Pyridazinic Bioisosteres with Potential Applications in Medicinal Chemistry and Agriculture
Abstract
1. Introduction
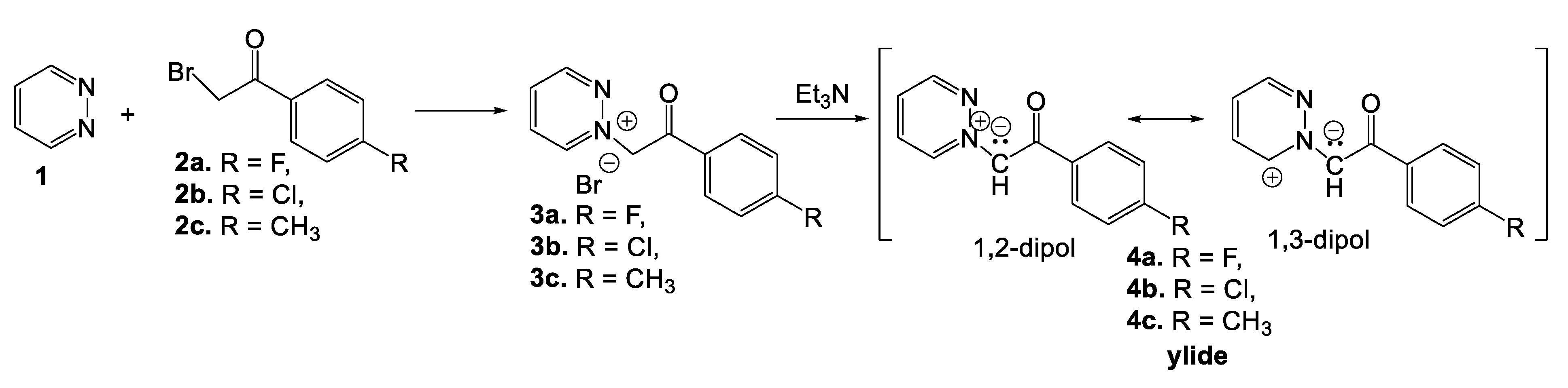
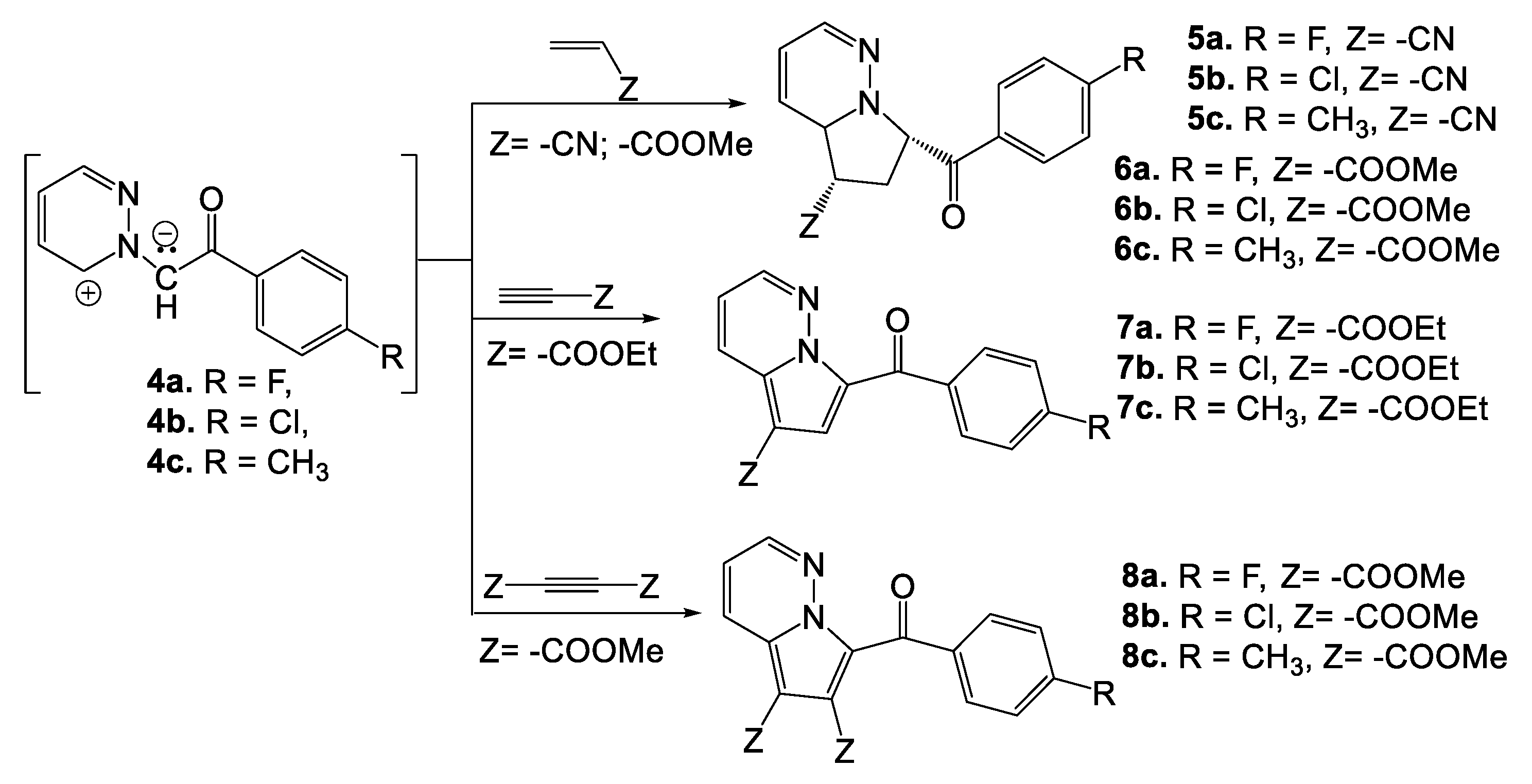
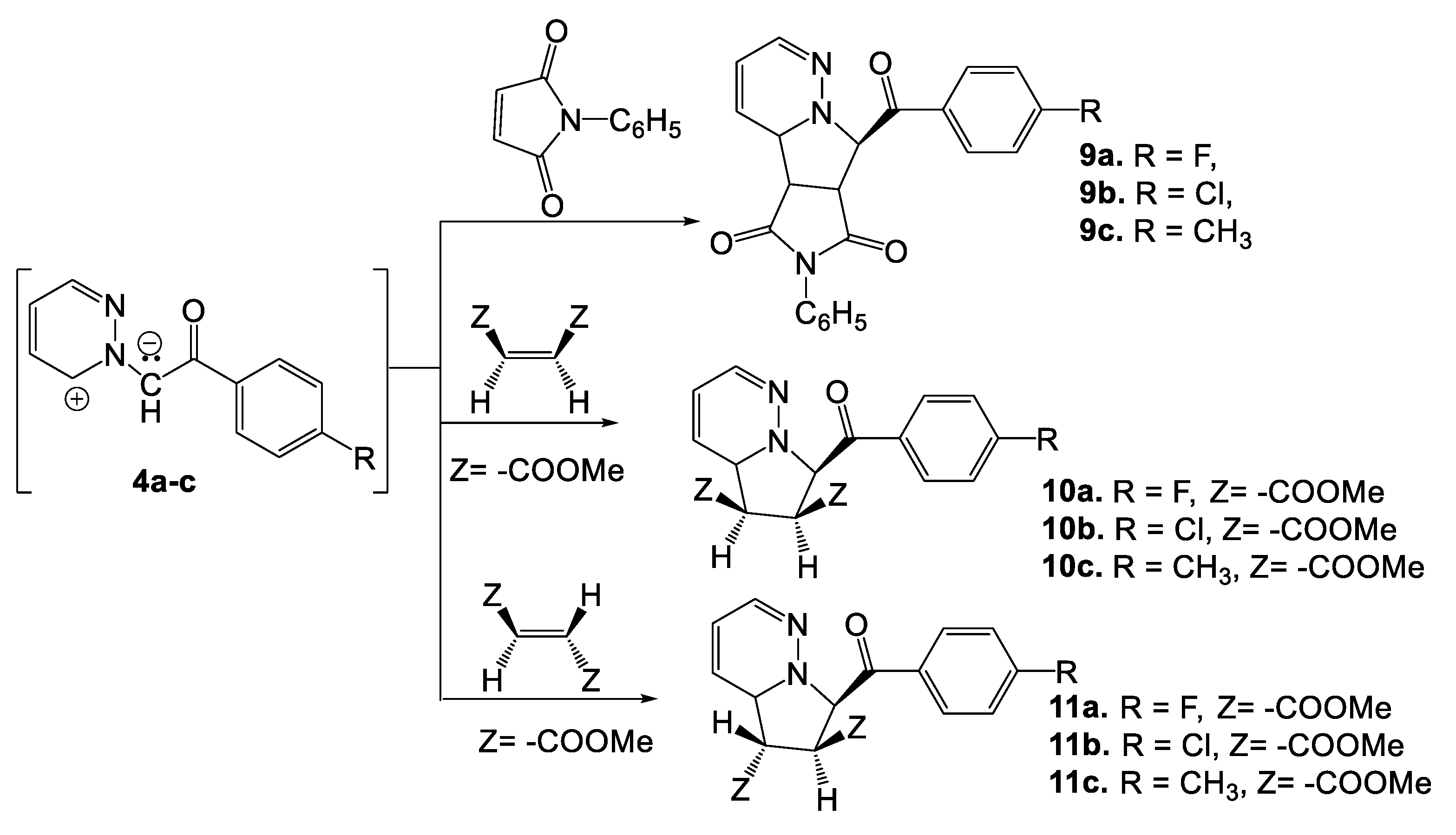
2. Synthesis of Pyridazine Bioisosteres
3. Characterization of Pyridazine Bioisosteres and Investigation of Their Effects
3.1. The Spectral Characterization of Pyridazine Bioisosteres
3.2. The Biological Activity of Pyridazine Bioisosteres
3.3. Tables
3.4. Antibacterial Activity
3.5. Antifungal Activity
3.6. The Biologic Effect on Wheat Germination and Seedling Growth
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nathan, B. Bioisosteres in Medicinal Chemistry; Wiley-VCH: Weinheim, Germany, 2012; Volume XVIII, p. 237. [Google Scholar]
- Silverman, R.B.; Holladay, M.W. The Organic Chemistry of Drug Design and Drug Action, 3rd ed.; Academic Press: London, UK, 2014; pp. 54–93. ISBN 9780123820303. [Google Scholar]
- Lima, L.M.; Barreiro, E. Bioisosterism: A Useful Strategy for Molecular Modification and Drug Design. Curr. Med. Chem. 2005, 12, 23–49. [Google Scholar] [CrossRef]
- Mangalagiu, I.I. Recent achievements in the chemistry of 1,2-diazines. Curr. Org. Chem. 2011, 15, 730–752. [Google Scholar] [CrossRef]
- Malik, A.; Mishra, R.; Mazumder, R.; Mazumder, A.; Mishra, P.S. A comprehensive study on synthesis and biological activities of pyridazine derivatives. Res. J. Pharm. Technol. 2021, 14, 3423–3429. [Google Scholar] [CrossRef]
- Amariucai-Mantu, D.; Mangalagiu, V.; Mangalagiu, I.I. [3 + n] Cycloaddition Reactions: A Milestone Approach for Elaborating Pyridazine of Potential Interest in Medicinal Chemistry and Optoelectronics. Molecules 2021, 26, 3359. [Google Scholar] [CrossRef]
- Amariucai-Mantu, D.; Mangalagiu, V.; Danac, R.; Mangalagiu, I.I. Microwave assisted reactions of azaheterocycles for medicinal chemistry applications. Molecules 2020, 25, 716. [Google Scholar] [CrossRef]
- Zbancioc, G.; Mangalagiu, I.I.; Moldoveanu, C. A Review on the Synthesis of Fluorescent Five- and Six-Membered Ring Azaheterocycles. Molecules 2022, 27, 6321. [Google Scholar] [CrossRef]
- Moldoveanu, C.; Mangalagiu, I.I.; Zbancioc, G. Fluorescent Azasteroids through Ultrasound Assisted Cycloaddition Reactions. Molecules 2021, 26, 5098. [Google Scholar] [CrossRef]
- Cheng, Y.; Ma, B.; Wudl, F. Synthesis and Optical Properties of a Series of Pyrrolopyridazine Derivatives: Deep Blue Organic Luminophors for Electroluminescent Devices. J. Mater. Chem. 1999, 9, 2183–2188. [Google Scholar] [CrossRef]
- Mangalagiu, I.I.; Baban, C.; Mardare, D.; Rusu, G.I. On the electrical properties of some new stable disubstituted ylides in thin films. Appl. Surf. Sci. 1997, 108, 205–210. [Google Scholar] [CrossRef]
- Tikkinen, M.; Riikonen, J.; Luoranen, J. Covering Norway spruce container seedlings with reflectiveshading cloth during field storage affects seedling post-planting growth. New For. 2022, 53, 627–642. [Google Scholar] [CrossRef]
- Butnariu, R.; Risca, I.M.; Caprosu, M.; Drochioiu, G.; Mangalagiu, I.I. Biological activity of some new pyridazine derivatives in wheat germination experiments. Rom. Biotechnol. Lett. 2008, 13, 3837–3842. [Google Scholar]
- Mantu, D.; Antoci, V.; Nicolescu, A.; Delenu, C.; Vasilache, V.; Mangalagiu, I.I. Synthesis, stereochemical studies and antimycobacterial activity of new acetyl-hydrazinespyridazinone. Curr. Org. Synth. 2017, 14, 112–119. [Google Scholar] [CrossRef]
- Aricu, A.; Ciocarlan, A.; Lungu, L.; Barba, A.; Shova, S.; Zbancioc, G.; Mangalagiu, I.I.; D’Ambrosio, M.; Vornicu, N. Synthesis of new antibacterial and antifungal drimane sesquiterpenoids with azaheterocyclic units. Med. Chem. Res. 2016, 25, 2316–2323. [Google Scholar] [CrossRef]
- Balan, A.M.; Miron, A.; Tuchilus, C.; Rotinberg, P.; Mihai, C.T.; Mangalagiu, I.I.; Zbancioc, G. Synthesis and in vitro analysis of novel dihydroxyacetophenone derivatives with antimicrobial and antitumor activities. Med. Chem. 2014, 10, 476–483. [Google Scholar]
- Kuchkova, K.; Aricu, A.; Barba, A.; Vlad, P.; Shova, S.; Secara, E.; Ungur, N.; Tuchilus, C.; Zbancioc, G.; Mangalagiu, I.I. Design, syntheses and antimicrobial activity of some novel homodrimane sesquiterpenoids with diazine skeleton. Med. Chem. Res. 2014, 23, 1559–1568. [Google Scholar] [CrossRef]
- Antoci, V.; Mantu, D.; Cozma, D.G.; Usru, C.; Mangalagiu, I.I. Hybrid anticancer 1,2-diazine derivatives with multiple mechanism of action. Part 3. Med. Hyp. 2014, 82, 11–15. [Google Scholar] [CrossRef]
- Tucaliuc, R.; Cotea, V.; Niculaua, M.; Tuchilus, C.; Mantu, D.; Mangalagiu, I.I. New pyridazine—Fluorine derivatives: Synthesis, chemistry and biological activity. Part II. Eur. J. Med. Chem. 2013, 67, 367–372. [Google Scholar] [CrossRef]
- Mantu, D.; Antoci, V.; Mangalagiu, I.I. Design, synthesis and antituberculosis activity of some new pyridazine derivatives: Bis-pyridazine. Part IV. Infect. Disord. Drug Targets 2013, 13, 344–351. [Google Scholar] [CrossRef]
- Mantu, D.; Luca, M.C.; Moldoveanu, C.; Zbancioc, G.; Mangalagiu, I.I. Synthesis and antituberculosis activity of some new pyridazine derivatives. Part II. Eur. J. Med. Chem. 2010, 45, 5164–5168. [Google Scholar] [CrossRef]
- Zbancioc, A.M.; Zbancioc, G.; Tanase, C.; Miron, A.; Ursu, C.; Mangalagiu, I.I. Design, synthesis and in vitro anticancer activity of a new class of dual DNA intercalators. Lett. Drug. Des. Discov. 2010, 7, 644–649. [Google Scholar] [CrossRef]
- Luca, M.C.; Tura, V.; Mangalagiu, I.I. Considerations concerning design and mechanism of action of a new class of dual DNA intercalators. Med. Hyp. 2010, 75, 627–629. [Google Scholar] [CrossRef]
- Balan, A.M.; Florea, O.; Moldoveanu, C.; Zbancioc, G.; Iurea, D.; Mangalagiu, I.I. Diazinium salts with dihydroxyacetophenone skeleton: Syntheses and antimicrobial activity. Eur. J. Med. Chem. 2009, 44, 2275–2279. [Google Scholar] [CrossRef] [PubMed]
- Butnariu, R.; Mangalagiu, I.I. New pyridazine derivatives: Synthesis, chemistry and biological activity. Bioorg. Med. Chem. 2009, 17, 2823–2829. [Google Scholar] [CrossRef]
- Butnariu, R.; Caprosu, M.; Bejan, V.; Ungureanu, M.; Poiata, A.; Tuchilus, C.; Florescu, M.; Mangalagiu, I.I. Pyridazine and Phthalazine Derivatives with Potential Antimicrobial Activity. J. Heterocyclic Chem. 2007, 44, 1149–1152. [Google Scholar] [CrossRef]
- Moldoveanu, C.; Mangalagiu, G.; Drochioiu, G.; Caprosu, M.; Petrovanu, M.; Mangalagiu, I.I. New Antituberculosis Compounds Derived from Diazine. An. Stiint. Univ. “Al. I. Cuza” Iasi Chem. 2003, 11, 367–374. [Google Scholar]
- Ungureanu, M.; Mangalagiu, I.I.; Grosu, G.; Petrovanu, M. Antimicrobial activity of some new pyridazine derivatives. Ann. Pharm. Fr. 1997, 55, 69–72. [Google Scholar]
- Zbancioc, G.; Mangalagiu, I.I. Microwave-Assisted Synthesis of Highly Fluorescent Pyrrolopyridazine Derivatives. Synlett 2006, 5, 804–806. [Google Scholar] [CrossRef]
- Zbancioc, G.; Huhn, T.; Groth, U.; Deleanu, C.; Mangalagiu, I.I. Pyrrolodiazine derivatives as blue organic luminophores: Synthesis and properties. Part 3. Tetrahedron 2010, 66, 4298–4306. [Google Scholar] [CrossRef]
- Zbancioc, G.; Mangalagiu, I.I. Pyrrolopyridazine derivatives as blue organic luminophores: Synthesis and properties. Part 2. Tetrahedron 2010, 66, 278–282. [Google Scholar] [CrossRef]
- Maftei, D.; Zbancioc, G.; Humelnicu, I.; Mangalagiu, I.I. Conformational effects on the lowest excited states of benzoyl-pyrrlopyridazine: Insights from PCM time-dependent DFT. J. Phys. Chem. A 2013, 117, 3165–3175. [Google Scholar] [CrossRef]
- Moldoveanu, C.; Amariucai-Mantu, D.; Mangalagiu, V.; Antoci, V.; Maftei, D.; Mangalagiu, I.I.; Zbancioc, G. Microwave Assisted Reactions of Fluorescent Pyrrolodiazine Building Blocks. Molecules 2019, 24, 3760. [Google Scholar] [CrossRef] [PubMed]
- Zbancioc, G.; Ciobanu, C.I.; Mangalagiu, I.I.; Moldoveanu, C. Ultrasound-Assisted Synthesis of Fluorescent Azatetracyclic Derivatives: An Energy-Efficient Approach. Molecules 2022, 27, 3180. [Google Scholar] [CrossRef] [PubMed]
- Risca, I.M.; Zbancioc, G.; Moldoveanu, C.; Drochioiu, G.; Mangalagiu, I.I. Effect of some new monoquaternary salts of diazine on germination and seedling growth of Norway spruce (Picea abies (L.) Karsten). Rom. Biotechnol. Lett. 2006, 11, 2563–2568. [Google Scholar]
- Mangalagiu, I.I.; Risca, I.; Maftei, M.; Moldoveanu, C.; Murariu, M.; Drochioiu, G. Effect of some new monoquaternary salts of pyridazine on germination and seedling growth of wheat. Rom. Biotech. Lett. 2005, 10, 2495–2501. [Google Scholar]
- Caprosu, M.; Butnariu, R.; Mangalagiu, I.I. Synthesis and antimicrobial activity of some new pyridazine derivatives. Heterocycles 2005, 65, 1871–1879. [Google Scholar]
- Tucaliuc, R.; Cotea, V.V.; Drochioiu, G.; Mangalagiu, I.I. Synthesis and the effect of some pyridazine derivatives in germination and seedling growth of wheat. Lucr. Ştiinţifice Ser. Hortic. USAMV Iaşi 2011, 54, 109–114. Available online: https://www.uaiasi.ro/revista_horti/files/arhiva/Vol-54-2_2011.pdf (accessed on 27 April 2023).
- Begue, J.P.; Bonnet-Delpon, D. Recent advances in fluorinated pharmaceuticals based on natural products. J. Fluor. Chem. 2006, 127, 992–1012. [Google Scholar] [CrossRef]
- Molteni, M.; Pesenti, C.; Sani, M.; Volonterio, A.; Zanda, M. Fluorinated peptidomimetics: Synthesis, conformational and biological features. J. Fluor. Chem. 2004, 125, 1735–1743. [Google Scholar] [CrossRef]
- Snedecor, G.W. Statistical Methods Applied to Experiments in Agriculture and Biology; The Iowa Stat University Press: Ames, IA, USA, 1994; pp. 255–274. ISBN 978-0813815619. [Google Scholar]

| Comp. | S. aureus (1) | S. Lutea (2) | B. subtillis (3) | P. aeruginosa (4) | E. coli (5) | C. albicans (6) |
|---|---|---|---|---|---|---|
| Chloram phenicol 30 μg/disc | 30 | 40 | 26 | 19 | 25 | - |
| Nystatin, 100 μg/disc | - | - | - | - | - | 29 |
| 3a. | 38 | 61 | 31 | 20 | 31 | 19 |
| 3b. | 36 | 57 | 32 | 20 | 36 | 29 |
| 3c. | 47 | 81 | 40 | 20 | 25 | 27 |
| 5a. | 26 | 50 | 27 | 14 | 20 | 23 |
| 5b. | 30 | 57 | 29 | 14 | 28 | 35 |
| 5c. | 46 | 67 | 39 | 19 | 23 | 30 |
| 7a. | 27 | 48 | 22 | 20 | 23 | 22 |
| 7b. | 25 | 48 | 25 | 20 | 33 | 30 |
| 7c. | 40 | 56 | 37 | 18 | 25 | 28 |
| 8a. | 27 | 52 | 23 | 15 | 21 | 21 |
| 8b. | 30 | 57 | 26 | 18 | 31 | 24 |
| 8c. | 46 | 65 | 26 | 19 | 25 | 29 |
| 9a. | 27 | 63 | 28 | 16 | 25 | 25 |
| 9b. | 28 | 54 | 28 | 18 | 28 | 34 |
| 9c. | 31 | 73 | 37 | 18 | 24 | 29 |
| 10a. | 29 | 60 | 37 | 18 | 35 | 24 |
| 10b. | 33 | 56 | 39 | 18 | 32 | 25 |
| 10c. | 41 | 62 | 39 | 19 | 37 | 30 |
| 11a. | 29 | 51 | 35 | 17 | 35 | 27 |
| 11b. | 23 | 54 | 36 | 15 | 31 | 27 |
| 11c. | 47 | 59 | 38 | 17 | 37 | 29 |
| Comp. | S. aureus | S. lutea | B. subtillis | P. aeruginosa | E. coli | C. albicans |
|---|---|---|---|---|---|---|
| Chloram phenicol 30 μg/disc | 25 | 30 | 25 | 19 | 29 | - |
| Nystatin, 100 μg/disc | - | - | - | - | - | 29 |
| 12a. | 30 | 41 | 31 | 16 | 34 | 29 |
| 12b. | 29 | 44 | 38 | 18 | 42 | 26 |
| 12c. | 49 | 60 | 36 | 19 | 40 | 32 |
| 13a. | 28 | 55 | 31 | 19 | 26 | 27 |
| 13b. | 30 | 58 | 33 | 20 | 35 | 37 |
| 13c. | 30 | 59 | 39 | 20 | 28 | 30 |
| 14a. | 88 | 58 | 38 | 21 | 31 | 29 |
| 14b, b’ | 35 | 61 | 42 | 19 | 38 | 39 |
| 14c. | 29 | 61 | 38 | 21 | 27 | 31 |
| Compound | Germination Rate (GR, %) | Number of Plantlets in the Lot |
|---|---|---|
| 3a. | 64 ± 5 | 26 ± 1 |
| 3b. | 38 ± 4 | 14 ± 5 |
| 3c. | 69 ± 4 | 9 ± 2 |
| 5a. | 61 ± 4 | 26 ± 4 |
| 5b. | 54 ± 5 | 20 ± 3 |
| 7a. | 81 ± 3 | 34 ± 4 |
| 7b. | 54 ± 4 | 20 ± 4 |
| 8a. | 73 ± 5 | 32 ± 2 |
| 8b. | 80 ± 4 | 24 ± 3 |
| 9a. | 72 ± 4 | 34 ± 3 |
| 9b. | 72 ± 4 | 25 ± 1 |
| 10a. | 73 ± 5 | 30 ± 3 |
| 10b. | 61 ± 5 | 17 ± 2 |
| 10c. | 59 ± 6 | 22 ± 1 |
| 11a. | 0 ± 0 | 0 ± 0 |
| 11b. | 79 ± 4 | 26 ± 6 |
| 11c. | 40 ± 5 | 28 ± 5 |
| Compound | H, cm | Hm, cm | W, g | Wm, mg |
|---|---|---|---|---|
| 3a. | 121 ± 15 | 5 ± 1 | 0.89 ± 0.12 | 40 ± 5 |
| 3b. | 52 ± 13 | 5 ± 0.4 | 0.47 ± 0.19 | 42 ± 17 |
| 3c. | 52 ± 13 | 5 ± 0.9 | 0.47 ± 0.41 | 40 ± 3 |
| 5a. | 178 ± 17 | 6 ± 0.6 | 1 ± 0.12 | 34 ± 2 |
| 5b. | 120 ± 15 | 7 ± 0.8 | 0.8 ± 0.13 | 44 ± 0.07 |
| 7a. | 239 ± 17 | 6 ± 0.4 | 1.38 ± 0.16 | 35 ± 4 |
| 7b. | 117 ± 6 | 27 ± 0.4 | 0.75 ± 0.07 | 43 ± 4 |
| 8a. | 233 ± 26 | 7 ± 0.7 | 1.37 ± 0.14 | 37 ± 4 |
| 8b. | 133 ± 3 | 6 ± 0.1 | 0.87 ± 0.17 | 42 ± 8 |
| 9a. | 198 ± 7 | 5 ± 0.6 | 1.11 ± 0.15 | 29 ± 13 |
| 9b. | 149 ± 8 | 7 ± 0.3 | 0.95 ± 0.14 | 45 ± 4 |
| 10a. | 186 ± 32 | 6 ± 0.9 | 1.11 ± 0.19 | 32 ± 5 |
| 10b. | 77 ± 10 | 6 ± 0.7 | 0.64 ± 0.30 | 42 ± 2 |
| 10c. | 11 ± 0.3 | 0.55 ± 0.01 | 0.08 ± 0.01 | 40 ± 0.01 |
| 11a. | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| 11b. | 185 ± 19 | 8 ± 0.8 | 1.06 ± 0.19 | 47 ± 8.48 |
| 11c. | 136 ± 31 | 6 ± 1.3 | 0.98 ± 0.08 | 42 ± 3.36 |
| W (water) | 224 ± 23 | 7 ± 0.7 | 1.42 ± 0.19 | 42 ± 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tucaliuc, R.A.; Mangalagiu, V.; Mangalagiu, I.I. Pyridazinic Bioisosteres with Potential Applications in Medicinal Chemistry and Agriculture. Processes 2023, 11, 2306. https://doi.org/10.3390/pr11082306
Tucaliuc RA, Mangalagiu V, Mangalagiu II. Pyridazinic Bioisosteres with Potential Applications in Medicinal Chemistry and Agriculture. Processes. 2023; 11(8):2306. https://doi.org/10.3390/pr11082306
Chicago/Turabian StyleTucaliuc, Roxana Angela, Violeta Mangalagiu, and Ionel I. Mangalagiu. 2023. "Pyridazinic Bioisosteres with Potential Applications in Medicinal Chemistry and Agriculture" Processes 11, no. 8: 2306. https://doi.org/10.3390/pr11082306
APA StyleTucaliuc, R. A., Mangalagiu, V., & Mangalagiu, I. I. (2023). Pyridazinic Bioisosteres with Potential Applications in Medicinal Chemistry and Agriculture. Processes, 11(8), 2306. https://doi.org/10.3390/pr11082306







