.12.1. Extraction of Total Polyphenols from Yogurt
2.12.2. Determination of Total Polyphenols (TPs)
2.13. Determination of Antioxidant Activity
2.13.1. Antioxidant Activity of Strain GA5 and Yogurt Samples
- As is the absorbance of the sample (DPPH and sample);
- Ab is the absorbance of the blank (sample and ethanol);
- Ac is the absorbance of the control (deionized water and DPPH).
2.13.2. ABTS Radical Cation Scavenging Assay
2.14. Statistical Analysis
3. Results and Discussion
3.1. Isolation and Investigation of Antimicrobial Activity
3.2. Morphomolecular Identification of Isolate GA5
3.3. Effect of Enzymes on Antimicrobial Activity
3.4. Safety Assessment of L. plantarum Strain GA5
3.5. Probiotic Properties of L. plantarum Strain GA5
3.5.1. Stress Tolerance
3.5.2. Cell Surface Hydrophobicity of L. plantarum Strain GA5
3.5.3. Antioxidant Activity of the CFS of L. plantarum Strain GA5
3.6. Prebiotic Properties of Hydnora Extract
3.7. Quantification of Polyphenols in H. abyssinica Extract
3.8. Mean Particle Size, Polydispersity Index, and Zeta Potential (ζ) of H. abyssinica Phytosomes
3.9. Properties of Yogurt Fortified with H. abyssinica
3.9.1. Physicochemical Properties of Yogurt
3.9.2. Viscosity Evaluation
3.9.3. Texture Profile Analysis of Yogurt Fortified with H. abyssinica
3.9.4. Antioxidant Activity of Yogurt Fortified with H. abyssinica
3.9.5. Sensory Evaluation
3.9.6. Storage and Microbial Quality of Fermented Yogurt Made Using L. plantarum GA5 and Fortified with H. abyssinica
4. Conclusions
5. Patents
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gao, J.; Li, X.; Zhang, G.; Sadiq, F.A.; Simal-Gandara, J.; Xiao, J.; Sang, Y. Probiotics in the dairy industry—Advances and opportunities. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3937–3982. [Google Scholar] [CrossRef]
- Khan, S.; Nosheen, A.; Correa, P.; Mendy, P.A. Probiotic: A Sustainable Approach towards Healthy Food. In Microbiome-Gut-Brain Axis; Springer: Singapore, 2022; pp. 281–296. [Google Scholar] [CrossRef]
- Latif, A.; Shehzad, A.; Niazi, S.; Zahid, A.; Ashraf, W.; Iqbal, M.W.; Rehman, A.; Riaz, T.; Aadil, R.M.; Khan, I.M.; et al. Probiotics: Mechanism of action, health benefits and their application in food industries. Front. Microbiol. 2023, 14, 1216674. [Google Scholar] [CrossRef]
- Subramanian, P.; Anandharamakrishnan, C. Introduction to functional foods and nutraceuticals. In Industrial Application of Functional Foods, Ingredients and Nutraceuticals; Academic Press: Cambridge, MA, USA, 2023; pp. 3–43. [Google Scholar] [CrossRef]
- Timothy, B.; Iliyasu, A.H.; Anvikar, A.R. Bacteriocins of lactic acid bacteria and their industrial application. Curr. Top. Lact. Acid Bact. Probiotics 2021, 7, 1–13. [Google Scholar] [CrossRef]
- Gu, Q. Application of Bacteriocins in the Food Industry. In Bacteriocins; Springer: Singapore, 2023; pp. 63–83. [Google Scholar] [CrossRef]
- Bolin, J.F.; Musselman, L.J. Epitypification and ecological notes for the Malagasy holoparasite Hydnora esculenta (Hydnoraceae). Nord. J. Bot. 2013, 31, 286–290. [Google Scholar] [CrossRef]
- Tennakoon, K.U.; Bolin, J.F.; Musselman, L.J.; Maass, E. Structural attributes of the hypogeous holoparasite Hydnora triceps Drège and Meyer (Hydnoraceae). Am. J. Bot. 2007, 94, 1439–1449. [Google Scholar] [CrossRef]
- Naumann, J.; Der, J.P.; Wafula, E.K.; Jones, S.S.; Wagner, S.T.; Honaas, L.A.; Ralph, P.E.; Bolin, J.F.; Maass, E.; Neinhuis, C.; et al. Detecting and characterizing the highly divergent plastid genome of the nonphotosynthetic parasitic plant Hydnora visseri (Hydnoraceae). Genome Biol. Evol. 2016, 8, 345–363. [Google Scholar] [CrossRef]
- Al-Fatimi, M.; Ali, N.A.; Kilian, N.; Franke, K.; Arnold, N.; Kuhnt, C.; Schmidt, J.; Lindequist, U. Ethnobotany, chemical constituents and biological activities of the flowers of Hydnora abyssinica A. Br. (Hydnoraceae). Die Pharm. Int. J. Pharm. Sci. 2016, 71, 222–226. [Google Scholar] [CrossRef]
- Belayneh, A.; Asfaw, Z.; Demissew, S.; Bussa, N.F. Medicinal plants potential and use by pastoral and agro-pastoral communities in Erer Valley of Babile Wereda, Eastern Ethiopia. J. Ethnobiol. Ethnomed. 2012, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Plaatjie, M.T.; Onyiche, T.E.; Ramatla, T.; Bezuidenhout, J.J.; Legoabe, L.; Nyembe, N.I.; Thekisoe, O. A scoping review on efficacy and safety of medicinal plants used for the treatment of diarrhea in sub-Saharan Africa. Trop. Med. Health 2024, 52, 6. [Google Scholar] [CrossRef]
- Kudamba, A.; Kasolo, J.N.; Bbosa, G.S.; Lugaajju, A.; Wabinga, H.; Niyonzima, N.; Ocan, M.; Damani, A.M.; Kafeero, H.M.; Ssenku, J.E.; et al. Medicinal plants used in the management of cancers by residents in the Elgon Sub-Region, Uganda. BMC Complement. Med. Ther. 2023, 23, 450. [Google Scholar] [CrossRef]
- Mkala, E.M.; Mutungi, M.M.; Mutinda, E.S.; Oulo, M.A.; Wanga, V.O.; Mwachala, G.; Hu, G.W. Understanding the Ethnobotany, Chemistry, Pharmacology, and Distribution of Genus Hydnora (Aristolochiaceae). Plants 2021, 10, 494. [Google Scholar] [CrossRef]
- Onyancha, J.M.; Cherongis, C.N.; Nzivo, J.M. Phytochemical screening and evaluation of antioxidant activity of methanolic extract of Kenyan Hydnora abyssinica A. Braun (Hydnoraceae). J. Innov. Pharm. Biol. Sci. 2015, 2, 022–033. [Google Scholar] [CrossRef]
- Kim, J.H.; Jang, M.J.; Park, Y.J. In Vitro α-Amylase, α-Glucosidase, Pancreatic Lipase, Xanthine Oxidase Inhibiting Activity of Agaricus bisporus Extracts. Mycobiology 2023, 51, 60–66. [Google Scholar] [CrossRef]
- Guerin-Danan, C. Storage of intestinal bacteria in samples frozen with glycerol. Microb. Ecol. Health Dis. 1999, 11, 180–182. [Google Scholar] [CrossRef]
- Tanasupawat, S.; Okada, S.; Komagata, K. Lactic acid bacteria found in fermented fish in Thailand. J. Gen. Appl. Microbiol. 1998, 44, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Zendo, T.; Eungruttanagorn, N.; Fujioka, S.; Tashiro, Y.; Nomura, K.; Sera, Y.; Kobayashi, G.; Nakayama, J.; Ishizaki, A.; Sonomoto, K. Identification and production of a bacteriocin from Enterococcus mundtii QU 2 isolated from soybean. J. Appl. Microbiol. 2005, 99, 1181–1190. [Google Scholar] [CrossRef]
- Todorov, S.D. Bacteriocin production by Lactobacillus plantarum AMA-K isolated from Amasi, a Zimbabwean fermented milk product and study of the adsorption of bacteriocin AMA-K to Listeria sp. Braz. J. Microbiol. 2008, 39, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, A.; Gatti, M.; Rizzotti, L.; Torriani, S.; Andrighetto, C.; Giraffa, G. Characterization of Streptococcus macedonicus strains isolated from artisanal Italian raw milk cheeses. Int. Dairy J. 2004, 14, 967–976. [Google Scholar] [CrossRef]
- CLSI (Clinical and Laboratory Standards Institute). Performance Standards for Antimicrobial Disk Susceptibility Tests, 9th ed.; Approved Standard; NCCLS: Wayne, PA, USA, 2006; pp. M2–A9. [Google Scholar]
- Daba, G.M.; Negm El-Dien, A.; Saleh, S.A.; Elkhateeb, W.A.; Awad, G.; Nomayama, T.; Yamashiro, K.; Zendo, T. Evaluation of Enterococcus strains newly isolated from Egyptian sources for bacteriocin production and probiotic potential. Biocatal. Agric. Biotechnol. 2021, 35, 102058. [Google Scholar] [CrossRef]
- Parente, E.; Ciocia, F.; Ricciardi, A.; Zotta, T.; Felis, G.E.; Torriani, S. Diversity of stress tolerance in Lactobacillus plantarum, Lactobacillus pentosus, Lactobacillus paraplantarum: A multivariate screening study. Int. J. Food Microbiol. 2010, 144, 270–279. [Google Scholar] [CrossRef]
- Vinderola, C.G.; Reinheimer, J.A. Lactic acid starter and probiotic bacteria: A comparative “in vitro” study of probiotic characteristics and biological barrier resistance. Food Res. Int. 2003, 36, 895–904. [Google Scholar] [CrossRef]
- Tadayoni, M.; Sheikh-Zeinoddin, M.; Soleimanian-Zad, S. Isolation of bioactive polysaccharide from acorn and evaluation of its functional properties. Int. J. Biol. Macromol. 2015, 72, 179–184. [Google Scholar] [CrossRef] [PubMed]
- El-Hawary, S.S.; Ibrahim, R.M.; Hamed, A.R.; El-Halawany, A.M. Nutritional Evaluation, Chemical Investigation of Phenolic Content and Antioxidant Activity of Ferocactus Glaucescens Ripe Fruits. Egypt. J. Chem. 2020, 63, 2435–2444. [Google Scholar] [CrossRef]
- Direito, R.; Reis, C.; Roque, L.; Gonçalves, M.; Sanches-Silva, A.; Gaspar, M.M.; Pinto, R.; Rocha, J.; Sepodes, B.; Rosário Bronze, M.; et al. Phytosomes with persimmon (diospyros kaki l.) extract: Preparation and preliminary demonstration of in vivo tolerability. Pharmaceutics 2019, 11, 296. [Google Scholar] [CrossRef] [PubMed]
- Darwish, A.M.; Soliman, T.; Elhendy, H.; El-Kholy, W. Nano-encapsulated iron and folic acid fortified functional yogurt enhance anemia in albino rats. Front. Nutr. 2021, 8, 135. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1990. [Google Scholar] [CrossRef]
- Hamed, S.F.; Soliman, T.; Hassan, L.; Abo-Elwafa, G. Preparation of functional yogurt fortified with fish oil-in-water nanoemulsion. Egypt. J. Chem. 2019, 62, 301–314. [Google Scholar] [CrossRef]
- Shehata, S.H.; Soliman, T.N. Preparation and characterization of functional yogurt using incorporated encapsulated curcumin by caseinate. Int. J. Dairy Sci. 2021, 16, 11–17. [Google Scholar] [CrossRef]
- Nasser, S.A. The Addition of lemon peel powder affects the properties of yogurt. J. Food Dairy Sci. 2022, 13, 65–70. [Google Scholar] [CrossRef]
- Moldovan, B.; Iasko, B.; David, L. Antioxidant Activity and Total Phenolic Content of Some Commercial Fruit-Flavoured Yogurts; Studia Universitatis Babes-Bolyai, Chemia: Cluj-Napoca, Romania, 2016; Volume 61, p. 101. [Google Scholar] [CrossRef]
- Fiori, L.; De Faveri, D.; Casazza, A.; Perego, P. Grape by-products: Extraction of polyphenolic compounds using supercritical CO2 and liquid organic solvent–a preliminary investigation Subproductos de la uva: Extracción de compuestos polifenólicos usando CO2 supercrítico y disolventes orgánicos líquidos–una investigación preliminar. Cyta-J. Food 2009, 7, 163–171. [Google Scholar] [CrossRef]
- Lee, B.J.; Kim, J.S.; Kang, Y.M.; Lim, J.H.; Kim, Y.M.; Lee, M.S.; Jeong, M.H.; Ahn, C.B.; Je, J. Antioxidant activity and γ-aminobutyric acid (GABA) content in sea tangle fermented by Lactobacillus brevis BJ20 isolated from traditional fermented foods. Food Chem. 2010, 122, 271–276. [Google Scholar] [CrossRef]
- Pannala, A.S.; Chan, T.S.; O’Brien, P.J.; Rice-Evans, C.A. Flavonoid B-ring chemistry and antioxidant activity: Fast reaction kinetics. Biochem. Biophys. Res. Commun. 2001, 282, 1161–1168. [Google Scholar] [CrossRef] [PubMed]
- Ennahar, S.; Sashihara, T.; Sonomoto, K.; Ishizaki, A. Class IIa bacteriocins: Biosynthesis, structure and activity. FEMS Microbiol. Rev. 2000, 24, 85–106. [Google Scholar] [CrossRef] [PubMed]
- Goel, A.; Halami, P.M. Structural and biosynthetic diversity of plantaricins from Lactiplantibacillus. Appl. Microbiol. Biotechnol. 2023, 107, 5635–5649. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, B.; Bangar, S.P.; Echegaray, N.; Suri, S.; Tomasevic, I.; Manuel Lorenzo, J.; Melekoglu, E.; Rocha, J.M.; Ozogul, F. The impacts of Lactiplantibacillus plantarum on the functional properties of fermented foods: A review of current knowledge. Microorganisms 2022, 10, 826. [Google Scholar] [CrossRef] [PubMed]
- Selegård, R.; Musa, A.; Nyström, P.; Aili, D.; Bengtsson, T.; Khalaf, H. Plantaricins markedly enhance the effects of traditional antibiotics against Staphylococcus epidermidis. Future Microbiol. 2019, 14, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Kothari, D.; Patel, S.; Kim, S.K. Probiotic supplements might not be universally-effective and safe: A review. Biomed. Pharmacother. 2019, 111, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, B.M.; Stanton, C.; Fitzgerald, G.F.; Ross, R. Survival of probiotic lactobacilli in acidic environments is enhanced in the presence of metabolizable sugars. Appl. Environ. Microbiol. 2005, 71, 3060–3067. [Google Scholar] [CrossRef] [PubMed]
- Won, G.; Choi, S.I.; Park, N.; Kim, J.E.; Kang, C.H.; Kim, G.H. In Vitro Antidiabetic, Antioxidant Activity, and Probiotic Activities of Lactiplantibacillus plantarum and Lacticaseibacillus paracasei Strains. Curr. Microbiol. 2021, 78, 3181–3191. [Google Scholar] [CrossRef] [PubMed]
- Campaniello, D.; Speranza, B.; Petruzzi, L.; Bevilacqua, A.; Corbo, M.R. How to routinely assess transition, adhesion and survival of probiotics into the gut: A case study on propionibacteria. Int. J. Food Sci. Technol. 2018, 53, 484–490. [Google Scholar] [CrossRef]
- Nath, S.; Sikidar, J.; Roy, M.; Deb, B. In Vitro screening of probiotic properties of Lactobacillus plantarum isolated from fermented milk product. Food Qual. Saf. 2020, 4, 213–223. [Google Scholar] [CrossRef]
- Wang, Y.; Ren, X.; Song, X.; Yu, T.; Lu, H.; Wang, P.; Wang, J.; Zheng, X.D. Control of postharvest decay on cherry tomatoes by marine yeast Rhodosporidium paludigenum and calcium chloride. J. Appl. Microbiol. 2010, 109, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Bubnov, R.V.; Babenko, L.P.; Lazarenko, L.M.; Mokrozub, V.V.; Spivak, M.Y. Specific properties of probiotic strains: Relevance and benefits for the host. EPMA J. 2018, 9, 205–223. [Google Scholar] [CrossRef] [PubMed]
- de Souza, B.M.; Borgonovi, T.F.; Casarotti, S.N.; Todorov, S.D.; Penna, A.L. Lactobacillus casei and Lactobacillus fermentum strains isolated from mozzarella cheese: Probiotic potential, safety, acidifying kinetic parameters and viability under gastrointestinal tract conditions. Probiotics Antimicrob. Proteins 2019, 11, 382–396. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.; Prasad, D.N. Application of in vitro methods for selection of Lactobacillus casei strains as potential probiotics. Int. J. Food Microbiol. 2005, 103, 109–115. [Google Scholar] [CrossRef]
- Saini, K.; Tomar, S.K. In Vitro evaluation of probiotic potential of Lactobacillus cultures of human origin capable of selenium bioaccumulation. LWT 2017, 84, 497–504. [Google Scholar] [CrossRef]
- Jang, H.J.; Lee, N.K.; Paik, H.D. Probiotic characterization of Lactobacillus brevis KU15153 showing antimicrobial and antioxidant effect isolated from kimchi. Food Sci. Biotechnol. 2019, 28, 1521–1528. [Google Scholar] [CrossRef] [PubMed]
- De Giani, A.; Bovio, F.; Forcella, M.E.; Lasagni, M.; Fusi, P.; Di Gennaro, P. Prebiotic effect of Maitake extract on a probiotic consortium and its action after microbial fermentation on colorectal cell lines. Foods 2021, 10, 2536. [Google Scholar] [CrossRef] [PubMed]
- Badhani, B.; Sharma, N.; Kakkar, R. Gallic acid: A versatile antioxidant with promising therapeutic and industrial applications. Rsc. Adv. 2015, 5, 27540–27557. [Google Scholar] [CrossRef]
- Grzesik, M.; Naparło, K.; Bartosz, G.; Sadowska-Bartosz, I. Antioxidant properties of catechins: Comparison with other antioxidants. Food Chem. 2018, 241, 480–492. [Google Scholar] [CrossRef]
- Bratovcic, A.; Suljagic, J. Micro-and nano-encapsulation in food industry. Croat. J. Food Sci. Technol. 2019, 11, 113–121. [Google Scholar] [CrossRef]
- Permana, A.D.; Utami, R.; Courtenay, A.; Manggau, M.; Donnelly, R.; Rahman, L. Phytosomal nanocarriers as platforms for improved delivery of natural antioxidant and photoprotective compounds in propolis: An approach for enhanced both dissolution behaviour in biorelevant media and skin retention profiles. J. Photochem. Photobiol. B Biol. 2020, 205, 111846. [Google Scholar] [CrossRef] [PubMed]
- El Badawy, A.M.; Silva, R.; Morris, B.; Scheckel, K.; Suidan, M.; Tolaymat, T. Surface charge-dependent toxicity of silver nanoparticles. Environ. Sci. Technol. 2011, 45, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Kraft, J.C.; Freeling, J.; Wang, Z.; Ho, R. Emerging research and clinical development trends of liposome and lipid nanoparticle drug delivery systems. J. Pharm. Sci. 2014, 103, 29–52. [Google Scholar] [CrossRef] [PubMed]
- Santillán-Urquiza, E.; Ruiz-Espinosa, H.; Angulo-Molina, A.; Ruiz, J.F.; Méndez-Rojas, M.A. Applications of nanomaterials in functional fortified dairy products: Benefits and implications for human health. In Nutrient Delivery; Academic Press: Cambridge, MA, USA, 2017; pp. 293–328. [Google Scholar] [CrossRef]
- Salama, H.H.; Elsaid, M.M.; Abdel Hamid, S.M.; Abozed, S.S.; Mounier, M. Effect of fortification with sage loaded liposomes on the chemical, physical, microbiological properties and cytotoxicity of yogurt. Egypt. J. Chem. 2020, 63, 3879–3890. [Google Scholar] [CrossRef]
- Tamine, A.Y.; Robinson, R.K. Yogurt: Science and Technology; CRC Press: Boca Raton, FL, USA, 1999. [Google Scholar] [CrossRef]
- Dabija, A.; Codină, G.G.; Ropciuc, S.; Gâtlan, A.M.; Rusu, L. Assessment of yogurt’s antioxidant activity and quality attributes enhanced with wild herbs extracts. J. Food Qual. 2018, 2018, 5329386. [Google Scholar] [CrossRef]
- Nishino, T.; Shibahara-Sone, H.; Kikuchi-Hayakawa, H.; Ishikawa, F. Transit of radical scavenging activity of milk products prepared by maillardreaction and Lactobacillus casei Strain Shirota fermentation through the hamster intestine. J. Dairy Sci. 2000, 83, 915–922. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, G.; Agrawal, R. Antioxidant activity and fatty acid profile of fermented milk prepared by Pediococcus pentosaceus. J. Food Sci. Technol. 2014, 51, 4138–4142. [Google Scholar] [CrossRef]
- Plessas, S.; Mantzourani, I.; Terpou, A.; Bekatorou, A. Assessment of the Physicochemical, Antioxidant, Microbial, and Sensory Attributes of Yogurt-Style Products Enriched with Probiotic-Fermented Aronia melanocarpa Berry Juice. Foods 2023, 13, 111–125. [Google Scholar] [CrossRef]



| Indicator Species | Strain * | Activity (AU/mL) |
|---|---|---|
| Enterococcus faecalis | JCM 5803T | 800 |
| Enterococcus faecium | JCM 5804T | 400 |
| Latilactobacillus sakei | JCM 1157T | 400 |
| Leuconostoc mesenteroides | JCM 6124T | 200 |
| Listeria innocua | ATCC 33090T | 200 |
| Pediococcus pentosaceus | JCM 5885 | 200 |
| Lactococcus lactis ssp. lactis | IL1403 | 0 |
| Lactococcus lactis ssp. lactis | ATCC 19435T | 0 |
| Lactococcus lactis ssp. lactis | NCDO 497 | 0 |
| Weizmannia coagulans | JCM 2257T | 0 |
| Kocuria rhizophila | NBRC 12708 | 0 |
| Hydnora abyssinica Extract Conc. (mg/mL) | Prebiotic Activity (%) |
|---|---|
| 25 | 106.20 ± 0.42 |
| 50 | 106.42 ± 0.03 |
| 75 | 107.22 ± 0.57 |
| 100 | 107.54 ± 1.48 |
| 200 | 113.14 ± 1.76 |
| Phenolic Standard | Area | Conc. (µg/g) |
|---|---|---|
| Gallic acid | 489.94 | 1926.98 |
| Chlorogenic acid | 384.83 | 2385.18 |
| Catechin | 452.82 | 5343.29 |
| Methyl gallate | 44.48 | 139.72 |
| Caffeic acid | 21.64 | 79.04 |
| Syringic acid | 1.62 | 5.32 |
| Ellagic acid | 27.24 | 850.33 |
| Coumaric acid | 5.85 | 8.43 |
| Cinnamic acid | 37.24 | 33.53 |
| Kaempferol | 5.86 | 31.66 |
| Phytosomes (Molar Ratio) | Before Sonication | After Sonication | EE% | ||||
|---|---|---|---|---|---|---|---|
| Average Particle Size ± SD (nm) | Polydispersity Index (PI) | Zeta (mV) | Average Particle Size ± SD (nm) | Polydispersity Index (PI) | Zeta (mV) | ||
| 1:1 | 380 a ± 56 | 0.283 | −29.5 a ± 0.19 | 328.0 a ± 58 | 0.163 | −29.9 a ± 0.09 | 90.68 a ± 3.27 |
| 1:2 | 310 b ± 49 | 0.328 | −34.0 b ± 0.01 | 222.9 b ± 35 | 0.273 | −35.0 b ± 0.11 | 93.87 a ± 2.79 |
| 1:3 | 251 a ± 37 | 0.398 | −35.1 b ± 1.1 | 72.56 b ± 9 | 0.323 | −37.8 bc ±0.05 | 95.45 a ± 3.20 |
| Samples | pH | Titratable Acidity (TA) | ||||
|---|---|---|---|---|---|---|
| Fresh | 7 Days | 14 Days | Fresh | 7 Days | 14 Days | |
| C | 4.60 Aa ± 0.01 | 4.55 ABa ± 0.03 | 4.47 Ca ± 0.02 | 0.86 Cb ± 0.02 | 0.89 ABa ± 0.02 | 0.92 Aa ± 0.01 |
| T1 | 4.58 Aa ± 0.02 | 4.55 ABa ± 0.01 | 4.48 Ca ± 0.01 | 0.86 Cb ± 0.01 | 0.88 ABa ± 0.03 | 0.92 Aa ± 0.03 |
| T2 | 4.59 Aa ± 0.01 | 4.56 ABa ± 0.02 | 4.47 Ca ± 0.02 | 0.86 Bb ± 0.03 | 0.88 Ba ± 0.02 | 0.92 Aa ± 0.04 |
| T3 | 4.54 Ab ± 0.02 | 4.51 ABa ± 0.01 | 4.43 Cab ± 0.01 | 0.88 aC ± 0.02 | 0.93 ABb ± 0.04 | 0.97 Aa ± 0.04 |
| T4 | 4.52 Abc ± 0.01 | 4.50 Aab ± 0.02 | 4.41 Cb ± 0.02 | 0.89 Ba ± 0.01 | 0.95 Abc ± 0.03 | 0.99 Aa ± 0.04 |
| Hardness (N) | Springiness (mm) | Cohesiveness | Gumminess (N) | Chewiness (N·mm) | |
|---|---|---|---|---|---|
| C | 1.61 d ± 0.10 | 0.64 a ± 0.01 | 0.40 a ± 0.01 | 0.64 c ± 0.02 | 0.41 b ± 0.01 |
| T1 | 2.60 b ± 0.15 | 0.57 b ± 0.02 | 0.34 b ± 0.01 | 0.88 a ± 0.01 | 0.51 a ± 0.03 |
| T2 | 3.00 a ± 0.10 | 0.38 cd ± 0.01 | 0.20 c ± 0.02 | 0.59 d ± 0.01 | 0.22 e ± 0.01 |
| T3 | 1.70 bc ±0.05 | 0.53 b ± 0.03 | 0.30 b ± 0.02 | 0.52 e ± 0.02 | 0.28 d ± 0.02 |
| T4 | 1.75 c ± 0.05 | 0.43 c ± 0.01 | 0.42 a ± 0.01 | 0.74 b ± 0.02 | 0.32 c ± 0.01 |
| Sample | DPPH | ABTS | ||||
|---|---|---|---|---|---|---|
| Fresh | 7 Days | 14 Days | Fresh | 7 Days | 14 Days | |
| C | 59.45 c ± 4.17 | 68.66 d ± 0.19 | 81.66 c ± 3.29 | 9.95 d ± 2.96 | 13.65 d ± 1.98 | 13.98 c ± 57 |
| T1 | 74.25 b ± 5.22 | 84.26 b ± 0.48 | 86.89 b± 0.99 | 46.07 ab ± 8.14 | 51.57 b ± 6.45 | 52.00 ab± 7.54 |
| T2 | 80.76 a ± 1.98 | 91.08 a ± 0.86 | 89.54 a ± 2.18 | 58.6 a ± 3.33 | 63.35 a ± 7.58 | 64.15 a ± 8.19 |
| T3 | 63.24 c ± 2.03 | 84.26 b ± 0.86 | 82.58 c ± 0.79 | 42.16 c ± 4.29 | 44.78 c ± 3.95 | 46.91 b ± 4.17 |
| T4 | 67.45 c ± 1.78 | 79.08 bc ± 0.96 | 89.19 a ± 1.09 | 55.13 a ± 3.29 | 56.75 ab ± 2.95 | 59.28 a ± 3.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daba, G.; Elkhateeb, W.; Soliman, T.N.; Negm El-Dein, A.; Zendo, T. Improving the Functionality of Yogurt after Fortification with a Synbiotic Combination of a Potential Probiotic and Bacteriocin-Producing Bacteria and Hydnora abyssinica Phytosomes. Processes 2024, 12, 727. https://doi.org/10.3390/pr12040727
Daba G, Elkhateeb W, Soliman TN, Negm El-Dein A, Zendo T. Improving the Functionality of Yogurt after Fortification with a Synbiotic Combination of a Potential Probiotic and Bacteriocin-Producing Bacteria and Hydnora abyssinica Phytosomes. Processes. 2024; 12(4):727. https://doi.org/10.3390/pr12040727
Chicago/Turabian StyleDaba, Ghoson, Waill Elkhateeb, Tarek Nour Soliman, Asmaa Negm El-Dein, and Takeshi Zendo. 2024. "Improving the Functionality of Yogurt after Fortification with a Synbiotic Combination of a Potential Probiotic and Bacteriocin-Producing Bacteria and Hydnora abyssinica Phytosomes" Processes 12, no. 4: 727. https://doi.org/10.3390/pr12040727
APA StyleDaba, G., Elkhateeb, W., Soliman, T. N., Negm El-Dein, A., & Zendo, T. (2024). Improving the Functionality of Yogurt after Fortification with a Synbiotic Combination of a Potential Probiotic and Bacteriocin-Producing Bacteria and Hydnora abyssinica Phytosomes. Processes, 12(4), 727. https://doi.org/10.3390/pr12040727







